What Do Fossil charophytes Whisper to Us? Palaeoecological and Palaeoenvironmental Reports from Pleistocene Continental Deposits of Umbria (Central Italy)
Abstract
1. Introduction
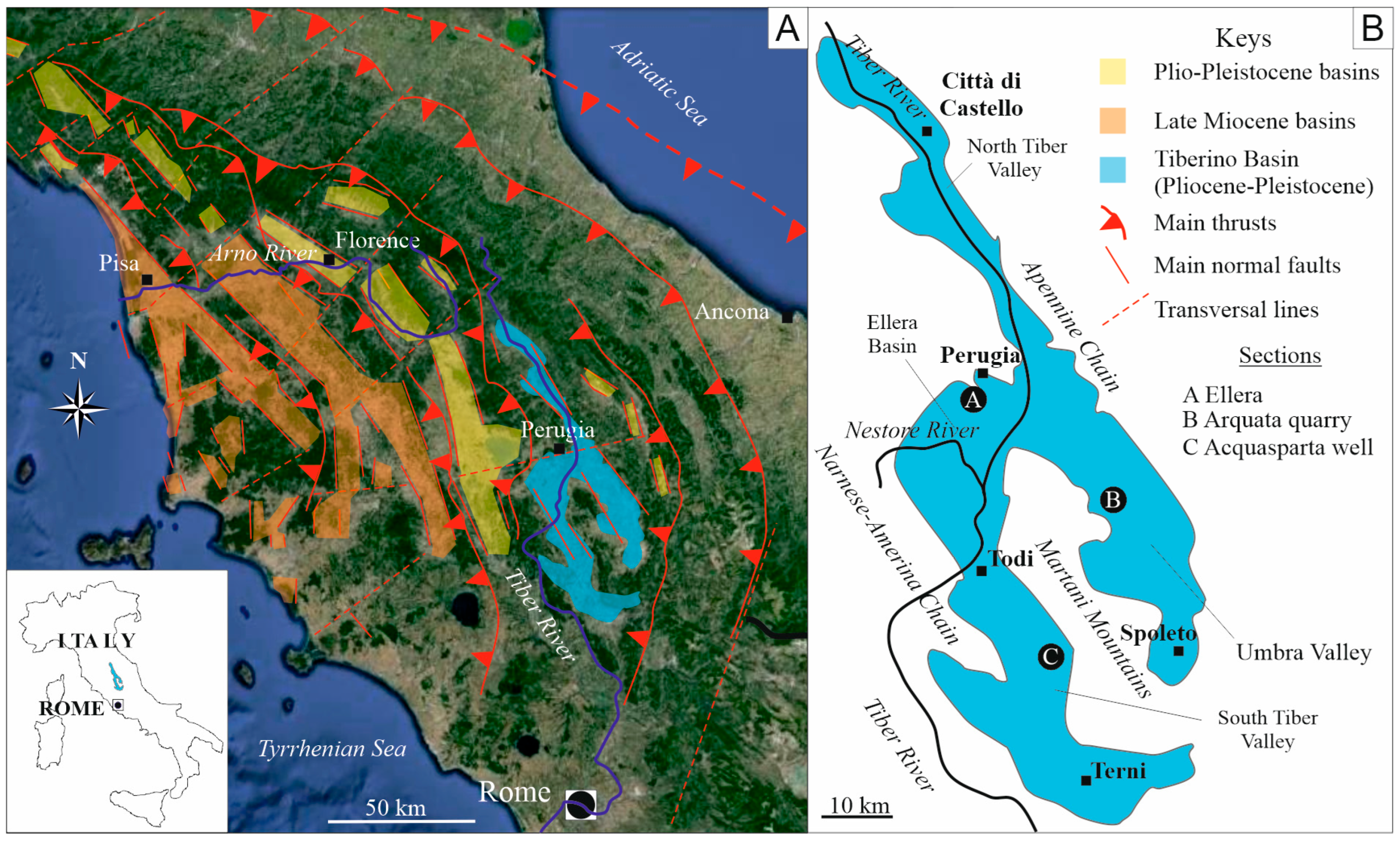
2. Geological Setting
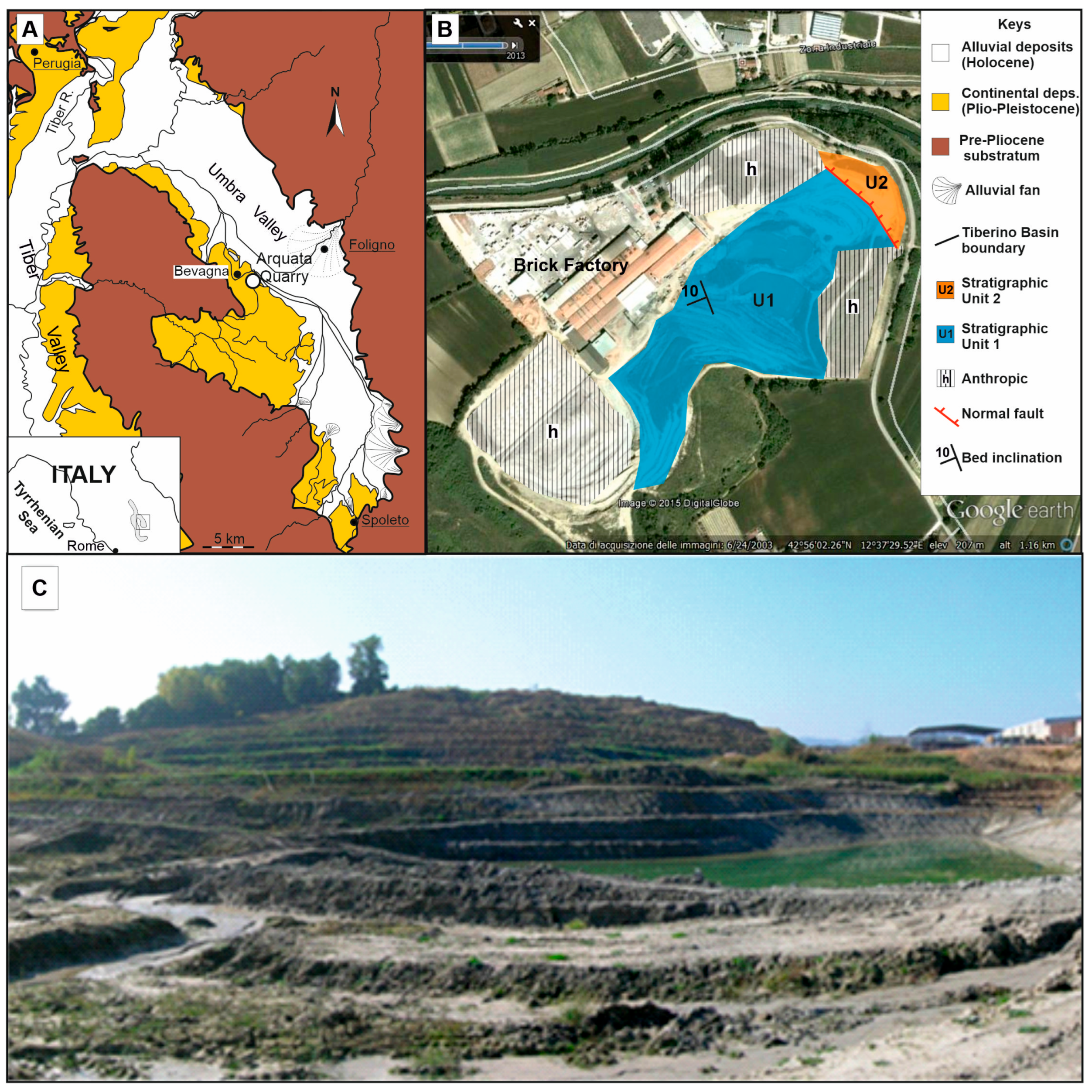
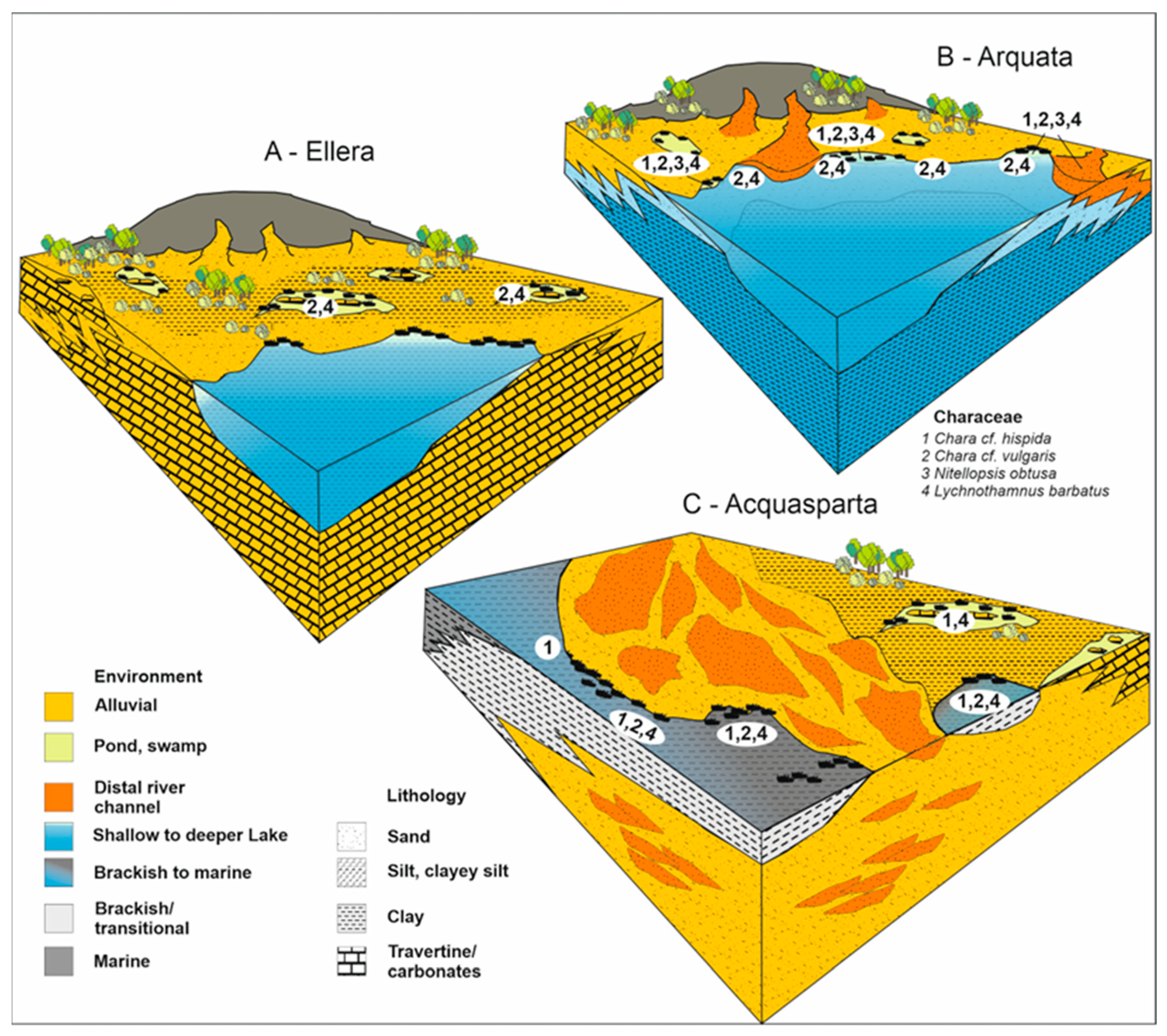
2.1. The Eastern Branch and the Arquata Quarry Section
