Depositional Environment as Main Controlling Factor for Low TOC Sediments in the Early Carboniferous Dawuba Formation of the Qiannan Depression
Abstract
1. Introduction
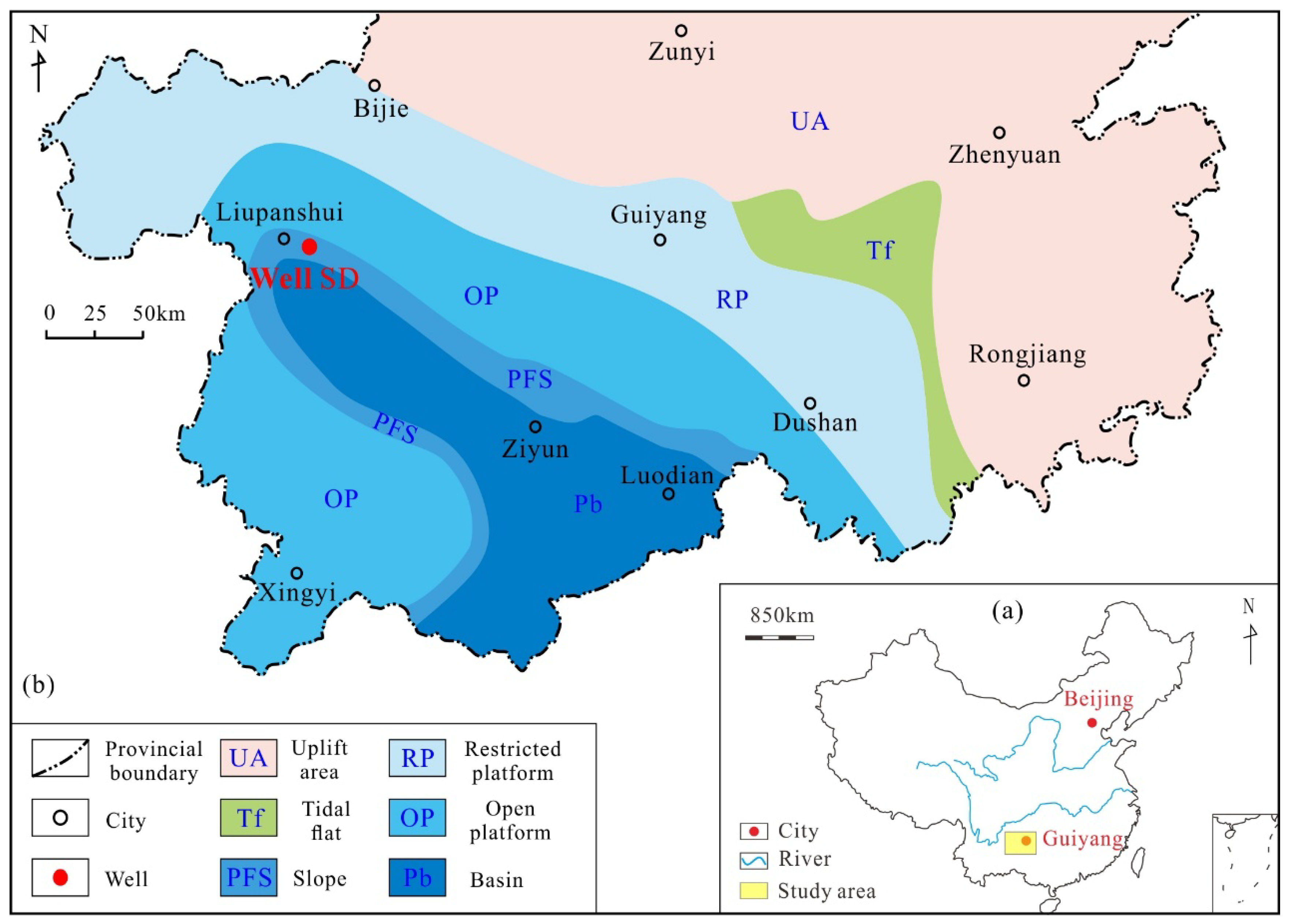
2. Geological Background
3. Samples and Methods
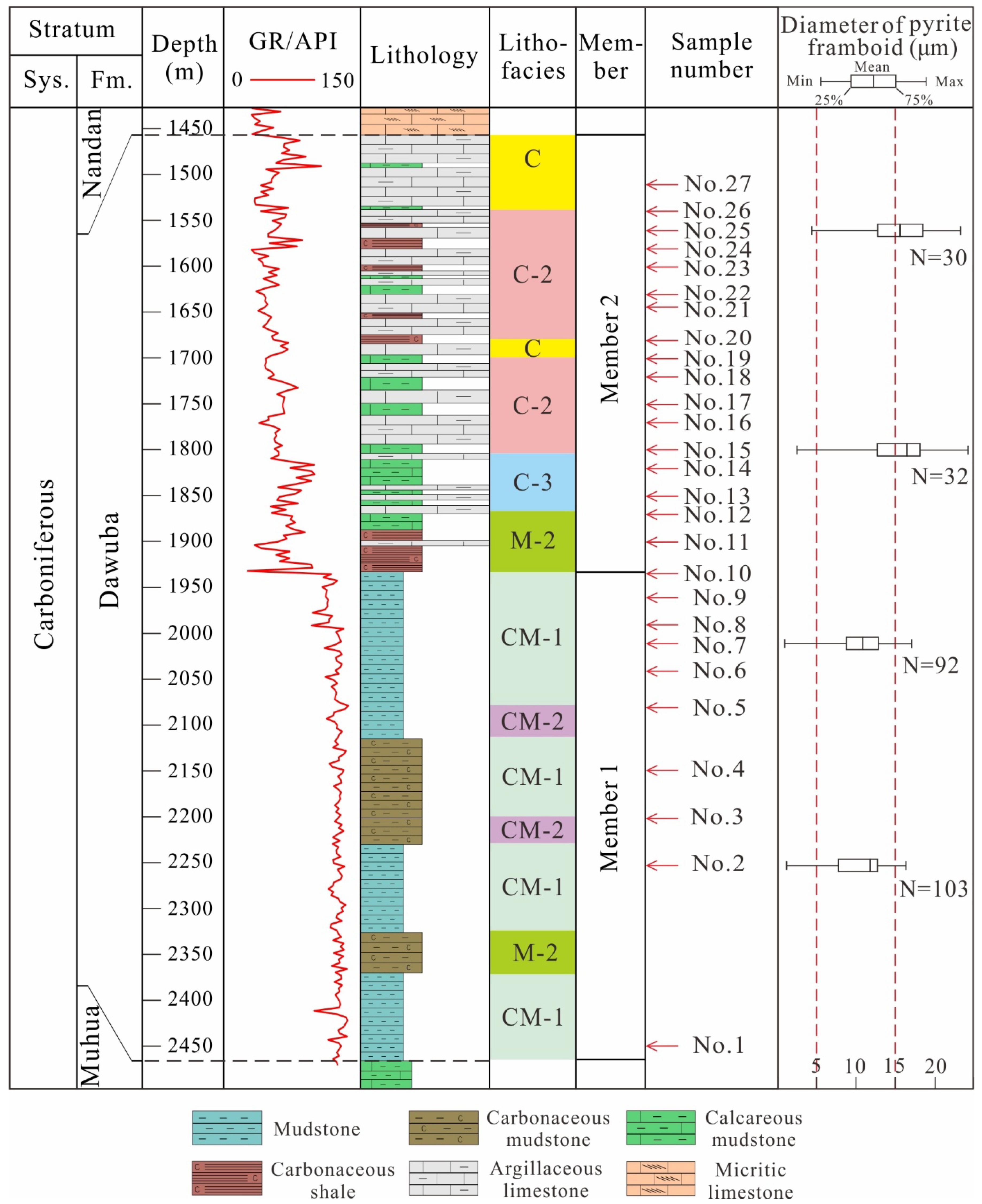
3.1. Core Samples
3.2. XRD, Thin Section Optical Microscopy and FE-SEM
3.3. TOC Content
3.4. Organic Petrography
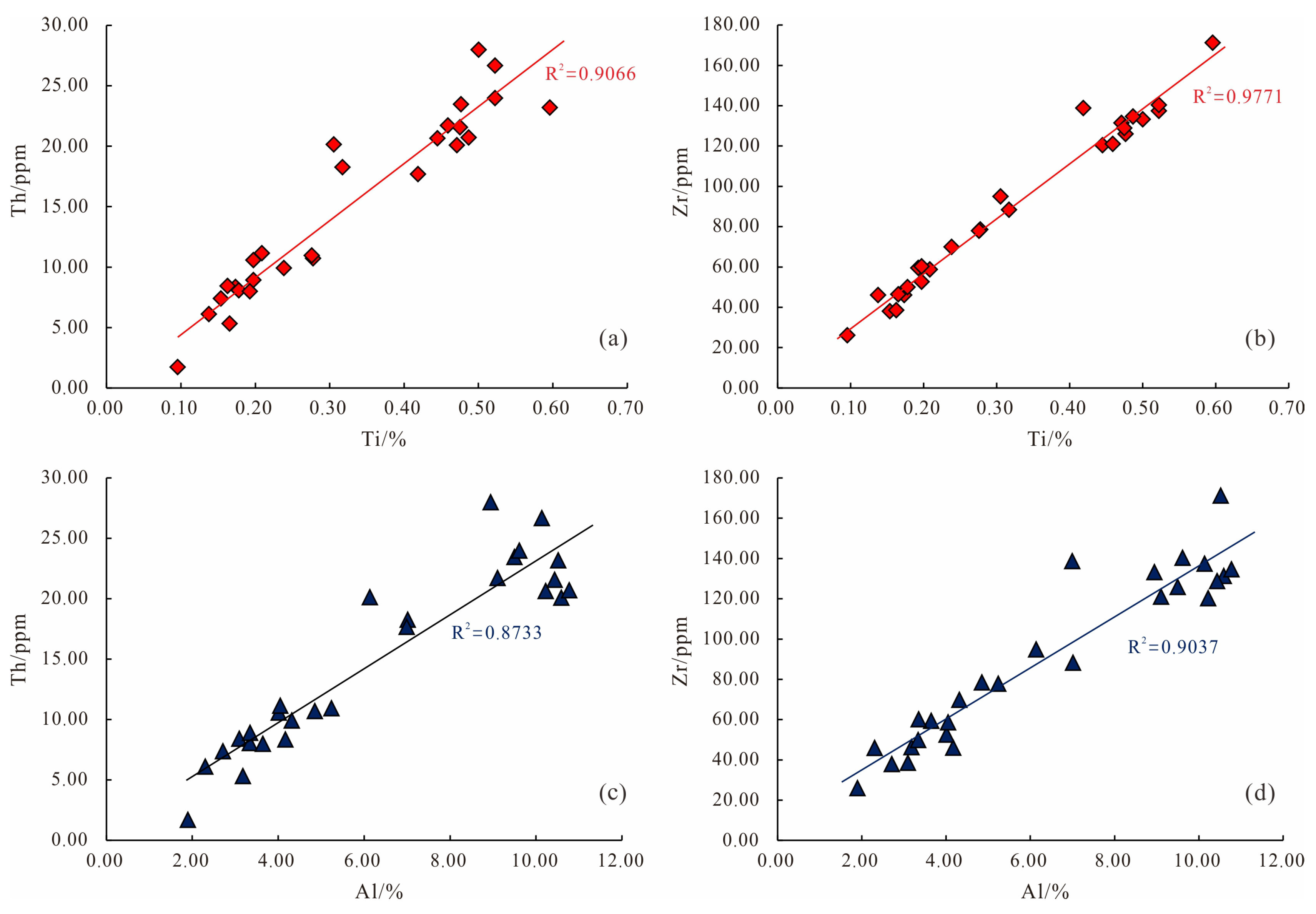
3.5. Major and Trace Elements
3.6. Data Processing
4. Results
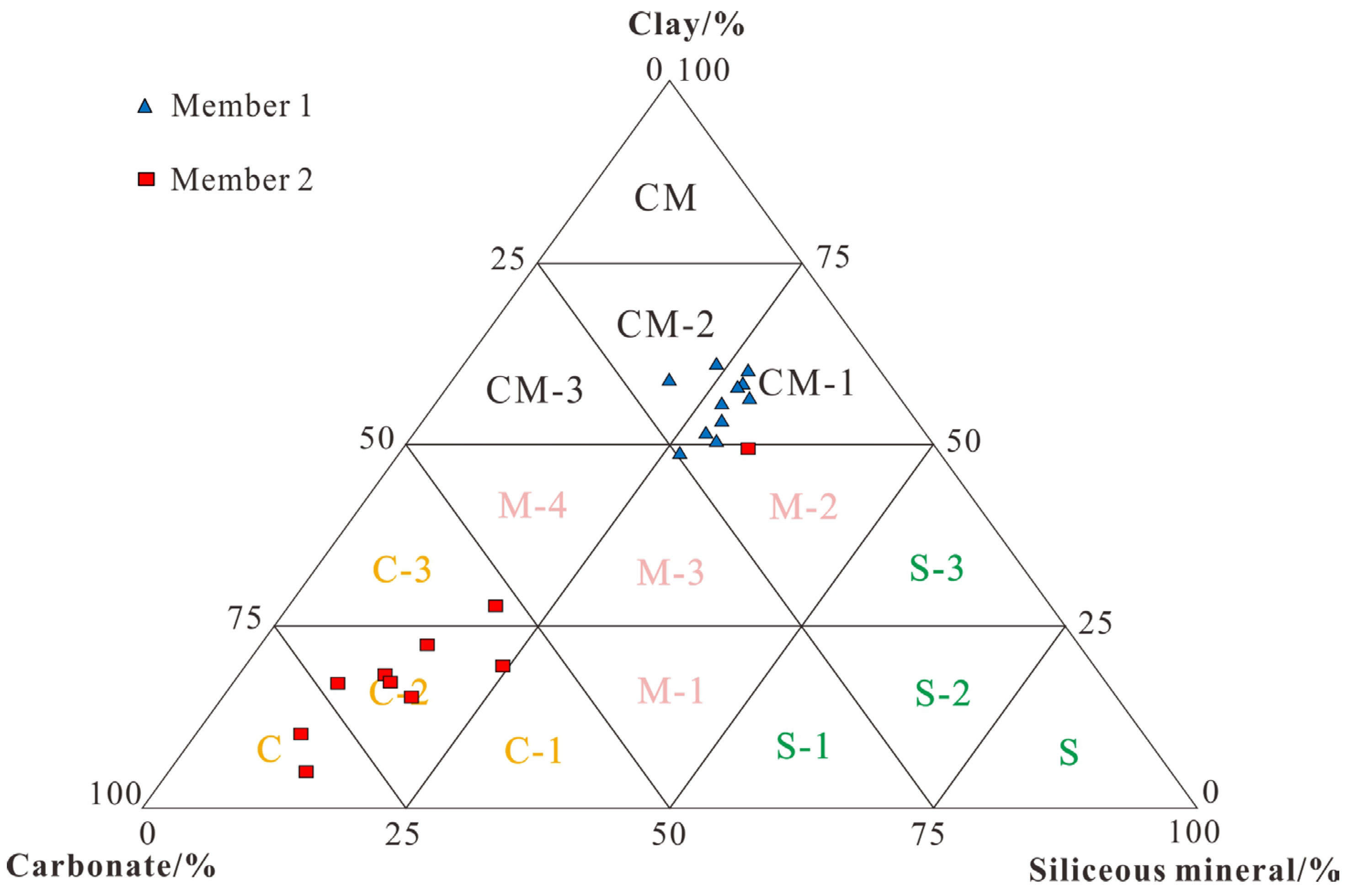
