Environmental Risk Assessment Based on High-Resolution Spatial Maps of Potentially Toxic Elements Sampled on Stream Sediments of Santiago, Cape Verde
Abstract
:1. Introduction
2. Cape Verde Archipelago and Santiago Island: Location and Geology
2.1. Settings of the Archipelago of Cape Verde and Santiago Island
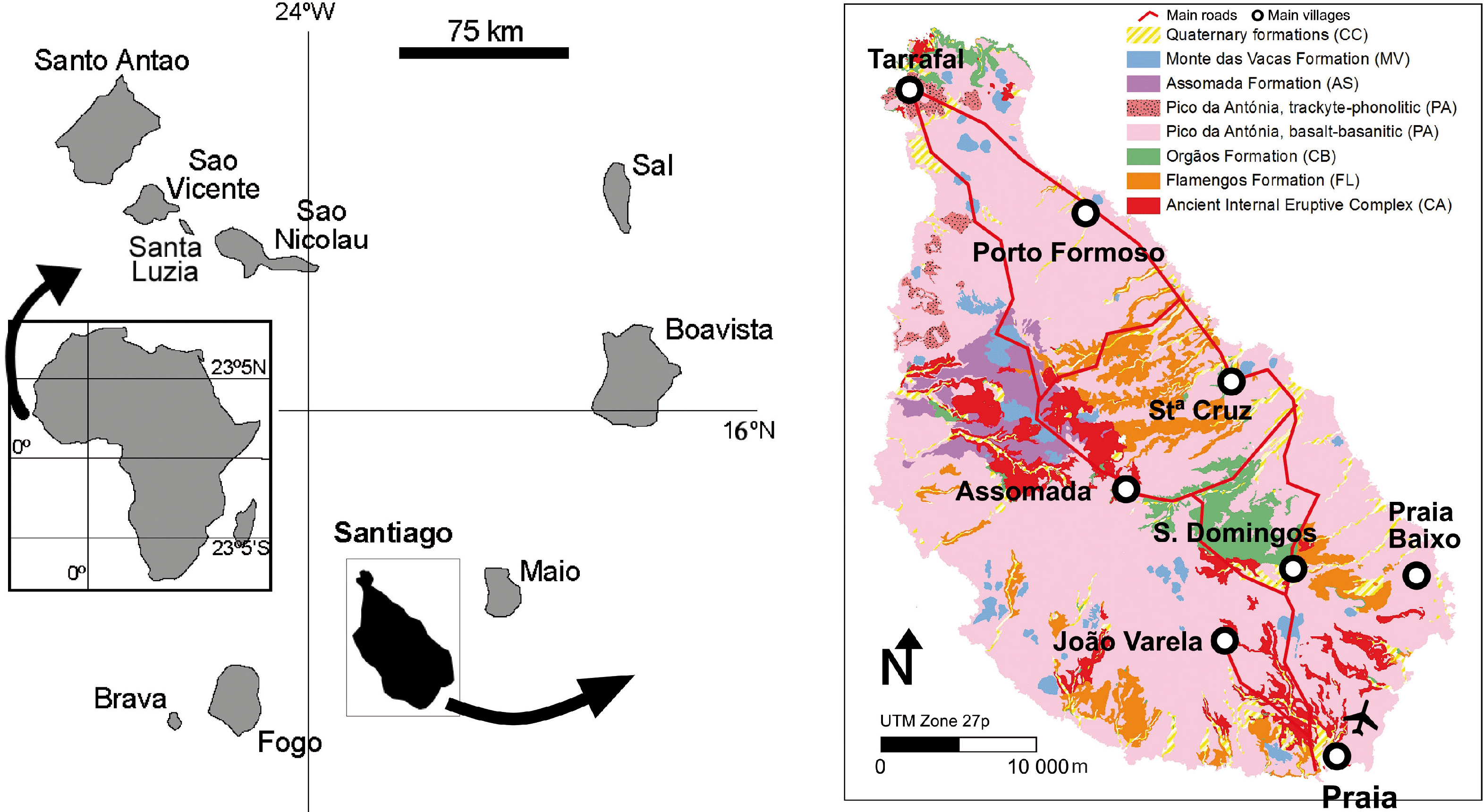
2.2. Geological Setting of Santiago Island
3. Materials and Methods
3.1. Sample Collection and Treatment
3.2. Chemical Analysis
3.3. Analytical Quality Control
 ) significantly representative, at a 0.01 significance level, of the spatial total observed variance (
) significantly representative, at a 0.01 significance level, of the spatial total observed variance (  ) quantified by an Analysis of Variance using field duplicates sampled at 26 locations evenly distributed over the island.
