Abstract
This research shows the results regarding the response to acidic condition of the sediment and Posidonia foraminiferal assemblages collected around the Panarea Island. The Aeolian Archipelago represents a natural laboratory and a much-promising study site for multidisciplinary marine research (carbon capture and storage, geochemistry of hydrothermal fluids and ocean acidification vs. benthic and pelagic organisms). The variability and the complexity of the interaction of the ecological factors characterizing extreme environments such as shallow hydrothermal vents did not allow us to carry out a real pattern of biota responses in situ, differently from those observed under controlled laboratory conditions. However, the study provides new insights into foraminiferal response to increasing ocean acidification (OA) in terms of biodiversity, faunal density, specific composition of the assemblages and morphological variations of the shells. The study highlights how the foraminiferal response to different pH conditions can change depending on different environmental conditions and microhabitats (sediments, Posidonia leaves and rhizomes). Indeed, mineral sediments were more impacted by acidification, whereas Posidonia microhabitats, thanks to their buffer effect, can offer “refugia” and more mitigated acidic environment. At species level, rosalinids and agglutinated group represent the most abundant taxa showing the most specific resilience and capability to face acidic conditions.
1. Introduction
Ocean acidification (OA) is one of the main consequences of climate change linked to the increasing atmospheric CO2 concentration, affecting current and future marine ecosystems [1,2,3]. OA associated with global warming of 1.5 °C to 2 °C [3], is projected to amplify its adverse effects on the growth, development, calcification and survival of marine organisms [4,5]. Reduced calcification, reduced rates of repair, and weakened calcified structures are among the most significant effects to the future levels of OA expected by 2100. However, the responses are species-specific and some organisms have not shown high sensitivity to changes in CO2, pH and carbonate concentrations [6,7,8,9,10]. Among meiofaunal protists, foraminifers, single-celled protists, are by far the most useful meiofaunal group utilized by geoscientists in environmental and climatological reconstructions of recent and past geological record. Based upon their widespread distribution, short life and reproductive cycle, high biodiversity, and specific ecological requirements, foraminifers may quickly respond to environmental changes and thus can be used as bio-indicators in a wide range of marine environments, e.g., [11,12,13,14,15]. The calcification process in benthic foraminifers is sensitive both to ocean acidification and local chemical and physical features of seawater. Therefore, they can record specific environmental conditions and they can provide a possible response to the ongoing acidification trend giving a picture of the present day and future changes [16,17]. Shallow-water (less than 200 m water depth) hydrothermal fields provide the opportunity to study fluid impacts and the effects of elevated pCO2 on the benthic foraminiferal communities [1,18,19,20]. Among them, the seafloor around Panarea Island (Aeolian Archipelago) represents a natural laboratory and a much-promising study site for multidisciplinary marine research (carbon capture and storage, geochemistry of hydrothermal fluids and ocean acidification vs benthic and pelagic organisms). The seabottoms of Panarea Island offer a wide environmental variability linked to different degrees of hydrothermal activity varying from extreme environments to normal marine conditions. In addition, luxuriant Posidonia oceanica meadows host many ecological niches and different microhabitats useful to investigate the response of epiphytic community in which foraminifers represent one of the most conspicuous components [21,22,23,24]. Previous studies on benthic foraminiferal assemblages in Mediterranean areas characterized by underwater hydrothermal vents and CO2 fluid emissions, have indicated biodiversity loss, decrease in faunal density and variations in the test-type abundances (agglutinated, porcelaneous, hyaline) [25,26,27]. Moreover, experimental data showed how P. oceanica meadows metabolism can modulate the responses of epiphytic micro/meio-invertebrates to future OA, conferring more uniform pH regimes [28,29,30].
The main part of the experimental studies was carried out under controlled conditions in laboratory where it is possible to observe the response of microfauna (as a whole in foraminiferal assemblages or at single-species level) to the variations of each parameter (temperature, pH) [28,31]. The relationships between the environmental parameters (e.g., pH, temperature, salinity, CO2) and foraminiferal distribution in such environments in situ are yet poorly studied. Actually, the results obtained in the laboratory are very difficult to apply in nature because of the complexity of interaction of abiotic and biotic factors added to the temporal and spatial variability of these same factors.
On this perspective, the aim of this research is to present the results of the study of foraminiferal assemblages recorded both in sediments and Posidonia leaves off Panarea Island. Our purpose is to give new insights into the overall pattern of foraminiferal community response to the extreme environment of shallow hydrothermal vents in terms of biodiversity, specific composition of the assemblages, and morphological variations of the foraminiferal shells. These results could represent a background for biomonitoring the area in the future and to acquire data to elaborate short-time scenarios.
Panarea Island is part of the Aeolian Archipelago, a 200 km-long, ring-shaped partly submerged volcanic ridge, located in the SE sector of the Tyrrhenian Sea. The latter is a back-arc basin developed since the Neogene, as a consequence of the NW-directed subduction of the Ionian oceanic lithosphere below the Calabrian Arc [32,33,34,35,36]. Therefore, the archipelago is a subduction related volcanic arc [37,38], composed by stratovolcanoes formed by both explosive and effusive activity [39,40,41]. The volcanic products composition display calcalkaline, shoshonitic and potassic affinity, with an age ranging from 1.3 Ma to present [40,41,42]. Panarea Island and the surrounding islets represent the emerged portions of a wide, mostly submerged, volcanic edifice, more than 2000 m high, mainly dismantled by erosion and by neo- and volcano-tectonics activity [43,44,45]. The volcanic complex shows a conic shape, with a flat summit formed by a submerged shelf located at depths from 80 to 130 m b.s.l. To the east of Panarea, the islets of Basiluzzo, Panarelli, Dattilo, Bottaro, Lisca Bianca, Lisca Nera and Formiche represent an archipelago partly emerging from the flat eroded plateau, at a depth of less than 30 m [43]. The eruptive history of the volcanic system is formed by successive eruptive cycles of volcanic activity, separated by quiescent stages [46]. Panarea island is mostly composed of andesitic to dacitic lava domes, dated between 149 ± 5 and 127 ± 1.5 ka [46,47,48], interbedded with pyroclastic deposits. The islets are made of dacite and andesite lavas, the last of which were emplaced with the Basiluzzo endogenous dome (54 ± 8 ka) [47,49]. Currently, Panarea volcanic complex is characterized by local subsidence, observed in the Basiluzzo area [50] and by degassing from several fumarolic areas, both inland and offshore. Gas venting is frequent along a NE-SW trending extensional fault system, located NE of Panarea Island. Here a series of fault scarps and fresh volcanic rocks and mineralization are exposed, where gas venting has been observed [51,52]. These hydrothermal processes produced sediment-hosted sulphide deposits, Fe-rich crusts, bacterial mats and chimneys [51,53,54,55]. Hydrothermalism is also attested by diffuse degassing at the seafloor, and gas emissions and boiling of the seawater has been described since historical times at Panarea Island [51,56]. The active vents are characterized by discharge of CO2-dominated gases and thermal fluids with temperatures up to 140 °C [57,58]. An intense hydrothermal activity was reached in November 2002: a gas eruption occurred 125 km east of Panarea island, between 2 and 30 m b.s.l., localized on top of a shallow rise, surrounded by the islets of Panarelli, Lisca Bianca, Bottaro, Lisca Nera and Dattilo [56]. The most intense gas flux was measured to the west of the islet of Bottaro, where one vent was erupting at 108–109 lt/day [59,60]. According to [57], the total gas output under normal conditions is about 9 × 106 lt/day over the whole area. Direct measurements at the site of eruption reported values up to 110 °C, with pH between 5.0 and 5.5 [61,62]. The active degassing phase lasted several months, up to mid-2003, and had great influence on the biota and water properties in the proximity of vents, such as the death of benthic communities, high bacteria abundance and diversity, water salinity increasing, pH lowering, strong Cl- excess with respect to seawater [63,64,65]. Progressively, the hydrothermal activity reduced its intensity, and gas vents shifted their position with time [56]. Nowadays, most of the venting activity is concentrated within the submarine crater formed close to Bottaro islet during the paroxysmal phase of November 2002 [56].
2. Materials and Methods
2.1. Sampling Strategy
In the present paper the authors show the results of a sampling survey carried out in the frame of the Scientific Diving Summer School hosted in the ECCSEL-NatLab Italy laboratory of Panarea Island on September 2019, an ECCSEL-ERIC infrastructure of the National Institute of Oceanography and applied Geophysics. The samples were collected, by scuba divers of the diving center Amphibia, from a depth interval ranging from 4 to 24 m off the NE coastal sector of the Panarea Island. Four areas affected by submarine hydrothermal manifestations with CO2 dominated emissions (>90%) located at shallow depth around Panarea island were considered: Ditella areas (Hot and Cold), Bottaro crater, P21 and Black Point (Figure 1). These areas are well-known and documented and they are characterized by the presence of wide seagrasses patchy meadows, e.g., [57,58,65,66,67,68,69]. Study on the Panarea bottoms showed that P. oceanica shoot and leaf density seems to be unaffected by OA [70].