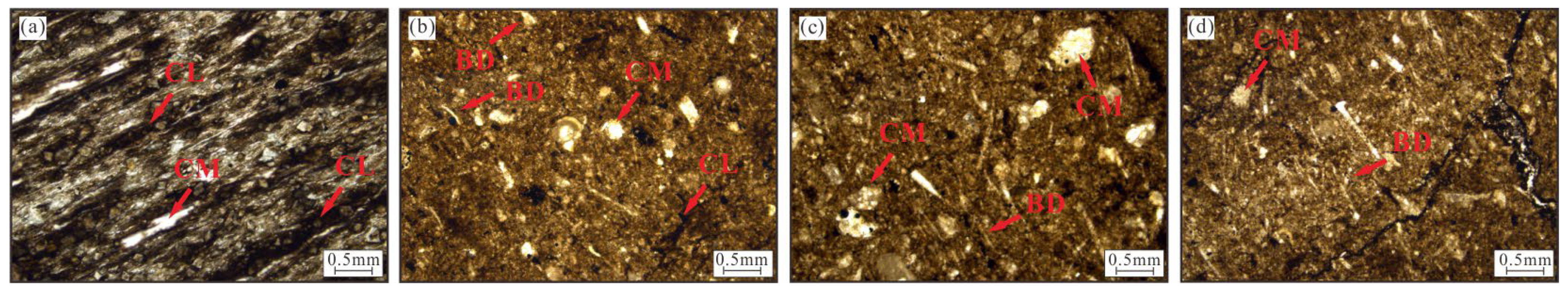
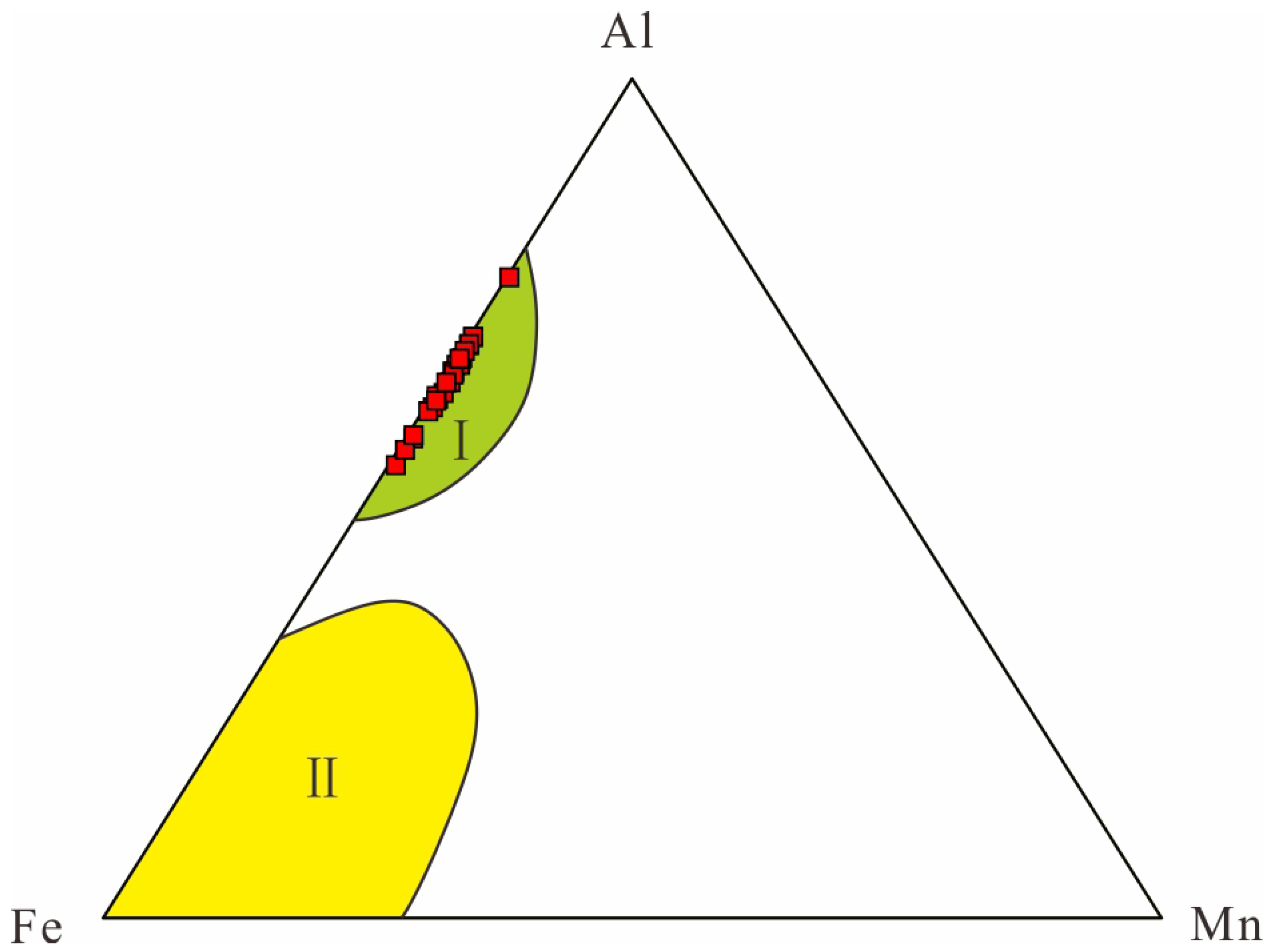
4.1. Mineral Components and Lithofacies
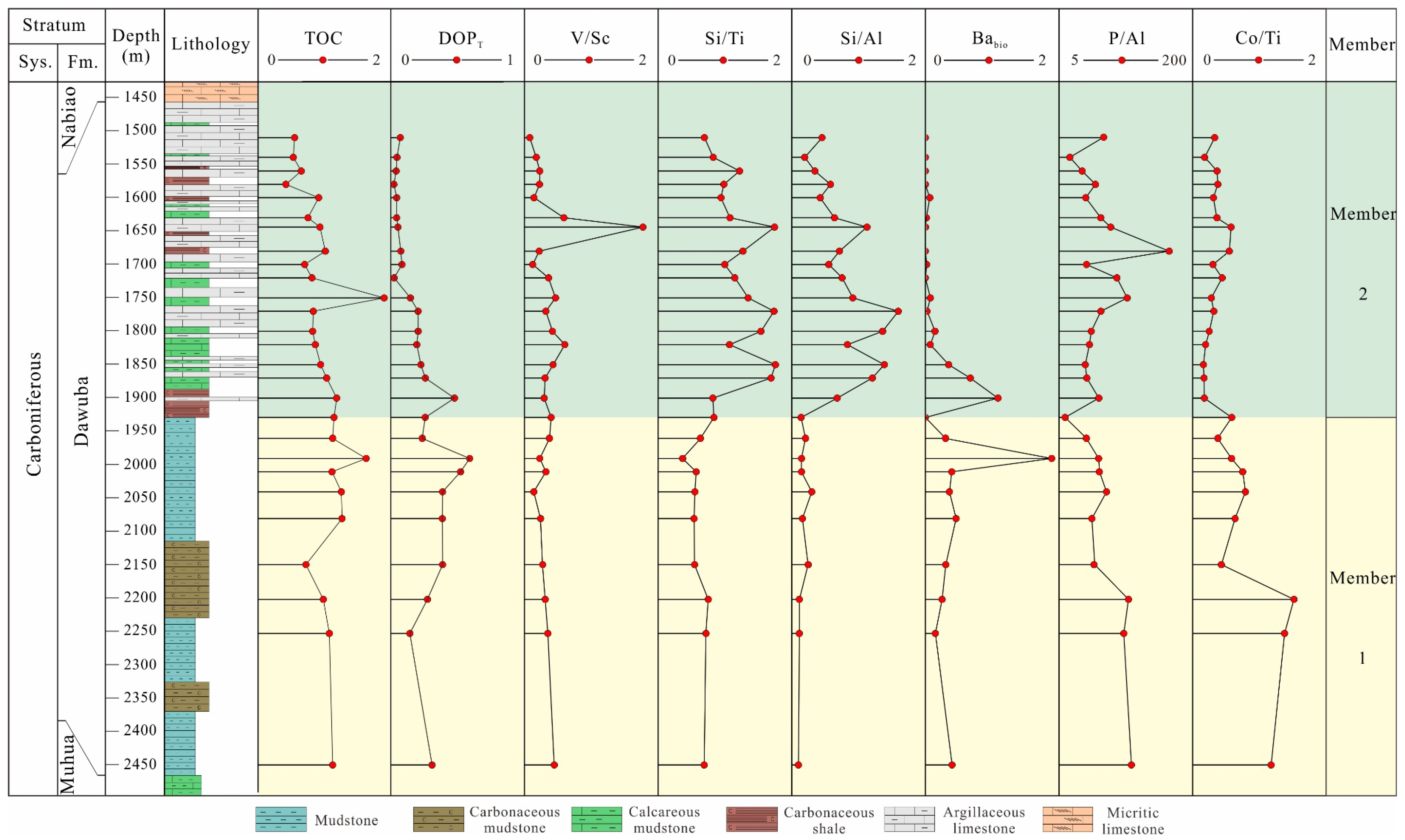
4.2. Total Organic Carbon Content
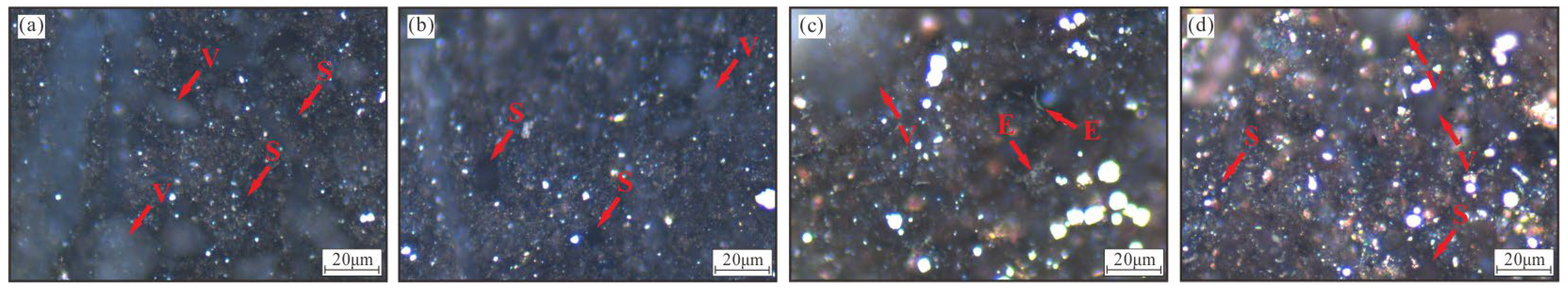
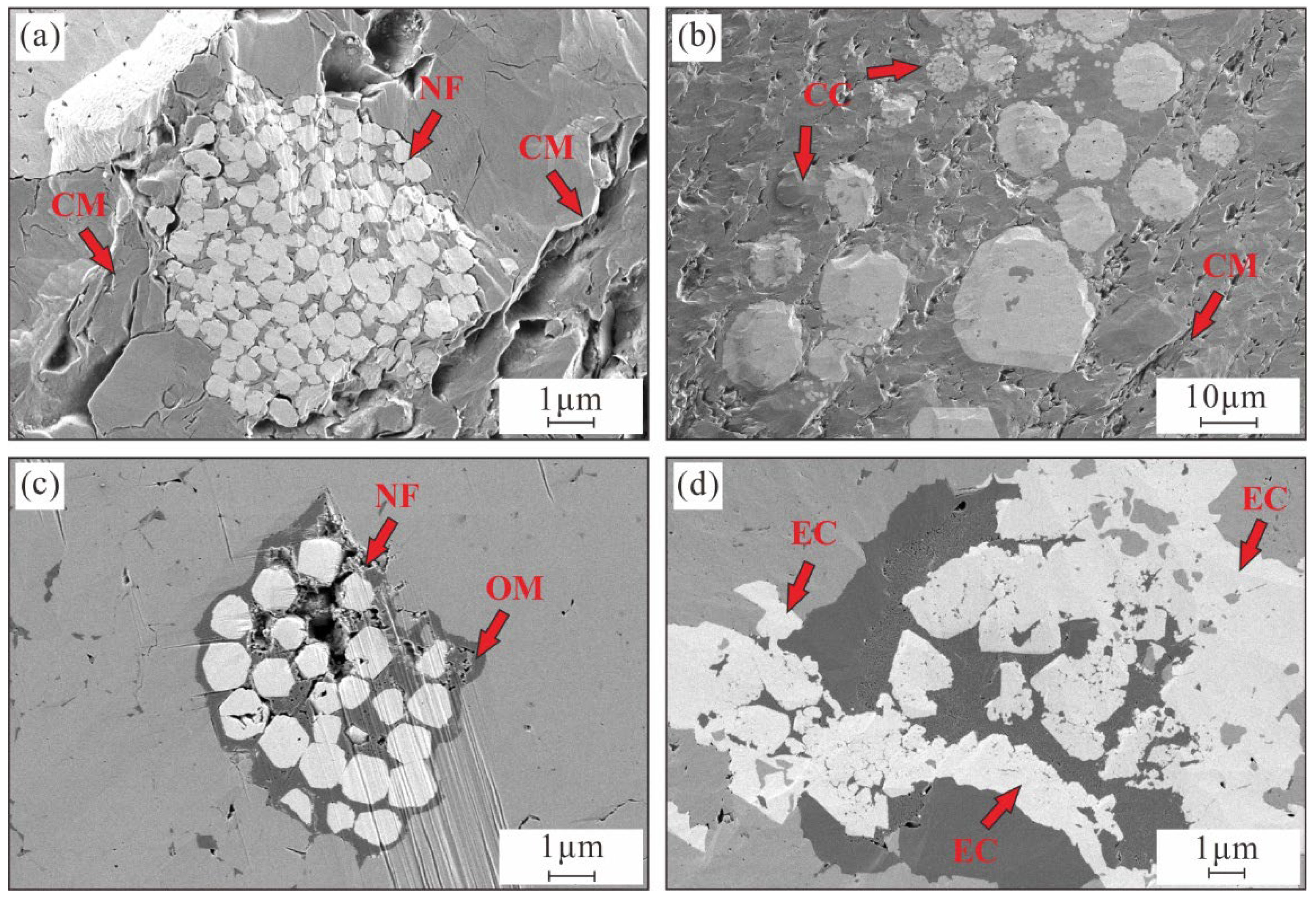
4.3. Petrographic Characteristics
4.4. Organic Matter Petrography
| Depth/m | Member | Sapropelinite | Exinite | Vitrinite | TI | Kerogen Type |
|---|---|---|---|---|---|---|
| 1755.00 | 2 | 45.00 | 0.00 | 55.00 | 3.75 | II2 |
| 1782.50 | 2 | 55.00 | 7.00 | 38.00 | 30.00 | II2 |
| 2062.00 | 1 | 70.00 | 0.00 | 30.00 | 47.50 | II1 |
