Abstract
Many freshwater bivalves restore themselves to the sediment water interface after burial by upward escape burrowing. We studied the escape burrowing capacity of two modern unionoids, Elliptio complanata and Pyganodon cataracta and the invasive freshwater venerid Corbicula fluminea, in a controlled laboratory setting varying sediment grain size and burial depth. We found that the relatively streamlined E. complanata is a better escape burrower than the more obese P. cataracta. E. complanata is more likely to escape burial in both fine and coarse sand, and at faster rates than P. cataracta. However, successful escape from 10 cm burial, especially in fine sand, is unlikely for both unionoids. The comparatively small and obese C. fluminea outperforms both unionoids in terms of escape probability and escape time, especially when body size is taken into consideration. C. fluminea can escape burial depths many times its own size, while the two unionoids rarely escape from burial equivalent to the length of their shells. E. complanata, and particularly P. cataracta, are morphological paradigms for the extinct Devonian unionoid bivalve Archanodon catskillensis, common in riverine facies of the Devonian Catskill Delta Complex of the eastern United States. Our observations suggest that the escape burrowing capability of A. catskillensis was no better than that of P. cataracta. Archanodon catskillensis was likely unable to escape burial of more than a few centimeters of anastrophically deposited sediment. The long (up to 1 meter), vertical burrows that are associated with A. catskillensis, and interpreted to be its escape burrows, represent a response to episodic, small-scale sedimentation events due to patterns of repetitive hydrologic or weather-related phenomena. They are not a response to a single anastrophic event involving the influx of massive volumes of sediment.
1. Introduction
Archanodon is a genus of bivalves familiar to those who have worked on rocks of the Devonian Catskill Delta Complex in New York and Pennsylvania, and in the Carboniferous rocks of Atlantic Canada and northwestern Europe. However, from its initial designation as Cypricardites by Lardner Vanuxem in his monumental 1842 work on the geology and paleontology of New York State [1], Archanodon has also been at the center of considerable controversy regarding its taxonomy, paleoecology and paleoethology. Our focus here is on paleoethology, and on burrowing in particular. Our aim is to assess the upward escape burrowing capacity of Archanodon in terms of the functional morphology and burrowing behavior of modern Archanodon bivalve proxies. However, it is helpful to first outline some current issues in the taxonomy and paleoecology of this enigmatic bivalve.
1.1. Taxonomy
Vanuxem linked his Cypricardites to the common modern unionoid Anodonta in terms of shell form and freshwater habitat. Assignment of Archanodon to the bivalve order, Unionoida, was the accepted view during the years following (e.g., Hall [2]; Clarke [3]). Therein is the first of the taxonomic issues—is Archanodon actually a unionoid? In his survey of Devonian and Carboniferous freshwater bivalves, Weir [4] found it difficult to accept this idea because he saw the highly disjunct geographic distribution of Archanodon as atypical of what one would expect of a true unionoid with glochidial larval dispersal. However, using occurrences of Archanodon catskillensis discovered since 1969, Chamberlain and Chamberlain [5] argued that this species of Archanodon is no more disjunct in its pattern of occurrence in Catskill Magnafacies rocks than are many modern unionoid bivalves in modern New York State waterways. They suggested that unknown larval developmental strategy should not be seen as weighing against a unionoid assignment for Archanodon. In addition, Chamberlain et al. [6] show that A. catskillensis shells appear to have had numerous adventitious organic layers within the shell material as is typical of modern unionoids. In this paper we take the view, therefore, that Archanodon is indeed a unionoid, a view consistent with the bivalve taxonomic surveys of Newell [7] and Watters [8], who interpret Archanodon as a member of the Archanodontacea, a Paleozoic unionoid superfamily most probably unrelated to Post-Paleozoic and modern unionoid clades comprising the superfamily Unionacea.
The second taxonomic issue is the status of the name Archanodon. Vanuxem [1] identified two species, Cypricardites catskillensis and C. angustata, based on differences in overall shell shape. In his monograph on New York paleontology, Hall [2] united Vanuxem’s species under the name Amnigenia catskillensis because he felt that the shape of Vanuxem’s C. angustata, of which there was only one specimen, was altered during orogenic events later in the Paleozoic. More recently, Chamberlain and Chamberlain [5] have argued that Vanuxem’s specimen has not been stretched tectonically since there is no evidence of such distortion in the beds from which the sample was recovered, and that there are, in fact, many specimens shaped like Vanuxem’s C. angustata from a variety of locales in the states of New York and Pennsylvania. However, they refrained from re-establishing C. angustata as a species because of the well-known variability in shell shape within many modern unionoid species. Weir [4] subsumed Hall’s Amnigenia by uniting all Devonian and Carboniferous Anodonta-like freshwater bivalves under the name Archanodon. The name was first used by Howse [9] in his comparative analysis of his Lower Carboniferous Northumbrian form and the Irish Anodonta jukesii of Forbes [10] of roughly equivalent age. Also included in Weir’s [4] amalgamation was Amnigenia rhenana Beushausen [11] from the middle Devonian Schiefergebirge of northwest Germany and Aesthenodonta westoni Whiteaves [12] from the Carboniferous coal measures at Joggins, Nova Scotia. Although Chamberlain & Chamberlain [5] felt that Weir’s [4] taxonomic framework for Devonian and Carboniferous Anodonta-like freshwater bivalves of one genus (Archanodon) containing four species (catskillensis, rhenana, jukesii, and westoni) was unlikely to hold up as research on these bivalves progressed, it is currently in use and we adopt it in the present work.
1.2. Paleoecology
Vanuxem’s [1] identification of Archanodon catskillensis as an inhabitant of freshwater environments was accepted for more than a century [2,3,13] as were similar habitat assignments for A. jukesii and A. westoni [4,9,10,12]. Beushausen [11], however, interpreted A. rhenana as an inhabitant of brackish, coastal waters because of its occurrence in a greenish, greywacke sandstone lacking fully marine or terrestrial fossils, but containing abundant plant debris. Archanodon catskillensis is commonly associated with plant debris also [1,14,15], and it sometimes occurs in stratigraphic sequences representing delta front or tidal flat settings [14,15,16]. In addition, A. catskillensis occasionally occurs in association with animals of known brackish or marine affinities [13,15,17]. Such fossil associations are often difficult to assess, however. For example, at the A. catskillensis locality at East Windham, NY (Knox and Gordon [17]), beds containing these bivalves are not the same as beds containing marine or brackish water fossils [6,15]. However, the A. catskillensis beds at East Windham do contain freshwater ostracodes [18], and thus the paleoenvironment of this population of A. catskillensis appears to be freshwater rather than brackish. Nevertheless, it is clear that A. catskillensis inhabited lowland and deltaic areas close to, and perhaps in, bodies of brackish water, and that A. catskillensis probably had some brackish water tolerance (Friedman & Chamberlain [15]). It also seems apparent that as time progressed Archanodon lost its association with lowland, coastal areas because the later occurrences of the genus are in purely fluvial deposits. For example, the Pennsylvanian A. westoni inhabited an upland, fluvial environment periodically prone to drought and flooding [19,20,21].
1.3. Paleoethology
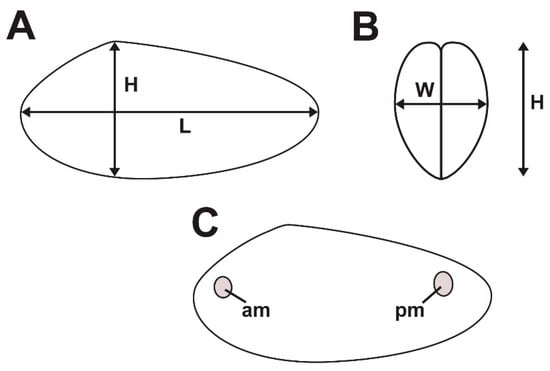
Two aspects of bivalve behavior have been a focus of discussion on Archanodon—living position and burrowing habits. Although most occurrences of Archanodon body fossils are post-mortem hydrodynamic accumulations—sometimes with valves imbricated and convex-up (Figure 1B)—a few examples are known in which the animals were preserved in life position. An elegant example is that of Bridge et al. [13] (Plate 3C) who show a bedding plane containing a cluster of A. catskillensis internal molds, all oriented preferentially and some with hydrodynamic scour marks on what must have been the upstream margin of the shell. From this, as Bridge et al. [13] suggest, it is evident that these specimens of A. catskillensis lived in a group of similar-sized (and aged?) individuals, shells partly nestled in the sediment with the hinge uppermost and inclined downward anteriorly so that the posterior shell margin extended well above the sediment-water interface facing upstream. This arrangement of individuals is also occasionally seen in rock slabs containing multiple specimens (Figure 1C). This is the life orientation adopted by the modern unionoid, Margaritifera margaritifera [22], and many other modern unionoids as well [23,24]. It anchors the animal in the sediment and exposes the siphonal openings of the posterior mantle margin to the oncoming flow (Figure 2). Nevertheless, many examples of departures from this posterior-upstream orientation—perhaps due to variation in small-scale, microenvironmental flow conditions surrounding individual animals—have been reported [25,26,27].
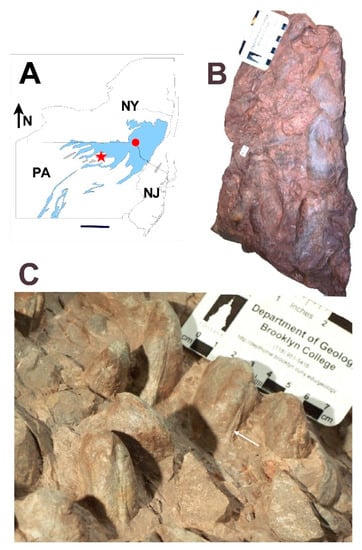
Figure 1.
Preservation styles of Archanodon catskillensis. (A) Map of New York State (NY), Pennsylvania (PA), and New Jersey (NJ) showing collection locality of the Paleontological Research Institute (PRI) specimen illustrated in B (red dot), and the Academy of Natural Sciences of Drexel University (ANDU) specimen illustrated in C (red star), respectively. Blue shading shows the outcrop area of terrestrial Catskill facies of Johnson and Friedman [30]. Scale bar = 100 km; (B) Siltstone slab showing post-mortem transported accumulation of A. catskillensis. All are internal molds; Downsville Arch, Downsville, Delaware County, NY; PRI, Trumansburg, NY, specimen #94-03-10. Scale divisions = 1 cm; (C) Siltstone slab showing A. catskillensis shell impressions in original life position steeply inclined to bedding. Shell casts have a subparallel orientation, most with hinge facing the camera. White arrow points to the hinge claustrum in specimen below the scale bar. Other specimens can also be seen to have a claustrum within their hinge lines. Specimen comes from the Catskill Formation exposed in a road cut, US Highway 15, near Powys, Lycoming County, PA. Slab is an unnumbered specimen held in the fossil collection of ANDU, Philadelphia, PA. Scale divisions = 1 cm.
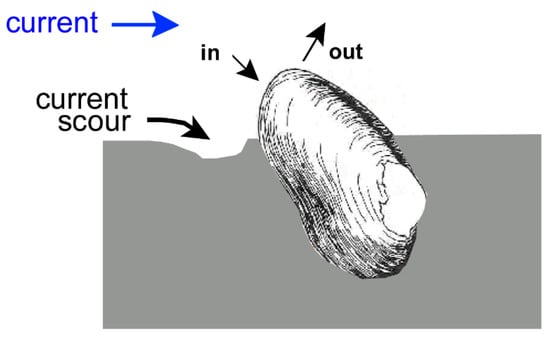
Figure 2.
Life position of Archanodon catskillensis. Hinge up, commissure down, shell inclined to sediment water interface so that the posterior remains free of sediment and the inhalant (in) and exhalant (out) siphonal openings are exposed to the flow. After Figure 9 of Chamberlain and Chamberlain [29].
Interest in A. catskillensis burrowing has primarily focused on the vertical meniscate burrows present in rocks of the Catskill deltaic complex (Figure 3).
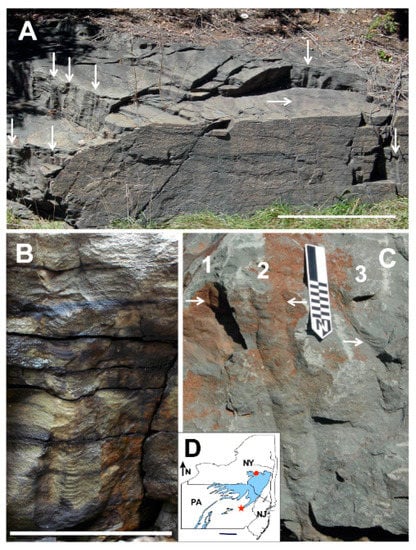
Figure 3.
Vertical Catskill Magnafacies burrows. (A) Outcrop of the Towamensing Member of the Catskill Formation, near Harrity, Pennsylvania, which exposes vertical burrows associated with shell impressions of Archanodon catskillensis body fossils found at the same site. Most burrows at this site show well developed menisci. Vertical arrows indicate individual burrows oriented perpendicular to bedding and seen in longitudinal view. Horizontal arrow indicates a cluster of about 15 burrows oriented perpendicular to bedding seen on a bedding surface in cross-sectional view. Scale bar represents 2 m. From Figure 6D of Chamberlain and Chamberlain 2007 [5]; (B) Single Harrity burrow with concave up menisci in the burrow core and down-turned bedding planes in the burrow halo. Scale bar represents 25 cm; (C) Three vertical burrows (numbered) in the Oneonta Formation, Jewett Quarry, Jewett, New York. Burrows do not show well developed menisci, although some are present (arrows). These burrows are not associated with A. catskillensis body fossils. Small scale divisions represent 1 cm. From Figure 6C of Chamberlain and Chamberlain 2007 [5]. (D) Map of New York State (NY), Pennsylvania (PA), and New Jersey (NJ) showing location of the Harrity, PA, outcrop (red star), and the Jewett, NY, quarry site (red dot). Blue shading shows the outcrop area of terrestrial Catskill facies of Johnson and Friedman [30].
