Groundwater Quality Variations in Multiple Aquifers: A Comprehensive Evaluation for Public Health and Agricultural Use
Abstract
1. Introduction
2. Materials and Methods
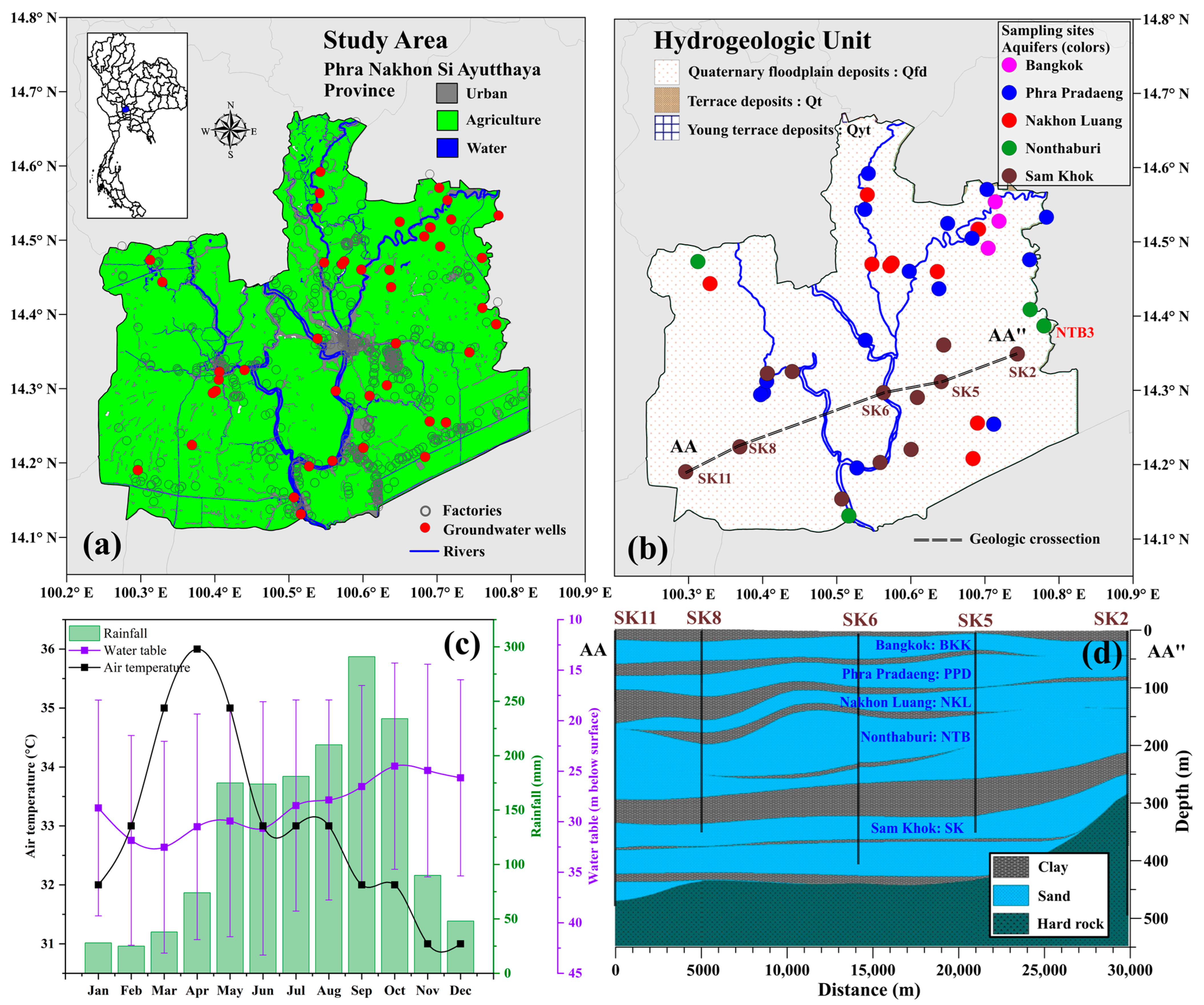
2.1. Study Area and Climate Conditions
2.2. Hydrogeological Settings
2.3. Sampling Collection
2.4. Hydrogeochemical Analyses
3. Results and Discussion
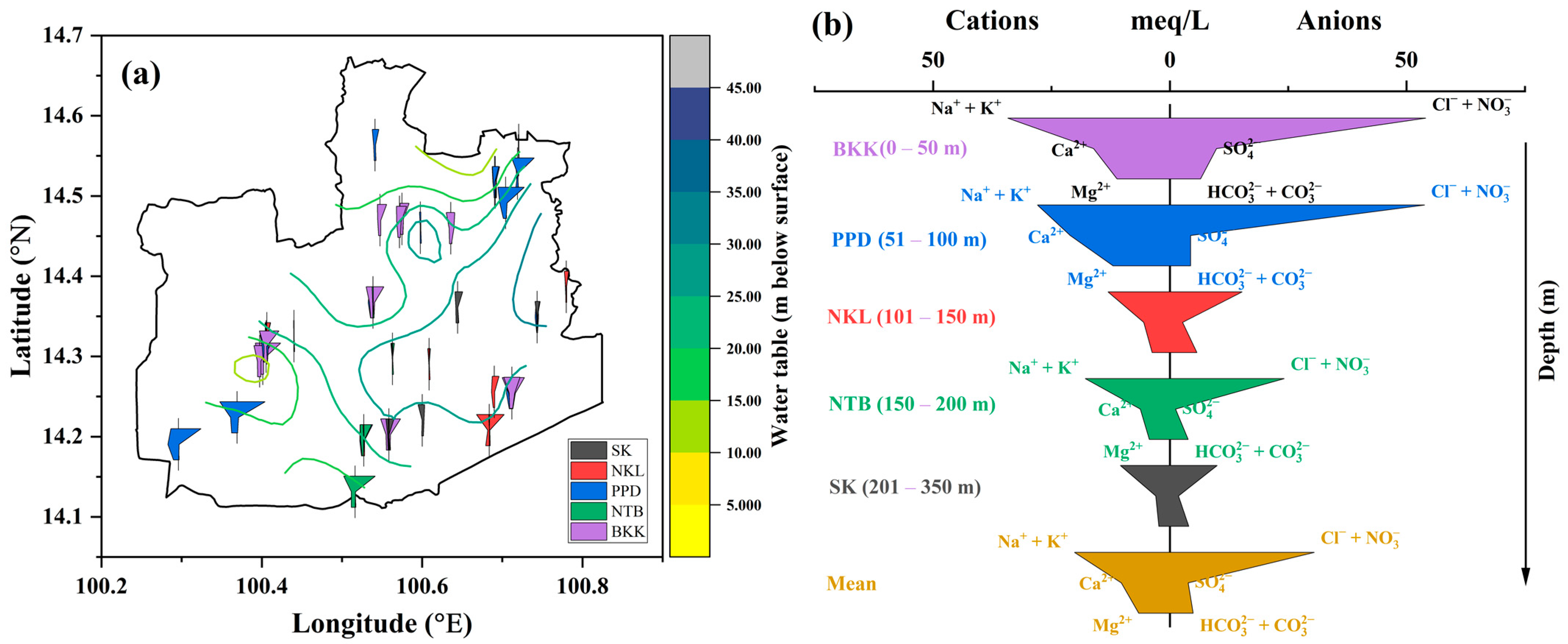
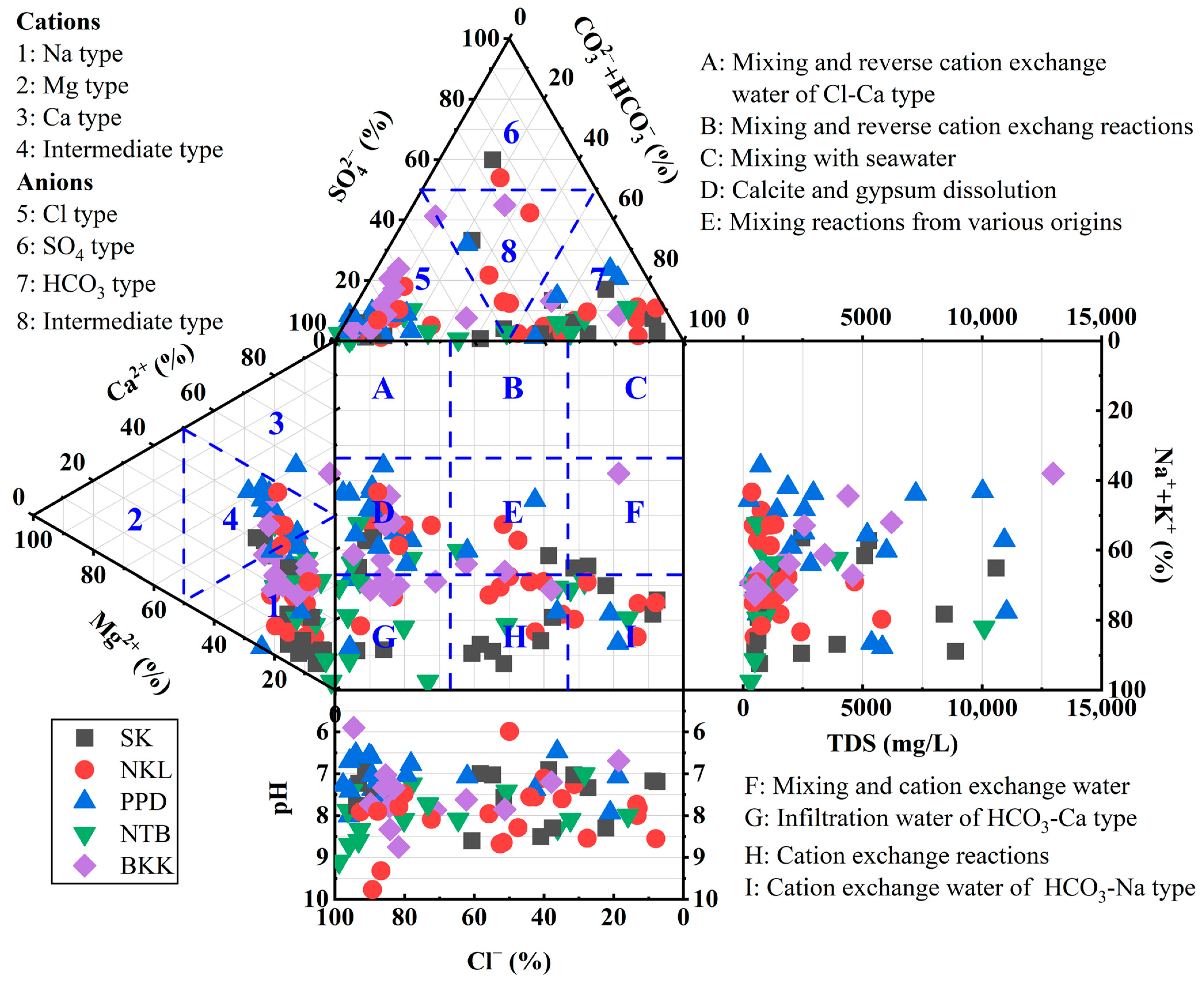
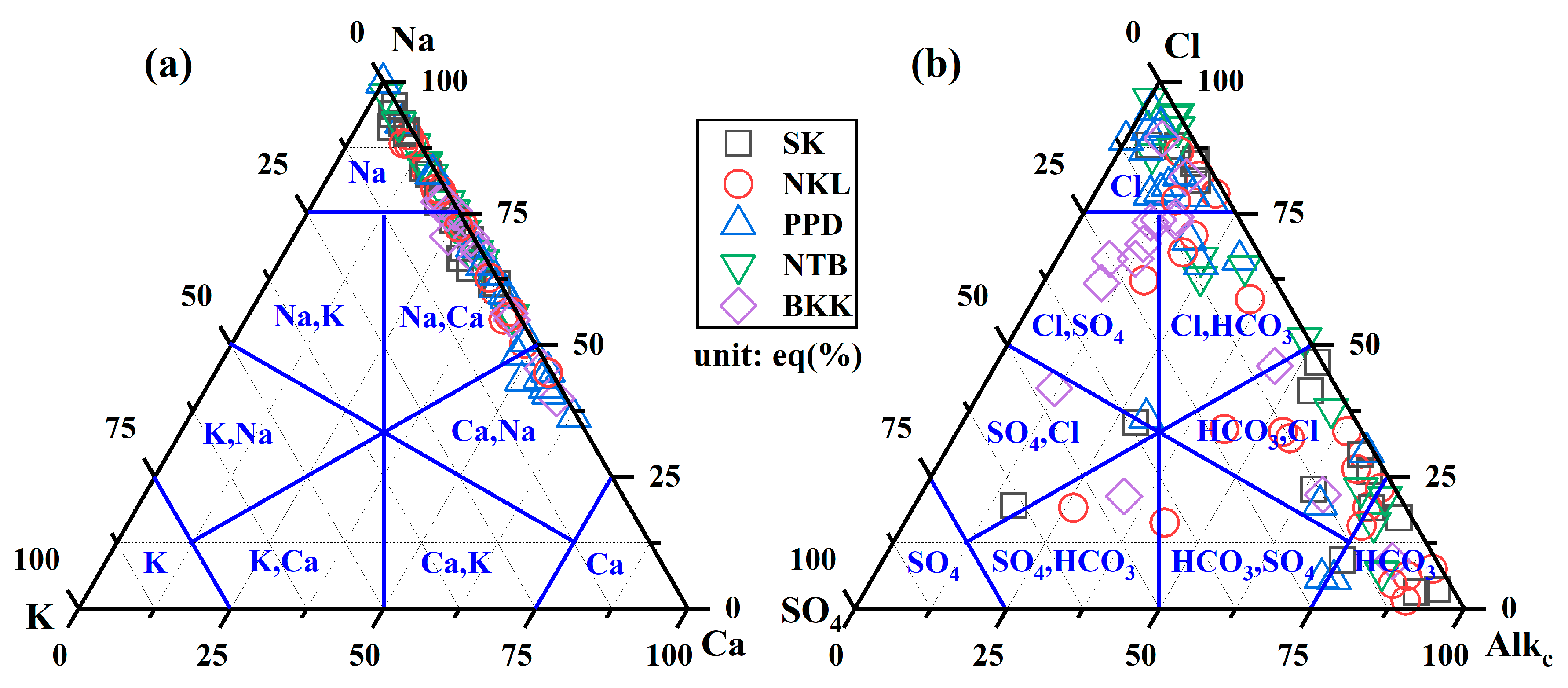
3.1. Hydrochemical Facies in Groundwater
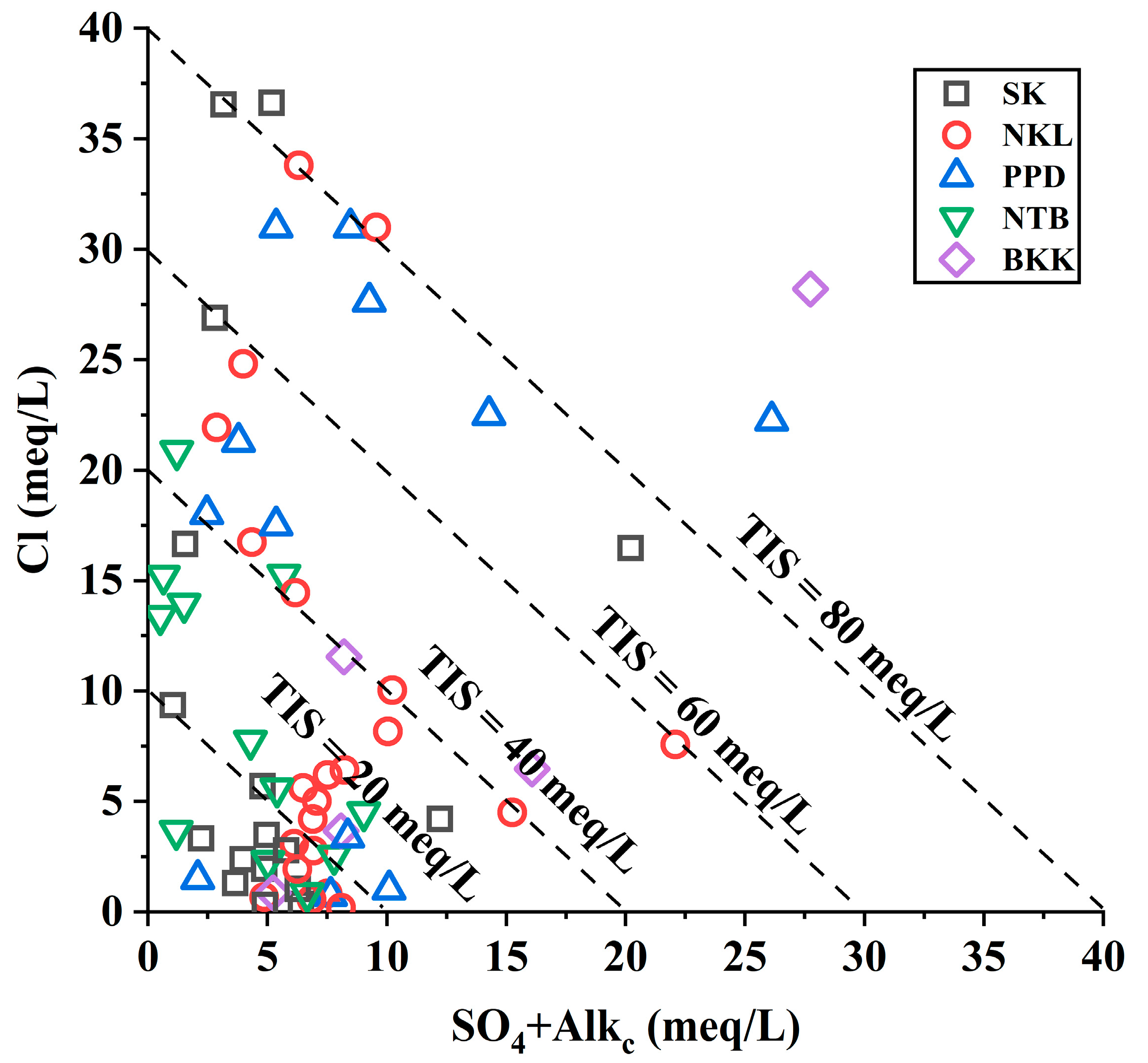
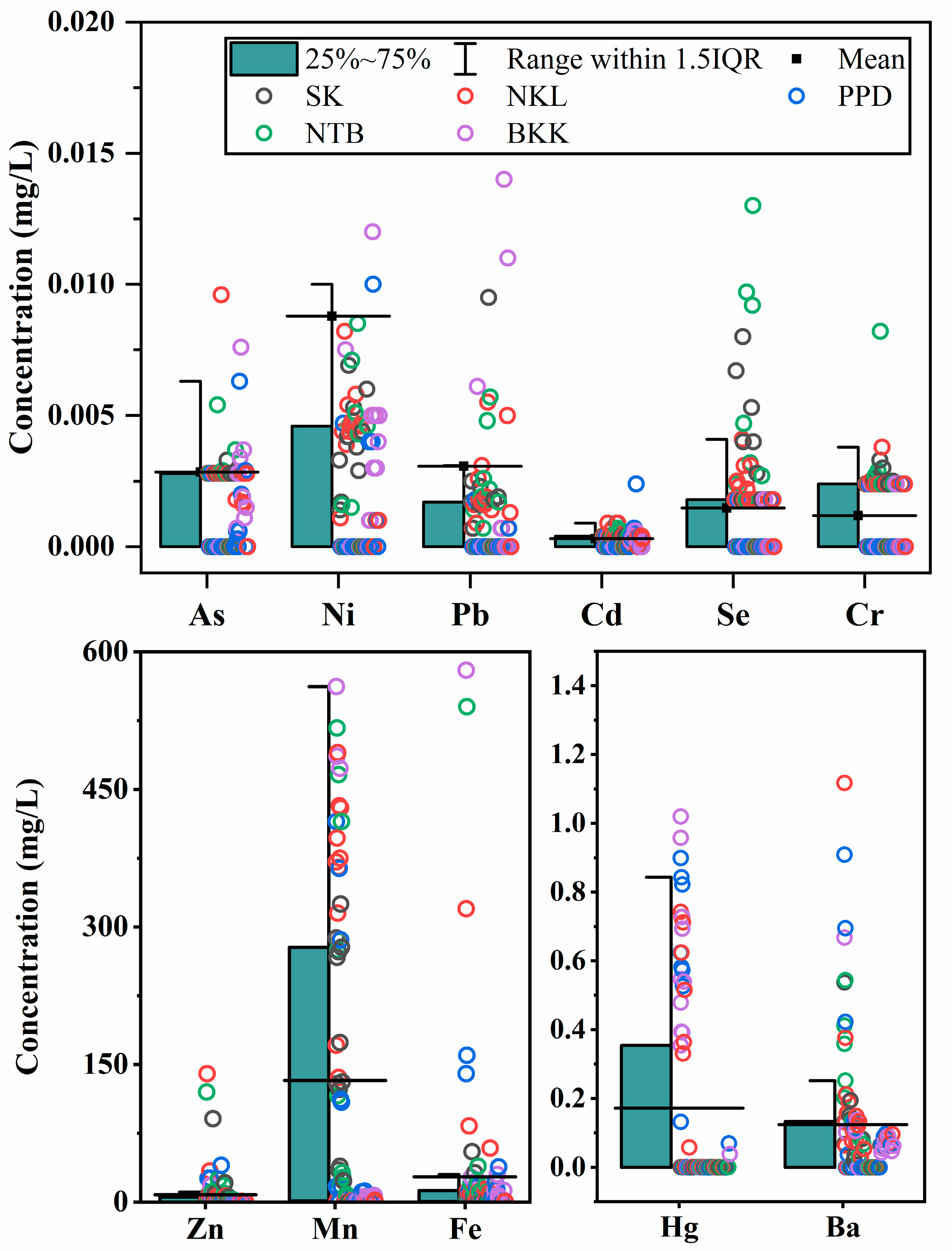
3.2. Groundwater Characterization
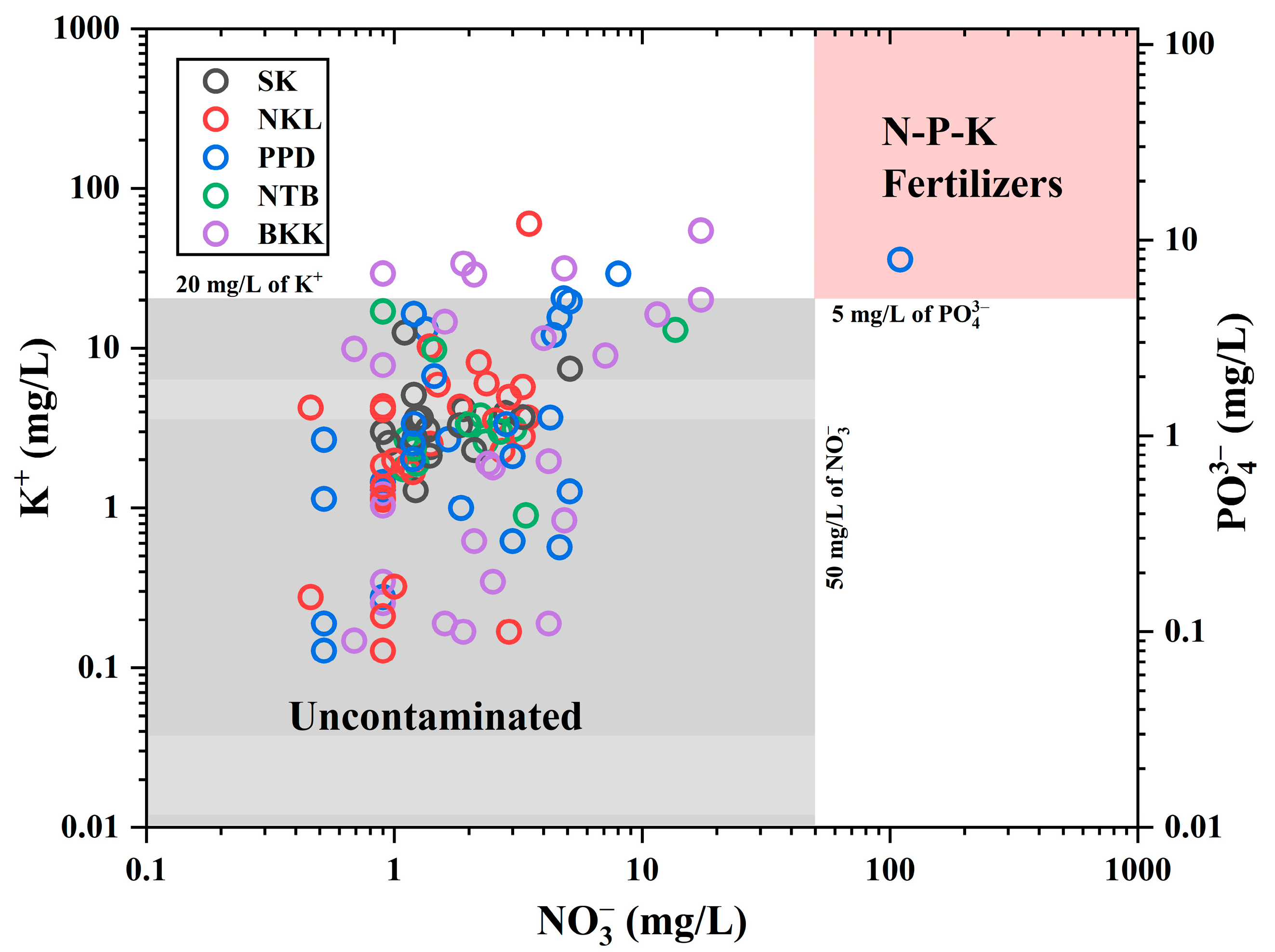
3.3. Groundwater Quality for Human Consumption
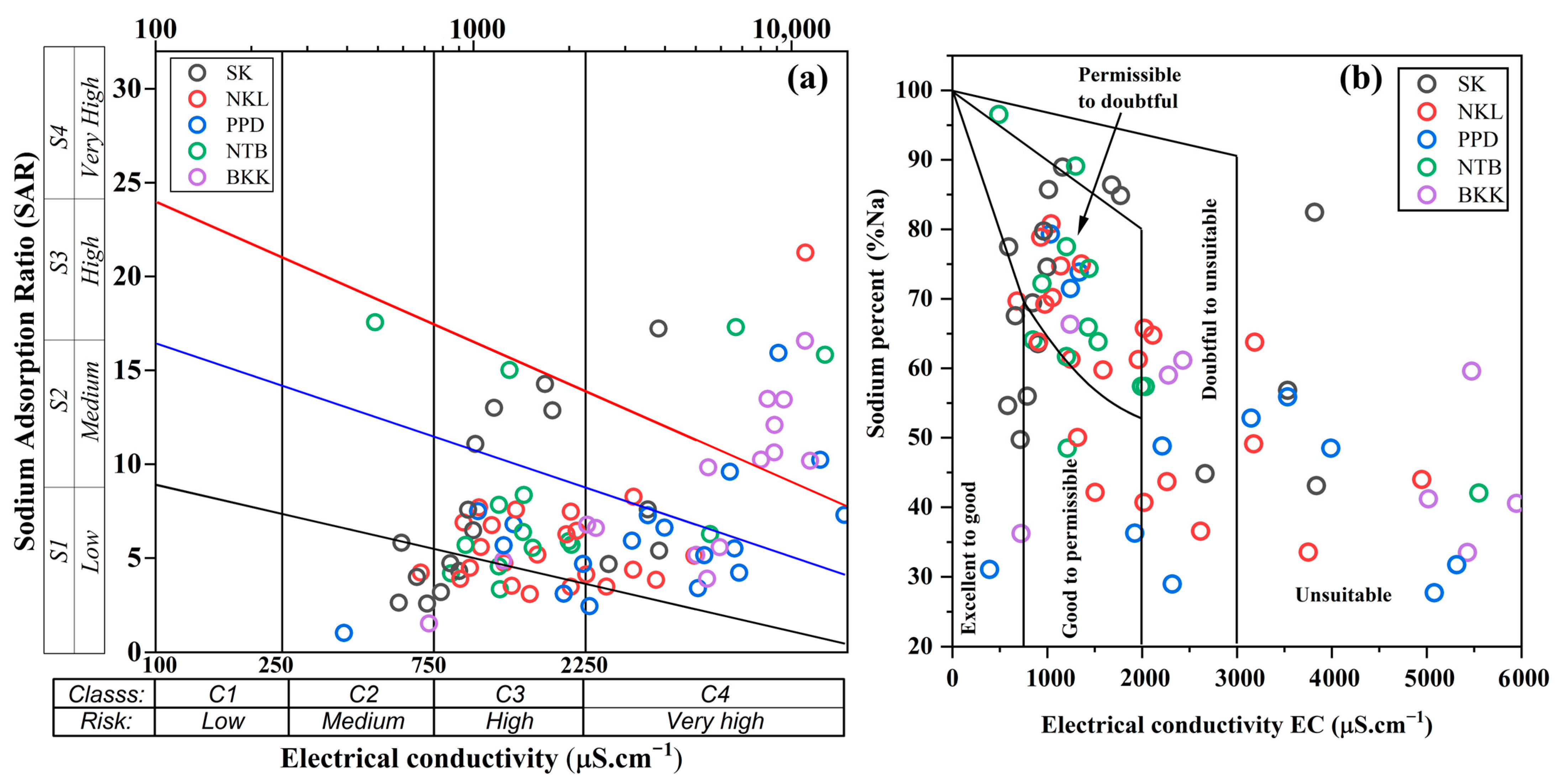
3.4. Groundwater Quality for Irrigation Purposes
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Elements | Values (mg/L) | Aquifers | |||||
|---|---|---|---|---|---|---|---|
| BKK | PPD | NKL | NTB | SK | Overall | ||
| As | Max | 0.0158 | 0.0253 | 0.0329 | 0.0054 | 0.0033 | 0.0329 |
| Min | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | |
| Mean | 0.0028 | 0.0021 | 0.0047 | 0.0019 | 0.0017 | 0.0028 | |
| StdDev | 0.0040 | 0.0058 | 0.0083 | 0.0017 | 0.0014 | 0.0053 | |
| Zn | Max | 18.0000 | 40.0000 | 140.0000 | 120.0000 | 91.0000 | 140.0000 |
| Min | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | |
| Mean | 1.4549 | 5.3316 | 9.7915 | 12.7844 | 9.1000 | 7.8058 | |
| StdDev | 4.4426 | 11.6740 | 29.2966 | 29.4649 | 21.1045 | 21.8650 | |
| Hg | Max | 1.0200 | 0.8990 | 0.7430 | 0.0002 | 0.0003 | 1.0200 |
| Min | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | |
| Mean | 0.3840 | 0.2669 | 0.1773 | 0.0001 | 0.0001 | 0.1663 | |
| StdDev | 0.3527 | 0.3493 | 0.2834 | 0.0001 | 0.0001 | 0.2913 | |
| Ni | Max | 0.0120 | 0.0100 | 0.5160 | 0.0425 | 0.0069 | 0.5160 |
| Min | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | |
| Mean | 0.0032 | 0.0015 | 0.0252 | 0.0051 | 0.0025 | 0.0085 | |
| StdDev | 0.0033 | 0.0027 | 0.1070 | 0.0103 | 0.0024 | 0.0537 | |
| Pb | Max | 0.0140 | 0.0018 | 0.0055 | 0.0057 | 0.1590 | 0.1590 |
| Min | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | |
| Mean | 0.0021 | 0.0001 | 0.0014 | 0.0015 | 0.0103 | 0.0030 | |
| StdDev | 0.0044 | 0.0004 | 0.0015 | 0.0017 | 0.0372 | 0.0166 | |
| Mn | Max | 562.0000 | 415.0000 | 490.0000 | 830.0000 | 325.0000 | 830.0000 |
| Min | 0.1910 | 0.1000 | 0.2000 | 0.0000 | 0.1000 | 0.0000 | |
| Mean | 98.1132 | 72.0928 | 164.5378 | 187.4125 | 115.9472 | 128.3652 | |
| StdDev | 203.6324 | 131.9233 | 189.1197 | 252.4662 | 121.7922 | 184.3860 | |
| Cd | Max | 0.0006 | 0.0024 | 0.0009 | 0.0007 | 0.0009 | 0.0024 |
| Min | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | |
| Mean | 0.0002 | 0.0003 | 0.0004 | 0.0003 | 0.0003 | 0.0003 | |
| StdDev | 0.0002 | 0.0006 | 0.0003 | 0.0003 | 0.0003 | 0.0003 | |
| Fe | Max | 580.0000 | 160.0000 | 320.0000 | 540.0000 | 55.0000 | 580.0000 |
| Min | 0.5000 | 0.0000 | 0.0460 | 0.1000 | 0.2000 | 0.0000 | |
| Mean | 61.7444 | 22.5959 | 23.3658 | 42.1406 | 6.6250 | 29.8712 | |
| StdDev | 153.3979 | 46.0680 | 67.7370 | 133.2024 | 14.1562 | 93.2930 | |
| Ba | Max | 0.6680 | 0.9090 | 1.1176 | 0.5436 | 0.5375 | 1.1176 |
| Min | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | |
| Mean | 0.1005 | 0.1382 | 0.1477 | 0.1420 | 0.0796 | 0.1232 | |
| StdDev | 0.1564 | 0.2563 | 0.2279 | 0.1715 | 0.1305 | 0.1958 | |
| Se | Max | 0.0018 | 0.0018 | 0.0041 | 0.0130 | 0.0080 | 0.0130 |
| Min | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | |
| Mean | 0.0004 | 0.0002 | 0.0015 | 0.0031 | 0.0022 | 0.0014 | |
| StdDev | 0.0007 | 0.0006 | 0.0012 | 0.0041 | 0.0025 | 0.0024 | |
