Synchrotron-Based Micro-CT Investigation of Oxic Corrosion of Copper-Coated Carbon Steel for Potential Use in a Deep Geological Repository for Used Nuclear Fuel
Abstract
:1. Introduction
2. Materials and Methods
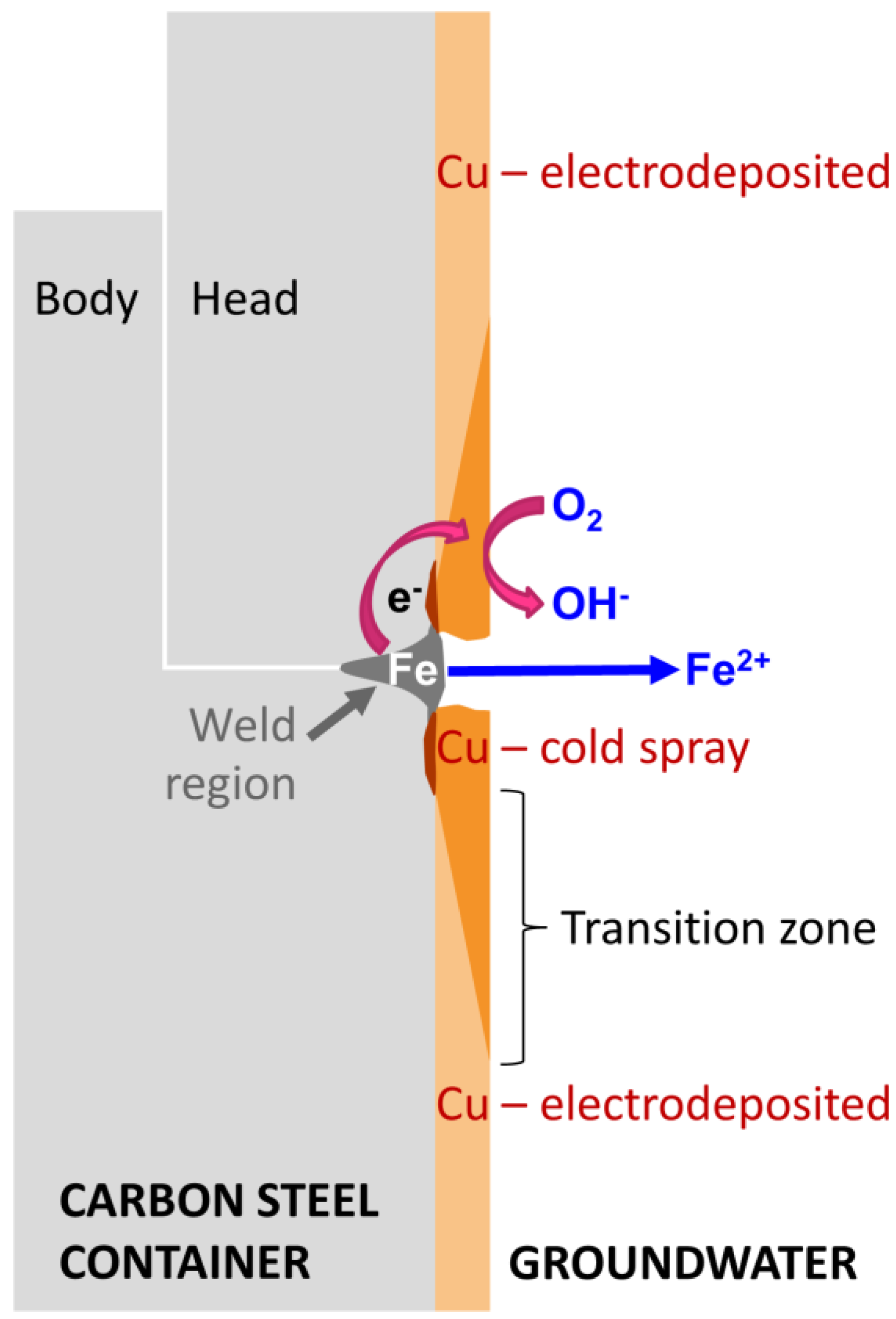
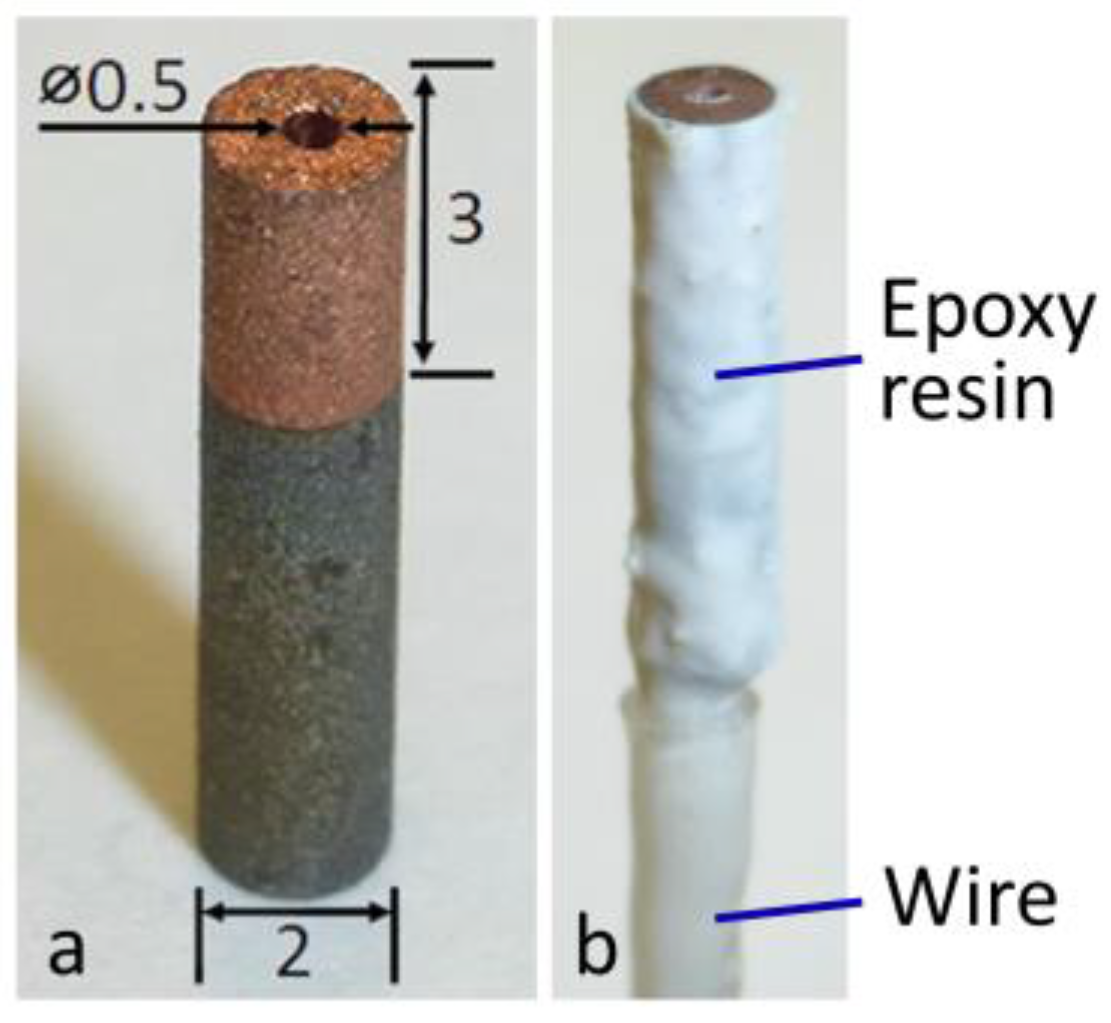
2.1. Cu-Coated Carbon Steel Specimens
2.2. Electrochemical Setup
2.3. Synchrotron X-ray Micro Computed Tomography (Micro-CT)
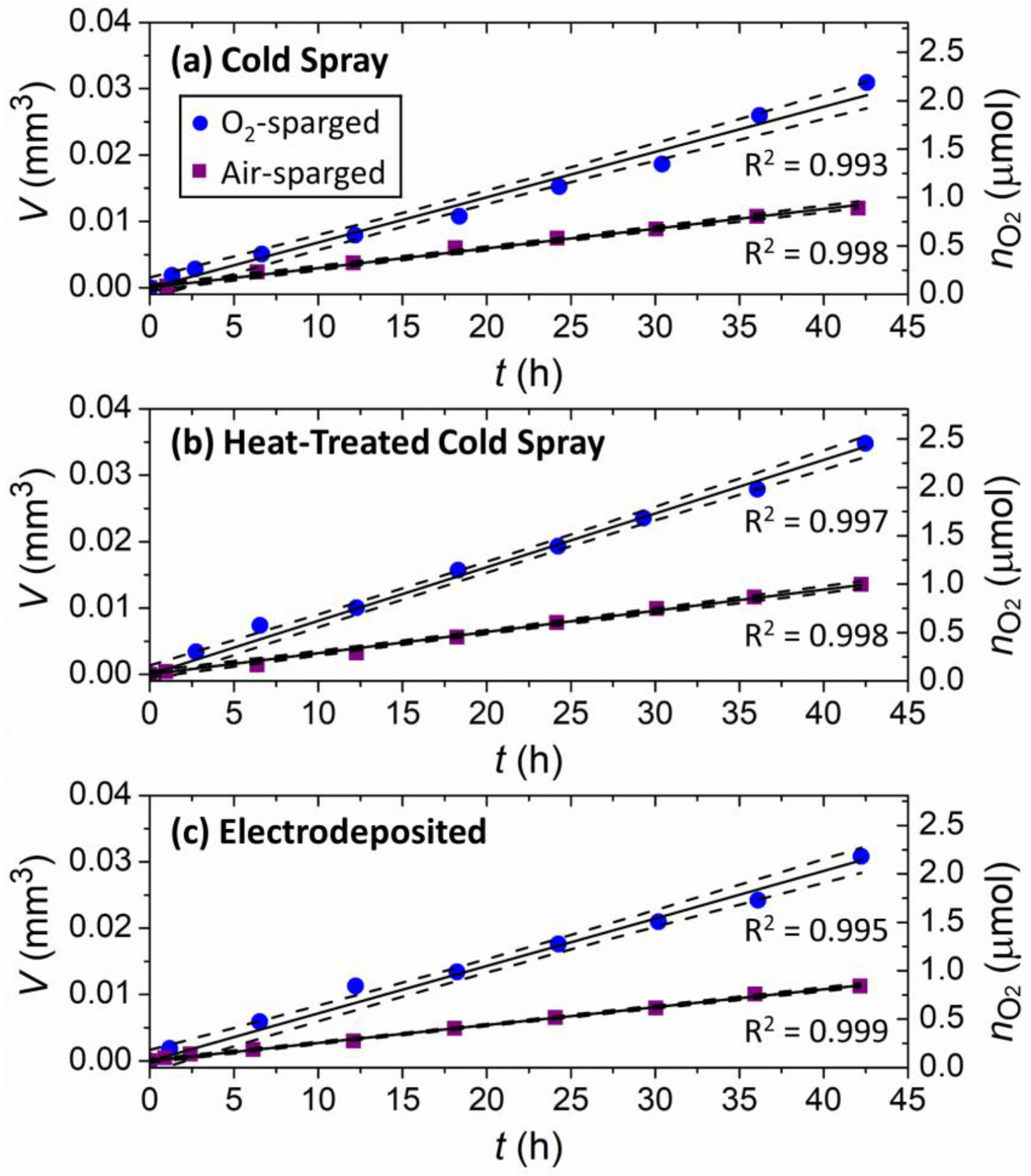
2.4. Damage Volume Measurements
3. Results and Discussion
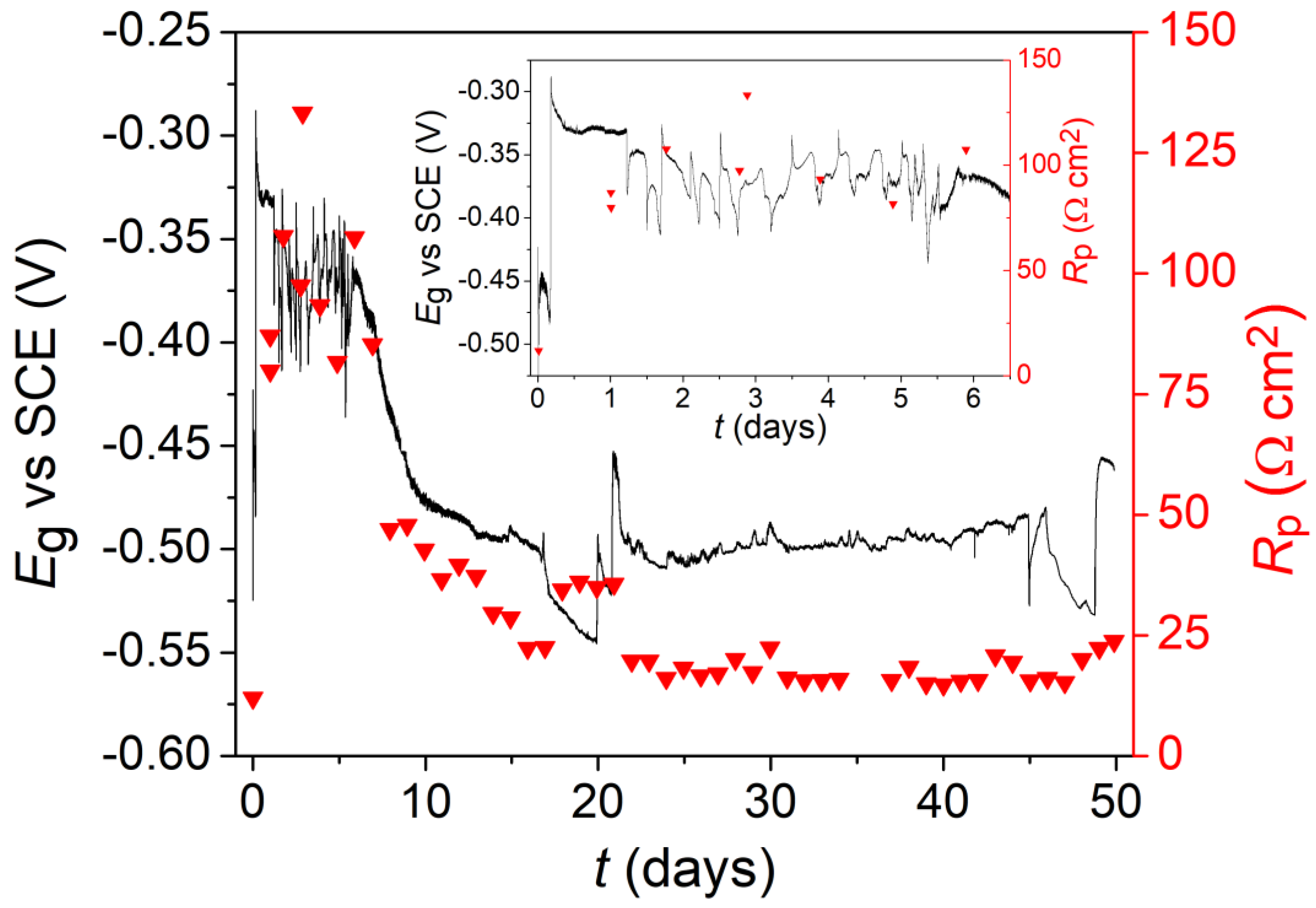
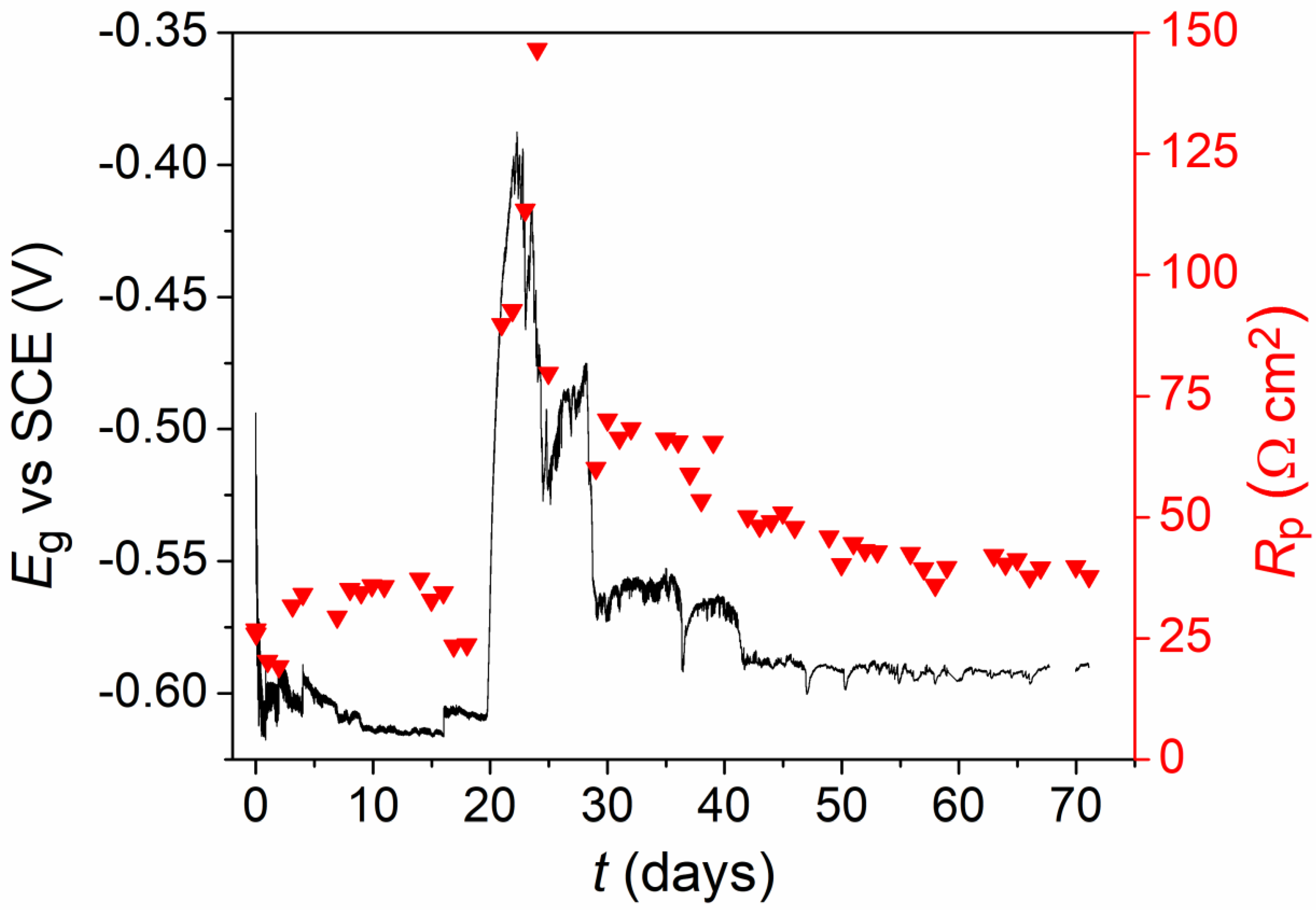
3.1. Electrochemistry
3.1.1. Oxygenated Conditions
3.1.2. Aerated Conditions
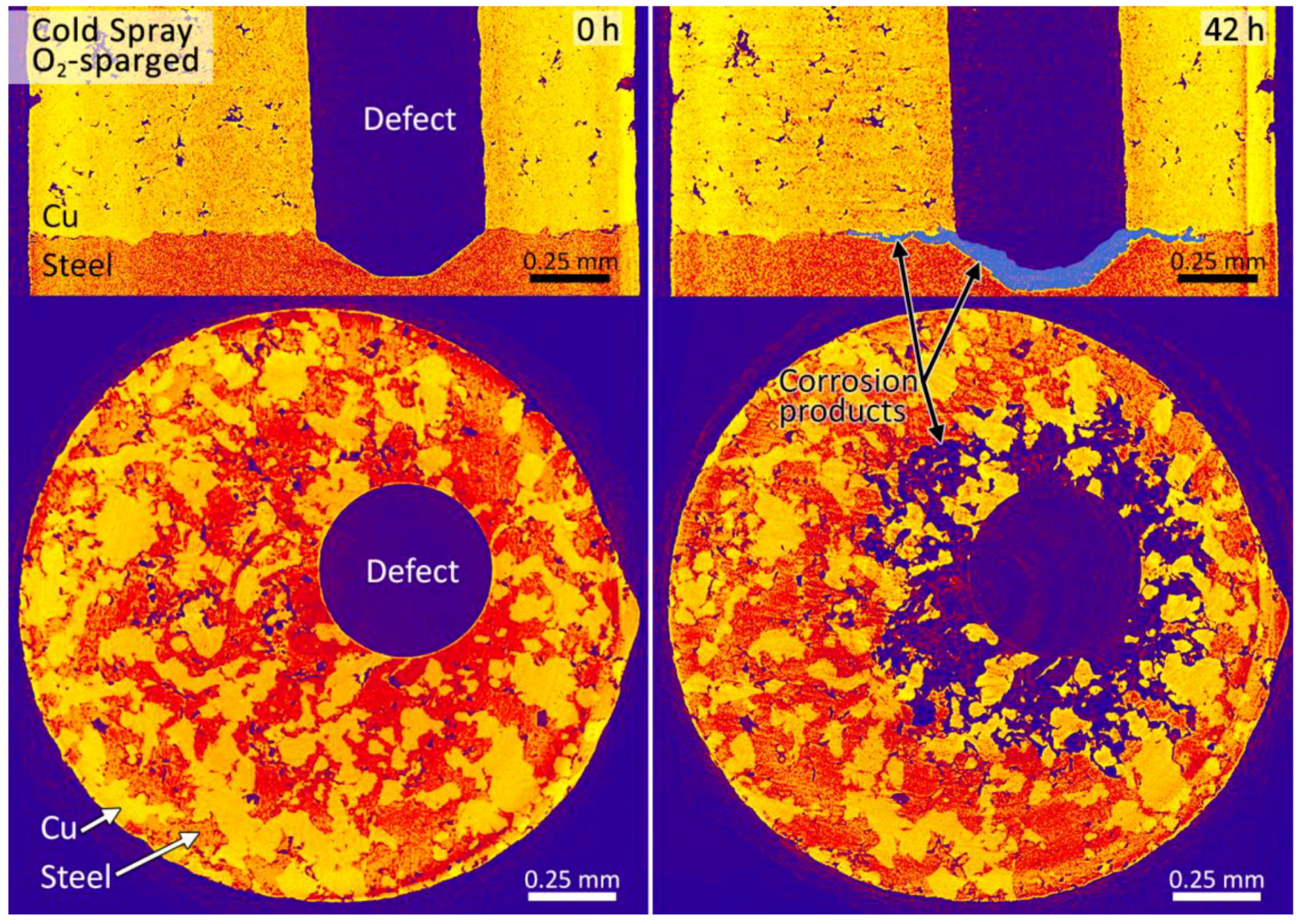
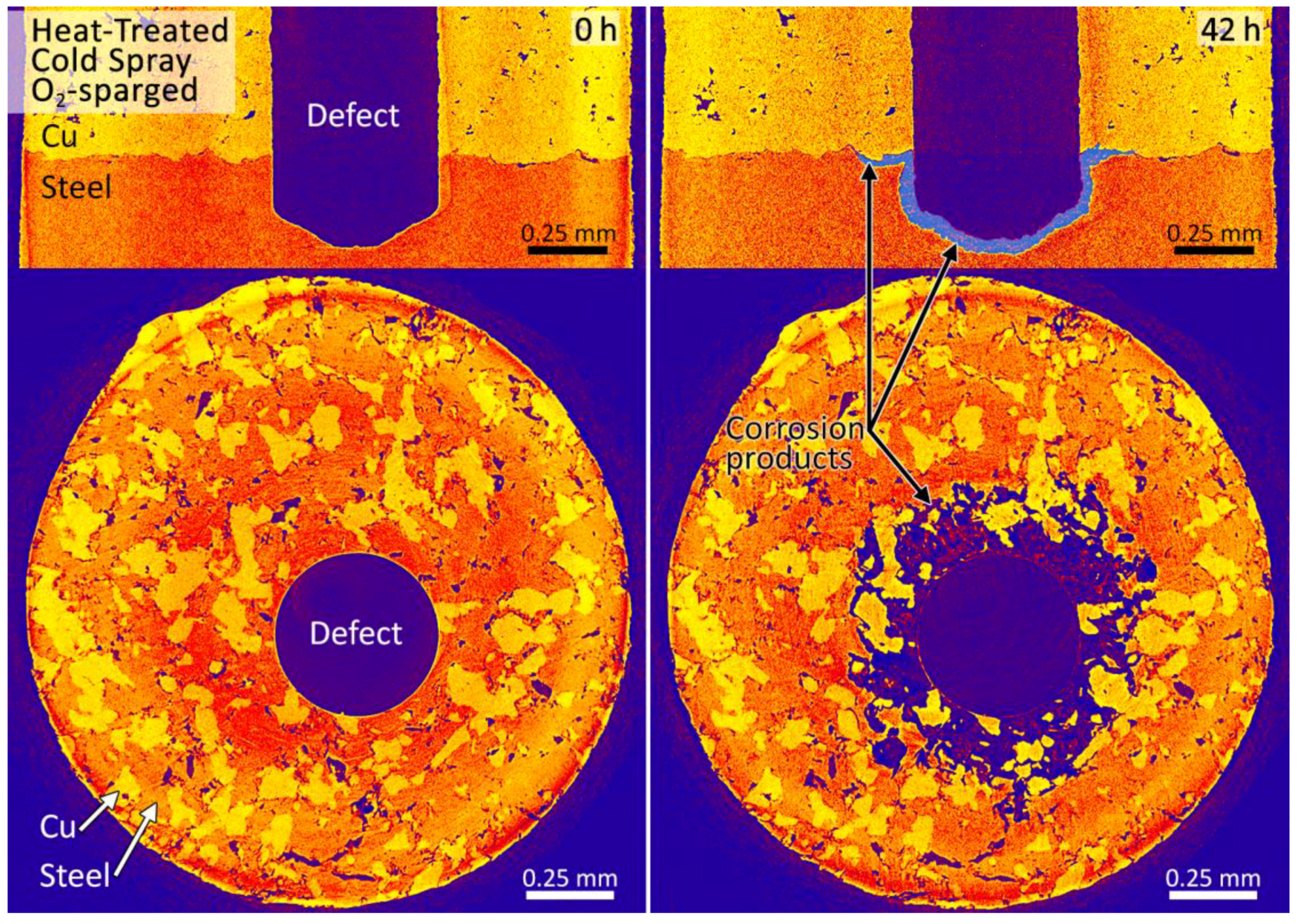
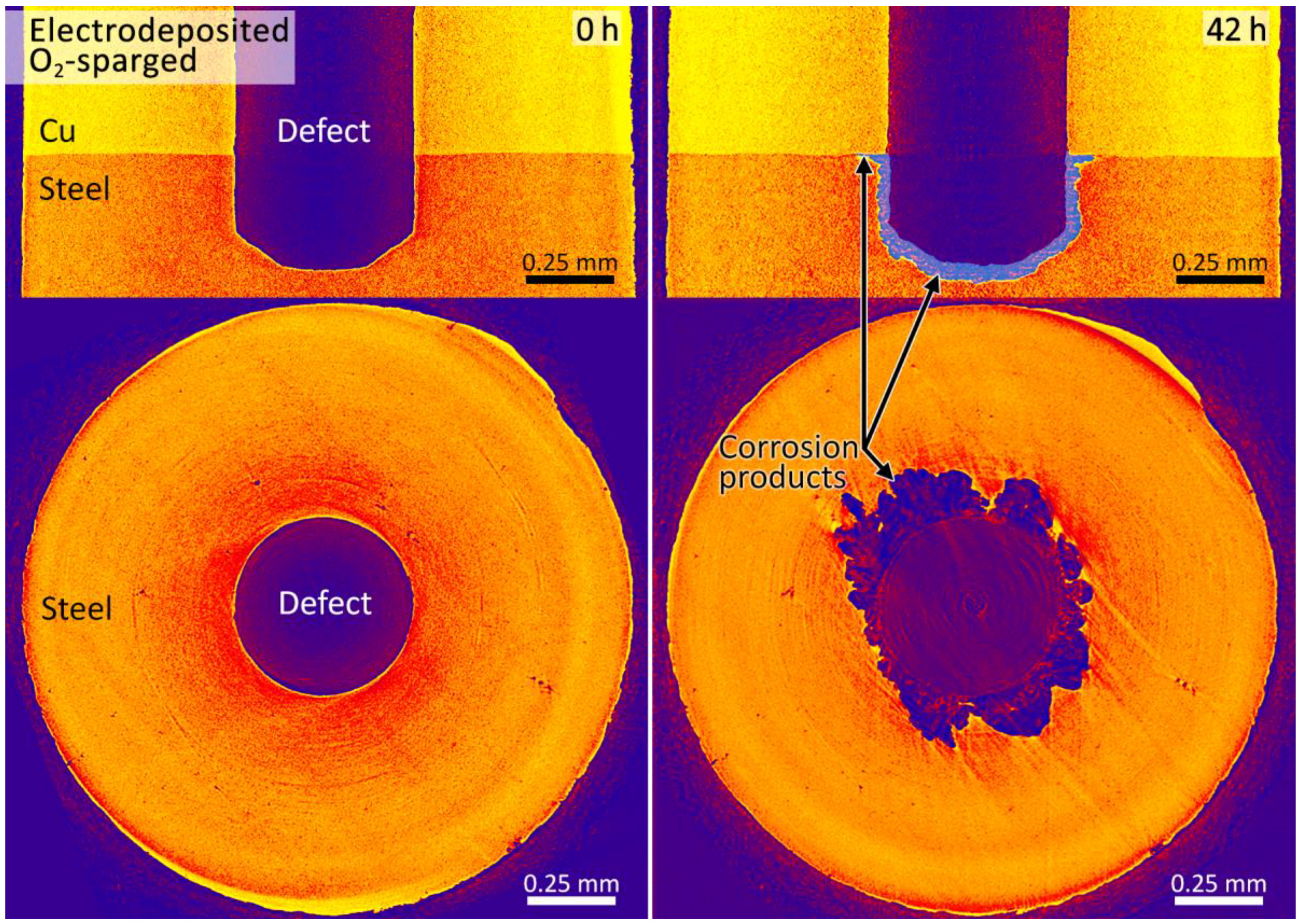
