Quantitative Biofacies Analysis of Upper Oligocene Reef-Coral Neritic Carbonates (Southern Pakistan)
Abstract
1. Introduction
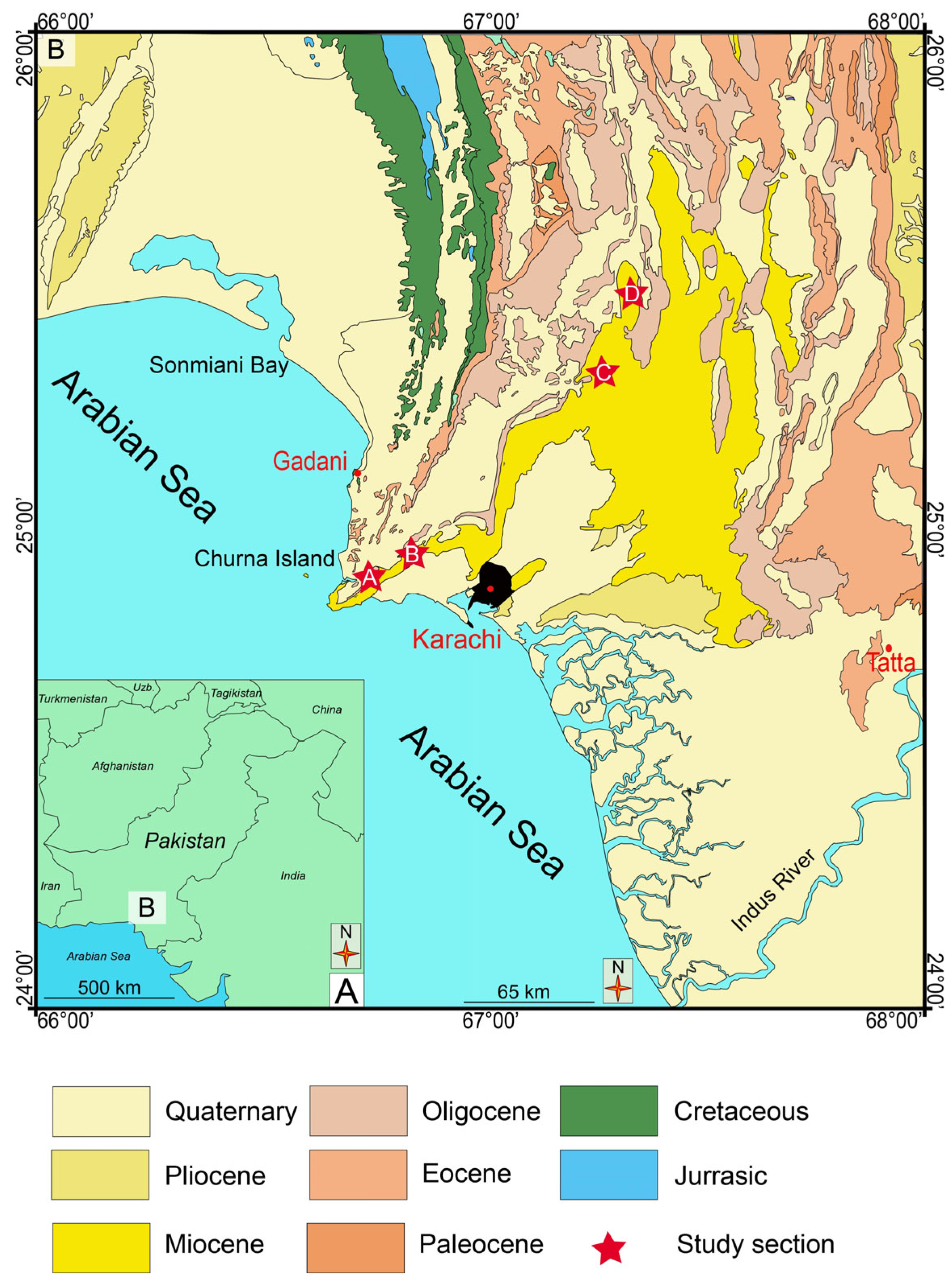
2. Geological Setting
3. Materials and Methods
4. Results
4.1. Description of the Stratigraphic Sections
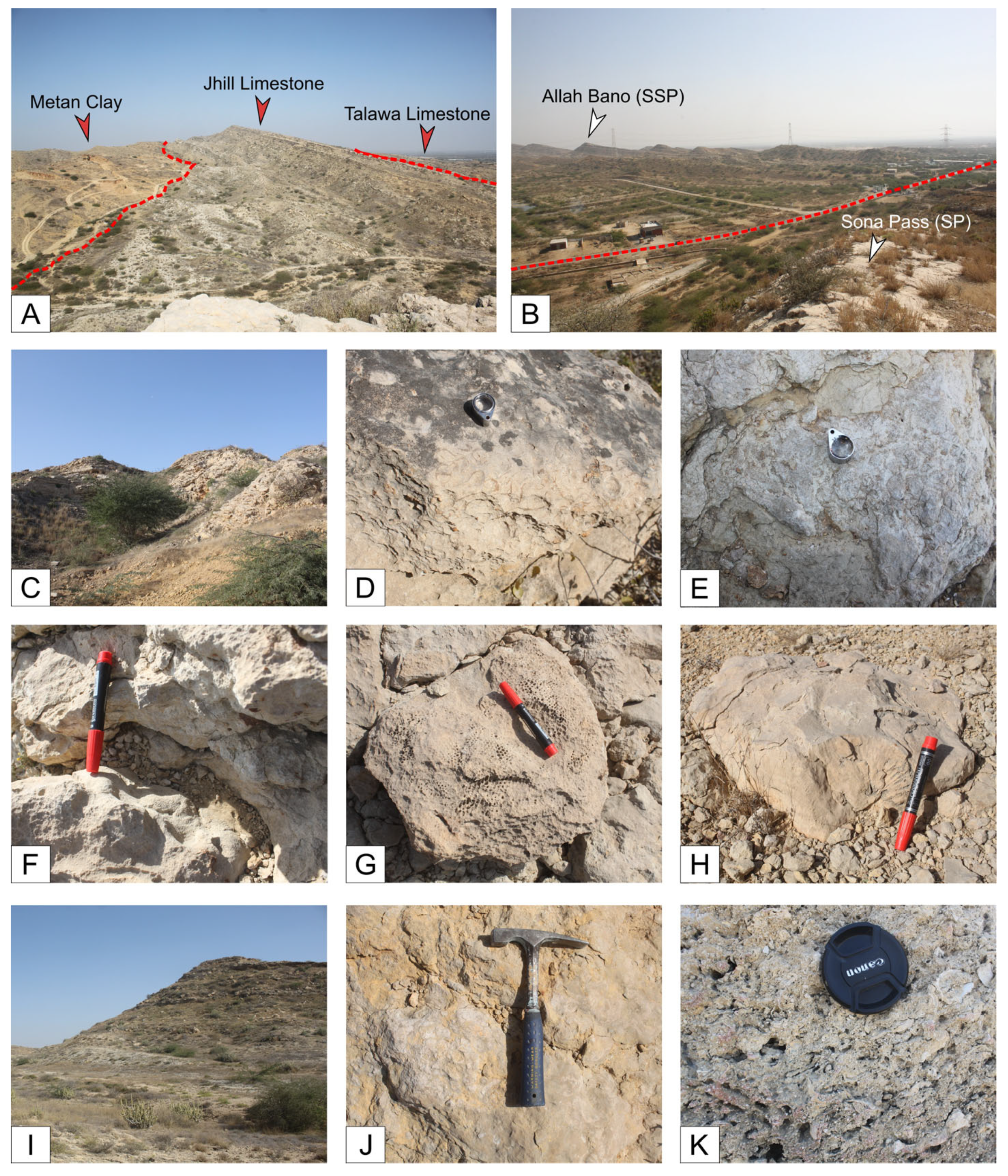
4.1.1. Sona Pass Section (SP)
4.1.2. Allah Bano (Lal Bakkar) Section (SSP)
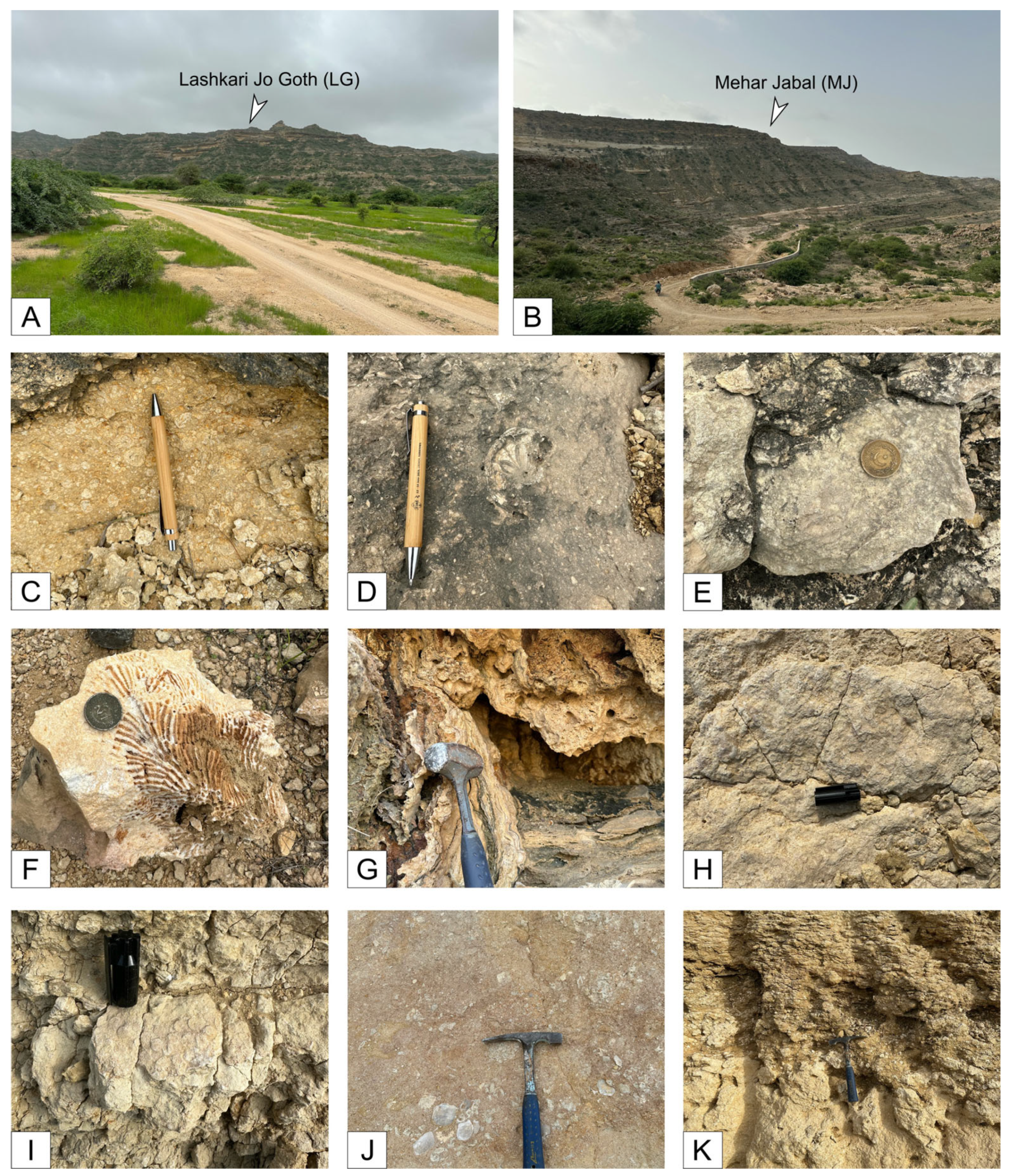
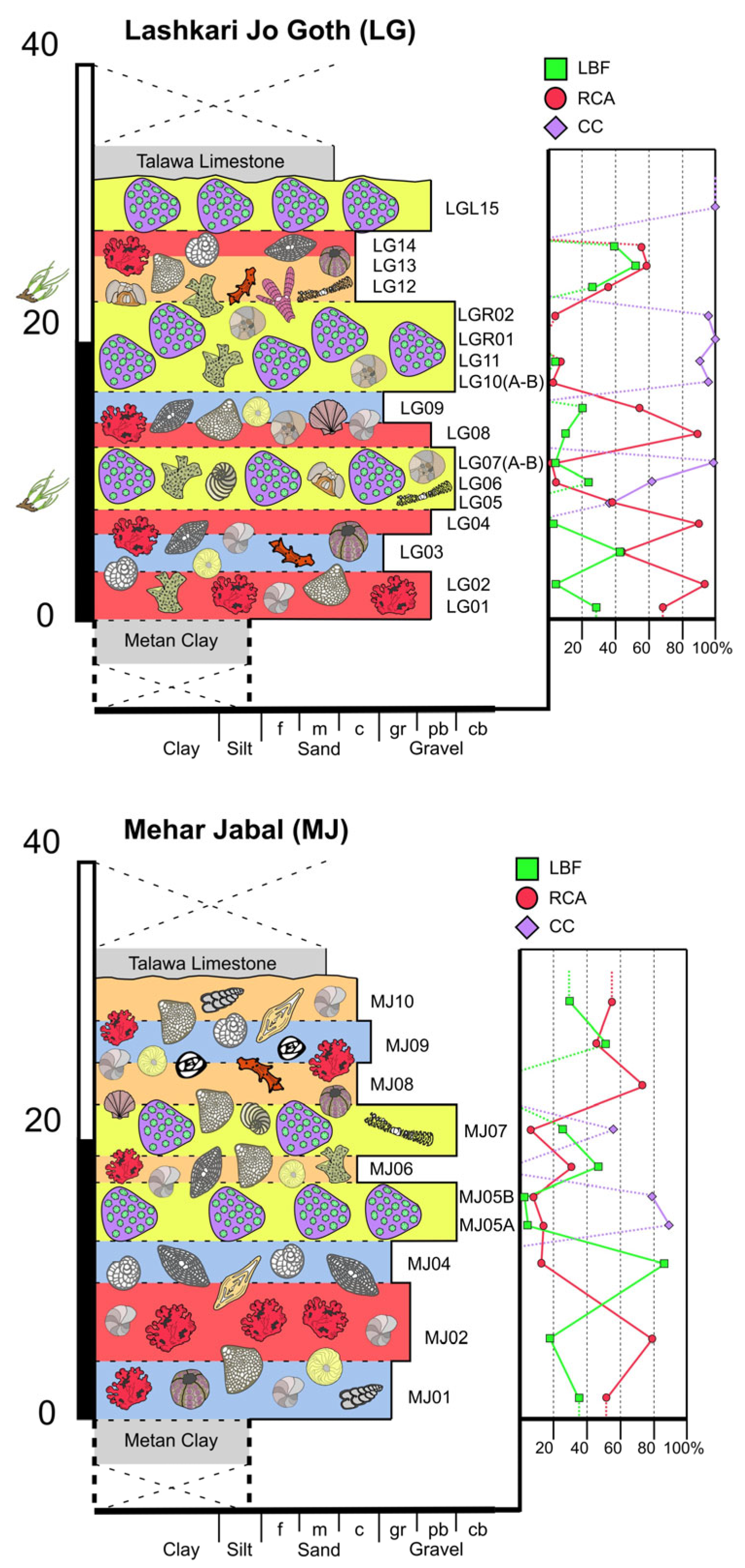
4.1.3. Lashkari Jo Goth (LG)
4.1.4. Mehar Jabal (MJ)
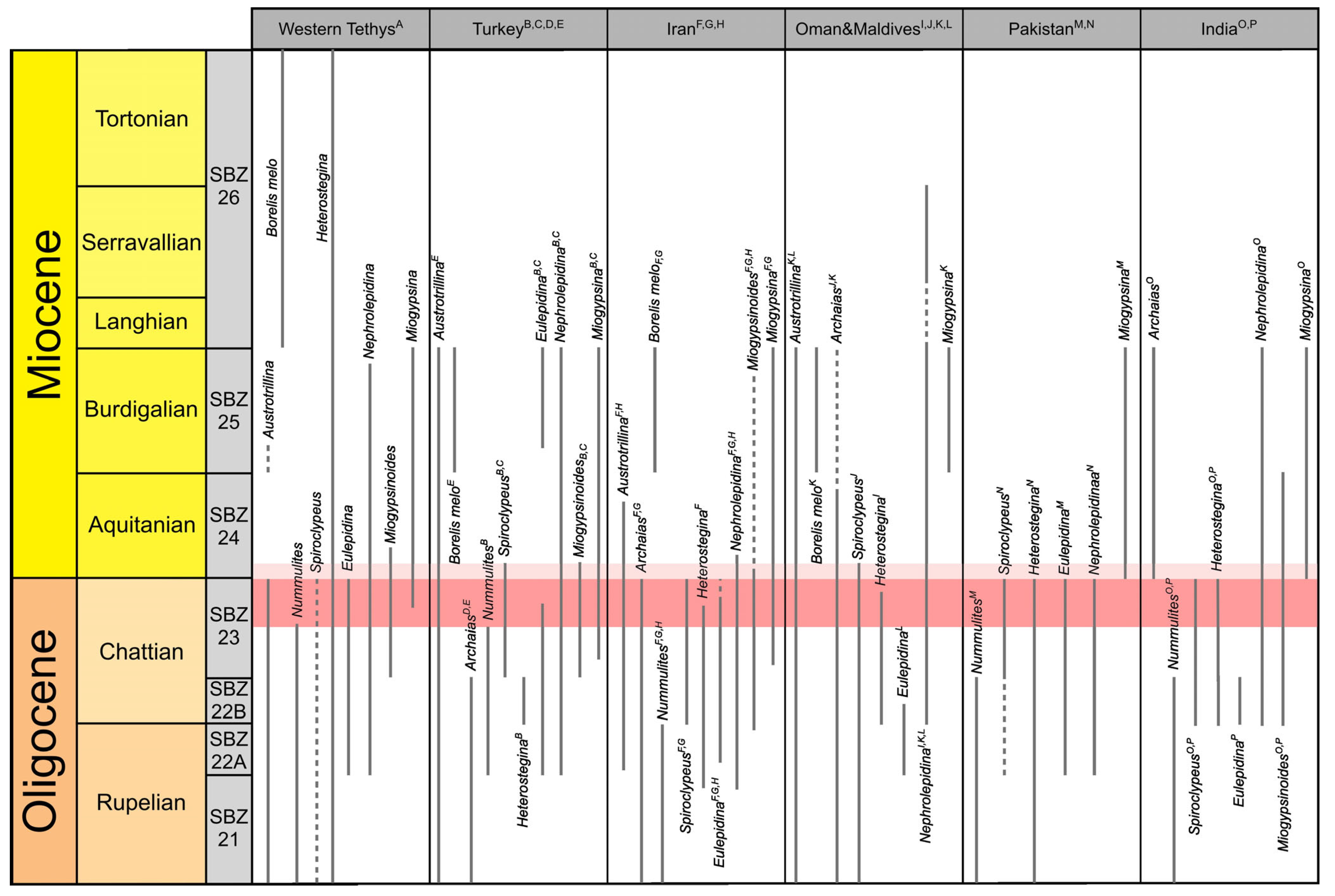
4.2. Biostratigraphy
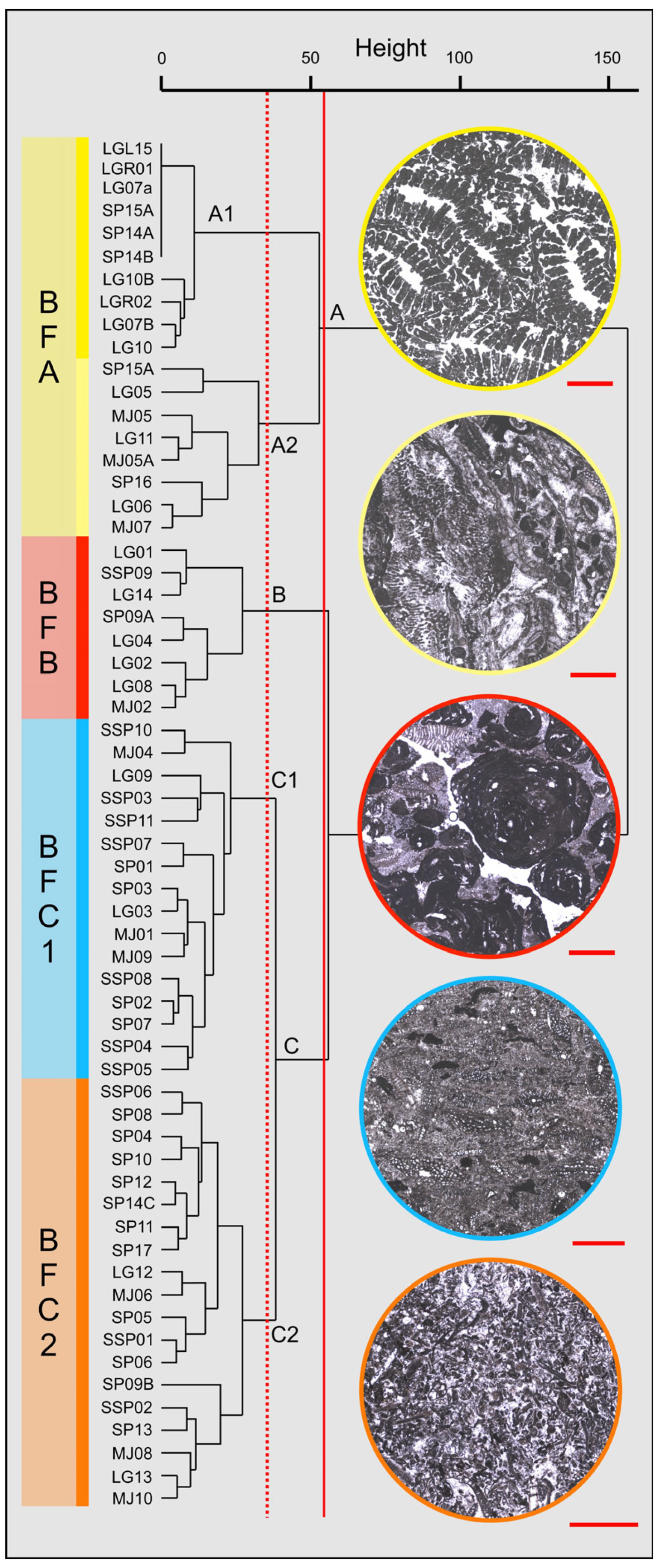
4.3. Quantitative Biofacies Analysis
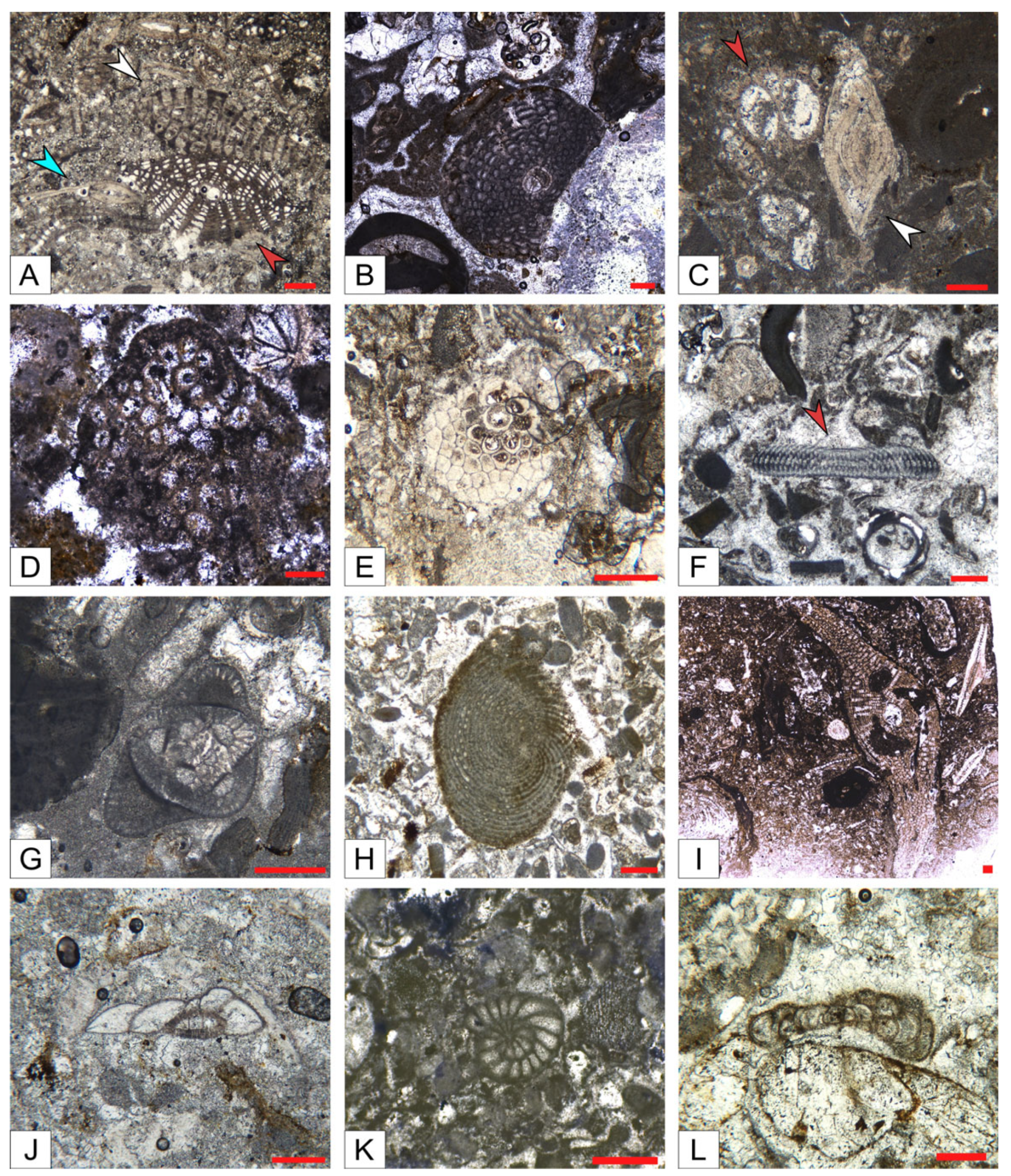
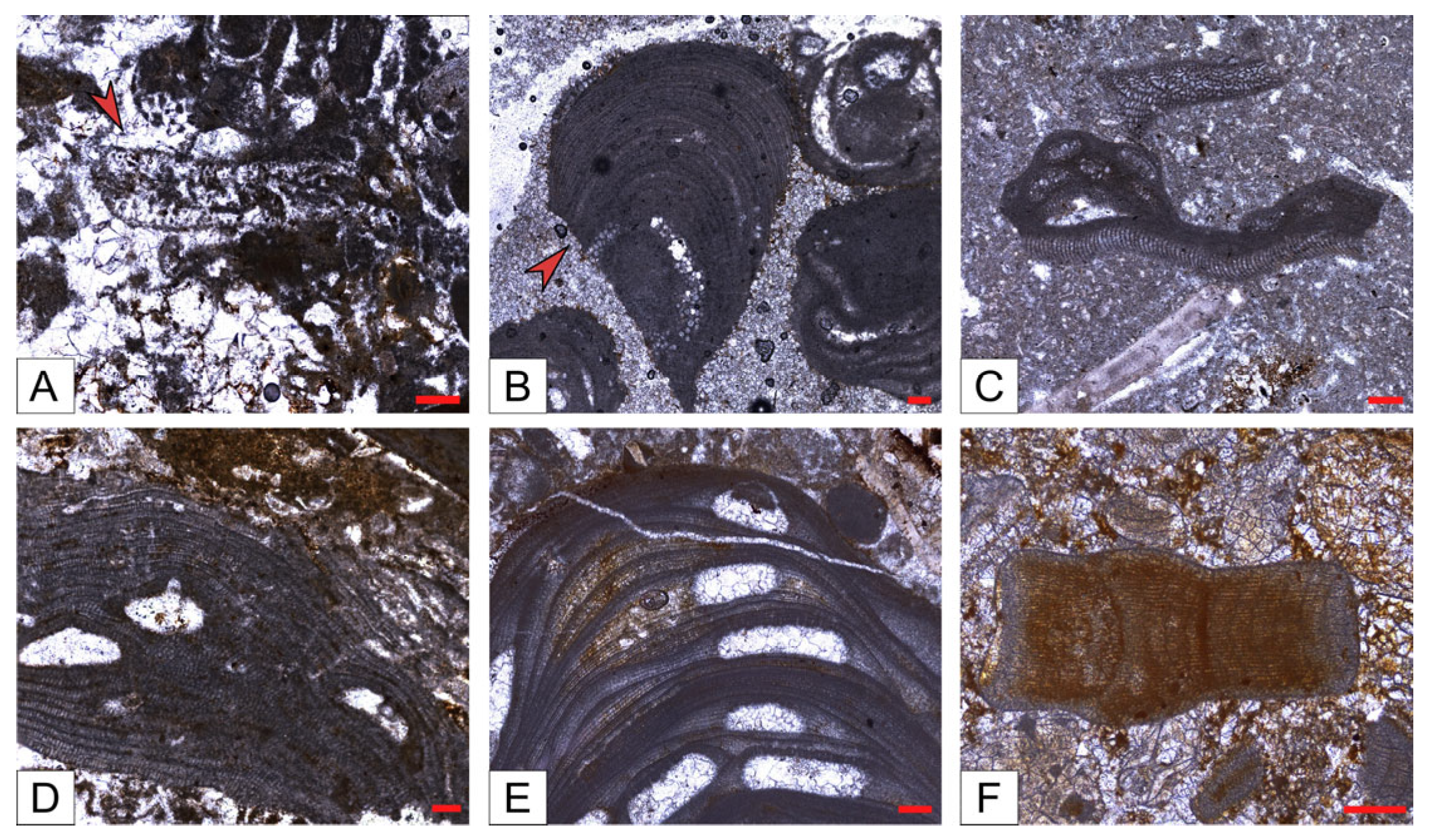
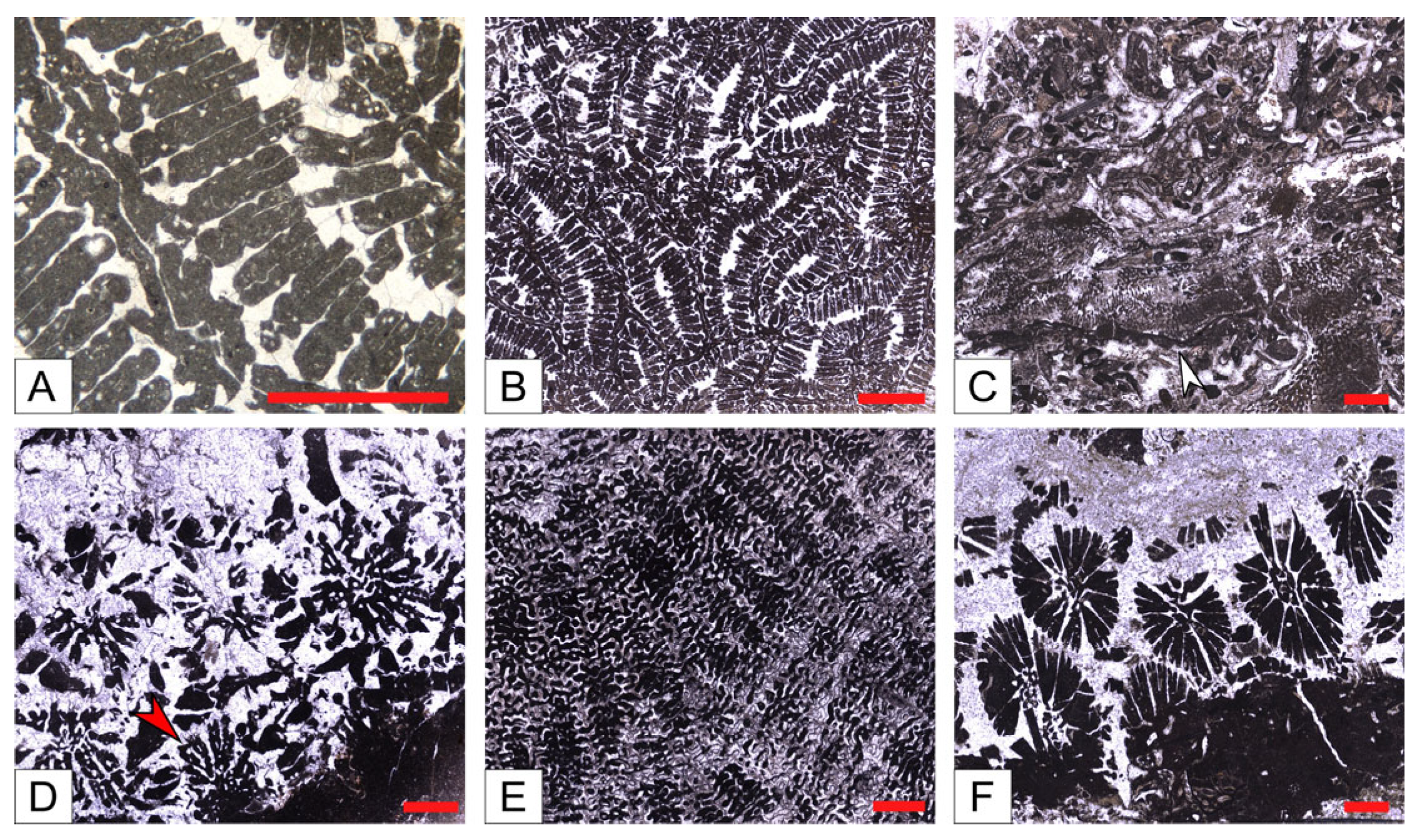
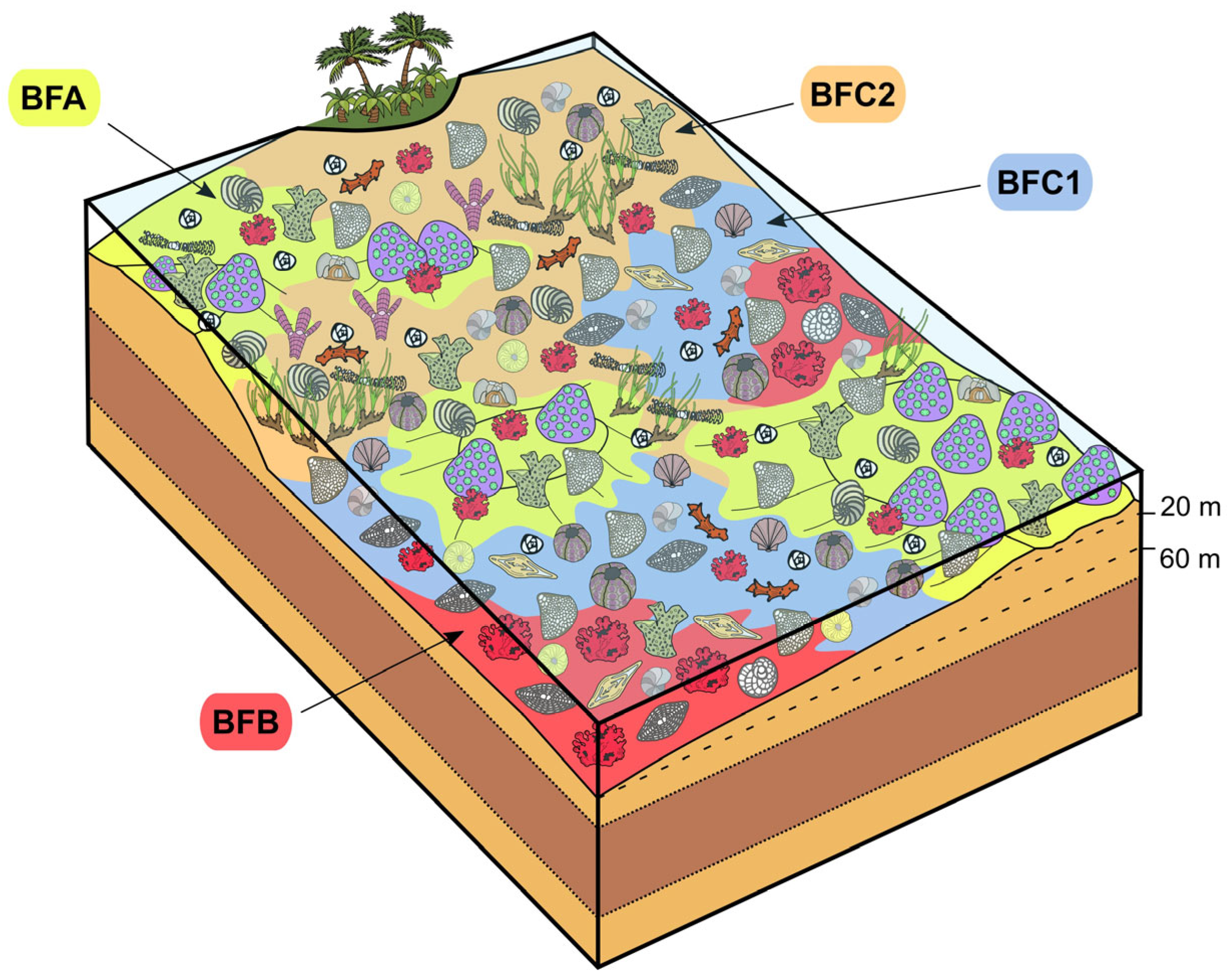
- Reef coral biofacies (BFA): this biofacies is recorded in different intervals within the SP, LG, and MJ sections, for a total thickness of about 22 m (Figure 3 and Figure 5). In SP, it is recorded from 39 to 41 m abs (SP14A-B), and from 43 to 46 m abs (SP15A-B; SP16), for a total thickness of 5 m. In LG, it is recorded from 8 to 12 m abs (LG05; LG06; LG07A-B), from 18 to 23 m abs (LG10A-B; LG11; LGR01; LGR02), and from 29 to 32 m abs (top of the sequence; LGL15), for a total thickness