| Cr | Max | 0.0024 | 0.0024 | 0.0038 | 0.0082 | 0.0033 | 0.0082 |
| Min | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | |
| Mean | 0.0005 | 0.0003 | 0.0016 | 0.0018 | 0.0016 | 0.0012 | |
| StdDev | 0.0010 | 0.0008 | 0.0013 | 0.0021 | 0.0013 | 0.0014 | |
References
- Arya, S.; Kumar, V.; Sharma, S. Analysis of water quality parameters of groundwater in and around Diamond Cement Industry, Jhansi, Central India. Int. J. Curr. Res. 2012, 4, 75–77. [Google Scholar]
- Kaviarasan, M.; Geetha, P.; Soman, K.P. GIS-based ground water quality monitoring in Thiruvannamalai District, Tamil Nadu, India. In Proceedings of the International Conference on Soft Computing Systems: ICSCS 2015, Chennai, India, 20–21 April 2015; pp. 685–700. [Google Scholar]
- Putthividhya, A.; Laonamsai, J. Assessment of Surface and Ground-water Interactions using Stable Isotope Fingerprinting Technique in Thailand. In Proceedings of the World Environmental and Water Resources Congress, Austin, TX, USA, 17–21 May 2015; pp. 464–474. [Google Scholar]
- Babiker, I.S.; Mohamed, M.A.A.; Hiyama, T. Assessing groundwater quality using GIS. Water Resour. Manag. 2007, 21, 699–715. [Google Scholar] [CrossRef]
- Zhang, Y.; Wu, J.; Xu, B. Human health risk assessment of groundwater nitrogen pollution in Jinghui canal irrigation area of the loess region, northwest China. Environ. Earth Sci. 2018, 77, 273. [Google Scholar] [CrossRef]
- Raza, M.; Hussain, F.; Lee, J.-Y.; Shakoor, M.B.; Kwon, K.D. Groundwater status in Pakistan: A review of contamination, health risks, and potential needs. Crit. Rev. Environ. Sci. Technol. 2017, 47, 1713–1762. [Google Scholar] [CrossRef]
- Shankar, S.; Shanker, U. Arsenic contamination of groundwater: A review of sources, prevalence, health risks, and strategies for mitigation. Sci. World J. 2014, 2014, 304524. [Google Scholar] [CrossRef]
- Ullah, R.; Malik, R.N.; Qadir, A. Assessment of groundwater contamination in an industrial city, Sialkot, Pakistan. Afr. J. Environ. Sci. Technol. 2009, 3, 429–446. [Google Scholar]
- Dawood, F.; Akhtar, M.M.; Ehsan, M. Evaluating urbanization impact on stressed aquifer of Quetta Valley, Pakistan. Desalin. Water Treat. 2021, 222, 103–113. [Google Scholar] [CrossRef]
- Rapant, S.; Krčmová, K. Health risk assessment maps for arsenic groundwater content: Application of national geochemical databases. Environ. Geochem. Health 2007, 29, 131–141. [Google Scholar] [CrossRef]
- Li, P.; Karunanidhi, D.; Subramani, T.; Srinivasamoorthy, K. Sources and consequences of groundwater contamination. Arch. Environ. Contam. Toxicol. 2021, 80, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Kumar, M.; Borah, P.; Devi, P. Priority and emerging pollutants in water. In Inorganic Pollutants in Water; Elsevier: Amsterdam, The Netherlands, 2020; pp. 33–49. [Google Scholar]
- Fuoco, I.; Marini, L.; De Rosa, R.; Figoli, A.; Gabriele, B.; Apollaro, C. Use of reaction path modelling to investigate the evolution of water chemistry in shallow to deep crystalline aquifers with a special focus on fluoride. Sci. Total Environ. 2022, 830, 154566. [Google Scholar] [CrossRef]
- Alarcón-Herrera, M.T.; Gutiérrez, M. Geogenic arsenic in groundwater: Challenges, gaps, and future directions. Curr. Opin. Environ. Sci. Health 2022, 27, 100349. [Google Scholar] [CrossRef]
- Cho, B.W.; Choo, C.O. Geochemical behavior of uranium and radon in groundwater of Jurassic granite area, Icheon, Middle Korea. Water 2019, 11, 1278. [Google Scholar] [CrossRef]
- Shannon, M.C.; Grieve, C.M. Tolerance of vegetable crops to salinity. Sci. Hortic. 1998, 78, 5–38. [Google Scholar] [CrossRef]
