Superdeep Diamond Genesis Through Fe-Mediated Carbonate Reduction
Abstract
1. Introduction
2. Materials and Methods
2.1. Starting Materials and Multi-Anvil Press Experiments
2.2. Microanalyses of the Run Products
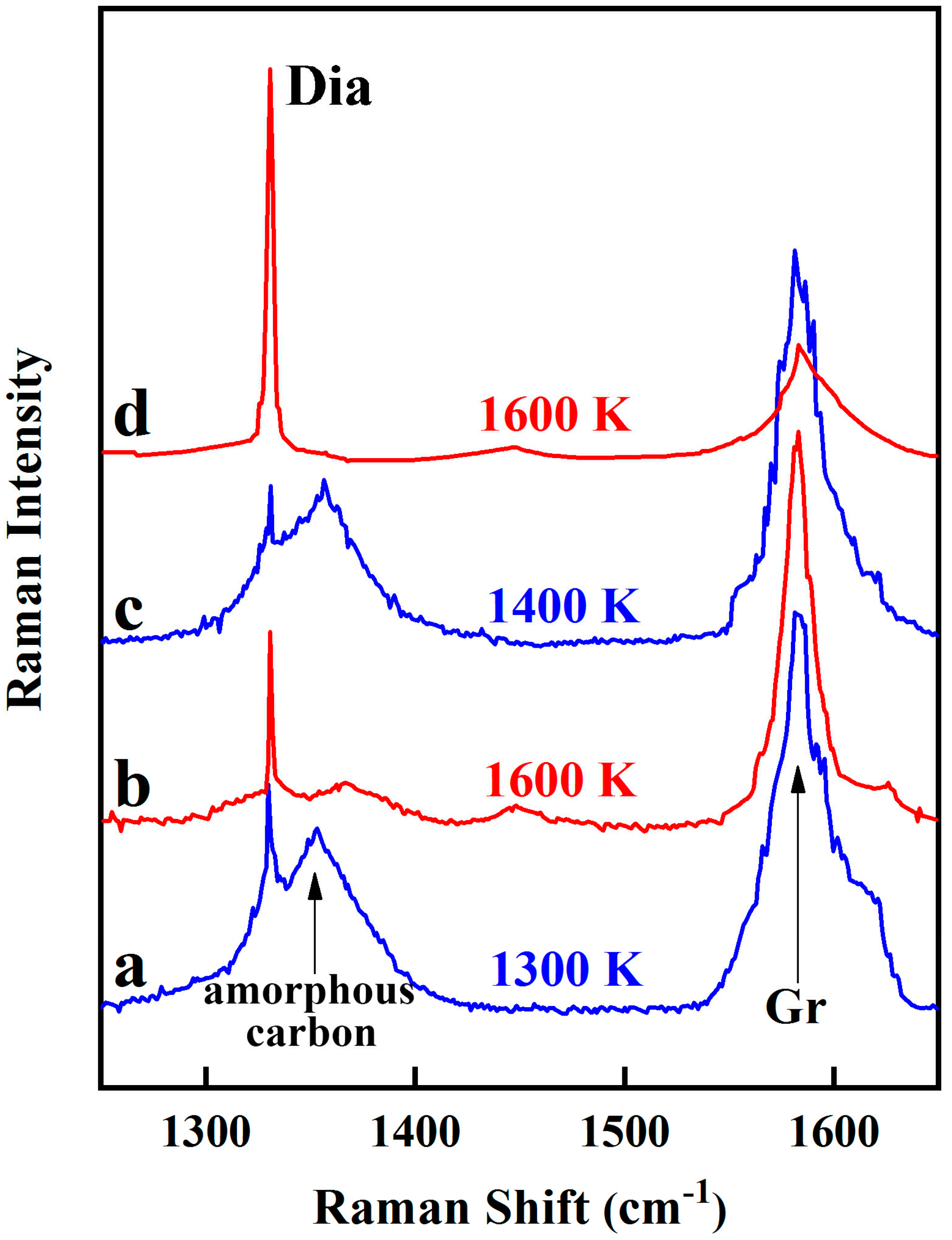
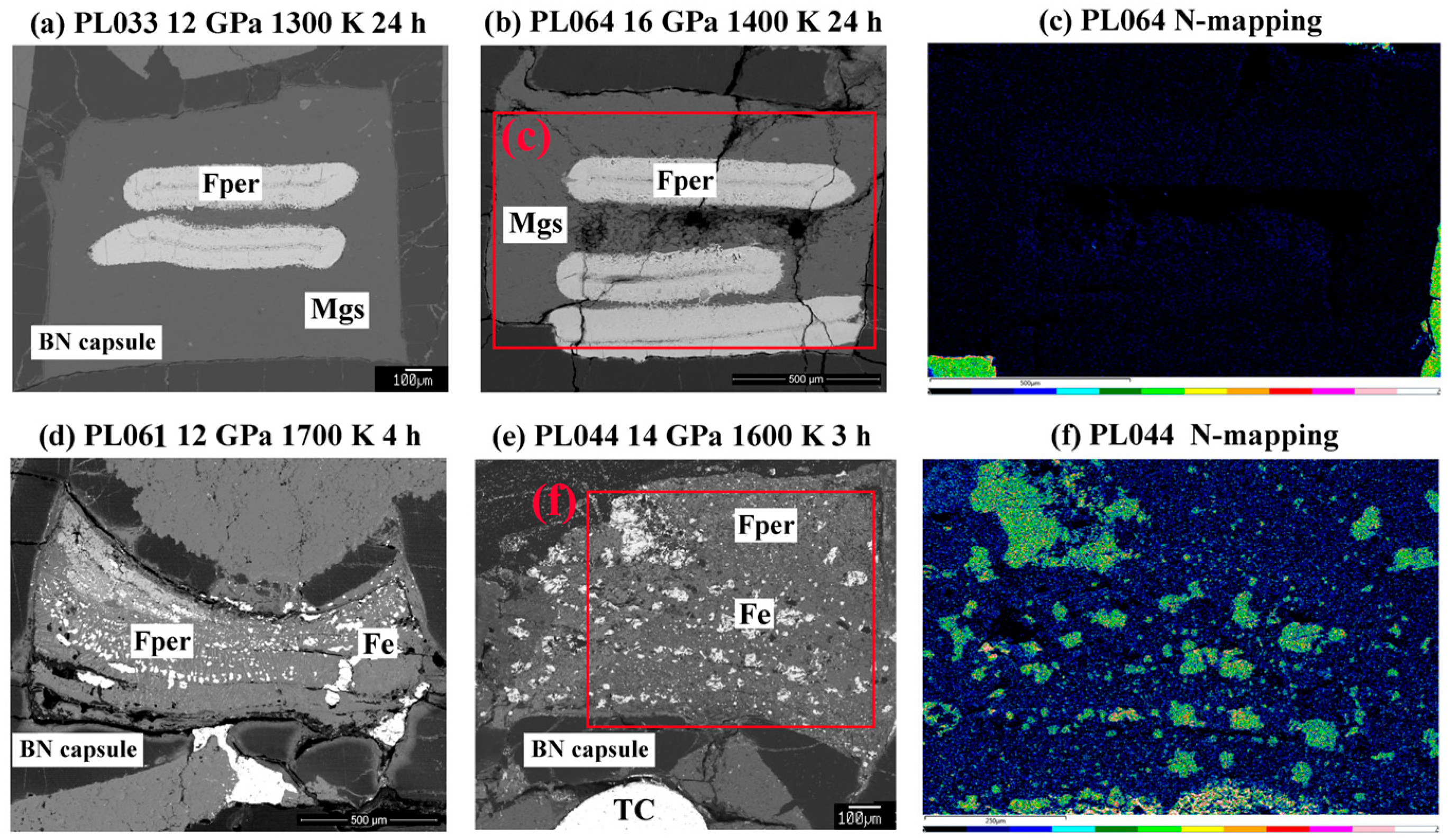
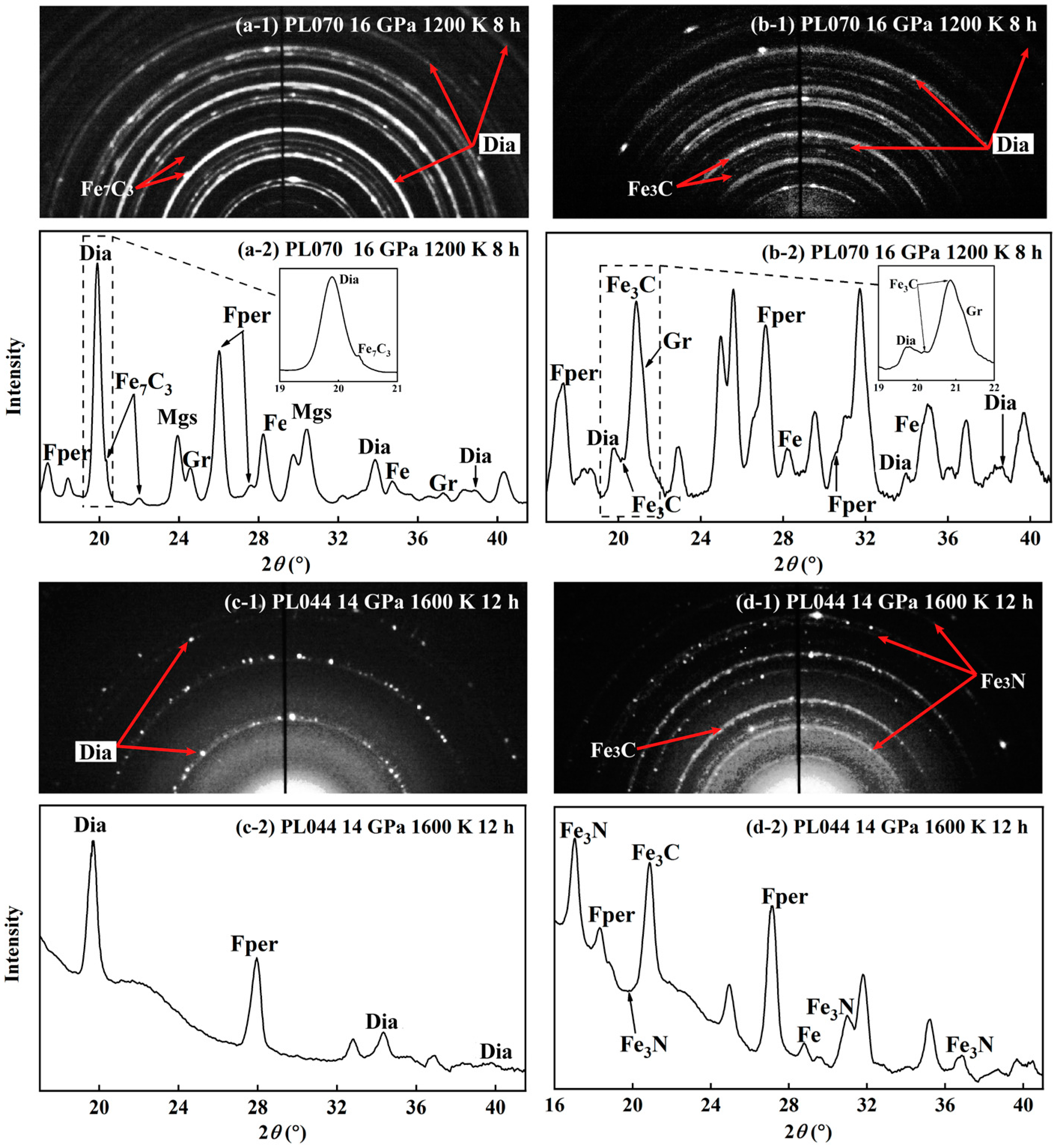
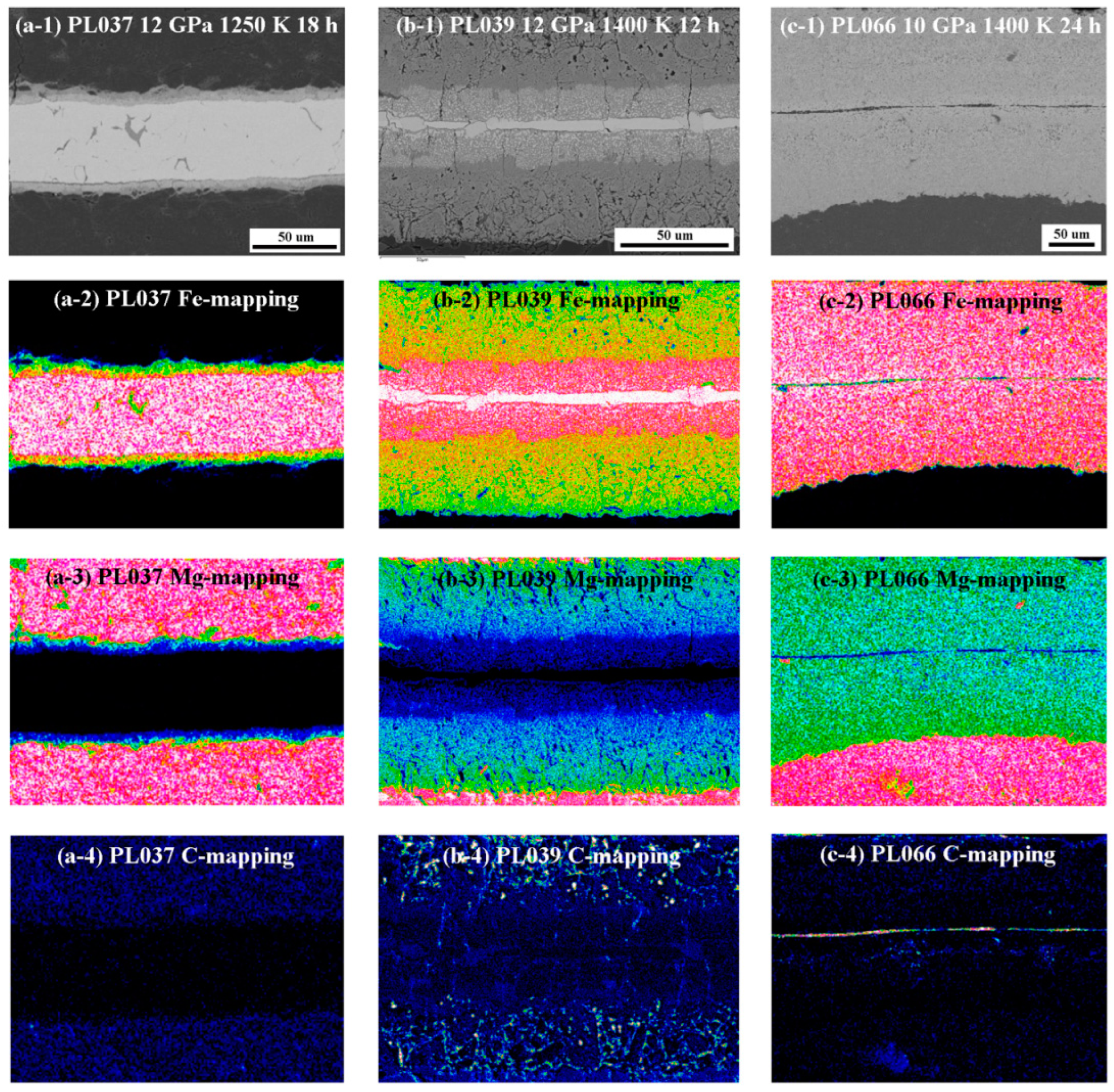
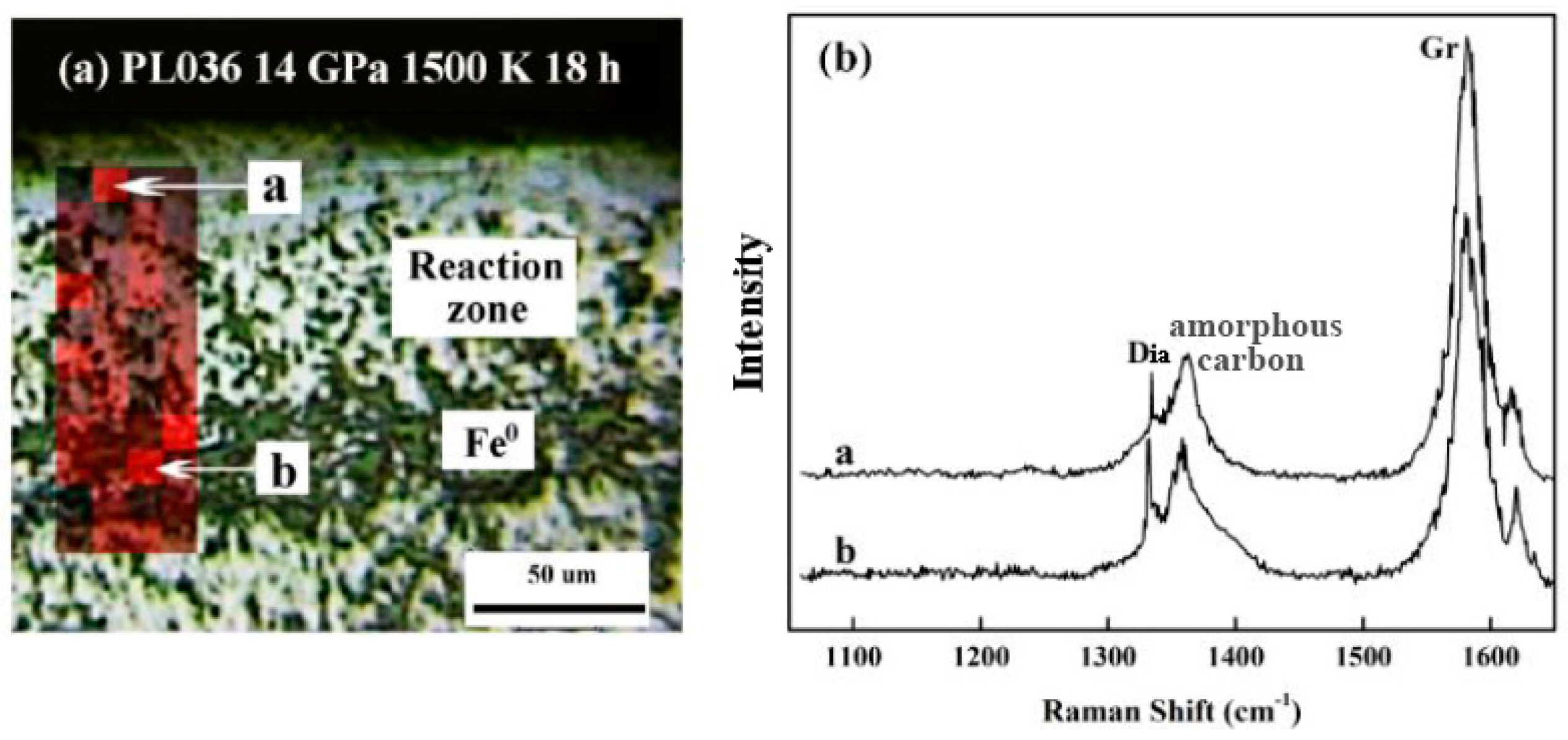
3. Results
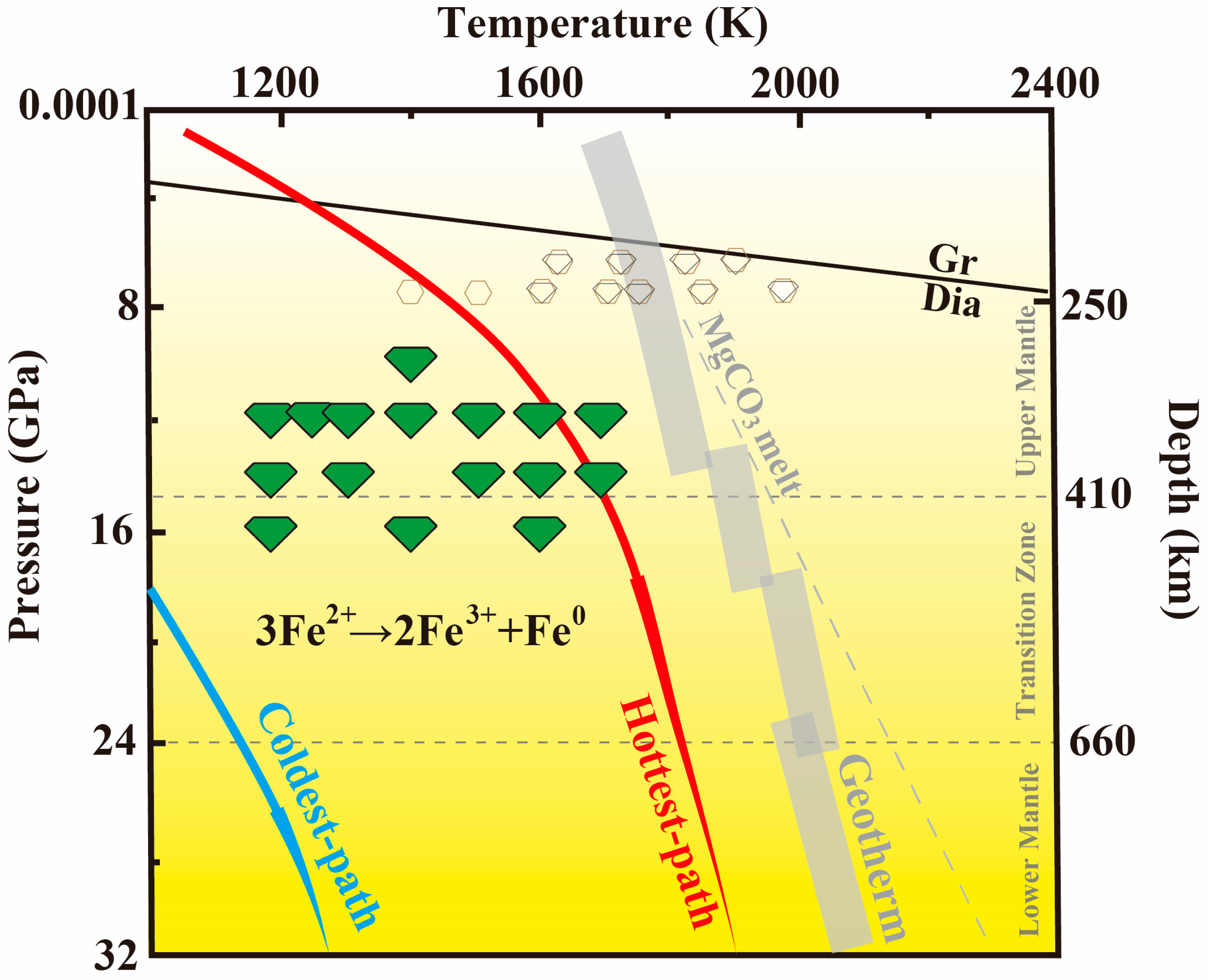
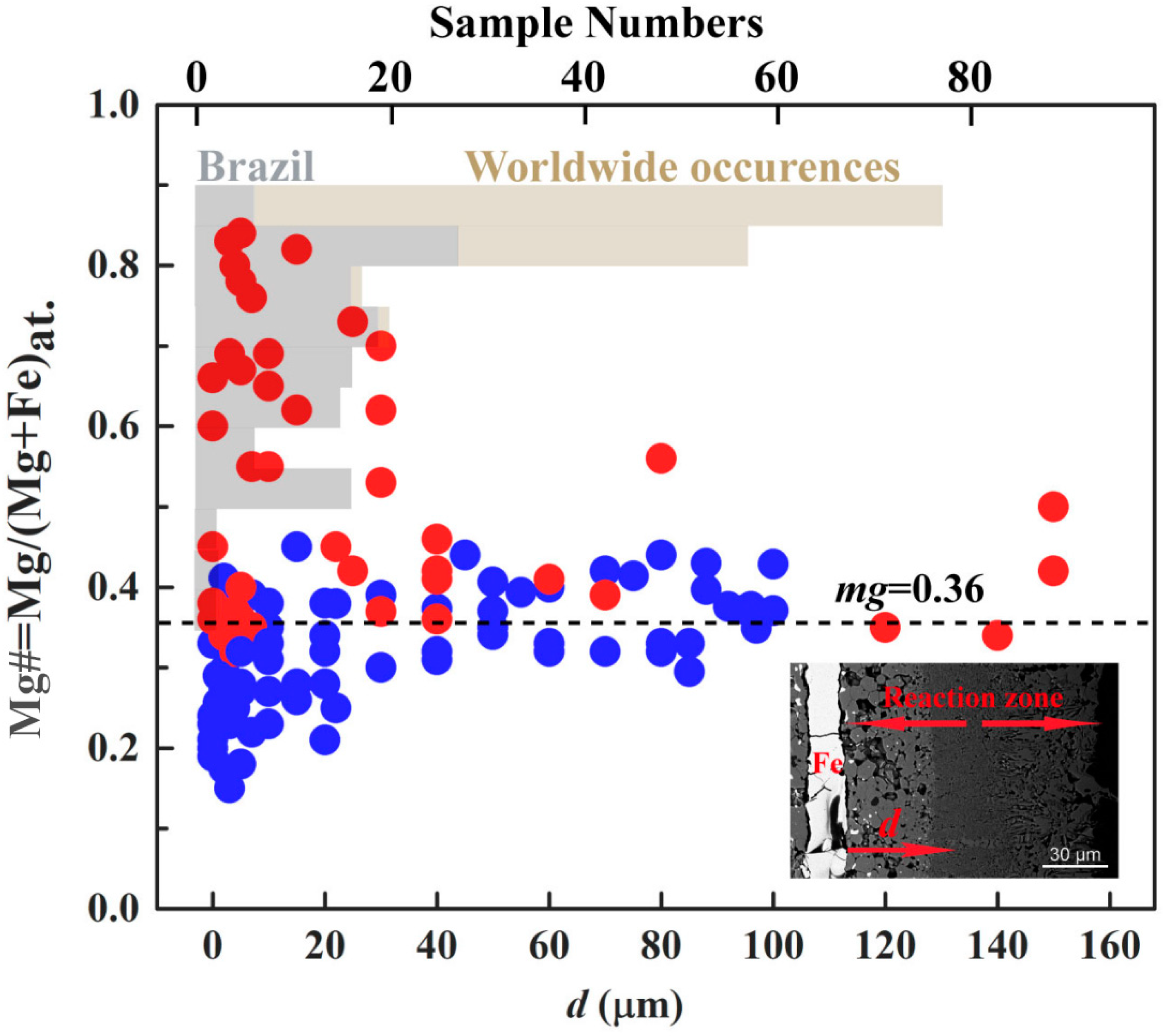
4. Discussion
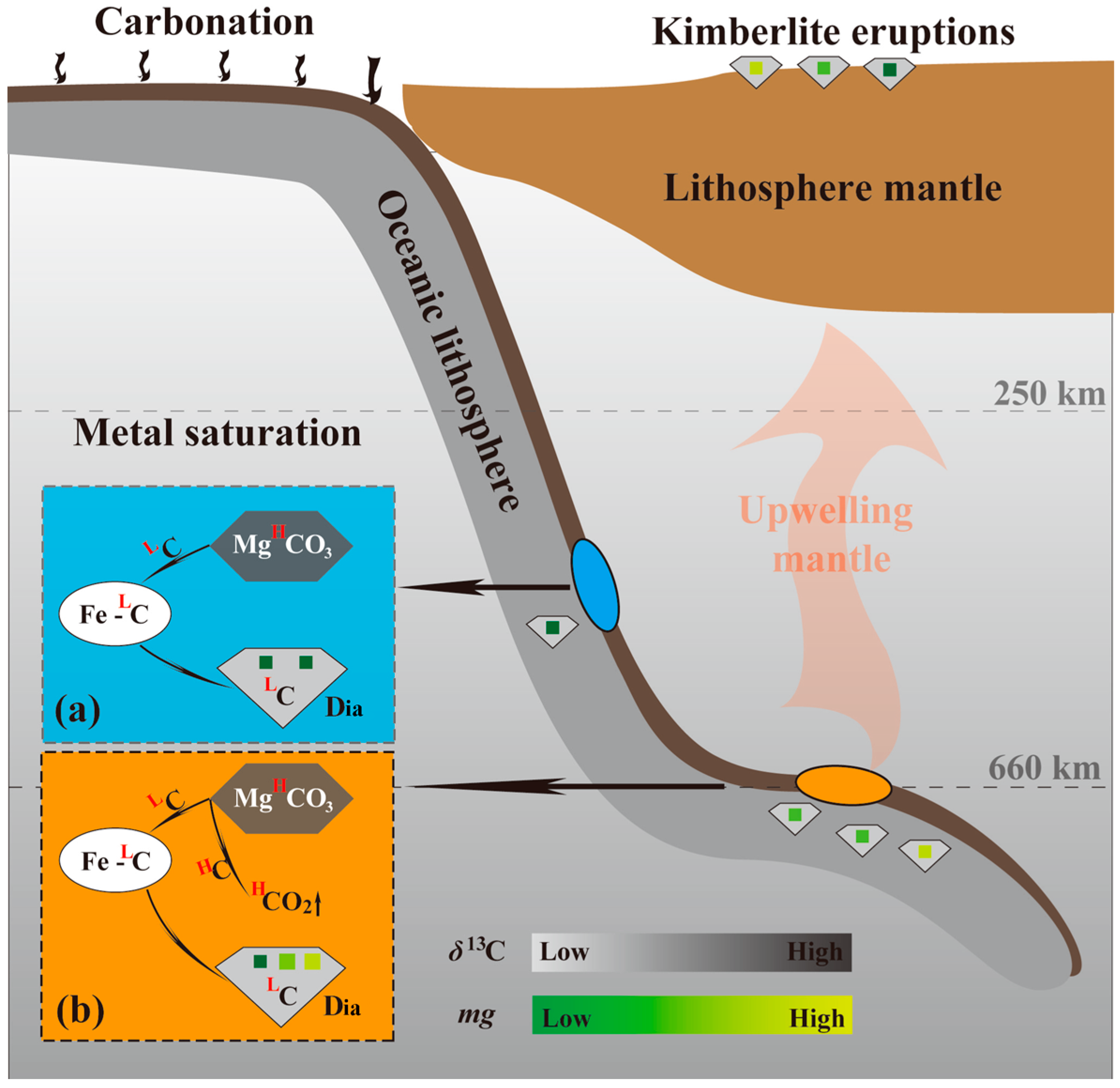
4.1. Redox Pathways in Diamond Formation
4.2. Role of Intermediate Fe-C Phases in Diamond Genesis
4.3. Temperature Dependence of (MG,Fe)O Compositions
5. Implications
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Run No. | Starting Materials | P (GPa) | T (K) | Duration (hr) |
|---|---|---|---|---|
| PL062 | Mgs+3Fe | 12 | 1200 | 15 |
| PL051 | Mgs+3Fe | 14 | 1200 | 12 |
| PL070 | Mgs+3Fe | 16 | 1200 | 8 |
| PL037 | Mgs+2Fe | 12 | 1250 | 18 |
| PL033 | Mgs+2Fe | 12 | 1300 | 24 |
| PL041 | Mgs+2Fe | 14 | 1300 | 12 |
| PL066 | Mgs+2Fe | 10 | 1400 | 24 |
| PL039 | Mgs+2Fe | 12 | 1400 | 12 |
| PL064 | Mgs+3Fe | 16 | 1400 | 24 |
| PL036 | Mgs+2Fe | 14 | 1500 | 18 |
| PL043 | Mgs+3Fe | 12 | 1500 | 5 |
| PL045 | Mgs+3Fe | 12 | 1600 | 3 |
| PL044 | Mgs+3Fe | 14 | 1600 | 12 |
| PL065 | Mgs+3Fe | 16 | 1600 | 5 min |
| PL061 | Mgs+2Fe | 12 | 1700 | 4 |
| PL060 | Mgs+3Fe | 14 | 1700 | 20 |
| C | O | Mg | Fe | Total | Phase | |
|---|---|---|---|---|---|---|
| spectrum1 | 17.20 | 46.20 | 11.74 | 23.56 | 98.70 | (Mg0.35Fe0.65)O |
| spectrum2 | 11.53 | 50.01 | 12.67 | 24.99 | 99.20 | (Mg0.36Fe0.64)O |
| spectrum3 | 9.52 | 52.95 | 11.99 | 23.21 | 97.67 | (Mg0.36Fe0.64)O |
| spectrum4 | 17.57 | 49.20 | 11.23 | 21.69 | 99.69 | (Mg0.35Fe0.65)O |
| spectrum5 | 6.66 | 52.88 | 12.13 | 27.44 | 99.11 | (Mg0.32Fe0.68)O |
| spectrum6 | 8.03 | 51.21 | 13.18 | 25.68 | 98.10 | (Mg0.34Fe0.66)O |
| spectrum7 | 6.65 | 53.40 | 13.17 | 25.68 | 98.90 | (Mg0.34Fe0.66)O |
