Hydrodynamic Shear-Induced Densification of Bacteriogenic Iron Oxides: Mechanisms and Implications
Abstract
1. Introduction
2. Materials and Methods
2.1. Site Description and Sample Collection
2.2. Shear Stress Application
2.3. Batch Column Settling Trials
2.4. Gravimetric and Volumetric Analyses
2.5. Optical Microscopy and Scanning Electron Microscopy (SEM)
3. Results
3.1. Shear Stress Application
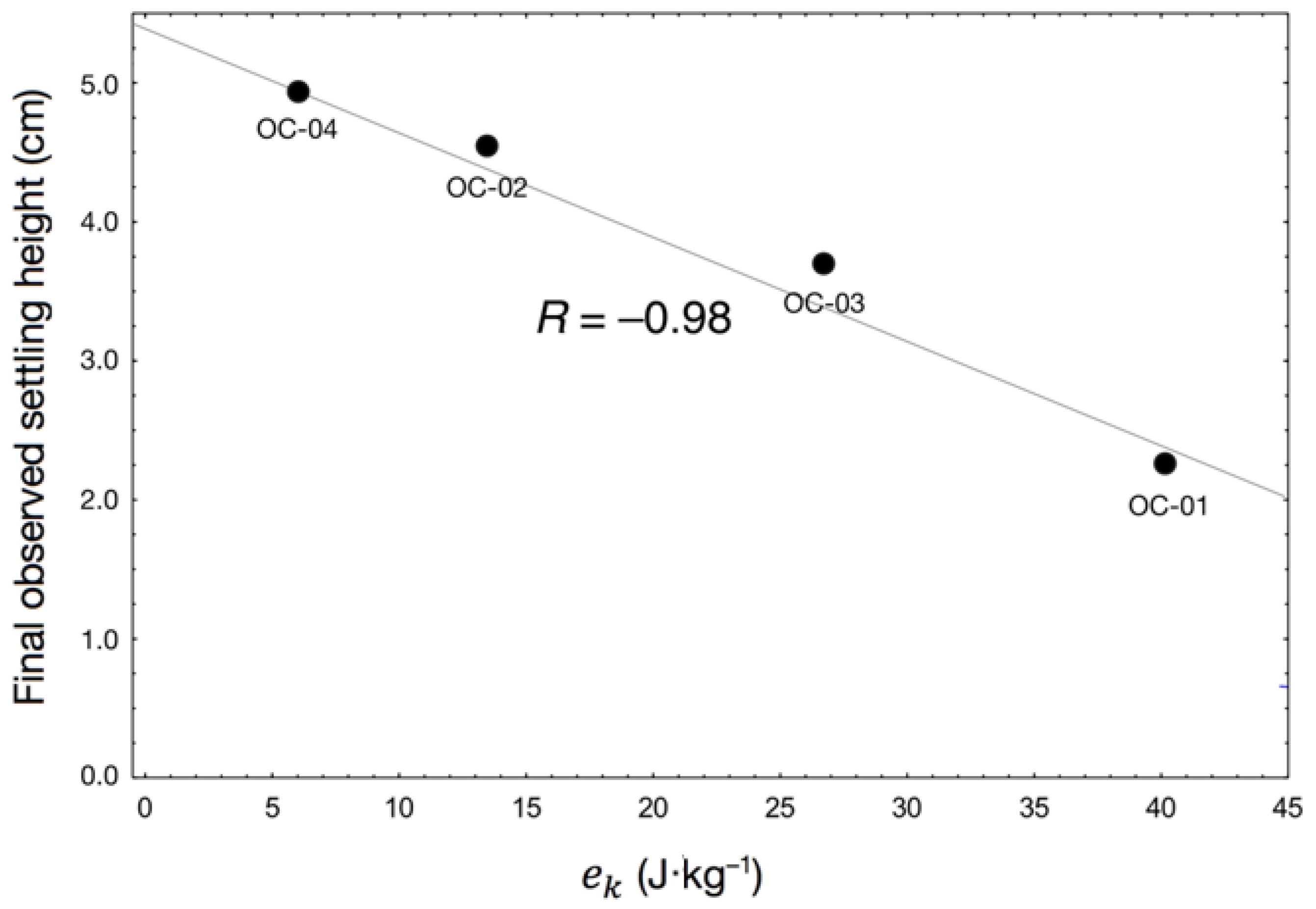
3.2. Batch Column Settling Trials
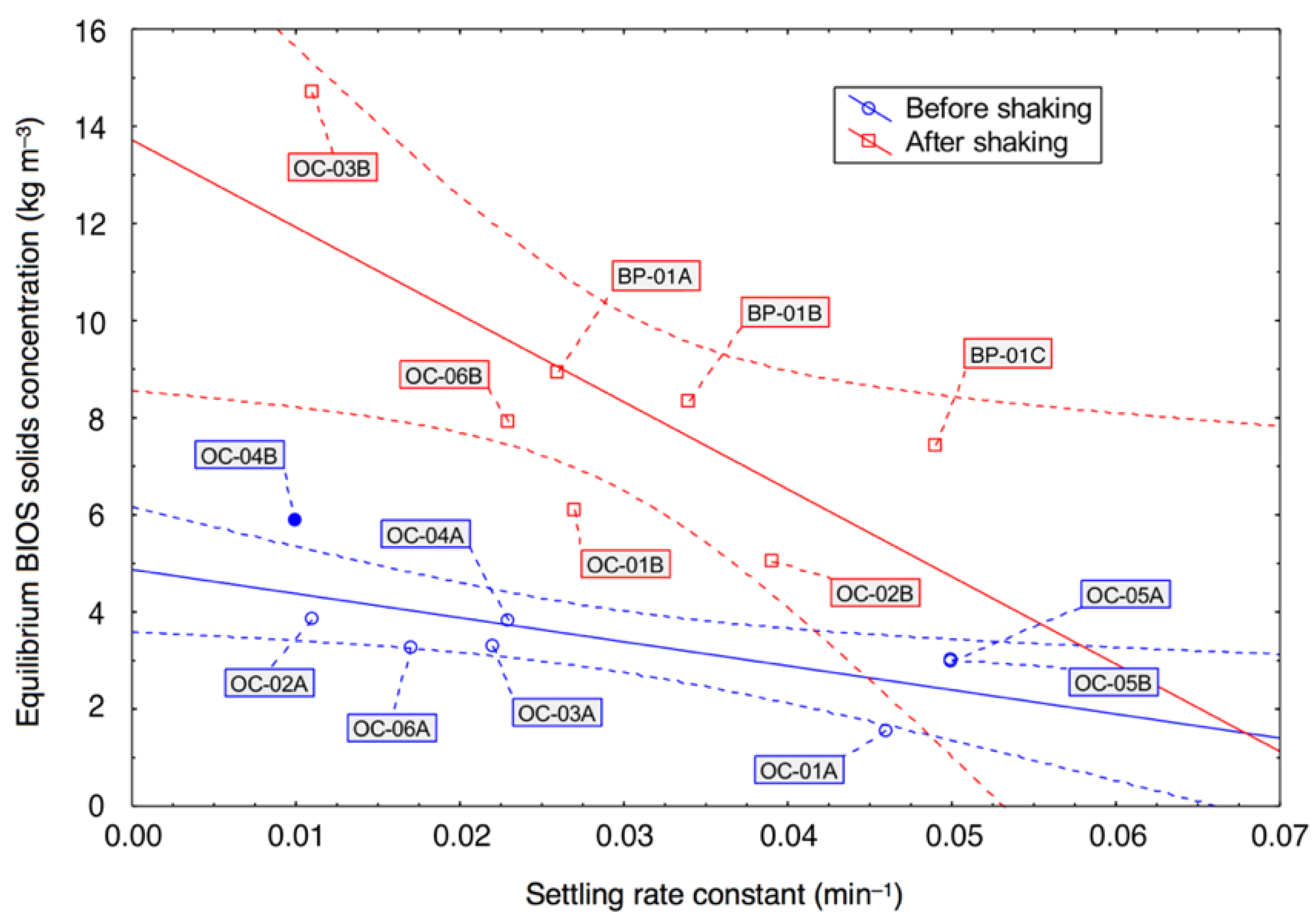
3.3. Gravimetric and Volumetric Analyses
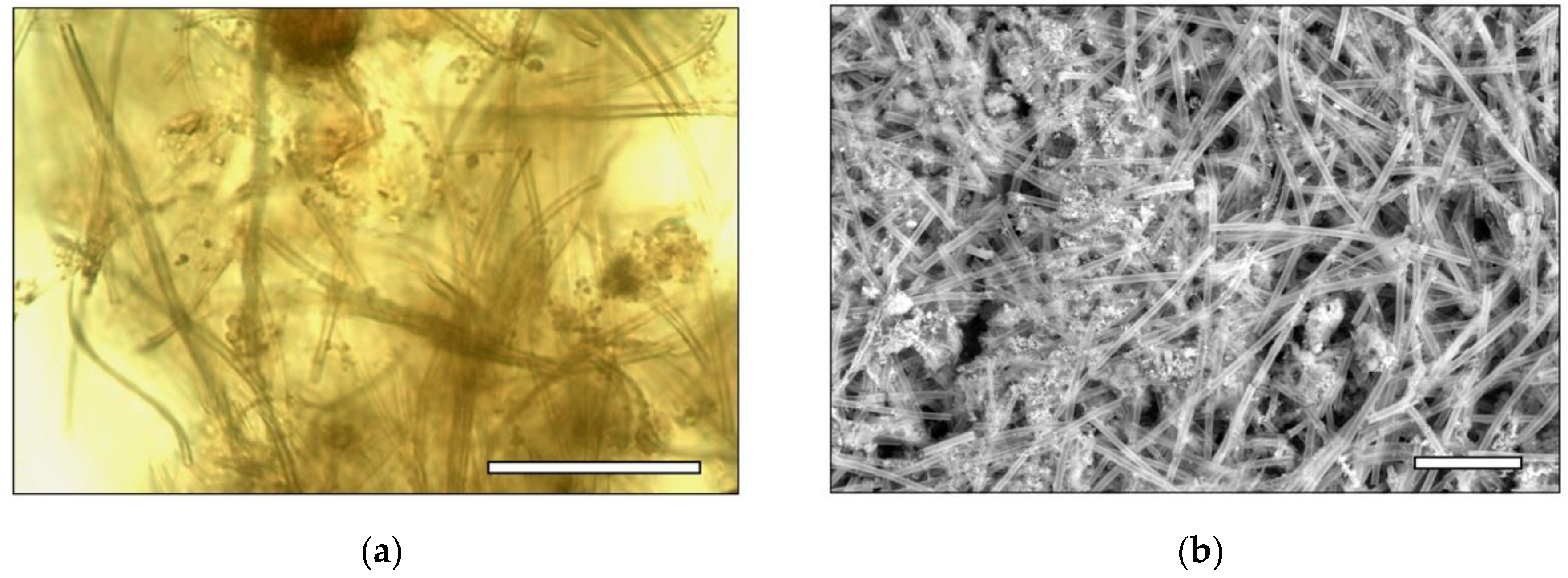
3.4. Optical and SEM Microscopy
4. Discussion
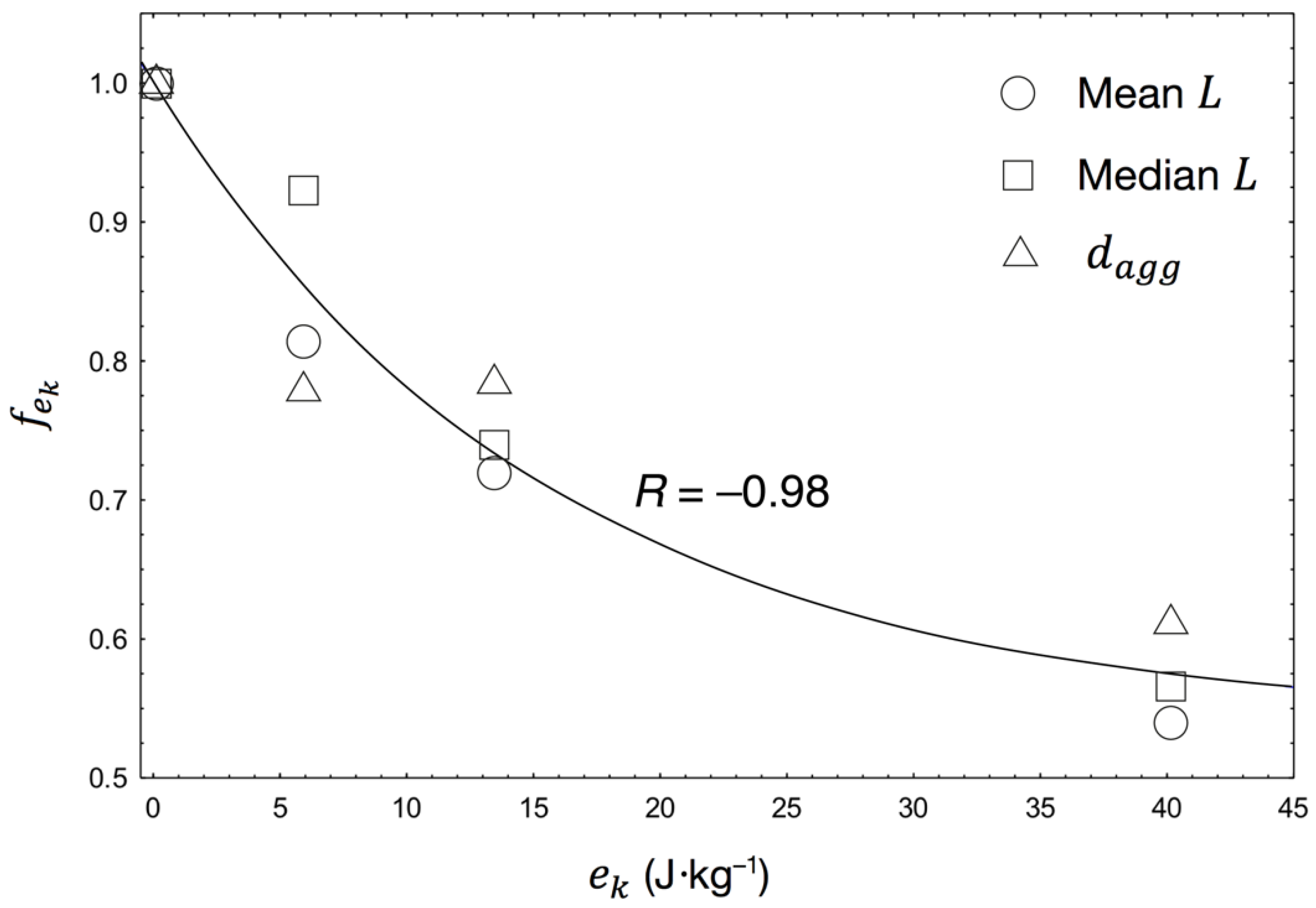
4.1. Effects of Hydrodynamic Shear on Settling Behaviour
4.2. Physical Changes to Bacterial–Mineral Aggregates
4.3. Broader Implications of Aggregate Densification
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Du, H.H.; Lin, Y.P.; Chen, W.L.; Cai, P.; Rong, X.M.; Shi, Z.H.; Huang, Q.Y. Copper adsorption on composites of goethite, cells of Pseudomonas putida and humic acid. Eur. J. Soil Sci. 2017, 68, 514–523. [Google Scholar] [CrossRef]
- Moon, E.M.; Peacock, C.L. Adsorption of Cu(II) to ferrihydrite and ferrihydrite–bacteria composites: Importance of the carboxyl group for Cu mobility in natural environments. Geochim. Cosmochim. Acta 2012, 92, 203–219. [Google Scholar] [CrossRef]
- Anderson, C.R.; James, R.E.; Fru, E.C.; Kennedy, C.B.; Pedersen, K. In situ ecological development of a bacteriogenic iron oxide-producing microbial community from a subsurface granitic rock environment. Geobiology 2006, 4, 29–42. [Google Scholar] [CrossRef]
- Gault, A.G.; Ibrahim, A.; Langley, S.; Renaud, R.; Takahashi, Y.; Boothman, C.; Lloyd, J.R.; Clark, I.D.; Ferris, F.G.; Fortin, D. Microbial and geochemical features suggest iron redox cycling within bacteriogenic iron oxide-rich sediments. Chem. Geol. 2011, 281, 41–51. [Google Scholar] [CrossRef]
- Posth, N.R.; Huelin, S.; Konhauser, K.O.; Kappler, A. Size, density and composition of cell–mineral aggregates formed during anoxygenic phototrophic Fe(II) oxidation: Impact on modern and ancient environments. Geochim. Cosmochim. Acta 2010, 74, 3476–3493. [Google Scholar] [CrossRef]
- Li, Y.; Long, X.; Chong, Y.; Yu, G.; Huang, Z. Characterization of the cell–Fe mineral aggregate from nitrogen removal employing ferrous and its adsorption features to heavy metal. J. Clean. Prod. 2017, 156, 538–548. [Google Scholar] [CrossRef]