) quantified by an Analysis of Variance using field duplicates sampled at 26 locations evenly distributed over the island. ) into the spatial geochemical variance (
) into the spatial geochemical variance (  ) and the variance associated to errors due to field sampling and chemical analysis (
) and the variance associated to errors due to field sampling and chemical analysis (  ):
):




 , thus the spatial geochemical variability,
, thus the spatial geochemical variability,  , can only be represented by the total variability,
, can only be represented by the total variability,  , if
, if  <<
<<  . To address this issue the following hypothesis test is performed:
. To address this issue the following hypothesis test is performed:


3.4. Estimated Background Value and Statistical Analysis
| ID | Model | Main Direction | C0 | C1 | Length | Anisotropy Ratio | RMSE | MAD |
|---|---|---|---|---|---|---|---|---|
| Co | exponential | 0 | 30 | 150 | 4,000 | 1.27 | 2.38 | 1.58 |
| Cr | exponential | 45 | 2,100 | 1,900 | 2,000 | 1.64 | 3.45 | 2.01 |
| Cu | exponential | 0 | 160 | 200 | 5,000 | 1.22 | 1.05 | 0.98 |
| Ni | exponential | 30 | 2,600 | 2,800 | 3,500 | 1.83 | 4.82 | 2.99 |
| V | exponential | 0 | 1,200 | 600 | 4,000 | 1.98 | 3.56 | 2.33 |
| PC1 | exponential | 90 | 7,000,000 | 9,000,000 | 5,000 | 1.36 | 2.53 | 1.87 |
| PC2 | exponential | 90 | 1,000,000 | 7,500,000 | 2,000 | 1.23 | 1.95 | 1.54 |
3.5. Environmental Risk Assessment
4. Results and Discussion
4.1. Estimated Background Values
| Var | Min | Med | Me | Max | SD | CV | Sk | Krt | P5–P95 | Tukey Range | EBV-S |
|---|---|---|---|---|---|---|---|---|---|---|---|
| As | 0.3 | 0.3 | 0.6 | 7.2 | 0.62 | 1.07 | 5.18 | 43.79 | 0.3–1.6 | 0.3–1.4 | 0.25 |
| Cd | 0.05 | 0.10 | 0.14 | 1.00 | 0.09 | 0.64 | 3.82 | 30.16 | 0.05–0.30 | 0.05–0.35 | 0.10 |
| Co | 3.1 | 44.7 | 45.1 | 139.9 | 13.86 | 0.31 | 1.21 | 7.79 | 26.4–66.1 | 15.8–73.4 | 44.65 |
| Cr | 8.0 | 114.0 | 123.7 | 463.1 | 68.03 | 0.55 | 1.49 | 4.48 | 20.0–251.5 | 8.0–264.0 | 111.00 |
| Cu | 3.2 | 48.8 | 48.6 | 141.6 | 17.99 | 0.37 | 0.52 | 2.38 | 17.6–77.8 | 9.4–87.6 | 48.70 |
| Hg | 0.01 | 0.01 | 0.01 | 0.08 | 0.01 | 0.74 | 2.13 | 7.24 | 0.15–0.54 | 0.07–0.61 | 0.26 |
| Mn | 197.0 | 1191.0 | 1259.9 | 4210.0 | 441.65 | 0.35 | 2.07 | 8.87 | 737.1–2043.5 | 255.1–2162.1 | 1182.00 |
| Ni | 6.8 | 155.2 | 160.5 | 477.0 | 76.02 | 0.47 | 0.50 | 1.13 | 21.3–286.2 | 6.8–337.5 | 152.85 |