- Barakat, M.A. New trends in removing heavy metals from industrial wastewater. Arab. J. Chem. 2011, 4, 361–377. [Google Scholar] [CrossRef]
- Fu, Z.; Xi, S. The effects of heavy metals on human metabolism. Toxicol. Mech. Methods 2020, 30, 167–176. [Google Scholar] [CrossRef]
- Mohankumar, K.; Hariharan, V.; Rao, N.P. Heavy metal contamination in groundwater around industrial estate vs residential areas in Coimbatore, India. J. Clin. Diagn. Res. JCDR 2016, 10, BC05. [Google Scholar] [CrossRef]
- Mehmood, A.; Qadir, A.; Ehsan, M.; Ali, A.; Raza, D.; Aziz, H. Hydrogeological studies and evaluation of surface and groundwater quality of Khyber Pakhtunkhwa, Pakistan. Desalin. Water Treat. 2021, 244, 41–54. [Google Scholar] [CrossRef]
- Bhutiani, R.; Kulkarni, D.B.; Khanna, D.R.; Gautam, A. Geochemical distribution and environmental risk assessment of heavy metals in groundwater of an industrial area and its surroundings, Haridwar, India. Energy Ecol. Environ. 2017, 2, 155–167. [Google Scholar] [CrossRef]
- Chanpiwat, P.; Lee, B.-T.; Kim, K.-W.; Sthiannopkao, S. Human health risk assessment for ingestion exposure to groundwater contaminated by naturally occurring mixtures of toxic heavy metals in the Lao PDR. Environ. Monit. Assess. 2014, 186, 4905–4923. [Google Scholar] [CrossRef]
- Rehman, K.; Fatima, F.; Waheed, I.; Akash, M.S.H. Prevalence of exposure of heavy metals and their impact on health consequences. J. Cell. Biochem. 2018, 119, 157–184. [Google Scholar] [CrossRef]
- Rezaei, H.; Zarei, A.; Kamarehie, B.; Jafari, A.; Fakhri, Y.; Bidarpoor, F.; Karami, M.A.; Farhang, M.; Ghaderpoori, M.; Sadeghi, H. Levels, distributions and health risk assessment of lead, cadmium and arsenic found in drinking groundwater of Dehgolan’s villages, Iran. Toxicol. Environ. Health Sci. 2019, 11, 54–62. [Google Scholar] [CrossRef]
- Ghosh, G.C.; Khan, M.J.H.; Chakraborty, T.K.; Zaman, S.; Kabir, A.H.M.E.; Tanaka, H. Human health risk assessment of elevated and variable iron and manganese intake with arsenic-safe groundwater in Jashore, Bangladesh. Sci. Rep. 2020, 10, 5206. [Google Scholar] [CrossRef]
- Batayneh, A.T. Toxic (aluminum, beryllium, boron, chromium and zinc) in groundwater: Health risk assessment. Int. J. Environ. Sci. Technol. 2012, 9, 153–162. [Google Scholar] [CrossRef]
- Laonamsai, J.; Ichiyanagi, K.; Patsinghasanee, S. Isotopic temporal and spatial variations of tropical rivers in Thailand reflect monsoon precipitation signals. Hydrol. Process. 2021, 35, e14068. [Google Scholar] [CrossRef]
- Laonamsai, J.; Putthividhya, A. Preliminary assessment of groundwater and surface water characteristics in the upper Chao Phraya River Basin land using a stable isotope fingerprinting technique. In Proceedings of the World Environmental and Water Resources Congress, West Palm Beach, FL, USA, 22–26 May 2016; pp. 367–386. [Google Scholar]
- Venter, Z.S.; Barton, D.N.; Chakraborty, T.; Simensen, T.; Singh, G. Global 10 m Land Use Land Cover Datasets: A Comparison of Dynamic World, World Cover and Esri Land Cover. Remote Sens. 2022, 14, 4101. [Google Scholar] [CrossRef]
- Peel, M.C.; Finlayson, B.L.; McMahon, T.A. Updated world map of the Köppen-Geiger climate classification. Hydrol. Earth Syst. Sci. 2007, 11, 1633–1644. [Google Scholar] [CrossRef]
- Laonamsai, J.; Ichiyanagi, K.; Kamdee, K.; Putthividhya, A.; Tanoue, M. Spatial and temporal distributions of stable isotopes in precipitation over Thailand. Hydrol. Process. 2021, 35, e13995. [Google Scholar] [CrossRef]
- Tevapitak, K.; Helmsing, A.H.J.B. The interaction between local governments and stakeholders in environmental management: The case of water pollution by SMEs in Thailand. J. Environ. Manag. 2019, 247, 840–848. [Google Scholar] [CrossRef] [PubMed]