- Schädler, S.; Burkhardt, C.; Hegler, F.; Straub, K.L.; Miot, J.; Benzerara, K.; Kappler, A. Formation of Cell-Iron-Mineral Aggregates by Phototrophic and Nitrate-Reducing Anaerobic Fe(II)-Oxidizing Bacteria. Geomicrobiol. J. 2009, 26, 93–103. [Google Scholar] [CrossRef]
- Elliott, A.V.C.; Plach, J.M.; Droppo, I.G.; Warren, L.A. Collaborative microbial Fe-redox cycling by pelagic floc bacteria across wide ranging oxygenated aquatic systems. Chem. Geol. 2014, 366, 209–216. [Google Scholar] [CrossRef]
- Hao, L.; Li, J.; Kappler, A.; Obst, M. Mapping of Heavy Metal Ion Sorption to Cell-Extracellular Polymeric Substance-Mineral Aggregates by Using Metal-Selective Fluorescent Probes and Confocal Laser Scanning Microscopy. Appl. Environ. Microb. 2013, 79, 6524–6534. [Google Scholar] [CrossRef] [PubMed]
- Kappler, A.; Straub, K.L. Geomicrobiological Cycling of Iron. Rev. Mineral. Geochem. 2005, 59, 89–108. [Google Scholar] [CrossRef]
- Kennedy, C.B.; Gault, A.G.; Fortin, D.; Clark, I.D.; Ferris, F.G. Retention of iodide by bacteriogenic iron oxides. Geomicrobiol. J. 2011, 28, 387–395. [Google Scholar] [CrossRef]
- Langley, S.; Gault, A.G.; Ibrahim, A.; Takahashi, Y.; Renaud, R.; Fortin, D.; Clark, I.D.; Ferris, F.G. Sorption of Strontium onto Bacteriogenic Iron Oxides. Environ. Sci. Technol. 2009, 43, 1008–1014. [Google Scholar] [CrossRef] [PubMed]
- Bauld, J.; D’Amelio, E.; Farmer, J.D. Modern Microbial Mats. In The Proterozoic Biosphere: A Multidisciplinary Study; Schopf, J.W., Klein, C., Eds.; Cambridge University Press: Cambridge, UK, 1992; pp. 261–270. ISBN 0521366151. [Google Scholar]
- Schieber, J.; Glamoclija, M. Microbial mats built by iron bacteria: A modern example from southern Indiana. In Atlas of Microbial Mat Features Preserved Within the Siliciclastic Rock Record; Schieber, J., Bose, P., Eriksson, P., Banerjee, S., Sarkar, S., Altermann, W., Catuneanu, O., Eds.; Elsevier: Amsterdam, The Netherlands, 2007; pp. 233–244. ISBN 9780444528599. [Google Scholar]
- Kapellos, G.E.; Alexiou, T.S.; Payatakes, A.C. Theoretical modeling of fluid flow in cellular biological media: An overview. Math. Biosci. 2010, 225, 83–93. [Google Scholar] [CrossRef] [PubMed]
- Oyen, M.L. Mechanical characterisation of hydrogel materials. Int. Mater. Rev. 2014, 59, 44–59. [Google Scholar] [CrossRef]
