Geochemical Features of Ultramafic Rocks and Formation of Magnesium–Bicarbonate Groundwaters in the Kraka Massif Area (Southern Urals)
Abstract
1. Introduction
2. History of Geological and Hydrogeologic Studies
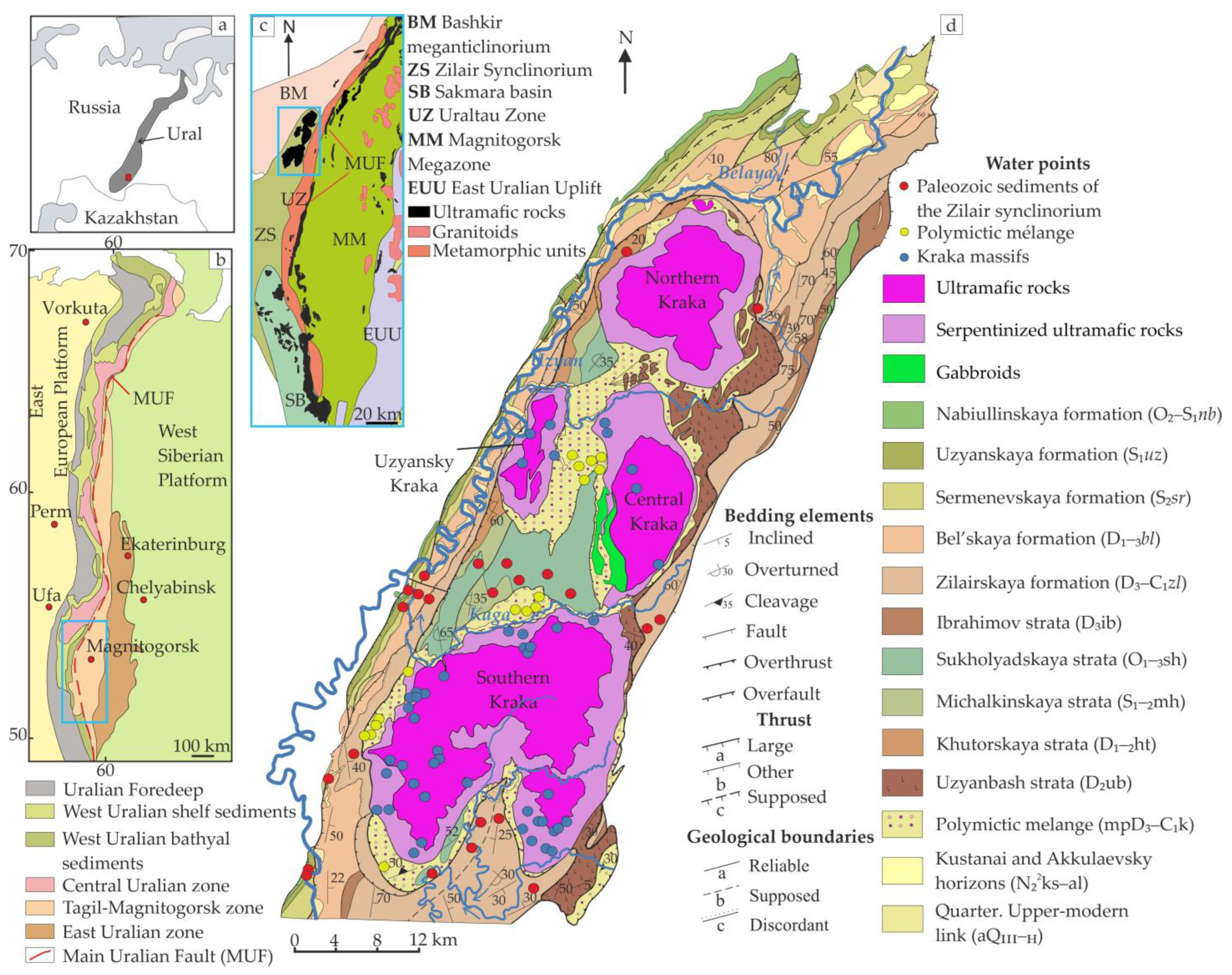
3. Geological and Hydrogeological Characteristics
3.1. Stratigraphy of Host Rocks
- The carbonate–terrigenous section, represented by sediments from the Nabiullinskaya (O2–S1nb) to the Zilair formation (D3–C1zl), and located in the western part of the synclinorium;
- The terrigenous–siliceous section composed of rocks from the Sukholyadskaya strata (O1–3sh) to the Zilair formation (D3–C1zl) in the eastern part of the synclinorium (Figure 1).
3.2. Geologic Structure of the Kraka Massifs
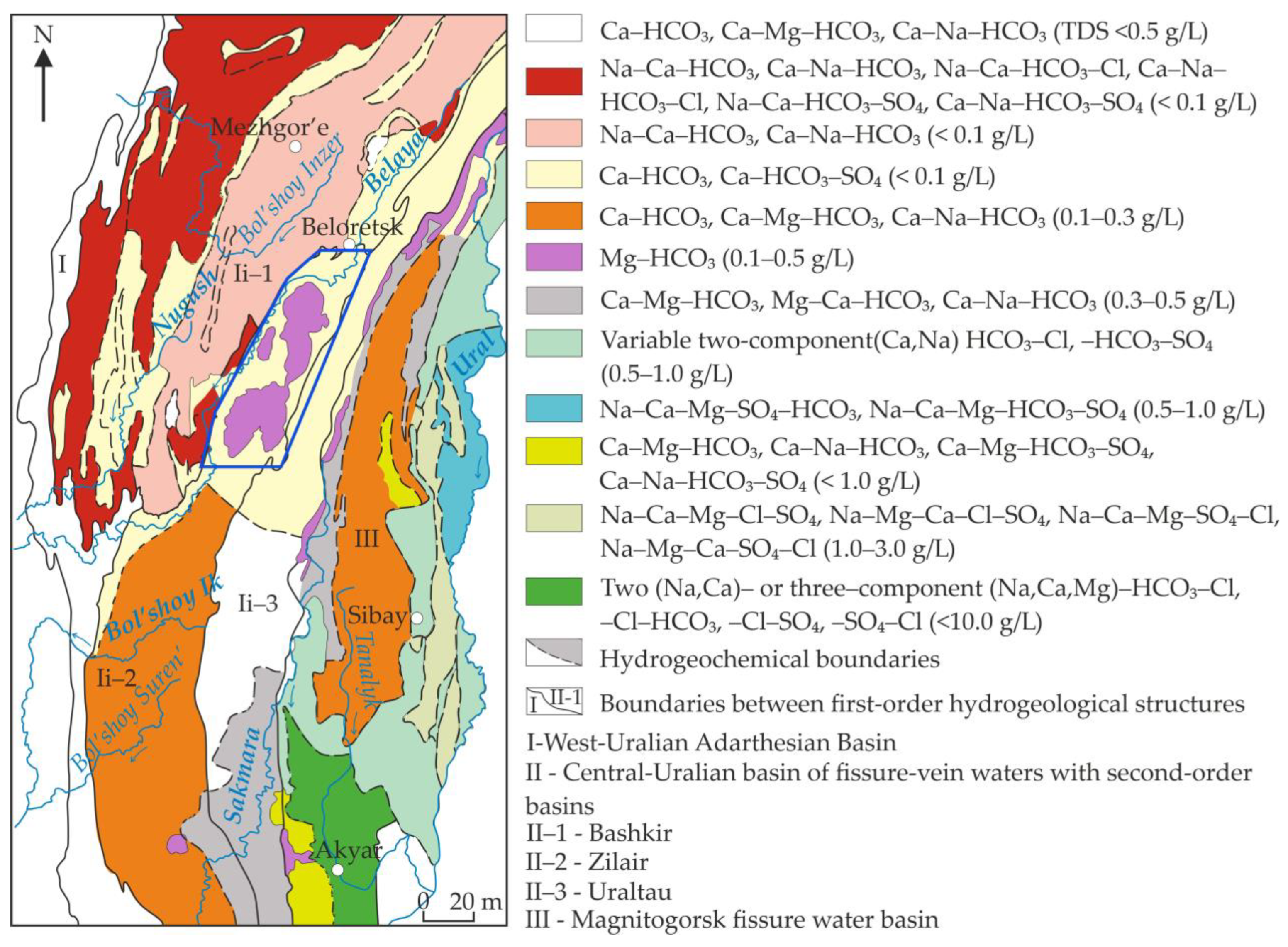
3.3. Hydrogeological Characteristics of the Northern Part of Zilair Synclinorium
4. Materials and Methods
5. Results
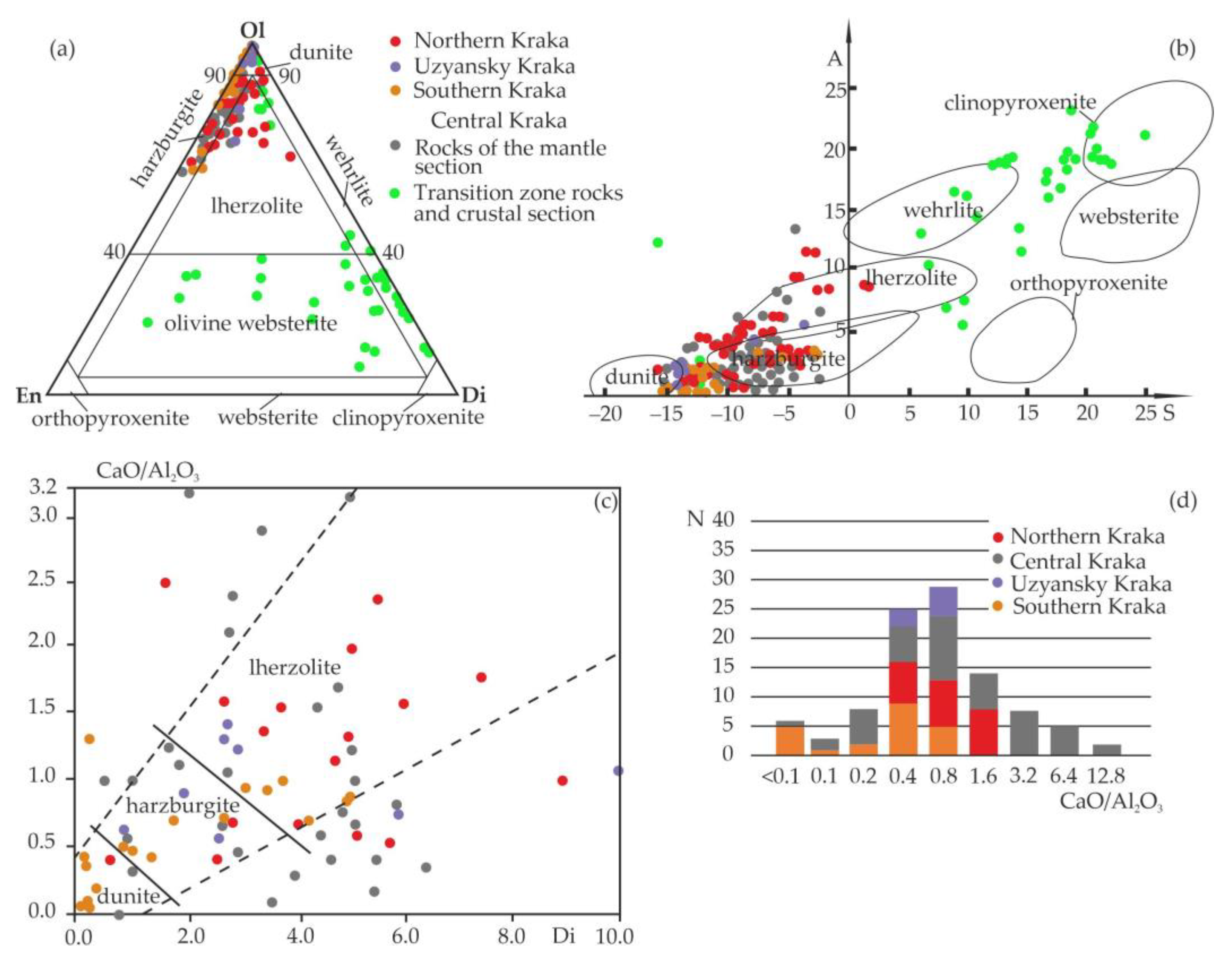
5.1. Analysis of the Chemical Composition of Rocks from the Kraka Massifs
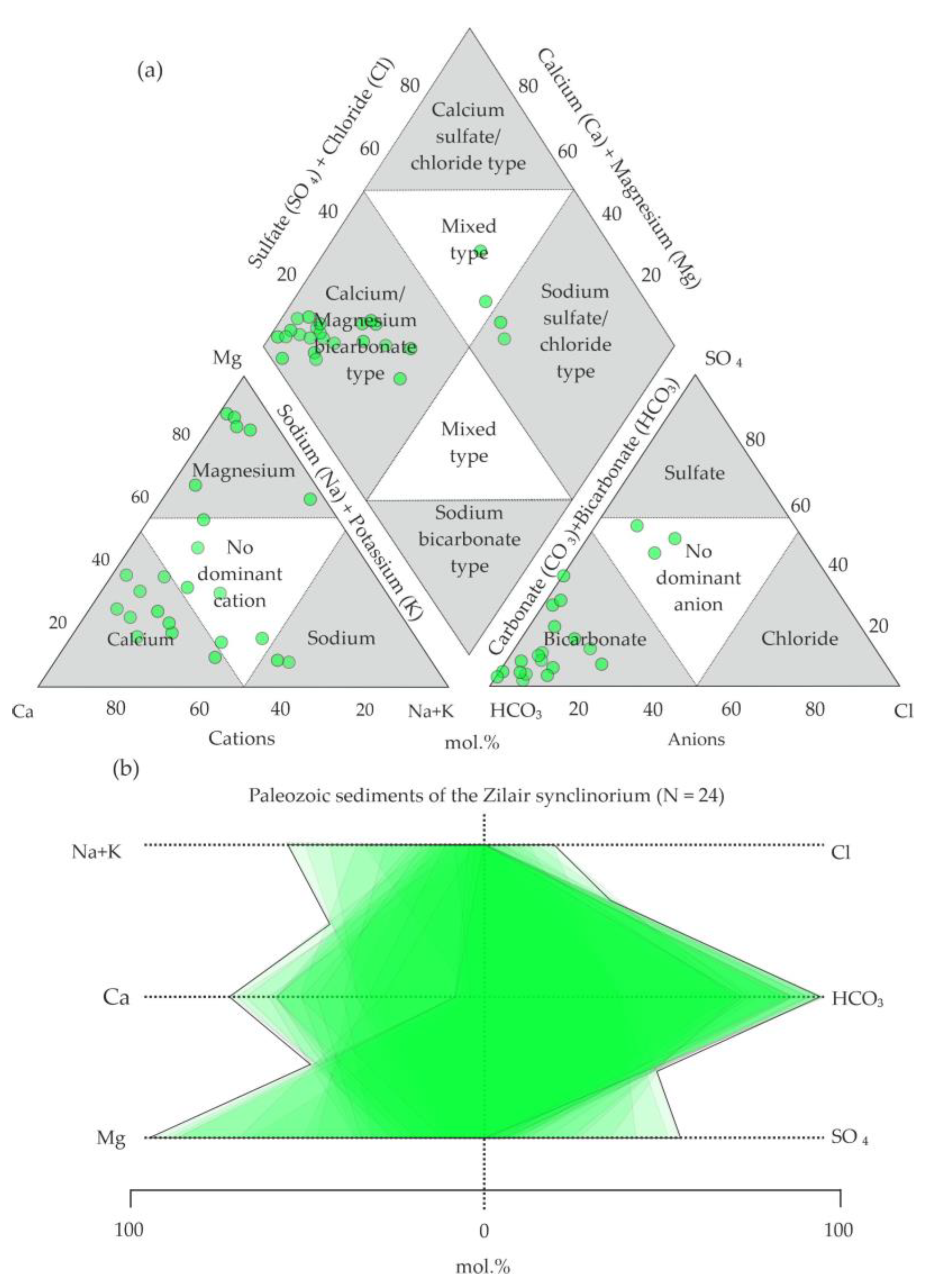
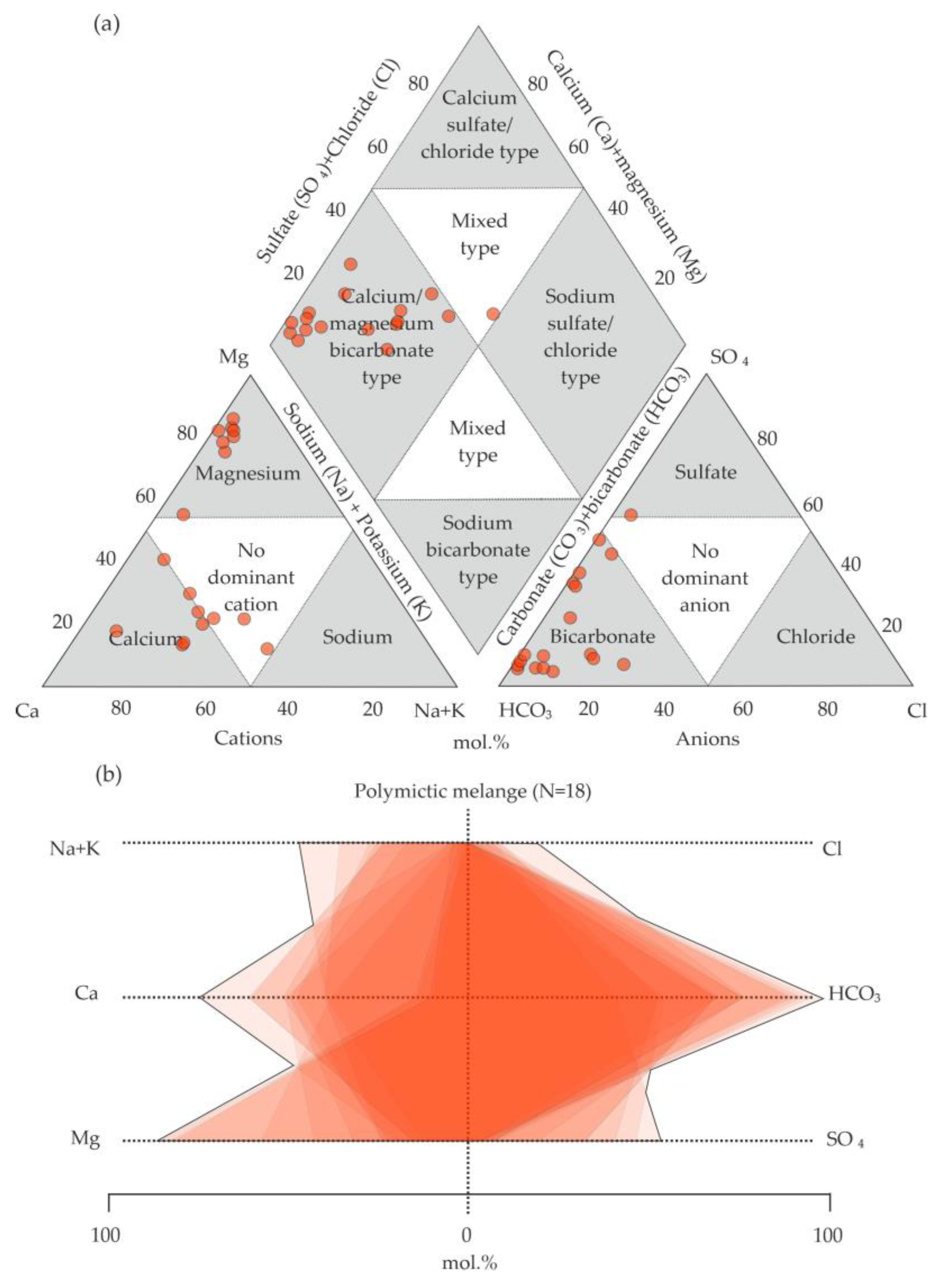
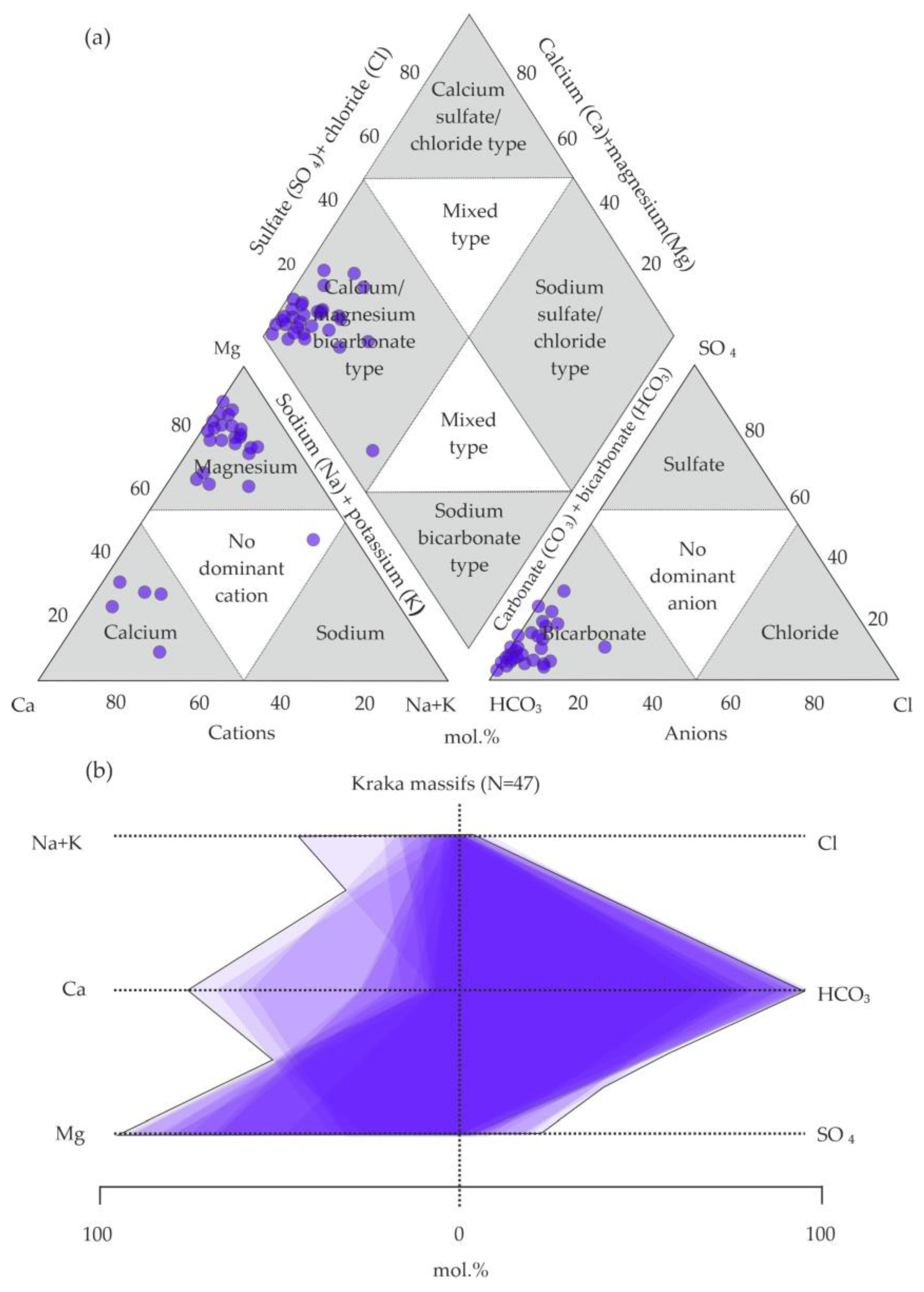
5.2. Analysis of the Chemical Composition of Groundwaters on the Territory
6. Discussion
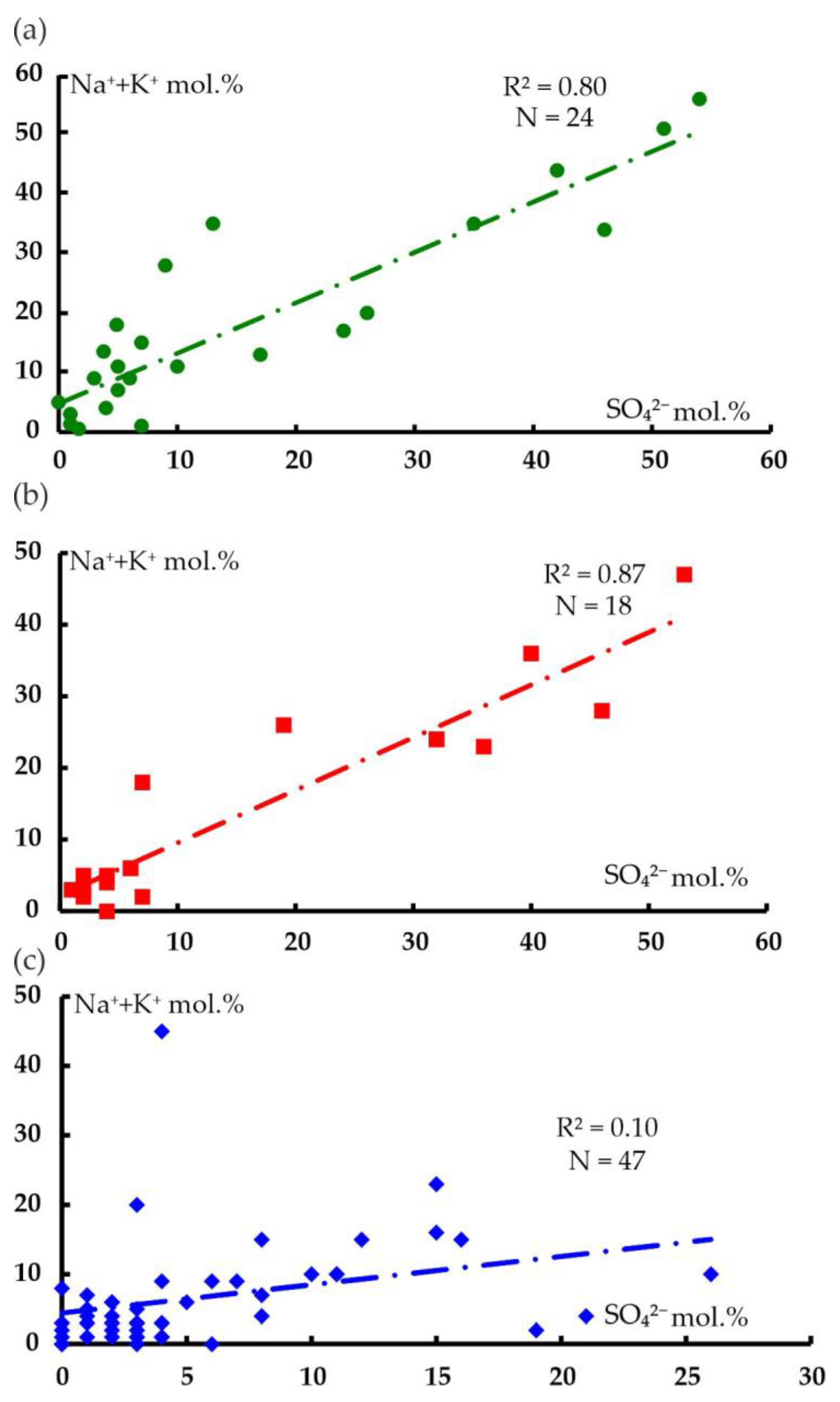
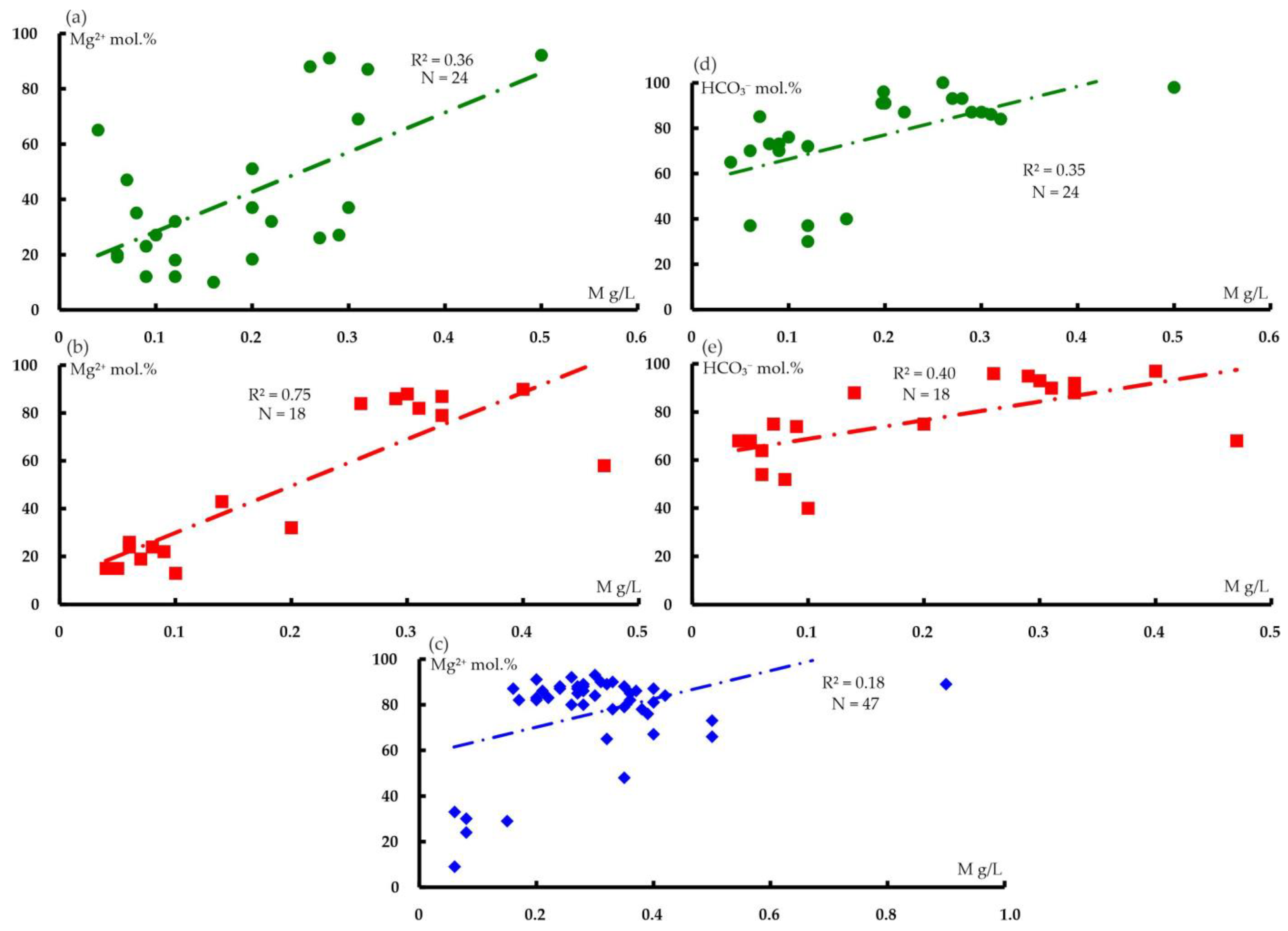
6.1. Hydrogeochemical Zonation and Conditions of Formation of Magnesium–Bicarbonate Waters of the Kraka Massifs
6.2. Comparison of the Magnesium–Bicarbonate Waters of the Kraka Massifs with Other Objects
6.3. Assessment of the Quality of Groundwaters on the Territory of the Kraka Massifs and the Host Rocks for Drinking Purposes
7. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yadav, P.; Sreekesh, S.; Nandimandalam, J.R. Groundwater Quality and Its Suitability in the Semi-Arid River Basin in India: An Analysis of Hydrogeochemical Processes Using Multivariate Statistics. Environ. Model. Assess. 2025, 30, 625–646. [Google Scholar] [CrossRef]
- Lapworth, D.J.; Boving, T.B.; Kreamer, D.K.; Kebede, S.; Smedley, P.L. Groundwater Quality: Global Threats, Opportunities and Realising the Potential of Groundwater. Sci. Total Environ. 2022, 811, 152471. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, I.; Singh, U.K. Hydrogeochemical Characterizations and Quality Evaluation of Groundwater in the Major River Basins of a Geologically and Anthropogenically Driven Semi-Arid Tract of India. Sci. Total Environ. 2022, 805, 150323. [Google Scholar] [CrossRef]
- Sekar, S.; Surendran, S.; Roy, P.D.; Kamaraj, J. Groundwater Geochemistry and Irrigation Suitability in the Semi-Arid Melur Block of Madurai District, South India. Environ. Earth Sci. 2025, 84, 139. [Google Scholar] [CrossRef]
- Medhi, S.; Choudhury, R. Elevated groundwater arsenic in deeper aquifers of the Brahmaputra floodplains in Assam, India. J. Environ. Sci. Health Sustain. 2025, 1, 131–143. [Google Scholar] [CrossRef]
- Werner, A.D.; Bakker, M.; Post, V.E.A.; Vandenbohede, A.; Lu, C.; Ataie-Ashtiani, B.; Simmons, C.T.; Barry, D.A. Seawater intrusion processes, investigation and management: Recent advances and future challenges. Adv. Water Resour. 2013, 51, 3–26. [Google Scholar] [CrossRef]