- Phien-wej, N.; Giao, P.H.; Nutalaya, P. Land subsidence in bangkok, Thailand. Eng. Geol. 2006, 82, 187–201. [Google Scholar] [CrossRef]
- Stoecker, F.; Babel, M.S.; Gupta, A.D.; Rivas, A.A.; Evers, M.; Kazama, F.; Nakamura, T. Hydrogeochemical and isotopic characterization of groundwater salinization in the Bangkok aquifer system, Thailand. Environ. Earth Sci. 2013, 68, 749–763. [Google Scholar] [CrossRef]
- Gupta, A.D. Simulated Salt-Water Movement in the Nakhon Luang Aquifer, Bangkok, Thailand. Groundwater 1985, 23, 512–522. [Google Scholar] [CrossRef]
- Nutalaya, P.; Yong, R.N.; Chumnankit, T.; Buapeng, S. Land subsidence in Bangkok during 1978–1988. Sea-Level Rise Coast. Subsid. Causes Conseq. Strateg. 1996, 2, 105–130. [Google Scholar]
- Putthividhya, A.; Laonamsai, J. SWAT and MODFLOW modeling of spatio-temporal runoff and groundwater recharge distribution. In Proceedings of the World Environmental and Water Resources Congress, Sacramento, CA, USA, 21–25 May 2017; pp. 51–65. [Google Scholar]
- Deborah, C. Water Quality Assessments: A Guide to Use of Biota, Sediments and Water in Environmental Monitoring; UNESCO/WHO/UNEP: Cambridge, UK, 1996. [Google Scholar]
- Miner, G. Standard methods for the examination of water and wastewater. Am. Water Work. Assoc. J. 2006, 98, 130. [Google Scholar] [CrossRef]
- Richards, L.A. Diagnosis and Improvement of Saline and Alkali Soils; LWW: Philadelphia, PA, USA, 1954; Volume 78. [Google Scholar]
- World Health Organization. Guidelines for Drinking-Water Quality: Incorporating the First and Second Addenda; World Health Organization: Geneva, Switzerland, 2022. [Google Scholar]
- Rabeiy, R.E. Assessment and modeling of groundwater quality using WQI and GIS in Upper Egypt area. Environ. Sci. Pollut. Res. 2018, 25, 30808–30817. [Google Scholar] [CrossRef] [PubMed]
- Magesh, N.S.; Chandrasekar, N. Evaluation of spatial variations in groundwater quality by WQI and GIS technique: A case study of Virudunagar District, Tamil Nadu, India. Arab. J. Geosci. 2013, 6, 1883–1898. [Google Scholar] [CrossRef]
- Unigwe, C.O.; Egbueri, J.C. Drinking water quality assessment based on statistical analysis and three water quality indices (MWQI, IWQI and EWQI): A case study. Environ. Dev. Sustain. 2023, 25, 686–707. [Google Scholar] [CrossRef]
- Ram, A.; Tiwari, S.K.; Pandey, H.K.; Chaurasia, A.K.; Singh, S.; Singh, Y.V. Groundwater quality assessment using water quality index (WQI) under GIS framework. Appl. Water Sci. 2021, 11, 46. [Google Scholar] [CrossRef]
- Loh, Y.S.A.; Akurugu, B.A.; Manu, E.; Aliou, A.-S. Assessment of groundwater quality and the main controls on its hydrochemistry in some Voltaian and basement aquifers, northern Ghana. Groundw. Sustain. Dev. 2020, 10, 100296. [Google Scholar] [CrossRef]
- Ravikumar, P.; Somashekar, R.K.; Prakash, K.L. A comparative study on usage of Durov and Piper diagrams to interpret hydrochemical processes in groundwater from SRLIS river basin, Karnataka, India. Elixir Earth Sci 2015, 80, 31073–31077. [Google Scholar]
- Wilcox, L. Classification and Use of Irrigation Waters; US Department of Agriculture: Washington, DC, USA, 1955. [Google Scholar]
- Cioni, R.; Marini, L. A Thermodynamic Approach to Water Geothermometry; Springer: Berlin/Heidelberg, Germany, 2020. [Google Scholar]
- Procesi, M.; Marini, L.; Cinti, D.; Sciarra, A.; Basile, P.; Mazzoni, T.; Zarlenga, F. Preliminary fluid geochemical survey in Tete Province and prospective development of geothermics in Mozambique. Geotherm. Energy 2022, 10, 2. [Google Scholar] [CrossRef]