| spectrum8 | 74.03 | 17.28 | 3.16 | 4.93 | 99.40 | C |
| C | O | Mg | Fe | Total | Phase | |
|---|---|---|---|---|---|---|
| spectrum1 | 2.18 | 54.43 | 28.33 | 14.97 | 99.91 | (Mg0.65Fe0.35)O |
| spectrum2 | 2.69 | 53.11 | 27.35 | 16.10 | 99.25 | (Mg0.62Fe0.38)O |


References
- Gorman, P.J.; Kerrick, D.M.; Connolly, J.A.D. Modeling open system metamorphic decarbonation of subducting slabs. Geochem. Geophy. Geosy. 2006, 7, Q04007. [Google Scholar] [CrossRef]
- Plank, T.; Manning, C.E. Subducting carbon. Nature 2019, 574, 343–352. [Google Scholar] [CrossRef]
- Regier, M.E.; Pearson, D.G.; Stachel, T.; Luth, R.W.; Stern, R.A.; Harris, J.W. The lithospheric-to-lower-mantle carbon cycle recorded in superdeep diamonds. Nature 2020, 585, 234–238. [Google Scholar] [CrossRef]
- Li, K.; Li, L.; Pearson, D.G.; Stachel, T. Diamond isotope compositions indicate altered igneous oceanic crust dominates deep carbon recycling. Earth Planet. Sci. Lett. 2019, 516, 190–201. [Google Scholar] [CrossRef]
- Walter, M.J.; Kohn, S.C.; Araujo, D.; Bulanova, G.P.; Smith, C.B.; Gaillou, E.; Wang, J.; Steele, A.; Shirey, S.B. Deep mantle cycling of oceanic crust: Evidence from diamonds and their mineral inclusions. Science 2011, 334, 54–57. [Google Scholar] [CrossRef]
- Pearson, D.G.; Brenker, F.E.; Nestola, F.; McNeill, J.; Nasdala, L.; Hutchison, M.T.; Matveev, S.; Mather, K.; Silversmit, G.; Schmitz, S.; et al. Hydrous mantle transition zone indicated by ringwoodite included within diamond. Nature 2014, 507, 221–224. [Google Scholar] [CrossRef] [PubMed]
- Tschauner, O.; Huang, S.; Greenberg, E.; Prakapenka, V.B.; Ma, C.; Rossman, G.R.; Shen, A.H.; Zhang, D.; Newville, M.; Lanzirotti, A.; et al. Ice-VII inclusions in diamonds: Evidence for aqueous fluid in Earth’s deep mantle. Science 2018, 359, 1136–1139. [Google Scholar] [CrossRef]
- Miyawaki, R.; Hatert, F.; Pasero, M.; Mills, S.J. IMA commission on new minerals, nomenclature and classification (CNMNC)- Newsletter 58. Eur. J. Mineral. 2020, 32, 645–651. [Google Scholar] [CrossRef]