- Gutierrez, M.; Sunkari, E.D.; Mickus, K.; Okyere, M.B. Groundwater salinization in inland basins of arid and semiarid climates: An overview. J. Environ. Sci. Health Sustain. 2025, 1, 167–184. [Google Scholar] [CrossRef]
- Vörösmarty, C.; McIntyre, P.; Gessner, M.; Dudgeon, D.; Prusevich, A.; Green, P.; Glidden, S.; Bunn, S.E.; Sullivan, C.A.; Liermann, C.R.; et al. Global threats to human water security and river biodiversity. Nature 2010, 467, 555–561. [Google Scholar] [CrossRef]
- Tzanakakis, V.A.; Paranychianakis, N.V.; Angelakis, A.N. Water Supply and Water Scarcity. Water 2020, 12, 2347. [Google Scholar] [CrossRef]
- Wang, M.; Bodirsky, B.L.; Rijneveld, R.; Beier, F.; Bak, M.P.; Batool, M.; Droppers, B.; Popp, A.; van Vliet, M.T.H.; Strokal, M.A. Triple Increase in Global River Basins with Water Scarcity Due to Future Pollution. Nat. Commun. 2024, 15, 880. [Google Scholar] [CrossRef]
- Singh, A.K.; Kumar, S.R. Quality assessment of groundwater for drinking and irrigation use in semi-urban area of Tripura, India. Eco. Env. Cons. 2015, 21, 97–108. [Google Scholar]
- Alam, K.; Mohammad, N.; Ali, W.; Muhammad, S.; Raziq, A. Geogenic Contamination of Groundwater in a Highland Watershed: Hydrogeochemical Assessment, Source Apportionment, and Health Risk Evaluation of Fluoride and Nitrate. Hydrology 2025, 12, 70. [Google Scholar] [CrossRef]
- Ruskeeniemi, T.; Blomqvist, R.; Lindberg, A.; Ahonen, L.; Frape, S.; Posiva, O. Hydrogeochemistry of Deep Groundwaters of Mafic and Ultramafic Rocks in Finland; Posiva Oy: Helsinki, Finland, 1996; p. 123. [Google Scholar]
- Pacheco, R.R. Hydrology of ultramafic rocks in Moa (Cuba). In Proceedings of the XXXIII Congress IAH&7 Congress ALHSUD ”Groundwater Flow Understanding from Regional and Local Scales”, Zacatecas City, Mexico, 11–15 October 2004; pp. 1–4. [Google Scholar]
- Dewandel, B.; Lachassagne, P.; Bouldier, F.; Al-Hattali, S.; Ladouche, B.; Pinault, J.-L.; Al-Suleimani, Z. A conceptual hydrogeological model of ophiolite hard-rock aquifers in Oman based on a multiscale and a multidisciplinary approach. Hydrogeol. J. 2005, 13, 708–726. [Google Scholar] [CrossRef]
- Begum, S.; Shah, M.T.; Muhammad, S.; Khan, S. Role of mafic and ultramafic rocks in drinking water quality and its potential health risk assessment, Northern Pakistan. J. Water Health 2015, 13, 1130–1142. [Google Scholar] [CrossRef]
- Segadelli, S.; Vescovi, P.; Ogata, K.; Chelli, A.; Zanini, A.; Boschetti, T.; Petrella, E.; Toscani, L.; Gargini, A.; Celico, F. A conceptual hydrogeological model of ophiolitic aquifers (serpentinised peridotite): The test example of Mt. Prinzera (Northern Italy). Hydrol. Process. 2016, 31, 1058–1073. [Google Scholar] [CrossRef]
- Giampouras, M.; Garrido, C.J.; Zwicker, J.; Vadillo, I.; Smrzka, D.; Bach, W.; Peckmann, J.; Jimenez, P.; Benavente, J.; Garcia-Ruiz, J.M. Geochemistry and mineralogy of serpentinization-driven hyperalkaline springs in the Ronda peridotites. Lithos 2019, 350–351, 105215. [Google Scholar] [CrossRef]
