Cellular Immunotherapy: Using Alloreactivity to Induce Anti-Leukemic Responses without Prolonged Persistence of Donor Cells
Abstract
:1. Introduction
2. Cellular Therapy; Clinical Experiences
3. Cellular Therapy; Mechanism of Action
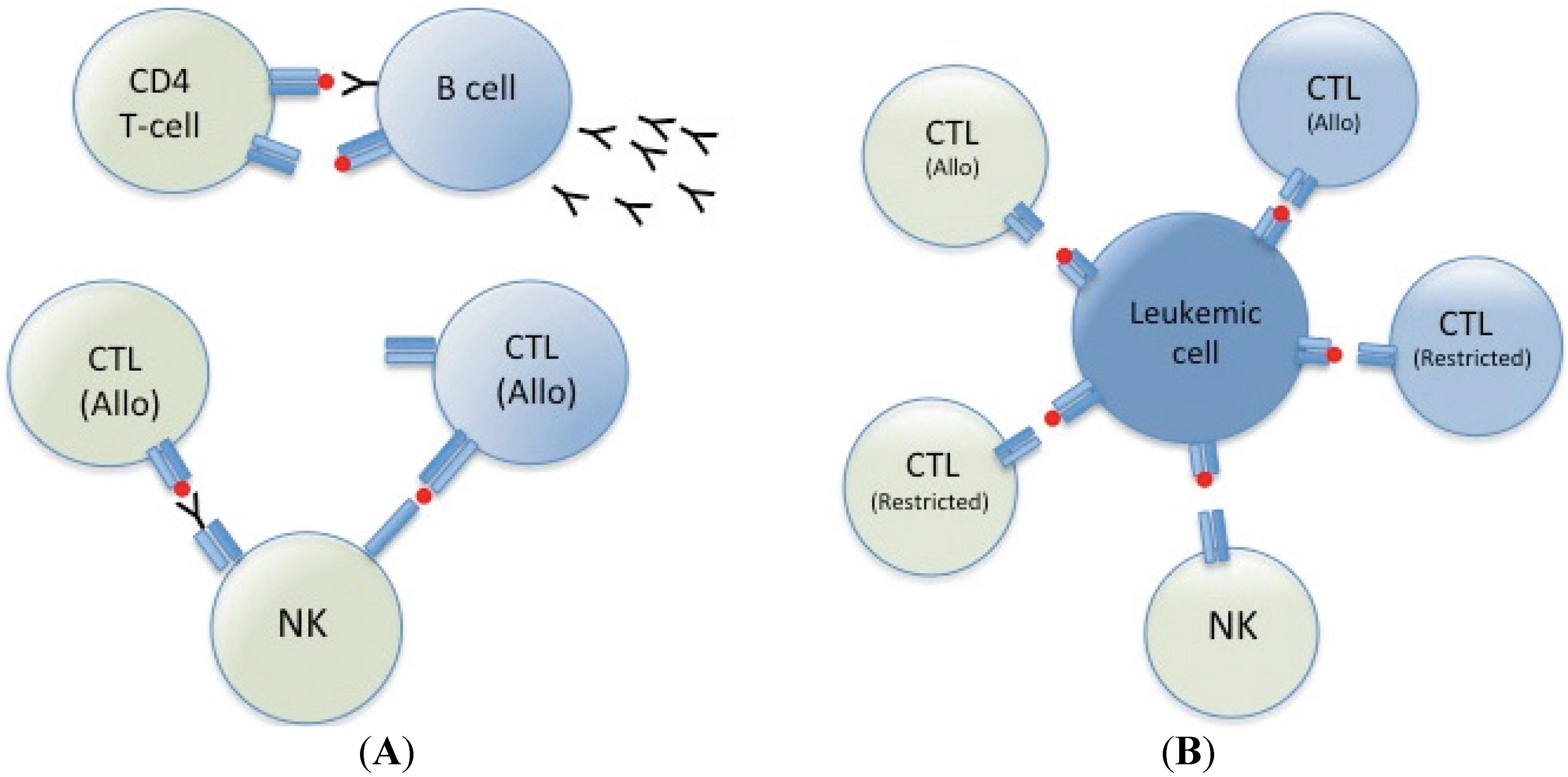
4. Conclusions
Conflicts of Interest
References
- D’Orsogna, L.J.; Nguyen, T.H.; Claas, F.H.; Witt, C.; Mifsud, N.A. Endogenous-peptide-dependent alloreactivity: New scientific insights and clinical implications. Tissue Antigens 2013, 81, 399–407. [Google Scholar] [CrossRef]
- Blazar, B.R.; Murphy, W.J.; Abedi, M. Advances in graft-versus-host disease biology and therapy. Nat. Rev. Immunol. 2012, 12, 443–458. [Google Scholar] [CrossRef]
- Weiden, P.L.; Flournoy, N.; Thomas, E.D.; Prentice, R.; Fefer, A.; Buckner, C.D.; Storb, R. Antileukemic effect of graft-versus-host disease in human recipients of allogeneic-marrow grafts. N. Engl. J. Med. 1979, 300, 1068–1073. [Google Scholar] [CrossRef]
- Kolb, H.J. Graft-versus-leukemia effects of transplantation and donor lymphocytes. Blood 2008, 112, 4371–4383. [Google Scholar] [CrossRef]
- Bar, M.; Sandmaier, B.M.; Inamoto, Y.; Bruno, B.; Hari, P.; Chauncey, T.; Martin, P.J.; Storb, R.; Maloney, D.G.; Storer, B.; et al. Donor lymphocyte infusion for relapsed hematological malignancies after allogeneic hematopoietic cell transplantation: Prognostic relevance of the initial CD3+ T cell dose. Biol. Blood Marrow Transplant. 2013, 19, 949–957. [Google Scholar] [CrossRef]
- Dey, B.R.; McAfee, S.; Colby, C.; Cieply, K.; Caron, M.; Saidman, S.; Preffer, F.; Shaffer, J.; Tarbell, N.; Sackstein, R.; et al. Anti-tumour response despite loss of donor chimaerism in patients treated with non-myeloablative conditioning and allogeneic stem cell transplantation. Br. J. Haematol. 2005, 128, 351–359. [Google Scholar] [CrossRef]
- Rubio, M.T.; Kim, Y.M.; Sachs, T.; Mapara, M.; Zhao, G.; Sykes, M. Antitumor effect of donor marrow graft rejection induced by recipient leukocyte infusion in mixed chimeras prepared with nonmyeloablative conditioning: Critical role for recipient-derived IFN-gamma. Blood 2003, 102, 2300–2307. [Google Scholar] [CrossRef]
- Rubio, M.T.; Saito, T.I.; Kattleman, K.; Zhao, G.; Buchli, J.; Sykes, M. Mechanisms of the antitumor responses and host-versus-graft reactions induced by recipient leukocyte infusions in mixed chimeras prepared with nonmyeloablative conditioning: A critical role for recipient CD4+ T cells and recipient leukocyte infusion-derived IFN-gamma-producing CD8+ T cells. J. Immunol. 2005, 175, 665–676. [Google Scholar]
- Saito, T.I.; Li, H.W.; Sykes, M. Invariant NKT cells are required for antitumor responses induced by host-versus-graft responses. J. Immunol. 2010, 185, 2099–2105. [Google Scholar] [CrossRef]
