Microbiological Study of Yamal Lakes: A Key to Understanding the Evolution of Gas Emission Craters
Abstract
1. Introduction
2. Study Area
3. Materials and Methods
3.1. Sample Collection and Characterization
3.2. Analytical Techniques
3.3. Isotopic Composition of Carbon Compounds
3.4. Radiotracer Experiments
3.5. Cell Counts
3.6. DNA Extraction and Sequencing and Read-Centric Analysis
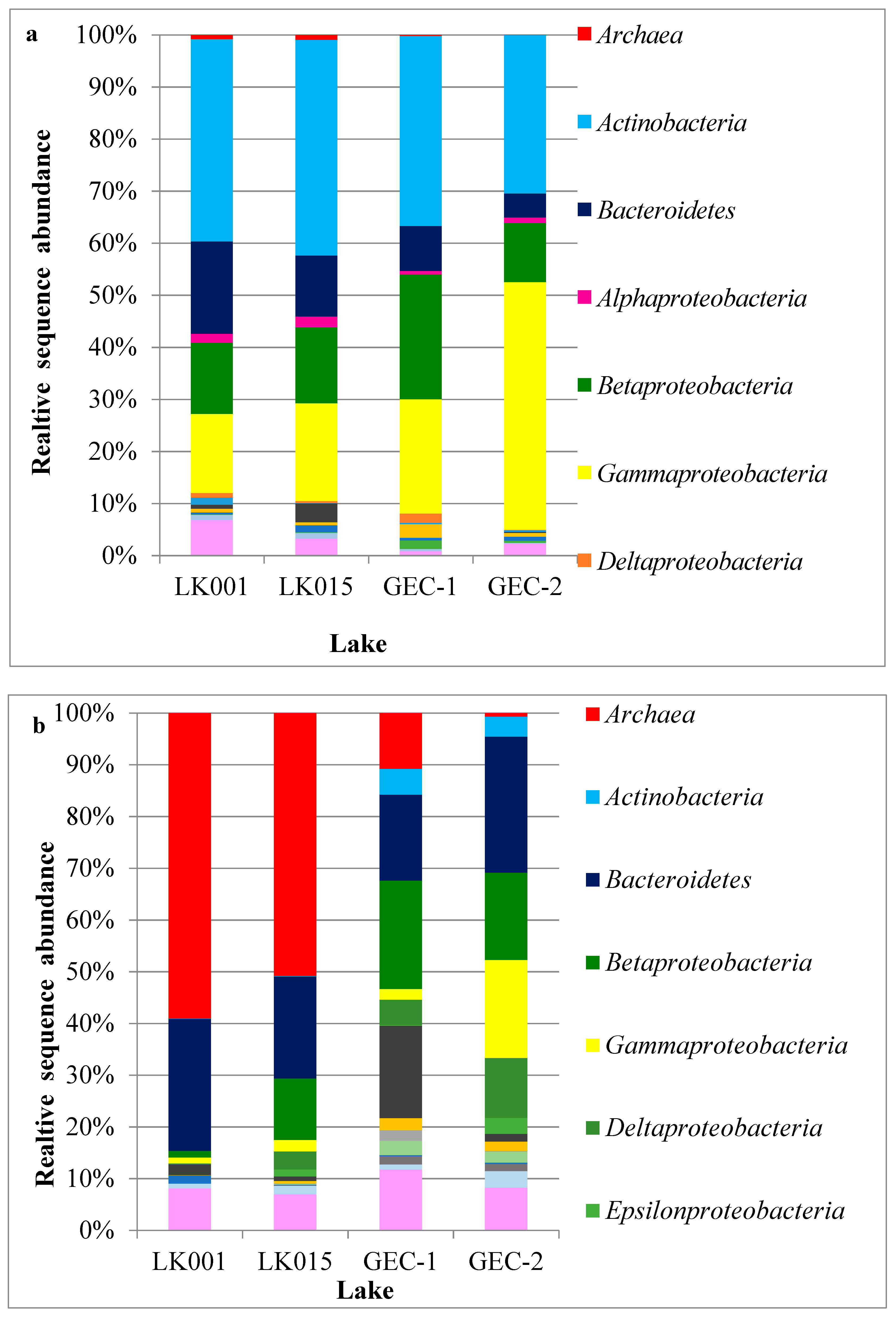
4. Results
5. Discussion
6. Conclusions
- Yamal lakes share similarities with the tundra and boreal lakes located in other areas of the permafrost zone. Slow-flowing microbial processes are characteristic of both types of lakes (GEC and background), which is expected for the cold climate of the studied area. The bacterial communities of both types of studied lakes were dominated by the taxa typically found in thermokarst lakes and other permafrost-affected habitats. Both types of studied lakes were inhabited by aerobic methanotrophs of the genus Methylobacter: the most frequently detected methanotrophs in various freshwater environments, including boreal thermokarst and non-thermokarst lakes. The nitrate-dependent ANME 2d detected in both GEC and background lakes were also found previously in other thermokarst lakes. Methane concentrations in the sediments of background lakes were within the wide range of concentrations known for mesotrophic and dystrophic lakes of the boreal zone, and representatives of methanogenic archaea in background lakes were similar to those found in other boreal basins.
- At the same time, the GEC lakes essentially differed from the background ones by low rates of anaerobic processes (methanogenesis and sulfate reduction), a reliably lower methane concentration, and low diversity and abundance of methanogenic archaea in the sediments. Archaea in the GEC lakes were probably allochthonous microbiota from the surface and active-layer runoff. Thus, GEC lakes could be distinguished from other exogenous lakes based on their weak methanogenic population and activity.