- Shah, M.T.; Ara, A.; Muhammad, S.; Khan, S.; Tariq, S. Health risk assessment via surface water and sub-surface water consumption in the mafic and ultramafic terrain, Mohmand agency, northern Pakistan. J. Geochem. Explor. 2012, 118, 60–67. [Google Scholar] [CrossRef]
- Shah, M.T.; Ara, J.; Muhammad, S.; Khan, S.; Asad, S.A.; Ali, L. Potential heavy metal accumulation of indigenous plant species along the mafic and ultramafic terrain in the Mohmand agency, Pakistan. Clean-Soil Air Water 2014, 42, 339–346. [Google Scholar] [CrossRef]
- Khan, K.; Lu, Y.; Khan, H.; Zakir, S.; Ihsanullah, S.; Khan, A.A.; Wei, L.; Wang, T. Health risks associated with heavy metals in the drinking water of Swat, northern Pakistan. J. Environ. Sci. 2013, 25, 2003–2013. [Google Scholar] [CrossRef] [PubMed]
- Hussain, M.; Muhammad, S.; Malik, R.N.; Khan, M.U.; Farooq, U. Status of heavy metal residues in fish species of Pakistan. Rev. Environ. Contam. Toxicol. 2014, 230, 111–131. [Google Scholar] [CrossRef]
- Monteiro, L.C.; Vieira, L.C.G.; Bernardi, J.V.E.; Pereira, T.A.M.; Walkimar, A.C.J.; Silva, W.P.; Santos, L.A.G.; Goncalves, J.F., Jr.; Nabout, J.C.; Diniz-Filho, J.A.F.; et al. Combined effects of land use and geology on potentially toxic elements contamination in lacustrine sediments from the Araguaia River floodplain, Brazilian Savanna. Environ. Monit. Assess. 2025, 197, 683. [Google Scholar] [CrossRef]
- Danilov-Danilyan, V.I.; Katsov, V.M.; Porfiryev, B.N. Ecology and climate: Where we are now and where we will be in two to three decades. The situation in Russia. Her. Russ. Acad. Sci. 2023, 93, 1032–1046. (In Russian) [Google Scholar] [CrossRef]
- Zakutin, V.P.; Golitsyn, M.S.; Shvets, V.M. Current problems of studying and assessing the quality of underground drinking waters. Water Resour. 2012, 39, 485–495. (In Russian) [Google Scholar]
- Abdrakhmanov, R.F.; Poleva, A.O.; Nosareva, S.P.; Durnaeva, V.N. Technogenesis and its impact on fresh groundwater resources of the Southern Urals and the Cis-Urals. Geol. Bull. 2025, 144–158. (In Russian) [Google Scholar] [CrossRef]
- Abdrakhmanov, R.F.; Popov, V.G. Geochemistry and Formation of Groundwater in the Southern Urals; Academy of Sciences of the Republic of Bashkortostan, Gilem: Ufa, Russia, 2010; p. 420. (In Russian) [Google Scholar]
- Krainov, S.R.; Ryzhenko, B.N.; Shvets, V.M. Geochemistry of Groundwater: Theoretical, Applied, and Environmental Aspects; TsentrLitNefteGaz: Moscow, Russia, 2012; p. 213. (In Russian) [Google Scholar]
- Moskaleva, S.V. Hyperbasites and Their Chromite-Bearing Potential; Nedra: Leningrad, Russia, 1974; p. 279. (In Russian) [Google Scholar]
- Savelieva, G.N. Gabbro–Ultrabasic Complexes of Ural Ophiolites and Their Analogues in Modern Oceanic Crust; Nauka: Moscow, Russia, 1987; p. 246. (In Russian) [Google Scholar]
- Snachev, V.I.; Saveliev, D.E.; Rykus, M.V. Petrogeochemical Features of Rocks and Ores of the Kraka Gabbro–Hyperbasite Massifs; Bashkir State University: Ufa, Russia, 2001; p. 212. (In Russian) [Google Scholar]