- Tonani, F.B.; Nagao, K.; Moore, J.; Natale, G.; Sperry, T. Water and gas geochemistry of the Cove-Fort Sulphurdale geothermal system. In Proceedings of the Twenty-Third Workshop on Geothermal Reservoir Engineering, Stanford, CA, USA, 26–28 January 1998; pp. 26–28. [Google Scholar]
- Chegbeleh, L.P.; Aklika, D.K.; Akurugu, B.A. Hydrochemical characterization and suitability assessment of groundwater quality in the Saboba and Chereponi Districts, Ghana. Hydrology 2020, 7, 53. [Google Scholar] [CrossRef]
- Sahu, P.; Sikdar, P.K. Hydrochemical framework of the aquifer in and around East Kolkata Wetlands, West Bengal, India. Environ. Geol. 2008, 55, 823–835. [Google Scholar] [CrossRef]
- Sadashivaiah, C.; Ramakrishnaiah, C.R.; Ranganna, G. Hydrochemical analysis and evaluation of groundwater quality in Tumkur Taluk, Karnataka State, India. Int. J. Environ. Res. Public Health 2008, 5, 158–164. [Google Scholar] [CrossRef] [PubMed]
- Sinsakul, S. Late quaternary geology of the lower central plain, Thailand. J. Asian Earth Sci. 2000, 18, 415–426. [Google Scholar] [CrossRef]
- Laonamsai, J.; Ichiyanagi, K.; Patsinghasanee, S.; Kamdee, K.; Tomun, N. Application of Stable Isotopic Compositions of Rainfall Runoff for Evaporation Estimation in Thailand Mekong River Basin. Water 2022, 14, 2803. [Google Scholar] [CrossRef]
- Stanienda, K.J. Carbonate phases rich in magnesium in the Triassic limestones of the eastern part of the Germanic Basin. Carb. Evaporites 2016, 31, 387–405. [Google Scholar] [CrossRef]
- Krawczyk, W.E.; Ford, D.C. Correlating specific conductivity with total hardness in limestone and dolomite karst waters. Earth Surf. Process. Landf. 2006, 31, 221–234. [Google Scholar] [CrossRef]
- Ahmed, A.A.; Shabana, A.R.; Saleh, A.A. Using hydrochemical and isotopic data to determine sources of recharge and groundwater evolution in arid region from Eastern Desert, Egypt. J. Afr. Earth Sci. 2019, 151, 36–46. [Google Scholar] [CrossRef]
- Bucher, K.; Grapes, R. Petrogenesis of Metamorphic Rocks; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2011. [Google Scholar]
- Park, S.-C.; Yun, S.-T.; Chae, G.-T.; Yoo, I.-S.; Shin, K.-S.; Heo, C.-H.; Lee, S.-K. Regional hydrochemical study on salinization of coastal aquifers, western coastal area of South Korea. J. Hydrol. 2005, 313, 182–194. [Google Scholar] [CrossRef]
- Li, P.; Qian, H.; Wu, J.; Zhang, Y.; Zhang, H. Major ion chemistry of shallow groundwater in the Dongsheng Coalfield, Ordos Basin, China. Mine Water Environ. 2013, 32, 195. [Google Scholar] [CrossRef]
- Hosono, T.; Nakano, T.; Shimizu, Y.; Onodera, S.i.; Taniguchi, M. Hydrogeological constraint on nitrate and arsenic contamination in Asian metropolitan groundwater. Hydrol. Process. 2011, 25, 2742–2754. [Google Scholar] [CrossRef]
- Lucena, J.J. Effects of bicarbonate, nitrate and other environmental factors on iron deficiency chlorosis. A review. J. Plant Nutr. 2000, 23, 1591–1606. [Google Scholar] [CrossRef]
- Ebrahimi, M.; Kazemi, H.; Ehtashemi, M.; Rockaway, T.D. Assessment of groundwater quantity and quality and saltwater intrusion in the Damghan basin, Iran. Geochemistry 2016, 76, 227–241. [Google Scholar] [CrossRef]
- Chae, G.-T.; Yun, S.-T.; Kim, K.; Mayer, B. Hydrogeochemistry of sodium-bicarbonate type bedrock groundwater in the Pocheon spa area, South Korea: Water–rock interaction and hydrologic mixing. J. Hydrol. 2006, 321, 326–343. [Google Scholar] [CrossRef]
- Schofield, S.; Jankowski, J. Hydrochemistry and isotopic composition of Na–HCO3-rich groundwaters from the Ballimore region, central New South Wales, Australia. Chem. Geol. 2004, 211, 111–134. [Google Scholar] [CrossRef]