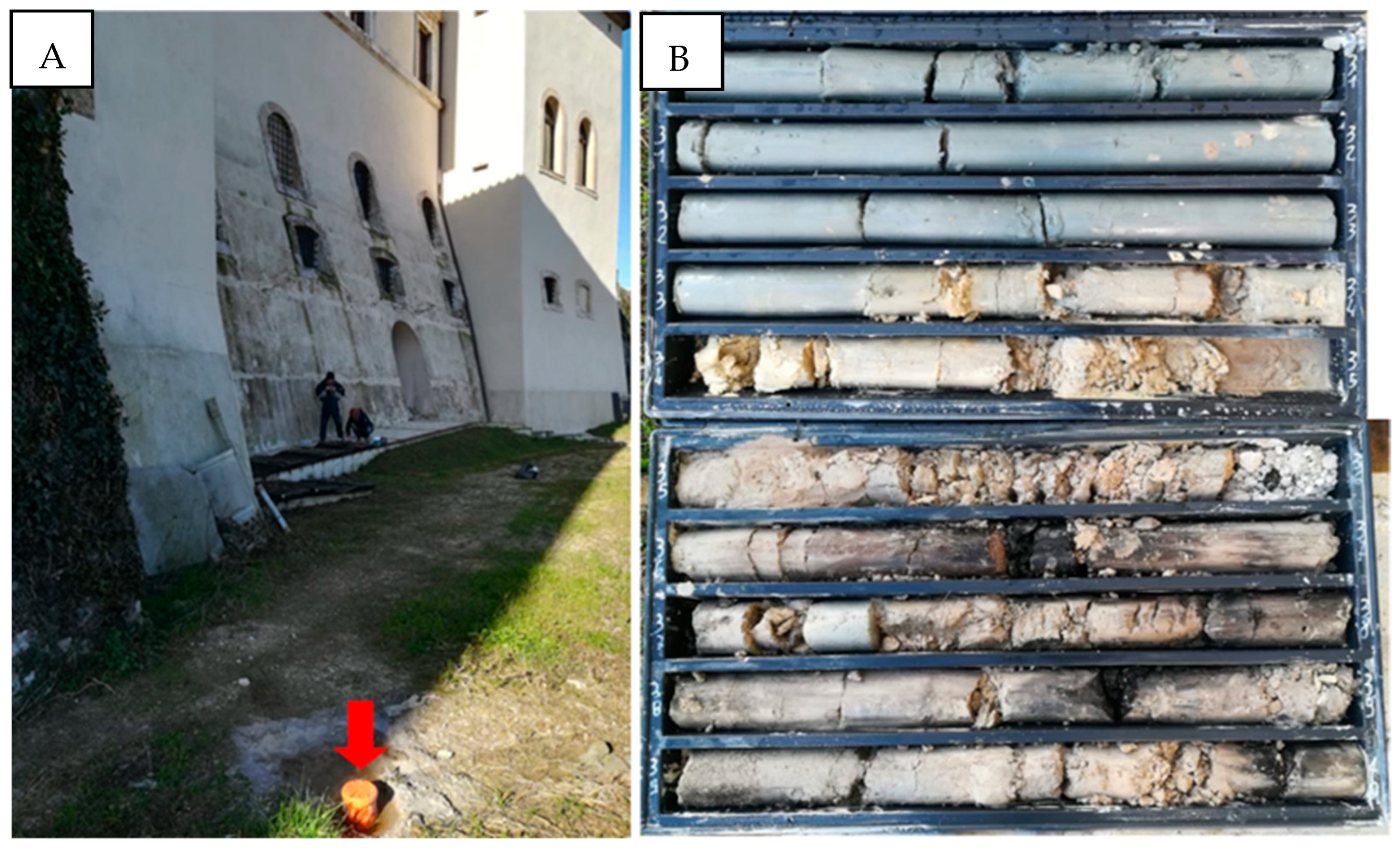
2.2. The Western Branch and the Acquasparta Borehole
2.3. The Minor Ellera Basin and Its Sedimentary Succession
3. Materials and Methods
4. Results
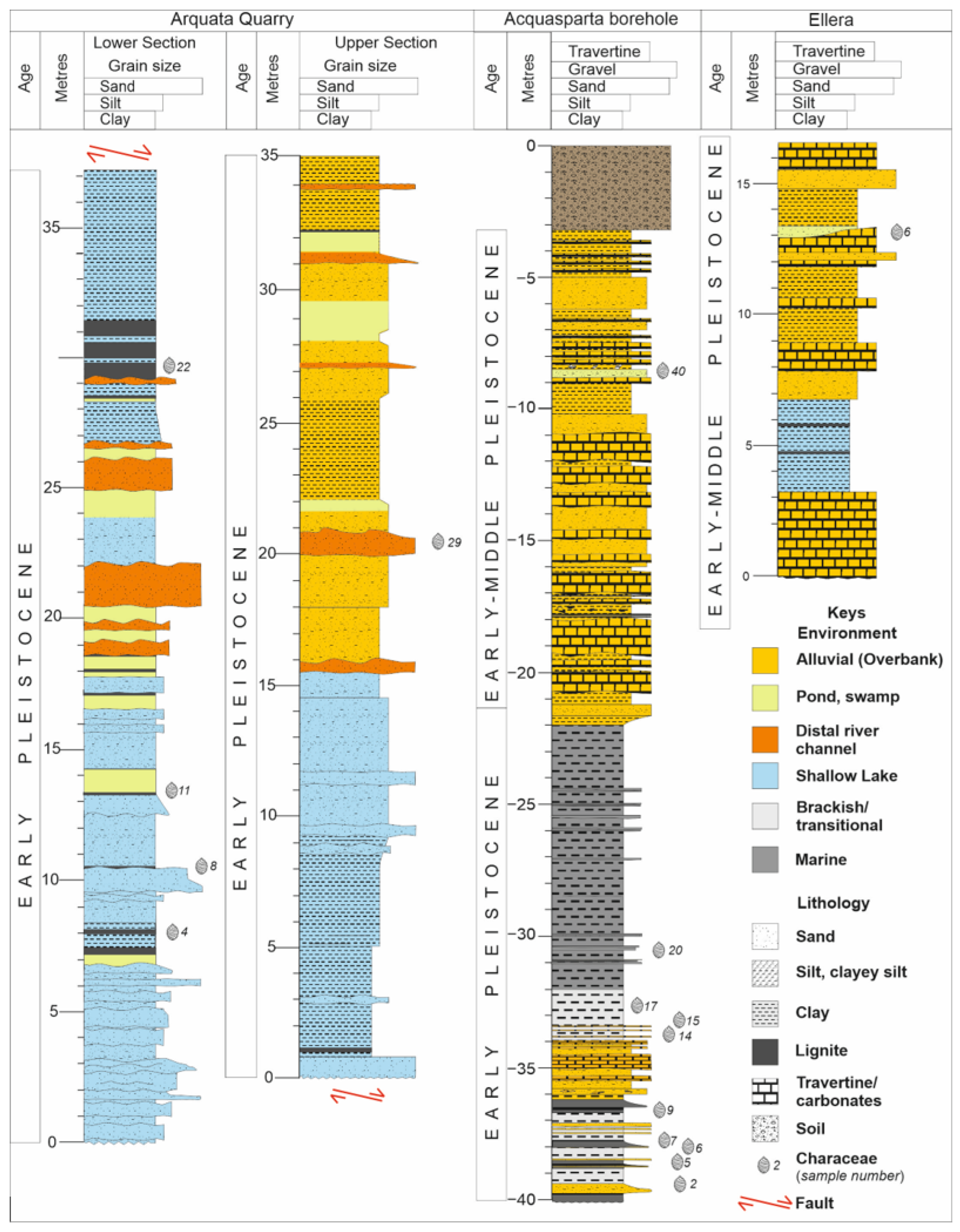
4.1. Charophyte Assemblages at the Arquata Quarry (Bevagna)
4.2. Charophyte Assemblages at Ellera—Ex Quasar Section
4.3. Charophyte Assemblages at Acquasparta Borehole
5. The Charophytes and Their Fossil Record
5.1. Genus Chara Linnaeus, 1753
5.2. Genera Nitellopsis and Lychnothamnus
5.3. Comparison of Tiberino Basin and Coeval Ilgin Basin Morphometric Data (Türkiye)
6. Discussion
6.1. Environmental Interpretations Supported by Charophyte Assemblages
6.2. Charophytes as Palaeoenvironmental Proxies
6.3. Insights from Comparative Palaeolimnology
7. Conclusions
- (1)
- Laboratory preparation of additional samples from the three sites with the aim of increasing the number of specimens. This approach will facilitate the collection of more data on morphological parameters and potential variations in relation to hydrological/climatic changes.