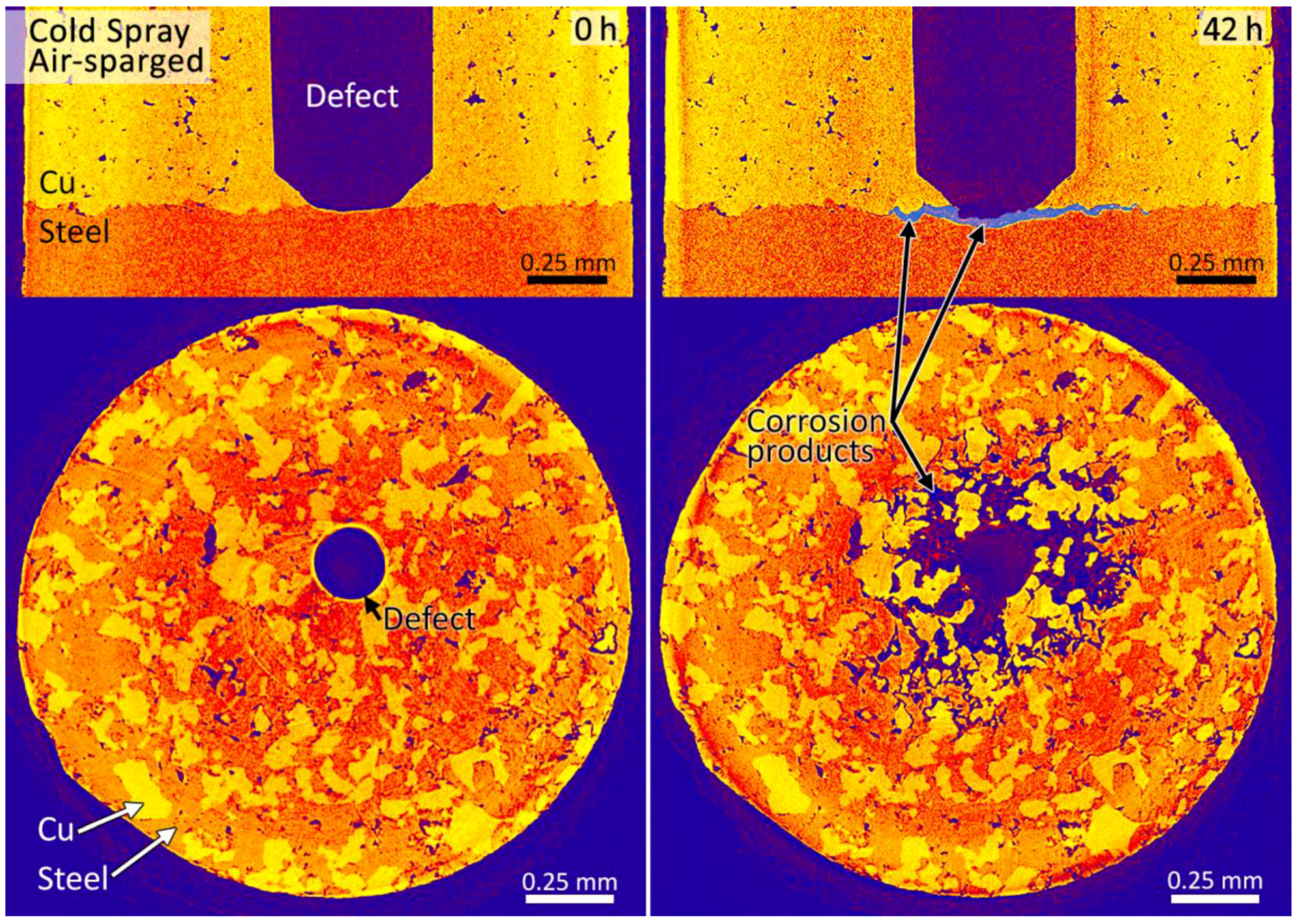
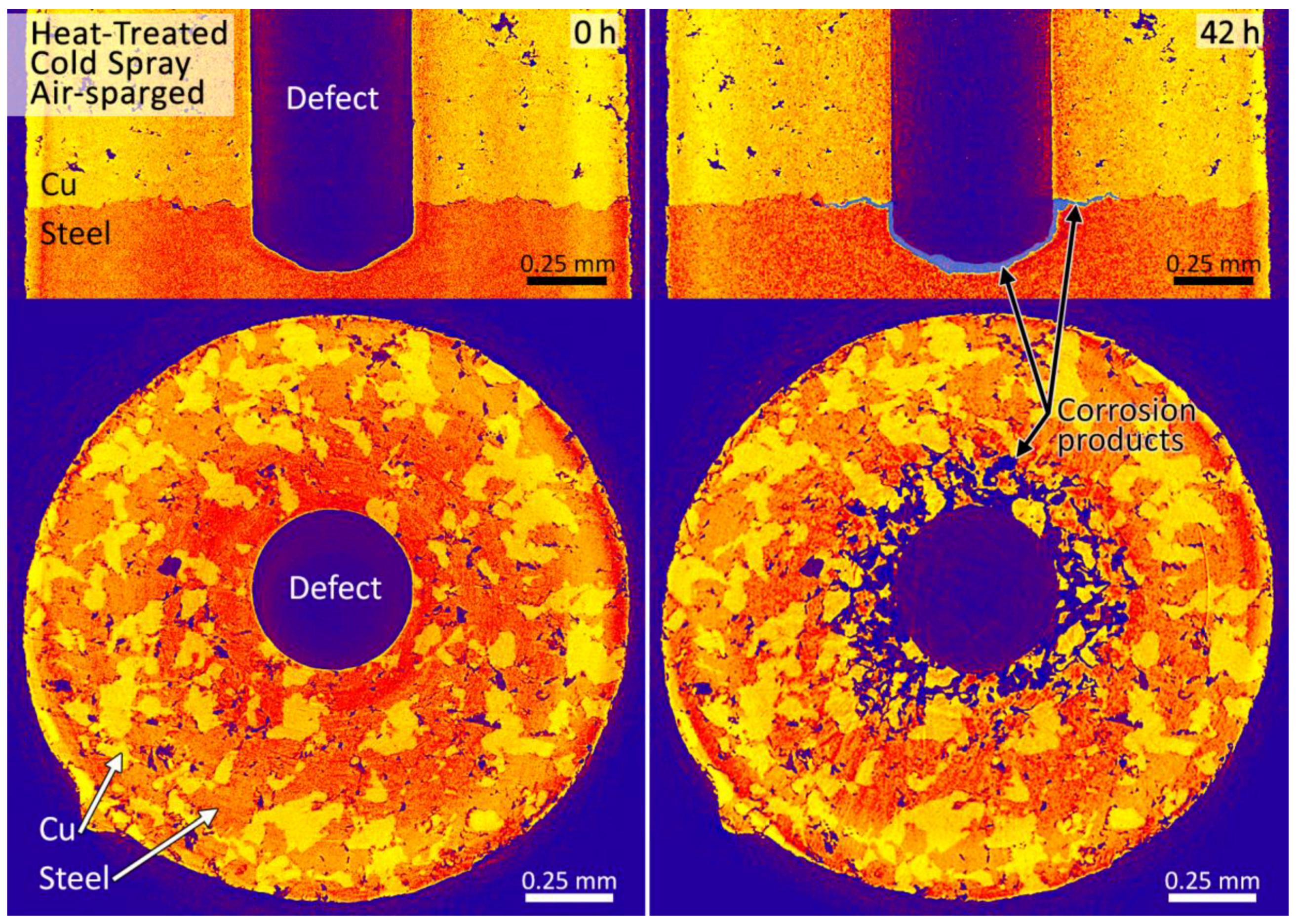
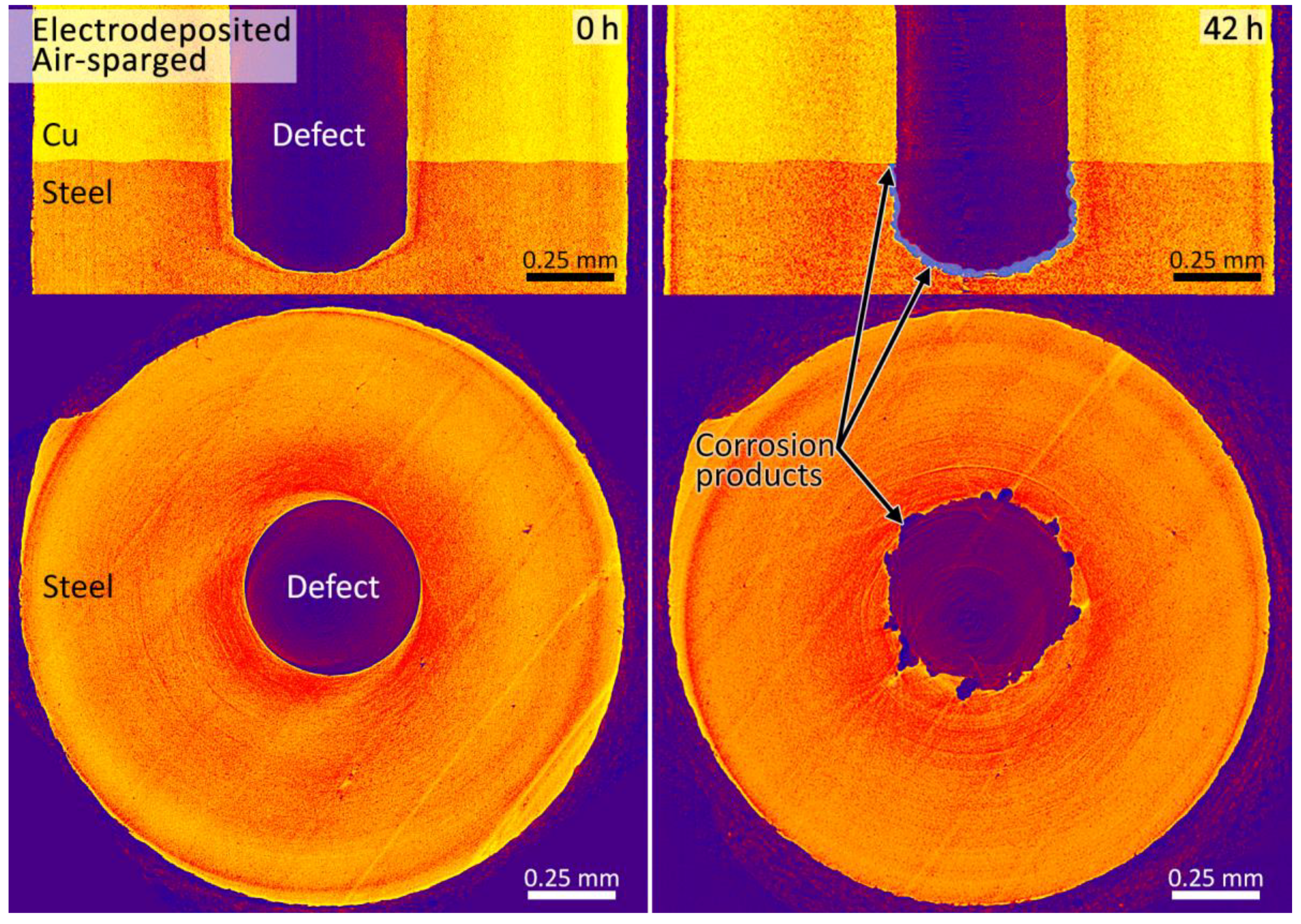
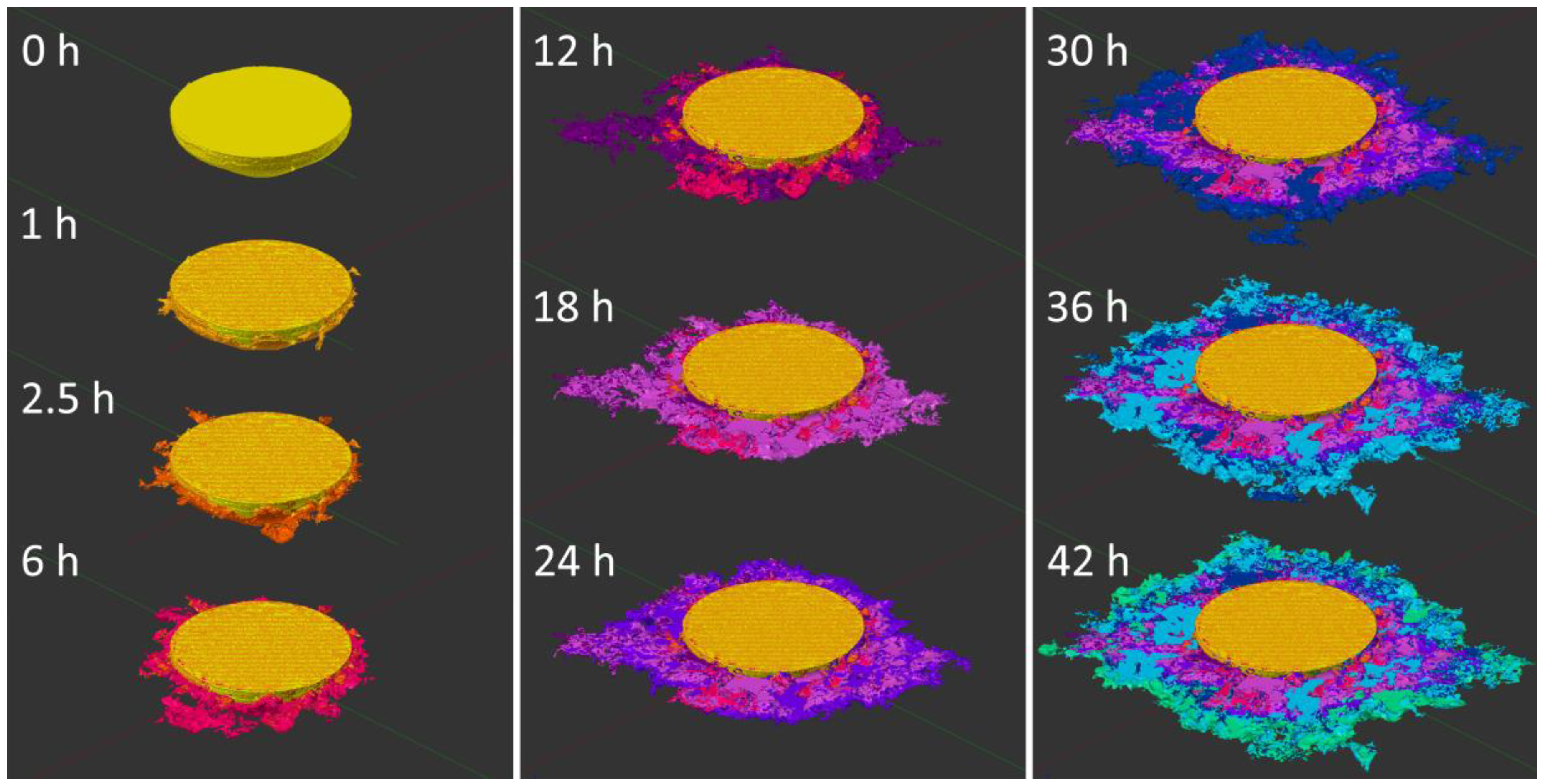
3.2. Synchrotron Micro-CT
4. Discussion
5. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Standish, T.; Chen, J.; Jacklin, R.; Jakupi, P.; Ramamurthy, S.; Zagidulin, D.; Keech, P.; Shoesmith, D. Corrosion of copper-coated steel high level nuclear waste containers under permanent disposal conditions. Electrochim. Acta 2016, 211, 331–342. [Google Scholar] [CrossRef]
- Standish, T.E.; Zagidulin, D.; Ramamurthy, S.; Keech, P.G.; Noël, J.J.; Shoesmith, D.W. Galvanic corrosion of copper-coated carbon steel for used nuclear fuel containers. Corros. Eng. Sci. Technol. 2017, 52, 65–69. [Google Scholar] [CrossRef]