- Nestola, F.; Korolev, N.; Kopylova, M.; Rotiroti, N.; Pearson, D.G.; Pamato, M.G.; Alvaro, M.; Peruzzo, L.; Gurney, J.J.; Moore, A.E.; et al. CaSiO3 perovskite in diamond indicates the recycling of oceanic crust into the lower mantle. Nature 2018, 555, 237–241. [Google Scholar] [CrossRef]
- Zhang, Q.W.; Timmerman, S.; Stachel, T.; Chinn, I.; Stern, R.A.; Davies, J.; Nestola, F.; Luth, R.; Pearson, D.G. Sublithospheric diamonds extend Paleoproterozoic record of cold deep subduction into the lower mantle. Earth Planet. Sci. Lett. 2024, 634, 118675. [Google Scholar] [CrossRef]
- Shatsky, V.S.; Ragozin, A.L.; Logvinova, A.M.; Wirth, R.; Kalinina, V.V.; Sobolev, N.V. Diamond-rich placer deposits from iron-saturated mantle beneath the northeastern margin of the Siberian Craton. Lithos 2020, 364–365, 105514. [Google Scholar] [CrossRef]
- Smith, E.M.; Ni, P.; Shirey, S.B.; Richardson, S.H.; Wang, W.Y.; Shahar, A. Heavy iron in large gem diamonds traces deep subduction of serpentinized ocean floor. Sci. Adv. 2021, 7, eabe9773. [Google Scholar] [CrossRef] [PubMed]
- Kaminsky, F.V.; Wirth, R. Iron carbide inclusions in lower-mantle diamond from Juina, Brazil. Can. Mineral. 2011, 49, 555–572. [Google Scholar] [CrossRef]
- Stachel, T.; Harris, J.W.; Brey, G.P. Rare and unusual mineral inclusions in diamonds from Mwadui, Tanzania. Contrib. Mineral. Petrol. 1998, 132, 34–47. [Google Scholar] [CrossRef]
- Smith, E.M.; Kopylova, M.G. Implications of metallic Fe for diamonds and nitrogen in the sublithospheric mantle. Can. J. Earth Sci. 2014, 51, 510–516. [Google Scholar] [CrossRef]
- Smith, E.M.; Shirey, S.B.; Nestola, F.; Bullock, E.S.; Wang, J.H.; Richardson, S.H.; Wang, W.Y. Large gem diamonds from metallic liquid in Earth’s deep mantle. Science 2016, 354, 1403–1405. [Google Scholar] [CrossRef]
- Frost, D.J.; Liebske, C.; Langenhorst, F.; Mccammon, C.A.; Tronnes, R.G.; Rubie, D.C. Experimental evidence for the existence of iron-rich metal in the earth’s lower mantle. Nature 2004, 428, 409–412. [Google Scholar] [CrossRef]