Two questions present themselves: (1) were these burrows actually made by A. catskillensis; and (2) if so, what do the burrows signify with respect to the animal’s behavior? With regard to the first question, the structural similarity of the meniscate Catskill delta burrows to those made by modern bivalves and the association of many such burrows with A. catskillensis body fossils, including the rare occurrences of A. catskillensis body fossils preserved within them, has suggested to many workers that they are indeed burrows made by these animals [5,13,16]. However, not all vertical burrows in Catskill Delta rocks are likely to be the product of A. catskillensis, particularly those lacking menisci, or those with one or more branches, or those of sizes exceeding the dimensions of these bivalves [28]. Chamberlain and Chamberlain [29] surveyed characteristics of vertical Catskill Magnafacies burrows, as well as similar burrows in Atlantic Canada and northern Europe, and produced the listing of Archanodon burrow characteristics seen in Table 1. The second question noted above is the focus of the present paper.

Table 1.
Physical, environmental, and temporal characteristics of burrows constructed by Archanodon catskillensis. After Figure 13 of Chamberlain and Chamberlain [29].
1.4. Aims of the Research Discussed Here
Missing in the discussion on A. catskillensis burrowing is an understanding of the burrowing behavior and capabilities of unionoid bivalves. That is what we address here. We selected three modern bivalves, two of them unionoids, which we use as morphological and behavioral models for A. catskillensis. We examined the burrowing of these two modern A. catskillensis proxies as a means of illuminating the burrowing ability of their Devonian predecessor. The third species, an invasive venerid bivalve, was selected to compare against the burrowing behavior of the unionoids. We are primarily interested in the burrowing potential of these modern bivalves as a function of sediment grain size and burial depth below the sediment-water interface. We are also interested in the morphological attributes of the burrows that they construct, and the biomechanical processes they use to move through the sediment. Finally, we use the data gained to interpret the paleoenvironmental and paleoethological significance of the ancient burrows associated with A. catskillensis. We would emphasize that using the behavior of modern animals to help interpret the behavior of fossil animals is a widely applied paleobiological approach, as, for example, inferring swimming behavior of fossil nautiloids and ammonoids from that of modern Nautilus [31,32,33]. This is particularly true in paleoichnology where fossil burrowing behavior is understood primarily via deductions from modern burrowers and the burrowing patterns that they create [34,35,36,37,38,39].
One particularly exciting new research avenue in the study of bivalve burrowing is the use of operational, inanimate robots to interpret burrowing behavior. This approach has evolved from the lever-controlled, aluminum-epoxy, “burrowing” shell models of Stanley [40] to sophisticated, self-propelled, bivalve robots that mimic the size, shape, and burrowing movements of their live counterparts [41,42,43,44,45,46]. The application of robotics to problems in ichnology has great potential for providing valuable data unobtainable by other means, for example in evaluating burrowing of hypothetical animals having morphologies or behaviors not seen in nature. As emphasized by Raup [47,48,49] in his work on theoretical morphology and by McGhee [50], it is often as illuminating to learn why certain morphologies or behaviors do not occur in nature as it is to learn why others do occur.
2. Bivalve Burrowing Behavior
Although bivalves are largely sedentary, many forms are equipped for locomotion across the sediment surface, for downward burrowing to attain an optimal living position and for upward burrowing to escape burial. Marine forms have been the preferred target of studies on bivalve locomotion; relatively little attention has been directed toward freshwater bivalves.
2.1. Downward Burrowing
The typical bivalve burrowing sequence for some littoral venerid species involves repetitive actions of the foot, adductors and retractors [51,52]. The animal usually positions itself with its plane of symmetry vertically inclined to the sediment water interface, or nearly so, and usually descends with its anterior shell margin leading the way into the sediment. First, the animal’s foot penetrates the substrate and dilates to anchor the animal in the substrate. The siphons close, and the adductor muscles contract to quickly close the valves. The adduction of the valves ejects water from the mantle margins near the foot to fluidize sediment beneath the anterior margin of the shell, thus making the substrate here less cohesive, and more readily penetrable. Subsequently, first the anterior, then the posterior retractor muscles contract, which pulls the shell downward into the sediment, often with a rocking motion. Once this movement is concluded, the adductor muscles relax and the cycle begins anew.
Stanley [53] found that in marine bivalves downward burrowing rate is dependent on animal size and shape. Small animals burrow more rapidly than large animals in proportion to their size, because as Stanley [53] noted, the contraction time of a muscle is proportional to its length, and muscle strength increases at an increasingly slower rate relative to its size as the animal grows. This means that without allometric muscle growth, a bivalve’s muscles operate more slowly and become weaker relative to body mass as the animal increases in size. In addition to animal anatomy, shell morphology determines the relative ease with which individuals of different species can penetrate the substrate. Trueman et al. [54], for example, were able to assess the relationship between shell width of some common marine bivalves and force needed for penetration into the substrate, and concluded that species with slender, streamlined shells minimize the force needed to burrow.
Downward burrowing in freshwater bivalves is similar to that observed for marine species [55]. In a series of downward burrowing experiments on the unionoid Margaritifera margaritifera, Trueman [22] discovered that Margaritifera, and perhaps unionoids generally, exhibit a digging cycle similar to that of marine bivalves in consisting of muscle adduction, foot dilation and retraction, and water ejection from the mantle cavity to fluidize sediment below the shell. Lewis and Riebel [56] found that downward burrowing in Elliptio complanata and Pyganodon grandis was achieved using the same patterns of muscle and shell movements as in other unionoids and marine bivalves generally, and that sediment type influenced burrowing speed in these animals. Watters [57] showed that some aspects of shell shape and sculpture influence unionoid burrowing ability.
2.2. Escape Burrowing
Among modern bivalves, vertical, meniscate burrows of the kind associated with A. catskillensis are produced when animals burrow upward in an effort to restore their normal living position relative to the sediment-water interface following sediment deposition events [34,35,37]. This allows for the siphons or siphonal openings to be in contact with the water for respiration, feeding, excretion and reproduction [58]. Because animals are attempting to escape new sedimentary overburden, this behavioral process is referred to as escape burrowing. In as much as A. catskillensis burrows show this burrow structure, this is the burrowing mode in which we are primarily interested here.
2.2.1. Marine Bivalves
Escape burrowing has been widely studied among modern marine bivalves [51,52,53,54,59,60,61,62,63,64], particularly those bivalves with some economic value. A parameter of prime interest has been escape potential—i.e., the ability of an animal to escape a given depth of burial. Field experiments conducted by Glude [59] showed that the escape potential of the commercially important infaunal softshell clam, Mya arenaria, is largely dependent on depth of burial (the deeper the burial, the lower are survival chances), initial animal position, animal size (the larger the animal, the better is the chance of survival) and grain size (silt and coarse sand limit survival changes relative to fine sand). In a similar study, Shulenberger [62], who investigated escape behavior of the small saltwater clam Gemma gemma, observed that the animal’s escape potential is higher in sand than in silt. He also found that G. gemma is able to survive burial for a maximum of six days, and that it almost always burrows vertically, especially at greater depths.
Kranz [63] assessed the escape potential of modern marine bivalves typical of many life-habit groups. His experiments suggested that escape potential is largely determined by three factors: (1) the animals’ foot shape, which functions in anchorage or leverage in the sediment; (2) the volume of interstitial and mantle cavity water available for sediment fluidization; and (3) the degree of mantle fusion, which influences the hydrostatic pressure used to create the water jets that drive sediment fluidization. According to Kranz [63], shell orientation during escape burrowing can be the same as the downward burrowing orientation, i.e., the animal “backs” out of the overlying sediment with the shell posterior leading the way upward (Figure 4A). This requires that the animal use its foot to push off the underlying substrate. It is the periodic compression by the foot of the sediment directly beneath it that produces the characteristic meniscate structures seen in bivalve burrows. Sediment fluidization near the foot, critical in downward burrowing, would seem to be counter-productive in escape burrowing because it would act to degrade the solid footing the animal needs for its foot to push it upward. Sediment fluidization would also destroy menisci. In contrast, some bivalves burrow upward using the same orientation in which they burrow downward, i.e., shell anterior leading the way (Figure 4B). This requires shell rotation in the substrate to bring the shell anterior above the posterior, but once achieved, it enables the animal to pull itself out of the sediment [63]. This mode of escape burrowing does not produce meniscate burrows [37]. Kranz [63] observed that the mode of escape burrowing is chiefly determined by the type of foot and by shell shape. Extensible, flexible feet are associated with pulling the shell upward (e.g., Donax and Phacoides), whereas animals with large muscular feet push themselves upward (e.g., Clinocardium and Ensis). Due to the energy required for sub-surface rotation, most bivalves adopt the pushing orientation for escaping shallow burial [63]. Only those with a roughly ovate, equidimensional shell shape can rotate sufficiently within the sediment to bring the shell into an anterior up position needed to pull themselves upward.
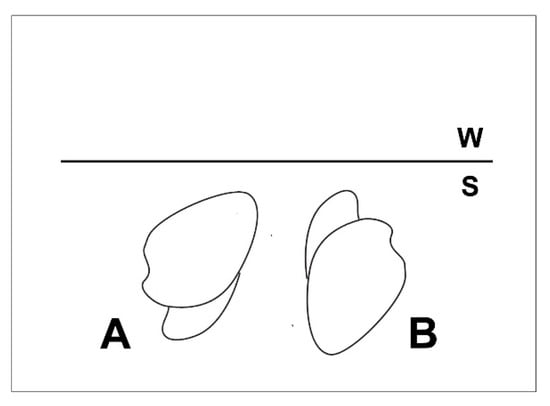
Figure 4.
Generalized clam orientation during upward escape burrowing. (A) Shell anterior downward; foot pushes animal upward; (B) Shell anterior upward; foot pulls animal upward. S—sediment; W—water.
Kranz [63] also observed that rapid downward burrowers are also rapid escape burrowers, although as noted by Stanley [53], escape rates are generally slower than downward burrowing rates. In addition, Kranz [63] suggests that upon burial bivalves are able to determine which way is up based on their balance sensory receptor, or statocyst, located near the pedal ganglia. Upward movement, as Eagar [55] found, is triggered once the protruding siphons are cut off from the overlying water mass by accumulating sediment.
2.2.2. Freshwater Bivalves
Although it would seem that freshwater bivalves would also have a need to contend with shifting sediment and periodic burial, work on their escape burrowing is practically non-existent. To be sure, attention has been given to vertical movement in freshwater bivalves relating to shell size, reproductive status, seasonal temperature variation, predation and desiccation avoidance [65,66,67,68,69,70], but not escape burrowing as defined here.
Modern unionoids have siphons reduced in size and complexity compared to venerids and many other bivalve groups. Many lack true siphons altogether. Consequently, they usually burrow very shallowly with their posterior partially protruding from the sediment or buried just beneath so that water flow into the mantle cavity is not fouled by sediment [71]. Trueman [52] and Yeager et al. [72] deal with the mechanics of downward, non-escape burrowing, and find that in general it mirrors the downward burrowing mechanisms of marine bivalves. Horizontal, surface crawling is also commonly observed among modern unionoids [69,70,72] and has been attributed to avoiding environmental stresses such as low oxygen and inadequate food availability, and to enhancing reproductive success [68,69]. While these studies provide valuable information on unionoid motility, they do not address the issue of escape burrowing in these animals.
3. Comparative Shell Form
Our approach to the issue of escape burrowing in modern and fossil unionoids involves experimentation on the burrowing ethology of four different bivalve species having different shell morphology. We describe and evaluate these morphological differences here as a means of setting forth the experimental basis for our work and to give a sense of the important morphological variables that we investigated in our experiments.
3.1. Shell Form of Archanodon Catskillensis
As originally noted by Vanuxem [1] Archanodon catskillensis typically has a subelliptical, inequilateral, equivalved shell morphology (Figure 5) similar to that of some modern unionoids [5,15,73]. There is also a much rarer elongate form [5] (Figure 5B), which Hall [2] mistook for a tectonically altered normal shell. In this paper, we deal with the more common, normal, subelliptical morph.
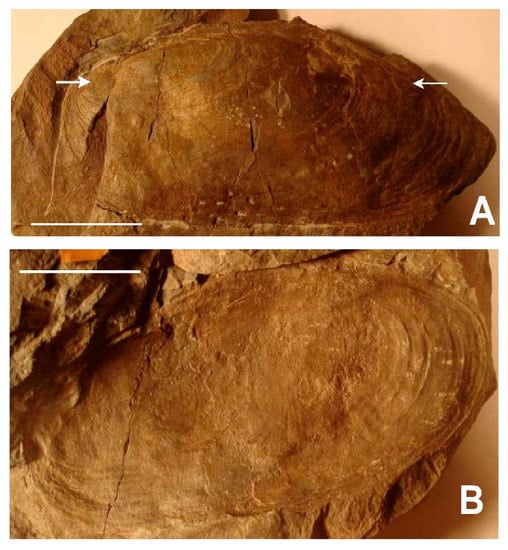
Figure 5.
Shell form of Archanodon catskillensis. Two different specimens preserved on a slab of hard siltstone now held in the invertebrate fossil collections of the American Museum of Natural History (AMNH), New York, which Hall [2] used to define his Amnigenia catskillensis. The slab is labeled AMNH FI 006331, and is noted as having been collected near Oneonta, New York. (A) Shell impression with adductor muscle scars indicated by white arrows; (B) Shell impression with exposed hinge line lacking obvious hinge teeth. Anterior to the left in both specimens. Scale bars = 3 cm in both (A,B).
Figure 5 also shows that in subelliptical morphs, the posterior region of the shell is enlarged and inflated relative to the short, rounded shell anterior. This is a feature typically seen in modern unionoid bivalves in which it is linked to the enlarged mantle cavity needed to house the glochidial brood chamber. Posterior inflation sometimes produces a shallow ventral sinus as in Figure 5A, the magnitude of which varies considerably intraspecifically (the shell in Figure 5B does not have a ventral sinus). There is no shell ornamentation apart from shallow concentric growth lines (Figure 5). As noted by Weir [4], the hinge is long and relatively straight and lacks hinge teeth (Figure 5B). However, many specimens show a pronounced claustrum (Figure 1C), a structure which probably assumes to some extent the function of hinge teeth. Adductor muscle scars are rarely seen. Among the specimens (locality and stratigraphic setting detailed in Figure 1, Figure 3 and Figure 5; and from the Gilboa Formation in East Windham, NY, which is not depicted here), both whole and fragmentary, upon which this study is based (approximately 100), we found only one, which showed a visible adductor muscle scar (Figure 5A). Absence of muscle scars is a taphonomic phenomenon, and is not due to an actual absence of adductor muscles in these animals. Adductor scars are ovate and relatively small compared to the size of the shell (see Table 2).

Table 2.