| Pb | 1.4 | 3.9 | 5.2 | 81.4 | 6.61 | 1.26 | 7.53 | 70.27 | 2.0–10.1 | 1.4–10.1 | 3.80 |
| V | 24.0 | 160.0 | 161.0 | 372.0 | 45.68 | 0.28 | 0.64 | 2.57 | 92.4–237.3 | 50.5–262.5 | 159.00 |
| Zn | 15.0 | 81.0 | 82.7 | 189.0 | 19.14 | 0.23 | 1.23 | 5.35 | 57.0–111.0 | 34.0–130.0 | 81.00 |
| Variable | EBV-CA | EBV-FL | EBV-CB | EBV-PA | EB-AS | EBV-MV | EBV-CC | EBV-AL |
|---|---|---|---|---|---|---|---|---|
| (n = 41) | (n = 21) | (n = 28) | (n = 118) | (n = 15) | (n = 18) | (n = 9) | (n = 87) | |
| As | 0.3 | 0.3 | 0.3 | 0.3 | 0.3 | 0.6 | 0.7 | 0.3 |
| Cd | 0.10 | 0.10 | 0.10 | 0.10 | 0.20 | 0.20 | 0.10 | 0.10 |
| Co | 43.6 | 41.3 | 40.3 | 48.8 | 35.8 | 42.6 | 42.2 | 44.9 |
| Cr | 116.0 | 112.5 | 99.0 | 122.5 | 20.5 | 76.0 | 93.5 | 119.3 |
| Cu | 53.8 | 57.6 | 56.7 | 46.6 | 26.4 | 34.2 | 47.4 | 48.9 |
| Hg | 0.01 | 0.01 | 0.01 | 0.02 | 0.02 | 0.03 | 0.01 | 0.02 |
| Mn | 1199 | 1036 | 980 | 1328 | 1612 | 1423 | 1027 | 1157 |
| Ni | 126.7 | 139.1 | 145.1 | 168.1 | 20.1 | 104.1 | 148.9 | 164.1 |
| Pb | 3.6 | 2.5 | 3.3 | 4.7 | 6.6 | 6.0 | 4.1 | 3.4 |
| V | 166.0 | 157.5 | 148.0 | 167.0 | 153.0 | 131.0 | 147.0 | 156.0 |
| Zn | 86.5 | 80.0 | 76.5 | 76.0 | 99.0 | 88.0 | 73.5 | 81.0 |
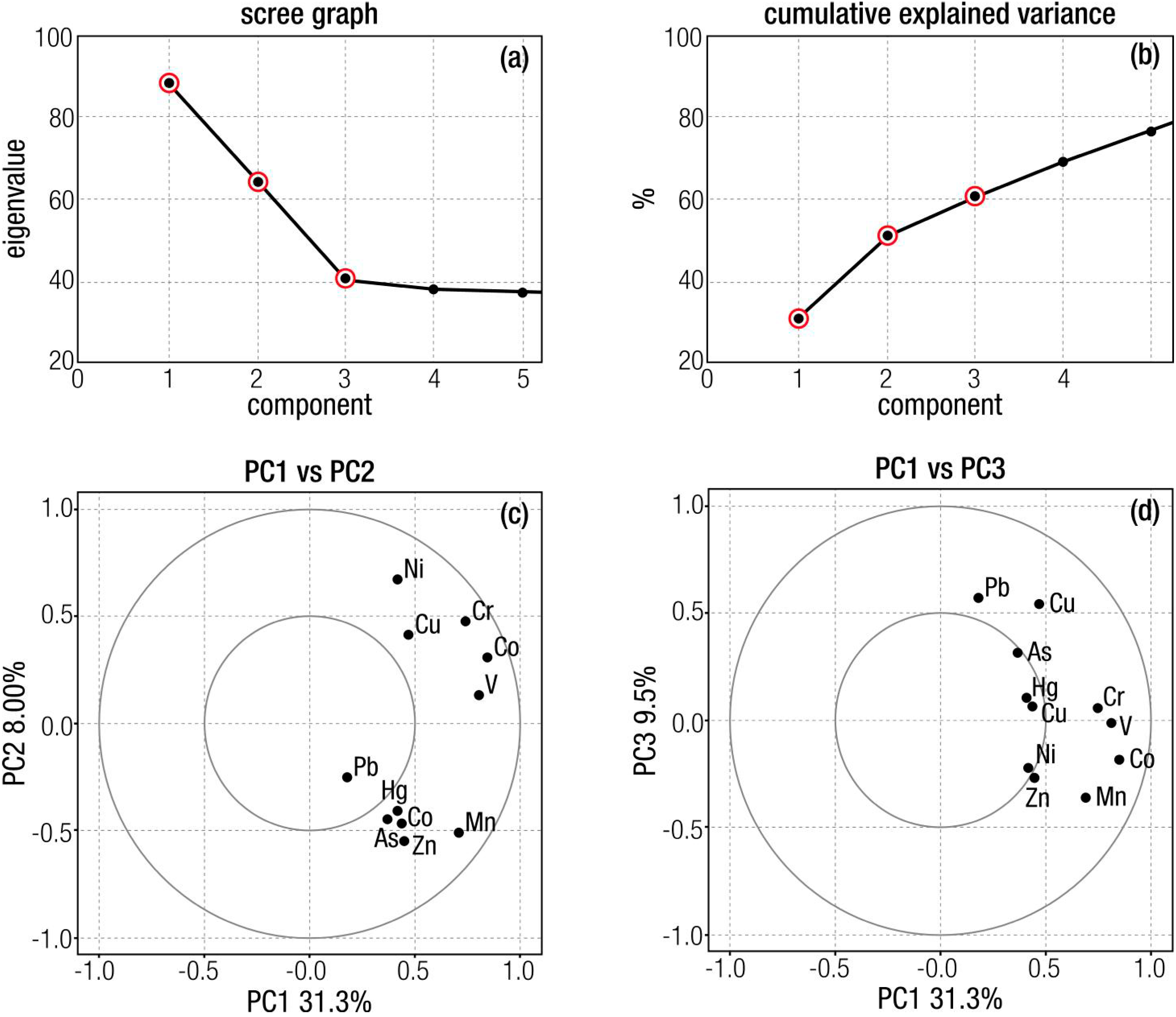
4.2. Multivariate Statistical Analysis

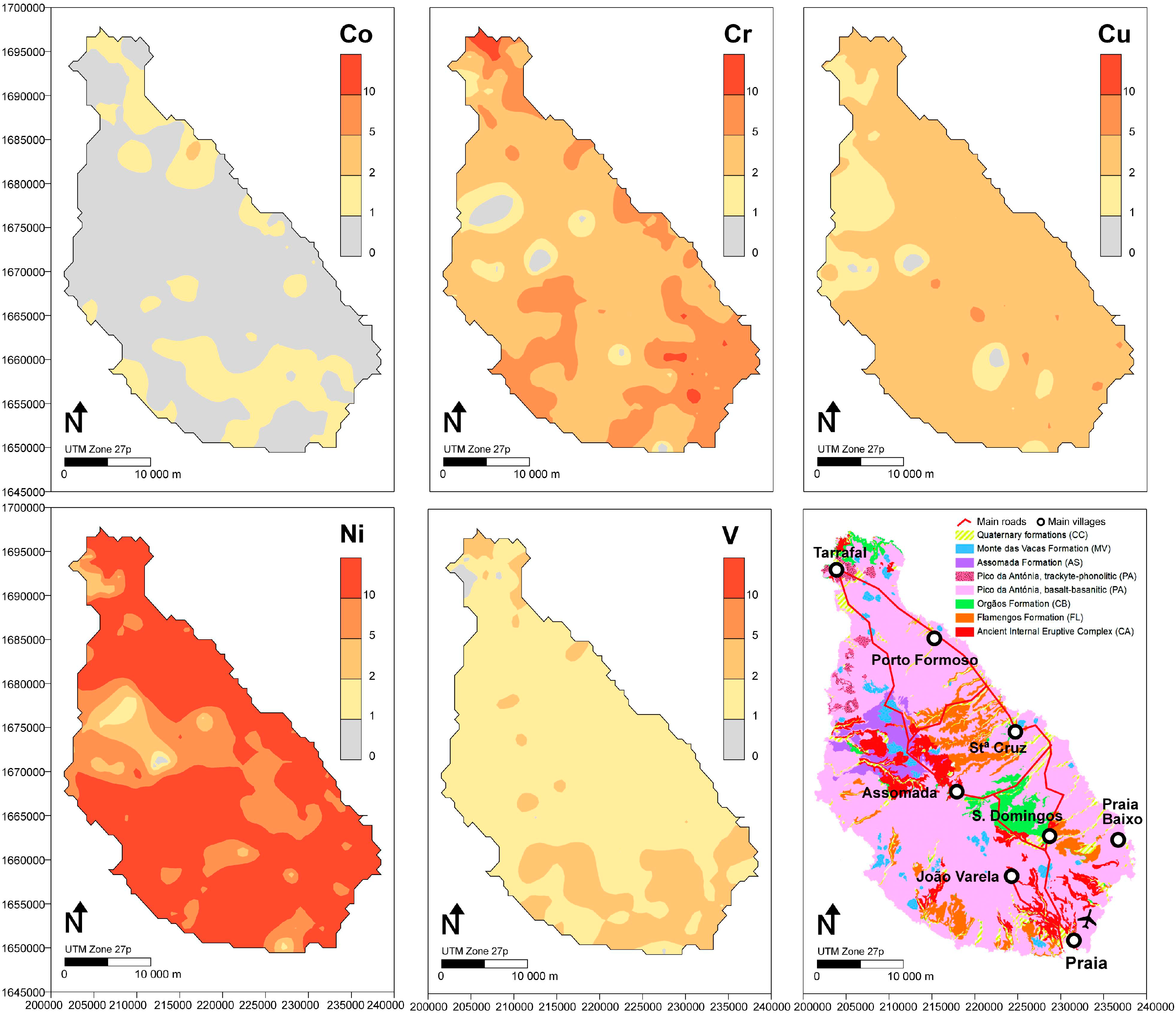
4.3. Environmental Risk Assessment
| EBV-S | Canadian Guidelines | Dutch Guidelines | United States [56] | Numerical Sediment Quality Guidelines for Freshwater Ecosystems | |
|---|---|---|---|---|---|
| All Types of Property Uses | Target Values | Mean Values | Probable Effect Concentrations: PEL; SEL; TET | ||
| As | 0.60 | 6 | 29 | 3.9 | 17; 33; 17 |
| Cd | 0.91 | 0.6 | 0.8 | - | 3.53; 10; 3 |
| Co | 46.4 | 50 | 9 | 9.1 | -; -; - |
| Cr | 118 | 26 | 100 | - | 90; 110; 100 |
| Cu | 50.8 | 16 | 36 | 14.2 | 197; 110;86 |
| Hg | 0.02 | 0.2 | 0.3 | - | 0.486; 2; - |
| Mn | 1293 | - | - | 760 | -; -; - |
| Ni | 136.1 | 16 | 36 | 15.1 | 36; 75; 61 |
| Pb | 5.00 | 31 | 85 | 14.1 | 91.3; 250; 170 |
| V | 169 | 90 * | - | 81.1 | -; -; - |
| Zn | 79 | 120 | 140 | 62.9 | 315; 820; 540 |
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Siegel, F.R. Environmental Geochemistry of Potentially Toxic Metals; Springer: Berlin, Germany, 2002. [Google Scholar]
- Pinto, M.M.S.C.; Silva, M.M.V.G.; Neiva, A.M.R. Pollution of water and stream sediments associated with the Vale de Abrutiga Uranium Mine, Central Portugal. Mine Water Environ. 2004, 23, 66–75. [Google Scholar]