- Usher, S.P.; Spehar, R.; Scales, P.J. Theoretical analysis of aggregate densification: impact on thickener performance. Chem. Eng. J. 2009, 151, 202–208. [Google Scholar] [CrossRef]
- Droppo, I.G.; Leppard, G.G.; Flannigan, D.T.; Liss, S.N. The freshwater floc: A functional relationship of water and organic and inorganic floc constituents affecting suspended sediment properties. Water Air Soil Pollut. 1997, 99, 43–54. [Google Scholar] [CrossRef]
- Emerson, D.; Fleming, E.J.; McBeth, J.M. Iron-Oxidizing Bacteria: An Environmental and Genomic Perspective. Ann. Rev. Microbiol. 2010, 64, 561–583. [Google Scholar] [CrossRef] [PubMed]
- Van Deventer, B.B.G.; Usher, S.P.; Kumar, A.; Rudman, M.; Scales, P.J. Aggregate densification and batch settling. Chem. Eng. J. 2011, 171, 141–151. [Google Scholar] [CrossRef]
- Scales, P.J.; Dixon, D.R.; Harbour, P.J.; Stickland, A.D. The fundamentals of wastewater sludge characterization and filtration. Water Sci. Technol. 2004, 49, 67–72. [Google Scholar] [CrossRef] [PubMed]
- Grassia, P.; Usher, S.P.; Scales, P.J. A simplified parameter extraction technique using batch settling data to estimate suspension material properties in dewatering applications. Chem. Eng. Sci. 2008, 63, 1971–1986. [Google Scholar] [CrossRef]
- Pearse, M.J. Historical use and future development of chemicals for solid–liquid separation in the mineral processing industry. Miner. Eng. 2003, 16, 103–108. [Google Scholar] [CrossRef]
- Ferris, F.G.; James, R.E.; Pedersen, K. Fragmentation of bacteriogenic iron oxides in response to hydrodynamic shear stress. Geomicrobiol. J. 2015, 32, 564–569. [Google Scholar] [CrossRef]
- Gerdes, G. Structures left by modern microbial mats in their host sediments. In Atlas of Microbial Mat Features Preserved Within the Siliciclastic Rock Record; Schieber, J., Bose, P., Eriksson, P., Banerjee, S., Sarkar, S., Altermann, W., Catuneanu, O., Eds.; Elsevier: Amsterdam, The Netherlands, 2007; pp. 5–38. ISBN 9780444528599. [Google Scholar]
- Konhauser, K.O.; Hamade, T.; Raiswell, R.; Morris, R.C.; Ferris, F.G.; Southam, G.; Canfield, D.E. Could bacteria have formed the Precambrian banded iron formations? Geology 2002, 30, 1079–1082. [Google Scholar] [CrossRef]
- Kennedy, C.B.; Scott, S.D.; Ferris, F.G. Hydrothermal phase stabilization of 2-line ferrihydrite by bacteria. Chem. Geol. 2004, 212, 269–277. [Google Scholar] [CrossRef]