- Keech, P.G.; Vo, P.; Ramamurthy, S.; Chen, J.; Jacklin, R.; Shoesmith, D.W. Design and development of copper coatings for long term storage of used nuclear fuel. Corros. Eng. Sci. Technol. 2014, 49, 425–430. [Google Scholar] [CrossRef] [Green Version]
- Jakupi, P.; Keech, P.G.; Barker, I.; Ramamurthy, S.; Jacklin, R.L.; Shoesmith, D.W.; Moser, D.E. Characterization of commercially cold sprayed copper coatings and determination of the effects of impacting copper powder velocities. J. Nucl. Mater. 2015, 466, 1–11. [Google Scholar] [CrossRef]
- Boyle, C.H.; Meguid, S.A. Mechanical performance of integrally bonded copper coatings for the long term disposal of used nuclear fuel. Nucl. Eng. Des. 2015, 293, 403–412. [Google Scholar] [CrossRef]
- Hall, D.S.; Keech, P.G. An overview of the Canadian corrosion program for the long-term management of nuclear waste. Corros. Eng. Sci. Technol. 2017, 52, 2–5. [Google Scholar] [CrossRef]
- Harinipriya, S.; Sangaranarayanan, M.V. Hydrogen evolution reaction on electrodes: Influence of work function, dipolar adsorption, and desolvation energies. J. Phys. Chem. B 2002, 106, 8681–8688. [Google Scholar] [CrossRef]
- Stoulil, J.; Kouřil, M.; Pavlova, L.; Dobrev, D.; Gondolli, J. 1D simulation of canister galvanic corrosion in saturated compacted bentonite. Mater. Corros. 2018, 1–7. [Google Scholar] [CrossRef]
- Vo, P.; Poirier, D.; Legoux, J.-G.; Keech, P.G.; Irissou, E. Application of copper coatings onto used fuel canisters for the Canadian nuclear industry. In High Pressure Cold Spray: Principles and