Shell shape and muscle scar size for Archanodon catskillensis and three modern species, used here as burrowing proxies for A. catskillensis. Definitions for shell elongation, obesity and muscle scar size are given in Figure 6. Size range is based on maximum and minimum shell lengths for the specimen populations measured. Standard deviations around the mean are indicated where calculated. Dashed lines indicate no data available.
3.2. Archanodon Catskillensis Analogues
As noted above, our approach to investigating burrowing in A. catskillensis involves evaluating the burrowing of modern bivalve analogues. We have selected three modern bivalves to examine in this study: two unionoids, Pyganodon cataracta and Elliptio complanata; and the freshwater venerid Corbicula fluminea, which we include not as a close proxy for A. catskillensis, but rather as a yardstick representing apex bivalve burrowers against which to measure the performance of the unionoids. All are abundant in waterways of the eastern United States and none are on state or federal endangered species lists. Typical examples of the shells of these species are shown in Figure 7.
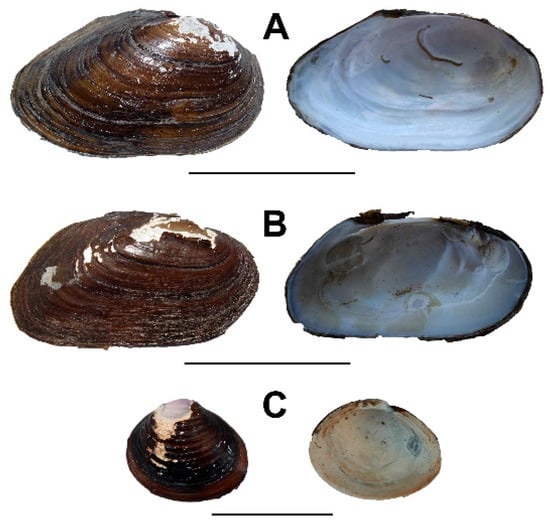
Figure 7.
Shell form for the three modern Archanodon catskillensis proxies studied here. (A) Pyganodon cataracta. Scale bar = 7 cm; (B) Elliptio complanata. Scale bar = 5 cm; (C) Corbicula fluminea. Scale bar = 3 cm.
3.2.1. Pyganodon Cataracta
This species, also known as the eastern floater, is one of the most common freshwater bivalves in the eastern United States and even occurs within New York City itself [74]. P. cataracta inhabits a wide range of freshwater habitats including marshes, lakes, and rivers, but is particularly common in quiet, protected water bodies [23,74]. It normally lives with only the shell anterior buried in the sediment at an acute angle, typically with the long axis of the shell at 45°–60° to the sediment-water interface. We have observed that in our maintenance tanks smaller specimens frequently burrow more deeply than larger ones. Usually, most of the shell is exposed above the sediment surface. In fact, we have often encountered animals in the wild, especially large ones, not buried at all. Instead, the animals are totally exposed on the sediment surface usually upright or nearly so, resting on the ventral margin of their shell. This too we observed in our maintenance tanks.
Of our two A. catskillensis analogues, P. cataracta is most similar to A. catskillensis. It is one of the very few modern unionoids to equal A. catskillensis in shell size, and is the only one of our three analogues to do so (Table 2). Both species have equivalved, elongated, slim shells, although shell shape in A. catskillensis is slightly more extreme in terms of elongation and obesity (Table 2). These differences are statistically significant (Table 3), and suggest that burrowing capacity in A. catskillensis would probably benefit from its somewhat greater streamlining. The shells of both species have a shallow ventral sinus, and like A. catskillensis, P. cataracta has a long, relatively straight hinge with no hinge teeth (Figure 7). However, P. cataracta appears to have larger adductor muscles relative to its size than A. catskillensis (Table 2), although the fact that we could find only one A. catskillensis shell showing discernable muscle scars, precludes statistical evaluation of this parameter. P. cataracta is thus a proxy of considerable similitude to A. catskillensis.

Table 3.
Results of t-tests for shell shape and muscle size parameters. 0 means no test; + means significantly different (p ≤ 0.001); — means not significantly different (p > 0.001). ARC—Archanodon catskillensis; PYG—Pyganodon cataracta; ELL—Elliptio complanata; COR—Corbicula fluminea.
Another unionoid species extremely common in waterways of the eastern United States is the eastern elliptio, E. complanata. This species inhabits a wide range of different freshwater environments from small streams to large rivers and lakes [23]. E. complanata often lives with most the shell positioned below the sediment-water interface, with only the posterior shell edge and siphonal openings exposed. Occasionally we have found animals in life position, particularly smaller ones, completely below the sediment surface. However, it can also be found totally exposed on the surface of the sediment, and we have sometimes observed it crawling slowly along the surface on its ventral margin. This species has an elongated, equivalved shell lacking prominent ornamentation, a shallow ventral sinus, and obesity values similar to those of A. catskillensis (Table 2 and Table 3). However, E. complanata is generally smaller in size (usually 5–8 cm) and infrequently slightly greater than 10 cm in shell length) [23]. In contrast, A. catskillensis reaches lengths exceeding 20 cm [4,5,15]. In addition, E. complanata differs markedly from A. catskillensis in possessing prominent hinge teeth (Figure 7) and probably much larger adductor muscles relative to its size (Table 2 and Table 3).
3.2.2. Corbicula Fluminea
Corbicula fluminea, known in North America as the Asiatic clam, is native to drainages in China and southeastern Asia. In recent decades, it has quickly become the most invasive freshwater venerid species in North America due to its tolerance for unstable habitats [75]. C. fluminea individuals live more frequently below the sediment surface, often several cm below the surface, than they do partly exposed at the sediment-water interface. In this, they differ from the two unionoid study species. C. fluminea has a small (<50 mm), thick, relatively rounded to trigonal shell, which is ornamented with distinct elevated concentric ridges (Figure 7). In these respects, its shell differs considerably from that of A. catskillensis (Table 2 and Table 3). Hinge dentition of C. fluminea consists of prominent cardinal and lateral teeth, and its umbo is inflated and extends above the dorsal shell margin. Unlike our other two proxies, C. fluminea has extensive mantle fusion, bears true siphons and is equipped with highly developed statocysts that help make it an effective burrower compared to many native American unionoids [75].
4. Experimental Methods
4.1. Specimen Collection
Live specimen collection was done in water up to 1 m in depth, either by hand-probing in turbid water, or in clear water by visually locating siphons or shell margins at the sediment-water interface. Animals were then extracted from the sediment by hand. Aquascopes (metal tubes with a clear plastic bottom) were used to scan the sediment surface when surface water agitation impeded clear viewing.
For this study, 82 live specimens of P. cataracta were collected in Willowbrook Pond and Clove Lakes Pond, Staten Island, NY; Millstone River, Kingston, NJ; and Brunnels Pond, Bridgeport, CT. Empty, dead shells were also collected at these localities and in Wolf Pond, Staten Island, NY, in the latter case following the catastrophic drainage in 2011 of the animals’ coastal pond due to Hurricane Irene. Sixty-three live specimens of Elliptio complanata were collected from the following localities: Susquehanna River, Millersburg, PA; Raritan River, New Brunswick and Piscataway, NJ; Lamington River, Readington, NJ; Delaware and Raritan Canal, East Millstone, NJ; Passaic River, Basking Ridge, NJ; Brunnels Pond, Bridgeport, CT; and Norwalk River, Norwalk, CT. Empty, dead shells were collected from most of these sites also. One hundred and forty-eight live specimens of Corbicula fluminea were collected from the following localities: Susquehanna River, Millersburg, PA; Raritan River, New Brunswick and Piscataway, NJ; Mill Pond, Plainsboro, NJ; Clove Lakes Pond, Staten Island, NY; Brunnels Pond, Bridgeport, CT; and Norwalk River, Norwalk, CT. Empty, dead shells were collected at these sites also.
4.2. Animal Maintenance
The animals were kept in maintenance aquaria of 0.038 to 0.114 m3 (10 to 30 US gallons) in the Aquatic Research and Environmental Assessment Center (AREAC) at Brooklyn College (http://www.brooklyn.cuny.edu/web/academics/centers/areac.php). Testing was also done using AREAC facilities. Maintenance aquaria were supplied with filtered, re-circulating water at 17 °C, and contained a thick layer of sand into which the animals could burrow. Animals were held for no longer than 3 months. Surviving animals were returned to their original collection sites after this time. The animals were fed twice a week with phytoplankton and zooplankton obtained from a commercial aquarium supplier. Water quality, diet and animal vitality (as indicated by immediate valve closure in reaction to external stimuli, e.g., touch, light variation) were strictly monitored to insure that only animals with no obvious decline in vitality were used for the burrowing experiments.
4.3. Escape Burrowing Tests
Escape burrow testing procedures used here were adapted from Shulenberger [62] and Kranz [63]. Testing was conducted in glass and Plexiglas aquaria large enough to allow testing of several animals to run simultaneously. Test aquaria were supplied with filtered, recirculating water. The base of each test aquarium was covered with a layer of sand deep enough for the animals to attain their normal living position when first placed in the aquarium (Figure 8).
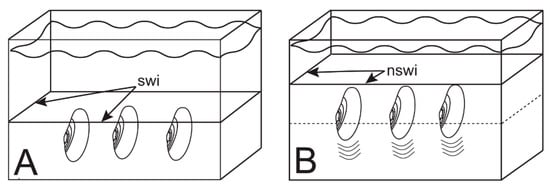
Figure 8.
Experimental setup. (A) Three animals in life position at start of burrowing test. swi—original sediment water interface; (B) Animals burrowing upward to escape addition of new sediment. nswi—new sediment water interface.
Test aquaria were partitioned into compartments using perforated plastic dividers that allowed for exchange of water between compartments. Partitions were spaced so that animals would not interfere with one another, and their burrowing movements would not be constrained by the walls of their enclosure. Sediment surface and water level positions were measured using a metric scale. Experiments were conducted using two size classes of sand: fine sand (grain size = 2Φ to 3Φ; 0.25 to 0.125 mm); and coarse sand (grain size = 0Φ to1Φ; 1 to 0.5 mm). These grain sizes were selected because they commonly dominate many fluvial, deltaic, and estuarine sedimentary environments. We tested animals in sand composed totally of one size class, and then ran a separate series of tests in which animals were tested in the other size class.
To start a test, animals of the same species and of similar size were placed in a test aquarium and allowed to burrow downward to attain their normal semi-infaunal (P. cataracta and E. complanata) or shallowly infaunal (C. fluminea) living positions. Animals that failed to do so were assumed unhealthy and replaced. Following Kranz [63], sand of a specific grain size was released above the test aquarium and allowed to settle gently to simulate natural sedimentation. This produced a layer of new sand through which a test specimen was forced to burrow to re-establish its normal life position with siphons and posterior shell edge exposed above the sediment-water interface as diagrammed in Figure 8. Burial depth is here defined as the vertical distance from the posterior shell margin prior to the addition of new sand to the new sediment-water interface.
Animal behavior was recorded using time-lapse and real-time photography. Animals that failed to move upward at all within 24–36 h (depending on burial depth; some mortalities occurred after 24 h), were deemed unable to extricate themselves, and were thus removed, placed back in the maintenance tanks to facilitate recuperation, and excluded from further experiments. Most animals were used only once as test specimens. Animals subject to more than one burrowing test were allowed ample time, usually more than one week, to recuperate between trials.
4.4. Burrow Structure Tests
To illuminate the structure of escape burrows, single specimens of P. cataracta and E. complanata underwent burial in vertically oriented PVC pipes. These pipes were 50 cm in length, 15 cm in internal diameter, and closed off at the base. Initially, a pipe was filled with water and had a layer of sand at its base into which an animal was allowed to burrow. When the test specimen had adopted its normal living position, layers of colored sand were successively added until a burial depth of 10 cm was achieved. This produced layers of differently colored sand through which the test animal would have to burrow to restore its position at the new sediment-water interface within the pipe. Upon successful escape, the animals were slowly removed so as not to disturb the sand; excess water was drained; and the wet, sand-filled pipe was frozen in a common trunk freezer. When frozen solid, the sand-filled pipe was sectioned using a rock saw to reveal burrowing structure as represented by the post-burrowing distribution of colored sand within the pipe.
5. Field Methods
Burrows made by the fossil bivalve Archanodon catskillensis are known from a large number of locations in New York, Pennsylvania and New Jersey [5,13,16,28]. Perhaps no single locality shows A. catskillensis burrows to better advantage than an outcrop of the Towamensing Member of the Catskill Formation near Harrity, Pennsylvania, first described by Epstein et al. [76], and illustrated here as Figure 3A. At this site, dozens of A. catskillensis burrows are revealed in both longitudinal section and cross section in a planar-bedded, fine-grained sandstone. Figure 3B shows one of these burrows. At the top of the outcrop are hundreds of A. catskillensis shell impressions. In order to get a better sense of the character of the sediment into which these animals had burrowed, we made thin sections from rock samples collected adjacent to burrows from this exposure, including the burrow seen in Figure 3B. From these thin sections, we quantified such lithologic attributes of the burrowed sediment as grain size and composition, and interpreted the original depositional environment.
6. Experimental Results
The testing procedures described above allowed us to generate evidence on the following burrowing parameters: escape orientation; escape potential; escape time; and escape burrow morphology.
6.1. Escape Orientation
Although the sediment used in this study is opaque, the orientation of escaping animals as they break through the new sediment surface can be readily observed. This is shown in Figure 9. From Figure 9A,B, it is evident that the escape burrowing orientation of both P. cataracta and E. complanata is the same as their living position, i.e., individuals of these species advance upward posterior shell margin first. This orientation was ubiquitous; it occurred in every test we ran for these two species. This mode of escape is the same as that observed by Kranz [63] for marine bivalves with elongate, inequilateral shells similar in shape to the shells of P. cataracta and E. complanata. In species with elongate shells, such as these two unionoids, the foot is situated anteriorly and thus emerges nearly parallel to the animals’ longitudinal axis. Rotation of the shell within the sediment is not normally possible, and when escaping they “back out” of the newly deposited layer of sediment, shell posterior leading the way. They do this by pushing on the sediment beneath the shell with the foot, so that they move upward slightly. The foot is then retracted and the space vacated by the foot fills with sediment sloughing inward from the edges of the burrow. The process is repeated until the animal regains its living position at the new sediment-water interface. Sand fluidization occurs just around the siphon openings in a quick apparent burst (Figure 9D). Whether posterior fluidization occurs while an animal remains below the surface of the sediment cannot be ascertained from our testing procedure, but it seems unlikely because, in the moments prior to the shell edge emerging, sand at and just below the surface arches upward and outward as it is pushed aside by the advancing animal. This “bow wave” is quite large in the case of P. cataracta (Figure 9A).