- Edwards, B.A.; Shirokova, V.L.; Enright, A.M.L.; Ferris, F.G. Dependence of In Situ Bacterial Fe(II)-Oxidation and Fe(III)-Precipitation on Sequential Reactive Transport. Geomicrobiol. J. 2018, 35, 503–510. [Google Scholar] [CrossRef]
- Ferris, F.G.; Enright, A.M.L.; Fortin, D.; Clark, I.D. Rates of Fe(II)-oxidation and solubility of bacteriogenic iron oxides. Geomicrobiol. J. 2016, 33, 237–242. [Google Scholar] [CrossRef]
- Gauger, T.; Byrne, J.M.; Konhauser, K.O.; Obst, M.; Crowe, S.; Kappler, A. Influence of organics and silica on Fe(II) oxidation rates and cell–mineral aggregate formation by the green-sulfur Fe(II)-oxidizing bacterium Chlorobium ferrooxidans KoFox—Implications for Fe(II) oxidation in ancient oceans. Earth Planet. Sci. Lett. 2016, 443, 81–89. [Google Scholar] [CrossRef]
- James, R.E.; Ferris, F.G. Evidence for microbial-mediated iron oxidation at a neutrophilic groundwater spring. Chem. Geol. 2004, 212, 301–311. [Google Scholar] [CrossRef]
- Shirokova, V.L.; Enright, A.M.L.; Kennedy, C.B.; Ferris, F.G. Thermal intensification of microbial Fe(II)/Fe(III) redox cycling in a pristine shallow sand aquifer on the Canadian Shield. Water Res. 2016, 106, 604–612. [Google Scholar] [CrossRef] [PubMed]
- Carr, S.D.; Easton, R.M.; Jamieson, R.A.; Culshaw, N.G. Geologic transect across the Grenville orogen of Ontario and New York. Can. J. Earth Sci. 2000, 37, 193–216. [Google Scholar] [CrossRef]
- Büchs, J. Introduction to advantages and problems of shaken cultures. Biochem. Eng. J. 2001, 7, 91–98. [Google Scholar] [CrossRef]
- Gaur, R.L.; Pai, M.; Banaei, N. Impact of Blood Volume, Tube Shaking, and Incubation Time on Reproducibility of QuantiFERON-TB Gold In-Tube Assay. J. Clin. Microbiol. 2013, 51, 3521–3526. [Google Scholar] [CrossRef] [PubMed]
- Cherng, W.-J.; Dong, Z.-S.; Chou, C.-C.; Yeh, C.-H.; Pan, Y.-H. Hydrodynamic Simulation of an Orbital Shaking Test for the Degradation Assessment of Blood-Contact Biomedical Coatings. Micromachines 2017, 8, 1–9. [Google Scholar] [CrossRef]
- Li, Z.J.; Shukla, V.; Wenger, K.; Fordyce, A.; Pedersen, A.G.; Marten, M. Estimation of Hyphal Tensile Strength in Production-Scale Aspergillus oryzae Fungal Fermentations. Biotechnol. Bioeng. 2002, 77, 601–613. [Google Scholar] [CrossRef] [PubMed]
- Shirokova, V.L.; Ferris, F.G. Microbial Diversity and Biogeochemistry of a Shallow Pristine Canadian Shield Groundwater System. Geomicrobiol. J. 2013, 30, 140–149. [Google Scholar] [CrossRef]