- Saito, T.I.; Rubio, M.T.; Sykes, M. Clinical relevance of recipient leukocyte infusion as antitumor therapy following nonmyeloablative allogeneic hematopoietic cell transplantation. Exp. Hematol. 2006, 34, 1271–1277. [Google Scholar]
- Symons, H.J.; Levy, M.Y.; Wang, J.; Zhou, X.; Zhou, G.; Cohen, S.E.; Luznik, L.; Levitsky, H.I.; Fuchs, E.J. The allogeneic effect revisited: Exogenous help for endogenous, tumor-specific T cells. Biol. Blood Marrow Transplant. 2008, 14, 499–509. [Google Scholar] [CrossRef]
- Colvin, G.A.; Berz, D.; Ramanathan, M.; Winer, E.S.; Fast, L.; Elfenbein, G.J.; Quesenberry, P.J. Nonengraftment haploidentical cellular immunotherapy for refractory malignancies: Tumor responses without chimerism. Biol. Blood Marrow Transplant. 2009, 15, 421–431. [Google Scholar] [CrossRef]
- Guo, M.; Hu, K.X.; Yu, C.L.; Sun, Q.Y.; Qiao, J.H.; Wang, D.H.; Liu, G.X.; Sun, W.J.; Wei, L.; Sun, X.D.; et al. Infusion of HLA-mismatched peripheral blood stem cells improves the outcome of chemotherapy for acute myeloid leukemia in elderly patients. Blood 2011, 117, 936–941. [Google Scholar] [CrossRef]
- Guo, M.; Hu, K.X.; Liu, G.X.; Yu, C.L.; Qiao, J.H.; Sun, Q.Y.; Qiao, J.X.; Dong, Z.; Sun, W.J.; Sun, X.D.; et al. HLA-mismatched stem-cell microtransplantation as postremission therapy for acute myeloid leukemia: Long-term follow-up. J. Clin. Oncol. 2012, 30, 4084–4090. [Google Scholar] [CrossRef]
- Byrd, J.C.; Mrozek, K.; Dodge, R.K.; Carroll, A.J.; Edwards, C.G.; Arthur, D.C.; Pettenati, M.J.; Patil, S.R.; Rao, K.W.; Watson, M.S.; et al. retreatment cytogenetic abnormalities are predictive of induction success, cumulative incidence of relapse, and overall survival in adult patients with de novo acute myeloid leukemia: Results from Cancer and Leukemia Group B (CALGB 8461). Blood 2002, 100, 4325–4336. [Google Scholar] [CrossRef]
- Fast, L.D. Recipient CD8+ cells are responsible for the rapid elimination of allogeneic donor lymphoid cells. J. Immunol. 1996, 157, 4805–4810. [Google Scholar]
- Fast, L.D. Recipient elimination of allogeneic lymphoid cells: Donor CD4(+) cells are effective alloantigen-presenting cells. Blood 2000, 96, 1144–1149. [Google Scholar]
- D’Orsogna, L.J.; van den Heuvel, H.; van der Meer-Prins, E.M.; Roelen, D.L.; Doxiadis, II; Claas, F.H. Stimulation of human EBV- and CMV-specific cytolytic effector function using allogeneic HLA molecules. J. Immunol. 2012, 189, 4825–4831. [Google Scholar] [CrossRef]
- Brehm, M.A.; Daniels, K.A.; Priyadharshini, B.; Thornley, T.B.; Greiner, D.L.; Rossini, A.A.; Welsh, R.M. Allografts stimulate cross-reactive virus-specific memory CD8 T cells with private specificity. Am. J. Transplant. 2010, 10, 1738–1748. [Google Scholar] [CrossRef]
- Morris, G.P.; Allen, P.M. Cutting edge: Highly alloreactive dual TCR T cells play a dominant role in graft-versus-host disease. J. Immunol. 2009, 182, 6639–6643. [Google Scholar] [CrossRef]