- Rohrbach, A.; Ballhaus, C.; Golla-Schindler, U.; Ulmer, P.; Kamenetsky, V.S.; Kuzmin, D.V. Metal saturation in the upper mantle. Nature 2007, 449, 456–458. [Google Scholar] [CrossRef]
- Palyanov, Y.N.; Bataleva, Y.V.; Sokol, A.G.; Borzdov, Y.M.; Kupriyanov, I.N.; Reutsky, V.N.; Sobolev, N.V. Mantle-slab interaction and redox mechanism of diamond formation. Proc. Natl. Acad. Sci. USA 2013, 110, 20408–20413. [Google Scholar] [CrossRef]
- Rohrbach, A.; Ghosh, S.; Schmidt, M.W.; Wijbrans, C.H.; Klemme, S. The stability of Fe-Ni carbides in the Earth’s mantle: Evidence for a low Fe-Ni-C melt fraction in the deep mantle. Earth Planet. Sci. Lett. 2014, 388, 211–221. [Google Scholar] [CrossRef]
- Lipschutz, M.E.; Anders, E. The record in the meteorites-IV: Origin of diamonds in iron meteorites. Geochim. Cosmochim. Acta 1961, 24, 83–88. [Google Scholar] [CrossRef]
- Yin, L.W.; Li, M.S.; Liu, Y.X.; Xu, B.; Liu, P.; Hao, Z.Y. Diamond homo-epitaxial growth from iron carbide under high temperature-high pressure. Chem. Phys. Lett. 2002, 363, 211–218. [Google Scholar] [CrossRef]
- Kesson, S.E.; Fitz Gerald, J.D. Partitioning of MgO, FeO, NiO, MnO and Cr2O3 between magnesian silicate perovskite and magnesiowustite: Implications for the origin of inclusions in diamond and the composition of the lower mantle. Earth Planet. Sci. Lett. 1991, 111, 229–240. [Google Scholar] [CrossRef]
- Lee, K.K.M.; O’Neill, B.; Panero, W.R.; Shim, S.H.; Benedetti, L.R.; Jeanloz, R. Equations of state of the high-pressure phases of a natural peridotite and implications for the Earth’s lower mantle. Earth Planet. Sci. Lett. 2004, 223, 381–393. [Google Scholar] [CrossRef]
- Kaminsky, F.V. Mineralogy of the lower mantle: A review of ‘superdeep’ mineral inclusions in diamond. Earth Sci. Rev. 2012, 110, 127–147. [Google Scholar] [CrossRef]
- Harte, B.; Fitzsimons, I.C.W.; Harris, J.W.; Otter, L. Carbon isotope ratios and nitrogen abundances in relation to cathodoluminescence characteristics for some diamonds from the Kaapvaal Province, S. Africa. Mineral. Mag. 1999, 63, 829–856. [Google Scholar] [CrossRef]