- Korson, L.; Drost-Hansen, W.; Millero, F.J. Viscosity of Water at Various Temperatures. J. Phys. Chem. 1969, 73, 34–39. [Google Scholar] [CrossRef]
- Peter, C.P.; Suzuki, Y.; Büchs, J. Hydromechanical stress in shake flasks: Correlation for the maximum local energy dissipation rate. Biotechnol. Bioeng. 2006, 93, 1164–1176. [Google Scholar] [CrossRef] [PubMed]
- Usher, S.P.; Scales, P.J.; White, L.R. Prediction of transient bed height in batch sedimentation at large times. AIChE J. 2006, 52, 986–993. [Google Scholar] [CrossRef]
- Zhang, Y.; Brassia, P.; Martin, A.; Usher, S.P.; Scales, P.J. Mathematical modeling of batch sedimentation subject to slow aggregate densification. Chem. Eng. Sci. 2015, 128, 54–63. [Google Scholar] [CrossRef]
- Lester, D.R.; Usher, S.P.; Scales, P.J. Estimation of the Hindered Settling Function R(ϕ) from Batch-Settling Tests. AIChE J. 2005, 51, 1158–1168. [Google Scholar] [CrossRef]
- Spehar, R.; Kiviti-Manor, A.; Fawell, P.; Usher, S.P.; Rudman, M.; Scales, P.J. Aggregate densification in the thickening of flocculated suspensions in an un-networked bed. Chem. Eng. Sci. 2015, 122, 585–595. [Google Scholar] [CrossRef]
- Benn, F.A.; Fawell, P.D.; Halewood, J.; Austin, P.J.; Costine, A.D.; Jones, W.G.; Francis, N.S.; Druett, D.C.; Lester, D. Sedimentation and consolidation of different density aggregates formed by polymer-bridging flocculation. Chem. Eng. Sci. 2018, 184, 111–125. [Google Scholar] [CrossRef]
- Takeno, H.; Kimura, Y.; Nakamura, W. Mechanical, Swelling, and Structural Properties of Mechanically Tough Clay-Sodium Polyacrylate Blend Hydrogels. Gels 2017, 3, 1–10. [Google Scholar] [CrossRef]
- Fleming, E.J.; Langdon, A.E.; Martinez-Garcia, M.; Stepanauskas, R.; Poulton, N.J.; Masland, E.D.P.; Emerson, D. What’s New Is Old: Resolving the Identity of Leptothrix ochracea Using Single Cell Genomics, Pyrosequencing and FISH. PLoS ONE 2011, 6, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Johnson, K.W.; Carmichael, M.J.; McDonald, W.; Rose, N.; Pitchford, J.; Windelspecht, M.; Karatan, E.; Bräuer, S.L. Increased Abundance of Gallionella spp., Leptothrix spp. and Total Bacteria in Response to Enhanced Mn and Fe Concentrations in a Disturbed Southern Appalachian High Elevation Wetland. Geomicrobiol. J. 2012, 29, 124–138. [Google Scholar] [CrossRef]
- Vesenka, J.; Havu, J.; Hruby, K.; Emerson, D. A Model for Sheath Formation Coupled to Motility in Leptothrix ochracea. Geomicrobiol. J. 2018, 35, 366–374. [Google Scholar] [CrossRef]