- (2)
- High-resolution stable isotope analyses of charophyte calcifications.
- (3)
- Palynological studies (at Acquasparta and Ellera sites) to reconstruct associated terrestrial vegetation.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Schneider, S.C.; García, A.; Martín-Closas, C.; Chivas, A.R. The role of charophytes (Charales) in past and present environments: An overview. Aquat. Bot. 2015, 120 Pt A, 2–6. [Google Scholar] [CrossRef]
- Strother, P.K.; Foster, C. A fossil record of land plant origins from charophyte algae. Science 2021, 373, 792–796. [Google Scholar] [CrossRef]
- García, A.; Chivas, A.R. Quaternary and extant euryhaline Lamprothamnium Groves (Charales) from Australia: Gyrogonite morphology and paleolimnological significance. J. Paleolimnol. 2004, 31, 321–341. [Google Scholar] [CrossRef]
- Soulié-Märsche, I.; García, A. Gyrogonites and oospores, complementary viewpoints to improve the study of the charophytes (Charales). Aquat. Bot. 2015, 120, 7–17. [Google Scholar] [CrossRef]
- Martín-Closas, C.; Soulié-Märsche, I.; Sanjuan, J. The Fossil Record and Evolution of Charophytes from European Data. In Charophytes of Europe; Schubert, H., Blindow, I., Nat, E., Korsch, H., Gregor, T., Denys, L., Stewart, N., van de Weyer, K., Romanov, R., Casanova, M.T., Eds.; Springer: Cham, Switzerland, 2024; pp. 153–175. [Google Scholar]
- Basilici, G. Sedimentary facies in an extensional and deep lacustrine depositional system: The Pliocene Tiberino basin, Central Italy. Sediment. Geol. 1997, 109, 73–94. [Google Scholar] [CrossRef]
- Coltorti, M.; Pieruccini, P. The southern East Tiber Basin (Spoleto, Central Italy): Geology and stratigraphy of the Plio-Pleistocene sediments. Il Quat. 1997, 10, 159–180. [Google Scholar]
- Bizzarri, R.; Corrado, P.; Magri, D.; Martinetto, E.; Esu, D.; Caprai, V.; Colacicchi, R.; Napoleone, G.; Albianelli, A.; Baldanza, A. Palaeoenvironmental and climatic inferences from the late early Pleistocene lacustrine deposits in the eastern Tiberino Basin (central Italy). Quat. Res. 2018, 90, 201–221. [Google Scholar] [CrossRef]
- Ambrosetti, P.; Basilici, G.; Capasso-Barbato, L.; Carboni, M.G.; Di Stefano, G.; Esu, D.; Gliozzi, E.; Petronio, C.; Sardella, R.; Squazzini, E. Il Pleistocene inferiore nel ramo sudoccidentale del bacino tiberino (Umbria): Aspetti litostratigraficie biostratigrafici. Il Quat. 1995, 8, 19–36. [Google Scholar]
- Melelli, L.; Barchi, M.; Brozzetti, F.; Lupattelli, A.; Mirabella, F.; Pazzaglia, F.; Pucci, S.; Saccucci, L. Morphotectonic evolution of High Tiber Valley (Umbria, Italy) related to an active low angle normal fault segmentation. Rend. Online Soc. Geol. Ital. 2010, 11, 629–630. [Google Scholar]
- Martinetto, E.; Bertini, A.; Basilici, G.; Baldanza, A.; Bizzarri, R.; Cherin, M.; Gentili, S.; Pontini, M.R. The plant record of the Dunarobba and Pietrafitta sites in the Plio-Pleistocene palaeoenvironmental context of central Italy. AMQ 2014, 27, 29–72. [Google Scholar]
- Colacicchi, R.; Bizzarri, R. Correlation between environmental evolution, historical settlement and cultural heritage upgrading in Valle Umbra (Central Italy). Geogr. Fis. Dinam. Quat. 2008, 31, 107–118. [Google Scholar]
- Bizzarri, R.; Albianelli, A.; Argenti, P.; Baldanza, A.; Colacicchi, R.; Napoleone, G. The latest continental filling of Valle Umbra (Tiber Basin, central Italy) dated to one million years ago by magnetostratigraphy. Il Quat. 2011, 24, 51–65. [Google Scholar]
- Cherin, M.; Bizzarri, R.; Buratti, N.; Caponi, T.; Grossi, F.; Kotsakis, T.; Pandolfi, L.; Pazzaglia, F.; Barchi, M.R. Multidisciplinary study of a new Quaternary mammal-bearing site from Ellera di Corciano (central Umbria, Italy): Preliminary data. Rend. Online Soc. Geol. Ital. 2012, 21, 1075–1077. [Google Scholar]
- Soulié-Märsche, I. Comparative Study of Gyrogonites of Extant and Fossil charophytes and Phylogeny of Extant Genera; Printing House of the Lindens: Millau, France, 1989; pp. 1–237. [Google Scholar]