- Hayman, P.C.; Kopylova, M.G.; Kaminsky, F.V. Lower mantle diamonds from Rio Soriso (Juina area, Mato Grosso, Brazil). Contrib. Mineral. Petrol. 2005, 149, 430–445. [Google Scholar] [CrossRef]
- Liu, L. An alternative interpretation of lower mantle mineral associations in diamonds. Contrib. Mineral. Petrol. 2002, 144, 16–21. [Google Scholar] [CrossRef]
- Seitz, H.M.; Brey, G.P.; Harris, J.W.; Durali-Müller, S.; Ludwig, T.; Höfer, H.E. Ferropericlase inclusions in ultradeep diamonds from Sao Luiz (Brazil): High Li abundances and diverse Li-isotope and trace element compositions suggest an origin from a subduction mélange. Mineral. Petrol. 2018, 112, 291–300. [Google Scholar] [CrossRef]
- Stachel, T.; Harris, J.W.; Brey, G.P.; Joswig, W. Kankan diamonds (Guinea) II: Lower mantle inclusion parageneses. Contrib. Mineral. Petrol. 2000, 140, 16–27. [Google Scholar] [CrossRef]
- Thomson, A.R.; Walter, M.J.; Kohn, S.C.; Brooker, R.A. Slab melting as a barrier to deep carbon subduction. Nature 2016, 529, 76–79. [Google Scholar] [CrossRef] [PubMed]
- Harte, B. Diamond formation in the deep mantle: The record of mineral inclusions and their distribution in relation to mantle dehydration zones. Mineral. Mag. 2010, 74, 189–215. [Google Scholar] [CrossRef]
- Isshiki, M.; Irifune, T.; Hirose, K.; Ono, S.; Ohishi, Y.; Watanuki, T.; Nishibori, E.; Takata, M.; Sakata, M. Stability of magnesite and its high-pressure form in the lowermost mantle. Nature 2004, 427, 60–63. [Google Scholar] [CrossRef]
- Kaminsky, F.V.; Wirth, R.; Schreiber, A. Carbonatitic inclusions in deep mantle diamond from Juina, Brazil: New minerals in the carbonatehalide association. Can. Mineral. 2013, 51, 669–688. [Google Scholar] [CrossRef]
- Andersson, G.; Sundqvist, B.; Backstrom, G. A high-pressure cell for electrical resistance measurements at hydrostatic pressures up to 8 GPa: Results for Bi, Ba, Ni, and Si. J. Appl. Phys. 1989, 65, 3943–3950. [Google Scholar] [CrossRef]