- Chidambaram, S.; Karmegam, U.; Sasidhar, P.; Prasanna, M.V.; Manivannan, R.; Arunachalam, S.; Manikandan, S.; Anandhan, P. Significance of saturation index of certain clay minerals in shallow coastal groundwater, in and around Kalpakkam, Tamil Nadu, India. J. Earth Syst. Sci. 2011, 120, 897. [Google Scholar] [CrossRef]
- Nihalani, S.A.; Behede, S.N.; Meeruty, A.R. Groundwater suitability determination near solid waste dump site at Pune. Sustain. Water Resour. Manag. 2022, 8, 64. [Google Scholar] [CrossRef]
- Vespasiano, G.; Muto, F.; Apollaro, C. Geochemical, geological and groundwater quality characterization of a complex geological framework: The case study of the Coreca area (Calabria, South Italy). Geosciences 2021, 11, 121. [Google Scholar] [CrossRef]
- Williams, M.; Fordyce, F.; Paijitprapapon, A.; Charoenchaisri, P. Arsenic contamination in surface drainage and groundwater in part of the southeast Asian tin belt, Nakhon Si Thammarat Province, southern Thailand. Environ. Geol. 1996, 27, 16–33. [Google Scholar] [CrossRef]
- Li, B.; Song, X.; Yang, L.; Yao, D.; Xu, Y. Insights onto Hydrologic and Hydro-Chemical Processes of Riparian Groundwater Using Environmental Tracers in the Highly Disturbed Shaying River Basin, China. Water 2020, 12, 1939. [Google Scholar] [CrossRef]
- Alloway, B.J. Sources of heavy metals and metalloids in soils. Heavy Met. Soils Trace Met. Met. Soils Bioavailab. 2013, 22, 11–50. [Google Scholar]
- Nriagu, J.O. Zinc in the Environment. Part I: Ecological Sycling; John Wiley & Sons: New York, NY, USA, 1980. [Google Scholar]
- Nordberg, G.F.; Nordberg, M.; Costa, M. Toxicology of metals: Overview, definitions, concepts, and trends. Handb. Toxicol. Met. 2022, 1–14. [Google Scholar] [CrossRef]
- Das, S.; Green, A. Zinc in Crops and Human Health. In Biofortification of Food Crops; Singh, U., Praharaj, C.S., Singh, S.S., Singh, N.P., Eds.; Springer India: New Delhi, India, 2016; pp. 31–40. [Google Scholar]
- Wagner, S.E.; Burch, J.B.; Hussey, J.; Temples, T.; Bolick-Aldrich, S.; Mosley-Broughton, C.; Liu, Y.; Hebert, J.R. Soil zinc content, groundwater usage, and prostate cancer incidence in South Carolina. Cancer Causes Control 2009, 20, 345–353. [Google Scholar] [CrossRef]
- Haglund, B.; Ryckenberg, K.; Selinus, O.; Dahlquist, G. Evidence of a Relationship Between Childhood-Onset Type I Diabetes and Low Groundwater Concentration of Zinc. Diabetes Care 1996, 19, 873–875. [Google Scholar] [CrossRef]
- Nriagu, J.O. Zinc in the Environment. Part II: Health Effects; John Wiley & Sons: New York, NY, USA, 1980. [Google Scholar]
- Krok, B.; Mohammadian, S.; Noll, H.M.; Surau, C.; Markwort, S.; Fritzsche, A.; Nachev, M.; Sures, B.; Meckenstock, R.U. Remediation of zinc-contaminated groundwater by iron oxide in situ adsorption barriers—From lab to the field. Sci. Total Environ. 2022, 807, 151066. [Google Scholar] [CrossRef]
- Vukojević Medvidović, N.; Nuić, I.; Ugrina, M.; Trgo, M. Evaluation of Natural Zeolite as a Material for Permeable Reactive Barrier for Remediation of Zinc-Contaminated Groundwater Based on Column Study. Water Air Soil Pollut. 2018, 229, 367. [Google Scholar] [CrossRef]
- Ghezzi, L.; Arrighi, S.; Giannecchini, R.; Bini, M.; Valerio, M.; Petrini, R. The legacy of mercury contamination from a past leather manufacturer and health risk assessment in an urban area (Pisa Municipality, Italy). Sustainability 2022, 14, 4367. [Google Scholar] [CrossRef]
- González-Fernández, B.; Menéndez-Casares, E.; Meléndez-Asensio, M.; Fernández-Menéndez, S.; Ramos-Muñiz, F.; Cruz-Hernández, P.; González-Quirós, A. Sources of mercury in groundwater and soils of west Gijón (Asturias, NW Spain). Sci. Total Environ. 2014, 481, 217–231. [Google Scholar] [CrossRef]