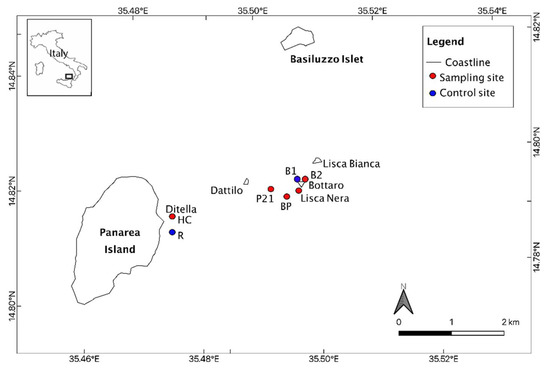
Figure 1.
Map of sampling sites. The triangle indicates the crater Bottaro zone. HC: Hot and Cold spots in Ditella area; R: Raya sites; P21 site; B1 and B2: Bottaro crater sites; BP: Black Point.
Two sites (Raya and B1) displaying normal marine pH and temperature values (pH = 8.15, 23–26 °C) were considered as control sites. Temperature and pH values reported in Table 1 are unpublished data (personal communication). For Ditella stations T and pH values reply the values reported in literature [68].

Table 1.
Environmental parameters for each study site. pH and Temperature were measured at the emission point. n.d.: not determined values.
Ditella area is located NE of Panarea Island at 10–12 m depth. This area is characterized by spots with very different temperatures at the distance of few meters from each other, which probably influence the distribution of the patchy meadow of P. oceanica seagrass. The hot spots are characterized by CO2 emissions with a temperature up to 60° and pH values ranging from 7 to 5.6 (parameters were measured at the emission points). The cold spots are characterized by reduced venting activity and display temperature of about 26 °C and pH of about 7.9. In this site Posidonia coverage appears continuous. Data collected during 2016 reported higher shoot density near the cold areas (365.6 ± 89.7 shoots/m2) than near the hot plots (273.2 ± 99.2 shoots/m2) [71].
Posidonia leaves and soft sediment samples were collected from each station to highlight differences between sediment and leaves foraminiferal assemblages.
The Bottaro crater is an area characterized by a depression (about 14 m wide, over 20 m long and about 11 m deep) located 3.3 km to the east of Panarea close to the Bottaro islet. This crater is the result of the massive gas eruption occurred in November 2002 [56,58]. In the Bottaro crater zone only Posidonia samples were considered (13 m depth) coming from two of the three sites (B1–B2). Literature data reported shoot density ranging from 674.7 ± 26.6 to 609.3 ± 91.8 shoots/m2 [70]. Considering a profile of the crater, starting from the central site inside the crater, to the margin of the depression (B3) any Posidonia coverage was recorded. Inside the crater pH values range from 6.0 to 7.8 with a temperature of about 22 °C. B2 is 7 m from the edge of the crater and represents the first site in which Posidonia were recorded along the crater profile; temperature is 22.6 °C, while pH is about 7.9. Finally, B1 is about 30 m from the edge of the crater and is characterized by temperature of 22.7 °C and pH of 8.15. It was considered representative of normal marine conditions.
Black Point and P21 stations are in the area nearby Dattilo, Bottaro and Lisca Nera islets in front of Panarea Island at 23 and 21 m depth, respectively. The zone is characterized by a rocky bottom with strong localized gas emissions adjacent to a tectonic fault [63,72]. The Posidonia cover is very patchy. These two sites represent the stations with the highest venting activity of all study sites with temperatures ranging from 55.7 °C (P21) to 133 °C (Black Point); pH value is 5.21 for P21 and ranges from 2.78 to 4.9 in the Black Point site. At these stations no sediment samples were collected due to the rocky feature of the bottom.
Finally, sediment samples coming from both the hot and cold sites and from an area far from the sites, have been mineralogically characterized by diffractometric analysis.
2.2. Benthic Microfauna
Two typologies of samples were considered: sediment and Posidonia (rhizomes and leaves). For each type of samples different treatments were adopted following, as far as possible, the standard procedures [21,24,73].
Sediment samples—Sediment was collected only in Ditella and Raya stations with three replicates for each site. Sediment samples were sampled by mean of the syringe technique (diameter 3 cm, 50 mL related to 10 cm top layer equivalent to 100 cc of sediment). The whole syringe content was analyzed because of the coarse grain size of the sediment that made difficult to slice 0.5 or 1 cm subsamples thick. To distinguish the living fauna, all sediment samples were stained and preserved in a solution of 90% ethanol with 2 g/L of Rose Bengal [73,74,75]. After 15 days, the samples were wet sieved through a 63 μm sieve and then dried at 40 °C. For each sample, stained foraminifers were counted, hand-picked, and identified using a binocular microscope. The Rose Bengal staining method has been widely used in ecological studies to distinguish live from dead foraminifers because it is inexpensive and easy to use [22,76,77]. However, under specific conditions (i.e., anoxic environments) the accuracy of this method may be affected by the presence of undecayed protoplasm, which can persist for weeks or months after death [76,77,78,79,80]. While the staining criteria are confidently applied to the superficial samples, ambiguities may arise in the case of deeper intervals [81], commonly consisting of a slight overestimation of the living assemblages [82]. However, the Rose Bengal method when used with care, permits the obtainment of results as reliable as those obtained by other techniques [75,80,83]. The quantitative analysis of benthic foraminifers was based on the count of all specimens present in the whole sample. In addition, the frequency of groups distinguished by their basic wall structure (agglutinated, porcelaneous and hyaline) was calculated in order to display their distribution in different pH conditions.
The scarce and irregular quantitative data didn’t allow us to carry out statistical analyses. Density in the sediment samples was calculated as number of individuals in 100 cc of sediment, while diversity was calculated as total number of taxa in the sediments (S) and also α-Fisher [84] and Shannon (H) indices [85]. All these parameters were calculated by mean of the PAST software (ver.4.03) [86].
Posidonia samples—Posidonia rhizomes were cut off the substrate approximately one centimeter above the sediment surface. Leaves and rhizomes were immediately stowed away under water in plastic bags, and later carefully washed with seawater over 63 µm sieve. Epiphytes were washed into larger bowls, washed with fresh water and dried. Plant fragments were examined under the microscope to remove living specimens that may have remained glued to the plant surface. All epiphytic foraminifers were picked from each sample and identified at species level. Posidonia samples comprise material from approximately 27 rhizomes and 172 leaves (Table 2). Similar length leaves were considered, and the ratio between number of specimens recorded and number of leaves analyzed (F/P) was calculated to have an indicative estimation of epifaunal density.

Table 2.
Summary of samples collected at different sites.
A scheme for all the samples is reported in Table 2.
The classification at the genus level was made according to the most used taxonomical study on foraminiferal genera [87], while species were determined according to some important studies on the Mediterranean area [25,88,89], and to the World Modern Foraminifera Database. Neoconorbina spp. includes mainly N. posidonicola and little specimens (possible juvenile stage) very difficult to distinguish at specific level. Frequent agglutinated subcircular encrusted tests attached to phytal surface (rhizomes or leaves) were grouped under Daitrona sp. [88].
The foraminifers were hand-picked and photographed using FEIQUANTA400 scanning electron microscope at the SEM Laboratory of Earth Sciences Department, Sapienza University of Rome.
3. Results
3.1. General Characters of Sediment and Posidonia Samples
All sediment samples consist mainly of coarse sandy volcanic grains and are characterized by the dominance of plagioclase (andesine) and amphibole (pargasite), which are the most common phenocryst phases in the andesitic and dacitic lava widely outcropping in Panarea and in the islets [40] (Figure 2 and Figure 3).
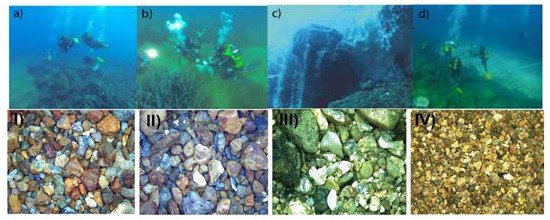
Figure 2.
