Assessing Shallow Groundwater Quality Around the Sheba Leather Tannery Area, Wikro, North Ethiopia: A Geophysical and Hydrochemical Study
Abstract
1. Introduction
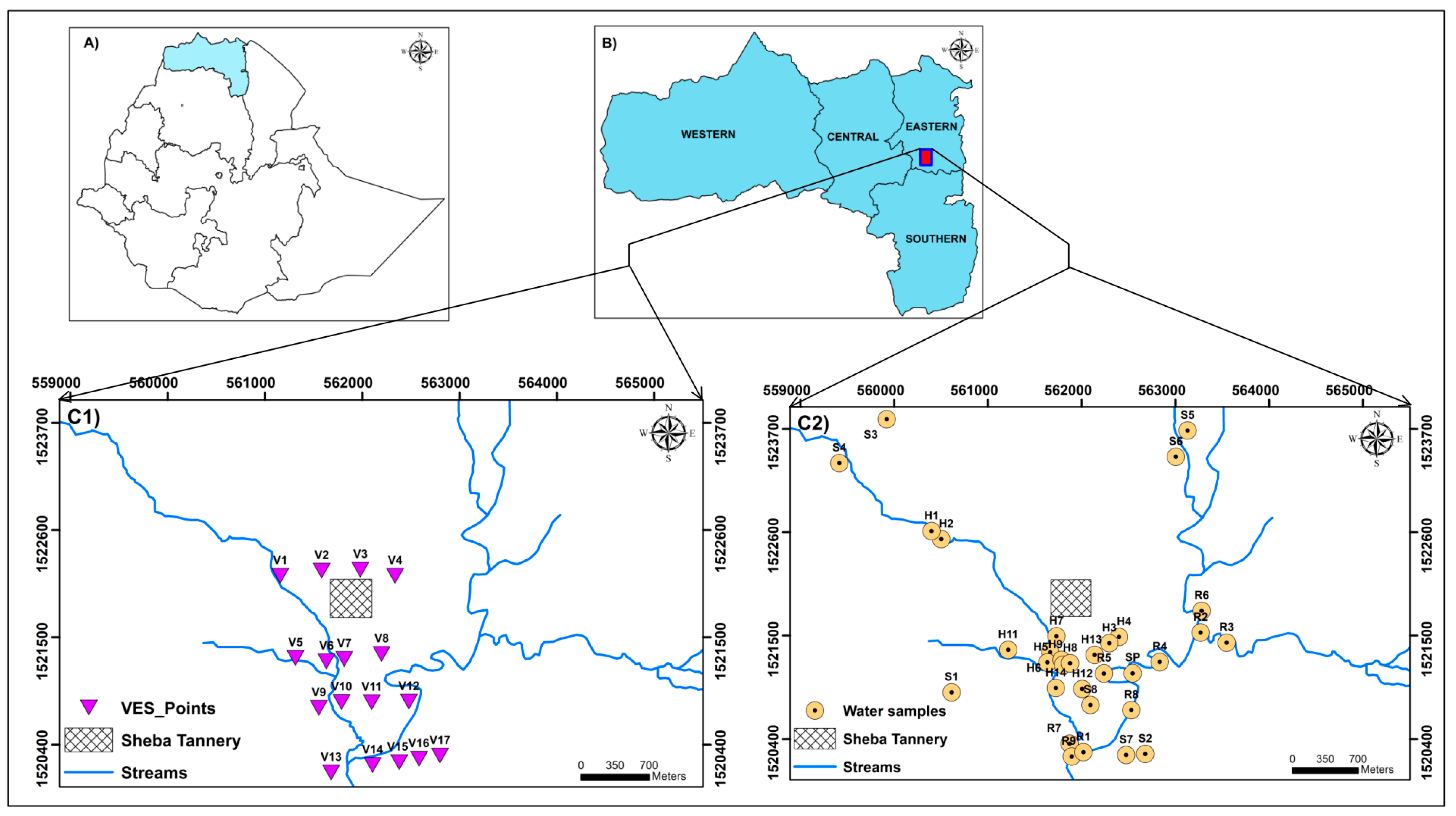
2. Study Area Description
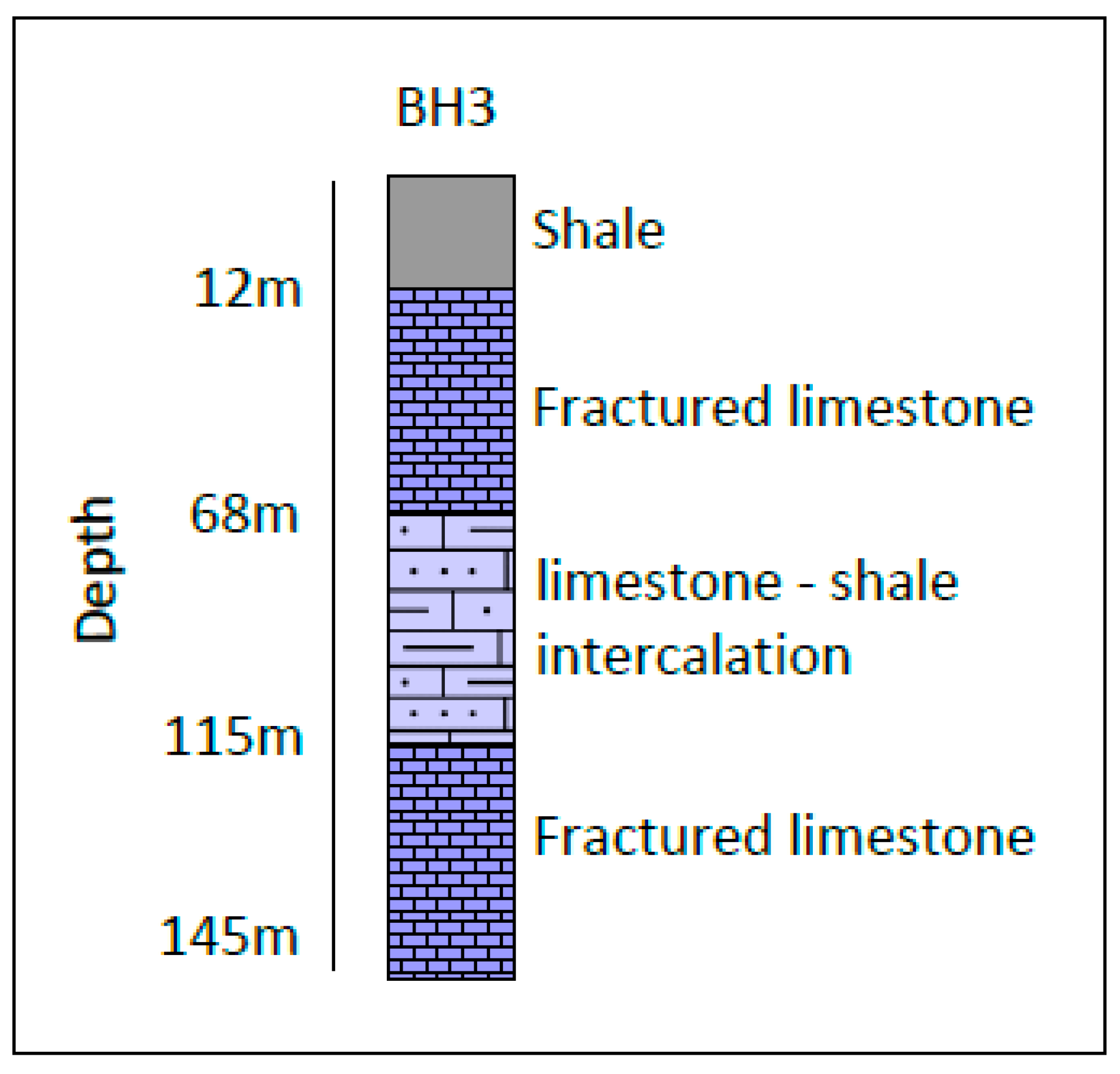
3. Geological and Hydrogeological Settings
4. Materials and Methods
4.1. Geophysical Survey Using Vertical Electrical Soundings (VES)
4.2. Water Samples, Analyses, and Suitability Parameters
5. Results and Discussion
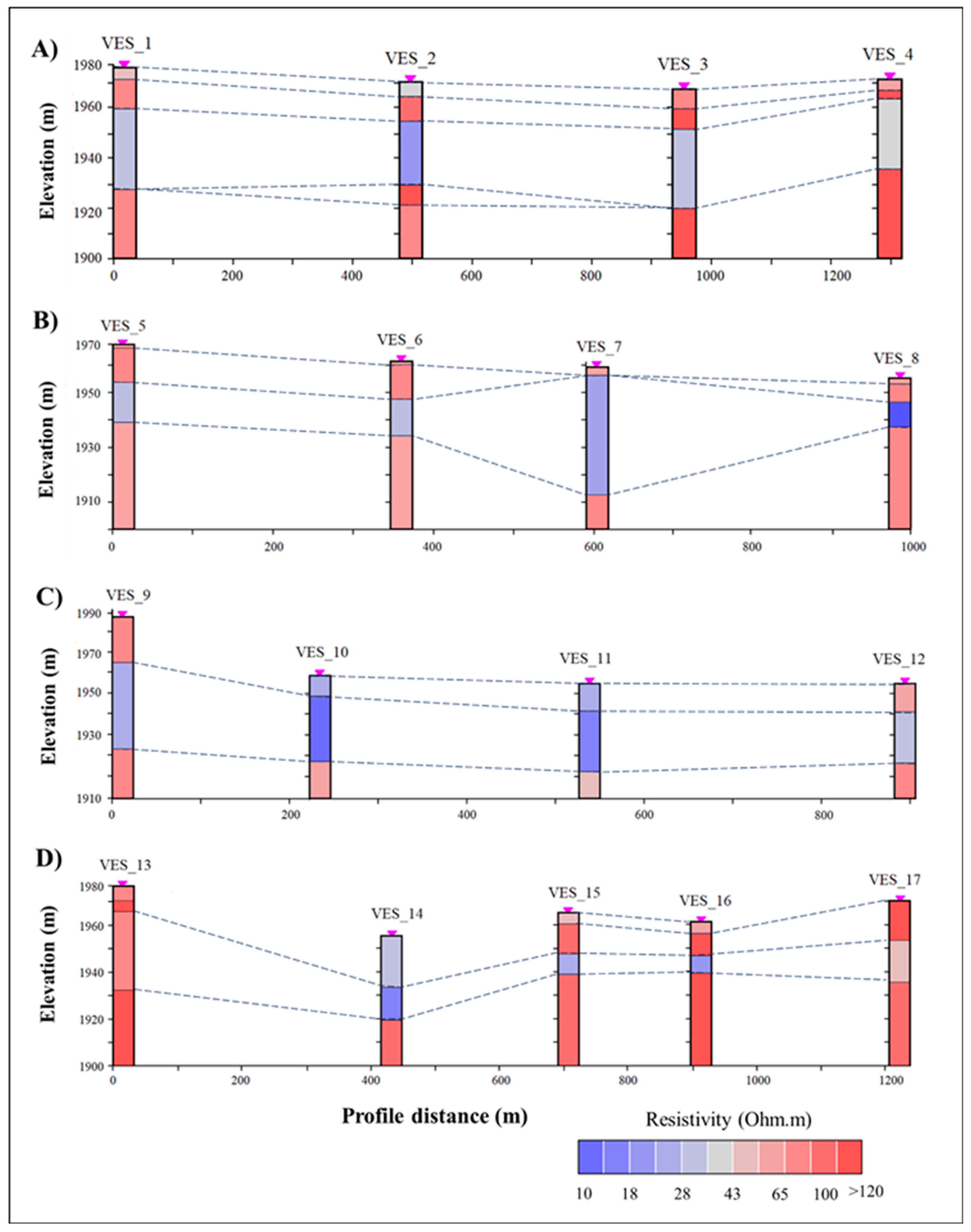
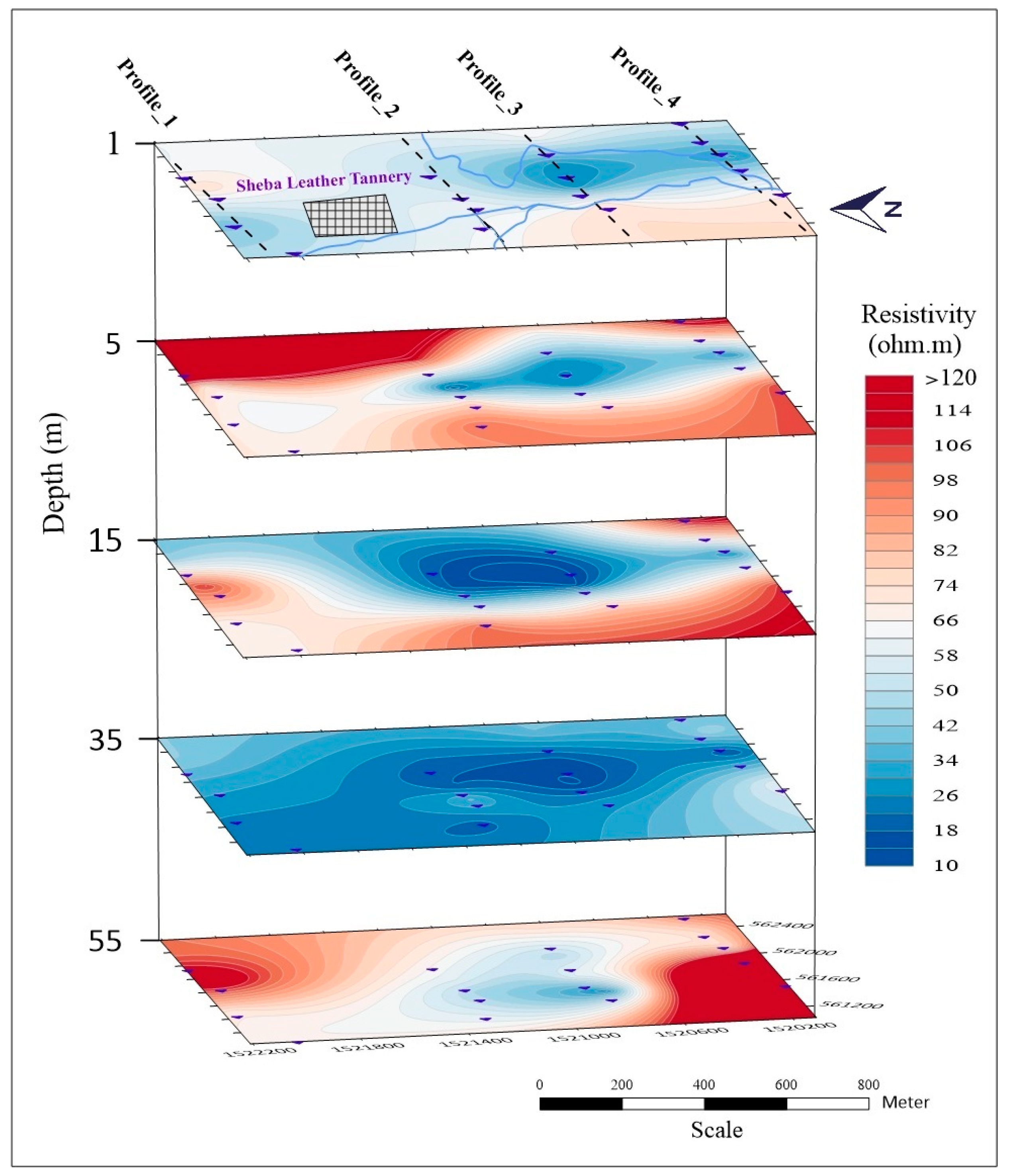
5.1. Geophysical Resistivity Survey
5.1.1. The Subsurface Resistivity Model of the Study Area
5.1.2. The 3D Resistivity Slice Model
5.2. Hydrochemical Characterization
5.2.1. Physicochemical Characteristics of the Water Samples
5.2.2. Hydrogeochemical Processes Interpretation
5.2.3. Correlation Among Selected Parameters
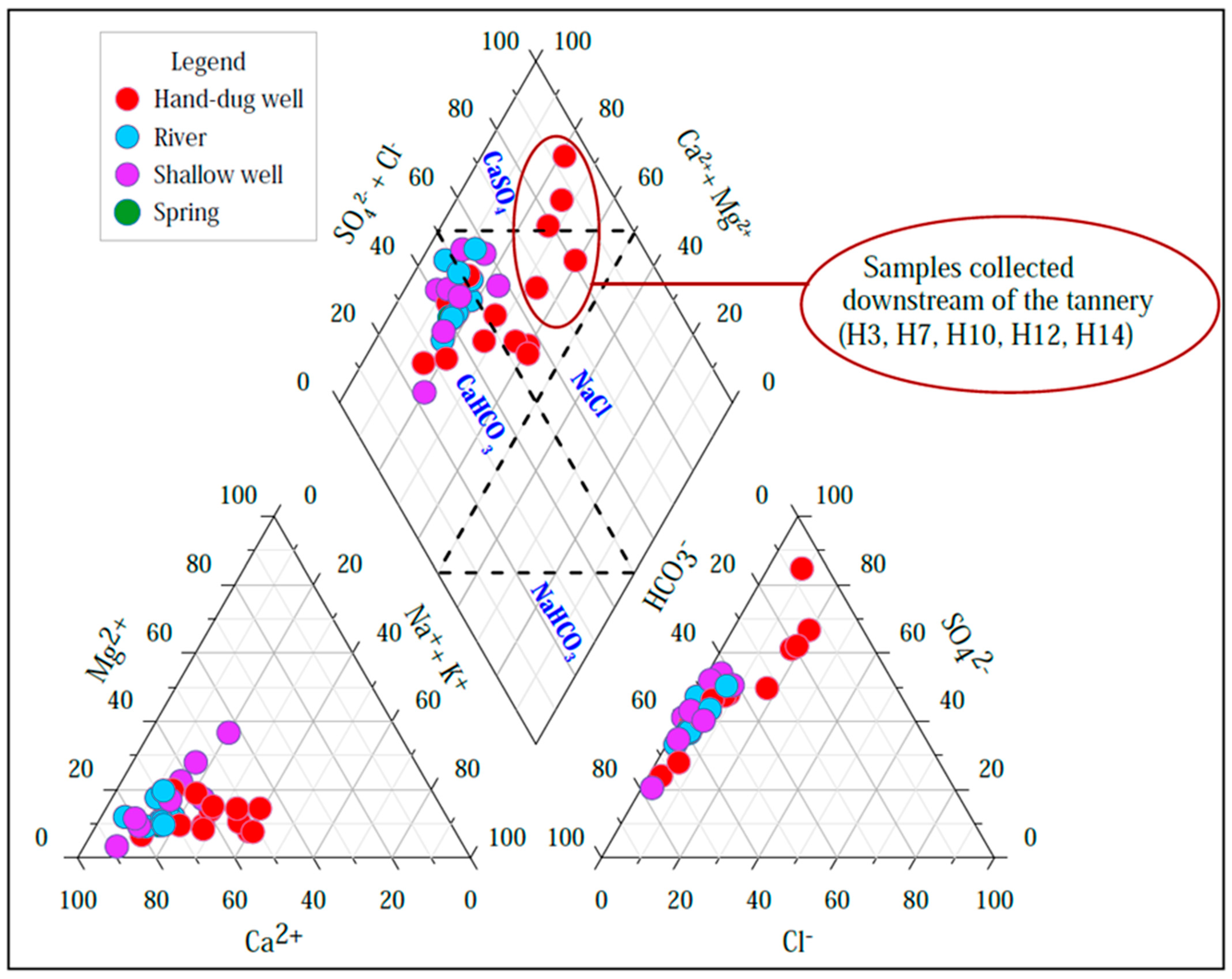
5.2.4. Hydrochemical Facies
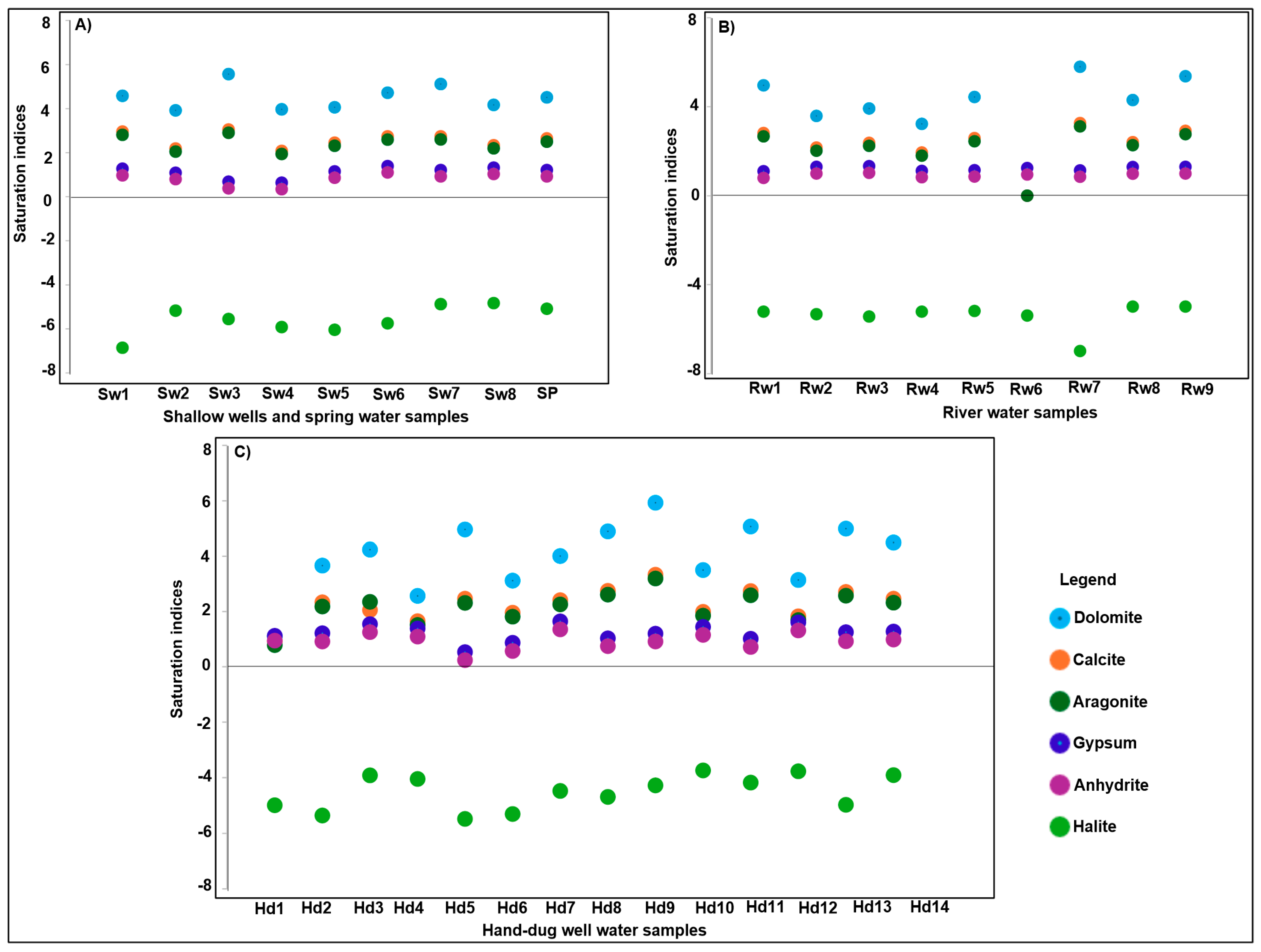
5.2.5. Saturation Indices (SI)
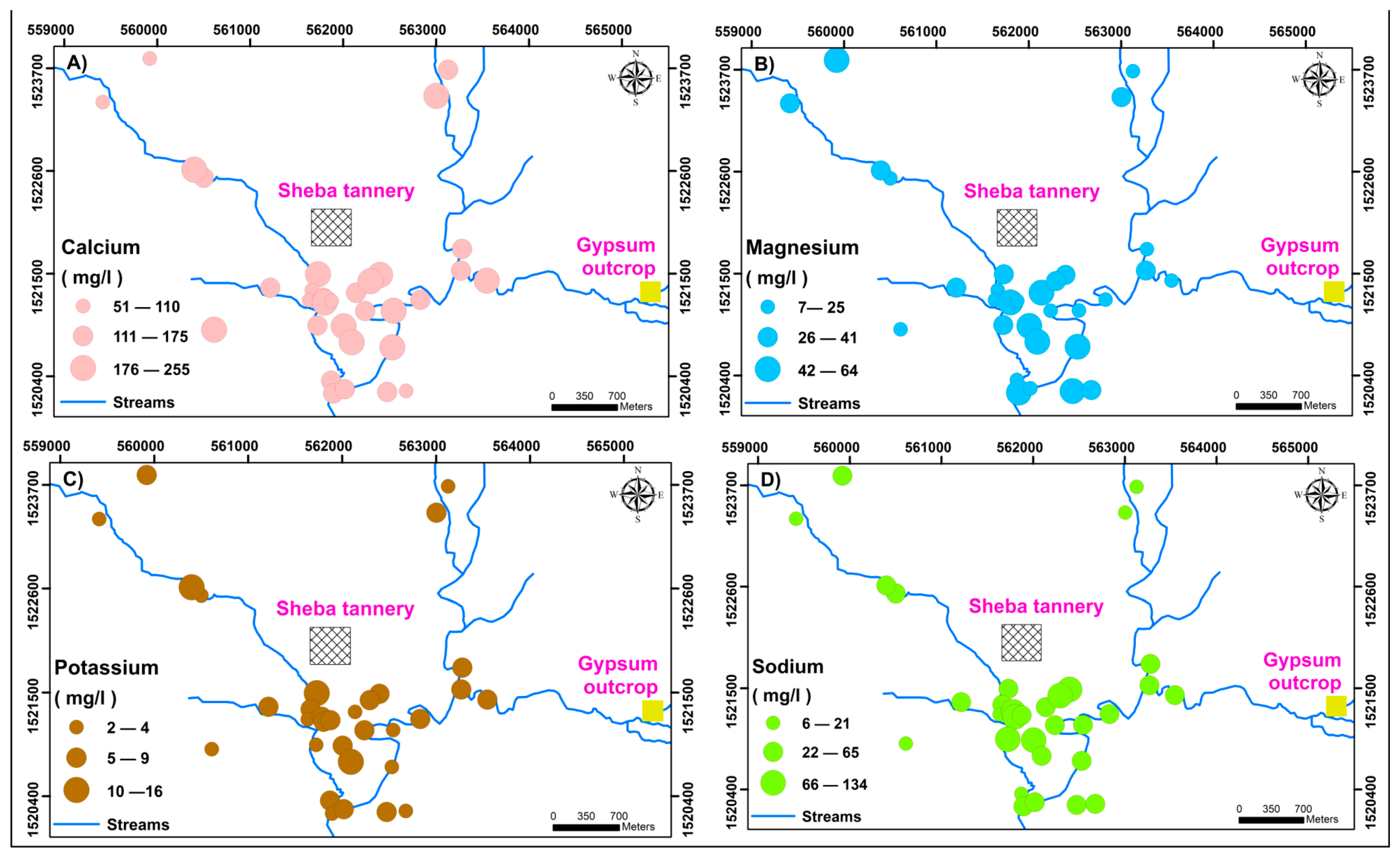
5.2.6. Evaluation of Pollution Status Using Major Cations Concentration
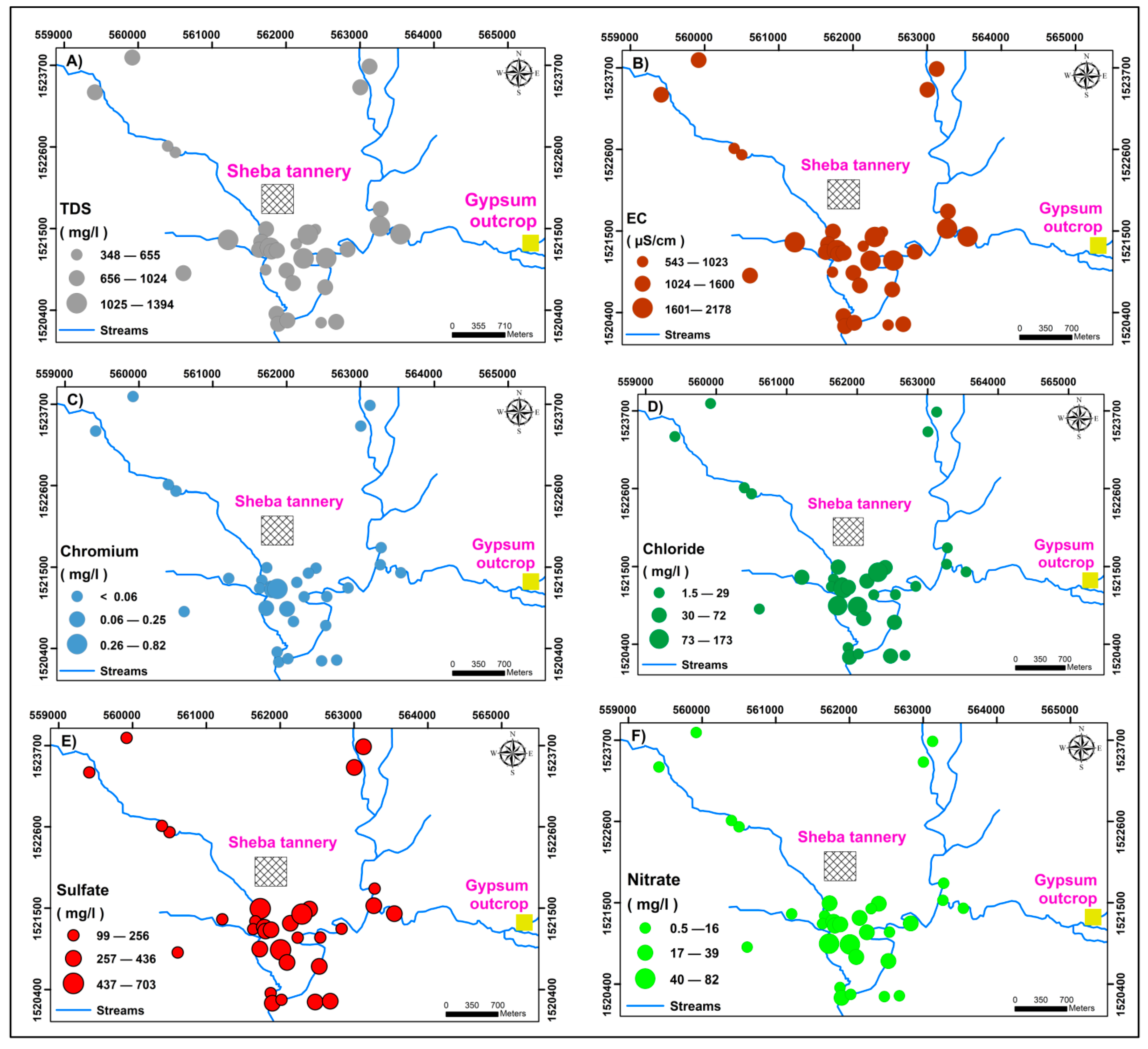
5.2.7. Evaluation of Pollution Status Using EC, TDS, Anions, and Selected Heavy Metals
5.3. Water Quality Index (WQI)
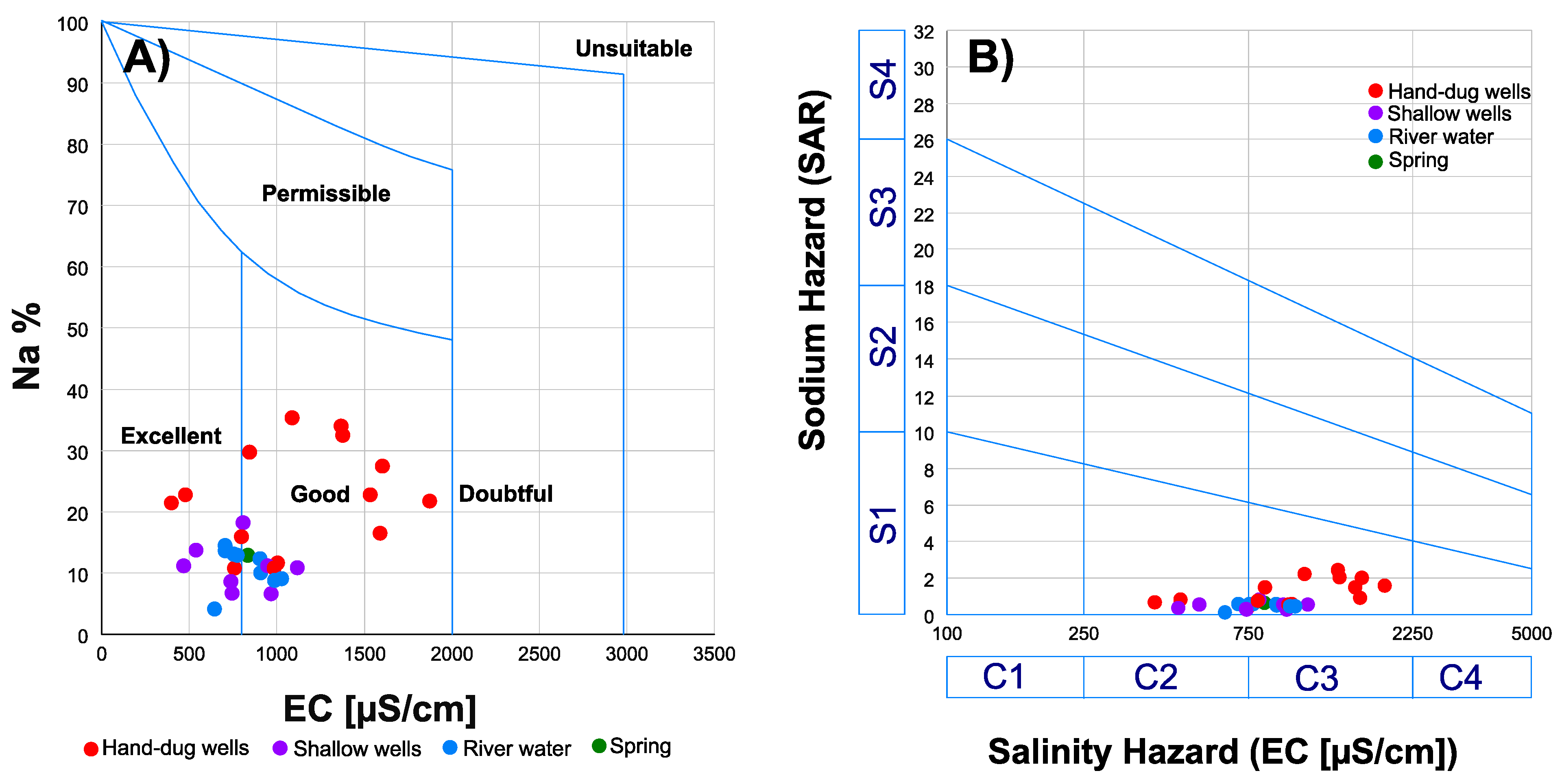
5.4. Quality Suitability Assessment for Irrigation
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lofrano, G.; Meriç, S.; Zengin, G.E.; Orhon, D. Chemical and biological treatment technologies for leather tannery area chemicals and wastewaters: A review. Sci. Total Environ. 2013, 461, 265–281. [Google Scholar] [CrossRef]
- Magesh, N.S.; Chandrasekar, N.; Elango, L. Trace element concentrations in the groundwater of the Tamiraparani river basin, South India: Insights from human health risk and multivariate statistical techniques. Chemosphere 2017, 185, 468–479. [Google Scholar] [CrossRef] [PubMed]
- Vignati DA, L.; Ferrari, B.J.; Roulier, J.L.; Coquery, M.; Szalinska, E.; Bobrowski, A.; Czaplicka, A.; Kownacki, A.; Dominik, J. Chromium bioavailability in aquatic systems impacted by tannery area wastewaters. Part 1: Understanding chromium accumulation by indigenous chironomids. Sci. Total Environ. 2019, 653, 401–408. [Google Scholar] [CrossRef]
- Tadesse, G.L.; Guya, T.K.; Walabu, M. Impacts of tannery area effluent on environments and human health: A review article. Adv. Life Sci. Technol. 2017, 54, 58–67. [Google Scholar]
- Hammons, A.S.; Huff, J.E.; Braunstein, H.M.; Drury, J.S.; Shriner, C.R.; Lewis, E.B.; Whitfield, B.L.; Towill, L.E. Reviews of the Environmental Effects of Pollutants: IV. Cadmium; Oak Ridge National Lab: Oak Ridge, TN, USA, 1978. [Google Scholar]
- Schwingl, P.J.; Lunn, R.M.; Mehta, S.S. A tiered approach to prioritizing registered pesticides for potential cancer hazard evaluations: Implications for decision making. Environ. Health 2021, 20, 13. [Google Scholar] [CrossRef] [PubMed]
- McCance, W.; Jones, O.; Cendón, D.; Edwards, M.; Surapaneni, A.; Chadalavada, S.; Currell, M. Decoupling wastewater impacts from hydrogeochemical trends in impacted groundwater resources. Sci. Total Environ. 2021, 774, 145781. [Google Scholar] [CrossRef]
- Aristone, C.; Mehdi, H.; Hamilton, J.; Bowen, K.L.; Currie, W.J.; Kidd, K.A.; Balshine, S. Impacts of wastewater treatment plants on benthic macroinvertebrate communities in summer and winter. Sci. Total Environ. 2022, 820, 153224. [Google Scholar] [CrossRef] [PubMed]
- López Arias, T.R.; Franco, D.; Medina, L.; Benítez, C.; Villagra, V.; McGahan, S.; Duré, G.M.; Kurita-Oyamada, H.G. Removal of Chromium (III) and reduction in toxicity in a primary tannery effluent using two floating macrophytes. Toxics 2024, 12, 152. [Google Scholar] [CrossRef] [PubMed]
- Urbina-Suarez, N.A.; Ayala-González, D.D.; Rivera-Amaya, J.D.; Barajas-Solano, A.F.; Machuca-Martínez, F. Evaluation of the light/dark cycle and concentration of tannery wastewater in the production of biomass and metabolites of industrial interest from microalgae and cyanobacteria. Water 2022, 14, 346. [Google Scholar] [CrossRef]