- Saveliev, D.E.; Snachev, V.I.; Savelieva, E.N.; Bazhin, E.A. Geology, Petrochemistry, and Chromite-Bearing Capacity of Gabbro–Hyperbasite Massifs of the Southern Urals; DesignPolygraphService: Ufa, Russia, 2008; p. 320. (In Russian) [Google Scholar]
- Saveliev, D.E.; Gataullin, R.A. Lherzolites of the Aznagulov area (Southern Urals): Composition and P–T–fO2 formation conditions. Bull. Acad. Sci. Repub. Bashkortostan 2021, 40, 15–25. (In Russian) [Google Scholar] [CrossRef]
- Saveliev, D.E. Ultramafic Massifs of Kraka (Southern Urals): Structure and Composition of Peridotite–Dunite–Chromite Associations; Bashkir Encyclopedia: Ufa, Russia, 2018; p. 204. (In Russian) [Google Scholar]
- Puchkov, V.N. Structure and geodynamics of the Uralian orogen. Geol. Soc. Lond. Spec. Publ. 1997, 121, 201–236. [Google Scholar] [CrossRef]
- Saveliev, D.E.; Shilovskikh, V.V.; Makatov, D.K.; Gataullin, R.A. Accessory Cr-spinel from peridotite massifs of the South Urals: Morphology, composition and origin. Miner. Petrol. 2022, 116, 401–427. [Google Scholar] [CrossRef]
- Knyazev, Y.G.; Knyazeva, O.Y. State Geological Map of the Russian Federation. Scale 1:200,000, 2nd ed.; South Ural Series; Sheet N-40-XXIII Beloretsk. Explanatory Note; Bashkirgeologiya: Ufa, Russia, 2006; p. 194. (In Russian) [Google Scholar]
- Larionov, N.N.; Bergazov, I.R. State Geological Map of the Russian Federation. Scale 1:200,000, 2nd ed.; South Ural Series; Sheet N-40-XXP (Tukan); Explanatory Note; VSEGEI: Moscow, Russia, 2015; p. 247. (In Russian) [Google Scholar]
- Knyazev, Y.G.; Knyazeva, O.Y. State Geological Map of the Russian Federation. Scale 1:200,000, 2nd ed.; South Ural Series; Sheet N-40-XXVIII; Explanatory Note; VSEGEI: Moscow, Russia, 2015; p. 237. (In Russian) [Google Scholar]
- Yakupov, R.R.; Mavrinskaya, T.M.; Abramova, A.N. Paleontological Justification of the Paleozoic Stratigraphy Scheme of the Northern Part of the Zilair Megasynclinorium; Institute of Geology, Ufa Scientific Center, RAS: Ufa, Russia, 2002; p. 160. (In Russian) [Google Scholar]
- Mavrinskaya, T.M.; Yakupov, R.R. Ordovician sediments of the western slope of the Southern Urals and their correlation based on conodonts and chitinozoans. Geol. Geophys. 2016, 57, 333–352. (In Russian) [Google Scholar] [CrossRef]
- Saveliev, D.E.; Biembetov, A.I.; Shabutdinov, T.D.; Samigullin, A.A.; Gataullin, R.A. Mineralogical and geochemical features of ultramafic rocks in the eastern part of the Southern Kraka massif (Southern Urals). Georesources 2024, 26, 248–259. (In Russian) [Google Scholar] [CrossRef]
- Saveliev, D.E.; Nugumanova, Y.N.; Gataullin, R.A.; Sergeev, S.N. Ultramafites of the Uzyansky Kraka massif (Southern Urals). Geol. Bull. 2018, 3, 79–97. (In Russian) [Google Scholar] [CrossRef]
- Sobolev, N.D. Ultrabasic Rocks of the Greater Caucasus; Gosgeolizdat: Moscow, Russia, 1952; p. 240. (In Russian) [Google Scholar]