- Morris, G.P.; Uy, G.L.; Donermeyer, D.; Dipersio, J.F.; Allen, P.M. Dual receptor T cells mediate pathologic alloreactivity in patients with acute graft-versus-host disease. Sci. Transl. Med. 2013, 5, 188ra174. [Google Scholar]
- Lask, A.; Goichberg, P.; Cohen, A.; Goren-Arbel, R.; Milstein, O.; Aviner, S.; Feine, I.; Ophir, E.; Reich-Zeliger, S.; Hagin, D.; et al. TCR-independent killing of B cell malignancies by anti-third-party CTLs: The critical role of MHC-CD8 engagement. J. Immunol. 2011, 187, 2006–2014. [Google Scholar] [CrossRef]
- Lask, A.; Ophir, E.; Or-Geva, N.; Cohen-Fredarow, A.; Afik, R.; Eidelstein, Y.; Reich-Zeliger, S.; Nathansohn, B.; Edinger, M.; Negrin, R.S.; et al. A new approach for eradication of residual lymphoma cells by host nonreactive anti-third-party central memory CD8 T cells. Blood 2013, 121, 3033–3040. [Google Scholar] [CrossRef]
- Tietze, J.K.; Wilkins, D.E.; Sckisel, G.D.; Bouchlaka, M.N.; Alderson, K.L.; Weiss, J.M.; Ames, E.; Bruhn, K.W.; Craft, N.; Wiltrout, R.H.; et al. Delineation of antigen-specific and antigen-nonspecific CD8(+) memory T-cell responses after cytokine-based cancer immunotherapy. Blood 2012, 119, 3073–3083. [Google Scholar] [CrossRef]
- Franciszkiewicz, K.; Le Floc’h, A.; Boutet, M.; Vergnon, I.; Schmitt, A.; Mami-Chouaib, F. CD103 or LFA-1 engagement at the immune synapse between cytotoxic T cells and tumor cells promotes maturation and regulates T-cell effector functions. Cancer Res. 2013, 73, 617–628. [Google Scholar] [CrossRef]
- Ruggeri, L.; Capanni, M.; Urbani, E.; Perruccio, K.; Shlomchik, W.D.; Tosti, A.; Posati, S.; Rogaia, D.; Frassoni, F.; Aversa, F.; et al. Effectiveness of donor natural killer cell alloreactivity in mismatched hematopoietic transplants. Science 2002, 295, 2097–2100. [Google Scholar] [CrossRef]
- Cooley, S.; Weisdorf, D.J.; Guethlein, L.A.; Klein, J.P.; Wang, T.; Le, C.T.; Marsh, S.G.; Geraghty, D.; Spellman, S.; Haagenson, M.D.; et al. Donor selection for natural killer cell receptor genes leads to superior survival after unrelated transplantation for acute myelogenous leukemia. Blood 2010, 116, 2411–2419. [Google Scholar] [CrossRef]
- Sivori, S.; Carlomagno, S.; Falco, M.; Romeo, E.; Moretta, L.; Moretta, A. Natural killer cells expressing the KIR2DS1-activating receptor efficiently kill T-cell blasts and dendritic cells: Implications in haploidentical HSCT. Blood 2011, 117, 4284–4292. [Google Scholar]
- Waggoner, S.N.; Cornberg, M.; Selin, L.K.; Welsh, R.M. Natural killer cells act as rheostats modulating antiviral T cells. Nature 2012, 481, 394–398. [Google Scholar]
- Cook, K.D.; Whitmire, J.K. The depletion of NK cells prevents T cell exhaustion to efficiently control disseminating virus infection. J. Immunol. 2013, 190, 641–649. [Google Scholar] [CrossRef]
- Rabinovich, B.A.; Li, J.; Shannon, J.; Hurren, R.; Chalupny, J.; Cosman, D.; Miller, R.G. Activated, but not resting, T cells can be recognized and killed by syngeneic NK cells. J. Immunol. 2003, 170, 3572–3576. [Google Scholar]