(a,b) Hot and Cold site at Ditella station; (c) P21site (modified from [90]); (d) Bottaro crater (modified from [90]); (I) Cold site sediment, bar = 1 cm; (II) Hot site sediment, bar = 1 cm; (III) Black Point site sediment, bar = 1 cm; (IV) Raya Control site sediment, bar = 0.1 mm).
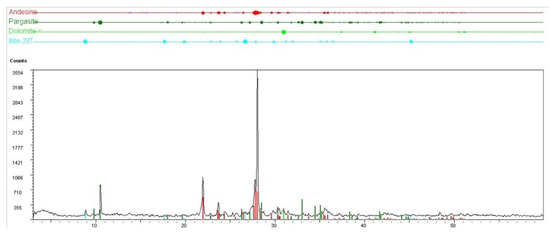
Figure 3.
XRD spectrum of a sample representative of the mineral assemblage constituting the sediments in hot site.
Additional phases recognized in the sediments are dolomite, which is present in minor amounts in almost all samples and an argillitic component (illite) that occurs mainly in the hot sample HFT (Figure 3).
The biogenic fraction of the sediment floor is characterized by scarce or absent vegetal debris, mollusks (bivalves and gastropods), bryozoans, serpulids, diatoms, echinoids and ostracods in addition to foraminifers. Brachiopods, Joania cordata and Argyrotheca cuneata, two common species in the Mediterranean Sea [91] are recorded mainly in the rhizomes at the cold and Bottaro stations; in the control samples, rare individuals are found both in the sediment and attached on the Posidonia rhizomes.
Posidonia leaves and rhizomes are characterized by the presence of encrusting organisms such as serpulids, coralline algae, bryozoans, sponges and diatoms. They are very scarce or totally absent at the P21 station leaves, except for diatoms that are always abundant and highly diversified associated with siliceous sponge spicules.
3.2. Foraminiferal Content: General Features
Sediment samples—Dead assemblage is totally or almost absent in most of the samples. A total of 40 foraminiferal specimens were found in the sediment samples with a reduction in the number of species and individuals along a pH gradient from 8 to 6 (Table 3a). The most acidic conditions (pH < 6) were characterized by rare and exclusively agglutinated taxa.

Table 3.
(a) Quantitative data recorded in sediment samples for each site. Foraminiferal density is expressed as total number of individuals in 100 cc of sediment. Foraminiferal diversity is expressed as number of taxa (S), Shannon (H) and α-Fisher indices. Quantitative data expressed as number of individual related to each typology of test, are reported too: Agglutinated (Aggl.), Porcelaneous (Porcel.) and Hyaline. (b) Quantitative data on Posidonia rhizomes and leaves for each site. Foraminiferal density is expressed as number of number of individuals on 3 rhizomes and number of individual/number of leaves considered (F/P ratio). Foraminiferal diversity is expressed as number of taxa (S), Shannon (H) and α-Fisher indices. (c) Total number of individuals related to Agglutinated (Aggl.), Porcelaneous (Porcel.), and Hyaline tests recorded on Posidonia rhizomes and leaves are reported for each studied site.
Posidonia samples—A total of 1125 individuals were counted on the total Posidonia leaves and rhizomes. The number of individuals tends to increase along the pH gradient (Table 3b,c) except for the hot site, where an increase of density is recorded both in the leaves and in the rhizomal microhabitats. Generally, the highest density is concentrated on leaves particularly on the adult portion of them. Only in the most acidic areas (P21, BP and B2 sites) rhizomal density (mainly agglutinated taxa, Table 3b) is similar or higher than leaves one. The biodiversity is low in all sites. The greatest number of taxa (S) is recorded at the control site B1 (Table 3c). Diversity indices display α-Fisher index < 6 except for the Cold site where it reaches 6.45 value, and Shannon index (H) always <2. Agglutinated tests are clearly dominant in more acidic sites, while porcelaneous and hyaline shells reach their highest frequencies in normal marine conditions (control sites: Raya and B1). However, it is noteworthy that the hyaline taxa abundance and diversity increase on both Posidonia leaves and rhizomes at the Hot spot sites (Table 3a).
The most abundant species is Rosalina bradyi (25.4% of epiphytic total) followed by Neoconorbina spp. (18.9%) and Planorbulina mediterranensis (9.8%). All these hyaline species accounted the highest frequencies at Hot spots stations while decrease until to be totally absent at P21 and Black Point sites. The list of total species is reported in the Table S1. In all sites with pH values < 8, the SEM analyses highlight strong signs of decalcifications and alterations (variations in the pore distribution, cracks and fractures) resulting an increase of shell fragility for the most part of the tests (Figure 4 and Figure 5).
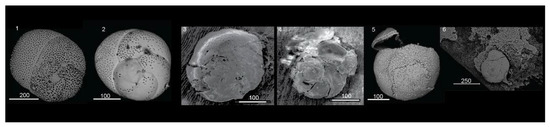
Figure 4.
Rosalina bradyi specimens collected at Ditella and Raya station:(1) Raya Control site pH > 8, normal test of Rosalina specimen; (2) Cold site at 7 < pH < 8, the test shows some morphological variations mainly in the pore distribution on the surface of the chambers; (3–6) Hot site at acidic conditions 6 < pH < 7.9 with high temperature, clear signs of decalcification are visible (3,4). The tests are extremely fragile with cracks and fractures affecting the wall of the chambers (5,6).
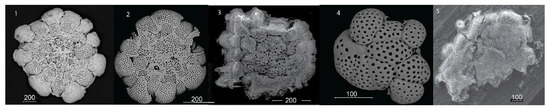
Figure 5.
Planorbulina mediterranensis specimens collected at Ditella station: (1,2) Cold site at 7 < pH < 8, similarly to Rosalina specimens, the test shows morphological variations mainly in the pore size and distribution on the surface; (3–5) Hot site at acidic conditions 6 < pH < 7.9 with high temperature, signs of decalcification (3,5), increase of pore size and morphological abnormalities are visible (4).
3.3. Foraminiferal Fauna Recorded at Each Study Site
3.3.1. Ditella Station
Hot Samples
Sediment samples—The foraminiferal assemblage is constituted exclusively of rare specimens of Lepidodeuterammina ochracea. Only diatoms and partly dissolved carbonate tests (serpulids, ostracods and foraminifers) are found in bottom samples.
Posidonia leaves—A total of 292 living foraminifers were found on 11 leaves (2 leaves were totally barren in foraminifers) with a F/P ratio of 24.43. The most part of specimens was attached in the mature part of the leaves, while only 9 specimens (Lobatula lobatula, Asterigerinata mamilla, P. mediterranensis, R. bradyi) were found also on the juvenile portion of them. The foraminiferal community is represented mainly by Neoconorbina spp. (40%) and R. bradyi (33%) which are clearly the most abundant taxa, P. mediterranensis follows with values of 15%, while the remaining species are <4% (Figure 6).
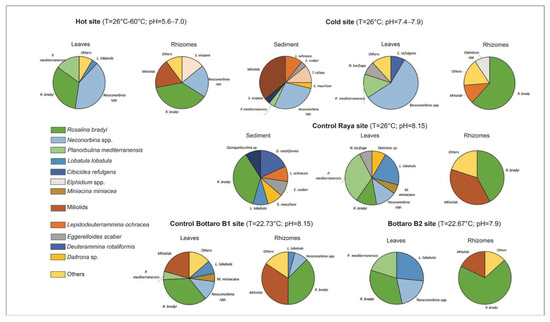
Figure 6.
Pie diagrams showing sediments, leaves and rhizomes foraminiferal composition recorded at each study sites. In the legend the most abundant taxa are reported.
With reference to the shell composition, the main part of foraminifers belongs to hyaline group (99%), while agglutinated and porcelaneous (miliolids) groups are about 1% (Figure 7).
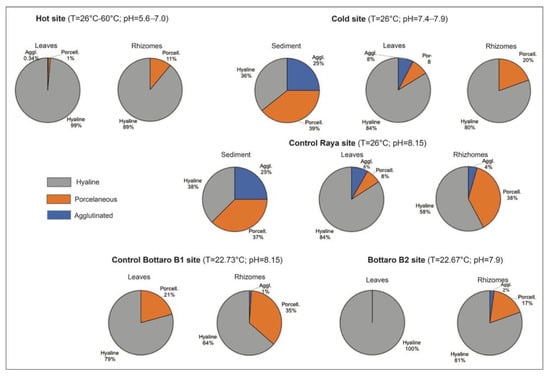
Figure 7.
Pie diagrams showing test composition of foraminifers recorded in sediments, leaves and rhizomes.