- Belay, A.A. Impacts of chromium from tannery effluent and evaluation of alternative treatment options. J. Environ. Prot. 2010, 1, 53. [Google Scholar] [CrossRef]
- Fernandez, M.; Pereira, P.P.; Agostini, E.; González, P.S. Impact assessment of bioaugmented tannery effluent discharge on the microbiota of water bodies. Ecotoxicology 2020, 29, 973–986. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.A.; Costa, F.B.; Fenton, O.; Jordan, P.; Fennell, C.; Mellander, P.-E. Using a multi-dimensional approach for catchment scale herbicide pollution assessments. Sci. Total Environ. 2020, 747, 141232. [Google Scholar] [CrossRef]
- Montalvão, M.F.; da Silva Castro, A.L.; de Lima Rodrigues, A.S.; de Oliveira Mendes, B.; Malafaia, G. Impacts of tannery effluent on development and morphological characters in a neotropical tadpole. Sci. Total Environ. 2018, 610, 1595–1606. [Google Scholar] [CrossRef]
- Comber, S.; Gardner, M.; Ansell, L.; Ellor, B. Assessing the impact of wastewater treatment works effluent on downstream water quality. Sci. Total Environ. 2022, 845, 157284. [Google Scholar] [CrossRef] [PubMed]
- De Sousa, A.; Wilhelm, C.M.; Da Silva, C.E.M.; Goldoni, A.; Rodrigues, M.A.S.; Da Silva, L.B. Treated tannery effluent and its impact on the receiving stream water: Physicochemical characterization and cytogenotoxic evaluation using the Allium cepa test. Protoplasma 2023, 260, 949–954. [Google Scholar] [CrossRef] [PubMed]
- Mengistie, E.; Ambelu, A.; Van Gerven, T.; Smets, I. Impact of tannery effluent on the self-purification capacity and biodiversity level of a river. Bull. Environ. Contam. Toxicol. 2016, 96, 369–375. [Google Scholar] [CrossRef]
- Ngobeni, P.V.; Mpofu, A.B.; Ranjan, A.; Welz, P.J. A Critical Review of Systems for Bioremediation of Tannery Effluent with a Focus on Nitrogenous and Sulfurous Species Removal and Resource Recovery. Processes 2024, 12, 1527. [Google Scholar] [CrossRef]
- Younas, F.; Bibi, I.; Afzal, M.; Al-Misned, F.; Niazi, N.K.; Hussain, K.; Shahid, M.; Shakil, Q.; Ali, F.; Wang, H. Unveiling distribution, hydrogeochemical behavior and environmental risk of chromium in tannery wastewater. Water 2023, 15, 391. [Google Scholar] [CrossRef]
- García-Valero, A.; Martínez-Martínez, S.; Faz, Á.; Terrero, M.A.; Muñoz, M.Á.; Gómez-López, M.D.; Acosta, J.A. Treatment of wastewater from the tannery industry in a constructed wetland planted with Phragmites australis. Agronomy 2020, 10, 176. [Google Scholar] [CrossRef]
- Gebrekidan, A.; Weldegebriel, Y.; Hadera, A.; Van der Bruggen, B. Toxicological assessment of heavy metals accumulated in vegetables and fruits grown in Ginfel river near Sheba Tannery, Tigray, Northern Ethiopia. Ecotoxicol. Environ. Saf. 2013, 95, 171–178. [Google Scholar] [CrossRef] [PubMed]
- Gebrekidan, A.; Gebresellasie, G.; Mulugeta, A. Environmental impacts of Sheba tannery (Ethiopia) effluents on the surrounding water bodies. Bull. Chem. Soc. Ethiop. 2009, 23. [Google Scholar] [CrossRef]
- Amabye, T.G. Plant, soil and water pollution due to tannery effluent a case study from Sheb Tannery PLC, Wukro Tigray, Ethiopia. Science 2015, 3, 47–51. [Google Scholar]
- Gebru, H.; Tadesse, N.; Konka, B. Impact of waste disposal from tannery on surface and groundwater, Sheba leather factory near Wurko, Tigray, Northern Ethiopia. Int. J. Earth Sci. Eng. 2012, 5, 665–672. [Google Scholar]
- Gebreyohannes, T.; De Smedt, F.; Walraevens, K.; Gebresilassie, S.; Hussien, A.; Hagos, M.; Amare, K.; Deckers, J.; Gebrehiwot, K. Regional groundwater flow modeling of the Geba basin, northern Ethiopia. Hydrogeol. J. 2017, 25, 639. [Google Scholar] [CrossRef]
- Alemayehu, T.; Leis, A.; Dietzel, M. Environmental isotope and hydrochemical characteristics of groundwater in central portion of Mekelle sedimentary outlier, northern Ethiopia. J. Afr. Earth Sci. 2020, 171, 103953. [Google Scholar] [CrossRef]