| 2280.00 | 1 | 75.00 | 0.00 | 25.00 | 56.25 | II1 |
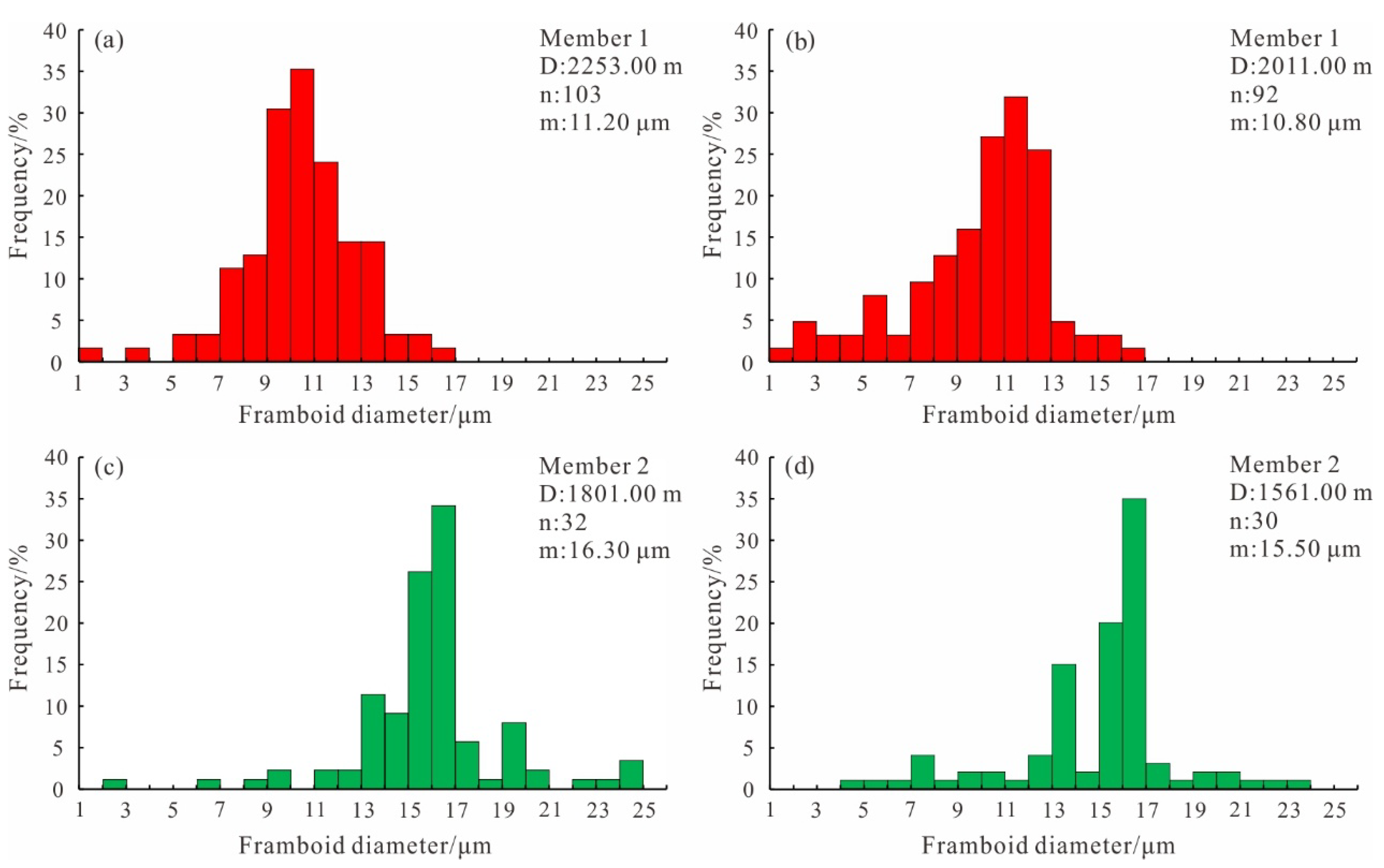
4.5. Petrographic Features of Pyrite
4.6. Elemental Geochemistry
4.6.1. Major Elements
4.6.2. Trace Elements
5. Discussion
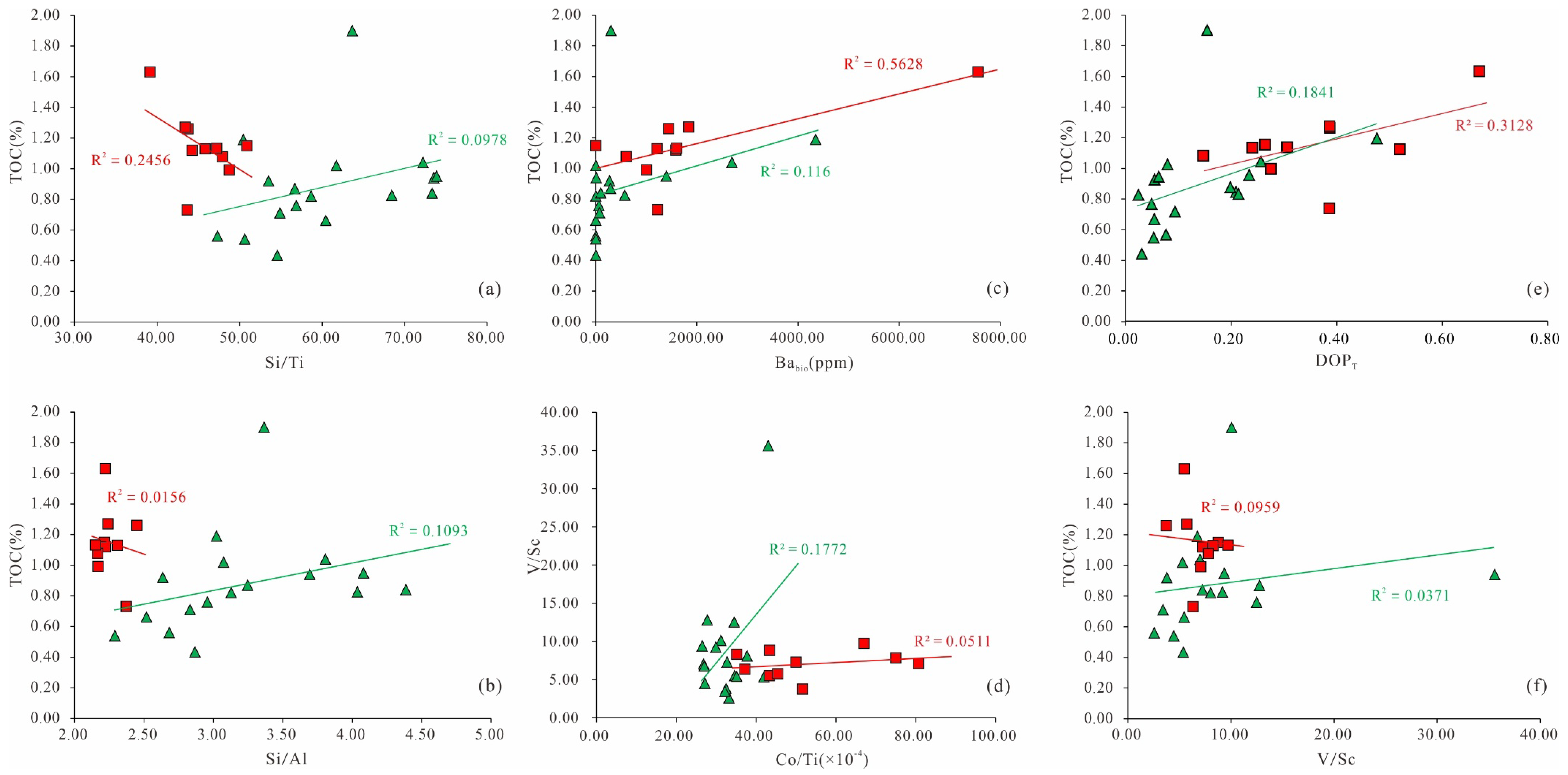
5.1. Terrestrial Detrital Influx
5.2. Evolution of Paleoredox Condition
5.3. Paleoproductivity
5.4. Controlling Factors of the Organic Matter Accumulation
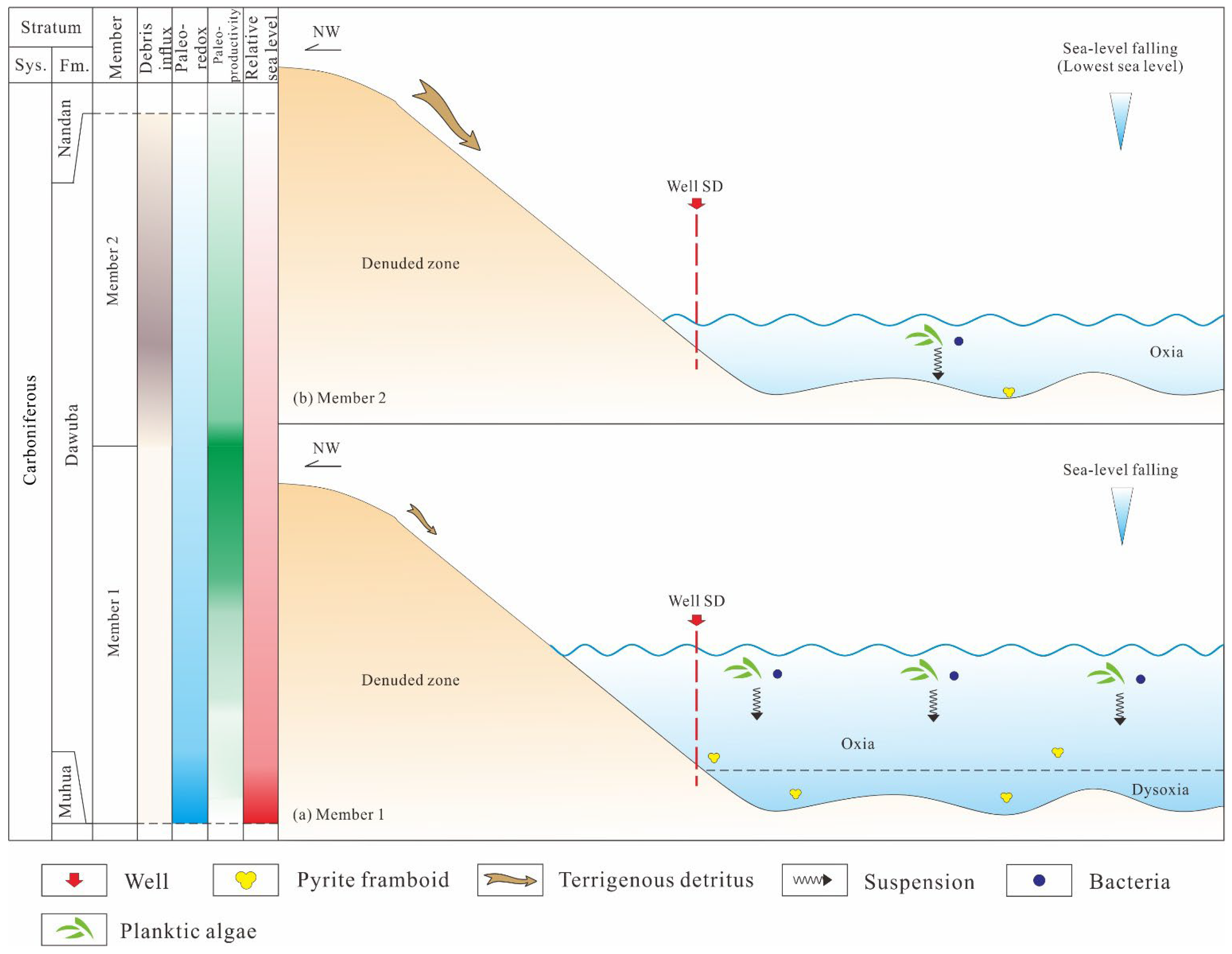
5.5. Model of Organic Matter Accumulation
6. Conclusions
- (1)
- The Early Carboniferous Dawuba Formation in the Qiannan Depression comprises two members: Member 1 is characterized by the CM-1 lithofacies (thick-bedded mudstone), while Member 2 is dominated by the C-2 lithofacies (marl).
- (2)
- Sediments of the Dawuba Formation are characterized by overall low TOC contents, comprising two distinct types: organic-containing sediments (1.00% < TOC < 2.00%) and organic-poor sediments (TOC < 1.00%). The accumulation of organic matter in these sediments is collectively controlled by three key factors: redox conditions, primary productivity, and terrigenous detrital flux.