Posidonia rhizomes—A total of 50 specimens were recorded on the rhizomes. Rosalina brady (38%) and Neoconorbina spp. (20%) dominate the assemblage followed by miliolids with 18%. The remaining taxa display values < 10% except for Spirillina vivipara which is recorded with frequencies of 14%. The agglutinated taxa are totally absent.
Cold Samples
Sediment samples—A total of 28 specimens are recorded.
Miliolids (36%), Neoconorbina spp. (29%) and agglutinated taxa such as L. ochracea and Trochammina inflata (11%) are the dominant species (Figure 6).
Posidonia leaves—A total of 96 foraminifers were found on a total of 9 leaves with a F/P ratio of 10.67. In addition, in this case the most part of the foraminiferal occurrences was recorded attached in the mature part of the leaves. Only 7 specimens (mainly Neoconorbina spp., C. refulgens and P. mediterranensis this last very damaged) were found on the juvenile portion of them. The recorded taxa are represented mainly by Neoconorbina posidonicola (57%) and P. mediterranensis (15%). Nubecularia lucifuga (8%), C. refulgens (8%), Miniacina miniacea (3%), R. bradyi (3%), Cymbaloporetta sp. (3%), S. vivipara (1%), and L. lobatula (1%) follow with frequencies < 10%. The most part of foraminifers belongs to hyaline group however agglutinated taxa, represented exclusively by N. lucifuga, show significant values. The miliolid (porcelaneous) group is present with frequencies of 8% (Figure 7).
Posidonia rhizomes—42 specimens were recorded on the rhizomes. The assemblage is dominated by R. bradyi (60%). Elphidium spp. follow with frequencies < 10%. In the cold stations, the rhizomes are lacking agglutinated taxa and the miliolids (20%) are constituted of rare specimens of Miliolinella subrotunda, Pseudotriloculina schreiberiana, Siphonaperta aspera, Quinqueloculina sp. and Q. disparilis (Figure 6 and Figure 7).
3.3.2. Raya Station (Control Sample)
Sediment samples—A total of 11 specimens were recorded in the sediments represented mainly by R. bradyi (45%); E. crispum, Quinqueloculina sp. and L. lobatula follow with lower frequencies (18%). Hyaline (38%) and porcelaneous 37(%) show similar frequencies while agglutinated taxa follow with percentages of 25% (Figure 7).
Posidonia leaves—a total of 144 specimens were found on 10 leaves; the F/P ratio is 14.4. The most part of the assemblages is represented by P. mediterranensis (33%), L. lobatula (21%), Neoconorbina spp. and R. bradyi (12%), Daitrona sp. and N. lucifuga (8%), and Miniacina miniacea (6%). Hyaline group dominates (84%).
Posidonia rhizomes—only 45 specimens were found mainly represented by miliolids (38%) and R. bradyi (42%); similarly to leaves microhabitat, other species follow with frequencies < 5% (Figure 6 and Figure 7). Hyaline and porcelaneous group show frequencies of 58% and 38%, respectively, followed by agglutinated (4%).
3.3.3. Bottaro Crater
Posidonia leaves—In B1 control site, 159 specimens were collected directly on the adult portion of leaves (15). The F/P ratio is 10.6. They are represented mainly by R. bradyi (37%), miliolids (19%), Neoconorbina spp. (17%), L. lobatula (9%) and P. mediterranensis (5%). Agglutinated taxa are absent. In B2 site, 15 specimens were found on the adult portion of 20 Posidonia leaves with a F/P ratio of 0.75. The assemblage consists exclusively of R. bradyi (33%), L. lobatula (27%), P. mediterranensis and Neoconorbina spp. (20%). The assemblage is dominated by hyaline taxa.
Posidonia rhizomes—B1 site shows 96 specimens, among them the most abundant are R. bradyi (38%) followed by miliolids (21%) that slightly increase, while Neoconorbina spp. (8%) decrease with respect to the leaf’s assemblages. In B2 rhizomes foraminiferal content displays 45 specimens among them the most abundant species is R. bradyi (69%). Porcelaneous are present with percentages of 17% while other species are very scarce with values <3% (Figure 6 and Figure 7). In both sites agglutinated taxa are present with low percentages <3%.
3.3.4. P21 and Black Point Site
Posidonia leaves—At the P21 site a total of 39 leaves was analyzed. The F/P ratio records its lowest value (0.18). The most part of them is barren in foraminiferal content; only 5 specimens of Daitrona sp., an agglutinated taxon, and one specimen of R. bradyi were recorded. At the Black Point site Posidonia leaves developed no closer than 10 m from emission points and exhibit very scarce foraminiferal content. The F/P is 2.5 and on 12 leaves analyzed, 30 specimens were found. They are represented mainly by agglutinated taxa such as A. globigeriniformis (33%), Eggerelloides scaber (20%), A. planorbis (7%), Trochammina inflata (7%) and Daitrona sp. (13%).
Posidonia rhizomes—At the P21 site a total of 29 specimens was found dominated by agglutinated species (A. globigeriniformis 48%, L. ochracea 12%, A. planorbis 20%, E. scaber 8%). Very scarce miliolids were recorded represented by Siphonaperta aspera, N. lucifuga and M. subrotunda. Black Point site displayed 31 specimens. They are dominated by A. globigeriniformis (38%) followed by Daitrona sp. (10%), A. planorbis (11%) and R. bradyi (10%).
3.4. Summary of Different pH Conditions
In Panarea sites, our observations allowed us to detect three main different pH conditions with respect to normal marine conditions with pH = 8.1 (control Raya and B1 sites): low acidic conditions (pH = 7.9), acidic conditions (Hot sites; pH=7.0–5.6) and strong acidic conditions (Black Point and P21 sites; pH = 3.8–5.2)
The normal marine conditions display assemblages typical of shallow water oligotrophic environment. On rhizomes, the assemblages are mainly characterized by relatively high frequencies of miliolids and R. brady, whereas on leaves L. lobatula and P. mediterranensis dominate. No deformities or abnormal test were observed. In the low acidic conditions, rosalinids increase significantly with R. bradyi mainly concentrated on the rhizomes, whereas Neoconorbina spp. are more frequent on sediments and leaves. In acidic conditions Rosalinids continue to dominate the epifaunal assemblages, while under strong acidic conditions, moving from the spot emission at not less than 10 m (5.2 < pH < 7), the first Posidonia shoots host only a typical agglutinated assemblage both on leaves and rhizomes. In addition, the rare miliolids recorded in the rhizomes, are covered by an agglutinated protective layer. Frequent deformities or abnormal test were evidenced starting from pH values < 7.
4. Discussion
4.1. Response of Foraminiferal Assemblages to CO2 Fluid Emission
The abiotic parameter pH is well known to influence the distribution, biodiversity and abundance of benthic foraminifers and, in case its value is far from the optimum, it acts as environmental stressor [22,26,31]. The microfaunal analysis conducted in sites under different pH and T conditions around the Panarea Island, allow us to make some considerations. The benthic foraminiferal response to increasing water acidification consists of reduction of biomineralization potentiality, loss of biodiversity and morphological modifications. In addition, environmental contexts may affect differently these responses to future OA [28,92,93]. It can be very variable on the basis of complex environmental interactions. Experimental studies showed as the exposure times and the temporal variations of the acidic conditions are among the most significant factors [28]. In the Panarea sites, the available geophysical data show a continuous hydrothermal acidic activity affecting the benthic ecosystem [55,69] with a pH decrease ranging from 0.2 to 4.3 units from natural marine conditions (pH = 8).
Differently from controlled laboratory conditions, the great variability of physical and chemical parameters of the Panarea sites makes very difficult to define a pattern of biota responses in situ. This is probably the reason of the observed different response of benthic fauna (e.g., Cold and B2 sites) under similar pH values. From this perspective, the obtained data should be considered as the results of lasting foraminiferal adaptation in response to the overall environmental conditions rather than the immediate response to the values measured at the sampling time.
Our observations show that the response of the Panarea foraminiferal assemblages displays different patterns in sediment and Posidonia (rhizomes and leaves) samples. Regarding the sediment assemblages, the scarcity of the microfaunal content (dead and living) and the lack of data from all analyzed sites, does not allow a reliable assessment of the foraminiferal response to the different acidic conditions. Based on our data and likewise to Pontine vent activity (central Tyrrhenian Sea [27]), strong and persistent CO2 emissions probably cause the complete dissolution of post-mortem calcareous tests at the sea bottom and the inhibition of the living assemblage. On the other hand, the calcareous component in the sediment is very low except for the presence of rare dolomite (low-temperature dolomite) that may be precipitate from the hydrothermal fluids which permeate the host rock (Figure 3) [94,95].