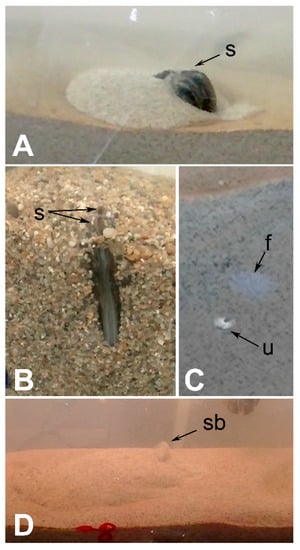
Figure 9.
Still images of video footage showing shell orientation during upward escape burrowing. (A) Pyganodon cataracta breaking through sediment surface (fine sand: 2Φ to 3Φ) as seen from the side of the test tank. Sand pushed aside by the escaping animal is visible as the mound of light-colored material surrounding the emerging shell; (B) Elliptio complanata breaking through the sediment surface (coarse sand: 0Φ to 1Φ) as viewed from above; (C) Corbicula fluminea in the process of escaping as seen from the side of the test tank. This view shows an animal at the inner edge of the glass wall of the aquarium burrowing upward. All that is visible is the shell umbo where it is in contact with the glass and the mucous trail of the foot where it lies just inside the glass. f—foot; s—siphon; u—shell umbo; (D) “Siphon blow” of sediment upon emergence of Elliptio complanata. This phenomon was observed in all three test species. sb—siphon blow.
The escape mode used by C. fluminea differs from that observed in the two unionoids. Rather than pushing itself off of the underlying substrate in a series of escape burrowing cycles, C. fluminea extends its foot upward while only minimally rotating its shell within the substrate (Figure 9C). This is readily facilitated by the relatively round shell shape of this species (Table 2). As Stanley [53] pointed out in species with rounded shells, such as C. fluminea, the foot extends perpendicularly to the longitudinal axis of the shell approximately opposite the hinge. Thus, once C. fluminea anchors its foot in the sediment, it can either pull or push itself upward depending on the direction in which the foot is flexed. As a result, C. fluminea may be able to both push and pull itself through anastrophic overburden. Where shell and foot were visible in our experiments, as in Figure 9C, locomotion via pulling was the primary means of escape. As was apparent in both unionoids, emergence at the surface was usually accompanied by an upward burst of fluidized sand from around the siphons (Figure 9D). We were unable to visually ascertain whether sediment was fluidized in front of the leading edge of the shell as the animal moved upward prior to emergence, but this observation suggests that such a strategy is possible.
6.2. Escape Potential
Escape potential is the probability that an animal will escape burial and re-establish its normal living position with the shell partly exposed above the sediment-water interface. We follow Kranz [63] in expressing this parameter as the percentage of test specimens successfully escaping anastrophic burial.
6.2.1. Fine Sand
Experimental results for escape potential in fine sand (2Φ ≥ grain size ≥ 3Φ) are given in Figure 10 and Table 4. Figure 10 indicates that in fine sand, all three test species have high escape probabilities at low burial depths. However, as burial depth increases escape potential declines markedly. P. cataracta has increasing difficulty successfully escaping a burial depth of 5 cm or more, whereas in C. fluminea and E. complanata lower escape probabilities become significant at burial depths of about 8 cm. C. fluminea, despite its small size, seems able to deal with deeper burial in fine sand relatively well. In contrast, both E. complanata and P. cataracta may be approaching their maximum escape capabilities at about 10 cm.
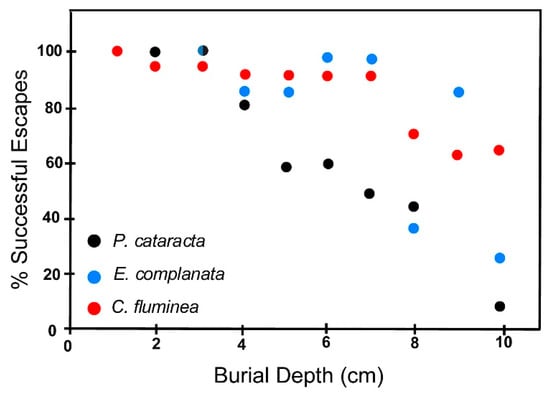
Figure 10.
Escape potential of the three test species in fine sand (2Φ ≥ grain size ≥ 3Φ) expressed as a percent of successful escapes as a function of burial depth. A successful escape is one in which an animal breaks through the new sediment-water interface associated with the addition of a blanket of sand to the test tank, and regains its normal life position. Burial depth is the vertical distance between the new sediment water interface and the posterior margin of the animal before it initiates upward escape burrowing. Two semicircles indicate a convergence of datapoints for two species. Bicolored dot symbols indicate overlapping datapoints for two species.

Table 4.
Escape potential data for fine sand (2Φ ≥ grain size ≥ 3Φ). PYG—Pyganodon cataracta; ELL—Elliptio complanata; COR—Corbicula fluminea. In columns 2–4, the number to the left of the comma in each paired entry refers to the number of animals used in the tests upon which Figure 10 is based. The number to the right of the comma refers to the number of animals that died in the process of escape burrowing within our 24–36 h time limit. A dash means that no tests were made.
Thus, in terms of body size (which we define as shell length), C. fluminea can escape from anastrophic events producing burial depths many times its body size reasonably well, while P. cataracta and E. complanata show seriously compromised escape potential at depths equivalent to only about one body length. A complicating consideration here is the fact that C. fluminea individuals often live completely buried in the sediment, as noted above, so that escape to the surface would not seem to be as critical for them as for the other two species.
Table 4 shows that there is an added dimension to escape potential—at deeper burial depths some animals that are unable to free themselves die within the 24 to 36 h exposure limit of our testing regimen. P. cataracta has a higher mortality than does E. complanata. C. fluminea, on the other hand, shows no mortality at all as a function of long intervals of time within the sediment, an observation that is consonant with their predilection for subsurface life.
6.2.2. Coarse Sand
The escape capabilities of the test species in coarse sand (0Φ ≥ grain size ≥ 1Φ) are shown in Figure 11 and Table 5. The same general pattern of decreasing escape probability and increasing mortality with increasing burial depth is observed here as for tests in finer sand outlined above. However, there are differences, among which the most obvious is that there is a wider scatter to the data we report for the coarser sand tests. This makes interpretation of these results difficult. Nevertheless, E. complanata seems to be more likely to free itself from burial in coarse-grained sand than either P. cataracta or C. fluminea. As in fine sand, escape capabilities of P. cataracta are poorest in burial depths greater than 6 cm. Generally, both unionoids, but especially P. cataracta, are more likely to escape burial in coarse-grained sand than in fine grand sand, whereas C. fluminea appears to be more successful in fine sand.
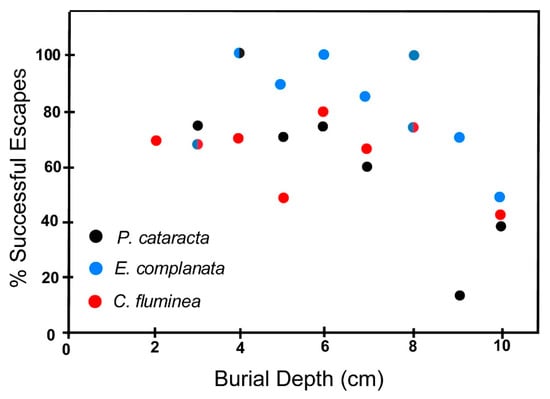
Figure 11.
Escape potential of the three test species in coarse sand (0Φ ≥ grain size ≥ 1Φ) expressed as a percent of successful escapes as a function of burial depth. Successful escape and burial depth defined as in Figure 10. Bicolored dot symbols indicate overlapping data points for two species.

Table 5.
Escape potential data for coarse sand (0Φ ≥ grain size ≥ 1Φ). PYG—Pyganodon cataracta; ELL—Elliptio complanata; COR—Corbicula fluminea. In columns 2–4, the number to the left of the comma in each paired entry refers to the number of animals used in the tests upon which Figure 11 is based. The number to the right of the comma refers to the number of animals that died in the process of escape burrowing within our 24–36 h time limit. A dash means that no tests were made.
6.3. Effect of Shell Size on Escape Potential
The size of a bivalve can be expected to influence escape potential. For example, large animals buried anastrophically to a particular depth may have less difficulty escaping than smaller animals of the same species buried to the same depth because, being larger, they are, relative to their body size, closer to the new sediment surface than a small conspecific. In the large animals, this would presumably require fewer digging cycles of the type observed in Margaritifera by Trueman [22,52]. In this regard, both Glude [59] and Kranz [63] observed that in the marine species they studied generally larger animals are more likely to escape burial than smaller animals of the same species. They interpreted this as the result of large animals needing to travel fewer body lengths to restore their living depth than do small animals.
On the other hand, it seems reasonable to expect that this size-escape relationship may not be so clear-cut. Muscle strength scales with size as the cross-sectional area of a muscle [77]. This would be the adductor and pedal muscles in burrowing bivalves. In contrast, the mass to be moved scales with volume, which for escaping bivalves is the animal itself plus the sediment and water that must be pushed aside. Volume increases more rapidly with increasing size than does area, so that a burrowing bivalve growing isometrically will become relatively weaker and less able to burrow effectively as it grows. This negative effect of size increase can be offset by allometric growth of the propulsive anatomy relative to the rest of the animal. As shown in Figure 12, however, allometry in propulsive musculature does not occur in our three test species, at least with respect to muscle scars that record the cross sectional area of the adductor muscles. In all three species, muscle scar area scales linearly with shell area rather than as an allometric power function of shell area (shell area1.5) needed for muscle strength to keep pace with size increase of the animal. In addition, larger muscle systems operate more slowly than smaller ones so that the digging cycle in burrowing bivalves should operate more slowly in large animals than in small ones. Trueman [61] and Stanley [53] observed this to be the case in several different marine bivalves.
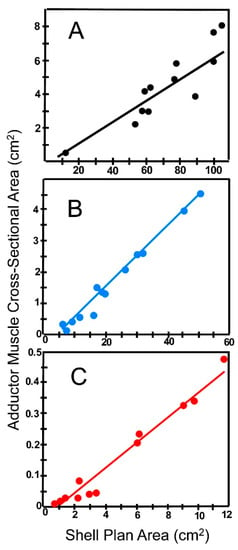
Figure 12.
Sum of the cross-sectional area of the anterior and posterior muscle scars regressed as a function of plan area of the shell (plan area is the area of a flat surface defined by the periphery of the shell). Specimens selected randomly from among our total collection. (A) Pyganodon cataracta; (B) Elliptio complanata; (C) Corbicula fluminea. Note that three the graphs have different units on the X and Y axes. Regression parameters: P. cataracta: Y = 0.076X − 0.906; R = 0.906. E. complanata: Y = 0.101X − 0.485; R = 0.993. C. fluminea: Y = 0.043X − 0.030; R = 0.982.
We evaluated the effect of shell size on escape potential in terms of escape to the sediment surface. Our observations indicate that in escape burrowing the siphons of C. fluminea typically reach the sediment-water interface. Nevertheless, because C. fluminea individuals often live below the sediment water interface, as noted above, it is not clear that for this species failure to reach the surface when escaping from anastrophic burial is actually failure. For this reason, we do not consider C. fluminea further with regard to this parameter. With respect to P. cataracta and E. complanata we evaluated our results in three ways. We analyzed shell size and escape for our total data set, i.e., all test runs. We then divided our data into two depth categories, shallow burial (1–5 cm burial depth) and deep burial (6–10 cm burial depth); and evaluated each separately. This gives us a sense of whether shell size influences success at different depths. Our results for the three testing regimens are shown in Table 6.

Table 6.
Escape from anastrophic burial as a function of shell size, burial depth, and sediment grain size for Pyganodon cataracta and Elliptio complanata. L—Shell length in cm. σ—standard deviation. Grain size; Coarse—0Φ to1Φ; Fine—2Φ to 3Φ. Total data set—results for all tests conducted from 1 to 10 cm burial depth. Shallow Burial —results for tests conducted only from 1 to 5 cm burial depth. Deep Burial—results for tests conducted only from 6 to 10 cm burial depth.
The data in Table 6 do not strongly support either alternative. In coarse sand, larger P. cataracta individuals have less success in escaping anastrophic burial, but the relationship is not uniform across depth categories. In fine sand, the data are not sufficiently discriminating to confidently distinguish the effect of shell size. It is apparent that more precise testing, testing which allows one to compare performance at finer depth intervals and with many more specimens than were available to us, particularly at the extremes of the size range in these two species, is needed to resolve this issue more clearly because the sizes of the individuals tested represent only a relatively small portion of the actual size range of the two test species.
6.4. Escape Time
We measured the time required for animals escaping anastrophic burial to reach the new sediment-water interface. Our results are shown in Figure 13 for both coarse sand (Figure 13A,C,E) and fine sand (Figure 13B,D,F).
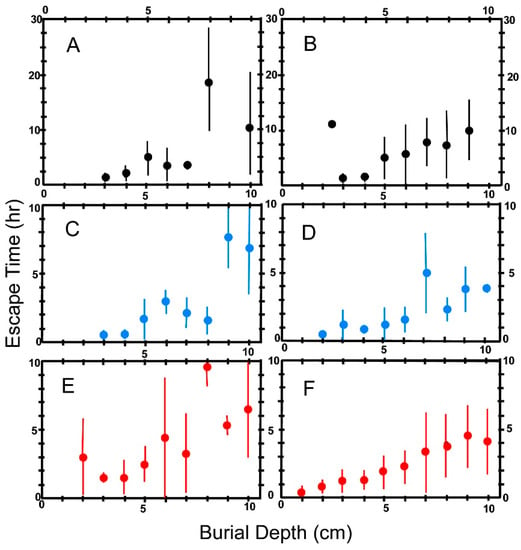
Figure 13.
Escape times for the three test species plotted as a function of burial depth. Escape time is the time required for an animal to reach the new sediment-water interface after anastrophic burial. Burial depth defined as in Figure 10. Each point plotted on the graphs represents the mean of escape times for animals buried at the indicated depth. The vertical bars give ±1σ. (A,B) Pyganodon cataracta; (C,D) Elliptio complanata; (E,F) Corbicula fluminea; (A,C,E): Burial in coarse sand (0Φ ≥ grain size ≥ 1Φ). (B,D,F): Burial in fine sand (2Φ ≥ grain size ≥ 3Φ).