- Pinto, M.M.S.C.; Silva, M.M.V.G. Contemporary reviews of mine water studies in Europe—Portugal. In Mine Water and the Environment; Wolkersdorfer, C., Bowell, R., Eds.; Springer: Berlin, Germany, 2005; pp. 50–53. [Google Scholar]
- Komatina, M.M. Medical Geology: Effects of Geological Environments on Human Health; Developments in Earth and Environmental Sciences 2; Elsevier Science: Amsterdam, The Netherlands, 2004. [Google Scholar]
- Kozlowski, H.; JanickaKlosb, A.; Brasunb, J.; Gaggelli, E.; Valensinc, D.; Valensinc, J. Copper, iron, and zinc ions homeostasis and their role in neurodegenerativedisorders (metal uptake, transport, distribution and regulation). Coord. Chem. Rev. 2009, 253, 2665–2685. [Google Scholar]
- Gorell, J.M.; Johnson, C.C.; Rybicki, B.A.; Peterson, E.L.; Kortsha, G.X.; Kortsha, G.G.; Richardson, R.J. Occupational exposure to manganese, copper, lead, iron, mercury and zinc and the risk of Parkinson’s disease. Neurotoxicology 1999, 20, 239–248. [Google Scholar]
- Zatta, P.; Lucchini, R.; van Rensburg, S.J.; Taylor, A. The role of metals in neurodegenerative processes: aluminum, manganese, and zinc. Brain Res. Bull. 2003, 62, 15–28. [Google Scholar]
- Elsner, R.; Spangler, J. Neurotoxicity of inhaled manganese: Public health danger in the shower? Med. Hypotheses 2005, 65, 607–616. [Google Scholar]
- Erikson, K.M.; Syversen, T.; Aschner, J.L.; Aschner, M. Interactions between excessive manganese exposures and dietary iron-deficiency in neurodegeneration. Environ. Toxicol. Pharmacol. 2005, 19, 415–421. [Google Scholar]
- Schneider, J.S.; Decamp, E.; Koser, A.J.; Fritz, S.; Gonczi, H.; Syversen, T.; Guilarte, T.R. Effects of chronic manganese exposure on cognitive and motor functioning in non-human primates. Brain Res. 2006, 1118, 222–231. [Google Scholar]
- Cersosimo, M.G.; Koller, W.C. The diagnosis of manganese-induced parkinsonism. NeuroToxicology 2006, 27, 340–346. [Google Scholar]
- Santamaria, A.B.; Cushing, C.A.; Antonini, J.M.; Finley, B.L.; Mowat, F.S. State-of-the-science review: Does manganese exposure during welding pose a neurological risk? J. Toxicol. Environ. Health 2007, 10, 417–465. [Google Scholar]
- Flyn, M.R.; Susi, P. Neurological risks associated with manganese exposure from welding operations—A literature review. Int. J. Hyg. Environ. Health 2009, 212, 459–469. [Google Scholar]