- Kusaba, K.; Galoisy, L.; Wang, Y.B.; Weidner, D.J. Determination of phase transition pressures of ZnTe under quasi-hydrostatic conditions. Pure Appl. Geophys. 1993, 141, 643–652. [Google Scholar] [CrossRef]
- Leinenweber, K.D.; Tyburczy, J.A.; Sharp, T.G.; Soignard, E.; Diedrich, T.; Petuskey, W.B.; Wang, Y.; Mosenfelder, J.L. Cell assemblies for reproducible multi-anvil experiments (the COMPRES assemblies). Am. Mineral. 2012, 97, 353–368. [Google Scholar] [CrossRef]
- Martirosyan, N.S.; Litasov, K.D.; Shatskiy, A.; Ohtani, E. The reactions between iron and magnesite at 6 GPa and 1273-1873 K: Implication to reduction of subducted carbonate in the deep mantle. J. Miner. Petrol. Sci. 2015, 110, 49–59. [Google Scholar] [CrossRef]
- Syracuse, E.M.; van Keken, P.E.; Abers, G.A. The global range of subduction zone thermal models. Earth Planet. Sci. Lett. 2010, 183, 73–90. [Google Scholar] [CrossRef]
- Katsura, T.; Yoneda, A.; Yamazaki, D.; Yoshino, T.; Ito, E. Adiabatic temperature profile in the mantle. Phys. Earth Planet. Inter. 2010, 183, 212–218. [Google Scholar] [CrossRef]
- Day, H.W. A revised diamond-graphite transition curve. Am. Mineral. 2012, 97, 52–62. [Google Scholar] [CrossRef]
- Zhu, F.; Li, J.; Liu, J.C.; Lai, X.J.; Chen, B.; Meng, Y. Kinetic control on the depth distribution of superdeep diamonds. Geophys. Res. Lett. 2018, 46, 1984–1992. [Google Scholar] [CrossRef]
- Frezzotti, M.L.; Huizenga, J.M.; Compagnoni, R.; Selverstone, J. Diamond formation by carbon saturation in C-O-H fluids during cold subduction of oceanic lithosphere. Geochim. Cosmochim. Acta 2014, 143, 68–86. [Google Scholar] [CrossRef]
- Fei, Y.W.; Brosh, E. Experimental study and thermodynamic calculations of phase relations in the Fe-C system at high pressure. Earth Planet. Sci. Lett. 2014, 408, 155–162. [Google Scholar] [CrossRef]
- Oganov, A.R.; Ono, S.; Ma, Y.M.; Glass, C.W.; Garcia, A. Novel high-pressure structures of MgCO3, CaCO3 and CO2 and their role in Earth’s lower mantle. Earth Planet. Sci. Lett. 2008, 273, 38–47. [Google Scholar] [CrossRef]