of 12 m. In MJ, it is recorded from 13 to 15 abs (MJ05A-B), and from 19 to 22 m abs (MJ07), for a total thickness of 5 m. This biofacies is primarily defined by the presence of coral colonies in growth position, which form small-scale, patchy bioconstructions, while those transported are clearly subordinate. Overall, it is dominated by colonial corals (82% average abundance) (Table 1) and, based on their abundance together with the percentage of colonies in growth position, two types of coral fabrics, corresponding to two sub-biofacies, can be recognized (Figure 7) (Table 1): coral boundstones (BFA1) and coral floatstones (BFA2). The coral boundstones are constituted almost entirely (99% average abundance) of colonies in growth position. Among the latter, the genera Hydnophora, Porites, Agathiphyllia, Montastrea, and Favites have been recognized (Figure 9). The only attempt at identification at the species level was carried out on sample LGR01, from the LG section, that has been assigned to Hydnophora cf. provincialis on the basis of clear morphometric diagnostic characters [45] (Figure 9A,B). The floatstones display a skeletal assemblage dominated by fragments of branching corals (62% on average; among the recognized genera there are Acropora and Stylophora), together with less abundant coral colonies in growth position, and also includes other carbonate producers, particularly encrusting RCA (15%), miogypsinids (6%), large miliolids (3.5%), and encrusting benthic foraminifera and victoriellids (4%). Regarding the area-counting analysis of the foraminiferal assemblage (Table 2), BFA is characterized predominantly by hyaline SBF (62%) (particularly of the morphotype B e.g., Cibicides, Lobatula, see [60,61,62]), porcelaneous SBF (12.5%) (e.g., Quinqueloculina, Triloculina, Spiroloculina), miogypsinids (11%) (Miogypsina, Miogypsinoides), and porcelaneous LBF (8.5%) (Sorites, Archaias, and other peneroplids). With regards to the calcareous algal assemblage, crustose RCA largely dominate and are mainly represented by Corallinales and Hapalidiales (in similar amounts). Sporolithales and articulated RCA are present. Rare fragments of Halimeda also occur (Figure 8A).
- Coralline algal biofacies (BFB): this biofacies is logged at distinct intervals within SP, SSP, LG, and MJ, for a total thickness of about 20 m (Figure 3 and Figure 5). Within SP, it is recorded from 27 to 30 m abs (SP09A-B), for a total thickness of 3 m. In SSP, it is recorded from 25 to 29 m abs (SSP09), for a thickness of 4 m. In LG, it is recorded from 0 to 2 m abs (LG01; LG02), from 6 to 8 m abs (LG04), from 13 to 15 m abs (LG08), and from 28 to 30 m abs (LG14), for a total thickness of 8 m. In MJ, it is recorded from 5 to 10 m abs (MJ02). This biofacies is characterized by rudstones and floatstones and its skeletal assemblage is largely dominated by encrusting RCA (77%), followed by lepidocyclinids (6%), flat and thin hyaline LBF (6%), miogypsinids (4%), and encrusting benthic foraminifera (2%) (Table 1). Based on area-counting, the foraminiferal assemblage is dominated by hyaline SBF (31%) and miogypsinids (26.5%) (Miogypsina, Miogypsinoides), Spiroclypeus and other flat-shaped nummulitids, e.g., Operculina and Heterostegina (14.5%; Spiroclypeus alone represent 12% of the assemblage), amphisteginids (Figure 6C) and other hyaline LBF (13.5%), and lepidocyclinids (6%) (Nephrolepidina, Eulepidina) (Table 2). BFB displays also the highest abundance of rounded acervulinids (although they only represent nearly 1% of the foraminiferal assemblage). Crustose RCA dominate the algal assemblage and are mainly represented by Hapalidiales (Figure 8E). However, Corallinales and Sporolithales are also present, albeit in smaller quantities.
- Large benthic foraminiferal and coralline algal biofacies (BFC): this biofacies is primarily characterized by the dominance of LBF and can be further subdivided based on the type of LBF.
- a.