- It can be gingerly assumed that the very slow rates of anaerobic microbial processes indicate a transformation of the newly formed water bodies (i.e., GEC lakes) into real lakes. This may relate not only to GEC lakes, but also to newly formed thermokarst lakes.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bogoyavlenskiy, V.I.; Bogoyavlenskiy, I.V.; Nikonov, R.A. Results of aerial, space and field investigations of large gas blowouts near Bovanenkovo field on Yamal Peninsula. Arct. Ecol. Econ. 2017, 3, 4–17. (In Russian) [Google Scholar] [CrossRef]
- Leibman, M.O.; Dvornikov, Y.A.; Khomutov, A.V.; Babkin, E.M.; Babkina, E.A.; Vanshtein, B.G.; Kizyakov, A.I.; Oblogov, G.E.; Semenov, P.B.; Streletskaya, I.D. Hydro-chemical features of water in lakes and gas-emission craters embedded in the marine deposits of West-Siberian north. In Proceedings of the XXII International Conference on Marine Geology, Moscow, Russia, 20–24 November 2017. (In Russian). [Google Scholar]
- Leibman, M.O.; Kizyakov, A.I.; Plehanov, A.V.; Streletskaya, I.D. New permafrost feature: deep crater in Central Yamal, West Siberia, Russia as a response to local climate fluctuations. Geogr. Environ. Sustain. 2014, 7, 68–80. [Google Scholar] [CrossRef]
- Kizyakov, A.I.; Sonyushkin, A.; Leibman, M.O.; Zimin, M.V.; Khomutov, A.V. Geomorphological conditions of the gas-emission crater and its dynamics in Central Yamal. Earth Cryosphere 2015, 2, 15–25. [Google Scholar]
- Olenchenko, V.V.; Sinitsky, A.I.; Antonov, E.Y.; Eltsov, I.N.; Kushnarenko, O.N.; Plotnikov, A.E.; Potapov, V.V.; Epov, M.I. Results of geophysical researches of the area of new geological formation “Yamal crater”. Kriosf. Zemli 2015, 4, 94–106. [Google Scholar]
- Khilimonyuk, V.Z.; Ospennikov, E.N.; Buldovich, S.N.; Gunar, A.Y.; Gorshkov, E.I. Geocryological conditions of Yamal crater location. In Proceedings of the Fifth Conference of Russian Geocryologists, Moscow, Russia, 14–17 June 2016; University Book: Moscow, Russia, 2016; Volume 2, pp. 245–255. (In Russian). [Google Scholar]
- Sizov, O.V. Remote Sensing Analysis of the Consequences of Surface Gas Emissions in the North of Western Siberia. Geomatics 2015, 1, 53–68. (In Rusian) [Google Scholar]
- Kizyakov, A.; Zimin, M.; Sonyushkin, A.; Dvornikov, Y.; Khomutov, A.; Leibman, M. Comparison of Gas Emission Crater Geomorphodynamics on Yamal and Gydan Peninsulas (Russia), Based on Repeat Very-High-Resolution Stereopairs. Remote Sens. 2017, 9, 1023. [Google Scholar] [CrossRef]