- (3)
- The organic-containing sediments of the Early Carboniferous Dawuba Formation developed in a deep-water suboxic-oxic transitional environment, characterized by intermittent weakly redox conditions, moderate primary productivity, and limited terrigenous detrital input, which sustained partial organic carbon preservation in Member 1. In Member 2, the organic-poor sediments were deposited in a shallow water column induced by sea-level fall, exhibiting persistent oxic conditions, decreased productivity, and enhanced terrestrial detrital influx. Notably, the decrease in organic matter exposure time within the oxic water column-driven by rapid terrigenous detrital accumulation-represents a critical factor for the formation of organic-poor sediments.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dong, D.Z.; Shi, Z.S.; Guan, Q.Z.; Jiang, S.; Zhang, M.Q.; Zhang, C.C.; Wang, S.Y.; Sun, S.S.; Yu, R.Z.; Liu, D.X.; et al. Progress, challenges and prospects of shale gas exploration in the Wufeng–Longmaxi reservoirs in the Sichuan Basin. Nat. Gas Ind. B 2018, 5, 415–424. [Google Scholar] [CrossRef]
- Guo, T.L.; Deng, H.C.; Zhao, S.; Wel, L.M.; He, J.H. Formation mechanisms and exploration breakthroughs of new type of shale gas in Cambrian Qiongzhusi Formation, Sichuan Basin, SW China. Pet. Explor. Dev. 2025, 52, 64–78. [Google Scholar] [CrossRef]
- Zhao, Z.; Yang, W.; Zhao, Z.Y.; Xu, W.L.; Gong, D.Y.; Jin, H.; Song, W.; Liu, G.; Zhang, C.L.; Huang, S.P. Research progresses in geological theory and key exploration areas of coal-formed gas in China. Pet. Explor. Dev. 2024, 51, 1435–1450. [Google Scholar] [CrossRef]
- Cunningham, C.M.; Arnott, R.W.C. Organic matter deposition and preservation in ancient deep-sea levée sediments: Implications for Neoproterozoic trends in carbon burial. Sedimentology 2023, 70, 475–504. [Google Scholar] [CrossRef]
- Liu, Z.S.; Zhang, Y.; Zhou, Q.H.; Wu, Z.B.; Wang, Y.X. In-situ Technologies for Controlling Sediment Phosphorus in Eutrophic Shallow Lakes: A Review. J. Earth Sci. 2025, 36, 113–133. [Google Scholar] [CrossRef]
- Yuan, K.; Huang, W.H.; Fang, X.X.; Wang, T.; Lin, T.; Chen, R. Evaluation of Favorable Shale Gas Intervals in Dawuba Formation of Ziyun Area, South Qian Depression. Geofluids 2021, 2021, 6688141. [Google Scholar] [CrossRef]
- Wang, T.; Xu, Z.J.; Yuan, K.; Wang, X.L.; Ge, M.N. Paleoenvironment and shale gas potential of the Carboniferous Dawuba and the Cambrian Niutitang shales in the Upper Yangtze Platform, South China. Front. Earth Sci. 2024, 12, 1404178. [Google Scholar] [CrossRef]
- Niu, J.L.; Zhang, J.C.; Tang, X.; Yuan, K.; Lin, T.; Liu, Y.; Niu, Y.J.; Li, P.; Liang, Y.T. Main Controlling Factors and Models of Organic Matter Accumulation in Lower Carboniferous Dawuba Formation Shale in Southern Guizhou, China. ACS Omega 2021, 6, 32441–32459. [Google Scholar] [CrossRef]
- Zheng, F.Z.; Tang, X.; Yuan, K.; Lin, T.; You, M.X.; Niu, J.L.; Zi, Y.Y.; Liang, Y.T. Sedimentation models and development mechanisms of organic-rich shales of the lower carboniferous dawuba formation: A case study in the yaziluo rift trough, south of Guizhou province, southern China. Acs Omega 2022, 7, 29054–29071. [Google Scholar] [CrossRef]
- Liang, Y.T.; Tang, X.; Zhang, J.C.; Liu, Y.; Zhang, Y.; Yuan, K.; Lin, T.; Ding, J.H.; Wang, Y. Origin of lower carboniferous cherts in southern Guizhou, south China. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2022, 590, 110863. [Google Scholar] [CrossRef]
- Zhang, H.Q.; Zhang, Q.; Zhou, Y.X.; Lan, B.F.; Feng, X.T.; Chen, Y.; Yu, Q.; Cheng, J.X.; Men, Y.P.; Zhao, A.K. Geochemical characteristics and sedimentary environment of black mudstone in the early Carboniferous Dawuba Formation in the Middle and Upper Yangtze region. Front. Earth Sci. 2023, 11, 1277359. [Google Scholar] [CrossRef]
- Sun, C.X.; Nie, H.K.; Dang, W.; Chen, Q.; Zhang, G.R.; Li, W.P.; Lu, Z.Y. Shale gas exploration and development in China: Current status, geological challenges, and future directions. Energy Fuels 2021, 35, 6359–6379. [Google Scholar] [CrossRef]
- Lu, Y.B.; Jiang, S.; Lu, Y.C.; Xu, S.; Shu, Y.; Wang, Y.X. Productivity or preservation? The factors controlling the organic matter accumulation in the late Katian through Hirnantian Wufeng organic-rich shale, South China. Mar. Pet. Geol. 2019, 109, 22–35. [Google Scholar] [CrossRef]
- Liu, W.; Gao, P.; Xiao, X.M.; Zhao, Y.M.; Xing, Y.J.; Li, J.K. Variable depositional environments and organic matter enrichment of Early Cambrian shales in the Middle Yangtze region, South China. J. Asian Earth Sci. 2024, 259, 105874. [Google Scholar] [CrossRef]
- Ross, D.J.K.; Bustin, R.M. Investigating the use of sedimentary geochemical proxies for paleoenvironment interpretation of thermally mature organic-rich strata: Examples from the Devonian-Mississippian shales, Western Canadian Sedimentary Basin. Chem. Geol. 2009, 260, 1–19. [Google Scholar] [CrossRef]
- Liu, Y.Z.; Shi, W.Z.; Hu, Q.H.; Yuan, K.; Xu, X.F.; Zhang, X.M.; Wang, R.; Chen, X.L.; Gingras, M.K.; Konhauser, K.O. The mechanism of organic matter accumulation in the archipelago marine sediments: Insights from the Middle Devonian Givetian mudstone with low TOC in the Youjiang Basin, South China. Mar. Pet. Geol. 2024, 160, 106626. [Google Scholar] [CrossRef]
- Brumsack, H.J. The trace metal content of recent organic carbon-rich sediments: Implications for Cretaceous black shale formation. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2006, 232, 344–361. [Google Scholar] [CrossRef]
- Li, P.; Liu, Z.B.; Bi, H.; Jiang, T.; Bian, R.K.; Wang, P.W.; Shang, X.Y. Differences in and factors controlling organic matter enrichment in the Ziliujing Formation shale in the Sichuan Basin. Pet. Sci. 2024, 21, 77–86. [Google Scholar] [CrossRef]
- Liu, Q.Y.; Li, P.; Jin, Z.J.; Sun, Y.W.; Hu, G.; Zhu, D.Y.; Huang, Z.K.; Liang, X.P.; Zhang, R.; Liu, J.Y. Organic-rich formation and hydrocarbon enrichment of lacustrine shale strata: A case study of Chang 7 Member. Sci. China Earth Sci. 2022, 65, 118–138. [Google Scholar] [CrossRef]
- Zhao, Z.F.; Zou, C.N.; Dai, S.F.; Guo, Z.J.; Li, Y.; Nielsen, A.T.; Schovsbo, N.H.; Jing, Z.H.; Liu, H.L.; Yuan, M.; et al. Weathering-induced organic matter enrichment in marine-continental transitional shale: A case study on the early Permian Taiyuan Formation in the Ordos Basin, China. Int. J. Coal Geol. 2024, 291, 104562. [Google Scholar] [CrossRef]
- Yuan, K.; Huang, W.H.; Fang, X.X.; Li, S.Z.; Wang, T.; Lin, T.; Liu, G.H. Geochemical characteristics and sedimentary environment of the middle Devonian organic-rich shales in the northwest of guizhong depression, southwest China. China Geol. 2020, 3, 567–574. [Google Scholar] [CrossRef]
- Yu, C.M.; Qie, W.K.; Lu, J.F. Emsian (early devonian) yujiang event in southsouth China. Palaeoworld 2018, 27, 53–65. [Google Scholar] [CrossRef]
- Mei, M.X.; Ma, Y.S.; Deng, J.; Chu, H.M.; Zheng, K.B. Sequence-stratigraphic frameworks and their palaeogeographic patterns for the Permian Lopingian of the Dianqiangui Basin and its adjacent areas of Southwestern China. Sci. China Ser. D: Earth Sci. 2007, 50, 869–885. [Google Scholar] [CrossRef]
- Qie, W.K.; Sun, Y.L.; Guo, W.; Nie, T.; Chen, B.; Song, J.J.; Liang, K.; Yin, B.A.; Han, S.P.; Chang, J.Y.; et al. Devonian-Carboniferous boundary in China. Palaeobiodiversity Palaeoenvironments 2021, 101, 589–611. [Google Scholar] [CrossRef]
- International Committee for Coal and Organic Petrology (ICCP). The new vitrinite classification (ICCP systems 1994). Fuel 1998, 77, 349–358. [Google Scholar] [CrossRef]