It is apparent from Figure 13 that in all three test species escape time increases dramatically with burial depth. One might expect that escape time would vary linearly with depth since the weight of sand and water that must be pushed aside by an ascending bivalve should vary linearly with depth. However, the very large standard deviations for some tests, due to a combination of small numbers of test runs and/or wide variation in burrowing speed among individual animals, mask any obvious pattern with respect to time increase. Nevertheless, it is apparent that there are major differences in burrowing time among the three test species. All three species seem to more slowly extract themselves from coarse sand than from the fine fraction. In addition, at most depths, P. cataracta appears to require greater time to extract itself from burial than do the other two species. The performance of E. complanata and C. fluminea do not seem to differ widely.
We tested these observations more quantitatively by dividing burial depth into two categories: shallow (1–5 cm); and deep (6–10 cm) and comparing the results of inter- and intraspecific performance using t-tests (Table 7).

Table 7.
Average Escape Time (time required to escape anastrophic burial by burrowing upward to the new sediment surface) for each test species expressed in hours. Shallow Burial—results for escape tests conducted from 1 to 5 cm burial depth. Deep Burial—results for escape tests conducted from 6 to 10 cm burial depth. Data shown here compare escape performance at shallow versus deep burial depth for each test species. Top half of the table compares average performance at constant grain size for shallow versus deep burial. Bottom half of the table compares average performance at constant depth in coarse versus fine sand. σ—standard deviation. Coarse sand: 0Φ ≥ grain size ≥ 1Φ. Fine sand: 2Φ ≥ grain size ≥ 3Φ. PYG: Pyganodon cataracta. ELL: Elliptio complanata. COR: Corbicula fluminea.
Table 7 (top half) shows that, as one would expect, on average, specimens of each species require significantly longer times in each type of sand to extract themselves from deep burial than for shallow burial. The data in Table 7 (bottom half) also indicate that there are major differences in escape time performance among the three species. For example, C. fluminea appears to escape from fine sand significantly faster than from coarse sand. Although P. cataracta and E. complanata also have shorter escape times in fine sand, the disparities relative to coarse sand in these species are smaller, and do not differ significantly from their performance in fine sand. We also tested the performance of each species relative to the other two species, and report results in Table 8.

Table 8.
Average Escape Time defined as in Table 7. Data shown here compare performance of the three test species to one another at different burial depths and in different sediments. Shallow Burial—results for escape tests conducted from 1 to 5 cm burial depth. Deep Burial—results for escape tests conducted from 6 to 10 cm burial depth. Top half of the table compares the species burrowing in coarse sand. Bottom half of the table compares the species burrowing in fine sand. ET—escape time in hours. σ—standard deviation. Coarse sand: 0Φ ≥ grain size ≥ 1Φ. Fine sand: 2Φ ≥ grain size ≥ 3Φ. PYG: Pyganodon cataracta. ELL: Elliptio complanata. COR: Corbicula fluminea.
From the data given in Table 8, it is clear that when burrowing in coarse sand E. complanata has significantly, or nearly significantly, shorter escape times than the other two species, and that the performance or P. cataracta is about the same as that of C. fluminea. In fine sand at shallow depths, all three species appear to have about the same escape times, but at greater depth, E. complanata again outperforms the other two species. P. cataracta is significantly slower than either of the other two species in extricating itself if buried deeply.
While additional data would probably be useful in distinguishing escape capacity in these three species, it seems nevertheless apparent that in general, of the three, E. complanata performs most effectively in escaping anastrophic burial; however, if animal length is considered, this picture changes.
6.5. Effect of Body Size on Escape Time
Our three test species differ considerably in size (Table 2). Among other animals, differences in overall body size can lead to great disparities in locomotor output and performance as noted above. To test whether size difference among our specimens is a significant factor in determining escape time, we adopt an approach commonly used when studying animal locomotion; we examine performance in terms of body size. This may be most conveniently done by expressing distance travelled in terms of body size. In burrowing clams shell length is the operative component of body size, and distance travelled is burial depth, so what we have done is examine escape time as a function of burial depth expressed in terms of body length (shell length) of the animals being tested. Results of this analysis are shown in Figure 14.
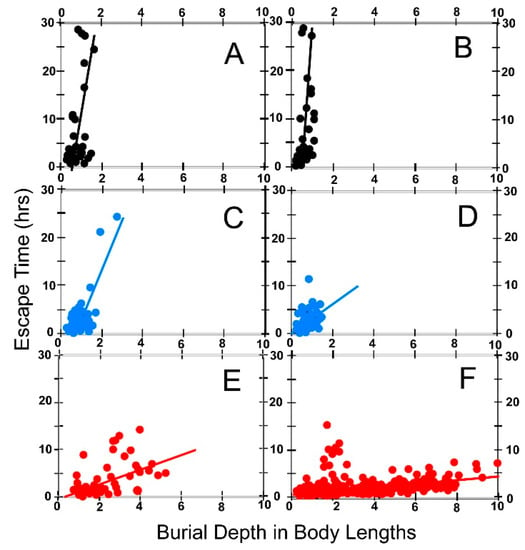
Figure 14.
Escape times for the three test species plotted as a function of body length. Escape time is defined as in Figure 13. Burial depth in body lengths is the distance between the uppermost point on the shell of the anastrophically buried animal and the new sediment-water interface expressed in body lengths as defined in the text (i.e., body length = shell length). Each point plotted on the graphs represents the escape time for a single animal buried at the indicated depth. (A,B) Pyganodon cataracta; (C,D) Elliptio complanata; (E,F) Corbicula fluminea; (A,C,E): Burial in coarse sand (0Φ ≥ grain size ≥ 1Φ); (B,D,F): Burial in fine sand (2Φ ≥ grain size ≥ 3Φ). Best fit linear regressions shown in red for each data set. (A): Y = 10.586X − 1.976 (corr coef = 0.414); (B): Y = 11.537X − 2.602 (corr coef = 0.618); (C): Y = 4.184X − 0.350 (corr coef = 0.268); (D): Y = 2.948X + 0.029 (corr coef = 0.423); (E): Y = 1.359X + 0.190 (corr coef = 0.445); (F): Y = 0.347X + 0.787 (corr coef = 0.395).
We opt to illustrate these results using common units on graph axes. This compresses the data field along the X-axis in graphs depicting P. cataracta and E. complanata (Figure 14A–D) because the animals of these species dealt with in this study are large relative to burial depth. The advantage of this approach is that there is no distortion in data fields or best fit lines of the graphs relative to one another. These lines are an index of a species burrowing capability with respect to size because slope steepness expresses increase in size-compensated escape time with burial depth. Steep slopes as in Figure 14A,B, for P. cataracta mean that size-compensated escape times increase dramatically with small increase in burial depth. It also implies that relative to body size there is undoubtedly a shallow limit to the depths from which such animals can escape burial at all. This supports the inferences drawn above in Figure 13. In contrast, for C. fluminea successful escape in fine sand is not greatly attenuated by greater burial depths up to at least 10 body lengths.
The correlation coefficients for the regressions plotted in Figure 14 are low (see caption for Figure 14). This is due to the wide differences in performance among some individuals as noted above. We tested the slopes of the regression lines using Zar’s procedure ([78]; p. 228) to determine whether the regression slopes can be distinguished from one another given these low correlation coefficients. Our results are tabulated in Table 9. It is evident from the t-test results reported in the top tier of Table 9 that when accounting for body size, the performance of P. cataracta in coarse sand and fine sand as expressed by these regressions cannot be perceived as different. The same holds true for E. complanata. In contrast to the two unionoids, C. fluminea performs better in fine sand than in coarse sand, i.e., size-compensated escape times in fine sand are of significantly shorter duration than in coarse sand.

Table 9.
Comparisons of slopes of linear regression best fit lines for data sets plotted in Figure 14. t-test results given here indicate whether the slopes of the BL burial depths vs ET regressions are significantly different from one another using the procedure of Zar (1974, p. 228). ET—escape time in hours. BL—body length. Coarse sand: 0Φ ≥ grain size ≥ 1Φ. Fine sand: 2Φ ≥ grain size ≥ 3Φ. PYG: Pyganodon cataracta. ELL: Elliptio complanata. COR: Corbicula fluminea. Top tier of the table compares the performance of each species in coarse and fine sand. The middle tier compares the performance of the three species in coarse sand. The lower tier compares the performance of the three species in fine sand.
When comparing the performance of the three species to one another, Table 9 indicates that in both coarse sand and fine sand, E. complanata performs significantly more effectively than P. cataracta. However, the slopes of their regressions are so high that it seems reasonable to suggest that individuals of the size range we studied here could not escape from anastrophic sedimentation events which buried them to depths exceeding much more than one shell length. Table 9 indicates that the slopes of the escape regressions of size-scaled C. fluminea are significantly smaller than for either unionoid. This implies that C. fluminea is a much more effective, i.e., faster, escaper of anastrophic sedimentation events than the two unionoids, particularly in fine sand. It is also clear from this analysis that C. fluminea can escape burial depths many more times its shell length, particularly in fine sand than can the two unionoids.
6.6. Burrow Structure
Figure 15 shows a typical result of our PVC pipe escape burrow experiments with E. complanata. As for marine bivalves, the experimental E. complanata burrows show a digging core, which represents the track of the upwardly burrowing animal, and a surrounding region of downward sloping beds, the digging aureole or halo, in which material is apparently pulled downward into the space vacated directly underneath the upwardly advancing animal.
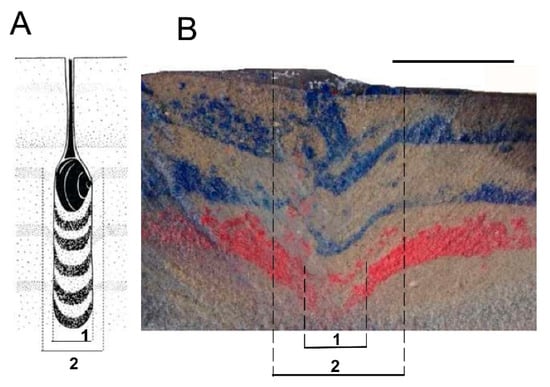
Figure 15.
Structure of bivalve escape burrows. (A) Structure of a typical escape burrow made by a marine venerid bivalve as interpreted by Reineck [34]. 1—digging core; 2—digging aureole; (B) Cross-section of typical escape burrow made by Elliptio complanata inside a PVC pipe originally filled with layers of anastrophically deposited colored sand. 1—digging core; 2—digging aureole. Scale bar in (B) equals 5 cm.
7. Field Results
Figure 16A shows a thin section typical of those we made from samples of the Harrity, PA, A. catskillensis burrow outcrop described above. It is evident that the rock here is a lithic sandstone having a framework of detrital quartz grains dispersed in a very fine-grained dark-colored matrix. Random point counts indicate that the rock consists of 73% framework quartz grains and 27% matrix. The framework fraction consists of well-sorted, fine-grained sand and coarse silt (Figure 16B) predominantly in the 2Φ to 4Φ range. Thus, the framework grains are about the same size as the sand grains we used in our escape experiments, particularly for our fine sand tests.
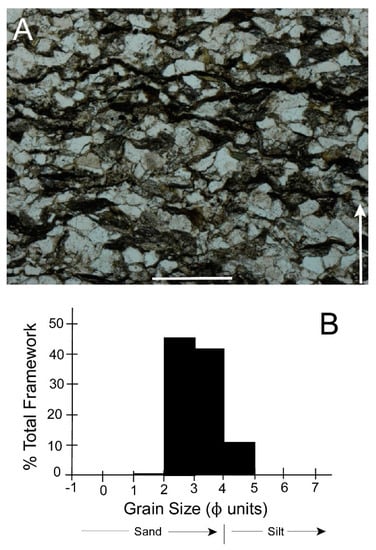
Figure 16.
Sedimentologic character of the Towamensing Member of the Catskill Formation, Harrity, PA. (A) Thin section of a rock sample collected a few centimeters from the burrow shown in Figure 3A. Arrow shows stratigraphic up direction. Scale bar = 0.5 mm; (B) Grain size distribution of framework quartz grains.
Framework grains show prominent, convoluted inter-grain contact surfaces typical of extensive quartz pressure-solution often associated with matrix-rich quartz sandstones [79]. This suggests the probability that framework quartz grains were originally somewhat larger than we now see in Figure 16A. Thus, the size distribution seen in Figure 16B would probably shift slightly toward coarser sizes (to the left), that is in the direction of the coarse sand we used in our experiments.
Matrix material is organized into dark, sinuous stylolitic lenses and layers (Figure 16A). Such structures in sandstones showing pressure-solution features are generally viewed as the remains of original siliceous muddy laminae present in the original quartz-rich sand [79]. Figure 16A indicates that these stylolitic laminae are separated from one another by about 0.5 mm and are oriented roughly perpendicular to the stratigraphic up direction. The latter observation suggests that pressure solution was probably due to post-depositional sediment compaction and was not related to the tectonic overprint in these rocks that derives from either the later phases of the Devonian Acadian Orogeny or the Late Carboniferous to Permian Alleghanian Orogeny. However, compaction was not extensive because the burrows do not appear greatly distorted (Figure 3).
8. Discussion
8.1. Escape Capacity of Modern Archanodon catskillensis Analogues
Among modern bivalves, escape capacity is influenced by a wide variety of factors, including: burial depth; sediment characteristics (e.g., grain size distribution; sediment cohesion [59,62]; shell characteristics (e.g., size, shape, hinge dentition, [53,54,57,63]); soft anatomy (e.g., adductor muscle size and mantle fusion [53,54]); life orientation; the availability of energy reserves [63]; and the reaction time of the animal upon burial [63]. The effects of such a multiplicity of constrains to burrowing performance can be expected to be complex, and to obfuscate the contributions of a single factor. That this difficulty is present in our study is seen in the large standard deviations we obtained in some of our experiments and in our inability to distinguish performance parameters of different test populations in some cases. Nevertheless, several important points deriving from our work can be recognized.
8.1.1. Escape Potential
The data presented (Figure 10 and Figure 11, Table 4 and Table 5) indicate that the probability of a successful escape from an anastrophic burial event decreases with increasing burial depth for all three test species. This inverse relationship between depth and escape potential is more strongly expressed in fine sand than in coarse sand. In coarse sand, escape potential varies across shallow and moderate burial depths for all three species due primarily to differences in the performance of individual test animals escaping from these depths. Nevertheless, in both fine and coarse sand escape probability markedly plummets at burial depths of 9 and 10 cm. Attenuation of successful escape with increasing burial depth has also been demonstrated previously for some marine bivalves [59]. In the paragraphs below we consider how a variety of factors influence this inverse relationship between escape potential and burial depth.