- Chan, C.S.; McAllister, S.M.; Leavitt, A.H.; Glazer, B.T.; Krepski, S.T.; Emerson, D. The Architecture of Iron Microbial Mats Reflects the Adaptation of Chemolithotrophic Iron Oxidation in Freshwater and Marine Environments. Front. Microbiol. 2016, 7, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Nan, J.; Yao, M.; Chen, T.; Wang, Z.; Li, Q.; Zhan, D. Experimental and numerical characterization of floc morphology: role of changing hydraulic retention time under flocculation mechanisms. Environ. Sci. Pollut. Res. 2016, 23, 3596–3608. [Google Scholar] [CrossRef] [PubMed]
- Droppo, I.G. Structural controls on floc strength and transport. Can. J. Civil Eng. 2004, 31, 569–578. [Google Scholar] [CrossRef]
- Markussen, T.N.; Andersen, T.J. Flocculation and floc break-up related to tidally induced turbulent shear in a low-turbidity, microtidal estuary. J. Sea Res. 2014, 89, 1–11. [Google Scholar] [CrossRef]
- Manning, A.J.; Dyer, K.R. A laboratory examination of floc characteristics with regard to turbulent shearing. Mar. Geol. 1999, 160, 147–170. [Google Scholar] [CrossRef]
- Kachhwal, L.K.; Yanful, E.K.; Rennie, C.D. A semi-empirical approach for estimation of bed shear stress in a tailings pond. Environ. Earth Sci. 2012, 66, 823–834. [Google Scholar] [CrossRef]
- Bale, A.J.; Widdows, J.; Harris, C.B.; Stephens, J.A. Measurements of the critical erosion threshold of surface sediments along the Tamar Estuary using a mini-annular flume. Cont. Shelf Res. 2006, 26, 1206–1216. [Google Scholar] [CrossRef]
- Jamieson, R.C.; Joy, D.M.; Lee, H.; Kostaschuk, R.; Gordon, R.P. Resuspension of Sediment-Associated Escherichia coli in a Natural Stream. J. Environ. Qual. 2005, 34, 581–589. [Google Scholar] [CrossRef] [PubMed]
- Van Leussen, W. Estuarine Macroflocs and Their Role in Fine-grained Sediment Transport. Ph.D. Thesis, University of Utrecht, Utrecht, The Netherlands, June 1994. [Google Scholar]
- Marchioli, C.; Soldati, A. Turbulent breakage of ductile aggregates. Phys. Rev. E 2015, 91, 053003. [Google Scholar] [CrossRef] [PubMed]
- Shanks, A.L.; Trent, J.D. Marine snow: Sinking rates and potential role in vertical flux. Deep-Sea Res. 1980, 27 Pt 1, 137–143. [Google Scholar] [CrossRef]
- Emerson, D.; Revsbech, N.P. Investigation of an Iron-Oxidizing Microbial Mat Community Located near Aarhus, Denmark: Field Studies. Appl. Environ. Microb. 1994, 60, 4022–4031. [Google Scholar]