- Miogypsinid, thin and flat large benthic foraminiferal and coralline algal sub-biofacies (BFC1): this biofacies is recorded in distinct intervals within SP, SSP, LG, and MJ, for a total thickness of about 44 m (Figure 3 and Figure 5). In SP, it is logged from 0 to 11 m abs (SP01; SP02; SP03). In SSP, it is logged from 9 to 17 m abs (SSP03; SSP04; SSP05), from 19 to 26 m abs (SSP07; SSP08), and from 30 to 33 m abs (SSP09; SSP10), for a total thickness of 18 m. In LG, it is logged from 3 to 5 m abs (LG03), and from 15 to 17 m abs (LG09), for a total thickness of 4 m. In MJ, it is recorded from 0 to 4 m abs (MJ01), from 9 to 13 m abs (MJ04), and from 27 to 30 m abs (MJ09), for a total thickness of 11 m. This biofacies is mainly composed of grainstones (particularly at the base of the SP section) and rudstones. The skeletal assemblage (Table 1) is dominated by encrusting RCA (29.8%), miogypsinids (25.40%), low T/D (test’s thickness/diameter ratio) hyaline LBF (14.3%), mollusks (7.45%), lepidocyclinids (5.40%), bryozoans (4%), and echinoderms (3.2%). An increase in the abundance of articulated RCA (2.40%) is reported. The foraminiferal association is dominated by miogypsinids (45.29%) (Miogypsina, Miogypsinoides), followed by hyaline SBF (18.2%), Spiroclypeus and other flat-shaped nummulitids, e.g., Heterostegina, Operculina (11.3%), small miliolids (7.36%) (Quinqueloculina, Triloculina), and lepidocyclinids (4.67%) (Nephrolepidina) (Table 2). The calcareous algal assemblage includes crustose and articulated RCA associated with rare green calcareous algae. Among crustose RCA, Hapalidiales (Figure 8C) and Corallinales (Figure 8D) occur in similar amounts.
- b.
- Miogypsinid and coralline algal sub-biofacies (BFC2): this biofacies is logged in different intervals along SP, SSP, LG, and MJ and is overall the most represented in the study area, for a total thickness of about 57 m (Figure 3 and Figure 5). In SP, it is recorded from 11 to 27 m abs (from SP04 to SP08), from 30 to 39 m abs (from SP10 to SP13), from 41 to 43 m abs (SP14C), and from 46 to 48 m abs (SP17), for a total thickness of 29 m. In SSP, it is logged from 0 to 9 m abs (SSP01; SSP02), and from 17 to 19 m abs (SSP06), for a thickness of 11 m. In LG, it is recorded from 23 to 28 m abs (LG12; LG13), for a thickness of 5 m. In MJ, it is logged from 15 to 19 m abs (MJ06), from 22 to 27 m abs (MJ08), and from 30 to 33 m abs (MJ10), for a total thickness of 12 m. This biofacies consists primarily of packstones and rudstones. Its skeletal assemblage is characterized by crustose RCA (24%), miogypsinids (20%), echinoderms (11%), bryozoans (9%), hyaline SBF (7%), and lepidocyclinids (4%) (Table 1). Among all the recognized biofacies, BFC2 hosts the highest abundance of articulated RCA (4%), of porcelaneous foraminifera (6%), and of green calcareous algae (although they only represent less than 1% of the assemblage of the biofacies) (Table 1). The foraminiferal assemblage comprises primarily hyaline SBF (38%) (mostly Cibicides, Lobatula, Rosalina, Neoconorbina, keeled Elphidium, and infaunal taxa, such as bolivinids) (Figure 6J), miogypsinids (31%) (Miogypsinoides, Miogypsina), small miliolids (10.5%) (Quinqueloculina, Spiroloculina, Triloculina), agglutinated SBF (8.5%) (Textularia, Bigenerina), and porcelaneous LBF (4.1%) (Sorites, Archaias, other peneroplids, Austrotrillina) (Figure 6F,G,H,K) (Table 2). Similarly to BFC1, the algal assemblage of BFC2 includes crustose RCA (Hapalidiales, Corallinales, and, in this case, Sporolithales) (Figure 8B), articulated RCA (Figure 8F) and rare green calcareous algae. Locally, crustose RCA are represented by thin crusts with a hooked morphology.
5. Discussion
5.1. Biostratigraphic and Paleoclimatic Considerations
5.2. Facies Interpretation
5.3. Depositional Model
5.4. Effectiveness of Foraminiferal-Based Paleobathymetric Parameters
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| LBF | Large benthic foraminifera |
| RCA | Red calcareous algae |
| CC | Reef corals |
| LOWE | Late Oligocene Warming Event |
| SBF | Small benthic foraminifera |
| SP | Sona Pass |
| SSP | Allah Bano |
| LG | Lashkari Jo Goth |
| MJ | Mehar Jabal |
References
- Bialik, O.M.; Coletti, G.; Mariani, L.; Commissario, L.; Desbiolles, F.; Meroni, A.N. Availability and type of energy regulate the global distribution of neritic carbonates. Sci. Rep. 2023, 13, 19687. [Google Scholar]
- Andruleit, H.; Freiwald, A.; Schäfer, P. Bioclastic carbonate sediments on the southwestern Svalbard shelf. Mar. Geol. 1996, 134, 163–182. [Google Scholar]
- Henrich, R.; Freiwald, A.; Betzler, C.; Bader, B.; Schäfer, P.; Samtleben, C.; Brachert, T.C.; Wehrmann, A.; Zankl, H.; Kühlmann, D.H.H. Controls on modern carbonate sedimentation on warm-temperate to arctic coasts, shelves and seamounts in the northern hemisphere: Implications for fossil counterparts. Facies 1995, 32, 71–108. [Google Scholar]
- Frank, T.D.; James, N.P.; Bone, Y.; Malcolm, I.; Bobak, L.E. Late Quaternary carbonate deposition at the bottom of the world. Sediment. Geol. 2014, 305, 1–16. [Google Scholar]
- Civitelli, G.; Brandano, M. Atlante delle litofacies e modello deposizionale dei Calcari a Briozoi e Litotamni nella Piattaforma carbonatica laziale-abruzzese. Boll. Soc. Geol. Ital. 2005, 124, 611. [Google Scholar]
- Benisek, M.F.; Betzler, C.; Marcano, G.; Mutti, M. Coralline-algal assemblages of a Burdigalian platform slope: Implications for carbonate platform reconstruction (northern Sardinia, western Mediterranean Sea). Facies 2009, 55, 375–386. [Google Scholar]
- Pomar, L. Types of carbonate platforms: A genetic approach. Basin Res. 2001, 13, 313–334. [Google Scholar]
- Reolid, J.; Betzler, C.; Braga, J.C.; Martín, J.M.; Lindhorst, S.; Reijmer, J.J. Reef slope geometries and facies distribution: Controlling factors (Messinian, SE Spain). Facies 2014, 60, 737–753. [Google Scholar]
- Chesnel, V.; Rodríguez, E. Facies analysis of a Bartonian–Aquitanian siliciclastic-carbonate system, Costa Rica. Sed. Geol. 2021, 417, 105884. [Google Scholar]
- Ager, D.V. The Nature of the Stratigraphical Record; Wiley & Sons: Hoboken, NJ, USA, 1973; p. 151. [Google Scholar]
- Kiessling, W.; Flügel, E.; Golonka, J. Paleoreef maps: Evaluation of a comprehensive database on Phanerozoic reefs. AAPG Bull. 1999, 83, 1552–1587. [Google Scholar]
- Johnson, K.G.; Jackson, J.B.; Budd, A.F. Caribbean reef development was independent of coral diversity over 28 million years. Science 2008, 319, 1521–1523. [Google Scholar] [PubMed]
- Coletti, G.; Commissario, L.; Mariani, L.; Bosio, G.; Desbiolles, F.; Soldi, M.; Bialik, O.M. Palaeocene to Miocene southern Tethyan carbonate factories: A meta-analysis of the successions of South-western and Western Central Asia. Dep. Rec. 2022, 8, 1031–1054. [Google Scholar]
- Benedetti, A.; Papazzoni, C.A.; Bosellini, F.R. Unparallel resilience of shallow-water tropical calcifiers (foraminifera and scleractinian reef corals) during the early Paleogene global warming intervals. Palaeogeogr. Palaeoclimat. Palaeoecol. 2024, 651, 112393. [Google Scholar]
- Ahmed, W.; Subhani, A.M.; Abidi, S.S.H. Geological map of Karachi; Geological Survey of Pakistan: Quetta, Pakistan, 1983. [Google Scholar]
- Naseem, S.; Sheikh, S.A.; Qadeeruddin, M. Geochemistry and sedimentology of Jhill limestone of Gaj formation, in Cape Monze and adjoining area, Karachi. Chin. J. Geochem. 1996, 15, 213–227. [Google Scholar]
- Shah, S.K. Indus ophiolite belt and the tectonic setting of the Malia Johar-Kiogad exotics in the Himalaya. Himalaya Sci. Terre Coil. Int. C.N.R.S., Paris 1977, 268, 361–368. [Google Scholar]
- Sahni, M.R.; Khan, E.J. Stratigraphy, structure and correlation of the Upper Shiwaliks, East of Chandigarh. J. Palaeontol. Soc. India 1968, 5–9, 61–74. [Google Scholar]
- Lawrence, R.D.; Yeats, R.S.; Khan, S.H.; Subhani, A.M.; Bonelli, D. Crystalline rocks of the Spinatizha area, Pakistan. J. Struct. Geol. 1981, 3, 449–457. [Google Scholar]
- Bannert, D.; Cheema, A.; Ahmed, A.; Schaffer, U. The structural development of the Western Fold Belt Pakistan. Geol. Jahrbuch 1992, B80, 3–60. [Google Scholar]
- Hu, X.; Garzanti, E.; Wang, J.; Huang, W.; An, W.; Webb, A. The timing of India-Asia collision onset–Facts, theories, controversies. Earth Sci. Rev. 2016, 160, 264–299. [Google Scholar]