- GOST 31867–2012; Drinking Water. Determination of Anion Content by Chromatography and Capillary Electrophoresis. Interstate Council for Standardization, Metrology and Certification (ISC): Moscow, Russia, 2019; p. 19. (In Russian)
- GOST 31869–2012; Water. Methods for Determination of Cation Content (Ammonium, Barium, Potassium, Calcium, Lithium, Magnesium, Sodium, Strontium) Using Capillary Electrophoresis. Interstate Council for Standardization, Metrology and Certification (ISC): Moscow, Russia, 2019; p. 23. (In Russian)
- M 01-58–2018; Quantitative Chemical Analysis of Water. Measurement of Mass Concentration of Chloride, Nitrite, Sulfate, Nitrate, Fluoride, and Phosphate Ions in Samples of Natural, Drinking, and Wastewater Using the “Kapel” Capillary Electrophoresis System. Federal Service for Accreditation of Measurement Procedures: Moscow, Russia, 2018. Available online: https://meganorm.ru/mega_doc/norm/metodika/3/pnd_f_14_1_2_4_157-99_kolichestvennyy_khimicheskiy_analiz.html (accessed on 14 December 2025). (In Russian)
- (ERD F) 14.1:2:3:4.121–97; Method for Measuring pH of Water Samples by Potentiometric Method (2018 Edition). Federal State Budgetary Institution “Federal Center for Analysis and Assessment of Technogenic Impact”: Moscow, Russia, 2018; p. 25. (In Russian)
- (ERD F) 14.1:2:3:98–97; Method for Measuring Total Hardness in Natural and Wastewater Samples by Titrimetric Method. Federal Center for Analysis and Assessment of Technogenic Impact: Moscow, Russia, 2016; p. 24. (In Russian)
- (ERD F) 14.1:2:4.167–2000; Method for Determining the Mass Concentration of Cations (Ammonium, Potassium, Sodium, Lithium, Magnesium, Strontium, Barium, and Calcium) in Drinking, Natural (Including Mineral), and Wastewater by Capillary Electrophoresis “Kapel”. Federal Center for Analysis and Assessment of Technogenic Impact: Moscow, Russia, 2000; p. 16. (In Russian)
- (ERD F) 14.1:2:4.261–2010; Method for determining the mass concentration of dry and calcined residues in drinking, natural, and wastewater by gravimetric method. Federal Center for Analysis and Assessment of Technogenic Impact: Moscow, Russia, 2015; p. 14. (In Russian)
- Piper, A.M. A graphic procedure in geochemical interpretation of water analyses. Am. Geophys. Union Trans. 1944, 25, 914–923. [Google Scholar]
- Stiff, H.A., Jr. The interpretation of chemical water analysis by means of patterns. J. Pet. Technol. 1951, 3, 15. [Google Scholar] [CrossRef]
- Dmitriev, L.V.; Ukhanov, A.V.; Sharaskin, L.Y. On the composition of upper mantle material. Geochemistry 1972, 10, 1155–1167. (In Russian) [Google Scholar]
- Bazylev, B.A. Petrological and Geochemical Evolution of Mantle Material in the Lithosphere: Comparative Study of Oceanic and Alpine-Type Spinel Peridotites. Ph.D. Thesis, Institute of Geochemistry of the Russian Academy of Sciences, Moscow, Russia, 26 November 2003; p. 371. (In Russian). [Google Scholar]
- Hatari Labs. What Is a Piper Diagram for Water Chemistry Analysis and How to Create one? Available online: https://hatarilabs.com/ih-en/what-is-a-piper-diagram-and-how-to-create-one (accessed on 17 December 2025).