- Feist, M.; Grambast-Fessard, N.; Guerlesquin, M.; Karol, K.; Lu, H.; McCourt, R.M.; Wang, Q.; Shenzen, Z. Charophyta. Treatise on Invertebrate Paleontology; The Geological Society of America: Boulder, CO, USA, 2005; Volume 1. [Google Scholar]
- Horn Af Rantzien, H. An annotated check-list of Genera of fossil Charophyta. Micropaleontology 1956, 2, 243–256. [Google Scholar] [CrossRef]
- Demirci, E.; Sanjuan, J.; Tunoğlu, C. Early Pleistocene charophyte flora from Dursunlu (Ilgın Basin, Turkey): Palaeoecological implications. Rev. Palaeobot. Palynol. 2023, 311, 104848. [Google Scholar] [CrossRef]
- Baldanza, A.; Bizzarri, R.; Posati, F.; Ravoni, M. Evidence of predation on early Pleistocene freshwater ostracods (Umbria, central Italy). Geosciences 2020, 10, 416. [Google Scholar] [CrossRef]
- Sanjuan, J.; Matamoros, D.; Casanovas-Vilar, I.; Vicente, A.; Moreno-Bedmar, J.A.; Martín Closas, C. Palaeoecology of Middle Miocene charophytes from the Vallès–Penedès and Vilanova basins (Catalonia, Spain). Hist. Biol. 2022, 35, 1665–1685. [Google Scholar] [CrossRef]
- Tuncer, A.; Tunoğlu, C. Early Pleistocene (Calabrian) Ostracoda assemblage and paleoenvironmental characteristics of the Fevzipaşa Formation, western Anatolia. Micropaleontology 2025, 61, 69–83. [Google Scholar] [CrossRef]
- Corillion, R. The Charophyceae of France and Western Europe; Works of the Botany Laboratory of the Faculty of Sciences of Angers: Angers, France, 1957. [Google Scholar]
- Krause, W. Charales (Charophyceae). In Süßwasserflora von Mitteleuropa, Band. 18; Ettl, H., Gärtner, G., Heynig, H., Mollenhauer, D., Eds.; Fischer: Stuggart, Germany, 1997; pp. 1–202. [Google Scholar]
- Soulié-Märsche, I.; Benkaddour, A.; El Khiatic, N.; Gemayel, P.; Ramdani, M. Charophytes, indicators of paleobathymetry of lake Tigalmamine (Middle Atlas, Morocco). Geobios 2008, 41, 435–444. [Google Scholar] [CrossRef]
- Mouronval, J.B.; Baoudouin, S.; Borel, N.; Soulie-Marche, I.; Grillas, P. Guide to the Characeae of Mediterranean France; National Office for Hunting and Wildlife: Paris, France, 2015; pp. 1–214. [Google Scholar]
- Barinova, S.; Romanov, R.; Nadir Solak, C. New Record of Chara hispida (L.) Hartm. (Streptophyta: Charophyceae, Charales) from the Işıklı Lake (Turkey) and Critical Checklist of Turkish Charophytes. Nat. Resour. Conserv. 2014, 2, 33–42. [Google Scholar] [CrossRef]
- Bellino, A.; Baldantoni, D. Biodiversity, ecology and distribution of Mediterranean Charophytes in Southern Italy. Plants 2023, 12, 3434. [Google Scholar] [CrossRef]
- Soulié-Märsche, I. Charophytes as lacustrine biomarkers during the Quaternary in North Africa. J. Afr. Earth Sci. 1991, 12, 341–351. [Google Scholar] [CrossRef]
- Soulié-Märsche, I. Apport des Charophytes fossiles à la recherche de phénomènes climatiques abrupts. Bull. De La Société Géologique De Fr. 1993, 164, 123–130. [Google Scholar]
- Baciu, C.; Negru, A.; Nicorici, E. Données préliminaires sur le contenu paléobotanique (Charophytes, fruits et graines) du Quaternaire récent de Cluj-Napoca (Roumanie). Stud. Univ. Babes-Bol. Geol. 1996, 41, 87–92. [Google Scholar]
- Becker, R.; Blindow, I.; Doege, A.; Franke, T.; Gregor, T.; Hamann, U.; Jäger, D.; Jorda, C.; Kabus, T.; Korsch, H.; et al. Kapitel 12: Beschreibung der characeen-arten Deutschlands. In Armleuchteralgen—Die Characeen Deutschlands; Arbeitsgruppe Characeen Deutschlands Lehrstuhl für Ökologie der Universität, Ed.; Springer Spektrum: Berlin/Heidelberg, Germany, 2016; pp. 209–572. [Google Scholar]
- Soulié-Märsche, I.; Benammi, M.; Gemayel, P. Biogeography of living and fossil Nitellopsis (Charophyta) in relationship to new finds from Morocco. J. Biogeogr. 2002, 29, 1703–1711. [Google Scholar] [CrossRef]
- Gianolla, D.; Negri, M.; Basso, D.; Sciunnach, D. Malacological response to Pleistocene sea-level change in the Northern Po Plain, N. Italy: Detailed palaeoenvironmental reconstructions from two Lombardian cores. Riv. Ital. Paleontol. Stratigr. 2010, 116, 79–102. [Google Scholar]