- Kizyakov, A.; Khomutov, A.; Zimin, M.; Khairullin, R.; Babkina, E.; Dvornikov, Y.; Leibman, M. Microrelief associated with gas emission craters: Remote-sensing and field-based study. Remote Sens. 2018, 10, 677. [Google Scholar] [CrossRef]
- Bogoyavlensky, V.I.; Bogoyavlensky, I.V.; Nikonov, R.A.; Sizov, O.S. Remote identification of areas of surface gas and gas emissions in the Arctic: Yamal Peninsula. Arct. Ecol. Econ. 2016, 3, 4–15. (In Russian) [Google Scholar]
- Bogoyavlenskij, V.I.; Garagash, I.A. Substantiation of the Gas Emission Craters Formation Process in the Arctic by Mathematical Modeling. Arct. Ecol. Econ. 2015, 3, 12–17. (In Russian) [Google Scholar]
- Dvornikov, Y.A.; Leibman, M.O.; Heim, B.; Khomutov, A.V.; Roessler, S.; Gubarkov, A.A. Thermodenudation on Yamal peninsula as a source of the dissolved organic matter increase in thaw lakes. Earth Cryosphere 2017, 21, 28–37. [Google Scholar]
- Streletskaya, I.D.; Leibman, M.O.; Kizyakov, A.I.; Oblogov, G.E.; Vasiliev, A.A.; Khomutov, A.V.; Dvornikov, Y.A. Ground ice and its role in the formation of the gas-emission crater in the Yamal peninsula. Geography 2017, 2, 91–99. [Google Scholar]
- Buldovicz, S.N.; Khilimonyuk, V.Z.; Bychkov, A.Y.; Ospennikov, E.N.; Vorobyev, S.A.; Gunar, A.Y.; Gorshkov, E.I.; Chuvilin, E.M.; Cherbunina, M.Y.; Kotov, P.I.; et al. Cryovolcanism on the Earth: Origin of a spectacular crater in the Yamal peninsula (Russia). Sci. Rep. 2018, 8, 13534. [Google Scholar] [CrossRef]
- Kraev, G.N.; Schultze, E.D.; Rivkina, E.M. Cryogenesis as a factor of methane distribution in layers of permafrost. Dokl. Earth Sci. 2013, 451, 882–885. [Google Scholar] [CrossRef]
- Sassen, R.; MacDonald, I.R. Hydrocarbons of experimental and natural gas hydrates, Gulf of Mexico continental slope. Org. Geochem. 1997, 26, 289–293. [Google Scholar] [CrossRef]
- Hamdan, L.J.; Gillevet, P.M.; Pohlman, J.P.; Sikaroodi, M.; Greinert, J.; Coffin, R.B. Diversity and biogeochemical structuring ofbacterial communities across the Porangahau ridge accretionary prism, New Zealand. FEMS Microbiol. Ecol. 2011, 77, 518–532. [Google Scholar] [CrossRef]
- Heuer, V.B.; Pohlman, J.W.; Torres, M.E.; Elvert, M.; Hinrichs, K.-U. The stable isotope biogeochemistry of acetate and other dissolved carbon species in deep subseafloor sediments at the northern Cascadia Margin. Geochem. Cosmochim. Acta 2009, 73, 3323–3336. [Google Scholar] [CrossRef]