- Barnes, I.; O’Neil, J.R. The relationship between fluids in some fresh alpinetype ultramafics and possible modern serpentinization, western United States. Geol. Soc. Am. Bull. 1969, 80, 1947–1960. [Google Scholar] [CrossRef]
- Neal, C.; Stanger, G. Past And Present Serpentinisation of Ultramafic Rocks; An Example from the Semail Ophiolite Nappe of Northern Oman. In The Chemistry of Weathering. Nato ASI Series; Drever, J.I., Ed.; Springer: Dordrecht, The Netherlands, 1985; Volume 149, pp. 249–275. [Google Scholar] [CrossRef]
- Bruni, J.; Canepa, M.; Chiodini, G.; Cioni, R.; Cipolli, F.; Longinelli, A.; Marini, L.; Ottonello, G.; Zuccolini, M.V. Irreversible watererock mass transfer accompanying the generation of the neutral, Mg-HCO3 and high-pH, Ca-OH spring waters of the Genova province, Italy. Appl. Geochem. 2002, 17, 455–474. [Google Scholar] [CrossRef]
- Boschetti, T.; Toscani, L. Springs and streams of the Taro-Ceno Valleys (Northern Apennine, Italy): Reaction path modeling of waters interacting with serpentinized ultramafic rocks. Chem. Geol. 2008, 257, 76–91. [Google Scholar] [CrossRef]
- Palandri, J.L.; Reed, M.H. Geochemical models of metasomatism in ultramafic systems: Serpentinization, rodingitization, and sea floor carbonate chimney precipitation. Geochem. Cosmochim. Acta 2004, 68, 1115–1133. [Google Scholar] [CrossRef]
- Kelemen, P.B.; Matter, J. In situ carbonation of peridotite for CO2 storage. Proc. Natl. Acad. Sci. USA 2008, 105, 17295–17300. [Google Scholar] [CrossRef]
- Paukert, A.N.; Matter, J.M.; Kelemen, P.B.; Shock, E.L.; Havig, J.R. Reaction path modeling of enhanced in situ CO2 mineralization for carbon sequestration in the peridotite of the Samail Ophiolite, Sultanate of Oman. Chem. Geol. 2012, 330, 86–100. [Google Scholar] [CrossRef]
- Guidelines for Drinking-Water Quality; World Health Organization: Geneva, Switzerland, 2011; Volume 2, p. 541.
- Boudibi, S. Modeling the Impact of Irrigation Water Quality on Soil Salinization in an Arid Region, Case of Biskra. Ph.D. Thesis, Université Mohamed Khider – Biskra, Biskra, Algeria, 2021. [Google Scholar] [CrossRef]
- SanPiN 1.2.3685–21; Hygienic Standards and Requirements for Ensuring Safety and/or Harmlessness to Humans of Environmental Factors. Chief State Sanitary Doctor of the Russian Federation, Federal Service for Surveillance on Consumer Rights Protection and Human Well-Being (Rospotrebnadzor): Moscow, Russia, 2021; p. 1025. (In Russian)
- GOST R 54316–2020; Natural Mineral Drinking Waters. Standartinform: Moscow, Russia, 2020; pp. 32–33. (In Russian)
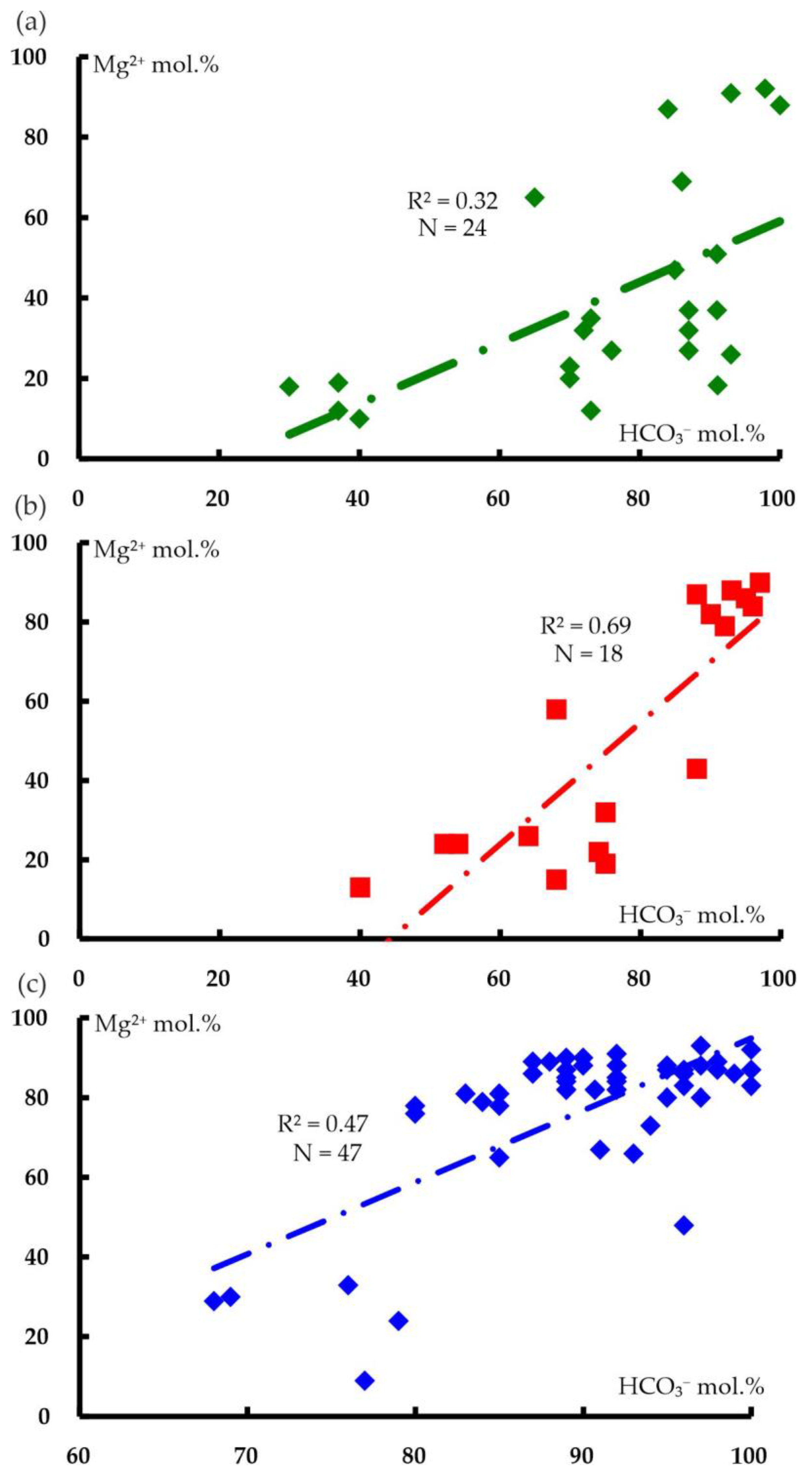
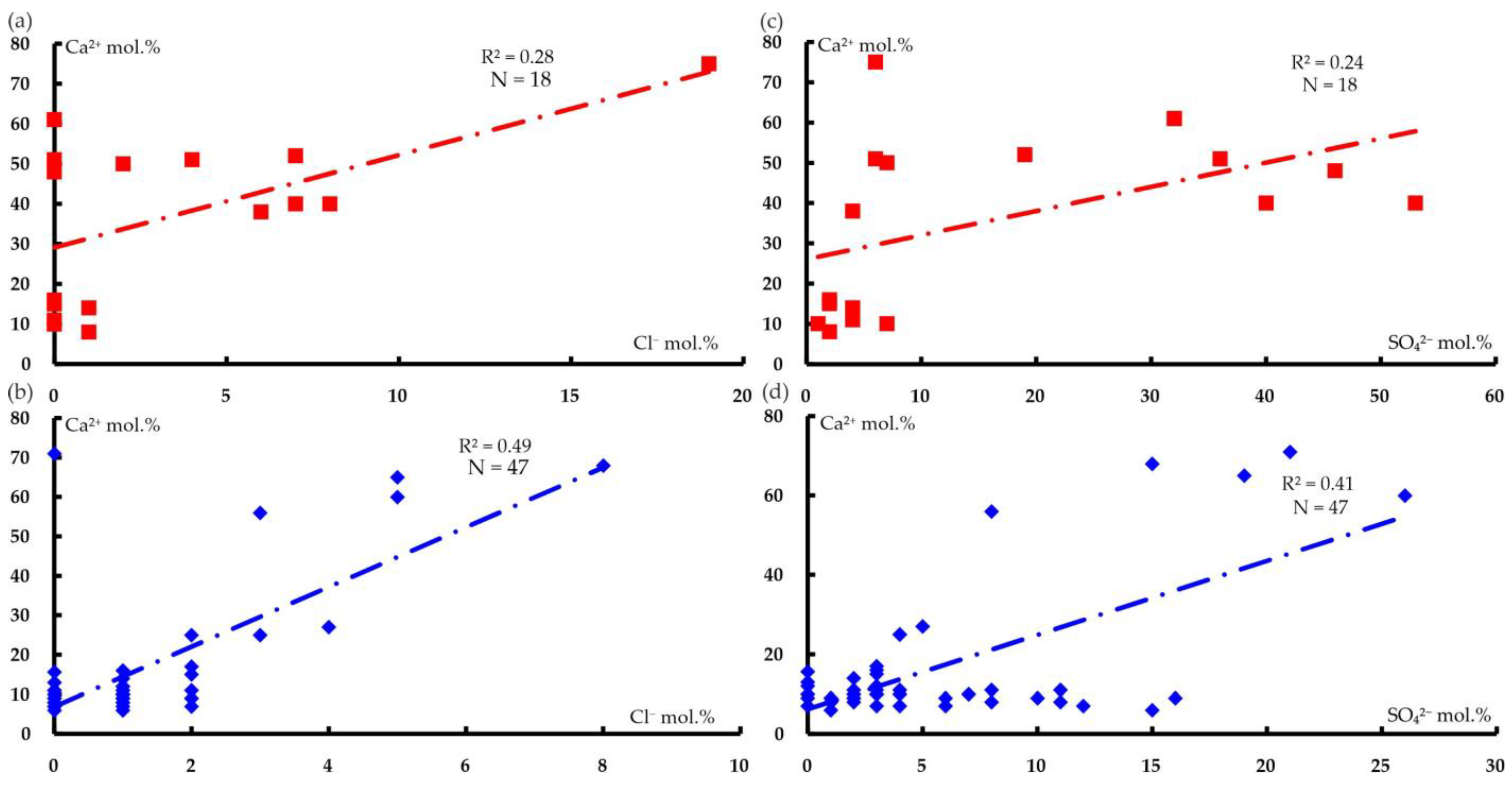
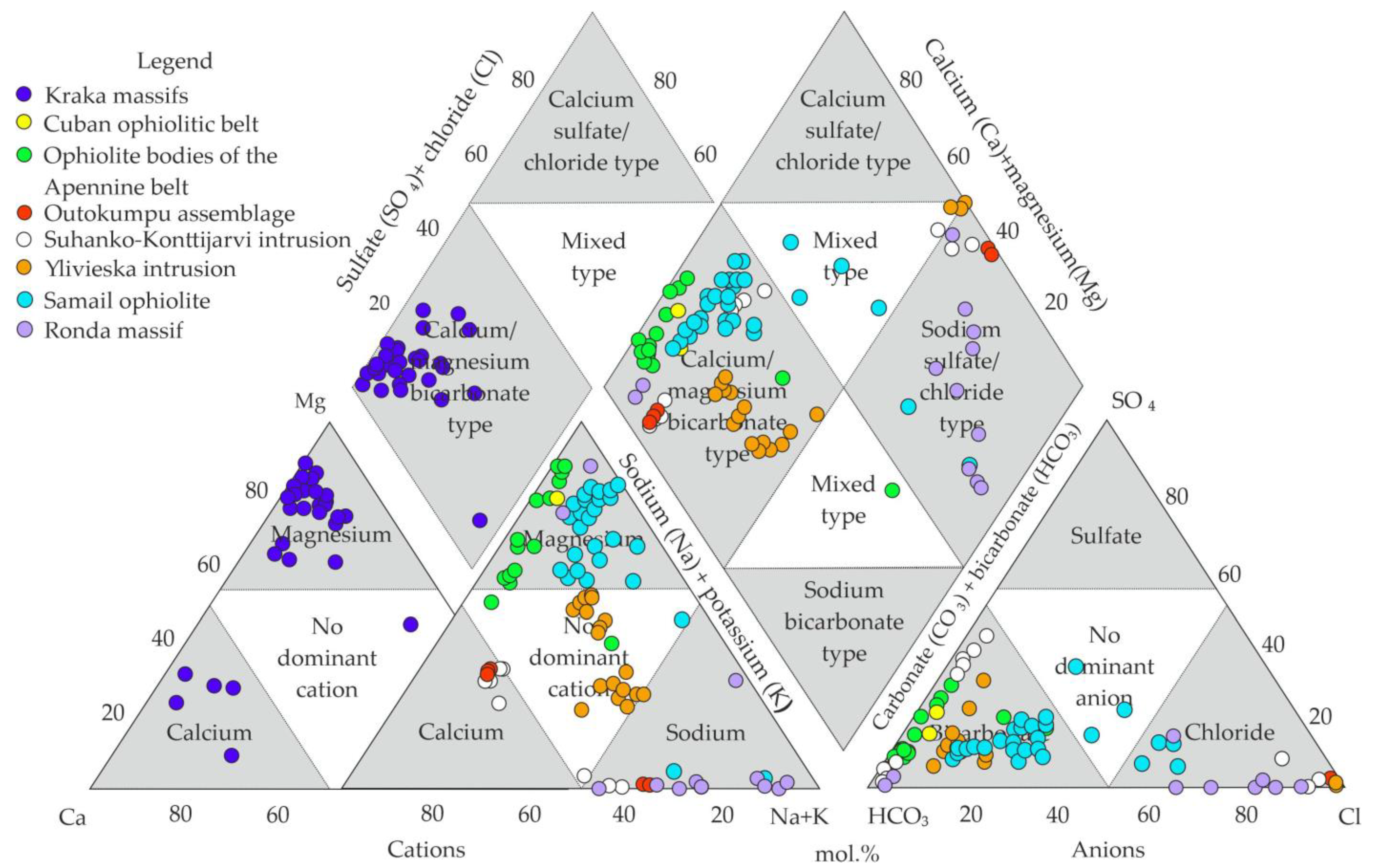
| wt.% | NK-78 | NK-79 | NK-84 | CK-64 | CK-512 | CK-45 | UK-1803 | SK-2331 | SK-2342-2 |
|---|---|---|---|---|---|---|---|---|---|
| SiO2 | 39.60 | 42.00 | 39.00 | 38.90 | 40.00 | 36.00 | 39.58 | 44.00 | 34.87 |
| TiO2 | 0.07 | 0.07 | 0.06 | 0.08 | 0.30 | 0.05 | 0.08 | 0.15 | 0.04 |
| Al2O3 | 0.50 | 3.60 | 0.20 | 0.20 | 2.40 | 2.80 | 2.46 | 1.89 | 0.21 |
| Fe2O3 | 2.40 | 2.40 | 8.00 | 5.80 | 4.80 | 5.70 | 7.80 | 8.21 | 8.09 |
| FeO | 5.70 | 5.70 | 4.30 | 0.70 | 3.10 | 2.80 | bdl | 3.59 | bdl |
| MnO | 0.12 | 0.14 | 0.11 | 0.13 | 0.13 | 0.11 | 0.17 | bdl | 0.14 |
| CaO | 0.20 | 1.90 | 0.50 | 1.50 | 1.40 | 1.10 | 1.83 | 0.85 | 0.01 |
| MgO | 47.00 | 42.00 | 40.00 | 38.60 | 40.00 | 40.50 | 39.37 | 40.00 | 40.55 |
| Na2O | 1.30 | 0.20 | 0.13 | 0.07 | 0.40 | 0.07 | 0.20 | 0.12 | 0.10 |
| K2O | 0.10 | 0.06 | 0.06 | 0.03 | 0.02 | 0.06 | 0.02 | bdl | 0.01 |
| LOI | 2.80 | 2.00 | 7.80 | 13.30 | 8.00 | 11.00 | 8.31 | 1.20 | 16.29 |