- Abera, K.A.; Gebreyohannes, T.; Abrha, B.; Hagos, M.; Berhane, G.; Hussien, A.; Belay, A.S.; Van Camp, M.; Walraevens, K. Vulnerability mapping of groundwater resources of mekelle city and surroundings, tigray region, Ethiopia. Water 2022, 14, 2577. [Google Scholar] [CrossRef]
- Damo, R.; Icka, P. Evaluation of Water Quality Index for Drinking Water. Pol. J. Environ. Stud. 2013, 22, 1045–1051. [Google Scholar]
- Gupta, S.; Gupta, S.K. A critical review on water quality index tool: Genesis, evolution and future directions. Ecol. Inform. 2021, 63, 101299. [Google Scholar] [CrossRef]
- Tyagi, S.; Sharma, B.; Singh, P.; Dobhal, R. Water quality assessment in terms of water quality index. Am. J. Water Resour. 2013, 1, 34–38. [Google Scholar] [CrossRef]
- Khan, I.; Zakwan, M.; Pulikkal, A.K.; Lalthazula, R. Impact of unplanned urbanization on surface water quality of the twin cities of Telangana state, India. Mar. Pollut. Bull. 2022, 185, 114324. [Google Scholar] [CrossRef]
- Arkin, Y.; Beyth, M.; Dow, D.; Levitte, D.; Temesgen Haile, T.H. Geological Map of Mekele Sheet Area ND 37-11, Tigre Province, 1: 250.000; Imperial Ethiopian Governement, Ministry of Mines, Geological Survey: Addis Ababa, Ethiopia, 1971. [Google Scholar]
- Kazmin, V.; Shifferaw, A.; Balcha, T. The Ethiopian basement: Stratigraphy and possible manner of evolution. Geol. Rundsch. 1978, 67, 531–546. [Google Scholar] [CrossRef]
- Tadesse, T. Structure across a possible intra-oceanic suture zone in the low-grade Pan-African rocks of northern Ethiopia. J. Afr. Earth Sci. 1996, 23, 375–381. [Google Scholar] [CrossRef]
- Alene, M.; Ruffini, R.; Sacchi, R. Geochemistry and geotectonic setting of Neoproterozoic rocks from northern Ethiopia (Arabian-Nubian Shield). Gondwana Res. 2000, 3, 333–347. [Google Scholar] [CrossRef]
- Kazmin, V. The geology of Ethiopia. Ethiop. Inst. Geol. Surv. Note 1972, 821012–821051. [Google Scholar]
- Abdelsalam, M.G.; Stern, R.J. Sutures and shear zones in the Arabian-Nubian Shield. J. Afr. Earth Sci. 1996, 23, 289–310. [Google Scholar] [CrossRef]
- Hagos, M.; Gebreyohannes, T.; Amare, K.; Hussien, A.; Berhane, G.; Walraevens, K.; Koeberl, C.; van Wyk de Vries, B.; Cavalazzi, B. Tectonic link between the Neoproterozoic dextral shear fabrics and Cenozoic extension structures of the Mekelle basin, Northern Ethiopia. Int. J. Earth Sci. 2020, 109, 1957–1974. [Google Scholar] [CrossRef]
- Kaczor, D.; Sotysiak, M. Geophysical investigation in contamination hazard studies. Ecohydrol. Hydrobiol. 2006, 6, 223–232. [Google Scholar] [CrossRef]
- Lopes, D.D.; Silva, S.M.; Fernandes, F.; Teixeira, R.S.; Celligoi, A.; Dall’Antônia, L.H. Geophysical technique and groundwater monitoring to detect leachate contamination in the surrounding area of a landfill–Londrina (PR–Brazil). J. Environ. Manag. 2012, 113, 481–487. [Google Scholar] [CrossRef]
- Donohue, S.; McCarthy, V.; Rafferty, P.; Orr, A.; Flynn, R. Geophysical and hydrogeological characterisation of the impacts of on-site wastewater treatment discharge to groundwater in a poorly productive bedrock aquifer. Sci. Total Environ. 2015, 523, 109–119. [Google Scholar] [CrossRef]
- Bello, A.; Muztaza, N.M.; Abir, I.A.; Zakaria, M.T.; Bello, J. Geophysical performance of subsurface characterization for site suitability in construction purpose. Phys. Chem. Earth Parts A/B/C 2022, 128, 103296. [Google Scholar] [CrossRef]
- Ihbach, F.-Z.; Kchikach, A.; Jaffal, M.; El Azzab, D.; Khadiri Yazami, O.; Jourani, E.-S.; Peña Ruano, J.A.; Olaiz, O.A.; Dávila, L.V. Geophysical prospecting for groundwater resources in phosphate deposits (Morocco). Minerals 2020, 10, 842. [Google Scholar] [CrossRef]
- Gong, S.; Zhao, K.; Wang, M.; Yan, S.; Li, Y.; Yang, J. Application of Integrated Geological and Geophysical Surveys on the Exploration of Chalcedony Deposits: A Case Study on Nanhong Agate in Liangshan, China. Minerals 2024, 14, 677. [Google Scholar] [CrossRef]
- Ahmed II, J.B.; Pradhan, B.; Mansor, S.; Yusoff, Z.M.; Ekpo, S.A. Aquifer potential assessment in termites manifested locales using geo-electrical and surface hydraulic measurement parameters. Sensors 2019, 19, 2107. [Google Scholar] [CrossRef]