- McAuliffe, C. Gas chromatographic determination of solutes by multiple phase equilibrium. Chem. Technol. 1971, 1, 46–51. [Google Scholar]
- Pimenov, N.V.; Bonch-Osmolovskaya, E.A. 2 In Situ Activity Studies in Thermal Environments. In Methods in Microbiology; Rainey, F., Oren, A., Eds.; Academic Press: Cambridge, MA, USA, 2006; pp. 29–53. [Google Scholar]
- Klindworth, A.; Pruesse, E.; Schweer, T.; Peplies, J.; Quastm, C.; Horn, M.; Glockner, F.O. Evaluation of general 16S ribosomal RNA gene PCR primers for classical and next-generation sequencing-based diversity studies. Nucleic Acids Res. 2013, 41, 1. [Google Scholar] [CrossRef]
- Quast, C.; Pruesse, E.; Yilmaz, P.; Gerken, J.; Schweer, T.; Yarza, P.; Peplies, J.; Glöckner, F.O. The SILVA ribosomal RNA gene database project: improved data processing and web-based tools. Opens external link in new window. Nucleic Acids Res. 2013, 41, D590–D596. [Google Scholar] [CrossRef]
- Savvichev, A.; Kokryatskaya, N.; Zabelina, S.; Rusanov, I.; Zakharova, E.; Veslopolova, E.; Lunina, O.; Patutina, E.; Bumazhkin, B.; Gruzdev, D.; et al. Microbial processes of the carbon and sulfur cycles in an ice-covered, iron-rich meromictic lake Svetloe (Arkhangelsk oblast, Russia). Environ. Microbiol. 2017, 19, 659–672. [Google Scholar] [CrossRef]
- Neuenschwander, S.M.; Ghai, R.; Pernthaler, J.; Salcher, M.M. Microdiversification in genome-streamlined ubiquitous freshwater Actinobacteria. ISME 2018, 12, 185–198. [Google Scholar] [CrossRef]
- Wartiainen, I.; Hestnes, A.G.; McDonald, I.R.; Svenning, M.M. Methylobacter tundripaludum sp. nov., a methane-oxidizing bacterium from Arctic wetland soil on the Svalbard islands, Norway (78° N). Int. J. Syst. Evol. Microbiol. 2006, 56, 109–113. [Google Scholar] [CrossRef]
- Juutinen, S.; Rantakari, M.; Kortelainen, P.; Huttunen, J.T.; Larmola, T.; Alm, J.; Silvola, J.; Martikainen, P.J. Methane dynamics in different boreal lake types. Biogeosciences 2009, 6, 209–223. [Google Scholar] [CrossRef]
- Crump, B.C.; Amaral-Zettler, L.A.; Kling, G.W. Microbial diversity in arctic freshwaters is structured by inoculation of microbes from soils. ISME J. 2012, 6, 1629–1639. [Google Scholar] [CrossRef]
- Vonk, J.E.; Tank, S.E.; Bowden, W.B.; Laurion, I.; Vincent, W.F.; Alekseychik, P.; Amyot, M.; Billet, M.F.; Canário, J.; Cory, R.M.; et al. Reviews and syntheses: Effects of permafrost thaw on Arctic aquatic ecosystems. Biogeosciences 2015, 12, 7129–7167. [Google Scholar] [CrossRef]