- Olson, J.A.; Leveson-Gower, D.B.; Gill, S.; Baker, J.; Beilhack, A.; Negrin, R.S. NK cells mediate reduction of GVHD by inhibiting activated, alloreactive T cells while retaining GVT effects. Blood 2010, 115, 4293–4301. [Google Scholar] [CrossRef]
- Cobbold, M.; De La Pena, H.; Norris, A.; Polefrone, J.M.; Qian, J.; English, A.M.; Cummings, K.L.; Penny, S.; Turner, J.E.; Cottine, J.; et al. MHC class I-associated phosphopeptides are the targets of memory-like immunity in leukemia. Sci. Transl. Med. 2013, 5, 203ra125. [Google Scholar] [CrossRef]
- Katz, D.H.; Davie, J.M.; Paul, W.E.; Benacerraf, B. Carrier function in anti-hapten antibody responses. IV. Experimental conditions for the induction of hapten-specific tolerance or for the stimulation of anti-hapten anamnestic responses by “nonimmunogenic” hapten-polypeptide conjugates. J. Exp. Med. 1971, 134, 201–223. [Google Scholar] [CrossRef]
- Pardoll, D.M. The blockade of immune checkpoints in cancer immunotherapy. Nat. Rev. Cancer 2012, 12, 252–264. [Google Scholar] [CrossRef]
- Dolen, Y.; Esendagli, G. Myeloid leukemia cells with a B7-2(+) subpopulation provoke Th-cell responses and become immuno-suppressive through the modulation of B7 ligands. Eur. J. Immunol. 2013, 43, 747–757. [Google Scholar] [CrossRef]
- Corm, S.; Berthon, C.; Imbenotte, M.; Biggio, V.; Lhermitte, M.; Dupont, C.; Briche, I.; Quesnel, B. Indoleamine 2,3-dioxygenase activity of acute myeloid leukemia cells can be measured from patients’ sera by HPLC and is inducible by IFN-gamma. Leuk. Res. 2009, 33, 490–494. [Google Scholar] [CrossRef]
- Curti, A.; Aluigi, M.; Pandolfi, S.; Ferri, E.; Isidori, A.; Salvestrini, V.; Durelli, I.; Horenstein, A.L.; Fiore, F.; Massaia, M.; et al. Acute myeloid leukemia cells constitutively express the immunoregulatory enzyme indoleamine 2,3-dioxygenase. Leukemia 2007, 21, 353–355. [Google Scholar] [CrossRef]
- Fritsch, K.; Finke, J.; Grullich, C. Suppression of granzyme B activity and caspase-3 activation in leukaemia cells constitutively expressing the protease inhibitor 9. Ann. Hematol. 2013, 92, 1603–1609. [Google Scholar] [CrossRef]
- Zhang, L.; Chen, X.; Liu, X.; Kline, D.E.; Teague, R.M.; Gajewski, T.F.; Kline, J. CD40 ligation reverses T cell tolerance in acute myeloid leukemia. J. Clin. Invest. 2013, 123, 1999–2010. [Google Scholar] [CrossRef]
- Matsushita, H.; Vesely, M.D.; Koboldt, D.C.; Rickert, C.G.; Uppaluri, R.; Magrini, V.J.; Arthur, C.D.; White, J.M.; Chen, Y.S.; Shea, L.K.; et al. Cancer exome analysis reveals a T-cell-dependent mechanism of cancer immunoediting. Nature 2012, 482, 400–404. [Google Scholar] [CrossRef]
- Vago, L.; Perna, S.K.; Zanussi, M.; Mazzi, B.; Barlassina, C.; Stanghellini, M.T.; Perrelli, N.F.; Cosentino, C.; Torri, F.; Angius, A.; et al. Loss of mismatched HLA in leukemia after stem-cell transplantation. N. Engl. J. Med. 2009, 361, 478–488. [Google Scholar] [CrossRef]