- Khattak, S.A.; Rashid, A.; Tariq, M.; Ali, L.; Gao, X.; Ayub, M.; Javed, A. Potential risk and source distribution of groundwater contamination by mercury in district Swabi, Pakistan: Application of multivariate study. Environ. Dev. Sustain. 2021, 23, 2279–2297. [Google Scholar] [CrossRef]
- Blue, L.Y.; Van Aelstyn, M.A.; Matlock, M.; Atwood, D.A. Low-level mercury removal from groundwater using a synthetic chelating ligand. Water Res. 2008, 42, 2025–2028. [Google Scholar] [CrossRef]
- Shalhevet, J. Using water of marginal quality for crop production: Major issues. Agric. Water Manag. 1994, 25, 233–269. [Google Scholar] [CrossRef]
- Fu, F.; Dionysiou, D.D.; Liu, H. The use of zero-valent iron for groundwater remediation and wastewater treatment: A review. J. Hazard. Mater. 2014, 267, 194–205. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Lin, S.; Lu, X.-Q.; Chen, Z.-l. Removal of Pb(II) from water using synthesized kaolin supported nanoscale zero-valent iron. Chem. Eng. J. 2010, 163, 243–248. [Google Scholar] [CrossRef]
- Ahmad, W.; Alharthy, R.D.; Zubair, M.; Ahmed, M.; Hameed, A.; Rafique, S. Toxic and heavy metals contamination assessment in soil and water to evaluate human health risk. Sci. Rep. 2021, 11, 17006. [Google Scholar] [CrossRef]
- Levy, B.S.; Nassetta, W.J. Neurologic effects of manganese in humans: A review. Int. J. Occup. Environ. Health 2003, 9, 153–163. [Google Scholar] [CrossRef]
- Rajmohan, N.; Elango, L. Distribution of iron, manganese, zinc and atrazine in groundwater in parts of Palar and Cheyyar river basins, South India. Environ. Monit. Assess. 2005, 107, 115–131. [Google Scholar] [CrossRef]
- Podgorski, J.; Araya, D.; Berg, M. Geogenic manganese and iron in groundwater of Southeast Asia and Bangladesh—Machine learning spatial prediction modeling and comparison with arsenic. Sci. Total Environ. 2022, 833, 155131. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Huang, T.; Wen, G.; Cao, X. The simultaneous removal of ammonium and manganese from groundwater by iron-manganese co-oxide filter film: The role of chemical catalytic oxidation for ammonium removal. Chem. Eng. J. 2017, 308, 322–329. [Google Scholar] [CrossRef]
- Ellis, D.; Bouchard, C.; Lantagne, G. Removal of iron and manganese from groundwater by oxidation and microfiltration. Desalination 2000, 130, 255–264. [Google Scholar] [CrossRef]
- Babel, S.; Kurniawan, T.A. Various treatment technologies to remove arsenic and mercury from contaminated groundwater: An overview. Proc. Southeast Asian Water Environ. 2005, 1. [Google Scholar] [CrossRef]
- Hashim, M.A.; Mukhopadhyay, S.; Sahu, J.N.; Sengupta, B. Remediation technologies for heavy metal contaminated groundwater. J. Environ. Manag. 2011, 92, 2355–2388. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.K.; Rammohan, V.; Sahayam, J.D.; Jeevanandam, M. Assessment of groundwater quality and hydrogeochemistry of Manimuktha River basin, Tamil Nadu, India. Environ. Monit. Assess. 2009, 159, 341–351. [Google Scholar] [CrossRef] [PubMed]
- Saleh, A.; Al-Ruwaih, F.; Shehata, M. Hydrogeochemical processes operating within the main aquifers of Kuwait. J. Arid Environ. 1999, 42, 195–209. [Google Scholar] [CrossRef]
- Anandakumar, S.; Subramani, T.; Elango, L. Major ion groundwater chemistry of Lower Bhavani River Basin, Tamil Nadu, India. J. Appl. Geochem. 2009, 11, 92–101. [Google Scholar]
- Kaçaroğlu, F.; Günay, G. Groundwater nitrate pollution in an alluvium aquifer, Eskişehir urban area and its vicinity, Turkey. Environ. Geol. 1997, 31, 178–184. [Google Scholar] [CrossRef]
- Srivastav, A.L. Chemical fertilizers and pesticides: Role in groundwater contamination. In Agrochemicals Detection, Treatment and Remediation; Elsevier: Amsterdam, The Netherlands, 2020; pp. 143–159. [Google Scholar]