- Matheus Carnevali, P.B.; Herbold, C.W.; Hand, K.P.; Priscu, J.C.; Murray, A.E. Distinct microbial assemblage structure and archaeal diversity in sediments of Arctic thermokarst lakes differing in methane sources. Front. Microbiol. 2018, 9, 1192. [Google Scholar] [CrossRef]
- Negandhi, K.; Laurion, I.; Whiticar, M.J.; Galand, P.E.; Xu, X.; Lovejoy, C. Small thaw ponds: An unaccounted source of methane in the Canadian High Arctic. PLoS ONE 2013, 8, e78204. [Google Scholar] [CrossRef]
- Wei, S.; Cui, H.; Zhu, Y.; Lu, Z.; Pang, S.; Zhang, S.; Dong, H.; Su, X. Shifts of methanogenic communities in response to permafrost thaw results in rising methane emissions and soil property changes. Extremophiles 2018, 22, 447–459. [Google Scholar] [CrossRef]
- De Jong, A.E.E.; In’t Zandt, M.H.; Meisel, O.H.; Jetten, M.S.M.; Dean, J.F.; Rasigraf, O.; Welte, C.U. Increases in temperature and nutrient availability positively affect methane-cycling microorganisms in Arctic thermokarst lake sediments. Environ. Microbiol. 2018. [Google Scholar] [CrossRef]
- Rivkina, E.; Petrovskaya, L.; Vishnivetskaya, T.; Krivushin, K.; Shmakova, L.; Tutukina, M.; Meyers, A.; Kondrashov, F. Metagenomic analyses of the late Pleistocene permafrost-additional tools for reconstruction of environmental conditions. Biogeosciences 2016, 13, 2207–2219. [Google Scholar] [CrossRef]
- Schoell, M. Genetic characterization of Natural Gases. AAPG Bull. 1983, 67, 2225–2238. [Google Scholar]
- Quay, P.D.; King, S.L.; Landsdown, J.M.; Wilbur, D.O. Isotopic composition of methane released from wetlands: implications for the increase in atmospheric methane. Glob. Biogeochem. Cycles 1988, 2, 385–397. [Google Scholar] [CrossRef]
- Singleton, C.M.; McCalley, C.K.; Woodcroft, B.J.; Boyd, J.A.; Evans, P.N.; Hodgkins, S.B.; Chanton, J.P.; Frolking, S.; Crill, P.M.; Saleska, S.R.; et al. Methanotrophy across a natural permafrost thaw environment. ISME J. 2018, 12, 2544–2558. [Google Scholar] [CrossRef]
- Borrel, G.; Jézéquel, D.; Biderre-Petit, C.; Morel-Desrosiers, N.; Morel, J.P.; Peyret, P.; Fonty, G.; Lehours, A.C. Production and consumption of methane in freshwater lake ecosystems. Res. Microbiol. 2011, 162, 832–847. [Google Scholar] [CrossRef]