(a) Effect of Animal Size on Escape Potential
Some observation on marine bivalves [59,63] indicate that larger individuals of a species are more likely to escape burial than smaller conspecifics because large animals need to travel fewer body lengths to reach the surface. However, Kranz [63] found that in some deep-burrowing siphonate suspension feeders, adults have diminished burrowing abilities relative to younger, smaller animals of the same species—a situation that he suggested may be due to ontogenetic reduction in foot size relative to shell size. Our data (summarized in Table 6) suggest that size may influence escape potential in some cases, for example with respect to P. cataracta or E. complanata escaping coarse sand. However, there is little consistency or pattern seen among the different categories of shell size/grain size categories tested. Our view is not that there is no relationship between these variables but rather that the restricted size range of animals tested and wide variation in performance among individuals mask any underlying pattern that may exist. Further experiments involving a much larger test population and one containing a wider range in size of animals tested will be needed to shed additional light on this issue.
(b) Effect of Shell Characteristics on Escape Potential
All three test species have lower escape potentials at greater burial depths than they do at shallow depths. P. cataracta is less likely than the other two species to escape depths greater than 5 cm (Figure 10 and Figure 11, Table 4 and Table 5). In fact, the animal is more likely to die at these depths and not escape at all. Trueman et al. [54] observed, that obesity appears to be linked to the relative ease with which bivalves burrow. P. cataracta is inflated and more cylindrical than the relatively streamlined E. complanata (obesity ratio of 0.67 and 0.55, respectively; see Table 2). Thus, P. cataracta should encounter more resistance moving upward through the sediment than an equally sized E. complanata. This may in part account for P. cataracta’s poor burrowing performance relative to that of E. complanata. In addition, the shell of P. cataracta, lacks hinge teeth, while that of E. complanata, like many other modern unionoids, has prominent hinge teeth. Hinge teeth function to minimize shear between the valves of the shell, i.e., hinge teeth reduce the likelihood that in opening or closing, one valve will rotate relative to the opposing valve around an axis perpendicular to the hinge. This allows strong and fast-acting articulation around the hinge when the shell is opened and closed. For active burrowers hinge teeth are an important adaptive attribute [53,63]. The absence of prominent hinge teeth in P. cataracta may thus contribute to its lackluster performance with regard to escape potential.
(c) Effect of Soft Anatomy on Escape Potential
Two aspects of bivalve soft anatomy may influence our results: mantle fusion, and adductor muscle size and placement. A fused mantle margin minimizes leakage of water from the mantle cavity, and thus maximizes both the water volume available to fluidize sediment during burrowing and the hydrostatic pressure produced by contraction of the adductor muscles that drives mantle water ejection into the sediment [51,52]. C. fluminea, like other venerids, has a fused mantle margin whereas the two unionoids tested do not. We postulate therefore that the superior escape performance of C. fluminea relative to P. cataracta and E. complanata (Figure 10 and Figure 11, Table 4 and Table 5) may in part relate to this difference—i.e., that C. fluminea can displace via fluidization more sediment relative to its size than can the two unionoids. Burrowing behavior should be an important factor here. Our observations indicate the two unionoids push themselves upward (Figure 9A,B) and do not actually fluidize sediment when escape burrowing. In contrast, we observe that C. fluminea pulls itself upward (Figure 9C) and that the “siphon blow” phenomenon (similar to the one observed for both unionoids, see Figure 9D) apparent as it emerges from the sediment suggests the use by C. fluminea of fluidization during escape burrowing.
Adductor muscles are a key component in the toolkit of any bivalve escaping anastrophic burial. Contraction of the adductors powers the fluidization process and by closing the valves and thus reducing the shell profile presented to the sediment, the adductors minimize the drag the animal must overcome in driving itself into the sediment. In simple fibrous muscles, like those of bivalve adductors, muscle strength is proportional to the cross-sectional area of the muscle. Thus, effective burrowers have large adductors compared to the size of the shell [53]. We evaluated adductor cross sectional area relative to the plan area of the shell and found that both P. cataracta and E. complanata have muscles of equivalent relative cross-sectional area (Table 2 and Table 3), and should be capable of producing equivalent power output. We also found that these two unionoids have adductors that are about twice the relative size as those of C. fluminea (Table 2 and Table 3). This seems anomalous because C. fluminea is clearly the better escape burrower, a situation that should require relatively large, powerful adductors. This can be resolved, however, by noting in Figure 7 that the ovoid, equilateral shell shape of C. fluminea and its highly curved hinge result in the placement of its adductor about twice as far from the axis of valve rotation located at the umbo than is the case for the two unionoids. Thus, the moment arm over which C. fluminea adductors act is about twice that of the unionoids. The force produced by C. fluminea relative to its size should thus be about the same as the force produced by the two unionoids relative to their size. All three species are equivalently endowed with respect to this aspect of their burrowing mechanism.
(d) Effect of Life Habit on Escape Potential
As noted above, P. cataracta—although semi-infaunal like E. complanata—assumes a living position in which much of the shell posterior protrudes from the sediment. In fact, P. cataracta frequently lies completely exposed on the substrate surface anchored in place by its foot, which extends down into the sediment. This behavior has also been noted in Pyganodon-like Anodonta living in ponds by Eagar [55], who attributed this behavior to periods of rest. E. complanata is generally found buried deeper, with only a small portion of the shell posterior, or only the siphonal openings visible above the sediment-water interface. Because it lives with more of its shell exposed, P. cataracta is less likely to be buried by a given sedimentation event. Thus, P. cataracta‘s mode of life may require less upward escape mobility. Kranz [63], however, did not find a simple correlation between living depth and escape potential in marine bivalves.
8.1.2. Escape Time
We measured the time required to escape anastrophic burial for animals that successfully did so (some test specimens were unable to escape or died during testing; (Table 4 and Table 5)). Figure 13 shows our results for all three test species escaping from burial in both coarse and fine sand. As in our results for escape potential, wide variability in the performance of individual animals is considerable (hence the wide variance bars in some test configurations). It is nevertheless apparent that all six graphs in Figure 13 show the same result—escape time increases with increasing burial depth. It is also evident that there are differences in this relationship among the three test species.
A variety of factors may influence escape time; including foot size; reaction time and sediment compaction; oxygen availability; and sexual dimorphism, age of individual, and diurnal or other cycles. Although we did not explicitly test for the effects of such factors, they may have contributed to the high variance seen in the performance of our test animals. We treat these parameters briefly here.
(a) Effect of Foot Size on Escape Time
One interesting and unexpected observation deriving from Figure 13 is the fact that C. fluminea is considerably slower in extricating itself from coarse than it is in escaping burial in fine sand (Figure 13E,F; Table 7, lowermost rows). We suggest that foot size is a key parameter here. We postulate that it is not so much the absolute size of the foot that is important, but rather the size of the foot relative to the size the sand grains against which the foot acts. To generate the motive force for burrowing, the foot must be large enough to gain a solid lodgment in the sediment through which the animal is moving. This is possible only if the foot is large enough to spread out over a wide array of sedimentary grains in a burrowing equivalent of the “snowshoe effect” discussed for cnidarians [80], brachiopods [81,82,83], and many other organisms that maintain life position on soft sediment surfaces by spreading out their weight across a wide area. C. fluminea is much smaller than either of the two unionoids we tested (Table 2) and has a much smaller foot. We suggest that the difficulty C. fluminea has in escaping coarse sand compared to fine sand can be explained by its small foot, which we argue is too small to readily obtain a solid anchorage in sediment where the grains are large and easily moved. Pyganodon cataracta and E. complanata may not encounter anchoring difficulties during escape burrowing in coarse sand because their feet are large enough to gain the strong purchase in coarse sand needed for adequately anchoring the upward motion of the shell during the foot extension phase of the burrowing cycle. As a result, their performance in coarse versus fine sand is not nearly as disparate as that of their smaller venerid relative.
(b) Effect of Reaction Time and Sediment Compaction on Escape Time
Reaction time is the time needed for an animal to sense that it has been anastrophically buried and to initiate escape burrowing behavior. Reaction time will increase overall escape time, but in addition will possibly make escape more difficult and lengthy because of its role in controlling sediment compaction and cohesiveness [63]. The idea here is that the quicker a bivalve responds to anastrophic burial by initiating upward burrowing, the less time there is for sediment to compact, increase sediment resistance to burrowing, and draw out the escape process. We could not assess reaction time of our test animals to burial because the opaqueness of the sediment prevented observation of burrowing movements. However, in experimental runs in which specimens of E. complanata and P. cataracta were unable to extricate themselves, we often found them not to have moved upward to any significant degree. This implies that reaction time of these two unionoids may be lengthy in some cases.
Trueman et al. [54] were able to identify a positive correlation between time elapsed after sediment deposition and the physical resistance upon downward burrowing. This situation would undoubtedly apply to upward burrowing as well. On the other hand, Monte Carlo simulations of compaction rates in modern deltaic sediments indicate proximal post-depositional compaction rates of only a few mm/yr [84] for a variety of common sediment types, including the sandy types used by us. Downward burrowing could take place long after surficial sediment was deposited when sediment was well compacted, but upward escape burrowing would be initiated relatively soon after anastrophic deposition when compaction is relatively inconsequential, and thus not a major impediment to escape activity. Further escape burrowing experimentation in which animal subsurface behavior is visualized, possibly by X-ray or the use of transparent sediment, and sediment compaction is assessed quantitatively would be needed to resolve these issues.
(c) Effect of Oxygen Availability on Escape Rate
Our observations on resistance to burrowing-related vitality decline (Table 4 and Table 5) may reflect differences in the source of oxygen on which the animals rely to support their metabolic needs during escape burrowing. Although E. complanata usually burrows deeper into the sediment to establish its life position than does P. cataracta, both species typically maintain sufficient contact with the overlying water body to ensure that they have unrestricted access to oxygenated water via their siphonal openings. In contrast, C. fluminea, as our observations show, is evidently small enough to subsist on oxygen available in interstitial water within the sediment, because in establishing their living position they often descend well below the sediment-water interface, deeper than the length of their extended siphons, and remain there for long intervals.
Oxygen concentration in interstitial water in sandy riverine sediments usually is comparatively low, especially below a depth of a few centimeters [85,86]. Elliptio complanata and especially P. cataracta may be too large and have too high an oxygen demand for such a low volume oxygen resource as the reservoir of interstitial water within the sediment. They may need to have access to oxygenated water above the sediment-water interface. If they remain buried too long they may begin to go into oxygen debt and eventually expire. They may face a second problem: using interstitial water in sediment of small grain size, i.e., fine sand and silt, may prove challenging because smaller, more mobile particles can be more readily drawn into the mantle cavity along with any interstitial water an animal may take in. This foreign material could damage the ctenidia, and other organs. P. cataracta and E. complanata may thus be subject to the same effects: both may experience difficulties utililzing oxygen in fine sand relative to coarse sand. Neither of these problems would appear to be detrimental to C. fluminea. Clearly, a closer investigation into the function of interstitial water during burial and measurements of oxygen demand in these animals would be helpful to resolve these issues.
(d) Effect of Sexual Dimorphism on Escape Rate
Sexual dimorphism may also play a role in the burrowing behavior of unionoid bivalves. Recently a study revealed that downward burrowing in E. complanata is sexually dimorphic [87]. According to these authors, only about 43% of female test specimens burrowed while this figure was 61% for males. In addition, females burrowed at slower rates than males. Whether such sex-specific behavior applies to upward escape burrowing in E. complanata or other unionoids is unclear, but it is something that needs to be evaluated.
8.2. Which Species Is the Better Escape Burrower
Considering its small size relative to that of the two unionoids (the average length of C. fluminea is 2–3 times less than that of the unionoids; (Table 2)), C. fluminea is clearly the superior escape burrower. To escape anastrophic burial, this species can typically travel many more body lengths to reach the surface and in considerably less time than it takes P. cataracta and E. complanata to escape the same burial depths (Figure 14). Corbicula fluminea can successfully escape from burial at depths many times its own length (Figure 14), whereas the two unionoids appear to approach their escape limits at depths of one or two body lengths (Figure 10, Figure 11 and Figure 14). Also, the escape potential of this small venerid (expressed as a percentage of anastrophically buried animals reaching the sediment surface) is higher (Figure 10 and Figure 11), and its burrowing mortality is lower, than for the two unionoids (Table 4 and Table 5).
Looking now at the two unionoids, we can see that E. complanata is superior to P. cataracta, in terms of escaping anastrophic burial. Elliptio complanata can burrow upward on average twice as fast as P. cataracta (Table 7 and Table 8). In addition, E. complanata has a greater escape potential (Figure 10 and Figure 11), and lower burrowing mortality than does P. cataracta (Table 4 and Table 5).
8.3. Escape Capacity of Archanodon catskillensis
Morphologically, both P. cataracta and E. complanata are reasonable analogues of A. catskillensis—i.e., all three species are roughly equivalent with respect to the main attributes of shell form. All have slightly obese, inequilateral, ovoid shells (Figure 7; Table 2) that lack prominent surface ornamentation. These equivalencies imply that A. catskillensis, like its unionoid analogues, would have advanced shell posterior first (“backed out”) when escape burrowing, and would have experienced resistance by the sediment to its movement roughly equivalent to equally sized specimens of these two modern paradigms. But in terms of hinge structure, the two modern unionoids diverge considerably as paradigms for their Devonian relative. Both A. catskillensis and P. cataracta have hinges lacking significant hinge teeth, whereas E. complanata is characterized by a pseudoheterodont hinge exhibiting prominent hinge teeth (Figure 7). Another point to consider here is that both P. cataracta and E. complanata have adductor muscles that occupy about 6% to 7% of the plan area of their shells (Table 2). A. catskillensis, on the other hand, appears to have had much smaller adductors relative to its size. These observations imply that A. catskillensis probably could not have closed its valves as forcefully as its modern unionoid analogues, a situation which undoubtedly would have significantly compromised its burrowing capacity. These disparities suggest to us that the escape burrowing capacity of A. catskillensis was probably no better than that of P. cataracta, and may actually have been noticeably less. We postulate that A. catskillensis probably could not successfully extricate itself from more than about 5 cm of anastrophic overburden. Burial to depths greater than this would probably have resulted in death.