- International Agency for Research on Cancer. Chromium, Nickel and Welding; IARC Monographs on the Evaluation of Carcinogenic Risks to Humans Volume 49; International Agency for Research on Cancer: Lyon, France, 1990. [Google Scholar]
- Staessen, J.A.; Buchet, J.P.; Ginucchio, G.; Lauwerys, R.R.; Lijnen, P.; Roels, H.; Fagard, R. Public health implications of environmental exposure to Cadmium and Lead: An overview of epidemiological studies in Belgium. J. Cardiovasc. Risk 2007, 3, 26–41. [Google Scholar]
- Schroeder, H.A. Cadmium, chromium, and cardiovascular disease. Circulation 1967, 35, 570–582. [Google Scholar]
- Panaullah, G.M.; Alam, T.; Baktear Hossain, M.; Loeppert, R.H.; Lauren, J.G.; Meisner, C.A.; Ahmed, Z.U.; Duxbury, J.M. Arsenic toxicity to rice (Oryza sativa L.) in Bangladesh. Plant Soil 2009, 317, 31–39. [Google Scholar]
- Yao, Y.; Pei, F.; Kang, P. Selenium, iodine, and the relation with Kashin-Beck disease. Nutrition 2011, 27, 1095–1100. [Google Scholar]
- Plant, J.A.; Smith, D.; Smith, B.; Williams, L. Environmental geochemistry at the global scale. Appl. Geochem. 2001, 16, 1291–1308. [Google Scholar]
- Smith, D.B.; Smith, S.M.; Horton, J.D. History and evaluation of national-scale geochemical data sets for the United States. Geosci. Front. 2013, 4, 167–183. [Google Scholar]
- Salminen, R.; Batista, M.J.; Bidovec, M.; Demetriades, A.; de Vivo, B.; de Vos, W.; Duris, M.; Gilucis, A.; Gregorauskiene, V.; Halamic, J.; et al. Geochemical Atlas of Europe, Part 1: Background Information, Methodology and Maps; Geological Survey of Finland: Espoo, Finland, 2005. [Google Scholar]
- Johnson, C.C.; Breward, N.; Ander, E.L.; Ault, L. G-BASE: Baseline geochemical mapping of Great Britain and Northern Ireland. Geochem. Explor. Environ. Anal. 2005, 5, 347–357. [Google Scholar]
- Garret, R.G.; Reimann, C.; Smith, D.B.; Xie, X. From geochemical prospecting to international geochemical mapping: A historical overview. Geochem. Explor. Environ. Anal. 2008, 8, 205–217. [Google Scholar]
- Smith, D.B.; Reimann, C. Low-Density geochemical mapping and the robustness of geochemical patterns. Geochem. Explor. Environ. Anal. 2008, 8, 219–227. [Google Scholar]
- Appleton, J.D.; Ridgway, J. Regional geochemical mapping in developing countries and its application to environmental studies. Appl. Geochem. 1993, 2, 103–110. [Google Scholar]