- Almand, B.; Clark, J.I.; Nikitina, E.; van Beynen, J.; English, N.R.; Knight, S.C.; Carbone, D.P.; Gabrilovich, D.I. Increased production of immature myeloid cells in cancer patients: a mechanism of immunosuppression in cancer. J. Immunol. 2001, 166, 678–689. [Google Scholar]
- Shenghui, Z.; Yixiang, H.; Jianbo, W.; Kang, Y.; Laixi, B.; Yan, Z.; Xi, X. Elevated frequencies of CD4(+) CD25(+) CD127lo regulatory T cells is associated to poor prognosis in patients with acute myeloid leukemia. Int. J. Cancer. 2011, 129, 1373–1381. [Google Scholar] [CrossRef]
- Szczepanski, M.J.; Szajnik, M.; Czystowska, M.; Mandapathil, M.; Strauss, L.; Welsh, A.; Foon, K.A.; Whiteside, T.L.; Boyiadzis, M. Increased frequency and suppression by regulatory T cells in patients with acute myelogenous leukemia. Clin. Cancer Res. 2009, 15, 3325–3332. [Google Scholar] [CrossRef]
- Wang, X.; Zheng, J.; Liu, J.; Yao, J.; He, Y.; Li, X.; Yu, J.; Yang, J.; Liu, Z.; Huang, S. Increased population of CD4(+)CD25(high), regulatory T cells with their higher apoptotic and proliferating status in peripheral blood of acute myeloid leukemia patients. Eur. J. Haematol. 2005, 75, 468–476. [Google Scholar] [CrossRef]
- Grupp, S.A.; Kalos, M.; Barrett, D.; Aplenc, R.; Porter, D.L.; Rheingold, S.R.; Teachey, D.T.; Chew, A.; Hauck, B.; Wright, J.F.; et al. Chimeric antigen receptor-modified T cells for acute lymphoid leukemia. N. Engl. J. Med. 2013, 368, 1509–1518. [Google Scholar] [CrossRef]
- Teachey, D.T.; Rheingold, S.R.; Maude, S.L.; Zugmaier, G.; Barrett, D.M.; Seif, A.E.; Nichols, K.E.; Suppa, E.K.; Kalos, M.; Berg, R.A.; et al. Cytokine release syndrome after blinatumomab treatment related to abnormal macrophage activation and ameliorated with cytokine-directed therapy. Blood 2013, 121, 5154–5157. [Google Scholar] [CrossRef]
- Sliwkowski, M.X.; Mellman, I. Antibody therapeutics in cancer. Science 2013, 341, 1192–1198. [Google Scholar] [CrossRef]
- Houot, R.; Kohrt, H.; Levy, R. Boosting antibody-dependant cellular cytotoxicity against tumor cells with a CD137 stimulatory antibody. Oncoimmunology 2012, 1, 957–958. [Google Scholar] [CrossRef]
- Kohrt, H.E.; Houot, R.; Weiskopf, K.; Goldstein, M.J.; Scheeren, F.; Czerwinski, D.; Colevas, A.D.; Weng, W.K.; Clarke, M.F.; Carlson, R.W.; et al. Stimulation of natural killer cells with a CD137-specific antibody enhances trastuzumab efficacy in xenotransplant models of breast cancer. J. Clin. Invest. 2012, 122, 1066–1075. [Google Scholar] [CrossRef] [Green Version]
- Marabelle, A.; Kohrt, H.; Sagiv-Barfi, I.; Ajami, B.; Axtell, R.C.; Zhou, G.; Rajapaksa, R.; Green, M.R.; Torchia, J.; Brody, J.; et al. Depleting tumor-specific Tregs at a single site eradicates disseminated tumors. J. Clin. Invest. 2013, 123, 2447–2463. [Google Scholar] [CrossRef]