- Canzoneri, A.; Capizzi, P.; Martorana, R.; Albano, L.; Bonfardeci, A.; Costa, N.; Favara, R. Geophysical Constraints to Reconstructing the Geometry of a Shallow Groundwater Body in Caronia (Sicily). Water 2023, 15, 3206. [Google Scholar] [CrossRef]
- Benson, R.C. Geophysical techniques for subsurface site characterization. In Geotechnical Practice for Waste Disposal; Springer: Berlin/Heidelberg, Germany, 1993; pp. 311–357. [Google Scholar]
- Metwaly, M.; AlFouzan, F. Application of 2-D geoelectrical resistivity tomography for subsurface cavity detection in the eastern part of Saudi Arabia. Geosci. Front. 2013, 4, 469–476. [Google Scholar] [CrossRef]
- Maharaj, R.; Kumar, S.; Rollings, N.; Antoniou, A. Groundwater detection using resistivity at Nubutautau village in Viti Levu in Fiji. Water 2023, 15, 4156. [Google Scholar] [CrossRef]
- Mohamaden, M.I.; Ehab, D. Application of electrical resistivity for groundwater exploration in Wadi Rahaba, Shalateen, Egypt. NRIAG J. Astron. Geophys. 2017, 6, 201–209. [Google Scholar] [CrossRef]
- Raji, W.; Adeoye, T. Geophysical mapping of contaminant leachate around a reclaimed open dumpsite. J. King Saud Univ.-Sci. 2017, 29, 348–359. [Google Scholar] [CrossRef]
- Riwayat, A.I.; Nazri, M.A.A.; Abidin, M.H.Z. Application of electrical resistivity method (ERM) in groundwater exploration. J. Phys. Conf. Ser. 2018, 995, 012094. [Google Scholar] [CrossRef]
- Bernstone, C.; Dahlin, T.; Ohlsson, T.; Hogland, H. DC-resistivity mapping of internal landfill structures: Two pre-excavation surveys. Environ. Geol. 2000, 39, 360–371. [Google Scholar] [CrossRef]
- Ismail, E.; Alexakis, D.E.; Heleika, M.A.; Hashem, M.; Ahmed, M.S.; Hamdy, D.; Ali, A. Applying geophysical and Hydrogeochemical methods to evaluate groundwater potential and quality in middle Egypt. Hydrology 2023, 10, 173. [Google Scholar] [CrossRef]
- Association, A.P.H. Standard Methods for the Examination of Water and Wastewater; American Public Health Association: Washington, DC, USA, 1926; Volume 6. [Google Scholar]
- Piper, A.M. A graphic procedure in the geochemical interpretation of water-analyses. Eos Trans. Am. Geophys. Union 1944, 25, 914–928. [Google Scholar]
- Parkhurst, D.L.; Appelo, C. User’s Guide to PHREEQC (Version 2): A Computer Program for Speciation, Batch-Reaction, One-Dimensional Transport, and Inverse Geochemical Calculations; US Geological Survey: Reston, VA, USA, 1999. [Google Scholar]
- World Health Organization. Guidelines For Drinking-Water Quality: Incorporating the First and Second Addenda; World Health Organization: Geneva, Switzerland, 2022. [Google Scholar]
- Sahu, P.; Sikdar, P. Hydrochemical framework of the aquifer in and around East Kolkata Wetlands, West Bengal, India. Environ. Geol. 2008, 55, 823–835. [Google Scholar] [CrossRef]
- Şener, Ş.; Şener, E.; Davraz, A. Evaluation of water quality using water quality index (WQI) method and GIS in Aksu River (SW-Turkey). Sci. Total Environ. 2017, 584, 131–144. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.M.; Bodrud-Doza, M.; Siddiqua, M.T.; Zahid, A.; Islam, A.R.M.T. Spatiotemporal distribution of fluoride in drinking water and associated probabilistic human health risk appraisal in the coastal region, Bangladesh. Sci. Total Environ. 2020, 724, 138316. [Google Scholar] [CrossRef] [PubMed]
- Pennino, M.J.; Leibowitz, S.G.; Compton, J.E.; Hill, R.A.; Sabo, R.D. Patterns and predictions of drinking water nitrate violations across the conterminous United States. Sci. Total Environ. 2020, 722, 137661. [Google Scholar] [CrossRef] [PubMed]
- Qu, S.; Duan, L.; Shi, Z.; Liang, X.; Lv, S.; Wang, G.; Liu, T.; Yu, R. Hydrochemical assessments and driving forces of groundwater quality and potential health risks of sulfate in a coalfield, northern Ordos Basin, China. Sci. Total Environ. 2022, 835, 155519. [Google Scholar] [CrossRef]
- Khettouch, A.; Hssaisoune, M.; Maaziz, M.; Taleb, A.A.; Bouchaou, L. Characterization of groundwater in the arid Zenaga plain: Hydrochemical and environmental isotopes approaches. Groundw. Sustain. Dev. 2022, 19, 100816. [Google Scholar] [CrossRef]