Applications; Karthikeyan, J., Kay, C., Eds.; ASM International: Materials Park, OH, USA, 2016; pp. 253–276. ISBN 978-1-62708-096-5. [Google Scholar]
- Gürsoy, D.; De Carlo, F.; Xiao, X.; Jacobsen, C. TomoPy: A framework for the analysis of synchrotron tomographic data. J. Synchrotron Radiat. 2014, 21, 1188–1193. [Google Scholar] [CrossRef] [PubMed]
- Sherar, B.W.A.; Keech, P.G.; Shoesmith, D.W. Carbon steel corrosion under anaerobic-aerobic cycling conditions in near-neutral pH saline solutions. Part 2: Corrosion mechanism. Corros. Sci. 2011, 53, 3643–3650. [Google Scholar] [CrossRef]
- Wang, F.; Zhao, J.; Li, A.; Zhang, H. Effects of cutting conditions on microhardness and microstructure in high-speed milling of H13 tool steel. Int. J. Adv. Manuf. Technol. 2014, 73, 137–146. [Google Scholar] [CrossRef]
- Prakash, M.; Shekhar, S.; Moon, A.P.; Mondal, K. Effect of machining configuration on the corrosion of mild steel. J. Mater. Process. Technol. 2015, 219, 70–83. [Google Scholar] [CrossRef]
- Ghosh, S.; Kain, V. Microstructural changes in AISI 304L stainless steel due to surface machining: Effect on its susceptibility to chloride stress corrosion cracking. J. Nucl. Mater. 2010, 403, 62–67. [Google Scholar] [CrossRef]
- Lyon, K.N.; Marrow, T.J.; Lyon, S.B. Influence of milling on the development of stress corrosion cracks in austenitic stainless steel. J. Mater. Process. Technol. 2015, 218, 32–37. [Google Scholar] [CrossRef]
- Hall, D.S.; Standish, T.E.; Behazin, M.; Keech, P.G. Corrosion of copper-coated used nuclear fuel containers due to