- Kallistova, A.; Kadnikov, V.; Rusanov, I.; Kokryatskaya, N.; Beletsky, A.; Mardanov, A.; Savvichev, A.; Ravin, N.; Pimenov, N. Microbial communities involved in aerobic and anaerobic methane cycling in a meromictic ferruginous subarctic lake. Aquat. Microb. Ecol. 2018, 82, 1–18. [Google Scholar] [CrossRef]
- Martinez-Cruz, K.; Leewis, M.C.; Herriott, I.C.; Sepulveda-Jauregui, A.; Anthony, K.W.; Thalasso, F.; Leigh, M.B. Anaerobic oxidation of methane by aerobic methanotrophs in sub-Arctic lake sediments. Sci. Total Environ. 2017, 607–608, 23–31. [Google Scholar] [CrossRef]
- Crevecoeur, S.; Vincent, W.F.; Comte, J.; Matveev, A.; Lovejoy, C. Diversity and potential activity of methanotrophs in high methane-emitting permafrost thaw ponds. PLoS ONE 2017, 12, e0188223. [Google Scholar] [CrossRef]
- Chistoserdova, L. Methylotrophs in natural habitats: current insights through metagenomics. Appl. Microbiol. Biotechnol. 2015, 99, 5763–5779. [Google Scholar] [CrossRef]
- Pimenov, N.V.; Zakharova, E.E.; Bryukhanov, A.L.; Korneeva, V.A.; Tourova, T.P.; Kuznetsov, B.B.; Pogodaeva, T.V.; Zemskaya, T.I.; Kalmychkov, G.V. Activity and structure of the sulfate-reducing bacterial community in the sediments of the southern part of Lake Baikal. Microbiology 2014, 83, 47–55. [Google Scholar] [CrossRef]
- Karnachuk, O.V.; Frank, Y.A.; Pimenov, N.V.; Yusupov, S.K.; Ivanov, M.V.; Puhakka, Y.A. Distribution, diversity, and activity of sulfate-reducing bacteria in the water column in Gek-Gel lake, Azerbaijan. Microbiology 2006, 75, 82–89. [Google Scholar] [CrossRef]
- Negandhi, K.; Laurion, I.; Lovejoy, C. Temperature effects on net greenhouse gas production and bacterial communities in arctic thaw ponds. FEMS Microbiol. Ecol. 2016, 92, fiw117. [Google Scholar] [CrossRef]
- Negandhi, K.; Laurion, I.; Lovejoy, C. Bacterial communities and greenhouse gas emissions of shallow ponds in the High Arctic. Polar Biol. 2014, 37, 1669–1683. [Google Scholar] [CrossRef]
- Poindexter, J.S. Oligotrophy. In Advances in Microbial Ecology; Alexander, M., Ed.; Springer: Boston, MA, USA, 1981; pp. 63–89. [Google Scholar]
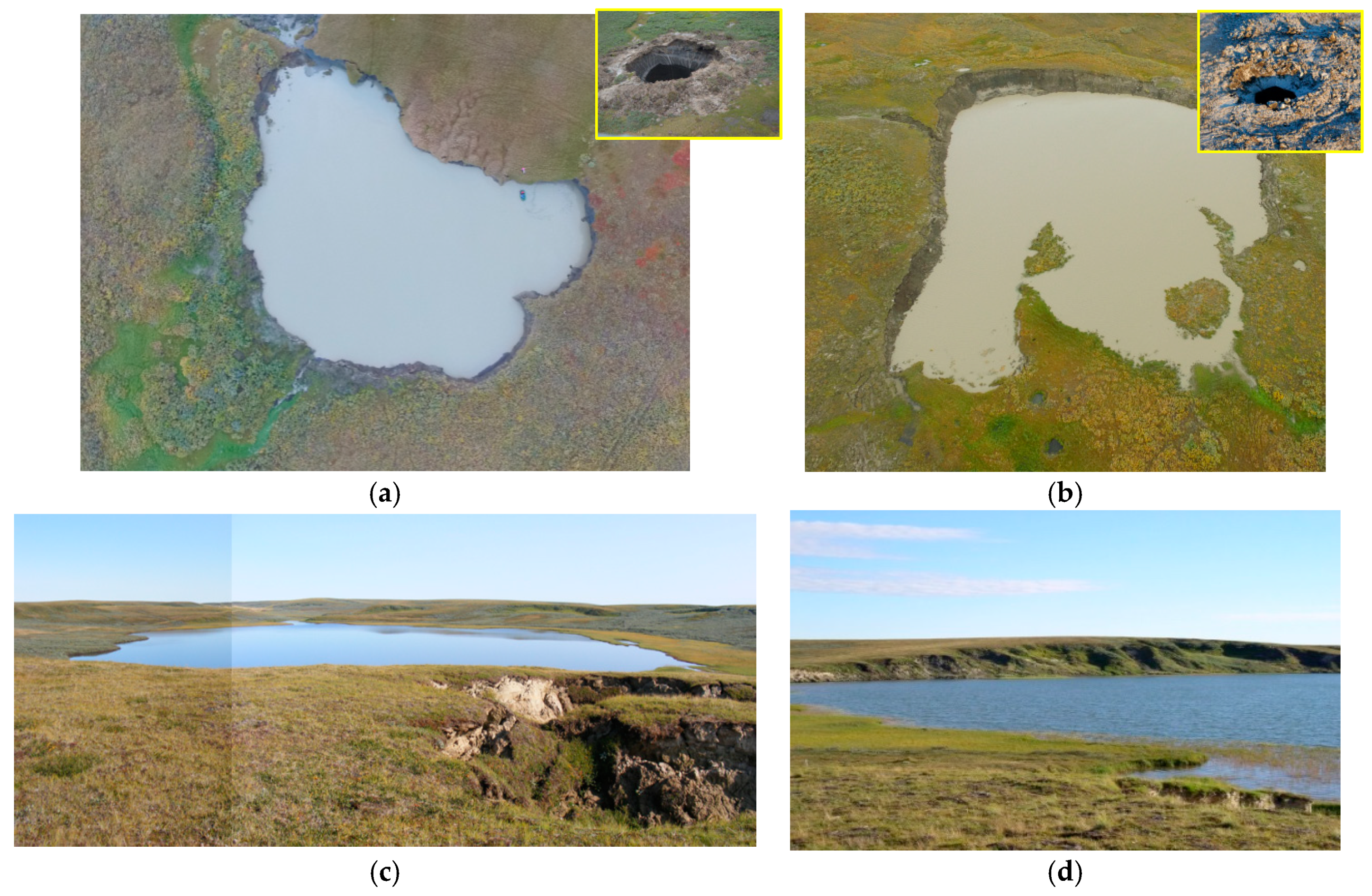

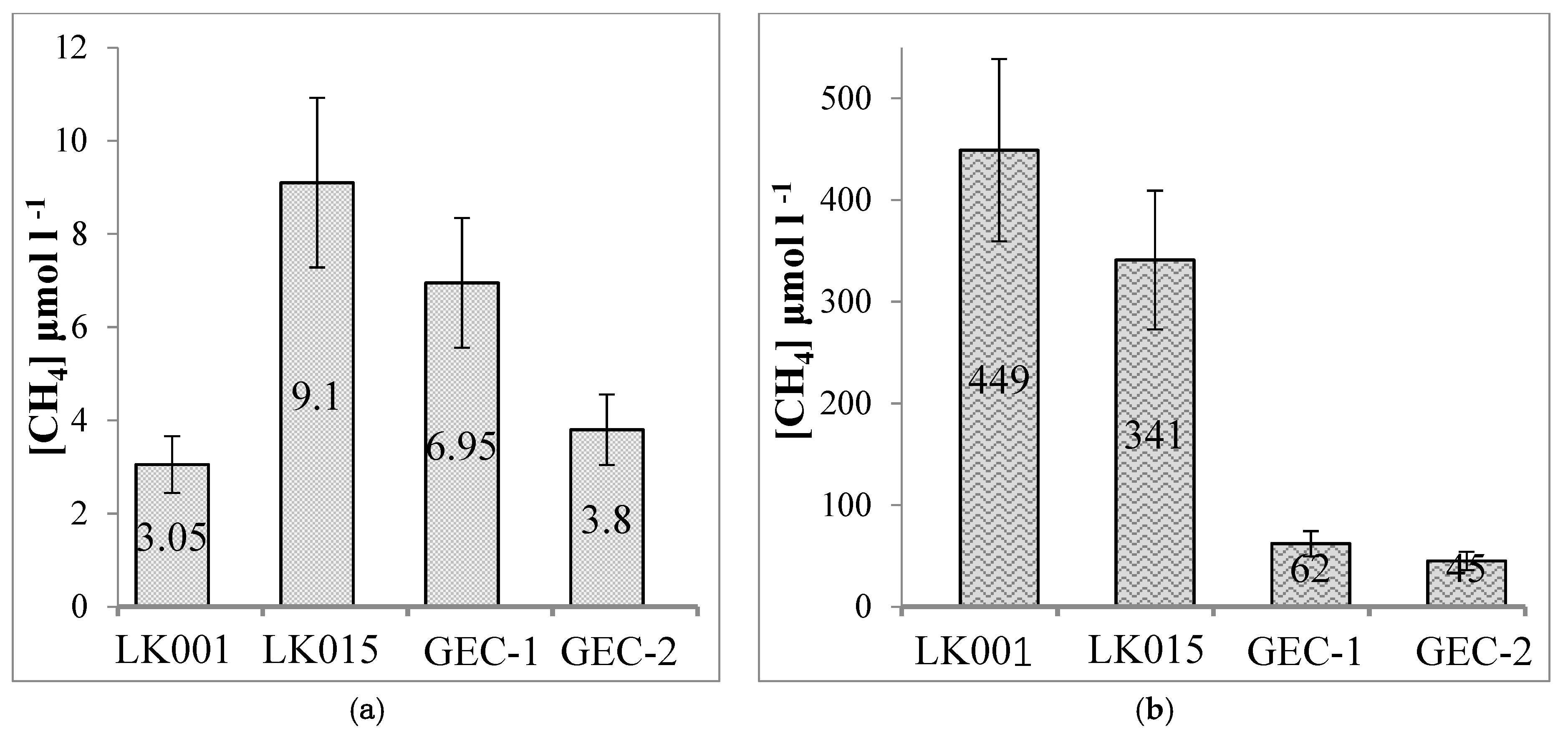
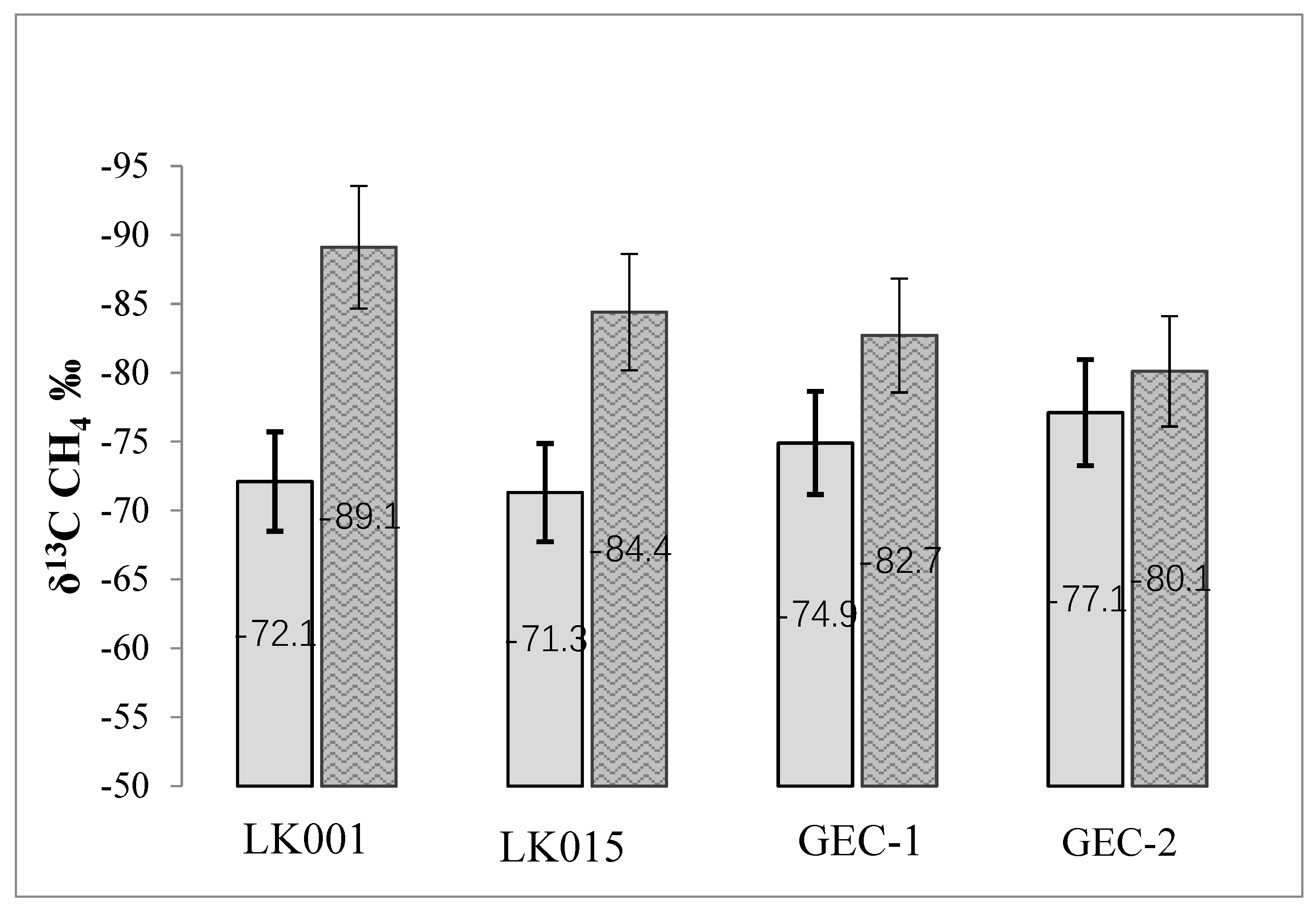
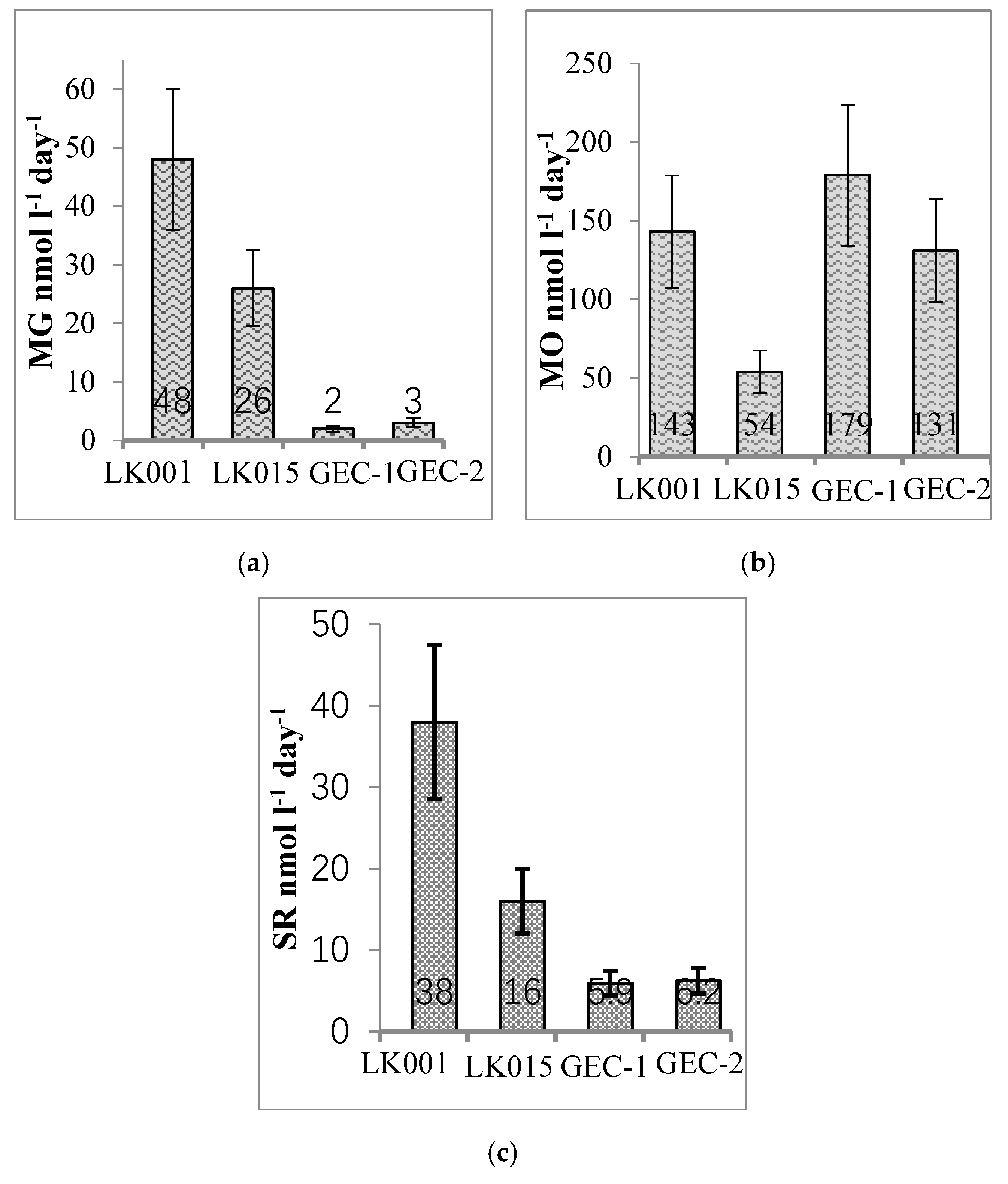
| Water Object | Total Concentration of Main Ions, mg∙L−1 | Na+ mg/eq % | Cl− mg/eq % | Ca2+ mg/eq % | HCO3− mg/eq % | DOC mg∙L−1 | t °C Winter/Summer | pH | CH4 μmol∙L−1 Winter/Summer |
|---|---|---|---|---|---|---|---|---|---|
| GEC lakes | 152–402 | 52–87 | 18–63 | 3–21 | 0–70 | 4.3–50.4 | (−0.08)/12.7 | 6.61–7.96 | 0.04–36.8/0.4–43.2 |