- Xia, L.; Han, Q.; Shang, L.; Wang, Y.; Li, X.; Zhang, J.; Yang, T.; Liu, J.; Liu, L. Quality assessment and prediction of municipal drinking water using water quality index and artificial neural network: A case study of Wuhan, central China, from 2013 to 2019. Sci. Total Environ. 2022, 844, 157096. [Google Scholar] [CrossRef]
- Swamee, P.K.; Tyagi, A. Improved method for aggregation of water quality subindices. J. Environ. Eng. 2007, 133, 220–225. [Google Scholar] [CrossRef]
- McGeorge, W. Diagnosis and Improvement of Saline and Alkaline Soils; By Staff of US Salinity Laboratory, Agriculture Handbook No.60; US Department of Agriculture; No. 60 US Dept. Agric., Supt. Documents; US Government Printing Office: Washington, DC, USA, 1954; 160p. [Google Scholar]
- Todd, D. Groundwater Hydrology; John Willey & Sons Inc.: New York, NY, USA, 1980; 535p. [Google Scholar]
- Wilcox, L.V. The Quality of Water for Irrigation Use; US Department of Agriculture: Washington, DC, USA, 1948. [Google Scholar]
- Paiva, I.; Cunha, L. Characterization of the hydrodynamic functioning of the Degracias-Sicó Karst Aquifer, Portugal. Hydrogeol. J. 2020, 28, 2613–2629. [Google Scholar] [CrossRef]
- Vaezihir, A.; Sepehripour, A.; Tabarmayeh, M. Identifying flow regime in the aquifer of fractured rock system in Germi Chai Basin, Iran. J. Mt. Sci. 2024, 21, 574–589. [Google Scholar] [CrossRef]
- Mohamed, A.; Othman, A.; Galal, W.F.; Abdelrady, A. Integrated Geophysical Approach of Groundwater Potential in Wadi Ranyah, Saudi Arabia, Using Gravity, Electrical Resistivity, and Remote-Sensing Techniques. Remote Sens. 2023, 15, 1808. [Google Scholar] [CrossRef]
- Shojaeian, A.; Bounds, T.; Muraleetharan, K.K.; Miller, G. Seismic Response of Pile Foundations in Clayey Soil Deposits Considering Soil Suction Changes Caused by Soil–Atmospheric Interactions. Geosciences 2024, 14, 234. [Google Scholar] [CrossRef]
- Mepaiyeda, S.; Madi, K.; Gwavava, O.; Baiyegunhi, C. Geological and geophysical assessment of groundwater contamination at the Roundhill landfill site, Berlin, Eastern Cape, South Africa. Heliyon 2020, 6, e04249. [Google Scholar] [CrossRef]
- Mas, P.; Calcagno, P.; Caritg-Monnot, S.; Beccaletto, L.; Capar, L.; Hamm, V. A 3D geomodel of the deep aquifers in the Orléans area of the southern Paris Basin (France). Sci. Data 2022, 9, 781. [Google Scholar] [CrossRef]
- Sanuade, O.A.; Arowoogun, K.I.; Amosun, J.O. A review on the use of geoelectrical methods for characterization and monitoring of contaminant plumes. Acta Geophys. 2022, 70, 2099–2117. [Google Scholar] [CrossRef]
- Meng, J.; Hu, K.; Wang, S.; Wang, Y.; Chen, Z.; Gao, C.; Mao, D. A framework for risk assessment of groundwater contamination integrating hydrochemical, hydrogeological, and electrical resistivity tomography method. Environ. Sci. Pollut. Res. 2024, 31, 28105–28123. [Google Scholar] [CrossRef] [PubMed]
- Wei, A.; Chen, Y.; Deng, Q.; Li, D.; Wang, R.; Jiao, Z. A Study on Hydrochemical Characteristics and Evolution Processes of Groundwater in the Coastal Area of the Dagujia River Basin, China. Sustainability 2022, 14, 8358. [Google Scholar] [CrossRef]
- Abascal, E.; Gómez-Coma, L.; Ortiz, I.; Ortiz, A. Global diagnosis of nitrate pollution in groundwater and review of removal technologies. Sci. Total Environ. 2022, 810, 152233. [Google Scholar] [CrossRef] [PubMed]
- Sarker, M.M.R.; Van Camp, M.; Hossain, D.; Islam, M.; Ahmed, N.; Karim, M.M.; Bhuiyan, M.A.Q.; Walraevens, K. Groundwater salinization and freshening processes in coastal aquifers from southwest Bangladesh. Sci. Total Environ. 2021, 779, 146339. [Google Scholar] [CrossRef]
- Shen, H.; Rao, W.; Tan, H.; Guo, H.; Ta, W.; Zhang, X. Controlling factors and health risks of groundwater chemistry in a typical alpine watershed based on machine learning methods. Sci. Total Environ. 2023, 854, 158737. [Google Scholar] [CrossRef]