- Squillace, P.J.; Scott, J.C.; Moran, M.J.; Nolan, B.T.; Kolpin, D.W. VOCs, pesticides, nitrate, and their mixtures in groundwater used for drinking water in the United States. Environ. Sci. Technol. 2002, 36, 1923–1930. [Google Scholar] [CrossRef]
- Boateng, T.K.; Opoku, F.; Acquaah, S.O.; Akoto, O. Groundwater quality assessment using statistical approach and water quality index in Ejisu-Juaben Municipality, Ghana. Environ. Earth Sci. 2016, 75, 489. [Google Scholar] [CrossRef]
- Czarnecki, S.; Düring, R.A. Influence of long-term mineral fertilization on metal contents and properties of soil samples taken from different locations in Hesse, Germany. Soil 2015, 1, 23–33. [Google Scholar] [CrossRef]
| Parameters | WHO (2022) Standard (Si, mg/L) | Weight (wi, mg/L) | Relative Weight (Wi, mg/L) |
|---|---|---|---|
| pH | 8.5 | 4 | 0.0533 |
| TDS | 1000 | 4 | 0.0533 |
| Ca | 100 | 2 | 0.0267 |
| Mg | 50 | 2 | 0.0267 |
| Na | 200 | 3 | 0.0400 |
| K | 20 | 2 | 0.0267 |
| HCO3 | 350 | 2 | 0.0267 |
| SO4 | 250 | 3 | 0.0400 |
| Cl | 250 | 3 | 0.0400 |
| NO3 | 50 | 2 | 0.0267 |
| PO4 | 5 | 4 | 0.0533 |
| F | 1 | 5 | 0.0667 |
| As | 0.01 | 5 | 0.0667 |
| Zn | 5 | 2 | 0.0267 |
| Pb | 0.01 | 5 | 0.0667 |
| Hg | 0.006 | 5 | 0.0667 |
| Fe | 0.3 | 3 | 0.0400 |
| Mn | 0.4 | 4 | 0.0533 |
| Cr | 0.05 | 5 | 0.0667 |
| Cd | 0.003 | 5 | 0.0667 |
| Ni | 0.02 | 5 | 0.0667 |
| Total | 75 | 1 | |
| Ions | Concentration (mg/L) | |||
|---|---|---|---|---|
| Maximum (Aquifer) | Minimum (Aquifer) | Average | Standard Deviation | |
| Ca | 1640.0 (BKK) | 1.0 (PPD) | 219.53 | 258.08 |
| Mg | 487.78 (PPD) | 0.7 (SK) | 46.97 | 101.52 |
| Na | 2888.89 (PPD) | 26.0 (BKK) | 590.62 | 507.81 |
| K | 60.0 (NKL) | 1.0 (PPD) | 10.94 | 10.99 |
| Cl | 9548.8 (BKK) | 7.2 (NTB) | 834.17 | 1463.07 |
| SO4 | 1109.33 (BKK) | 0.0 (PPD) | 343.88 | 254.79 |
| HCO3 | 788.0 (SK) | 0.0 (PPD) | 309.47 | 142.52 |
| CO3 | 370.25 (NKL) | 0.0 (PPD) | 216.83 | 11.10 |
| NO3 | 21.5 (BKK) | 0.0 (NTB and SK) | 2.68 | 11.69 |
| Aquifers | Water Quality Index (WQI) | ||
|---|---|---|---|
| Max | Min | Mean | |
| BKK | 14,314 | 28 | 2590 |
| PPD | 5752 | 22 | 1659 |
| NKL | 9251 | 55 | 2761 |
| NTB | 11,443 | 23 | 3314 |
| SK | 4414 | 26 | 1775 |
| Average | 9035 | 31 | 2419 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Laonamsai, J.; Pawana, V.; Chipthamlong, P.; Chomcheawchan, P.; Kamdee, K.; Kimmany, B.; Julphunthong, P. Groundwater Quality Variations in Multiple Aquifers: A Comprehensive Evaluation for Public Health and Agricultural Use. Geosciences 2023, 13, 195. https://doi.org/10.3390/geosciences13070195
Laonamsai J, Pawana V, Chipthamlong P, Chomcheawchan P, Kamdee K, Kimmany B, Julphunthong P. Groundwater Quality Variations in Multiple Aquifers: A Comprehensive Evaluation for Public Health and Agricultural Use. Geosciences. 2023; 13(7):195. https://doi.org/10.3390/geosciences13070195
Chicago/Turabian StyleLaonamsai, Jeerapong, Veeraphat Pawana, Phupha Chipthamlong, Phornsuda Chomcheawchan, Kiattipong Kamdee, Bounhome Kimmany, and Phongthorn Julphunthong. 2023. "Groundwater Quality Variations in Multiple Aquifers: A Comprehensive Evaluation for Public Health and Agricultural Use" Geosciences 13, no. 7: 195. https://doi.org/10.3390/geosciences13070195
APA StyleLaonamsai, J., Pawana, V., Chipthamlong, P., Chomcheawchan, P., Kamdee, K., Kimmany, B., & Julphunthong, P. (2023). Groundwater Quality Variations in Multiple Aquifers: A Comprehensive Evaluation for Public Health and Agricultural Use. Geosciences, 13(7), 195. https://doi.org/10.3390/geosciences13070195