- Xuejing, X.; Xuzhan, M.; Tianxiang, R. Geochemical mapping in China. J. Geochem. Explor. 1997, 60, 99–113. [Google Scholar]
- Reimann, C.; Caritat, P. Chemical Elements in the Environment—Factsheets for the Geochemist and Environmental Scientist; Springer-Verlag: Berlin, Germany, 2008. [Google Scholar]
- Lech, M.E.; Caritat, P. Regional Geochemical Study Paves Way for National Survey—Geochemistry of Near-Surface Regolith Points to New Resources. Available online: http://www.ga.gov.au/ausgeonews/ausgeonews200706/geochemical.jsp (accessed on 1 June 2007).
- Inácio, M.; Pereira, V.; Pinto, M. The soil geochemical Atlas of Portugal: Overview and applications. J. Geochem. Explor. 2008, 98, 22–33. [Google Scholar]
- Darnley, A. International geochemical mapping special issue. J. Geochem. Explor. 1990, 39, 1–13. [Google Scholar]
- Darnley, A.G.; Björklund, A.; Bølviken, B.; Gustavsson, N.; Koval, P.V.; Plant, J.A.; Steenfelt, A.; Tauchid, M.; Xie, X. A Global Geochemical Database for Environmental and Resource Management: Recommendations for International Geochemical Mapping; Final Report of IGCP Project 259; UNESCO: Paris, France, 1995. [Google Scholar]
- Albanese, S.; de Vivo, B.; Lima, A.; Cicchella, D. Geochemical background and baseline values oftoxic elements in stream sediments of Campania region (Italy). J. Geochem. Explor. 2007, 93, 21–34. [Google Scholar]
- Levinson, A.A. Introduction to Exploration Geochemistry; Applied Publishing Ltd.: Maywood, CA, USA, 1974. [Google Scholar]
- Beus, A.A.; Grigorian, V. Geochemical Exploration Methods for Mineral Deposits; Applied Publishing Ltd.: Wilmette, IL, USA, 1977. [Google Scholar]
- Reimann, C.; Siewers, U.; Tarvainen, T.; Bityukova, L.; Eriksson, J.; Gilucis, A.; Gregorauskiene, V.; Lukashev, V.K.; Matinian, N.N.; Pasieczna, A.; et al. Agricultural Soils in Northern Europe: A Geochemical Atlas; Schweizerbart Science Publishers: Stuttgart, Germany, 2003. [Google Scholar]
- Cabral Pinto, M.M.S. Geochemical Cartography, with a Low To-Median Density, of Santiago Island, Cape Verde. Ph.D. Thesis, University of Aveiro, Aveiro, Portugal, 2010. [Google Scholar]
- Desenfant, F.; Petrovský, E.; Rochette, P. Magnetic signature of industrial pollution of stream sediments and correlation with heavymetals: Case study from south France. Water Air Soil Pollut. 2004, 152, 297–312. [Google Scholar]
- INE 2010. Censo 2010. Available online: http://www.ine.cv (accessed on 21 March 2013).