- Taghavi, N.; Niven, R.K.; Kramer, M.; Paull, D.J. Comparison of DRASTIC and DRASTICL groundwater vulnerability assessments of the Burdekin Basin, Queensland, Australia. Sci. Total Environ. 2023, 858, 159945. [Google Scholar] [CrossRef] [PubMed]
- De, A.; Das, A.; Joardar, M.; Mridha, D.; Majumdar, A.; Das, J.; Roychowdhury, T. Investigating spatial distribution of fluoride in groundwater with respect to hydro-geochemical characteristics and associated probabilistic health risk in Baruipur block of West Bengal, India. Sci. Total Environ. 2023, 886, 163877. [Google Scholar] [CrossRef]
- Mao, H.; Wang, C.; Qu, S.; Liao, F.; Wang, G.; Shi, Z. Source and evolution of sulfate in the multi-layer groundwater system in an abandoned mine—Insight from stable isotopes and Bayesian isotope mixing model. Sci. Total Environ. 2023, 859, 160368. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Mei, Y.; Yu, K.; Li, Y.; Meng, X.; Hu, F. Anthropogenic effects on hydrogeochemical characterization of the Shallow Groundwater in an arid irrigated plain in Northwestern China. Water 2019, 11, 2247. [Google Scholar] [CrossRef]
- Gao, X.; Li, X.; Wang, W.; Li, C. Human activity and hydrogeochemical processes relating to groundwater quality degradation in the Yuncheng Basin, Northern China. Int. J. Environ. Res. Public Health 2020, 17, 867. [Google Scholar] [CrossRef] [PubMed]
- Gebreyohannes, T.; De Smedt, F.; Hagos, M.; Gebresilassie, S.; Amare, K.; Kabeto, K.; Hussein, A.; Nyssen, J.; Bauer, H.; Moeyersons, J. Large-Scale Geological Mapping of the Geba Basin, Northern Ethiopia; VLIR-Mekelle University IUC Programme: Mekelle, Ethiopia, 2010. [Google Scholar]
- Li, M.; Liu, Z.; Yu, Q.; Chen, Y. Exploratory analysis on spatio-seasonal variation patterns of hydro-chemistry in the upper Yangtze River basin. J. Hydrol. 2021, 597, 126217. [Google Scholar] [CrossRef]
- Awaleh, M.O.; Boschetti, T.; Adaneh, A.E.; Chirdon, M.A.; Ahmed, M.M.; Dabar, O.A.; Soubaneh, Y.D.; Egueh, N.M.; Kawalieh, A.D.; Kadieh, I.H. Origin of nitrate and sulfate sources in volcano-sedimentary aquifers of the East Africa Rift System: An example of the Ali-Sabieh groundwater (Republic of Djibouti). Sci. Total Environ. 2022, 804, 150072. [Google Scholar] [CrossRef] [PubMed]
- Naghavi, S.; Mirzaei, A.; Sardoei, M.A.; Azarm, H. Evaluation of water-energy-food-environment-agricultural economic growth nexus integrated approach to achieve sustainable production. Environ. Sci. Pollut. Res. 2023, 30, 96715–96725. [Google Scholar] [CrossRef]
- Ai, L.; Ma, B.; Shao, S.; Zhang, L. Heavy metals in Chinese freshwater fish: Levels, regional distribution, sources and health risk assessment. Sci. Total Environ. 2022, 853, 158455. [Google Scholar] [CrossRef]
- Dou, Y.; Yu, X.; Liu, L.; Ning, Y.; Bi, X.; Liu, J. Effects of hydrological connectivity project on heavy metals in Wuhan urban lakes on the time scale. Sci. Total Environ. 2022, 853, 158654. [Google Scholar] [CrossRef] [PubMed]
- Çiftçi, T.D. Determination of heavy metals and essential elements in nasal sprays and drops (Saline/Sea Water) and evaluation in terms of toxicity. Environ. Sci. Pollut. Res. 2023, 30, 96938–96947. [Google Scholar] [CrossRef] [PubMed]
- Besley, C.H.; Batley, G.E.; Cassidy, M. Tracking contaminants of concern in wet-weather sanitary sewer overflows. Environ. Sci. Pollut. Res. 2023, 30, 96763–96781. [Google Scholar] [CrossRef] [PubMed]
- Shrestha, A.; Shrestha, S.M.; Pradhan, A.M.S. Assessment of spring water quality of Khandbari Municipality in Sankhuwasabha District, Eastern Nepal. Environ. Sci. Pollut. Res. 2023, 30, 98452–98469. [Google Scholar] [CrossRef] [PubMed]
- Ayers, R.S.; Westcot, D.W. Water Quality for Agriculture; Food and Agriculture Organization of the United Nations: Rome, Italy, 1985; Volume 29. [Google Scholar]
- Wilcox, L. Classification and Use of Irrigation Waters; US Department of Agriculture: Washington, DC, USA, 1955. [Google Scholar]


| Parameters | Standards [58] | Weight of Each Parameter (wi) | Relative Weight (Wi) |
|---|---|---|---|
| pH | 6.5–8.5 | 4 | 0.09 |
| Iron | 0.5 mg/L | 3 | 0.07 |
| Calcium | 75 mg/L | 2 | 0.04 |
| Magnesium | 100 mg/L | 1 | 0.02 |