- Abdel-Gawad, M. Wrench movements in Baluchistan arc and relation to Himalayan and Indian Ocean tectonics. Bull. Geol. Soc. Am. 1971, 82, 1235. [Google Scholar]
- HSC. Reconnaissance Geology of Part West Pakistan: Colombo Plan Cooperative Project for Government of Pak; Government of Canada: Ottawa, ON, Canada, 1960; p. 550. [Google Scholar]
- Kazmi, A.H.; Rana, R.A. Tectonic Map of Pakistan; Geological Survey of Pakistan: Quetta, Pakistan, 1982. [Google Scholar]
- Iqbal, M.W.A. Oligo-Miocene bivalves and gastropods from Kirthar Province, Lower Indus Basin, Pakistan. Rec. Geol. Sur. Pak. 1980, 51, 59. [Google Scholar]
- Hasnain, S.M.; Farooqi, M.A.; Muhammad, M.J. Lithostratigraphy and microfauna of Cape Monze area. Karachi Kar. Uni. Jour. Sci. 1987, 15, 13–22. [Google Scholar]
- Dunham, R.J. Classification of carbonate rocks according to depositional texture. In Classification of Carbonate Rocks; Ham, W.E., Ed.; American Association of Petroleum Geologists: Tulsa, OK, USA, 1962; Volume 1, pp. 108–121. [Google Scholar]
- Embry, A.F.; Klovan, J.E. A Late Devonian reef tract on Northeastern Banks Island, NWT. Bull. Canad. Petrol. Geol. 1971, 19, 730–781. [Google Scholar]
- Lokier, S.W.; Al Junaibi, M. The petrographic description of carbonate facies: Are we all speaking the same language? Sedimentology 2016, 63, 1843–1885. [Google Scholar] [CrossRef]
- Özcan, E.; Less, G.; Báldi-Beke, M.; Kollányi, K.; Acar, F. Oligo-Miocene foraminiferal record (Miogypsinidae, Lepidocyclinidae and Nummulitidae) from the Western Taurides (SW Turkey): Biometry and implications for the regional geology. J. Asian Earth Sci. 2009, 34, 740–760. [Google Scholar] [CrossRef]
- Özcan, E.; Less, G.; Báldi-Beke, M.; Kollányi, K. Oligocene hyaline larger foraminifera from Kelereêdere Section (Muê, Eastern Turkey). Micropal. 2010, 56, 465–493. [Google Scholar] [CrossRef]
- van Buchem, F.S.P.; Allan, T.L.; Laursen, G.V.; Lotfpour, M.; Moallemi, A.; Monibi, S.; Motiei, H.; Pickard, N.A.H.; Tahmasbi, A.R.; Vedrenne, V.; et al. Regional stratigraphic architecture and reservoir types of the Oligo-Miocene deposits in the Dezful Embayment (Asmari and Pabdeh Formations) SW Iran. Geol. Soc. Lond. Spec. Pubs. 2010, 329, 219–263. [Google Scholar] [CrossRef]
- Ferràndez Cañadell, C.; Bover-Arnal, T. Late Chattian larger foraminifera from the prebetic domain (SE Spain): New data on Shallow Benthic Zone 23. Palaios 2017, 32, 83–109. [Google Scholar] [CrossRef]
- BouDagher-Fadel, M.K. Evolution and Geological Significance of Larger Benthic Foraminifera; University College London Press: London, UK, 2018; p. 693. [Google Scholar]
- Dill, M.A.; Vaziri-Moghaddam, H.; Seyrafian, A.; Behdad, A.; Shabafrooz, R. A review of the Oligo–Miocene larger benthic foraminifera in the Zagros basin, Iran; New insights into biozonation and palaeogeographical maps. Rev. Micropal. 2020, 66, 100408. [Google Scholar] [CrossRef]
- Benedetti, A.; Marino, M.; Pichezzi, R.M. Paleocene to lower Eocene larger foraminiferal assemblages from Central Italy: New remarks on biostratigraphy. Riv. Ital. Paleontol. Stratigr. 2018, 124, 73–90. [Google Scholar]
- Ali, M.; Coletti, G.; Mariani, L.; Benedetti, A.; Munawar, M.J.; Rehman, S.U.; Sternai, P.; Basso, D.; Malinverno, E.; Shahzad, K.; et al. Shallow-water carbonate facies herald the onset of the palaeocene-eocene thermal maximum (Hazara Basin, northern Pakistan). J. Asian Earth Sci. X 2024, 11, 100169. [Google Scholar] [CrossRef]
- Flügel, E. Microfacies of Carbonate Rocks, 2nd ed.; Springer: Berlin/Heidelberg, Germany, 2010; p. 976. [Google Scholar]
- Coletti, G.; Balmer, E.M.; Bialik, O.M.; Cannings, T.; Kroon, D.; Robertson, A.H.; Basso, D. Microfacies evidence for the evolution of Miocene coral-reef environments in Cyprus. Palaeogeogr. Palaeoclim. Palaeoecol. 2021, 584, 110670. [Google Scholar] [CrossRef]
- Ali, M.; Coletti, G.; Garzanti, E.; Adatte, T.; Castelltort, S.; Sternai, P.; Benedetti, A.; Malinverno, E.; Mariani, L.; Spangenberg, J.; et al. The Baroch Nala section (NE Pakistan): A new PETM standard for the eastern Tethys. Mar. Petrol. Geol. 2025, 171, 107183. [Google Scholar] [CrossRef]
- Coletti, G.; Mariani, L.; Garzanti, E.; Consani, S.; Bosio, G.; Vezzoli, G.; Hu, X.; Basso, D. Skeletal assemblages and terrigenous input in the Eocene carbonate systems of the Nummulitic Limestone (NW Europe). Sed. Geol. 2021, 425, 106005. [Google Scholar] [CrossRef]
- Mariani, L.; Coletti, G.; Bosio, G.; Mateu Vicens, G.; Ali, M.; Cavallo, A.; Mittempergher, S.; Malinverno, E. Tectonically-controlled biofacies distribution in the Eocene Foraminiferal Limestone (Pag, Croatia): A quantitative-based palaeontological analysis. Sed. Geol. 2024, 472, 106743. [Google Scholar] [CrossRef]
- Posit team. RStudio: Integrated Development Environment for R. Posit Software; PBC: Boston, MA, USA, 2024; Available online: http://www.posit.co/ (accessed on 1 April 2024).
- Murray, J.W. Ecology and Applications of Benthic Foraminifera; Cambridge University Press: Cambridge, UK, 2006; p. 426. [Google Scholar]
- Bosellini, F.R. The scleractinian genus Hydnophora (revision of Tertiary species). Paläontol. Zeit. 1999, 73, 217–240. [Google Scholar] [CrossRef]
- Schuster, F. Scleractinian corals from the Oligocene of the Qom Formation (Esfahan-Sirjan fore-arc basin, Iran), Cour. Forsch.-Inst. Senckenberg 2002, 239, 5–55. [Google Scholar]
- Schuster, F. Early Miocene scleractinian corals from the Qom and Asmari formations (central and southwest Iran). Cour. Forsch.-Inst. Senckenberg 2002, 239, 129–161. [Google Scholar]
- Budd, A.F.; Bosellini, F.R. Revision of Oligocene Mediterranean meandroid corals in the scleractinian families Mussidae, Merulinidae, and Lobophylliidae. J. System. Palaeont. 2016, 14, 771–798. [Google Scholar] [CrossRef]
- Bosellini, F.R.; Vescogni, A.; Budd, A.F.; Papazzoni, C.A. High coral diversity is coupled with reef-building capacity during the Late Oligocene Warming (Castro Limestone, Salento Peninsula, S. Italy). Riv. Ital. Paleontol. Stratigr. 2021, 127, 515–538. [Google Scholar]
- Serra-Kiel, J.; Hottinger, L.; Caus, E.; Drobne, K.; Ferrandez, C.; Jauhri, A.K.; Less, G.; Pavlovec, R.; Pignatti, J.; Samso, J.M.; et al. Larger forminiferal biostratigraphy on the Tethyan Paleocene and Eocene. Bulletin Societé Géologique France 1998, 2, 281–299. [Google Scholar]
- Pignatti, J.; Papazzoni, C.A. Oppelzones and their heritage in current larger foraminiferal biostratigraphy. Lethaia 2017, 50, 369–380. [Google Scholar]
- Papazzoni, C.A.; Fornaciari, B.; Giusberti, L.; Simonato, M.; Fornaciari, E. A new definition of the Paleocene Shallow Benthic Zones (SBP) by means of larger foraminiferal biohorizons, and their calibration with calcareous nannofossil biostratigraphy. Micropaleontology 2023, 69, 369–405. [Google Scholar]
- Özcan, E.; Less, G. First record of the co-occurrence of western Tethyan and Indo-Pacific larger foraminifera in the Burdigalian of the Mediterranean province. J. Foram. Res. 2009, 39, 23–39. [Google Scholar]
- BouDagher-Fadel, M.K.; David Price, G. The phylogenetic and palaeogeographic evolution of the miogypsinid larger benthic foraminifera. J. Geol. Soc. 2013, 170, 185–208. [Google Scholar]
- Coletti, G.; Stainbank, S.; Fabbrini, A.; Spezzaferri, S.; Foubert, A.; Kroon, D.; Betzler, C. Biostratigraphy of large benthic foraminifera from Hole U1468A (Maldives): A CT-scan taxonomic approach. Swiss J. Geosci. 2018, 111, 523–536. [Google Scholar]
- Cahuzac, B.; Poignant, A. Essai de biozonation de l’Oligo-Miocène dans les bassins européens à l’aide des grands foraminifères néritiques. Bull. Soc. Géol. France 1997, 168, 155–169. [Google Scholar]
- Kumar, A.; Saraswati, P.K. Response of larger foraminifera to mixed carbonate-siliciclastic environments: An example from the Oligocene-Miocene sequence of Kutch, India. Palaeogeogr. Palaeoclimatol. Palaeoecol. 1997, 136, 53–65. [Google Scholar]
- Less, G.; Frijia, G.; Özcan, E.; Saraswati, P.K.; Parente, M.; Kumar, P. Nummulitids, lepidocyclinids and Sr-isotope data from the Oligocene of Kutch (western India) with chronostratigraphic and paleobiogeographic evaluations. Geodinamica Acta 2018, 30, 183–211. [Google Scholar]