- Pickel, W.; Kus, J.; Flores, D.; Kalaitzidis, S.; Christanis, K.; Cardott, B.J.; Crosdale, P. Classification of liptinite-ICCP system 1994. Int. J. Coal. Geol. 2017, 169, 40–61. [Google Scholar] [CrossRef]
- International Committee for Coal and Organic Petrology (ICCP). The new inertinite classification (ICCP system 1994). Fuel 2001, 80, 459–471. [Google Scholar] [CrossRef]
- López, J.M.G.; Bauluz, B.; Fernández-Nieto, C.; Oliete, A.Y. Factors controlling the trace-element distribution in fine-grained rocks: The Albian kaolinite-rich deposits of the Oliete Basin (NE Spain). Chem. Geol. 2005, 214, 1–19. [Google Scholar] [CrossRef]
- Kang, Y.; Zhu, R.K.; Liu, K.Q.; Zhang, J.Y.; Zhang, S.R. Detrital and authigenic clay minerals in shales: A review on their identification and applications. Heliyon 2024, 10, e39239. [Google Scholar] [CrossRef]
- Xiao, B.; Liu, S.G.; Li, Z.W.; Ran, B.; Ye, Y.H.; Yang, D.; Li, J.X. Geochemical characteristics of marine shale in the Wufeng Formation-Longmaxi Formation in the northern Sichuan Basin, South China and its implications for depositional controls on organic matter. J. Pet. Sci. Eng. 2021, 203, 108618. [Google Scholar] [CrossRef]
- O’Connell, B.; Wallace, M.W. The palaeoenvironmental and biological significance of marine carbonate depositional surfaces. Geol. Soc. Lond. Spec. Publ. 2025, 556, 556–2024. [Google Scholar] [CrossRef]
- Taylor, S.R.; Mclennan, S.M. The Continental Crust: Its Composition and Evolution; Blackwell: London, UK, 1985. [Google Scholar]
- Reimann, C.; Caritat, P.D. Intrinsic flaws of element enrichment factors (EFs) in environmental geochemistry. Environ. Sci. Technol. 2000, 34, 5084–5091. [Google Scholar] [CrossRef]
- Liu, Y.; Wei, Y.; Liu, G.J.; Fu, B.; Chen, B.Y.; Zhang, J.M.; Gui, L.; Zhou, H.H.; Lu, M.Y. Fine chemical speciation and environmental impact capacity of trace elements with different enrichment levels in coal. Sci. Total Environ. 2023, 856, 158928. [Google Scholar] [CrossRef] [PubMed]
- Schoepfer, S.D.; Shen, J.; Wei, H.Y.; Tyson, R.V.; Ingall, E.; Algeo, T.J. Total organic carbon, organic phosphorus, and biogenic barium fluxes as proxies for paleomarine productivity. Earth Sci. Rev. 2015, 149, 23–52. [Google Scholar] [CrossRef]
- Zhong, N.N.; Lu, S.F.; Huang, Z.L.; Zhang, Y.S. TOC changes in the process of thermal evolution of source rock and its controls. Sci. China 2004, 47 (Suppl. S2), 141–149. [Google Scholar] [CrossRef]
- Wu, L.Y.; Hu, D.F.; Lu, Y.C.; Liu, R.B.; Liu, X.F. Advantageous shale lithofacies of Wufeng formation-longmaxi Formation in fuling gas field of Sichuan Basin, SW China. Petroleum. Explor. Dev. 2016, 43, 208–217. [Google Scholar] [CrossRef]
- Fontana, D. Detrital carbonate grains as provenance indicators in the Upper Cretaceous Pietraforte Formation (northern Apennines). Sedimentology 1991, 38, 1085–1095. [Google Scholar] [CrossRef]
- Ma, B.; Ji, L.M.; Zhou, Q.S.; Zhang, Y.; Fu, S.; Xiao, Y.Y.; Sun, H. Morphology and sulfur isotopes of pyrite in Chang73 shale: Indications for redox conditions and enrichment of organic matter and hydrocarbons. Mar. Pet. Geol. 2025, 180, 107484. [Google Scholar] [CrossRef]
- Qiao, J.Q.; Li, H.; Luo, Q.Y.; Liu, L.F.; Wang, D.D.; Shang, X.Q.; Xiao, F.; Zhang, T. Development conditions and factors controlling the formation of the Permian Pingdiquan source rocks in the Wucaiwan Sag, Junggar Basin, China: A comprehensively elemental, biomarker and isotopic perspective. J. Earth Sci. 2025, 36, 627–643. [Google Scholar] [CrossRef]
- Kang, J.H.; Wang, X.Z.; Huo, F.; Zeng, D.M.; Li, Y.; Huang, Z.S.; Zhu, Y.Q.; Li, B.; Xie, S.Y.; Yang, Y.R.; et al. Changes in lake sedimentary environment and characteristics of organic matter enrichment under the influence of geological events: The lower Jurassic (Sinemurian-Toarcian) organic-rich shale, Sichuan Basin, Upper Yangtze Plate. Mar. Pet. Geol. 2025, 177, 107390. [Google Scholar] [CrossRef]
- Tribovillard, N.P.; Desprairies, A.; Lallier-Vergès, E.; Bertrand, P.; Moureau, N.; Ramdani, A.; Ramanampisoa, L. Geochemical study of organic-matter rich cycles from the Kimmeridge Clay Formation of Yorkshire (UK): Productivity versus anoxia. Palaeogeogr. Palaeoclimatol. Palaeoecol. 1994, 108, 165–181. [Google Scholar] [CrossRef]
- Wilkin, R.T.; Barnes, H.L.; Brantley, S.L. The size distribution of framboidal pyrite in modern sediments: An indicator of redox conditions. Geochim. Et Cosmochim. Acta 1996, 60, 3897–3912. [Google Scholar] [CrossRef]
- Chang, J.Y.; Li, Y.Y.; Lu, H.L. The morphological characteristics of authigenic pyrite formed in marine sediments. J. Mar. Sci. Eng. 2022, 10, 1533. [Google Scholar] [CrossRef]
- Wilkin, R.T.; Barnes, H.L.; Brantley, S.L. Formation processes of framboidal pyrite. Geochim. Et Cosmochim. Acta 1997, 61, 323–339. [Google Scholar] [CrossRef]
- Wignall, P.B.; Bond, D.P.G.; Kuwahara, K.; Kakuwa, Y.; Newton, R.J.; Poulton, S.W. An 80-million-year oceanic redox history from Permian to Jurassic pelagic sediments of the Mino-Tamba terrane, SW Japan, and the origin of four mass extinctions. Glob. Planet. Change 2010, 71, 109–123. [Google Scholar] [CrossRef]
- Sawłowicz, Z. Framboids: From Their Origin to Application; Wydawnictwo Oddziału Polskiej Akademii Nauk: Warsaw, Poland, 2000; Volume 88. [Google Scholar]
- Ohfuji, H.; Rickard, D. Experimental syntheses of framboids-a review. Earth Sci. Rev. 2005, 71, 147–170. [Google Scholar] [CrossRef]
- Butler, I.B.; Rickard, D. Framboidal pyrite formation via the oxidation of iron (II) monosulfide by hydrogen sulphide. Geochim. Et Cosmochim. Acta 2000, 64, 2665–2672. [Google Scholar] [CrossRef]
- Duverger, A.; Berg, J.S.; Busigny, V.; Guyot, F.; Bernard, S.; Miot, J. Mechanisms of pyrite formation promoted by sulfate-reducing bacteria in pure culture. Front. Earth Sci. 2020, 8, 588310. [Google Scholar] [CrossRef]
- Algeo, T.J.; Liu, J.S. A re-assessment of elemental proxies for paleoredox analysis. Chem. Geol. 2020, 540, 119549. [Google Scholar] [CrossRef]
- Algeo, T.J.; Li, C. Redox classification and calibration of redox thresholds in sedimentary systems. Geochim. Et Cosmochim. Act 2020, 287, 8–26. [Google Scholar] [CrossRef]
- Lin, D.L.; Xi, Z.D.; Tang, S.H.; Lash, G.G.; Cheng, Y.; Zhang, Q.; Yang, X.X.; Jia, T.F. New insights into controlling factors of organic matter enrichment in Carboniferous–Permian shales based on machine learning. Geol. Soc. Am. Bull. 2025, 137, 2846–2866. [Google Scholar] [CrossRef]
- Zhou, D.S.; Dong, H.Z.; Huang, X.W.; Liang, Y.F.; Deng, Z.Y.; Huang, L.; Jiang, S. The paleo-oceanic environment and organic matter enrichment mechanism of the early Cambrian in the southern Lower Yangtze platform, China. Mar. Pet. Geol. 2024, 170, 107119. [Google Scholar] [CrossRef]
- Xu, H.Y.; Hou, D.J.; Löhr, S.C.; Liu, Q.Y.; Jin, Z.J.; Shi, J.Y.; Liang, X.P.; Niu, C.K.; George, S.C. Millimetre-scale biomarker heterogeneity in lacustrine shale identifies the nature of signal-averaging and demonstrates anaerobic respiration control on organic matter preservation and dolomitization. Geochim. Et Cosmochim. Acta 2023, 348, 107–121. [Google Scholar] [CrossRef]
- Doner, Z. Elemental geochemistry of upper Campanian shales in the western Pontides (Türkiye): Sedimentary environment controls on the organic matter accumulation and source rock potential. J. Earth Sci. 2025. [Google Scholar] [CrossRef]