- Liu, Y.; Tay, J.-H. The essential role of hydrodynamic shear force in the formation of biofilm and granular sludge. Water Res. 2002, 36, 1653–1665. [Google Scholar] [CrossRef]
- Thomas, K.; Herminghaus, S.; Porada, H.; Goehring, L. Formation of Kinneyia via shear induced instabilities in microbial mats. Phil. Trans. R. Soc. A 2013, 371, 20120362. [Google Scholar] [CrossRef] [PubMed]
- Okay, O. General Properties of Hydrogels. In Hydrogel Sensors and Actuators; Gerlach, G., Arndt, K.-F., Eds.; Springer: Berlin, Germany, 2010; pp. 1–14. ISBN 978-3-540-75645-3. [Google Scholar]
- Cockell, C.S. Geomicrobiology beyond Earth: microbe–mineral interactions in space exploration and settlement. Trends Microbiol. 2010, 18, 308–314. [Google Scholar] [CrossRef] [PubMed]
- Hofmann, B.A.; Farmer, J.D. Filamentous fabrics in low-temperature mineral assemblages: Are they fossil biomarkers? Implications for the search for a subsurface fossil record on the early Earth and Mars. Planet. Space Sci. 2000, 48, 1077–1086. [Google Scholar] [CrossRef]



| Sample | (g) | Description of Sample Treatment | |
|---|---|---|---|
| Cycle A | Cycle B | ||
| OC-01 | 0.043 | no shaking | shaken at a frequency = 2.69 s−1 for 60 s |
| OC-02 | 0.066 | no shaking | shaken at a frequency = 2.69 s−1 for 20 s |
| OC-03 | 0.084 | no shaking | shaken at a frequency = 2.69 s−1 for 40 s |
| OC-04 | 0.106 | no shaking | shaken at a frequency = 1.35 s−1 for 60 s |
| OC-05 | 0.066 | no shaking | no shaking, resuspension |
| OC-06 | 0.077 | no shaking | shaken at a frequency = 2.69 s−1 for 120 s |
| BP-01 | 0.079 | no shaking | no shaking, resuspension; repeated for cycle C trial |
| Sample 1 | (s−1) | (m2·s−3) | (N·m−2) | (s) | (J·kg−1) |
|---|---|---|---|---|---|
| OC-01 | 2.69 | 0.65 | 0.82 | 60 | 40.2 |
| OC-02 | 2.69 | 0.65 | 0.82 | 20 | 13.4 |
| OC-03 | 2.69 | 0.65 | 0.82 | 40 | 26.8 |
| OC-04 | 1.35 | 0.10 | 0.32 | 60 | 6.0 |
| OC-06 | 2.69 | 0.65 | 0.82 | 120 | 80.4 |
| Sample | Cycle | (cm·min−1) 1 | (cm) | (min−1) |
|---|---|---|---|---|
| OC-01 | A | 0.08 | 5.21 ± 0.07 | 0.046 ± 0.005 |
| OC-01 | B | 0.15 | 1.33 ± 0.24 | 0.027 ± 0.002 |
| OC-02 | A | 0.13 | 4.48 ± 0.29 | 0.015 ± 0.002 |
| OC-02 | B | 0.16 | 2.58 ± 0.71 | 0.01 ± 0.001 |
| OC-03 | A | 0.04 | 4.81 ± 0.19 | 0.022 ± 0.004 |
| OC-03 | B | 0.06 | 1.08 ± 0.52 | 0.011 ± 0.001 |