| Total | 99.79 | 100.07 | 100.16 | 99.31 | 100.55 | 100.19 | 99.81 | 100.01 | 100.30 |
| S | −15.69 | −8.31 | −13.47 | −6.41 | −8.33 | −13.16 | −7.83 | −7.95 | −13.94 |
| A | 2.1 | 5.76 | 0.89 | 1.8 | 4.22 | 4.03 | 4.51 | 2.86 | 0.33 |
| No Π/Π | Type of Water Point | Quantity of Samples | ||
|---|---|---|---|---|
| Paleozoic Sediments of the Zilair Synclinorium (O2–3 nb–D3–C1zl) | Polymictic Melange (mpD3–C1k) | Ultramafic Rocks of the Kraka Massifs | ||
| 1 | Streams | 11 | 11 | 31 |
| 2 | Springs | 11 | 6 | 16 |
| 3 | Boreholes | 1 | 0 | 0 |
| 4 | Pits | 0 | 1 | 0 |
| 5 | Wells | 1 | 0 | 0 |
| Total quantity | 24 | 18 | 47 | |
| Item No. | TDS g/L | pH | Type of Water Point | Components, mg/L, mmol/L, mol.% | Type of Water | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| HCO3− | SO42− | Cl− | Ca2+ | Mg2+ | Na+ | K+ | NO3− | CO32− | |||||
| 1 | 0.2 | – | Spring | 136.95 2.24 94.4 | 8.5 0.09 3.7 | 1.54 0.04 1.8 | 36.7 0.92 69.4 | 5.5 0.23 17.1 | 3.79 0.16 12.5 | 0.51 0.01 1.0 | - - 0.0 | - - 0.0 | Ca–HCO3 |
| 2 | 0.5 | – | Stream | 374.05 6.13 98.7 | 5.2 0.05 0.9 | 0.96 0.03 0.4 | 9.3 0.23 7.4 | 70.1 2.88 91.5 | 0.5 0.02 0.7 | 0.5 0.01 0.4 | - - 0.0 | - - 0.0 | Mg–HCO3 |
| 3 | 0.27 | 8.05 | Spring | - - 93.0 | - - 4.0 | - - 2.0 | - - 70.0 | - - 26.0 | - - 4.0 | - - 1.0 | - - 0.0 | Ca–Mg–HCO3 | |
| 4 | 0.12 | 7.90 | Spring | - - 72.0 | - - 9.0 | - - 16.0 | - - 40.0 | - - 32.0 | - - 28.0 | - - 3.0 | - - 0.0 | Ca–Mg–Na–HCO3 | |
| 5 | 0.10 | 7.30 | Spring | - - 40.0 | - - 53.0 | - - 7.0 | - - 40.0 | - - 13.0 | - - 47.0 | - - 0.0 | - - 0.0 | Na–Ca–SO4– HCO3 | |
| 6 | 0.20 | 7.70 | Stream | - - 75.0 | - - 7.0 | - - 2.0 | - - 50.0 | - - 32.0 | - - 18.0 | - - 0.0 | - - 16.0 | Ca–Mg–HCO3 | |
| 7 | 0.4 | 8.25 | Stream | 293.23 4.81 93.6 | 11.9 0.13 2.4 | 7.28 0.21 4.0 | 28.9 0.72 26.4 | 44.0 1.81 66.2 | 3.32 0.14 5.3 | 2.28 0.06 2.1 | - - 0.0 | - - 0.0 | Mg–Ca–HCO3 |
| 8 | 0.5 | 8.29 | Stream | 401.69 6.58 96.1 | 11.4 0.12 1.7 | 5.25 0.15 2.2 | 34.7 0.87 24.2 | 61.1 2.51 70.1 | 3.41 0.15 4.1 | 2.21 0.06 1.6 | - - 0.0 | - - 0.0 | Mg–Ca–HCO3 |
| 9 | 0.5 | 8.25 | Stream | 376.43 6.17 94.9 | 13.8 0.14 2.2 | 6.66 0.19 2.9 | 35.0 0.87 25.6 | 57.3 2.36 69.1 | 3.14 0.14 4.0 | 1.70 0.04 1.3 | - - 0.0 | - - 0.0 | Mg–Ca–HCO3 |
| 10 | 0.4 | 8.86 | Spring | - - 90.0 | - - 2.0 | - - 1.0 | - - 9.0 | - - 88.0 | - - 3.0 | - - 0.0 | - - 7.0 | Mg–HCO3 | |
| 11 | 0.4 | 8.76 | Spring | - - 87.0 | - - 2.0 | - - 1.0 | - - 8.0 | - - 86.0 | - - 6.0 | - - 0.0 | - - 10.0 | Mg–HCO3 | |
| 12 | 0.4 | 8.85 | Spring | - - 89.0 | - - 3.0 | - - 1.0 | - - 10.0 | - - 85.0 | - - 5.0 | - - 0.0 | - - 7.0 | Mg–HCO3 | |
| Object | TDS(g/L) | pH | Type of Water | Type of Rock |
|---|---|---|---|---|
| Kraka massifs | 0.06–0.9 | 7.00–8.88 | Mg–HCO3, Mg–Ca–HCO3, Ca–Mg–HCO3–SO4 | Ultramafic rock |
| Ophiolite bodies of the Apennine belt | 0.11–0.37 | 7.63–8.67 | Mg–HCO3, Mixed types | Ultramafic rock |
| Outokumpu assemblage | 0.02 9.2–9.5 | 7.50 9.40–10.20 | Ca–Mg–HCO3 Na–Cl | Tremolite-chlorite-actinolite and ultramafic rocks. |
| Suhanko–Konttijarvi intrusion | 0.18–0.20 0.41–0.51 | 7.10–7.41 9.65–9.81 | Ca–Mg–HCO3–(SO4) Na–Ca–Cl | Gabbro, ultramafic rock, quartz diorite |
| Ylivieska intrusion | 0.22–0.28 | 8.14–8.42 6.97–8.67 | Mg–Na–Ca–HCO3 Na–Mg–Ca–Cl | Ultramafic rock gabbro |
| Samali ophiolite | 0.11–1.29 0.46–5.54 | 7.40–9.60 7.4–11.5 | Mg–HCO3 Na–Cl | Ultramafic and mafic rocks |
| Ronda massif | 0.36–0.58 0.16–2.9 | 8.50–8.90 9.60–12.0 | Mg–HCO3, Na-Cl | Ultramafic rock |
| Mingora-Shangla mélange zone | 0.04–0.81 | 6.95–7.20 | Mg–HCO3, Mixed types | Ultramafic rock, green schist, talc-carbonate schist and metabasalts |