- Özcan, E.; Less, G.; Baydogan, E. Regional implications of biometric analysis of Lower Miocene larger foraminifera from Central Turkey. Micropaleontology 2009, 55, 559–588. [Google Scholar]
- Langer, M.R. Epiphytic foraminifera. Mar. Micropal. 1993, 20, 235–265. [Google Scholar]
- Mateu-Vicens, G.; Khokhlova, A.; Sebastián-Pastor, T. Epiphytic foraminiferal índices as bioindicators in Mediterranean seagrass meadows. J. Foram. Res. 2014, 44, 325–339. [Google Scholar] [CrossRef]
- Mariani, L.; Coletti, G.; Bosio, G.; Tentorio, C.; Mateu Vicens, G.; Bracchi, V.A.; Basso, D.; Malinverno, E. Benthic foraminifera as proxy for fossil seagrass from the Lower Pleistocene deposits of the Stirone River (Emilia-Romagna, Italy). Quat. Int. 2022, 640, 73–87. [Google Scholar] [CrossRef]
- Zachos, J.; Pagani, M.; Sloan, L.; Thomas, E.; Billups, K. Trends, Rhythms, and Aberrations in Global Climate 65 Ma to Present. Science 2011, 292, 686. [Google Scholar] [CrossRef]
- Zhang, Y.G.; Pagani, M.; Liu, Z.; Bohaty, S.M.; DeConto, R. A 40-million-year history of atmospheric CO2. Phil. Trans. Royal Soc. A 2013, 371, 20130096. [Google Scholar] [CrossRef]
- Wu, F.; Miao, Y.; Meng, Q.; Fang, X.; Sun, J. Late Oligocene Tibetan Plateau Warming and Humidity: Evidence from a Sporopollen Record. Geochem. Geophys. Geosyst. 2018, 20, 434–441. [Google Scholar] [CrossRef]
- Villa, G.; Persico, D. Late Oligocene climatic changes: Evidence from calcareous nannofossils at Kerguelen Plateau Site 784 (Southern Ocean). Paleogeogr. Paleoclimat. Paleoecol. 2006, 231, 110–119. [Google Scholar] [CrossRef]
- Alegret, L.; Cruz, L.E.; Fenero, R.; Molina, E.; Ortiz, S.; Thomas, E. Effects of the Oligocene climatic events on the foraminiferal record from Fuente Caldera section (Spain, western Tethys). Palaeog. Palaeoclim. Palaeoecol. 2008, 269, 94–102. [Google Scholar] [CrossRef]
- Bover-Arnal, T.; Ferràndez-Cañadell, C.; Aguirre, J.; Esteban, M.; Fernández-Carmona, J.; Albert-Villanueva, E.; Salas, R. Late Chattian platform carbonates from the SE Iberian Plate: An interglacial record from the western Tethys. Palaios 2017, 32, 61–82. [Google Scholar]
- Villa, G.; Florindo, F.; Persico, D.; Lurcock, P.; de Martini, A.P.; Jovane, L.; Fioroni, C. Integrated calcareous nannofossil and magnetostratigraphic record of ODP Site 709: Middle Eocene to late Oligocene paleoclimate and paleoceanography of the Equatorial Indian Ocean. Mar. Micropal. 2021, 169, 102051. [Google Scholar] [CrossRef]
- Bosellini, F.R.; Vescogni, A.; Briguglio, A.; Piazza, M.; Papazzoni, C.A.; Silvestri, G.; Morsilli, M. Resilient coral reef ecosystems: The case study of turbid-mesophotic coral buildups during the Late Oligocene Warming Event (Tertiary Piedmont Basin, NW Italy). Palaeogeogr. Palaeoclimatol. Palaeoecol. 2024, 649, 112330. [Google Scholar] [CrossRef]
- Frost, S.H. Oligocene Reef Coral Biofacies of the Vicentin, Northeast Italy. Eur. Foss. Reef Models 1981. [Google Scholar] [CrossRef]
- Budd, A.F. Diversity and extinction in the Cenozoic history of Caribbean reefs. Coral Reefs 2000, 19, 25–35. [Google Scholar] [CrossRef]
- Perrin, C. Tertiary: The emergence of modern reef ecosystems. In Phanerozoic Reef Patterns; Kiessling, W., Flügel, E., Golonka, J., Eds.; SEPM: Tulsa, OK, USA, 2002. [Google Scholar] [CrossRef]
- Hallock, P. Reefs and reef limestones in Earth history. In Life and Death of Coral Reefs; Birkeland, C., Ed.; Chapman and Hall: New York, NY, USA, 1997; pp. 13–42. [Google Scholar]
- Stanley, S.M. Influence of seawater chemistry on biomineralization throughout Phanerozoic time: Paleontological and experimental evidence. Palaeogeogr. Palaeoclim. Palaeoecol. 2006, 232, 214–236. [Google Scholar] [CrossRef]
- Sirel, E.; Özgen-Erdem, N.; Kangal, Ö. Systematics and biostratigraphy of Oligocene (Rupelian-Early Chattian) foraminifera from lagoonal-very shallow water limestone in the eastern Sivas Basin (central Turkey). Geol. Croat. 2013, 66, 83–109. [Google Scholar] [CrossRef]
- Gedik, F. Benthic foraminiferal fauna of Malatya Oligo-Miocene basin, (eastern Taurids, eastern Turkey). Bull. Min. Res. Expl. 2014, 149, 93–136. [Google Scholar] [CrossRef][Green Version]
- Amirshahkarami, M. Microfacies correlation analysis of the Oligocene-Miocene Asmari Formation, in the central part of the Rag-e-Safid anticlinal oil field, Zagros Basin, south-west Iran. Turk. J. Earth Sci. 2013, 22, 204–219. [Google Scholar] [CrossRef]
- Mattern, F.; Al-Sayigh, A.R.; Farfour, M.; Scharf, A.; Al-Amri, S.; Al-Omairi, J. Microfacies, Biostratigraphy, Depositional Environment, Seismic Refraction and Correlation of Coralline Limestones of the Barzaman Formation (Oligocene-Pliocene? Al-Khod, Muscat Area, Sultanate of Oman). SQU J. Sci. 2020, 25, 85–99. [Google Scholar]
- Afzal, J.; Racey, A. Early Miocene larger foraminifera from Suwadi Island, northern Oman. In Geology, Tectonics and Natural Resources of Arabia and its Surroundings; Geological Society: London, UK, 2025; p. 550. [Google Scholar] [CrossRef]
- Reuter, M.; Piller, W.E.; Harzhauser, M.; Mandic, O.; Berning, B.; Rögl, F.; Kroh, A.; Aubry, M.P.; Wielandt-Schuster, U.; Hamedani, A. The Oligo-/Miocene Qom Formation (Iran): Evidence for an early Burdigalian restriction of the Tethyan Seaway and closure of its Iranian gateways. Int. J. Earth Sci. 2009, 98, 627–650. [Google Scholar] [CrossRef]
- Kureshy, A.A. Large Tertiary Foraminiferal Biostratigraphy, Khirtar Province, Sind, Pakistan: ABSTRACT. AAPG Bull. 1982, 66, 591–592. [Google Scholar] [CrossRef]
- Kureshy, A.A. The Oligocene Foraminiferal Biostratigraphy of Pakistan. Riv. It. Paleont. Strat. 1984, 89, 395–406. [Google Scholar]
- Hallock, P.; Glenn, E.C. Larger foraminifera: A tool for paleoenvironmental analysis of Cenozoic carbonate depositional facies. Palaios 1986, 1, 55–64. [Google Scholar] [CrossRef]
- Beavington-Penny, S.J.; Racey, A. Ecology of extant nummulitids and other LBF: Application in palaeoenvironmental analysis. Earth Sci. Rev. 2004, 67, 219–265. [Google Scholar] [CrossRef]
- van der Zwaan, G.J.; Jorissen, F.J.; de Stigter, H.C. The depth dependency of planktonic/benthic foraminiferal ratios: Constraints and applications. Mar. Geol. 1990, 95, 1–16. [Google Scholar]
- Hohenegger, J. Depth estimation by proportions of living larger foraminifera. Mar. Micropal. 1995, 26, 31–47. [Google Scholar]
- Hohenegger, J. Coenoclines of larger foraminifera. Micropaleontology 2000, 46, 127–151. [Google Scholar]
- Hohenegger, J.; Yordanova, E.; Hatta, A. Remarks on west Pacific Nummulitidae (foraminifera). J. Foram. Res. 2000, 30, 3–28. [Google Scholar]
- Yamano, H.; Kayanne, H.; Matsuda, F.; Tsuji, Y. Lagoonal facies, ages, and sedimentation in three atolls in the Pacific. Mar. Geol. 2002, 185, 233–247. [Google Scholar]
- Renema, W. Larger foraminifera on reefs around Bali (Indonesia). Zool. Verh. 2003, 345, 337–366. [Google Scholar]
- Renema, W. Large benthic foraminifera from the deep photic zone of a mixed siliciclastic-carbonate shelf off East Kalimantan, Indonesia. Mar. Micropal. 2006, 58, 73–82. [Google Scholar]
- Ernst, S.; Janse, M.; Renema, W.; Kouwenhoven, T.; Goudeau, M.L.; Reichart, G.J. Benthic foraminifera in a large Indo-Pacific coral reef aquarium. J. Foram. Res. 2011, 41, 101–113. [Google Scholar]
- Goeting, S.; Briguglio, A.; Eder, W.; Hohenegger, J.; Roslim, A.; Kocsis, L. Depth distribution of modern larger benthic foraminifera offshore Brunei Darussalam. Micropaleontology 2018, 64, 299–316. [Google Scholar]
- Oron, S.; Friedlander, A.M.; Sala, E.; Goodman-Tchernov, B.N. Shallow water foraminifera from Niue and Beveridge Reef (South Pacific): Insights into ecological significance and ecosystem integrity. R. Soc. Open Sci. 2024, 11, 230997. [Google Scholar] [PubMed]
- Tomassetti, L.; Bosellini, F.R.; Brandano, M. Growth and demise of a Burdigalian coral bioconstruction on a granite rocky substrate (Bonifacio Basin, South Corsica). Facies 2013, 59, 703–716. [Google Scholar]
- Briguglio, A.; Vannucci, G.; Bruzzone, C.; Piazza, M. Stratigraphic development of a Late Oligocene Reef Complex under strong fluviatile influence in the Tertiary Piedmont Basin (Liguria, NW Italy). Micropaleontology 2021, 67, 315–339. [Google Scholar]
- Saint Martin, J.P.; Chaix, C.; Cahuzac, B.; Moissette, P.; André, J.P. Les faunes coralliennes de l’Oligocène de Malte: Biodiversité et paléoenvironnement. Ann. Paléontol. 2021, 107, 102508. [Google Scholar]