Burrows that have the features noted in Table 1 and illustrated in Figure 3, have long been interpreted as escape burrows made by A. catskillensis [5,14,16,29]. Some of these burrows are up to one meter in length, and most that we have seen in the field are demonstrably longer than 5 cm. We see no rationale by which one can argue that such burrows represent escape from a single anastrophic event, rather they must record successful escape from a series of small-scale anastrophic episodes, each one producing less than a few centimeters of new sediment. They were constructed by animals periodically migrating upward in response to relatively frequent deposition of thin coverings of new sediment, not by animals racing death by clawing their way upward through a single massive sedimentary blanket dumped on them via unusually severe storms, levee breaks, floods, turbidity flows or other such catastrophic events.
The burrows preserved at the Harrity, PA, site (Figure 3) illustrate this kind of situation reasonably well. As noted above, the Towamensing Member of the Catskill Formation exposed at this site is a lithic sandstone with prominent planar bedding. Epstein et al. [77], who first described this locality, interpreted the depositional environment as an estuarine bar subject to frequent depositional events. Thoms and Berg [16], who studied A. catskillensis burrows in equivalent beds of the Towamensing Member along the Delaware River 50 to 100 km northeast of Harrity, argued that the Towamensing Member was the product of deposition on river mouth bars associated with a delta front or delta plain. The results of our thin section study of Harrity beds described above are consistent with these views. We suggest that each dark mud lamina (Figure 16A), although now distorted by compaction and dissolution, forms a depositional couplet with the thin sandy bed lying beneath it. The sequence of couplets upward through the section represents repetitive small-scale depositional events generated by daily to seasonal hydrologic cycles or weather patterns. They could also represent current-driven sand bar or river bank migration. Whatever the cause, relatively frequent addition of thin laminations of new sediment would induce similarly frequent upward escape behavior of the A. catskillensis animals living there. The animals would keep pace with the shifting sediment-water interface with frequent, but small, upward movements.
Bromley [37] indicates that in some cases of upward escape movement over protracted periods, an escape burrow records the animal’s growth as an upward increase in burrow diameter and lengthening of the crescentic menisci. These features are not seen in the Harrity and Jewett Quarry burrows illustrated in Figure 3. Nor are such features observed at other burrow sites [5,14,16,29]. We postulate that this is the result of two factors: (1) such burrows were made by adult animals in which growth had essentially ceased; and (2) A. catskillensis may have been unusually long-lived so that the known burrows of these animals, even those of considerable length, may represent a time frame (decades, to a century or two) well within the lifespan of such modern unionoids as Margaritifera [88,89]. In contrast, in all of the examples of A. catskillensis preserved in life position that we have examined, such as that illustrated in Figure 1B, we have never observed a single escape burrow made by individual animals occurring in such clusters. We interpret this to mean that when A. catskillensis is preserved in life position it signifies anastrophic burial to depths greater than that from which the animals can readily escape.
8.4. Burrow Structure
The structure observed in the experimental burrow is consistent with escape burrows described by Reineck [34] for modern marine venerid bivalves. However, our experimental burrows and the idealized burrow structures sketched in Reineck ([34]; Figure 2) and Bromley [37] differ in some ways. Most obviously, the meniscate structures illustrated by these two authors in burrow digging cores of modern marine bivalves were not detected in the current experiments. This difference may relate to sediment property disparities in burrowing media. Menisci are more readily produced in situations where sediment immediately below the lower surface of the animal’s foot is easily compacted by the downward force the animal’s foot exerts on the sediment beneath it. The clean sand used here is undoubtedly considerably less compressible under the forces produced by bivalves than the soft mud characteristic of the studies of Reineck [34] and Bromley [37].
Bromley noted [37] that bivalves escaping with the foot leading (shell anterior up—shell posterior down), i.e., the foot pulling rather than pushing the animal up, construct structurally diffuse burrows. No menisci are produced in this style of escape, and the distortion of the beds is more extreme with little regularity in the relative extent of digging core and aureole. This is not unlike the structure of our experimental burrows (Figure 15B). However, in all our PVC pipe burrows, the test specimen always advanced upward with its posterior margin leading, and with the foot pushing downward onto sediment beneath the shell from the downward, trailing anterior shell margin. The irregularities seen in our experimental burrows, as compared to the sketches provided by Reineck [34] and Bromley [37], are more likely due to differences in the properties of the sand we used as compared to the muddy sediment they studied. It is evident that more extensive work than we attempt here is needed to resolve these questions.
One other difference is also of interest. Along the edges of the digging core, especially on the left side, small pods of red and blue sand separated and isolated from the red and blue layers are visible. These pods of colored sand may represent material lying against the outer shell surface that has been dragged upward by the ascending bivalve. This may indicate that at least in loosely bound sand, ascending animals may have a thin “boundary layer” of sediment adhering to the outer surface of their valves, which travels upward with them for short distances.
9. Conclusions
Using the two modern unionoids E. complanata, P. cataracta, as well as the invasive freshwater venerid C. fluminea, and simulating burial in a controlled laboratory setting, we determined the escape potential, escape duration and to some degree the escape burrowing structure of these animals. The two unionoids—but especially P. cataracta—are morphological analogues for the extinct Devonian freshwater bivalve A. catskillensis. E. complanata’s escape burrowing abilities are better than those of the more obese P. cataracta; the likelihood of successful escape from burial in both fine and coarse sand is greater for E. complanata, and the escape duration is shorter than that of P. cataracta. Nevertheless, both unionoids are rarely able to escape burial of 10 cm or more, especially in fine sand. The escape burrowing abilities of the smaller C. fluminea surpass that of both unionoids. The escape burrowing behavior observed here sheds light on the escape abilities of A. catskillensis and therefore the nature of the long, vertical burrows that are commonly associated with the animal. These burrows are interpreted to be escape structures, a response to anastrophic burial. Our observations demonstrate that A. catskillensis’ escape burrowing capabilities must have been relatively limited, and the animal, like its modern descendants, was unable to extricate itself from extensive burial. Thus, the escape structures attributed to A. catskillensis represent response to episodic, small-scale sedimentation, a single event of which deposited no more than 10 cm of new sediment.
Acknowledgments
We thank Annesia Lamb and Jack Lin, both of Brooklyn College, for their help in collecting the animals and for their support during the experimental phase of this study. We also thank Robert Dickie and Brett Branco, of Brooklyn College’s Aquatic Research and Environmental Assessment Center, for providing the necessary equipment and facility space needed to maintain animals in the lab and to conduct the necessary burrowing experiments. Jennifer Basil and Naomi Lewandowski, Brooklyn College, provided feedback regarding our experimental work, and shared their storage space with us. Bushra Hussain, American Museum of Natural History, allowed us access to Hall’s Archanodon collection. We also acknowledge the help of Alan Titus for constructive feedback and the help of D.L. Strayer, Cary Institute of Ecosystem Studies, in identifying P. cataracta. Two anonymous reviewers for this journal provided insightful comments which greatly improved the presentation of this work. This research was supported by PSC-CUNY awards to J.A.C. and R.B.C.
Author Contributions
K.K. performed the live animal experiments described here. K.K., R.B.C. and J.A.C. collected rock samples, burrows, and live specimens. All three co-authors also analyzed and interpreted the data, and all three wrote the paper.
Conflicts of Interest
The authors declare no conflict of interest. The funding sponsors had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, and in the decision to publish the results.
References
- Vanuxem, L. Geology of New York. Part 3. In Comprising the Survey of the Third Geological District; W. and A. White and J. Visscher: Albany, NY, USA, 1842; p. 306. [Google Scholar]
- Hall, J. Lamellibranchiata II. Dimyaria from the upper Helderberg, Hamilton, Portage, and Chemung groups. In Natural History of New York: Palaeontology; Part I.; Charles Van Benthuysen and Sons: Albany, NY, USA, 1885; Volume 5, pp. 516–518. [Google Scholar]
- Clarke, J.L. Value of Amnigenia as an indicator of fresh-water deposits during the Devonic of New York. N. Y. State Mus. Bull. 1901, 49, 199–203. [Google Scholar]
- Weir, J. Superfamily Archanodontacea. In Treatise on Invertebrate Paleontology; Part N, Mollusca 6; Moore, R.C., Ed.; Geological Society of America and University of Kansas: Lawrence, KS, USA, 1969; Volume 1, pp. 402–404. [Google Scholar]
- Chamberlain, J.A., Jr.; Chamberlain, R.B. The Devonian bivalve, Archanodon catskillensis: A status report on the first freshwater mussel from New Jersey. In Contributions to the Paleontology of New Jersey; Rainforth, E., Ed.; Geological Association of New Jersey: Trenton, NJ, USA, 2007; Volume 24, pp. 24–40. [Google Scholar]
- Chamberlain, J.A., Jr.; Friedman, G.M.; Chamberlain, R.B. Devonian archanodont unionoids from the Catskill Mountains of New York: Implications for the paleoecology and biogeography of the first freshwater bivalves. Northeast. Geol. Environ. Sci. 2004, 26, 211–229. [Google Scholar]
- Newell, N.D. Classification of the Bivalvia. Am. Mus. Nat. Hist. Novit. 1965, 2206, 1–25. [Google Scholar]
- Watters, G.T. The evolution of the Unionacea in North America, and its implications for the worldwide fauna. In Ecology and Evolution of the Freshwater Mussels Unionoida; Bauer, G., Wächtler, K., Eds.; Ecological Studies Springer: Berlin, Germany, 2001; Volume 145, pp. 281–307. [Google Scholar]
- Howse, R. Preliminary notice of the occurrence of Archanodon (Anodonta) jukesii, Forbes, in the Lower Carboniferous rocks of north Northumberland. Nat. Hist. Trans. Northumberl. Durh. Newctle.-Upon-Tyne 1878, 7, 173–175. [Google Scholar]
- Forbes, E. On the fossils of the Yellow Sandstone of the south of Ireland. Rep. Br. Assoc. Adv. Sci. (22nd Meet.) 1853, 22, 43. [Google Scholar]
- Beushausen, L. Amnigenia rhenana n. sp., ein Anodonta ähnlicher Zweischaler aus dem rheinsischen Mitteldevon. Jahrb. Königlich Preuss. Geol. Landesanst. Bergakad. Berlin Jahrb. Abhand. Mitarbeiten. 1890, Band XI, II, 1–10. [Google Scholar]
- Whiteaves, J.F. Note on the recent discovery of large Unio-like shells in the Coal Measures at South Joggins, Nova Scotia. Trans. R. Soc. Can. Sec. 4 1893, 11, 21–24. [Google Scholar]
- Bridge, J.S.; Gordon, E.A.; Titus, R.C. Non-marine bivalves and associated burrows in the Catskill magnafacies (Upper Devonian) of New York State. Palaeogeogr. Palaeoclimatol. Palaeoecol. 1986, 55, 65–77. [Google Scholar] [CrossRef]
- Berg, T.M. Bivalve burrow structures in the Bellvale Sandstone, New Jersey and New York. Bull. N. J. Acad. Sci. 1977, 22, 1–5. [Google Scholar]
- Friedman, G.M.; Chamberlain, J.A., Jr. Archanodon catskillensis (Vanuxem): Freshwater clams from one of the oldest back-swamp fluvial facies (upper Middle Devonian), Catskill Mountains, New York. Northeast. Geol. Environ. Sci. 1995, 17, 431–443. [Google Scholar]
- Thoms, R.E.; Berg, T.M. Interpretation of bivalve trace fossils in fluvial beds of the basal Catskill Formation (late Devonian), Eastern USA. In Biogenic Structures: Their Use in Interpreting Depositional Environments; Curran, H.A., Ed.; Society for Sedimentary Geology: Tulsa, OK, USA, 1985; Volume 35, pp. 13–20. [Google Scholar]
- Knox, L.; Gordon, E.L. Ostracodes as indicators of brackish water environments in the Catskill Magnafacies (Devonian) of New York State. Palaeogeogr. Palaeoclimatol. Palaeoecol. 1999, 148, 9–22. [Google Scholar] [CrossRef]
- Friedman, G.M.; Lundin, R.F. Freshwater ostracodes from Middle Devonian fluvial facies, Catskill Mountains, New York. J. Paleontol. 1998, 72, 485–490. [Google Scholar] [CrossRef]
- Falcon-Lang, H.J.; Rygel, M.C.; Calder, J.H.; Gibling, M.R. An early Pennsylvanian waterhole deposit and its fossil biota in a dryland alluvial plain setting, Joggins, Nova Scotia. J. Geol. Soc. Lond. 2004, 161, 209–222. [Google Scholar] [CrossRef]