| Parameters | Kraka Massifs (n = 47) | Paleozoic Sediments of the Zilair Synclinorium (n = 24) | WHO Standards |
|---|---|---|---|
| pH | 7.00–8.88 | 6.80–8.84 | 6.50–8.50 |
| TDS, g/L | 0.06–0.90 | 0.04–0.50 | 1.000 |
| Total hardness of water, mmol/L | 1.05–3.39 | 0.20–3.10 | <0.40 (soft) 0.40–1.30 (slightly hard) 1.30–2.60 (moderately hard) 2.60–3.90 (hard) >3.90 (very hard) |
| Ca mg/L | 28.90–35.00 | 9.30–36.70 | 100.00 |
| Mg mg/L | 44.00–57.30 | 5.50–70.10 | 50.00 |
| Na mg/L | 3.14–3.41 | 0.50–3.79 | 200.00 |
| K mg/L | 1.70–2.28 | 0.50–0.51 | 12.00 |
| HCO3 mg/L | 293.00–376.00 | 136.95–374.05 | - |
| SO4 mg/L | 11.40–13.80 | 0.96–1.54 | 250.00 |
| Cl mg/L | 5.25–7.28 | 5.20–8.50 | 250.00 |
| Parameters | Sanitary Rules and Regulations 1.2.3685–21 | Kraka Massifs (n = 47) | Polymictic Melange (mpD3–C1k) (n = 28) | Paleozoic Sediments Zilair Synclinorium(O2-3nb–D3-C1zl) (n = 24) |
|---|---|---|---|---|
| pH | 6.00–9.00 | 7.00–8.88 | 6.80–8.84 | 6.80–8.76 |
| TDS, g/L | 1.00 (1.50) | 0.06–0.90 | 0.04–0.50 | 0.04–0.50 |
| Total hardness of water, mEq/L, mmol/L | 7.00 (10.00) 3.50 (5.00) | 2.10–6.80 1.05–3.39 | 0.90–2.50 0.45–1.25 | 0.40–6.20 0.20–3.10 |
| Type of Water | Name of the Mineral Water Type and Its Location | Dominant Ions, %-Eq/L | TDS, g/L | mg/L | |||||
|---|---|---|---|---|---|---|---|---|---|
| HCO3− | SO42− | Cl− | Ca2+ | Mg2+ | (Na + + K+) | ||||
| Ca–Mg–HCO3, Mg–Ca–HCO3 | Senezh (boreholes 1/GVK-46240620, ZGVK-46247919). Senezh field, Moscow region | HCO3 > 70 Ca 40–75, Mg 20–55 | 0.3–0.7 | 250–450 | <15 | <10 | 40–90 | 10–50 | 10–40 |
| Ca–Mg–HCO3, Mg–Ca–HCO3 | Sineborie (boreholes 55-T). Vladimir Region | HCO3 > 80, Ca 50–80, Mg 30–50 | 0.2–0.5 | 100–400 | <25 | <15 | 20–80 | 10–40 | <15 |
| Ca–Mg–HCO3, Ca–Na–Mg–HCO3, Ca–Mg–Na–HCO3 | The Legend of the Arkhyz Mountains (boreholes No. 3). Nizhne-Arkhyzskoye field, Karachay-Cherkess Republic | HCO3 > 80, Ca 45–70, Mg 20–40, (Na + K) 15–30 | 0.1–0.25 | 50–200 | 5–15 | 5–15 | 5–50 | 2–20 | 5–50 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license.
Share and Cite
Shabutdinov, T.D.; Abdrakhmanov, R.F.; Saveliev, D.E.; Poleva, A.O.; Mashkova, E.A.; Snachev, A.V.; Gataullin, R.A.; Durnaeva, V.N.; Samigullin, A.A. Geochemical Features of Ultramafic Rocks and Formation of Magnesium–Bicarbonate Groundwaters in the Kraka Massif Area (Southern Urals). Geosciences 2026, 16, 8. https://doi.org/10.3390/geosciences16010008
Shabutdinov TD, Abdrakhmanov RF, Saveliev DE, Poleva AO, Mashkova EA, Snachev AV, Gataullin RA, Durnaeva VN, Samigullin AA. Geochemical Features of Ultramafic Rocks and Formation of Magnesium–Bicarbonate Groundwaters in the Kraka Massif Area (Southern Urals). Geosciences. 2026; 16(1):8. https://doi.org/10.3390/geosciences16010008
Chicago/Turabian StyleShabutdinov, Timur D., Rafil F. Abdrakhmanov, Dmitry E. Saveliev, Alexandra O. Poleva, Elena A. Mashkova, Alexander V. Snachev, Ruslan A. Gataullin, Vera N. Durnaeva, and Aidar A. Samigullin. 2026. "Geochemical Features of Ultramafic Rocks and Formation of Magnesium–Bicarbonate Groundwaters in the Kraka Massif Area (Southern Urals)" Geosciences 16, no. 1: 8. https://doi.org/10.3390/geosciences16010008
APA StyleShabutdinov, T. D., Abdrakhmanov, R. F., Saveliev, D. E., Poleva, A. O., Mashkova, E. A., Snachev, A. V., Gataullin, R. A., Durnaeva, V. N., & Samigullin, A. A. (2026). Geochemical Features of Ultramafic Rocks and Formation of Magnesium–Bicarbonate Groundwaters in the Kraka Massif Area (Southern Urals). Geosciences, 16(1), 8. https://doi.org/10.3390/geosciences16010008