| Potassium | 1.5 mg/L | 2 | 0.04 |
| TDS | 1000 mg/L | 4 | 0.09 |
| Turbidity | 5 NTU | 3 | 0.07 |
| Alkalinity | 200 mg/L | 4 | 0.09 |
| Nitrate | 50 mg/L | 5 | 0.12 |
| Sulfate | 250 mg/L | 5 | 0.12 |
| Chloride | 250 mg/L | 3 | 0.07 |
| Fluoride | 1.5 mg/L | 5 | 0.12 |
| Total | 41 | 0.94 |
| Parameters | Min | Max | Mean | Median | Mode | STD |
|---|---|---|---|---|---|---|
| Ca2+ | 51 | 255 | 158 | 162 | 142 | 51.4 |
| Mg2+ | 7 | 64 | 31.5 | 29 | 32 | 14.8 |
| Na+ | 5.8 | 134.1 | 44.8 | 30.8 | 14.8 | 34.4 |
| K+ | 2.03 | 16.4 | 5.4 | 4.81 | 4.81 | 3.2 |
| HCO3− | 54.9 | 652.7 | 296.8 | 304.9 | 396.5 | 114.6 |
| SO42− | 98.6 | 702.6 | 293.4 | 295.1 | 435.6 | 130.9 |
| Cl− | 1.52 | 172.5 | 42.2 | 22.6 | 12.6 | 44.73 |
| NO3− | 0.52 | 81.9 | 18.1 | 11.2 | 38.6 | 17.5 |
| Cr6+ | 0 | 0.82 | 0.07 | 0.01 | 0.01 | 0.14 |
| Cu2+ | 0.01 | 1.53 | 0.64 | 0.61 | 0.61 | 0.27 |
| Fe2+ | 0.21 | 0.97 | 0.57 | 0.56 | 0.61 | 0.19 |
| Mn2+ | 0 | 0.09 | 0.01 | 0.01 | 0 | 0.02 |
| Na+(%) | 4.09 | 35.15 | 16.11 | 12.87 | ---- | 8.17 |
| SAR | 0.12 | 2.4 | 0.86 | 0.58 | ---- | 0.59 |
| ID | pH | TDS | EC | Na+ | K+ | Ca2+ | Mg2+ | HCO3− | SO42− | Cl− | NO3− | Cr6+ | Cu2+ | Fe2+ | Mn2+ | WQI | SAR |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| S1 | 7.41 | 836 | 1306 | 14.80 | 2.74 | 186 | 7 | 365.9 | 256.4 | 1.52 | 0.86 | 0.01 | 0.61 | 0.61 | 0.00 | 80.14 | 0.28 |
| S2 | 6.91 | 731 | 1142 | 39.14 | 3.93 | 110 | 32 | 229.40 | 291.80 | 18.70 | 4.81 | 0.02 | 0.58 | 0.73 | 0.00 | 88.74 | 0.84 |
| S3 | 7.14 | 638 | 998 | 25.90 | 4.81 | 66 | 56 | 369.79 | 98.60 | 12.60 | 3.61 | 0.01 | 0.92 | 0.43 | 0.00 | 71.21 | 0.56 |
| S4 | 6.84 | 495 | 773 | 14.10 | 3.72 | 62 | 31 | 239.20 | 131.80 | 8.30 | 4.16 | 0.02 | 0.73 | 0.21 | 0.00 | 60.54 | 0.36 |
| S5 | 7.06 | 875 | 1367 | 14.80 | 2.84 | 128 | 15 | 396.50 | 306.40 | 7.90 | 2.18 | 0.00 | 0.81 | 0.61 | 0.00 | 76.89 | 0.32 |
| S6 | 7.24 | 951 | 1487 | 17.24 | 5.77 | 207 | 30 | 317.20 | 356.80 | 11.50 | 5.14 | 0.01 | 0.56 | 0.38 | 0.00 | 96.65 | 0.29 |
| S7 | 7.48 | 820 | 1281 | 29.87 | 4.39 | 142 | 51 | 237.89 | 291.40 | 46.70 | 15.60 | 0.03 | 0.87 | 0.51 | 0.03 | 94.64 | 0.54 |
| S8 | 6.71 | 1145 | 1789 | 33.60 | 11.63 | 204 | 51 | 436.20 | 325.80 | 47.80 | 33.70 | 0.06 | 0.73 | 0.72 | 0.04 | 167.45 | 0.54 |
| H1 | 5.02 | 1255 | 1960 | 37.50 | 16.44 | 255 | 36 | 652.70 | 214.80 | 28.90 | 12.80 | 0.01 | 0.88 | 0.38 | 0.01 | 157.81 | 0.58 |
| H2 | 6.81 | 801 | 1251 | 25.40 | 2.03 | 170 | 14 | 335.50 | 226.90 | 17.30 | 9.64 | 0.00 | 0.31 | 0.52 | 0.00 | 78.73 | 0.5 |
| H3 | 7.62 | 1186 | 1853 | 89.50 | 5.13 | 220 | 33 | 173.20 | 505.20 | 146.70 | 12.81 | 0.03 | 0.72 | 0.28 | 0.03 | 128.07 | 1.48 |
| H4 | 6.25 | 1255 | 1960 | 134.10 | 8.51 | 189 | 28 | 374.50 | 412.60 | 71.80 | 35.90 | 0.02 | 0.81 | 0.71 | 0.01 | 175.74 | 2.4 |
| H5 | 8.42 | 348 | 543 | 20.90 | 2.21 | 51 | 12 | 121.90 | 121.80 | 12.60 | 5.21 | 0.01 | 0.28 | 0.73 | 0.00 | 61.35 | 0.68 |
| H6 | 7.02 | 438 | 684 | 28.70 | 4.41 | 76 | 10 | 168.10 | 126.40 | 15.80 | 8.11 | 0.00 | 0.32 | 0.81 | 0.00 | 67.01 | 0.81 |
| H7 | 7.69 | 1195 | 1867 | 55.30 | 15.35 | 231 | 32 | 54.90 | 702.90 | 71.90 | 31.60 | 0.02 | 0.41 | 0.52 | 0.00 | 224.61 | 0.9 |
| H8 | 7.43 | 772 | 1206 | 64.50 | 6.91 | 107 | 21 | 254.30 | 261.80 | 33.70 | 20.80 | 0.82 | 0.49 | 0.81 | 0.01 | 122.63 | 1.48 |
| H9 | 8.05 | 986 | 1540 | 107.50 | 4.84 | 142 | 21 | 304.90 | 322.40 | 51.60 | 31.70 | 0.03 | 0.37 | 0.51 | 0.03 | 139.04 | 2.22 |
| H10 | 6.70 | 1311 | 2048 | 127.24 | 4.40 | 206 | 57 | 289.10 | 435.60 | 151.60 | 38.60 | 0.14 | 1.14 | 0.89 | 0.07 | 181.7 | 2.01 |
| H11 | 7.22 | 914 | 1428 | 39.84 | 5.19 | 131 | 41 | 457.49 | 192.70 | 39.20 | 6.53 | 0.04 | 0.73 | 0.63 | 0.06 | 87.14 | 0.77 |
| H12 | 6.69 | 1394 | 2178 | 108.53 | 5.39 | 246 | 64 | 122.00 | 591.50 | 172.50 | 81.90 | 0.25 | 1.53 | 0.95 | 0.09 | 269.26 | 1.58 |
| H13 | 7.28 | 930 | 1453 | 32.11 | 3.64 | 165 | 50 | 317.19 | 302.80 | 33.40 | 24.50 | 0.05 | 0.69 | 0.64 | 0.02 | 112.39 | 0.56 |
| H14 | 7.54 | 1024 | 1600 | 102.44 | 3.24 | 125 | 39 | 134.20 | 435.60 | 131.60 | 51.60 | 0.12 | 0.96 | 0.97 | 0.03 | 167.21 | 2.04 |