- Rutten, C.E.; van Luxemburg-Heijs, S.A.; Halkes, C.J.; van Bergen, C.A.; Marijt, E.W.; Oudshoorn, M.; Griffioen, M.; Falkenburg, J.H. Patient HLA-DP-specific CD4+ T cells from HLA-DPB1-mismatched donor lymphocyte infusion can induce graft-versus-leukemia reactivity in the presence or absence of graft-versus-host disease. Biol. Blood Marrow Transplant. 2013, 19, 40–48. [Google Scholar] [CrossRef]
- Stevanovic, S.; van Bergen, C.A.; van Luxemburg-Heijs, S.A.; van der Zouwen, B.; Jordanova, E.S.; Kruisselbrink, A.B.; van de Meent, M.; Harskamp, J.C.; Claas, F.H.; Marijt, E.W.; et al. HLA class II upregulation during viral infection leads to HLA-DP-directed graft-versus-host disease after CD4+ donor lymphocyte infusion. Blood 2013, 122, 1963–1973. [Google Scholar] [CrossRef]
- Jones-Mason, M.E.; Zhao, X.; Kappes, D.; Lasorella, A.; Iavarone, A.; Zhuang, Y. E protein transcription factors are required for the development of CD4(+) lineage T cells. Immunity 2012, 36, 348–361. [Google Scholar] [CrossRef]
- Rui, J.; Liu, H.; Zhu, X.; Cui, Y.; Liu, X. Epigenetic silencing of CD8 genes by ThPOK-mediated deacetylation during CD4 T cell differentiation. J. Immunol. 2012, 189, 1380–1390. [Google Scholar] [CrossRef]
- Mucida, D.; Husain, M.M.; Muroi, S.; van Wijk, F.; Shinnakasu, R.; Naoe, Y.; Reis, B.S.; Huang, Y.; Lambolez, F.; Docherty, M.; et al. Transcriptional reprogramming of mature CD4(+) helper T cells generates distinct MHC class II-restricted cytotoxic T lymphocytes. Nat. Immunol. 2013, 14, 281–289. [Google Scholar] [CrossRef]
- Hua, L.; Yao, S.; Pham, D.; Jiang, L.; Wright, J.; Sawant, D.; Dent, A.L.; Braciale, T.J.; Kaplan, M.H.; Sun, J. Cytokine-Dependent Induction of CD4+ T cells with Cytotoxic Potential during Influenza Virus Infection. J. Virol. 2013, 87, 11884–11893. [Google Scholar] [CrossRef]
- Reagan, J.L.; Fast, L.D.; Safran, H.; Nevola, M.; Winer, E.S.; Castillo, J.J.; Butera, J.N.; Quesenberry, M.I.; Young, C.T.; Quesenberry, P.J. Cellular immunotherapy for refractory hematological malignancies. J. Transl. Med. 2013, 11, 150. [Google Scholar] [CrossRef]
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Fast, L.D.; Reagan, J.; Quesenberry, P. Cellular Immunotherapy: Using Alloreactivity to Induce Anti-Leukemic Responses without Prolonged Persistence of Donor Cells. Med. Sci. 2013, 1, 37-48. https://doi.org/10.3390/medsci1010037
Fast LD, Reagan J, Quesenberry P. Cellular Immunotherapy: Using Alloreactivity to Induce Anti-Leukemic Responses without Prolonged Persistence of Donor Cells. Medical Sciences. 2013; 1(1):37-48. https://doi.org/10.3390/medsci1010037
Chicago/Turabian StyleFast, Loren D., John Reagan, and Peter Quesenberry. 2013. "Cellular Immunotherapy: Using Alloreactivity to Induce Anti-Leukemic Responses without Prolonged Persistence of Donor Cells" Medical Sciences 1, no. 1: 37-48. https://doi.org/10.3390/medsci1010037