| OC-04 | A | 0.04 | 5.24 ± 0.25 | 0.023 ± 0.006 |
| OC-04 | B | 0.04 | 3.41 ± 0.66 | 0.01 ± 0.003 |
| OC-05 | A | 0.11 | 4.20 ± 0.08 | 0.05 ± 0.004 |
| OC-05 | B | 0.11 | 4.14 ± 0.07 | 0.04 ± 0.002 |
| OC-06 | A | 0.24 | 4.46 ± 0.30 | 0.017 ± 0.003 |
| OC-06 | B | 0.72 | 1.84 ± 0.17 | 0.023 ± 0.002 |
| BP-01 | A | 0.15 | 1.67 ± 0.24 | 0.026 ± 0.002 |
| BP-01 | B | 0.19 | 1.79 ± 0.21 | 0.034 ± 0.003 |
| BP-01 | C | 0.27 | 2.01 ± 0.05 | 0.049 ± 0.002 |
| Sample | (mL) | (mL) | |
|---|---|---|---|
| OC-01 | 0.020 | 41 | 0.48 |
| OC-02 | 0.030 | 40 | 0.75 |
| OC-03 | 0.038 | 41 | 0.93 |
| OC-04 | 0.048 | 40 | 1.20 |
| OC-05 | 0.029 | 40 | 0.75 |
| OC-06 | 0.035 | 40 | 0.88 |
| BP-01 | 0.036 | 44 | 0.82 |
| Sample | Cycle | (mL) | (kg·m−3) | (µm) | ||
|---|---|---|---|---|---|---|
| OC-01 | A | 30.1 | 1.07 | 999 | 937 | 156 |
| OC-01 | B | 7.83 | 4.18 | 1003 | 240 | 95.3 |
| OC-02 | A | 26.4 | 1.90 | 1000 | 527 | 58.0 |
| OC-02 | B | 15.2 | 3.29 | 1002 | 304 | 45.5 |
| OC-03 | A | 28.3 | 2.25 | 1000 | 445 | 78.2 |
| OC-03 | B | 6.46 | 9.98 | 1010 | 100 | 39.1 |
| OC-04 | A | 30.9 | 2.60 | 1001 | 385 | 63.9 |
| OC-04 | B | 20.1 | 4.00 | 1003 | 250 | 49.8 |
| OC-05 | A | 24.7 | 2.01 | 1000 | 497 | 67.5 |
| OC-05 | B | 24.4 | 2.04 | 1000 | 491 | 61.5 |
| OC-06 | A | 26.3 | 2.23 | 1001 | 450 | 63.9 |
| OC-06 | B | 10.8 | 5.40 | 1005 | 185 | 69.4 |
| BP-01 | A | 9.84 | 6.08 | 1005 | 164 | 81.0 |
| BP-01 | B | 10.5 | 5.68 | 1005 | 176 | 91.2 |
| BP-01 | C | 11.8 | 5.05 | 1005 | 198 | 109 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Edwards, B.A.; Ferris, F.G. Hydrodynamic Shear-Induced Densification of Bacteriogenic Iron Oxides: Mechanisms and Implications. Geosciences 2018, 8, 423. https://doi.org/10.3390/geosciences8110423
Edwards BA, Ferris FG. Hydrodynamic Shear-Induced Densification of Bacteriogenic Iron Oxides: Mechanisms and Implications. Geosciences. 2018; 8(11):423. https://doi.org/10.3390/geosciences8110423
Chicago/Turabian StyleEdwards, Brock A., and F. Grant Ferris. 2018. "Hydrodynamic Shear-Induced Densification of Bacteriogenic Iron Oxides: Mechanisms and Implications" Geosciences 8, no. 11: 423. https://doi.org/10.3390/geosciences8110423
APA StyleEdwards, B. A., & Ferris, F. G. (2018). Hydrodynamic Shear-Induced Densification of Bacteriogenic Iron Oxides: Mechanisms and Implications. Geosciences, 8(11), 423. https://doi.org/10.3390/geosciences8110423