oxygen trapped in a Canadian deep geological repository a Canadian deep geological repository. Corros. Eng. Sci. Technol. 2018, 53, 309–315. [Google Scholar] [CrossRef]
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Standish, T.E.; Zagidulin, D.; Ramamurthy, S.; Keech, P.G.; Shoesmith, D.W.; Noël, J.J. Synchrotron-Based Micro-CT Investigation of Oxic Corrosion of Copper-Coated Carbon Steel for Potential Use in a Deep Geological Repository for Used Nuclear Fuel. Geosciences 2018, 8, 360. https://doi.org/10.3390/geosciences8100360
Standish TE, Zagidulin D, Ramamurthy S, Keech PG, Shoesmith DW, Noël JJ. Synchrotron-Based Micro-CT Investigation of Oxic Corrosion of Copper-Coated Carbon Steel for Potential Use in a Deep Geological Repository for Used Nuclear Fuel. Geosciences. 2018; 8(10):360. https://doi.org/10.3390/geosciences8100360
Chicago/Turabian StyleStandish, Thalia E., Dmitrij Zagidulin, Sridhar Ramamurthy, Peter G. Keech, David W. Shoesmith, and James J. Noël. 2018. "Synchrotron-Based Micro-CT Investigation of Oxic Corrosion of Copper-Coated Carbon Steel for Potential Use in a Deep Geological Repository for Used Nuclear Fuel" Geosciences 8, no. 10: 360. https://doi.org/10.3390/geosciences8100360
APA StyleStandish, T. E., Zagidulin, D., Ramamurthy, S., Keech, P. G., Shoesmith, D. W., & Noël, J. J. (2018). Synchrotron-Based Micro-CT Investigation of Oxic Corrosion of Copper-Coated Carbon Steel for Potential Use in a Deep Geological Repository for Used Nuclear Fuel. Geosciences, 8(10), 360. https://doi.org/10.3390/geosciences8100360