| GEC ice walls | 3–201 | 28–78 | 4–48 | 5–52 | 1–65 | 5.6–31.5 | - | - | 0.08–93.7 |
| Background lakes | 25–471 | 27–92 | 13–98 | 2–50 | 0–84 | 2.7–14.2 | 1.2/16.9 | 5.7–8.7 | 0.09–341/0–4.5 |
| Lake ice | - | - | - | - | - | - | - | - | 0.05–1.84 |
| Ponds | 1381–1946 | 47–71 | 87–94 | 11–23 | 0–4 | 7.6–10.9 | - | 5.9–7.43 | - |
| Rain | 5.2 | 8–31 | 41–50 | 45–55 | 0.2–0.4 | - | - | - | - |
| River | 141–147 | 55–58 | 56–91 | 14 | 6–42 | - | - | - | |
| Snow | 4.4–51 | 12–71 | 24–86 | 7–43 | 3–56 | - | - | 5.19–7.52 | - |
| Thermocirque | 988 | 45 | 24 | 22 | 66 | 243 | - | 7.6 | - |
| Parameter | LK001 | LK015 | GEC-1 | GEC-2 |
|---|---|---|---|---|
| Geology | IVth coastal-marine plain, clayey-silty deposits, altitude 40–41 m (Baltic) | Concave slope within IVth coastal-marine plain, clayey-silty deposits, altitude 40–43 m (Baltic) | ||
| lake surface’s average altitude (Baltic), m | 12.8 | 11.4 | 33 | |
| lake area, ha | 37.16 | 9.92 | 0.57 | 1.35 |
| lake depth mean/max | 4.4/16.9 | 7.7/23.2 | 2.6/4.9 | 1.0/2.5 |
| Coastal lithology | Clay, silt, sand, tabular ground ice covered by talus | Clay, silt, peat, ice wedges, tabular ground ice | Clay, silt, tabular ground ice | |
| Leading coastal process | Thermoerosion | Thermodenudation | Coastal thermoerosion | |
| DOC, mg∙L−1 (SF/BO) | 3.6/4.2 | 4.6/5.0 | 10.1/10.5 | 5.8 |
| Methane, μmol∙L−1 (SF/BO) | 0.25/6.1 | 0.13/62.8 | 16/21 ± 0.2 | 0.42 |
| Water temperature | 1.4–2.0 | 1.3–3.1 °C | 0.2 | 0.3 |
| Lake | TNM (103 Cell∙mL−1) | AOF (103 Cell∙mL−1) |
|---|---|---|
| LK001 | 200 ± 50 | 60 ± 20 |
| LK015 | 340 ± 70 | 70 ± 20 |
| GEC-1 | 420 ± 70 | 30 ± 10 |
| GEC-2 | 150 ± 40 | 25 ± 10 |
| Sample | Bottom Water | Bottom Sediments (0–14-cm Depth) | ||||||
|---|---|---|---|---|---|---|---|---|
| Lake | LK001 | LK015 | GEC-1 | GEC-2 | LK001 | LK015 | GEC-1 | GEC-2 |
| Chemoorganotrophic Actinobacteria (% to total 16S rRNA reads) | ||||||||
| Ilumatobacter | 9.1 | 8.5 | 15.5 | 11.9 | 0 | 0 | 0.1 | 0.6 |
| Ca. “Nanopelagicus” | 10.4 | 7.9 | 0.06 | 0 | 0.01 | 0 | 0 | 0 |
| Ca. “Planktophila” | 11.9 | 17.7 | 16.8 | 13.4 | 0.01 | 0.07 | 4.6 | 3.2 |
| Betaproteobacteria (% to total 16S rRNA reads) | ||||||||
| Methylotrophic | 3.5 | 8.1 | 1.7 | 1.4 | 0.16 | 0.16 | 0.2 | 0.7 |
| Involved in N cycle | 5.7 | 1.7 | 0.5 | 1.2 | 0.17 | 0.3 | 4.8 | 0.6 |
| Ferrous-iron-oxidizing | 2.0 | 0.7 | 0 | 0.01 | 0.08 | 3.9 | 9.98 | 1.1 |