- McCall, J.; Rosen, B.; Darrell, J. Carbonate deposition in accretionary prism settings: Early Miocene coral limestones and corals of the Makran Mountain Range in southern Iran. Facies 1994, 31, 141–178. [Google Scholar]
- Yazdi, M.; Shirazi, M.P.; Rahiminejad, A.H.; Motavalipoor, R. Paleobathymetry and paleoecology of colonial corals from the Oligocene-early Miocene (?) Qom Formation (Dizlu area, central Iran). Carbonates Evaporites 2021, 27, 395–405. [Google Scholar]
- Rahiminejad, A.H.; Yazdi, M.; Ashouri, A.R. Miocene scleractinian corals from a mixed siliciclastic-carbonate system: Bakhtiari succession, Zagros Basin (central-western Iran). Alcheringa 2011, 35, 571–592. [Google Scholar]
- Mohammadi, E.; Ghaedi, M. Diversity and paleoecological significance of zooxanthellate corals of Oligocene Qom Formation, SE Iran. Earth Sci. Res. J. 2024, 28, 127–137. [Google Scholar] [CrossRef]
- Wallace, C.C. Staghorn Corals of the World; a Revision of the Genus Acropora; CSIRO Press: Melbourne, Australia, 1999; p. 422. [Google Scholar]
- Schuster, F. Oligocene and Miocene examples of Acropora-dominated palaeoenvironments: Mesohellenic Basin (NW Greece) and northern Gulf of Suez (Egypt). In Proceedings of the 9th International Coral Reef Symposium, Bali, Indonesia, 23–27 October 2000; pp. 199–204. [Google Scholar]
- El-Azabi, M.H. Sedimentary facies and stratal architecture of the Middle Eocene Acropora-dominated succession in a storm-influenced ramp system, El-Ramliya area, north Eastern Desert, Egypt. Sed. Geol. 2023, 447, 106368. [Google Scholar] [CrossRef]
- Stolarski, J.; Bosellini, F.R.; Wallace, C.C.; Gothmann, A.; Mazur, M.; Domart-Coulon, I.; Gutner-Hoch, E.; Neuser, R.D.; Levy, O.; Shemesh, A.; et al. A unique coral biomineralization pattern has resisted 40 million years of major ocean chemistry change. Sci. Rep. 2016, 6, 27579. [Google Scholar] [CrossRef]
- Mohanti, M.; Srivastava, S.C. Oligocene reefal environment of Kutch Basin (Western India) with implications of the Mediterranean connection. Géologie Méditerranéenne 1994, 21, 3–4. [Google Scholar] [CrossRef]
- Hallock, P.; Hansen, H.J. Depth adaptation in Amphistegina: Change in· lamellar thickness. Bull. Geol. Soc. Den. 1978, 27, 99–104. [Google Scholar]
- Ćosović, V.; Drobne, K.; Moro, A. Paleoenvironmental model for Eocene foraminiferal limestones of the Adriatic Carbonate Platform (Istrian Peninsula). Facies 2004, 50, 61–75. [Google Scholar] [CrossRef]
- Baceta, J.I.; Mateu-Vicens, G. Seagrass development in terrigenous-influenced inner ramp settings during the middle Eocene (Urbasa-Andia Plateau, Western Pyrenees, North Spain). Sedimentology 2021, 69, 301–344. [Google Scholar] [CrossRef]
- Coletti, G.; Basso, D. Coralline algae as depth indicators in the Miocene carbonates of the Eratosthenes Seamount (ODP Leg 160, Hole 966F). Geobios 2020, 60, 29–46. [Google Scholar] [CrossRef]
- Hottinger, L. Shallow benthic foraminiferal assemblages as signals for depth of their deposition and their limitations. Bull. Société Géologique Fr. 1997, 168, 491–505. [Google Scholar]
- Brandano, M.; Cornacchia, I.; Raffi, I.; Tomassetti, L. The Oligocene-Miocene stratigraphic evolution of the Majella carbonate platform (Central Apennines, Italy). Sed. Geol. 2016, 333, 1–14. [Google Scholar] [CrossRef]
- Reich, S.; Di Martino, E.; Todd, J.A.; Wesselingh, F.P.; Renema, W. Indirect paleo-seagrass indicators (IPSIs): A review. Earth Sci. Rev. 2015, 143, 161–186. [Google Scholar] [CrossRef]
- Bilal, M.; Khan, A. Geotechnical Evaluation of Limestones from Cape Monze and Adjoining Areas, Karachi, Pakistan for Their Utilization as Road Aggregate. Int. J. Econ. Envir. Geol. 2019, 10, 1–8. [Google Scholar]
- Hakro, A.A.; Samtio, M.S.; Rajper, R.H.; Mastoi, A.S. Major Elements of Nari Formation Sandstone from Jungshahi Area of Southern Indus Basin, Pakistan. Pak. J. Sci. Ind. Res. Ser. A Phys. Sci. 2022, 65, 248–259. [Google Scholar]
- Karevan, M.; Vaziri-Moghaddam, H.; Mahboubi, A.; Moussavi-Harami, R. Biostratigraphy and paleo-ecological reconstruction on Scleractinian reef corals of Rupelian-Chattian succession (Qom Formation) in northeast of Delijan area. JGeope 2014, 4, 11–24. [Google Scholar]

| BFA | |||||
|---|---|---|---|---|---|
| Biofacies | BFA1 | BFA2 | BFB | BFC1 | BFC2 |
| CC | 99% | 62% | 0.0% | 1.1% | 0.23% |
| Green calcareous algae | 0.1% | 0.1% | 0.0% | 0.02% | 0.8% |
| RCA encrusting | 0.3% | 15.1% | 77.1% | 29.8% | 24.1% |
| RCA articulated | 0.0% | 0.8% | 0.0% | 2.4% | 4.3% |
| LBF miliolids | 0.0% | 3.5% | 0.0% | 2.2% | 3.4% |
| SBF miliolids | 0.02% | 0.5% | 0.1% | 0.9% | 2.8% |
| Miogypsinids | 0.1% | 6.3% | 4.2% | 25.4% | 20.6% |
| Lepidocyclinids | 0.0% | 0.0% | 6.1% | 5.4% | 4.3% |
| Amphisteginids | 0.0% | 0.7% | 0.4% | 0.6% | 1% |
| LBF hyaline low T/D | 0.0% | 0.3% | 5.8% | 14.3% | 0.9% |
| LBF hyaline others | 0.0% | 0.2% | 1.2% | 0.8% | 2.8% |
| SBF hyaline | 0.2% | 1.3% | 0.9% | 1.5% | 6.8% |
| Encrusting benthic foraminifera | 0.1% | 3.9% | 2.3% | 1% | 1.7% |
| SBF agglutinated | 0.0% | 0.1% | 0.04% | 0.1% | 1.2% |
| Planktic foraminifera | 0.0% | 0.0% | 0.0% | 0.0% | 0.1% |
| Mollusks | 0.3% | 1.6% | 0.2% | 7.5% | 3.7% |
| Bryozoans | 0.02% | 1.6% | 0.3% | 4.0% | 9.3% |
| Echinoderms | 0.0% | 1.9% | 1.4% | 3.2% | 11.2% |
| Arthropods | 0.0% | 0.1% | 0.0% | 0.0% | 0.4% |
| Serpulids | 0.0% | 0.0% | 0.0% | 0.02% | 0.04% |
| Ostracods | 0.01% | 0.1% | 0.02% | 0.0% | 0.5% |
| Biofacies | BFA | BFB | BFC1 | BFC2 |
|---|---|---|---|---|
| Soritids and peneroplids | 7.38% | 0% | 2.59% | 2.85% |
| Austrotrillina | 1.24% | 0% | 1.37% | 1.29% |
| SBF porcelaneous (small miliolids) | 12.57% | 1.81% | 7.36% | 10.41% |
| Miogypsinids | 11.31% | 26.38% | 45.29% | 30.65% |
| Lepidocyclinids | 0.18% | 5.94% | 4.80% | 0.87% |
| Spiroclypeus | 0% | 12.27% | 4.61% | 0% |
| Operculina and Heterostegina | 0.43% | 2.31% | 6.71% | 2.18% |
| Hyaline LBF (others) | 0.97% | 7.15% | 2.69% | 2.89% |
| Amphisteginids | 0.72% | 6.22% | 3.02% | 1.85% |
| Planorbulinids | 0.14% | 1.35% | 0.16% | 0.55% |
| Rounded acervulinids | 0% | 0.77% | 0.32% | 0.03% |
| SBF hyaline | 62.39% | 30.73% | 18.18% | 37.71% |
| SBF agglutinated | 2.13% | 5.07% | 2.76% | 8.35% |
| Planktic foraminifera | 0.44% | 0% | 0.10% | 0.21% |
| Calculated parameters | ||||
| LEPI/MIO | 0.02 | 0.23 | 0.1 | 0.03 |
| FN/MIO | 0.04 | 0.55 | 0.25 | 0.07 |
| (LEPI+FN)/MIO | 0.06 | 0.78 | 0.35 | 0.1 |
| LH/LP | 1.6 | / | 16.7 | 9.1 |
| H/P | 3.6 | 51.5 | 7.6 | 5.2 |
| Biofacies | BFA | BFB | BFC1 | BFC2 |
|---|---|---|---|---|
| Soritids and peneroplids | 1 | 0 | 0 | 1 |
| SBF porcelaneous (small miliolids) | 1 | 0 | 1 | 2 |
| SBF agglutinated | 0 | 0 | 0 | 1 |
| Miogypsinids | 1 | 2 | 8 | 5 |
| Lepidocyclinids | 0 | 0 | 1 | 0 |
| Spiroclypeus | 0 | 1 | 1 | 0 |
| Operculina and Heterostegina | 0 | 0 | 1 | 0 |
| Hyaline LBF (others) | 0 | 0 | 0 | 1 |
| Amphisteginids | 0 | 0 | 1 | 0 |
| SBF hyaline | 5 | 2 | 3 | 7 |
| TOT individuals expected | 7 | 6 | 17 | 18 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mariani, L.; Coletti, G.; Ali, M.; Iqbal, M.; Shumail, M.; Raza Hassan, H.A.; Bosellini, F.R. Quantitative Biofacies Analysis of Upper Oligocene Reef-Coral Neritic Carbonates (Southern Pakistan). Geosciences 2025, 15, 129. https://doi.org/10.3390/geosciences15040129
Mariani L, Coletti G, Ali M, Iqbal M, Shumail M, Raza Hassan HA, Bosellini FR. Quantitative Biofacies Analysis of Upper Oligocene Reef-Coral Neritic Carbonates (Southern Pakistan). Geosciences. 2025; 15(4):129. https://doi.org/10.3390/geosciences15040129
Chicago/Turabian StyleMariani, Luca, Giovanni Coletti, Mubashir Ali, Mahmood Iqbal, Muhammad Shumail, Hafiz Ahmed Raza Hassan, and Francesca R. Bosellini. 2025. "Quantitative Biofacies Analysis of Upper Oligocene Reef-Coral Neritic Carbonates (Southern Pakistan)" Geosciences 15, no. 4: 129. https://doi.org/10.3390/geosciences15040129
APA StyleMariani, L., Coletti, G., Ali, M., Iqbal, M., Shumail, M., Raza Hassan, H. A., & Bosellini, F. R. (2025). Quantitative Biofacies Analysis of Upper Oligocene Reef-Coral Neritic Carbonates (Southern Pakistan). Geosciences, 15(4), 129. https://doi.org/10.3390/geosciences15040129