- Dorfman, S.M.; Badro, J.; Nabiei, F.; Prakapenka, V.B.; Cantoni, M.; Gillet, P. Carbonate stability in the reduced lower mantle. Earth Planet. Sci. Lett. 2018, 489, 84–91. [Google Scholar] [CrossRef]
- Martirosyan, N.S.; Shatskiy, A.; Chanyshev, A.D.; Litasov, K.D.; Podborodnikov, I.V.; Yoshino, T. Effect of water on the magnesite-iron interaction, with implications for the fate of carbonates in the deep mantle. Lithos 2019, 326–327, 435–445. [Google Scholar] [CrossRef]
- Bulanova, G.P.; Walter, M.J.; Smith, C.B.; Kohn, S.C.; Armstrong, L.S.; Blundy, J.; Gobbo, L. Mineral inclusions in sublithospheric diamonds from Collier 4 kimberlite pipe, Juina, Brazil: Subducted protoliths, carbonated melts and primary kimberlite magmatism. Contrib. Mineral. Petrol. 2010, 160, 489–510. [Google Scholar] [CrossRef]
- Tappert, R.; Foden, J.; Stachel, T.; Muehlenbachs, K.; Tappert, M.; Wills, K. Deep mantle diamonds from South Australia: A record of Pacific subduction at the Gondwanan margin. Geology 2009, 37, 43–46. [Google Scholar] [CrossRef]
- Zedgenizov, D.A.; Kagi, H.; Shatsky, V.S.; Ragozin, A.L. Local variations of carbon isotope composition in diamonds from São-Luis (Brazil): Evidence for heterogenous carbon reservoir in sublithospheric mantle. Chem. Geol. 2014, 363, 114–124. [Google Scholar] [CrossRef]
- Galimov, E.M. Isotope fractionation related to kimberlite magmatism and diamond formation. Geochim. Cosmochim. Acta 1991, 55, 1697–1708. [Google Scholar] [CrossRef]
- Cartigny, P.; Harris, J.W.; Javoy, M. Eclogitic diamond formation at Jwaneng: No room for a recycled component. Science 1998, 280, 1421–1424. [Google Scholar] [CrossRef] [PubMed]
- Smith, E.M.; Shirey, S.B.; Richardson, S.H.; Nestola, F.; Bullock, E.S.; Wang, J.H.; Wang, W.Y. Blue boron-bearing diamonds from Earth’s lower mantle. Nature 2018, 560, 84–87. [Google Scholar] [CrossRef]
- Reutsky, V.; Borzdov, Y.; Palyanov, Y.; Sokol, A.; Izokh, O. Carbon isotope fractionation during experimental crystallisation of diamond from carbonate fluid at mantle conditions. Contrib. Mineral. Petrol. 2015, 170, 41. [Google Scholar] [CrossRef]