- Hebert, B.L.; Calder, J.H. On the discovery of a unique terrestrial faunal assemblage in the classic Pennsylvanian section at Joggins, Nova Scotia. Can. J. Earth Sci. 2004, 41, 247–254. [Google Scholar] [CrossRef]
- Rygel, M.C.; Gibling, M.R. Natural geomorphic variability recorded in a high-accommodation setting: Fluvial architecture of the Pennsylvanian Joggins Formation of Atlantic Canada. J. Sediment. Res. 2006, 76, 1230–1251. [Google Scholar] [CrossRef]
- Trueman, E.R. The Locomotion of the freshwater clam Margaritifera margaritifera (Unionacea: Margaritanidae). Malacologia 1968, 6, 401–410. [Google Scholar]
- Strayer, D.L.; Jirka, K.J. The Pearly Mussels of New York State. In New York State Museum; Memoir 26; New York State Education Department: Albany, NY, USA, 1997; p. 113. [Google Scholar]
- Haag, W.R. North American Freshwater Mussels; Cambridge University Press: Cambridge, UK, 2012; p. 505. [Google Scholar]
- Tevesz, M.J.S.; Cornelius, D.W.; Fisher, J.B. Life habits and distribution of riverine Lampsilis radiata luteola (Mollusca: Bivalvia). Kirtlandia 1985, 41, 27–33. [Google Scholar]
- Di Maio, J.; Corkum, L.D. Patterns of orientation in unionids as a function of rivers with differing hydrological variability. J. Molluscan Stud. 1997, 63, 531–539. [Google Scholar] [CrossRef]
- Perles, S.J.; Christian, A.D.; Berg, D.J. Vertical Migration, Orientation, Aggregation, and Fecundity of the Freshwater Mussel Lampsilis Siliquoidea. Ohio J. Sci. 2003, 103, 73–78. [Google Scholar]
- Gordon, E.A. Body and trace fossils from the Middle-Upper Devonian Catskill Magnafacies, southeastern New York, USA. In Devonian of the World, Volume II: Sedimentation; McMillan, N.J., Embry, A.F., Glass, D.J., Eds.; Canadian Society of Petroleum Geologists: Calgary, AB, Canada, 1988; pp. 139–155. [Google Scholar]
- Chamberlain, J.A., Jr.; Chamberlain, R.B. Archanodon catskillensis: The life and times of a bivalve pioneer. N. Am. Paleont. Conv. Halifax NS Can. Programme Abstr. PaleoBios 2005, 25 (Suppl. 2), 29. [Google Scholar]
- Johnson, K.G.; Friedman, G.M. The Tully clastic correlatives (Upper Devonian) of New York State: A model for the recognition of alluvial, dune (?), tidal, nearshore (bar and lagoon), and offshore sedimentary environments in a tectonic delta complex. J. Sediment. Petrol. 1969, 39, 451–485. [Google Scholar]
- Chamberlain, J.A., Jr. Hydromechanical design of fossil cephalopods. In The Ammonoidea; House, M.R., Senior, J.R., Eds.; Academic Press: New York, NY, USA, 1981; pp. 289–335. [Google Scholar]
- Chamberlain, J.A., Jr. Locomotion of Nautilus. In Nautilus: Biology and Paleobiology of a Living Fossil; Landman, N.H., Saunders, W.B., Eds.; Plenum Press: New York, NY, USA, 1987; pp. 489–525. [Google Scholar]
- Chamberlain, J.A., Jr. Jet propulsion of Nautilus: A surviving example of early Paleozoic cephalopod locomotor design. Can. J. Zool. 1990, 68, 806–814. [Google Scholar] [CrossRef]
- Reineck, H.-E. Wühlbau-Gefüge in Abhängigkeit von Sediment-Umlagerungen. Senckenberg. Lethaea 1958, 39, 1–24. [Google Scholar]
- Schäfer, W. Aktuo-Paläontologie Nach Studien in der Nordsee; Verlag Waldemar Kramer: Frankfurt am Main, Germany, 1962; p. 666. [Google Scholar]
- Raup, D.M.; Seilacher, A. Fossil foraging behavior: Computer simulation. Science 1969, 166, 994–995. [Google Scholar] [CrossRef] [PubMed]
- Bromley, R.G. Trace Fossils: Biology, Taphonomy and Applications; Chapman and Hall: New York, NY, USA, 1996; p. 361. [Google Scholar]
- Plotnick, R.E. Behavioral biology of trace fossils. Paleobiology 2012, 38, 459–473. [Google Scholar] [CrossRef]
- Sims, D.W.; Reynolds, A.M.; Humphries, N.E.; Southall, E.J.; Wearmouth, V.J.; Metcalfe, B.; Twitchett, R.J. Hierarchical random walks in trace fossils and the origin of optimal search behavior. Proc. Natl. Acad. Sci. USA 2014, 111, 11073–11078. [Google Scholar] [CrossRef] [PubMed]
- Stanley, S.M. Why clams have the shape they have. Paleobiology 1975, 1, 48–58. [Google Scholar] [CrossRef]
- Winter, A.G.; Hosois, A.E. Identification and evaluation of the Atlantic razor clam (Ensis directus) for biologically inspired subsea burrowing systems. Integr. Comp. Biol. 2011, 51, 151–157. [Google Scholar] [CrossRef] [PubMed]
- Winter, A.G.; Hosoi, A.E.; Slocum, A.H.; Deits, R.L.H. The design and testing of Roboclam: A machine used to investigate and optimize razor clam-inspired burrowing mechanisms for engineering applications. In Proceedings of the ASME 2009 International Design Engineering Technical Conferences and Computers and Information in Engineering Conference, San Diego, CA, USA, 30 August–2 September 2009; pp. 1–6. [Google Scholar]
- Winter, A.G.; Deits, R.L.H.; Dorsch, D.S.; Hosoi, A.E.; Slocum, A.H. Teaching Roboclam to dig: The design, testing, and genetic algorithm optimization of a Biomimetic robot. In Proceedings of the International Conference on Intelligent Robots and Systems (IROS), Taipei, Taiwan, 18–22 October 2009; pp. 4231–4235. [Google Scholar]
- Koller-Hodac, A.; Germann, D.P.; Gilgen, A.; Dietrich, K.; Hadorn, M.; Schatz, W.; EggenbergerHotz, P. Actuated bivalve robot study of the burrowing locomotion in sediment. In Proceedings of the International Conference on Robots and Automation (ICRA), Anchorage, AK, USA, 3–7 May 2010; pp. 1209–1214. [Google Scholar]
- Germann, D.P.; Schatz, W.; EggenbergerHotz, P. Artificial bivalves—The biomimetics of underwater burrowing. Procedia Comp. Sci. 2011, 7, 169–172. [Google Scholar] [CrossRef]
- Winter, A.G.; Deits, R.L.H.; Dorsch, D.S.; Slocum, A.H.; Hosoi, A.E. Razor clam to RoboClam: Burrowing drag reduction mechanisms and their robotic adaptation. Bioinspir. Biomim. 2014, 9, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Raup, D.M. Geometric analysis of shell coiling: General problems. J. Paleontol. 1966, 40, 1178–1190. [Google Scholar]
- Raup, D.M. Geometric analysis of shell coiling: Coiling in ammonoids. J. Paleontol. 1967, 41, 43–65. [Google Scholar]
- Raup, D.M.; Michelson, A. Theoretical morphology of the coiled shell. Science 1965, 147, 1294–1295. [Google Scholar] [CrossRef] [PubMed]
- McGhee, G.R., Jr. Theoretical Morphology: Its Concepts and Its Applications; Columbia University Press: New York, NY, USA, 1999; p. 323. [Google Scholar]
- Trueman, E.R. Bivalve molluscs: Fluid dynamics of burrowing. Science 1966, 153, 523–525. [Google Scholar] [CrossRef] [PubMed]
- Trueman, E.R. The burrowing activities of bivalves. Symp. Zool. Soc. Lond. 1968, 22, 167–186. [Google Scholar]
- Stanley, S.M. Relation of Shell Form to Life Habits of the Bivalvia (Mollusca); Geological Society of America: Boulder, CO, USA, 1970; p. 296. [Google Scholar]
- Trueman, E.R.; Brand, A.R.; Davis, P. Dynamics of burrowing of some common littoral bivalves. J. Exp. Biol. 1966, 44, 469–492. [Google Scholar]
- Eagar, R.M.C. Shape and function of the shell: A comparison of some living and fossil bivalve molluscs. Biol. Rev. Camb. Philos. Soc. 1978, 53, 169–210. [Google Scholar] [CrossRef]
- Lewis, J.B.; Riebel, P.N. The effect of substratum on burrowing in freshwater mussels (Unionidae). Can. J. Zool. 1984, 62, 2023–2025. [Google Scholar] [CrossRef]
- Watters, G.T. Form and function of unionoidean shell sculpture and shape Bivalvia). Am. Malacol. Bull. 1994, 11, 1–20. [Google Scholar]
- Campos, J.; Van der Veer, H.W. Autoecology of Crangon crangon (L.) with an emphasis on latitudinal trends. Oceanogr. Mar. Biol. Annu. Rev. 2008, 46, 65–104. [Google Scholar] [CrossRef]
- Glude, J.B. Survival of soft-shell clams, Mya arenaria, buried at various depths. Res. Bull. Maine Dept. Sea Shore Fish. 1954, 22, 1–26. [Google Scholar]
- Morton, J.E. Locomotion. In Physiology of Mollusca, 1; Wilbur, K.M., Yonge, C.M., Eds.; Academic Press: New York, NY, USA, 1964; pp. 383–423. [Google Scholar]
- Trueman, E.R. The Dynamics of Burrowing in Ensis (Bivalvia). Proc. R. Soc. Lond. Ser. B Biol. Sci. 1967, 166, 459–476. [Google Scholar] [CrossRef]
- Shulenberger, E. Responses of Gemma gemma (Mollusca: Pelecypoda) to a catastrophic burial. Veliger 1970, 13, 163–170. [Google Scholar]
- Kranz, P.M. The anastrophic burial of bivalves and its paleoecological significance. J. Geol. 1974, 82, 237–265. [Google Scholar] [CrossRef]
- Alexander, R.R.; Stanton, R.J., Jr.; Dodd, J.R. Influence of sediment grain size on the burrowing of bivalves: Correlation with distribution and stratigraphic persistence of selected Neogene clams. Palaios 1993, 8, 289–303. [Google Scholar] [CrossRef]
- Nichols, S.J. Burrowing saves Lake Erie clams. Nature 1997, 389, 921. [Google Scholar]
- Nichols, S.J. Factors protecting unionids from zebra-mussel induced mortality at Metzger Marsh, Lake Erie, Appendix 2. In Reestablishing the Freshwater Unionid Population of Metzger Marsh, Lake Erie; Nichols, S.J., Wilcox, D., Eds.; United States Geological Survey—Great Lakes Science Center: Ann Arbor, MI, USA, 2002. Available online: http://www.epa.gov/glnpo/ecopage/wetlands/metzger/apndx2.pdf (accessed on 2 October 2017).
- Amyot, J.-P.; Downing, J.A. Seasonal variation in vertical and horizontal movement of the freshwater bivalve Elliptio complanata (Mollusca: Unionidae). Freshw. Biol. 1997, 37, 345–354. [Google Scholar] [CrossRef]
- Amyot, J.-P.; Downing, J.A. Locomotion of Elliptio complanata (Mollusca: Unionidae): A reproductive function? Freshw. Biol. 1998, 39, 351–358. [Google Scholar] [CrossRef]
- Watters, G.T.; O’Dee, S.H.; Chordas, S. Patterns of vertical migration in freshwater mussels (Bivalvia: Unionoida). J. Freshw. Ecol. 2001, 16, 541–549. [Google Scholar] [CrossRef]
- Saarinen, M.; Taskinen, J. Burrowing and crawling behaviour of three species of Unionidae in Finland. J. Moll. Stud. 2003, 69, 81–86. [Google Scholar] [CrossRef]
- Allen, D.C.; Vaughn, C.C. Burrowing behavior of freshwater mussels in experimentally manipulated communities. J. N. Am. Benthol. Soc. 2009, 28, 93–100. [Google Scholar] [CrossRef]
- Yeager, M.M.; Cherry, D.S.; Neves, R.J. Feeding and burrowing behaviors of juvenile rainbow mussels, Villosa iris (Bivalvia: Unionidae). J. N. Am. Benthol. Soc. 1994, 13, 217–222. [Google Scholar] [CrossRef]
- Chamberlain, J.A., Jr. Two Catskill Freshwater clams, Archanodon (Devonian) and Margaritifera (Recent): What they tell us about the origin and evolution of unionoid bivalves. Northeast. Geol. Environ. Sci. 2004, 26, 5–21. [Google Scholar]
- Knoll, K.; Chamberlain, J.A., Jr.; Chamberlain, R.B. Escape burrowing of modern freshwater bivalves as a paradigm for escape capabilities of Archanodon catskillensis (Devonian), the oldest known freshwater bivalve. Geol. Soc. Am. Abstr. Progr. 2014, 46, 81. [Google Scholar]
- McMahon, R.F. Mollusca: Bivalvia. In Ecology and Classification of North American Freshwater Invertebrates; Thorp, J.H., Covich, A.P., Eds.; Academic Press: New York, NY, USA, 1991; pp. 315–399. [Google Scholar]
- Epstein, J.B.; Sevon, W.D.; Glaeser, J.D. Geology and mineral resources of the Lehighton and Palmerton Quadrangles, Carbon and Northampton Counties, Pennsylvania. In Atlas of the Geological Survey of Pennsylvania; Pennsylvania Geological Survey: Harrisburg, PA, USA, 1974; Volume 195, p. 460. [Google Scholar]
- Thompson, D.W. On Growth and Form; Cambridge University Press: Cambridge, UK, 1942; p. 1116. [Google Scholar]
- Zar, J.H. Biostatistical Analysis; Prentice-Hall: Englewood, NJ, USA, 1974; p. 620. [Google Scholar]
- Tada, R.; Siever, R. Pressure solution during diagenesis. Ann. Rev. Earth Planet. Sci. 1989, 17, 89–118. [Google Scholar] [CrossRef]
- Gibson, M.A.; Broadhead, T.W. Species specific growth responses of favositid corals to soft-bottom substrates. Lethaia 1989, 22, 287–299. [Google Scholar] [CrossRef]
- Alexander, R.R. Comparative hydrodynamic stability of brachiopod shells on current-scoured arenaceous substrates. Lethaia 1984, 17, 17–23. [Google Scholar] [CrossRef]
- Posenato, R. The gen. Comelicania Frech, 1901 (Brachiopoda) from the southern Alps: Morphology and classification. Riv. Italiana Paleontol. Stratigr. 1998, 104, 43–68. [Google Scholar] [CrossRef]
- Jin, J.; Copper, P. Evolution of the Early Silurian rhynchonellide brachiopod Stegerhynchus, Anticosti Island, eastern Canada. J. Paleontol. 2004, 78, 866–883. [Google Scholar] [CrossRef]
- Meckel, T.A.; Ten Brink, U.S.; Williams, S.J. Sediment compaction rates and subsidence in deltaic plains: Numerical constraints and stratigraphic influences. Basin Res. 2007, 19, 19–31. [Google Scholar] [CrossRef]
- Whitman, R.L.; Clark, W.J. Availability of oxygen in interstitial waters of a sandy Creek. Hydrobiologia 1982, 91, 651–658. [Google Scholar] [CrossRef]
- Chambers, P.A.; Flynn, E.E.; Gibson, K. Temporal and Spatial Dynamics in Riverbed Chemistry: The Influence of Flow and Sediment Composition. Can. J. Fish. Aquat. Sci. 1992, 49, 2128–2140. [Google Scholar]
- Flynn, K.; Belopolsky Wedin, M.; Bonventre, J.A.; Dillon-White, M.; Hines, J.; Weeks, B.S.; André, C.; Schreibman, M.P.; Gagné, F. Burrowing in the Freshwater Mussel Elliptio complanata is Sexually Dimorphic and Feminized by Low Levels of Atrazine. J. Toxicol. Environ. Health Part A 2013, 76, 1168–1181. [Google Scholar] [CrossRef] [PubMed]
- Hendelberg, J. The freshwater pearl mussel Margaritifera margaritifera (L.). Rept. Inst. Freshw. Res. Drottingholm 1961, 41, 149–171. [Google Scholar]
- Bauer, G. Variation in the life span and size of the freshwater Pearl Mussel. J. Anim. Ecol. 1992, 61, 425–436. [Google Scholar] [CrossRef]
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).