On the contrary, Posidonia epiphytic assemblages show a more complete picture. The distinction between leaves and/or rhizomes microhabitats allow us to carry out considerations not only on the microhabitat preference of foraminifers at the species level but also to highlight their functional aspect. The highest density is concentrated on leaves particularly on the adult portion of them, confirming the typical pattern of epiphytic colonization under normal marine condition [21]. Leaves represent “high” substrates (e.g., [96,97,98]) on which suspension feeders enjoy of a better exploitation of nutrients in the water mass around the plant, a greater degree of oxygenation and probably a better advantage of the Posidonia buffer effect [21,99,100]. Literature data demonstrated that effect of the phytal metabolism related to Posidonia activity may favor the development of foraminifers under acidic conditions [101,102,103,104,105]. Both controlled experiments conducted in laboratories and in situ studies, showed how little and fluctuating pH decrease (<0.4 unit) can be modulated by Posidonia oceanica conferring to the environment a major pH stability [28].
Under significant pH decrease (P21, BP and B2 sites), rhizomal foraminiferal density (mainly agglutinated taxa) become higher than foraminiferal density on leaves. These observations compel us to consider rhizomes as “refugia” with respect to more acidic surrounding environments. Rhizomes can trap sediment and with-it nutrients, and they can host fungi and bacteria providing food sources for many organisms including foraminifers [88,105,106,107].
No taxon at the species level is exclusive of the two Posidonia microhabitats (leaves and rhizomes). Rosalinids (R. bradyi and Neoconorbina spp.) are abundant in both microhabitats although Neoconorbina spp. show a preference for leaves. They are temporarily attached foraminifers with a great ability for detachment in response to changing environmental conditions moving from epifaunal (substrate and seagrass) to sediment microhabitats (or conversely) to find more advantageous life conditions [24,88,108,109,110,111,112]. Probably the motility and relatively short life span of rosalinids make these taxa more easily adapted to stress conditions, showing a typical opportunistic behavior (B morphotype after [21,24]).
On leaves rosalinids are frequently associated with cibicidids (L. lobatula, C. refulgens) which have similar ecological features. Although they are characterized by an attachment surface, they are predominantly motile species. These taxa are always associated with P. mediterranensis that, on the contrary, is considered a permanently attached species with long life spans and needs of optimal environmental conditions (A morphotype after [21,24,113,114,115]). This is testified by the high frequencies of this species in the control site (Raya site) and its clear decrease under acidic condition (except for warm hot site) indicating its preference of normal marine conditions and low tolerance towards stressed environment. The presence of P. mediterranensis in Panarea acidic sites may be explained by the mitigating effect of Posidonia meadow. The photosynthetic activity of the plant ensures the survival conditions for P. mediterranensis although evident test abnormalities and dissolution signs testify non-optimal conditions.
In the rhizomes, rosalinids are associated with miliolids and small agglutinated species that are permanently motile and have a very short life cycle (<3 months, D morphotype after [21,24], well adapted to stressed environment.
Moreover, it is noted that many agglutinated species (mainly A. globigeriniformis, L. ochracea, A. planorbis) are characterized by a very small (about few microns), agglutinated grains notwithstanding the coarse grain size of the sediments. This highlights their selective capability to exploit the scarce fine fraction deriving from the alteration of the volcanoclastic material. Under acidic/neutral conditions these alteration processes may result in the formation of clay minerals such as kaolinite and illite/smectite, respectively (Figure 3) [94,95]. Detailed considerations regarding different pH conditions follow.
4.1.1. Normal Marine Conditions (pH > 8)
The normal marine conditions sites (B1 and Raya) are characterized by epiphytic and epifaunal assemblages typical of shallow water oligotrophic environments [22,116]. In the sediment, although the sites are not affected by venting activity, the recorded faunal density is low, suggesting a possible indirect influence of CO2 emissions (testified by frequent agglutinated taxa, Figure 6), or other adverse factors (e.g., scarce food availability on and into the sediments, the nature of the bottom or water current), that may negatively influence the foraminiferal development in these areas. This is evidenced also by the low diversity indices of the epifaunal assemblages (leaves and rhizomes) that, although more diversified than sediment assemblage, is more similar to marginal environments rather than typical shelf ecosystems [22].
4.1.2. Low Acidic Conditions (Cold Sites, Bottaro B2; pH = 7.9)
Our observations carried out from low acidic conditions confirm literature data with slight changes in the foraminiferal assemblages [26]. The two sites associated with low acidic conditions, Cold and Bottaro B2, show mainly differences in terms of quantitative data. However, at both sites, the transition from normal marine (pH = 8.1) to low acidic conditions (decreasing of 0.2 unit) is marked by a general decrease in Posidonia foraminiferal content and diversity (mainly highlighted on leaves), although the carbonate tests are also well represented. A first sign of stressed conditions can be inferred by the significant reduction of the species with limited adaptative capability such as P. mediterranensis on leaves. Similarly, on the rhizomes, the porcelaneous group decreases significantly. Literature data suggest that their high Mg content make them more susceptible to dissolution in acidic conditions and to undergoing tests deformities in response to seawater pollution [26,117,118,119]. On the contrary, the sediment community (Cold site) displays an increase in terms of both density and diversity (H > 2 and Fisher indices > 9) proportionally for each test typology group with respect to the scarce foraminiferal content at the control site. As above mentioned, based on the available data, it is difficult to give an exhaustive explanation of that. However, a possible cause may be found in the more protected conditions of the sites that favor trapping of organic matter, a food source for foraminifers.
4.1.3. Acidic Conditions (Hot Sites; pH = 7.0–5.6)
The transition to acidic conditions is marked by a clear drop of foraminiferal content in the sediments with rare agglutinated taxa (L. ochracea). This finding confirms literature data indicating critical threshold pH values around 7.8 and 7.6 that prevent the building of foraminiferal carbonate test [26,31].
Regarding epiphytic foraminiferal content, contrary to what is expected, it accounts for the highest density values of all studied sites (high values of F/P ratio) and highly diversified assemblages (similar to control site and higher than low acidic conditions) probably explained by the buffer effect of Posidonia. This is testified by the increase of P. mediterranensis on leaves, and the re-occurrence of miliolids in the rhizomes although the most part of the tests recorded show clear signs of carbonate dissolution in terms of fragility, reductions of number of pores and increasing size of the single pores (L. lobatula, Rosalina bradyi, and P. mediterranensis, Figure 4 and Figure 5).
In addition, the presence of warm water due to the proximity of Hot sites in this zone seems to enhance the foraminiferal development amplifying the beneficial effect of vegetable activity. Temperature is an important factor controlling not only geographical distributions but also metabolism, growth rates and reproductive processes of foraminifers [120], however little is known about the relationship between a single species and this parameter. Literature data and laboratory experiments demonstrated that 45 °C is the lethal value threshold for most part of macroforaminifers [22,121]. In the analyzed samples, among cibicidids, L. lobatula shows a higher tolerance to increasing temperatures than C. refulgens that is more frequent at cold sites.
Rosalinids continue to dominate the assemblage showing their opportunistic behaviour. Rosalina species are considered to have a high tolerance to elevated temperatures resulting in greater adaptability to the future warming [122]. Its strong adaptative behavior is also demonstrated under a wide range of stressed conditions such as high euthrophic environments [123], pH fluctuations [28] or other extreme environments [124]. Little is known about the ecological characteristics of Neoconorbina species. Based on our observations their behavior is very similar to that of Rosalina spp.
4.1.4. Strong Acidic Conditions (Black Point and P21 Sites pH = 3.8–5.2)
According to literature data these conditions (low pH and very high temperatures up to 133 °C) do not allow any carbonate biomineralization process and prevent the development of carbonate foraminiferal tests [26,31,123] even in the presence of Posidonia seagrass.
At the species level, the association of A. globigeriniformis and E. scaber seems to be more resistant in strong acidic conditions confirming their opportunistic behavior in extreme environments such as submarine caves [123], other venting areas [27] or oil spill regions [124]. In addition, specimens attributable to Daitrona cf. Daitrona sp., a permanently attached taxon [21], seem to show tolerance to acidic conditions, because they agglutinate sediment particles to a proteinaceous or non-calcite matrix [125], a potential advantage under acidic conditions (Figure 8).
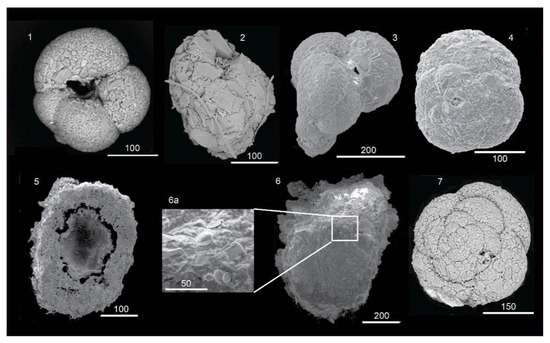
Figure 8.