- Stefaniak, K.; Kovalchuk, O.; Kotusz, J.; Stachowicz-Rybka, R.; Mirosław-Grabowska, J.; Winter, H.; Niska, M.; Sobczyk, A.; Barkaszi, Z.; Kotowski, A.; et al. Pleistocene freshwater environments of Poland: A comprehensive study of fish assemblages based on a multi-proxy approach. Boreas 2021, 50, 457–476. [Google Scholar] [CrossRef]
- Pokrzywinski, K.; Sartain, B.; Greer, M.; Getsinger, K.; Fields, M. Optimizing conditions for Nitellopsis obtusa (starry stonewort) growth and bulbil germination in a controlled environment. Aquat. Bot. 2020, 160, 103163. [Google Scholar] [CrossRef]
- Larkin, D.J.; Monfils, A.K.; Boissezon, A.; Sleith, R.S.; Skawinski, P.M.; Welling, C.H.; Cahill, B.C.; Karol, K.G. Biology, ecology, and management of starry stonewort (Nitellopsis obtusa; Characeae): A Red-listed Eurasian green alga invasive in North America. Aquat. Bot. 2018, 148, 15–24. [Google Scholar] [CrossRef]
- Brzozowski, M.; Pełechaty, M.; Bogawski, P. A winner or a loser in climate change? Modelling the past, current, and future potential distributions of a rare charophyte species. Glob. Ecol. Conserv. 2022, 34, e02038. [Google Scholar] [CrossRef]
- Brzozowski, M.; Palomares Cabanilles, M.; Kowalewski, G.; Pełechaty, M. Environmental factors responsible for the gyrogonite formation by an endangered macroalga, Lychnothamnus barbatus, a fertility indicator of past and present lacustrine ecosystems. Limnologica 2019, 77, 125686. [Google Scholar] [CrossRef]
- Casanova, M.T.; Garcia, A.; Feist, M. The ecology and conservation of Lychnothamnus barbatus (Characeae). Acta Micropalentol. Sin. 2023, 20, 118–128. [Google Scholar]
- Rey-Boissezon, A.; Auderset Joye, D. Habitat requirements of charophytes—Evidence of species discrimination through distribution analysis. Aquat. Bot. 2015, 120 Pt A, 84–91. [Google Scholar] [CrossRef]
- Karol, K.G.; Skawinski, P.M.; McCourt, R.M.; Nault, M.E.; Evans, R.; Barton, M.E.; Berg, M.S.; Perleberg, D.J.; Hall, J.D. First discovery of the charophycean green alga Lychnothamnus barbatus (Charophyceae) extant in the New World. Am. J. Bot. 2017, 7, 1108–1116. [Google Scholar] [CrossRef] [PubMed]
- Schneider, S.C.; Nowak, P.; Von Ammon, U.; Ballot, A. Species differentiation in the genus Chara (Charophyceae): Considerable phenotypic plasticity occurs within homogenous genetic groups. Eur. J. Phycol. 2016, 51, 282–293. [Google Scholar] [CrossRef]
- Kundal, S.N. Late Pliocene-Early Pleistocene Charophyte Gyroronites from Mudstone horizon Underlying volcanic ash beds of Northwest Himalaya: Palaeoecological and Palaeoenvironmental implications. JGSR 2022, 7, 172–187. [Google Scholar] [CrossRef]
- Blindow, I.; Romanov, R.; Schubert, H.; Stewart, N.; van de Weyer, K. Chara hispida. In Charophytes of Europe; Schubert, H., Blindow, I., Nat, E., Korsch, H., Gregor, T., Denys, L., Stewart, N., van de Weyer, K., Romanov, R., Casanova, M.T., Eds.; Springer: Cham, Switzerland, 2024; pp. 527–541. [Google Scholar]
- Soulié-Märsche, I.; Baumhauer, R.; Schütt, B. Palaeoenvironmental significance of charophyte gyrogonites from Laguna de Gallocanta (Aragón, Spain). Bot. Lett. 2018, 165, 35–44. [Google Scholar] [CrossRef]
- Détriché, S.; Bréhéret, J.-G.; Soulié-Märsche, I.; Karrat, L.; Macaire, J.-J. Late Holocene water level fluctuations of Lake Afourgagh (Middle-Atlas Mountains, Morocco) inferred from charophyte remains. Palaeogeogr. Palaeoclim. Palaeoecol. 2009, 283, 134–147. [Google Scholar] [CrossRef]
- Soulié-Märsche, I. Charophytes, indicators for low salinity phases in North African sebkhet. J. Afr. Earth Sci. 2008, 51, 69–76. [Google Scholar] [CrossRef]
- Demirci, E.; Sanjuan, J.; Tunoğlu, C.; Tuncer, A.; Bulut, Y. Late Miocene aquatic flora from the Yalvaç Basin (Central Anatolia, Türkiye): Biostratigraphy and paleoecology. Rev. Palaeobot. Palynol. 2025, 332, 105221. [Google Scholar] [CrossRef]
- Gibbard, P.L.; Head, M.J. Early–Middle Pleistocene transitions: An overview and recommendation for the defining boundary. Quatern. Int. 2020, 540, 3–7. [Google Scholar] [CrossRef]



| Taxa | Preferred Environmental Conditions | Palaeoecological Meaning |
|---|---|---|