| Chemoorganotrophic | 2.36 | 3.95 | 20.97 | 8.7 | 0.25 | 4.5 | 5.9 | 13.2 |
| Sulfur-oxidizing | 0 | 0 | 0.01 | 0 | 0.7 | 3.0 | 0.04 | 0.07 |
| Gammaproteobacteria (% to total 16S rRNA reads) | ||||||||
| Methanotrophic | 7.6 | 18.0 | 17.9 | 9.1 | 1.04 | 0.3 | 1.8 | 1.05 |
| Chemoorganotrophic | 7.0 | 0.03 | 3.5 | 38.2 | 0.01 | 1.9 | 0.1 | 17.8 |
| Deltaproteobacteria (% to total 16S rRNA reads) | ||||||||
| Sulfate-reducing | 0 | 0 | 0 | 0 | 0.11 | 2.53 | 2.2 | 4.3 |
| Sample | Near Bottom Water | Bottom Sediments (0–14-cm Depth) | ||||||
|---|---|---|---|---|---|---|---|---|
| Lake | LK001 | LK015 | GEC-1 | GEC-2 | LK001 | LK015 | GEC-1 | GEC-2 |
| % to Total 16S rRNA Reads | ||||||||
| Methanoregula | 0.12 | 0.16 | 0.05 | 0.02 | 22.93 | 15.27 | 0.06 | 0.08 |
| Methanosarcina | 0 | 0 | 0.01 | 0 | 0.04 | 0.78 | 2.91 | 0.13 |
| Methanosaeta | 0 | 0 | 0 | 0 | 3.61 | 5.69 | 0.64 | 0.01 |
| Methanomassiliicoccus | 0 | 0 | 0 | 0 | 1.18 | 0.88 | 0.06 | 0 |
| ANME-2d | 0 | 0 | 0 | 0.15 | 1.80 | 0.04 | 2.27 | 0.15 |
| Other archaea | 0.63 | 0.77 | 0.12 | 0.29 | 29.49 | 28.14 | 4.83 | 0.29 |
| Total archaea | 0.76 | 0.94 | 0.18 | 0.67 | 59.05 | 50.80 | 10.77 | 0.67 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Savvichev, A.; Leibman, M.; Kadnikov, V.; Kallistova, A.; Pimenov, N.; Ravin, N.; Dvornikov, Y.; Khomutov, A. Microbiological Study of Yamal Lakes: A Key to Understanding the Evolution of Gas Emission Craters. Geosciences 2018, 8, 478. https://doi.org/10.3390/geosciences8120478
Savvichev A, Leibman M, Kadnikov V, Kallistova A, Pimenov N, Ravin N, Dvornikov Y, Khomutov A. Microbiological Study of Yamal Lakes: A Key to Understanding the Evolution of Gas Emission Craters. Geosciences. 2018; 8(12):478. https://doi.org/10.3390/geosciences8120478
Chicago/Turabian StyleSavvichev, Alexander, Marina Leibman, Vitaly Kadnikov, Anna Kallistova, Nikolai Pimenov, Nikolai Ravin, Yury Dvornikov, and Artem Khomutov. 2018. "Microbiological Study of Yamal Lakes: A Key to Understanding the Evolution of Gas Emission Craters" Geosciences 8, no. 12: 478. https://doi.org/10.3390/geosciences8120478
APA StyleSavvichev, A., Leibman, M., Kadnikov, V., Kallistova, A., Pimenov, N., Ravin, N., Dvornikov, Y., & Khomutov, A. (2018). Microbiological Study of Yamal Lakes: A Key to Understanding the Evolution of Gas Emission Craters. Geosciences, 8(12), 478. https://doi.org/10.3390/geosciences8120478