- Serralheiro, A. A Geologia da ilha de Santiago (Cabo Verde). Boletim Museu Laboratório Mineralógico Geológico Faculdade de Ciências de Lisboa 1976, 14, 157–372. (In Portuguese) [Google Scholar]
- Instituto Nacional de Meteorologia e Geofisica (INMG). Climatologic Data of Some Stations in Santiago Island, Praia, Cabo Verde. Internal Report; INMG: Praia, Santiago Island, Cabo Verde, 2005. [Google Scholar]
- United Nations Development Program for Cape Verde; PNUD: New York, NY, USA, 1993.
- Matos Alves, C.A.; Macedo, J.R.; Celestino Silva, L.; Serralheiro, A.; Peixoto Faria, A.F. Estudo geológico, petrológico e vulcanológico da ilha de Santiago (Cabo Verde). Garcia de Orta Serviços Geológicos 1979, 3, 47–74. (In Portuguese) [Google Scholar]
- Ramalho, R.A.S. Building the Cape Verde Islands. Ph.D. Thesis, University of Bristol, Bristol, UK, 2011. [Google Scholar]
- Holm, P.M.; Grandvuinet, T.; Friis, J.; Wilson, J.R.; Barker, A.K.; Plesner, P. An 40Ar-39Ar study of the Cape Verde hot spot: Temporal evolution in a semistationary plate environment. J. Geophys. Res. 2008, 113. [Google Scholar] [CrossRef]
- Pina, A.F.L. Hydrochemistry and groundwater quality of the island of Santiago, Cape Verde (Hidroquímica e qualidade das águas subterrâneas da ilha de Santiago, Cabo Verde). Ph.D. Thesis, University of Aveiro, Aveiro, Portugal, 2009. [Google Scholar]
- Martins, S.; Mata, J.; Munhá, J.; Madeira, J.; Moreira, M. Evidências geológicas e geoquímicas para a existência de duas unidades estratigráficas distintas na Formação do Pico da Antónia (Ilha de Santiago, República de Cabo Verde). Memórias e Notícias Universidade de Coimbra 2008, 3, 123–128. (In Portuguese) [Google Scholar]
- Rose, A.W.; Hawkes, E.H.; Webb, J.S. Geochemistry in Mineral Exploration, 2nd ed.; Academic Press: London, UK, 1979. [Google Scholar]
- Thompson, M. Analytical methods in applied environmental geochemistry. In Applied Environmental Geochemistry; Thornton, I., Ed.; Academic Press: London, UK, 1983. [Google Scholar]
- Chao, T.T.; Sanzolone, R.F. Decomposition techniques. J. Geochem. Explor. 1992, 44, 65–106. [Google Scholar]
- Tukey, J.W. Exploratory Data Analysis; Addison-Wesley: Reading, UK, 1977. [Google Scholar]
- Reimann, C.; Filzmoser, P.; Garret, R.G.; Dutter, R. Statistical Data Analysis Explaned: Applied Environmental Statistics with R, 1st ed.; John Wiley & Sons: Chichester, UK, 2008. [Google Scholar]
- Minister of the Environment (Canada). Soil, Ground Water and Sediment Standards for Use under Part XV.1 of the Environmental Protection Act. Available online: http://www.mah.gov.on.ca/AssetFactory.aspx?did=8993 (accessed on 15 April 2011).