- Salocchi, A.C.; Krawielicki, J.; Eglinton, T.I.; Eglinton, T.I.; Fioroni, C.; Fontana, D.; Conti, S.; Picotti, V. Biomarker constraints on Mediterranean climate and ecosystem transitions during the Early-Middle Miocene. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2021, 562, 110092. [Google Scholar] [CrossRef]
- McManus, J.; Berelson, W.M.; Klinkhammer, G.P.; Johnson, K.S.; Coale, K.H.; Anderson, R.F.; Kumar, N.; Burdige, D.J.; Hammond, D.E.; Brumsack, H.J.; et al. Geochemistry of barium in marine sediments: Implications for its use as a paleoproxy. Geochim. Et Cosmochim. Acta 1998, 62, 3453–3473. [Google Scholar] [CrossRef]
- Griffith, E.M.; Paytan, A. Barite in the ocean–occurrence, geochemistry and palaeoceanographic applications. Sedimentology 2012, 59, 1817–1835. [Google Scholar] [CrossRef]
- Houk, R.S.; Fassel, V.A.; Flesch, G.D.; Svec, H.J.; Gray, A.L.; Taylor, C.E. Inductively coupled argon plasma as an ion source for mass spectrometric determination of trace elements. Anal. Chem. 1980, 52, 2283–2289. [Google Scholar] [CrossRef]
- Latimer, J.C.; Filippelli, G.M. Terrigenous input and paleoproductivity in the Southern Ocean. Paleoceanography 2001, 16, 627–643. [Google Scholar] [CrossRef]
- Jennerjahn, T.C. Biogeochemical response of tropical coastal systems to present and past environmental change. Earth Sci. Rev. 2012, 114, 19–41. [Google Scholar] [CrossRef]
- Hemmati, M.; Ahmadi, Y.; Vaferi, B.; Alibak, A.H.; Wood, D.A. Surveying Organic Matter, Thermal Maturity Level, and Paleo-Environmental Conditions by Considering Biomarker and Stable Carbon Isotopic Analysis. J. Earth Sci. 2025, 36, 428–440. [Google Scholar] [CrossRef]
- Li, Y.; Jiang, H.X.; Sun, C.H.; Yang, G.; Zhang, L.; Hu, Y.J. Comparison of end-Ediacaran to earliest Cambrian microbialites in the Tarim Basin, China: Diversities and paleo-environment. J. Earth Sci. 2025. [Google Scholar] [CrossRef]
- Ibach, L.E.J. Relationship between sedimentation rate and total organic carbon content in ancient marine sediments. AAPG Bull. 1982, 66, 170–188. [Google Scholar] [CrossRef]
- Wang, X.D.; Hu, K.Y.; Qie, W.K.; Sheng, Q.Y.; Chen, B.; Lin, W.; Yao, L.; Wang, Q.L.; Qi, Y.P.; Chen, J.T.; et al. Carboniferous integrative stratigraphy and timescale of China. Sci. China Earth Sci. 2019, 62, 135–153, (in Chinese with English abstract). [Google Scholar] [CrossRef]
| Depth (m) | Member | Quartz | Plagioclase | Calcite | Dolomite | Siderite | Pyrite | Clay |
|---|---|---|---|---|---|---|---|---|
| 1540.60 | 2 | 1.00 | 0.00 | 74.00 | 6.00 | 0.00 | 2.00 | 10.00 |
| 1561.00 | 2 | 1.00 | 0.00 | 55.00 | 13.00 | 0.00 | 2.00 | 18.00 |
| 1601.00 | 2 | 1.00 | 0.00 | 59.00 | 9.00 | 0.00 | 2.00 | 17.00 |
| 1645.00 | 2 | 1.00 | 0.00 | 63.00 | 10.00 | 0.00 | 1.00 | 17.00 |
| 1680.80 | 2 | 1.00 | 0.00 | 37.00 | 30.00 | 0.00 | 2.00 | 15.00 |
| 1701.00 | 2 | 1.00 | 0.00 | 62.00 | 20.00 | 0.00 | 1.00 | 5.00 |
| 1751.00 | 2 | 1.00 | 2.00 | 37.00 | 19.00 | 0.00 | 2.00 | 19.00 |
| 1801.00 | 2 | 1.00 | 2.00 | 29.00 | 33.00 | 0.00 | 2.00 | 22.00 |
| 1851.00 | 2 | 1.00 | 1.00 | 32.00 | 21.00 | 0.00 | 3.00 | 27.00 |
| 1901.00 | 2 | 1.00 | 1.00 | 14.00 | 7.00 | 10.00 | 3.00 | 43.00 |
| 1930.15 | 1 | 1.00 | 0.00 | 0.00 | 14.40 | 0.00 | 0.90 | 55.90 |
| 2011.00 | 1 | 1.00 | 1.00 | 13.00 | 5.00 | 3.00 | 0.00 | 54.00 |
| 2041.00 | 1 | 1.00 | 1.00 | 17.00 | 5.00 | 7.00 | 0.00 | 47.00 |
| 2103.00 | 1 | 1.00 | 0.00 | 16.00 | 6.00 | 5.00 | 0.00 | 56.00 |
| 2150.00 | 1 | 1.00 | 0.00 | 15.00 | 8.00 | 7.00 | 2.00 | 47.00 |
| 2202.00 | 1 | 1.00 | 0.00 | 11.00 | 7.00 | 6.00 | 4.00 | 55.00 |
| 2253.00 | 1 | 1.00 | 0.00 | 10.00 | 7.00 | 7.00 | 4.00 | 52.00 |
| 2303.00 | 1 | 1.00 | 0.00 | 13.00 | 8.00 | 7.00 | 3.00 | 48.00 |
| 2355.00 | 1 | 1.00 | 0.00 | 18.00 | 9.00 | 7.00 | 3.00 | 44.00 |
| 2399.00 | 1 | 1.00 | 0.00 | 11.00 | 5.00 | 8.00 | 4.00 | 53.00 |
| 2450.00 | 1 | 1.00 | 0.00 | 12.00 | 6.00 | 9.00 | 3.00 | 51.00 |
| Depth (m) | Member | TOC | SiO2 | Al2O3 | Fe2O3 | CaO | MgO | K2O | Na2O | TiO2 | P2O5 | MnO |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1511.00 | 2 | 0.43 | 15.59 | 5.13 | 1.96 | 39.62 | 1.85 | 0.74 | 0.27 | 0.26 | 0.08 | 0.01 |
| 1540.60 | 2 | 0.54 | 34.41 | 13.25 | 2.92 | 23.49 | 1.52 | 1.71 | 0.74 | 0.53 | 0.06 | 0.02 |
| 1561.00 | 2 | 0.56 | 22.48 | 7.87 | 2.50 | 34.40 | 1.78 | 0.75 | 0.50 | 0.29 | 0.07 | 0.01 |
| 1581.00 | 2 | 0.66 | 19.01 | 5.85 | 2.16 | 38.19 | 1.44 | 0.71 | 0.36 | 0.27 | 0.08 | 0.01 |
| 1601.00 | 2 | 0.71 | 22.64 | 7.57 | 2.40 | 35.05 | 1.49 | 0.80 | 0.46 | 0.33 | 0.08 | 0.01 |
| 1631.00 | 2 | 0.76 | 20.15 | 6.01 | 1.99 | 37.41 | 1.52 | 0.69 | 0.37 | 0.28 | 0.09 | 0.01 |
| 1645.00 | 2 | 0.82 | 15.03 | 3.59 | 1.57 | 42.77 | 1.55 | 0.47 | 0.25 | 0.16 | 0.07 | 0.02 |
| 1680.80 | 2 | 0.83 | 40.41 | 11.59 | 4.42 | 18.53 | 3.05 | 2.10 | 0.42 | 0.51 | 0.44 | 0.05 |
| 1701.00 | 2 | 0.84 | 24.56 | 7.64 | 2.70 | 33.19 | 1.96 | 1.08 | 0.40 | 0.35 | 0.08 | 0.03 |
| 1721.00 | 2 | 0.87 | 22.37 | 6.30 | 2.66 | 34.83 | 1.97 | 1.05 | 0.30 | 0.30 | 0.13 | 0.04 |
| 1751.00 | 2 | 0.92 | 26.28 | 6.88 | 2.52 | 32.37 | 2.16 | 1.07 | 0.46 | 0.32 | 0.16 | 0.04 |
| 1771.00 | 2 | 0.94 | 21.64 | 4.35 | 1.74 | 37.92 | 1.61 | 0.65 | 0.40 | 0.23 | 0.07 | 0.03 |
| 1801.00 | 2 | 0.95 | 28.95 | 6.32 | 2.44 | 31.75 | 1.86 | 0.97 | 0.52 | 0.33 | 0.08 | 0.03 |
| 1821.00 | 2 | 1.02 | 33.75 | 9.16 | 3.28 | 26.46 | 2.45 | 1.46 | 0.54 | 0.46 | 0.10 | 0.03 |
| 1851.00 | 2 | 1.04 | 37.71 | 8.15 | 2.91 | 25.04 | 2.79 | 1.16 | 0.52 | 0.40 | 0.08 | 0.02 |
| 1871.00 | 2 | 1.19 | 42.74 | 9.90 | 3.26 | 20.83 | 2.78 | 1.49 | 0.56 | 0.46 | 0.10 | 0.02 |
| 1901.00 | 2 | 1.90 | 45.29 | 13.21 | 4.53 | 16.52 | 2.48 | 1.79 | 0.66 | 0.70 | 0.19 | 0.02 |
| 1930.15 | 1 | 0.73 | 48.52 | 19.31 | 8.83 | 8.50 | 2.57 | 2.45 | 0.79 | 0.74 | 0.06 | 0.09 |
| 1961.00 | 1 | 0.99 | 45.07 | 17.20 | 6.16 | 13.47 | 2.24 | 2.20 | 0.78 | 0.76 | 0.18 | 0.03 |
| 1991.00 | 1 | 1.08 | 50.03 | 19.87 | 8.65 | 5.63 | 1.87 | 2.29 | 0.97 | 0.99 | 0.29 | 0.03 |
| 2011.00 | 1 | 1.12 | 45.20 | 17.94 | 8.41 | 12.11 | 2.06 | 2.08 | 0.79 | 0.79 | 0.26 | 0.05 |
| 2041.00 | 1 | 1.13 | 46.92 | 16.89 | 9.01 | 11.69 | 1.86 | 1.97 | 0.68 | 0.83 | 0.29 | 0.08 |
| 2081.00 | 1 | 1.13 | 48.61 | 19.14 | 8.48 | 9.24 | 1.94 | 2.43 | 0.85 | 0.83 | 0.23 | 0.07 |
| 2150.00 | 1 | 1.15 | 48.85 | 18.16 | 7.35 | 9.99 | 2.02 | 2.33 | 0.78 | 0.78 | 0.23 | 0.05 |
| 2202.00 | 1 | 1.26 | 49.25 | 20.00 | 12.18 | 5.83 | 1.92 | 2.18 | 0.88 | 0.81 | 0.49 | 0.08 |
| 2253.00 | 1 | 1.27 | 49.99 | 20.35 | 11.51 | 5.69 | 1.92 | 2.23 | 0.89 | 0.81 | 0.46 | 0.07 |
| 2450.00 | 1 | 1.63 | 48.08 | 19.71 | 10.39 | 7.16 | 1.85 | 2.20 | 0.86 | 0.79 | 0.50 | 0.06 |
| Depth(m) | Member | V | Cr | Ni | Cu | Sr | Li | Ba | Sc | Zn | Ga | As | Rb | Mo | Co | Cs | W | Tl | Pb | Th | U |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1511.00 | 2 | 24.89 | 22.96 | 13.43 | 5.33 | 837.57 | 27.23 | 102.39 | 9.73 | 12.87 | 9.69 | 5.30 | 61.67 | 0.82 | 4.29 | 3.45 | 1.03 | 0.19 | 8.18 | 7.38 | 1.91 |
| 1540.60 | 2 | 68.89 | 53.91 | 16.82 | 8.55 | 4262.17 | 92.18 | 336.11 | 15.43 | 25.30 | 20.71 | 20.10 | 162.13 | 0.42 | 7.92 | 10.39 | 1.30 | 0.35 | 14.68 | 18.26 | 2.11 |
| 1561.00 | 2 | 36.14 | 38.64 | 19.36 | 6.38 | 1333.56 | 65.17 | 161.46 | 6.61 | 23.76 | 7.92 | 7.40 | 69.50 | 1.26 | 5.77 | 4.75 | 0.72 | 0.20 | 10.47 | 8.35 | 1.91 |
| 1581.00 | 2 | 32.92 | 28.98 | 15.21 | 5.67 | 1105.26 | 39.91 | 93.66 | 6.12 | 27.79 | 5.95 | 8.00 | 65.09 | 1.19 | 4.02 | 4.10 | 0.66 | 0.20 | 8.96 | 8.42 | 2.05 |