- Polyakov, V.B.; Kharlashina, N.N. Effect of pressure on equilibrium isotopic fractionation. Geochim. Cosmochim. Acta 1994, 58, 4739–4750. [Google Scholar] [CrossRef]
- Horita, J.; Polyakov, V.B. Carbon-bearing iron phases and the carbon isotope composition of the deep Earth. Proc. Natl. Acad. Sci. USA 2015, 112, 31–36. [Google Scholar] [CrossRef]
- Harte, B.; Harris, J.W.; Hutchinson, M.T.; Watt, G.R.; Wilding, M.C. Lower Mantle Mineral Associations in Diamonds from Sao Luiz, Brazil; Special Publication; The Geochemical Society: Cape Town, South Africa, 1999; pp. 372–382. [Google Scholar]
- Palot, M.; Pearson, D.G.; Stern, R.A.; Stachel, T.; Harris, J.W. Isotopic constraints on the nature and circulation of deep mantle C-H-O-N fluids: Carbon and nitrogen systematics within ultra-deep diamonds from Kankan (Guinea). Geochim. Cosmochim. Acta 2014, 139, 26–46. [Google Scholar] [CrossRef]
- Stachel, T.; Brey, G.P.; Harris, J.W. Inclusions in sublithospheric diamonds: Glimpses of deep Earth. Elements 2005, 1, 73–78. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gao, J.; Chen, B.; Wu, X.; Lai, X.; Fan, C.; Liu, Y.; Zhang, J. Superdeep Diamond Genesis Through Fe-Mediated Carbonate Reduction. Geosciences 2025, 15, 163. https://doi.org/10.3390/geosciences15050163
Gao J, Chen B, Wu X, Lai X, Fan C, Liu Y, Zhang J. Superdeep Diamond Genesis Through Fe-Mediated Carbonate Reduction. Geosciences. 2025; 15(5):163. https://doi.org/10.3390/geosciences15050163
Chicago/Turabian StyleGao, Jing, Bin Chen, Xiang Wu, Xiaojing Lai, Changzeng Fan, Yun Liu, and Junfeng Zhang. 2025. "Superdeep Diamond Genesis Through Fe-Mediated Carbonate Reduction" Geosciences 15, no. 5: 163. https://doi.org/10.3390/geosciences15050163
APA StyleGao, J., Chen, B., Wu, X., Lai, X., Fan, C., Liu, Y., & Zhang, J. (2025). Superdeep Diamond Genesis Through Fe-Mediated Carbonate Reduction. Geosciences, 15(5), 163. https://doi.org/10.3390/geosciences15050163