| R1 | 7.54 | 655 | 1023 | 28.30 | 5.62 | 128 | 22 | 237.90 | 201.60 | 21.60 | 9.72 | 0.00 | 0.52 | 0.61 | 0.00 | 84.43 | 0.6 |
| R2 | 6.72 | 878 | 1371 | 31.40 | 5.86 | 175 | 26 | 304.90 | 308.80 | 16.80 | 8.72 | 0.02 | 0.48 | 0.36 | 0.01 | 98.25 | 0.58 |
| R3 | 6.81 | 926 | 1446 | 27.90 | 5.55 | 203 | 24 | 353.80 | 291.60 | 14.40 | 5.15 | 0.01 | 0.63 | 0.28 | 0.00 | 89.18 | 0.49 |
| R4 | 6.52 | 757 | 1182 | 28.20 | 5.32 | 142 | 25 | 305.00 | 208.10 | 21.70 | 21.70 | 0.00 | 0.48 | 0.43 | 0.00 | 100.95 | 0.57 |
| R5 | 7.10 | 788 | 1231 | 30.30 | 5.12 | 159 | 23 | 323.30 | 203.60 | 21.50 | 21.50 | 0.01 | 0.54 | 0.41 | 0.00 | 95.83 | 0.59 |
| R6 | 6.54 | 815 | 1273 | 28.80 | 4.81 | 144 | 19 | 396.50 | 203.60 | 13.60 | 4.98 | 0.01 | 0.01 | 0.41 | 0.00 | 66.56 | 0.59 |
| R7 | 8.02 | 636 | 993 | 5.81 | 4.34 | 137 | 20 | 243.90 | 220.60 | 2.86 | 0.52 | 0.01 | 0.61 | 0.69 | 0.02 | 78.71 | 0.12 |
| R8 | 6.89 | 959 | 1498 | 27.51 | 2.05 | 186 | 46 | 341.60 | 293.50 | 39.60 | 21.80 | 0.03 | 0.54 | 0.63 | 0.08 | 102.71 | 0.46 |
| R9 | 7.54 | 918 | 1434 | 26.89 | 3.75 | 174 | 50 | 268.40 | 314.90 | 41.50 | 38.60 | 0.01 | 0.38 | 0.51 | 0.02 | 126.14 | 0.46 |
| SP | 7.08 | 876 | 1368 | 35.50 | 2.99 | 180 | 24 | 372.10 | 231.70 | 23.60 | 5.27 | 0.00 | 0.61 | 0.35 | 0.01 | 80.71 | 0.65 |
| pH | TDS | EC | Na+ | K+ | Ca2+ | Mg2+ | Fe2+ | Cu2+ | Cr6+ | Mn2+ | Cl− | HCO3− | SO42− | NO3− | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| pH | 1 | ||||||||||||||
| TDS | −0.1 | 1 | |||||||||||||
| EC | −0.03 | 0.96 | 1 | ||||||||||||
| Na+ | 0.04 | 0.30 | 0.28 | 1 | |||||||||||
| K+ | −0.1 | −0.05 | −0.05 | 0.15 | 1 | ||||||||||
| Ca2+ | −0.22 | 0.09 | 0.07 | 0.32 | 0.49 | 1 | |||||||||
| Mg2+ | 0.01 | −0.05 | −0.11 | 0.32 | 0.16 | 0.30 | 1 | ||||||||
| Fe2+ | 0.20 | −0.03 | −0.08 | 0.53 | −0.11 | −0.1 | 0.17 | 1 | |||||||
| Cu2+ | −0.02 | −0.09 | −0.14 | 0.46 | 0.10 | 0.31 | 0.66 | 0.27 | 1 | ||||||
| Cr6+ | −0.08 | −0.11 | −0.12 | 0.17 | 0.57 | 0.44 | 0.27 | 0.02 | 0.41 | 1 | |||||
| Mn2+ | −0.06 | 0.15 | 0.05 | 0.41 | −0.06 | 0.37 | 0.70 | 0.42 | 0.60 | 0.21 | 1 | ||||
| Cl− | 0.10 | 0.25 | 0.21 | 0.83 | 0.11 | 0.44 | 0.56 | 0.43 | 0.62 | 0.22 | 0.66 | 1 | |||
| HCO3− | −0.25 | −0.02 | 0.01 | −0.22 | 0.24 | 0.23 | 0.01 | −0.3 | 0.01 | 0.43 | −0.05 | −0.38 | 1 | ||
| SO42− | 0.02 | 0.13 | 0.10 | 0.61 | 0.35 | 0.67 | 0.36 | 0.26 | 0.38 | 0.08 | 0.39 | 0.74 | −0.4 | 1 | |
| NO3− | −0.06 | 0.01 | −0.04 | 0.70 | 0.18 | 0.41 | 0.58 | 0.57 | 0.52 | 0.22 | 0.59 | 0.78 | −0.32 | 0.66 | 1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abera, K.A.; Asfaw, B.A.; Doyoro, Y.G.; Gebreyohanes, T.; Hussien, A.; Berhane, G.; Hagos, M.; Romha, A.; Walraevens, K. Assessing Shallow Groundwater Quality Around the Sheba Leather Tannery Area, Wikro, North Ethiopia: A Geophysical and Hydrochemical Study. Geosciences 2024, 14, 324. https://doi.org/10.3390/geosciences14120324
Abera KA, Asfaw BA, Doyoro YG, Gebreyohanes T, Hussien A, Berhane G, Hagos M, Romha A, Walraevens K. Assessing Shallow Groundwater Quality Around the Sheba Leather Tannery Area, Wikro, North Ethiopia: A Geophysical and Hydrochemical Study. Geosciences. 2024; 14(12):324. https://doi.org/10.3390/geosciences14120324
Chicago/Turabian StyleAbera, Kaleab Adhena, Berhane Abrha Asfaw, Yonatan Garkebo Doyoro, Tesfamichael Gebreyohanes, Abdelwassie Hussien, Gebremedhin Berhane, Miruts Hagos, Abadi Romha, and Kristine Walraevens. 2024. "Assessing Shallow Groundwater Quality Around the Sheba Leather Tannery Area, Wikro, North Ethiopia: A Geophysical and Hydrochemical Study" Geosciences 14, no. 12: 324. https://doi.org/10.3390/geosciences14120324
APA StyleAbera, K. A., Asfaw, B. A., Doyoro, Y. G., Gebreyohanes, T., Hussien, A., Berhane, G., Hagos, M., Romha, A., & Walraevens, K. (2024). Assessing Shallow Groundwater Quality Around the Sheba Leather Tannery Area, Wikro, North Ethiopia: A Geophysical and Hydrochemical Study. Geosciences, 14(12), 324. https://doi.org/10.3390/geosciences14120324