| 1601.00 | 2 | 40.47 | 33.92 | 17.91 | 6.89 | 1076.34 | 52.79 | 473.62 | 10.70 | 30.19 | 13.43 | 6.70 | 69.84 | 1.10 | 5.34 | 5.18 | 0.84 | 0.21 | 10.76 | 10.57 | 2.31 |
| 1631.00 | 2 | 38.72 | 31.09 | 14.87 | 5.53 | 2006.34 | 37.30 | 285.72 | 3.10 | 19.02 | 5.07 | 7.90 | 58.28 | 1.06 | 4.02 | 3.74 | 0.60 | 0.16 | 10.05 | 5.31 | 1.60 |
| 1645.00 | 2 | 24.14 | 26.49 | 12.16 | 3.55 | 1175.20 | 7.14 | 40.02 | 0.68 | 15.12 | 2.69 | 5.90 | 31.41 | 1.26 | 1.77 | 2.00 | 0.47 | 0.14 | 6.13 | 1.70 | 1.61 |
| 1680.80 | 2 | 87.52 | 62.59 | 39.05 | 10.86 | 395.55 | 42.20 | 105.49 | 16.49 | 22.68 | 19.89 | 12.30 | 173.50 | 1.34 | 16.75 | 8.79 | 1.52 | 0.40 | 34.09 | 20.13 | 3.84 |
| 1701.00 | 2 | 45.65 | 170.23 | 21.54 | 8.08 | 1480.53 | 36.61 | 295.84 | 13.43 | 44.25 | 16.39 | 8.60 | 89.47 | 32.41 | 6.68 | 5.30 | 1.00 | 0.25 | 14.92 | 11.14 | 2.39 |
| 1721.00 | 2 | 50.15 | 36.48 | 16.81 | 5.13 | 1143.18 | 31.12 | 257.73 | 6.24 | 31.12 | 6.26 | 8.90 | 87.12 | 0.84 | 7.43 | 4.74 | 0.76 | 0.23 | 15.10 | 8.06 | 1.56 |
| 1751.00 | 2 | 52.01 | 39.25 | 13.00 | 4.55 | 1372.56 | 20.99 | 629.36 | 5.16 | 38.47 | 5.64 | 7.20 | 79.19 | 1.30 | 4.61 | 3.85 | 0.72 | 0.19 | 13.78 | 7.98 | 1.87 |
| 1771.00 | 2 | 30.39 | 23.10 | 10.00 | 2.76 | 1538.35 | 11.06 | 223.31 | 4.20 | 25.18 | 3.51 | 9.80 | 44.95 | 1.35 | 2.58 | 2.37 | 0.48 | 0.14 | 6.48 | 6.10 | 2.23 |
| 1801.00 | 2 | 51.03 | 40.33 | 15.26 | 5.34 | 1249.15 | 18.11 | 940.43 | 5.56 | 42.90 | 5.26 | 7.40 | 77.95 | 1.87 | 4.50 | 3.51 | 0.80 | 0.19 | 13.46 | 8.90 | 2.51 |
| 1821.00 | 2 | 77.89 | 53.51 | 16.58 | 5.57 | 1116.92 | 26.26 | 698.36 | 6.10 | 51.77 | 7.32 | 9.30 | 109.94 | 2.26 | 5.81 | 4.69 | 1.02 | 0.23 | 18.33 | 10.71 | 2.27 |
| 1851.00 | 2 | 63.44 | 51.04 | 16.60 | 4.62 | 789.73 | 23.39 | 1872.88 | 6.78 | 50.29 | 6.22 | 5.00 | 83.33 | 1.56 | 4.61 | 3.99 | 0.79 | 0.20 | 14.58 | 9.91 | 2.11 |
| 1871.00 | 2 | 71.96 | 57.05 | 21.74 | 7.38 | 924.34 | 21.62 | 3742.07 | 10.28 | 74.29 | 12.77 | 6.10 | 110.63 | 1.54 | 5.81 | 5.23 | 0.97 | 0.30 | 17.89 | 10.94 | 2.65 |
| 1901.00 | 2 | 87.41 | 67.12 | 24.52 | 11.16 | 844.98 | 58.65 | 6748.43 | 12.96 | 78.03 | 15.34 | 9.10 | 134.47 | 1.49 | 8.81 | 7.31 | 1.45 | 0.33 | 23.24 | 17.67 | 2.91 |
| 1930.15 | 1 | 165.54 | 90.95 | 57.65 | 12.29 | 298.34 | 128.04 | 225.90 | 18.85 | 103.82 | 24.38 | 9.60 | 35.72 | 0.75 | 8.49 | 8.57 | 2.10 | 0.39 | 32.20 | 20.63 | 3.77 |
| 1961.00 | 1 | 138.83 | 87.12 | 59.49 | 12.77 | 639.26 | 157.03 | 2350.27 | 16.76 | 221.90 | 21.61 | 15.60 | 164.88 | 2.03 | 13.47 | 9.93 | 1.92 | 0.39 | 30.11 | 21.70 | 3.76 |
| 1991.00 | 1 | 140.87 | 82.50 | 54.98 | 12.23 | 299.51 | 137.81 | 6306.56 | 25.82 | 91.38 | 35.95 | 21.90 | 48.75 | 1.43 | 17.70 | 7.00 | 2.81 | 0.42 | 29.63 | 23.18 | 4.28 |
| 2011.00 | 1 | 134.62 | 83.97 | 50.48 | 14.77 | 661.06 | 159.58 | 2472.06 | 18.54 | 110.37 | 26.35 | 23.20 | 144.70 | 2.76 | 16.60 | 9.82 | 1.99 | 0.41 | 33.99 | 23.46 | 3.78 |
| 2041.00 | 1 | 114.79 | 76.54 | 52.61 | 17.38 | 463.25 | 145.74 | 2333.31 | 30.79 | 126.96 | 36.52 | 21.90 | 168.26 | 3.12 | 23.51 | 11.51 | 2.35 | 0.49 | 49.31 | 27.98 | 3.98 |
| 2081.00 | 1 | 121.82 | 70.72 | 62.24 | 34.32 | 344.49 | 170.47 | 1624.97 | 21.31 | 83.08 | 30.56 | 21.10 | 71.51 | 2.31 | 26.74 | 10.38 | 2.67 | 0.57 | 134.76 | 26.66 | 3.78 |
| 2150.00 | 1 | 123.78 | 70.47 | 42.17 | 14.42 | 838.56 | 130.10 | 1363.96 | 19.61 | 71.46 | 29.44 | 16.10 | 83.48 | 0.95 | 14.86 | 10.15 | 2.03 | 0.46 | 31.39 | 23.98 | 3.73 |
| 2202.00 | 1 | 131.07 | 74.62 | 48.17 | 19.60 | 255.23 | 142.87 | 1723.16 | 18.54 | 163.18 | 31.06 | 24.20 | 34.98 | 1.66 | 21.67 | 8.56 | 2.04 | 0.52 | 54.54 | 20.08 | 3.49 |
| 2253.00 | 1 | 135.18 | 76.45 | 56.27 | 18.50 | 262.02 | 154.45 | 1286.22 | 17.29 | 271.62 | 28.31 | 22.70 | 41.61 | 1.52 | 22.64 | 9.07 | 2.10 | 0.53 | 47.53 | 20.70 | 3.49 |
| 2450.00 | 1 | 158.83 | 88.60 | 60.56 | 17.62 | 333.91 | 188.06 | 2651.43 | 16.36 | 129.58 | 22.69 | 22.80 | 37.87 | 1.26 | 21.59 | 9.40 | 2.19 | 0.55 | 61.39 | 21.55 | 3.48 |
| Depth/m | Member | DOPT | V/Sc | Si/Ti | Si/Al | Babio(ppm) | P/Al | Sr/Ba | Co/Ti (×10−4) |
|---|---|---|---|---|---|---|---|---|---|
| 1511.00 | 2 | 0.08 | 2.56 | 47.34 | 2.68 | 0.00 | 69.33 | 6.84 | 33.18 |
| 1540.60 | 2 | 0.05 | 4.46 | 50.65 | 2.29 | 0.00 | 20.35 | 15.72 | 27.12 |
| 1561.00 | 2 | 0.05 | 5.47 | 60.48 | 2.52 | 0.00 | 38.47 | 7.78 | 34.59 |
| 1581.00 | 2 | 0.03 | 5.38 | 54.60 | 2.87 | 0.00 | 58.26 | 10.27 | 35.08 |
| 1601.00 | 2 | 0.05 | 3.78 | 53.54 | 2.64 | 268.60 | 43.82 | 2.22 | 32.43 |
| 1631.00 | 2 | 0.05 | 12.49 | 56.90 | 2.96 | 62.02 | 65.72 | 8.26 | 34.49 |
| 1645.00 | 2 | 0.06 | 35.58 | 73.57 | 3.69 | 9.68 | 80.15 | 10.31 | 42.99 |
| 1680.80 | 2 | 0.08 | 5.31 | 61.75 | 3.07 | 0.00 | 165.40 | 3.32 | 41.91 |
| 1701.00 | 2 | 0.09 | 3.40 | 54.93 | 2.83 | 74.37 | 44.78 | 4.89 | 32.12 |
| 1721.00 | 2 | 0.02 | 8.04 | 58.69 | 3.13 | 0.00 | 88.75 | 6.19 | 37.67 |
| 1751.00 | 2 | 0.15 | 10.07 | 63.69 | 3.37 | 297.34 | 104.47 | 2.70 | 31.16 |
| 1771.00 | 2 | 0.21 | 7.24 | 73.37 | 4.39 | 95.40 | 66.23 | 6.25 | 32.69 |
| 1801.00 | 2 | 0.21 | 9.18 | 68.47 | 4.04 | 572.53 | 52.41 | 1.58 | 29.90 |
| 1821.00 | 2 | 0.20 | 12.76 | 56.73 | 3.25 | 291.77 | 49.01 | 1.88 | 27.73 |
| 1851.00 | 2 | 0.23 | 9.35 | 73.90 | 4.08 | 1393.64 | 43.52 | 0.48 | 26.45 |
| 1871.00 | 2 | 0.26 | 7.00 | 72.26 | 3.81 | 2689.85 | 45.15 | 0.31 | 26.81 |
| 1901.00 | 2 | 0.48 | 6.75 | 50.49 | 3.02 | 4346.25 | 62.38 | 0.18 | 26.99 |
| 1930.15 | 1 | 0.26 | 8.78 | 50.90 | 2.21 | 0.00 | 13.84 | 0.84 | 43.38 |
| 1961.00 | 1 | 0.24 | 8.28 | 45.83 | 2.31 | 1207.47 | 44.88 | 0.37 | 35.08 |
| 1991.00 | 1 | 0.67 | 5.46 | 39.18 | 2.22 | 7558.23 | 63.05 | 0.04 | 43.29 |
| 2011.00 | 1 | 0.52 | 7.26 | 44.26 | 2.22 | 1578.28 | 63.48 | 0.31 | 49.93 |
| 2041.00 | 1 | 0.39 | 3.73 | 43.78 | 2.45 | 1441.22 | 74.45 | 0.23 | 51.59 |
| 2081.00 | 1 | 0.39 | 5.72 | 43.44 | 2.24 | 1836.77 | 52.90 | 0.14 | 45.38 |
| 2150.00 | 1 | 0.39 | 6.31 | 43.66 | 2.37 | 1218.39 | 55.76 | 0.47 | 37.16 |
| 2202.00 | 1 | 0.28 | 7.07 | 48.78 | 2.17 | 999.38 | 106.58 | 0.17 | 80.66 |
| 2253.00 | 1 | 0.15 | 7.82 | 47.92 | 2.17 | 598.62 | 99.41 | 0.23 | 74.99 |
| 2450.00 | 1 | 0.31 | 9.71 | 47.24 | 2.15 | 1595.88 | 110.54 | 0.16 | 66.95 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Y.; Wang, J.; Lin, T.; Li, D.; Chen, J.; Wang, S.; Shi, W.; Wang, R.; Zhang, X.; Xu, X.; et al. Depositional Environment as Main Controlling Factor for Low TOC Sediments in the Early Carboniferous Dawuba Formation of the Qiannan Depression. Geosciences 2025, 15, 442. https://doi.org/10.3390/geosciences15120442
Liu Y, Wang J, Lin T, Li D, Chen J, Wang S, Shi W, Wang R, Zhang X, Xu X, et al. Depositional Environment as Main Controlling Factor for Low TOC Sediments in the Early Carboniferous Dawuba Formation of the Qiannan Depression. Geosciences. 2025; 15(12):442. https://doi.org/10.3390/geosciences15120442
Chicago/Turabian StyleLiu, Yuzuo, Jiao Wang, Tuo Lin, Dongxiao Li, Jie Chen, Shengzhu Wang, Wanzhong Shi, Ren Wang, Xiaoming Zhang, Xiaofeng Xu, and et al. 2025. "Depositional Environment as Main Controlling Factor for Low TOC Sediments in the Early Carboniferous Dawuba Formation of the Qiannan Depression" Geosciences 15, no. 12: 442. https://doi.org/10.3390/geosciences15120442
APA StyleLiu, Y., Wang, J., Lin, T., Li, D., Chen, J., Wang, S., Shi, W., Wang, R., Zhang, X., Xu, X., & Liu, K. (2025). Depositional Environment as Main Controlling Factor for Low TOC Sediments in the Early Carboniferous Dawuba Formation of the Qiannan Depression. Geosciences, 15(12), 442. https://doi.org/10.3390/geosciences15120442