Agglutinated foraminifers collected at pH conditions <6 (Black Point and P21 sites), the bar is expressed in microns: (1,4) Ammoglobigerina globigeriniformis ventral view (1) and spiral view (4); (2,3) Eggerelloides scaber apertural view; (5,6) Specimen cf. Daitrona sp. ventral (5) and dorsal (6) view; 6a detail of the test with agglutinated diatom frustules; (7) Deuterammina rotaliformis spiral view.
5. Conclusions
The microfaunal analysis conducted at sites affected by CO2 venting activity around the Panarea Island, allow us to make some considerations on benthic foraminiferal response to increasing ocean acidification (OA). Under different pH conditions (from strong to low acidic conditions) the foraminiferal assemblage’s response can be very different depending on several environmental conditions and microhabitats. A decrease of pH from the normal marine values ranging between 2.9 and 1.1 units (strong acidic conditions) determines a strong decline of faunal density and biodiversity with the presence of only rare agglutinated taxa.
Under acidic conditions, although the sediment niches proved prohibitive for foraminiferal life, Posidonia leaves and rhizomes can offer a better condition for the epiphytic foraminiferal fauna that can take advantage from the buffer effect of Posidonia seagrass. Warm water conditions seem to amplify the beneficial effect of the Posidonia. However evident morphological abnormalities with reduction of biomineralization potentiality affect the foraminifers’ tests.
The threshold of pH = 7.9 determines the restoration of the foraminiferal community in the sediments and a more abundant and diversified epiphytic assemblages. Among the foraminiferal assemblages rosalinids and agglutinated taxa represent the most abundant taxa and the more resilient species capable of facing future OA scenarios.
Similar response could be expected by all epibiont calcifying community (mollusks, brachiopods but also weakly calcified such as crustaceans) that could be less disadvantaged from the effect of OA thanks to the photosynthesis processes and the consequent buffering role of seagrass. This study confirms the role of Posidonia oceanica as refuge and in the mitigation contrasting the future climate change.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/geosciences12050184/s1, Table S1: List of species.
Author Contributions
Conceptualization, L.D.B., S.B., M.I., A.C. and A.M.C.; methodology, M.G. and V.E.; formal analysis, M.I., L.D.B. and A.M.C.; resources, M.G., V.E. and S.B.; writing—original draft preparation, L.D.B., S.B., M.I., A.C., C.D.V. and A.M.C.; underwater sampling, M.G.; supervision, all the authors. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
Not applicable here.
Acknowledgments
We would like to thank Maria Cristina Gambi for the sampling of Posidonia materials and useful suggestions, the ECCSELNatLab Italy laboratory organizations, Francesco Italiano (INGV) and his collaborators for the pH and temperature data. M. Albano, Tania Ruspandini and S. Stellino are thanked for their contribution in SEM-EDS and XRD analyses. Special thanks are for Andrea Fogliuzzi and his scientific divers collaborators for their precious support in the sampling survey.
Conflicts of Interest
The authors declare no conflict of interest.
References
- IPCC. Climate Change 2007: The Physical Science Basis; Solomon, S., Qin, D., Manning, M., Chen, Z., Marquis, M., Averyt, K.B., Tignor, M., Miller, H., Eds.; Contribution of Working Group I to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change; Cambridge University Press: Cambridge, UK; New York, NY, USA, 2007. [Google Scholar]
- IPCC. Climate Change 2014: Synthesis Report; Core Writing Team, Pachauri, R.K., Meyer, L.A., Eds.; Contribution of Working Groups I, II and III to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change; IPCC: Geneva, Switzerland, 2014; 151p. [Google Scholar]
- IPCC. Climate Change 2018: Global Warming of 1.5 °C; Masson-Delmotte, V., Zhai, P., Pörtner, H.-O., Roberts, D., Skea, J., Shukla, P.R., Pirani, A., Moufouma-Okia, W., Péan, C., Pidcock, R., et al., Eds.; An IPCC Special Report on the Impacts of Global Warming of 1.5 °C above Pre-Industrial Levels and Related Global Greenhouse Gas Emission Pathways, in the Context of Strengthening the Global Response to the Threat of Climate Change, Sustainable Development, and Efforts to Eradicate Poverty; IPCC: Geneva, Switzerland, 2018. [Google Scholar]
- Kroeker, K.J.; Kordas, R.L.; Crim, R.N.; Singh, G.G. Meta-analysis reveals negative yet variable effects of ocean acidification on marine organisms. Ecol. Lett. 2010, 13, 1419–1434. [Google Scholar] [CrossRef] [PubMed]
- Rodolfo-Metalpa, R.; Houlbrèque, F.; Tambutté, E.; Boisson, F.; Baggini, C.; Patti, F.P.; Ross, J.; Fine, M.; Foggo, A.; Gattuso, J.P.; et al. Coral and mollusc resistance to ocean acidification adversely affected by warming. Nat. Clim. Chang. 2011, 1, 308–312. [Google Scholar] [CrossRef]
- Dove, S.G.; Kline, D.I.; Pantos, O.; Angly, F.E.; Tyson, G.W.; Hoegh-Guldberg, O. Future reef decalcification under a business-as-usual CO2 emission scenario. Proc. Nat. Acad. Sci. USA 2013, 110, 15342–15347. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fang, J.K.; Mello-Athayde, M.A.; Schönberg, C.H.; Kline, D.I.; Hoegh-Guldberg, O.; Dove, S. Sponge biomass and bioerosion rates increase under ocean warming and acidification. Glob. Chang. Biol. 2013, 19, 3581–3591. [Google Scholar] [CrossRef]
- Kroeker, K.J.; Kordas, R.L.; Crim, R.; Hendriks, I.E.; Ramajo, L.; Singh, G.S.; Duarte, C.; Gattuso, J.P. Impacts of ocean acidification on marine organisms: Quantifying sensitivities and interaction with warming. Glob. Chang. Biol. 2013, 19, 1884–1896. [Google Scholar] [CrossRef] [Green Version]
- Pörtner, H.-O.; Karl, D.M.; Boyd, P.W.; Cheung, W.W.L.; Lluch-Cota, S.E.; Nojiri, Y.; Schmidt, D.N.; Zavialov, P.O. Ocean systems. In Climate Change 2014: Impacts, Adaptation, and Vulnerability. Part A: Global and Sectoral Aspects. Contribution of Working Group II to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change; Field, C.B., Barros, V.R., Dokken, D.J., Mach, K.J., Mastrandrea, M.D., Bilir, T.E., Chatterjee, M., Ebi, K.L., Estrada, Y.O., Genova, R.C., et al., Eds.; Cambridge University Press: Cambridge, UK; New York, NY, USA, 2014; pp. 411–484. [Google Scholar]
- Gattuso, J.P.; Magnan, A.; Billé, R.; Cheung, W.W.; Howes, E.L.; Joos, F.; Allemand, D.; Bopp, L.; Cooley, S.R.; Eakin, C.M.; et al. Contrasting futures for ocean and society from different anthropogenic CO2 emissions scenarios. Science 2015, 349, aac4722. [Google Scholar] [CrossRef]
- Yanko, V.; Kronfeld, J.; Flexer, A. Response of benthic foraminifera to various pollution sources: Implications for pollution monitoring. J. Foraminifer. Res. 1994, 24, 1–17. [Google Scholar] [CrossRef]
- Alve, E. Benthic foraminiferal responses to estuarine pollution; a review. J. Foraminifer. Res. 1995, 25, 190–203. [Google Scholar] [CrossRef]
- Alve, E.; Murray, J.W. Marginal marine environments of the Skagerrak and Kattegat: A baseline study of living (stained) benthic foraminiferal ecology. Palaeogeogr. Palaeoclimatol. Palaeoecol. 1999, 146, 171–193. [Google Scholar] [CrossRef]
- Du Châtelet, É.A.; Debenay, J.P. The anthropogenic impact on the western French coasts as revealed by foraminifera: A review. Rev. Micropaléont. 2010, 53, 129–137. [Google Scholar] [CrossRef]
- Frontalini, F.; Coccioni, R. Benthic foraminifera as bioindicators of pollution: A review of Italian research over the last three decades. Rev. Micropaléont. 2011, 54, 115–127. [Google Scholar] [CrossRef]
- Caldeira, K.; Wickett, M.E. Anthropogenic carbon and ocean pH. Nature 2003, 425, 365. [Google Scholar] [CrossRef] [PubMed]
- Sabine, C.L.; Feely, R.A.; Gruber, N.; Key, R.M.; Lee, K.; Bullister, J.L.; Wanninkhof, R.; Wong, C.S.; Wallace, D.W.R.; Tilbrook, B.; et al. The oceanic sink for anthropogenic CO2. Science 2004, 305, 367–371. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Feely, R.A.; Sabine, C.L.; Lee, K.; Berelson, W.; Kleypas, J.; Fabry, V.J.; Millero, F.J. Impact of Anthropogenic CO2 on the CaCO3 System in the Oceans. Science 2004, 305, 362. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Caldeira, K.; Wickett, M.E. Ocean model predictions of chemistry changes from carbon dioxide emissions to the atmosphere and ocean. J. Geophis. Res. Oceans 2005, 110, C09S04. [Google Scholar] [CrossRef] [Green Version]
- Royal Society. Ocean Acidification Due to Increasing Atmospheric Carbon Dioxide. Policy Doc. 12/05, London. 2005. Available online: http://www.royalsoc.ac.uk (accessed on 22 November 2021).