| Nitellopsis obtusa | Deep, cold, stable, oligotrophic lakes | Stable low-energy lake |
| Lychnothamnus barbatus | Oligo- to mesotrophic lakes, low nutrient and energy levels, cold, phreatic waters | Stable lacustrine conditions |
| Chara cf. hispida | Oligo- to mesotrophic lakes, cold | Moderate trophic levels, decrease in water depth |
| Chara cf. vulgaris | Variable freshwater habitats, temporary ponds | Resilience to environmental change |
| Tiberino Basin Sites | Arquata Quarry | Ellera | Acquasparta Borehole | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Taxa\Samples | 4 | 8 | 11 | 22 | 29 | 6 | 2 | 5 | 6 | 7 | 9 | 14 | 15 | 17 | 20 | 40 |
| Chara cf. hispida | 10 | - | - | 20 | 25 | - | - | 2 | 75 | 29 | 36 | - | 1 | - | 1 | 5 |
| Chara cf. vulgaris | 6 | 4 | 2 | 10 | 16 | 10 | 4 | - | - | - | - | 28 | - | 1 | - | - |
| Nitellopsis obtusa | 2 | 50 | - | 120 | 10 | - | - | - | - | - | - | - | - | - | - | - |
| Lychnothamnus barbatus | 1 | - | 6 | 10 | 5 | 12 | 25 | - | 8 | 4 | - | 10 | - | - | - | 13 |
| Taxa | Height/Width | ISI | Convolutions |
|---|---|---|---|
| Chara cf. vulgaris (this paper) Chara vulgaris [18] | 800–760/700–520 | 107–146 | 7–11 |
| 746–414/465–256 | 115–209 | 6–15 | |
| Chara cf. hispida (this paper) Chara hispida [18] | 820–720/680–560 | 113–136 | 8–11 |
| 1143–721/707–432 | 126–197 | 8–14 | |
| Nitellopsis obtusa (this paper) Nitellopsis obtusa [18] | 1120–840/920–800 | 105–128 | 5–7 |
| 1198–836/1015–645 | 101–140 | 6–10 | |
| Lichnothamnus barbatus (this paper) Lichnothamnus barbatus [18] | 820–760/730–600 | 106–133 | 7–9 |
| 912–854/671–650 | 130–137 | 11 |
| Site | Dominant Taxa | Inferred Environment | Climatic Implications |
|---|---|---|---|
| Tiberino Basin (Arquata Quarry) | Nitellopsis obtusa, Lychnothamnus barbatus, Chara cf. hispida, Chara cf. vulgaris | Stable freshwater lakes | Humid cold phases |
| Tiberino Basin (Acquasparta borehole) | Chara cf. hispida, Chara cf. vulgaris, Lychnothamnus barbatus | Temporary ponds alternated with stable water level lake | Increased aridity |
| Ellera “satellite” Basin | Lichnothamnus barbatus, Chara cf. vulgaris | Ephemeral lakes | Increased aridity |
| Laguna de Gallocanta [45] | Chara vulgaris, Lamprothamnium papulosum | Ephemeral lakes | Semi-arid oscillations |
| Lake Afourgagh (Morocco) [46] | Chara aspera, Chara hispida, Chara globularis | Alternating lake water levels | Holocene climatic variability |
| Ilgin Basin (Türkiye) [18] | Chara vulgaris, Chara globularis (palustrine interval); Chara hispida, Nitellopsis obtusa (lake interval) | Very shallow eutrophic lake evolved to a shallow, stable, oligotrophic, alkaline, and oligohaline lake | Climatic oscillation during the Günz glaciation |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baldanza, A.; Angelini, P.; De Santis, A.M.; Nalli, I.; Bizzarri, R. What Do Fossil charophytes Whisper to Us? Palaeoecological and Palaeoenvironmental Reports from Pleistocene Continental Deposits of Umbria (Central Italy). Geosciences 2025, 15, 392. https://doi.org/10.3390/geosciences15100392
Baldanza A, Angelini P, De Santis AM, Nalli I, Bizzarri R. What Do Fossil charophytes Whisper to Us? Palaeoecological and Palaeoenvironmental Reports from Pleistocene Continental Deposits of Umbria (Central Italy). Geosciences. 2025; 15(10):392. https://doi.org/10.3390/geosciences15100392
Chicago/Turabian StyleBaldanza, Angela, Paola Angelini, Anna Maria De Santis, Isabella Nalli, and Roberto Bizzarri. 2025. "What Do Fossil charophytes Whisper to Us? Palaeoecological and Palaeoenvironmental Reports from Pleistocene Continental Deposits of Umbria (Central Italy)" Geosciences 15, no. 10: 392. https://doi.org/10.3390/geosciences15100392
APA StyleBaldanza, A., Angelini, P., De Santis, A. M., Nalli, I., & Bizzarri, R. (2025). What Do Fossil charophytes Whisper to Us? Palaeoecological and Palaeoenvironmental Reports from Pleistocene Continental Deposits of Umbria (Central Italy). Geosciences, 15(10), 392. https://doi.org/10.3390/geosciences15100392