- Italian Legislation: Decreto Ministeriale, n°471, 1999. Regolamento Recante Criteri, Procedure e Modalità per la Messa in Sicurezza, la Bonifica e il Ripristino Ambientale dei Siti Inquinati, ai Sensi Dell’artocolo 17 del Decreto Legislativo 5 Febbraio 1997, n.22, e Successive Modificazioni e Integrazioni; Gazzetta Ufficiale n°293 de 15 December 1999, Supplemento Ordinario n°218. Available online: http://www.eugris.info/FurtherDescription.asp?e=550&Ca=1&Cy=8&DocID=E&DocTitle=Management_administration&T=Italy (accessed on 15 October 2014). (In Italian)
- Sheng, J.; Wang, X.; Gong, P.; Tian, L.; Yao, T. Heavy metals of the Tibetan top soils—Level, source, spatial distribution, temporal variation and risk assessment. Environ. Sci. Pollut. Res. 2012, 19, 3362–3370. [Google Scholar]
- Spatial Planning and the Environment (VROM). Circular on Target Values and Intervention Values for Soil Remediation. The Netherlands Government Gazette, No. 39, Ministry of Housing, Spatial Planning and Environment, Directorate General for Environmental Protection, Department of Soil Protection. Available online: http://www.esdat.net/Environmental%20Standards/Dutch/annexS_I2000Dutch%20Environmental%20Standards.pdf (accessed on 16 October 2014).
- Cannon, W.F.; Woodruff, L.G.; Pimley, S. Some statistical relationships between stream sediment and soil geochemistry in northwestern Wisconsin—Can stream sediment compositions be used to predict compositions of soils in glaciated terranes? J. Geochem. Explor. 2004, 81, 29–46. [Google Scholar]
- MacDonald, D.D.; Ingersoll, C.G.; Berger, T.A. Development and evaluation of consensus-based sediment quality guidelines for freshwater ecosystems. Arch. Environ. Contam. Toxicol. 2000, 39, 20–31. [Google Scholar]
- Smith, S.L.; MacDonald, D.D.; Keenleyside, K.A.; Ingersoll, C.G.; Field, J. A preliminary evaluation of sediment quality assessment values for freshwater ecosystems. J. Gt. Lakes Res. 1996, 22, 624–638. [Google Scholar]
- Persaud, D.; Jaagumagi, R.; Hayton, A. Guidelines for the Protection and Management of Aquatic Sediment Quality in Ontario; Water Resources Branch, Ontario Ministry of the Environment: Toronto, Canada, 1993. [Google Scholar]
- Environment Canada. EC, MENVIQ (Environment Canada and Ministere de l’Envionnement du Quebec) Interim Criteria for Quality Assessment of St. Lawrence River Sediment; Environment Canada: Ottawa, Canada, 1992. [Google Scholar]
- Cabral Pinto, M.M.S.; da Silva, E.A.F.; Silva, M.M.V.G.; Melo-Gonçalves, P. Estimated backgroundvalues of some harmful metals in stream sediments of Santiago Island (Cape Verde). In Geochemistry: Earth’s System Processes; Dionisios, P., Ed.; InTech: Rijeka, Croatia, 2012; pp. 61–80. [Google Scholar]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pinto, M.M.S.C.; Silva, E.A.F.d.; Silva, M.M.V.G.; Melo-Gonçalves, P.; Candeias, C. Environmental Risk Assessment Based on High-Resolution Spatial Maps of Potentially Toxic Elements Sampled on Stream Sediments of Santiago, Cape Verde. Geosciences 2014, 4, 297-315. https://doi.org/10.3390/geosciences4040297
Pinto MMSC, Silva EAFd, Silva MMVG, Melo-Gonçalves P, Candeias C. Environmental Risk Assessment Based on High-Resolution Spatial Maps of Potentially Toxic Elements Sampled on Stream Sediments of Santiago, Cape Verde. Geosciences. 2014; 4(4):297-315. https://doi.org/10.3390/geosciences4040297
Chicago/Turabian StylePinto, Marina M. S. Cabral, Eduardo A. Ferreira da Silva, Maria M. V. G. Silva, Paulo Melo-Gonçalves, and Carla Candeias. 2014. "Environmental Risk Assessment Based on High-Resolution Spatial Maps of Potentially Toxic Elements Sampled on Stream Sediments of Santiago, Cape Verde" Geosciences 4, no. 4: 297-315. https://doi.org/10.3390/geosciences4040297
APA StylePinto, M. M. S. C., Silva, E. A. F. d., Silva, M. M. V. G., Melo-Gonçalves, P., & Candeias, C. (2014). Environmental Risk Assessment Based on High-Resolution Spatial Maps of Potentially Toxic Elements Sampled on Stream Sediments of Santiago, Cape Verde. Geosciences, 4(4), 297-315. https://doi.org/10.3390/geosciences4040297