- Langer, M.R. Epiphytic foraminifera. Mar. Micropaleontol. 1993, 20, 235–265. [Google Scholar] [CrossRef]
- Murray, J.W. Ecology and Applications of Benthic Foraminifera; Cambridge University Press: Cambridge, UK, 2006; p. 426. [Google Scholar]
- Mateu-Vicens, G.; Box, A.; Deudero, S.; Rodriguez, B. Comparative analysis of epiphytic foraminifera in sediments colonized by seagrass Posidonia oceanica and invasive macroalgae Caulerpa spp. J. Foraminifer. Res. 2010, 40, 134–147. [Google Scholar] [CrossRef]
- Mateu-Vicens, G.; Khokhlova, A.; Sebastián-Pastor, T. Epiphytic foraminiferal indices as bioindicators in Mediterranean seagrass meadows. J. Foraminifer. Res. 2014, 44, 325–339. [Google Scholar] [CrossRef]
- Panieri, G.; Gamberi, F.; Marani, M.; Barbieri, R. Benthic foraminifera from a recent, shallow-water hydrothermal environment in the Aeolian Arc (Tyrrhenian Sea). Mar. Geol. 2005, 218, 207–229. [Google Scholar] [CrossRef]
- Dias, B.B.; Hart, M.B.; Smart, C.W.; Hall-Spencer, J.M. Modern seawater acidification: The response of foraminifera to high-CO2 conditions in the Mediterranean Sea. J. Geol. Soc. 2010, 167, 843–846. [Google Scholar] [CrossRef] [Green Version]
- Di Bella, L.; Ingrassia, M.; Frezza, V.; Chiocci, F.L.; Martorelli, E. The response of benthic meiofauna to hydrothermal emissions in the Pontine Archipelago, Tyrrhenian Sea (central Mediterranean Basin). J. Mar. Syst. 2016, 164, 53–66. [Google Scholar] [CrossRef]
- Ramajo, L.; Lagos, N.A.; Duarte, C.M. Seagrass Posidonia oceanica diel pH fluctuations reduce the mortality of epiphytic forams under experimental ocean acidification. Mar. Pollut. Bull. 2019, 146, 247–254. [Google Scholar] [CrossRef] [PubMed]
- Mutalipassi, M.; Fink, P.; Maibam, C.; Porzio, L.; Buia, M.C.; Gambi, M.C.; Patti, F.P.; Scipione, M.B.; Lorenti, M.; Zupo, V. Ocean acidification alters the responses of invertebrates to wound-activated infochemicals produced by epiphytes of the seagrass Posidonia oceanica. J. Exp. Mar. Biol. Ecol. 2020, 530, 151435. [Google Scholar] [CrossRef]
- Boudouresque, C.F.; Pergent, G.; Pergent-Martini, C.; Ruitton, S.; Thibaut, T.; Verlaque, M. The necromass of the Posidonia oceanica seagrass meadow: Fate, role, ecosystem services and vulnerability. Hydrobiologia 2016, 781, 25–42. [Google Scholar] [CrossRef] [Green Version]
- Pettit, L.R.; Hart, M.B.; Medina-Sánchez, A.N.; Smart, C.W.; Rodolfo-Metalpa, R.; Hall-Spencer, J.M.; Prol-Ledesma, R.M. Benthic foraminifera show some resilience to ocean acidification in the northern Gulf of California, Mexico. Mar. Pollut. Bull. 2013, 73, 452–462. [Google Scholar] [CrossRef] [Green Version]
- Malinverno, A.; Ryan, W.B.F. Extension in the Tyrrhenian Sea and shortening in the Apennines as result of arc migration driven by sinking of the lithosphere. Tectonics 1986, 5, 227–245. [Google Scholar] [CrossRef]
- Faccenna, C.; Becker, T.W.; Lucente, F.P.; Jolivet, L.; Rossetti, F. History of subduction and back-arc extension in the central Mediterranean. Geophys. J. Int. 2001, 145, 809–820. [Google Scholar] [CrossRef] [Green Version]
- Doglioni, C. A proposal kinematic modelling for W-dipping subductions—Possible applications to the Tyrrhenian-Apennine systems. Terra Nova 1991, 3, 423–434. [Google Scholar] [CrossRef]
- Carminati, E.; Wortel, M.J.R.; Spakman, W.; Sabadini, R. The role of slab detachment processes in the opening of the western-central Mediterranean basins: Some geological and geophysical evidence. Earth Planet. Sci. Lett. 1998, 160, 651–665. [Google Scholar] [CrossRef]
- Goes, S.; Giardini, D.; Jenny, S.; Hollenstein, C.; Kahle, H.G.; Geiger, A. A recent tectonic reorganization in the south-central Mediterranean. Earth Planet. Sci. Lett. 2004, 226, 335–345. [Google Scholar] [CrossRef]
- Bortoluzzi, G.; Ligi, M.; Romagnoli, C.; Cocchi, L.; Casalbore, D.; Sgroi, T.; Cuffaro, M.; Tontini, F.C.; D’Oriano, F.; Ferrante, V.; et al. Interactions between volcanism and tectonics in the western Aeolian sector, southern Tyrrhenian Sea. Geophys. J. Int. 2010, 183, 64–78. [Google Scholar] [CrossRef] [Green Version]
- Barreca, G.; Bruno, V.; Cultrera, F.; Mattia, M.; Monacoa, C.; Scarfì, L. New insights in the geodynamics of the Lipari–Vulcano area (Aeolian Archipelago, southern Italy) from geological, geodetic and seismological data. J. Geodyn. 2014, 82, 150–167. [Google Scholar] [CrossRef]
- Gillot, P.Y. Histoire volcanique des Iles Eoliennes: Arc insulaire ou complexe orog’enique anulaire? Le detroit de Messine (Italie). Evolution tectono-sedimentaire recente (Pliocene et Quaternaire) et environment actuel. Doc. Trav. L’institut Géologique Albert Lapparent 1987, 11, 35–42. [Google Scholar]
- Peccerillo, A. The Aeolian arc. In Plio-Quaternary Volcanism in Italy. Petrology, Geochemistry, Geodynamics; Peccerillo, A., Ed.; Springer: Berlin/Heidelberg, Germany, 2005; pp. 173–213. [Google Scholar]
- De Astis, G.; Lucchi, F.; Dellino, P.; La Volpe, L.; Tranne, C.A.; Frezzotti, M.L.; Peccerillo, A. Geology, volcanic history and petrology of Vulcano (central AeolianArchipelago). In The Aeolian Islands Volcanoes, 37; Lucchi, F., Peccerillo, A., Keller, J., Tranne, C.A., Rossi, P.L., Eds.; Geological Society: London, UK, 2013; pp. 281–348. [Google Scholar]
- Gioncada, A.; Mazzuoli, R.; Bisson, M.; Pareschi, M.T. Petrology of volcanic products younger than 42 ka on the Lipari–Vulcano complex (Aeolian Islands, Italy): An example of volcanism controlled by tectonics. J. Volcanol. Geoth. Res. 2003, 122, 191–220. [Google Scholar] [CrossRef]
- Romagnoli, C.; Casalbore, D.; Bortoluzzi, G.; Bosman, A.; Chiocci, F.L.; D’oriano, F.; Gamberi, F.; Ligi, M.; Marani, M. Bathy-morphological setting of the Aeolian Islands. In: The Aeolian Islands Volcanoes. Geol. Soc. Lond. Mem. 2013, 37, 27–36. [Google Scholar] [CrossRef]
- Favalli, M.; Karatson, D.; Mazzuoli, R.; Pareschi, M.; Ventura, G. Volcanic geomorphology and tectonics of the Aeolian archipelago (Southern Italy) based on integrated DEM data. Bull. Volcanol. 2005, 68, 157–170. [Google Scholar] [CrossRef]
- Gabbianelli, G.; Romagnoli, C.; Rossi, P.; Calanchi, N. Marine geology of the Panarea-Stromboli area (Aeolian Archipelago, Southeastern Tyrrhenian Sea). Acta Vulcanol. 1993, 3, 11–20. [Google Scholar]
- Lucchi, F.; Tranne, C.A.; Calanchi, N.; Keller, J.; Rossi, P.L. Geological Map of Panarea and Minor Islets (Aeolian Islands). University of Bologna, University of Freiburg and INGV, 2003; L.A.C: Firenze, Italy, 2003. [Google Scholar]
- Gabbianelli, G.; Gillot, P.Y.; Lanzafame, G.; Romagnoli, C.; Rossi, P.L. Tectonic and volcanic evolution of Panarea (Aeolian Island, Italy). Mar. Geol. 1990, 92, 312–326. [Google Scholar] [CrossRef]
- Calanchi, N.; Tranne, C.A.; Lucchini, F.; Rossi, P.L.; Villa, I.M. Explanatory notes to the geological map (1:10.000) of Panarea and Basiluzzo islands (Aeolian arc. Italy). Acta Vulcanol. 1999, 11, 223–243. [Google Scholar]
- Gillot, P.Y. Datation par la Methode du Potassium-Argon de Roches Volcaniques Recentes (Pleistocenes et Holocenes). Contributions a L’etude Chronostratigraphique et Magmatique des Provinces Volcaniques de la Campania, des Iles Eoliennes, de Pantelleria (Italie du Sud) et de la Reunion (Ocean Indien). Ph.D. Thesis, Paris-Sud University, Bures-sur-Yvette, France, 1984; 249p. [Google Scholar]
- Tallarico, A.; Dragoni, M.; Anzidei, M.; Esposito, A. Modeling long-term round deformation due to the cooling of a magma chamber: Case of Basiluzzo island, Aeolian islands, Italy. J. Geophys. Res. 2003, 108, 2568. [Google Scholar] [CrossRef]
- Marani, M.P.; Gamberi, F.; Savelli, C. Shallow-water polymetallic sulfide deposits in the Aeolian island arc. Geology 1997, 25, 815–818. [Google Scholar] [CrossRef]
- Gamberi, F.; Marani, M.; Savelli, C. Tectonic, volcanic and hydrothermal features of a submarine portion of the Aeolian arc (Tyrrhenian Sea). Mar. Geol. 1997, 140, 167–181. [Google Scholar] [CrossRef]
- Gamberi, F.; Savelli, C.; Marani, M.P.; Ligi, M.; Bortoluzzi, G.; Landuzzi, V.; Costa, M. Contesto morfotettonico e depositi idrotermali di solfuri ed ossidi di ferro in una porzione sommersa dell’arco eoliano (in base ad indagini ad alta definizione). Boll. Soc. Geol. Ital. 1998, 117, 55–71. [Google Scholar]
- Savelli, C.; Marani, M.; Gamberi, F. Geochemistry of metalliferous, hydrothermal deposits in the Aeolian Arc (Tyrrhenian Sea). J. Volcanol. Geotherm. Res. 1999, 88, 305–323. [Google Scholar] [CrossRef]
- Esposito, V.; Andaloro, F.; Canese, S.; Bortoluzzi, G.; Bo, M.; Di Bella, M.; Italiano, F.; Sabatino, G.; Battaglia, P.; Consoli, P.; et al. Exceptional discovery of a shallow-water hydrothermal site in the SW area of Basiluzzo islet (Aeolian archipelago, South Tyrrhenian Sea): An environment to preserve. PLoS ONE 2018, 13, e0190710. [Google Scholar] [CrossRef]
- Esposito, A.; Giordano, G.; Anzidei, M. The 2002–2003 Submarine Gas Eruption at Panarea Volcano (Aeolian Islands, Italy): Volcanology of the Seafloor and Implications for the Hazard Scenario. Mar. Geol. 2006, 227, 119–134. [Google Scholar] [CrossRef]
- Italiano, F.; Nuccio, F. Geochemical investigations of submarine volcanic exhalations to the east of Panarea, Aeolian Islands, Italy. J. Volcanol. Geoth. Res. 1991, 46, 125–141. [Google Scholar] [CrossRef]
- Italiano, F. Hydrothermal fluids vented at shallow depths at the Aeolian islands: Relationships with volcanic and geothermal systems. In Research in Shallow Marine and Fresh Water Systems: 1st International Workshop—Proceedings; Geologisches Institut: Freiberg, Germany, 2009. [Google Scholar]
- Caracausi, A.; Ditta, M.; Italiano, F.; Longo, M.; Nuccio, P.M.; Paonita, A. Massive submarine gas output during the volcanic unrest off Panarea Island (Aeolian arc, Italy): Inferences for explosive conditions. Geochim. J. 2004, 39, 459–467. [Google Scholar] [CrossRef] [Green Version]
- Caliro, S.; Caracausi, A.; Chiodini, G.; Ditta, M.; Italiano, F.; Longo, M.; Minopoli, C.; Nuccio, P.M.; Paonita, A.; Rizzo, A. Evidence of a new magmatic input to the quiescent volcanic edifice of Panarea, Aeolian Islands, Italy. Geophys. Res. Lett. 2004, 31, L07619. [Google Scholar] [CrossRef]
- Capaccioni, B.; Rossi, P.M.L.; Tassi, F.; Tedesco, D.; Vaselli, O. Risultati Delle Osservazioni Geochimiche Presso l’isola di Panarea in Seguito all’evento di Degassamento Sottomarino del 3 Novembre 2002; Abstract; GNV General Assembly: Rome, Italy, 2003; p. 17. [Google Scholar]
- Caramanna, G.; Carapezza, M.L.; Cioni, R.; Cardellini, F.; Cinti, D.; Lelli, M.; Pizzino, L.; Quattrocchi, F.; Voltattorni, N. Primi Sei Mesi di Monitoraggio delle Diverse Fasi (Solida-Liquida e Gassosa) Presso le Esalazioni Sottomarine di Panarea a Partire dal Novembre 2002; Abstract; GNV General Assembly: Rome, Italy, 2003; p. 19. [Google Scholar]
- Gugliandolo, C.; Italiano, F.; Maugeri, T. The submarine hydrothermal system of Panarea (Southern Italy): Biogeochemical processes at the termal fluids—Sea bottom interface. Ann. Geophys. 2006, 49, 783–792. [Google Scholar] [CrossRef]
- Manini, E.; Luna, G.M.; Corinaldesi, C.; Zeppilli, D.; Bortoluzzi, G.; Caramanna, G.; Raffa, F.; Danovaro, R. Prokaryote diversity and virus abundance in shallow hydrothermal vents of the Mediterranean Sea (Panarea Island) and the Pacific Ocean (North Sulawesi—Indonesia). Microb. Ecol. 2008, 55, 626–639. [Google Scholar] [CrossRef] [PubMed]
- Tassi, F.; Capaccioni, B.; Caramanna, G.; Cinti, D.; Montegrossi, G.; Pizzino, L.; Quattrocchi, F.; Valselli, O. Low-pH waters discharging from sub marine vents at Panarea Island (Aeolian Islands, southern Italy) after the 2002 gas blast: Origin of hydrothermal fluids and implications for volcanic surveillance. Appl. Geochem. 2009, 24, 246–254. [Google Scholar] [CrossRef]
- Calanchi, N.; Capaccioni, B.; Martini, M.; Tassi, F.; Valentinio, L. Submarine gas-emission from Panarea Island (Aeolian Archipelago): Distribution of inorganic and organic compounds. Acta Vulcanol. 1995, 7, 43–48. [Google Scholar]
- Esposito, A.; Anzidei, M.; Atzori, S.; Devoti, R.; Giordano, G.; Pietrantonio, G. Modeling ground deformations of Panarea volcano hydrothermal/geothermal system (Aeolian Islands, Italy) from GPS data. Bull. Volcanol. 2010, 72, 609–621. [Google Scholar] [CrossRef]
- Rogelja, M.; Cibic, T.; Pennesi, C.; De Vittor, C. Microphytobenthic community composition and primary production at gas and thermal vents in the Aeolian Islands (Tyrrhenian Sea, Italy). Mar. Environ. Res. 2016, 118, 31–44. [Google Scholar] [CrossRef] [PubMed]
- Romano, D.; Gattuso, A.; Longo, M.; Caruso, C.; Lazzaro, G.; Corbo, A.; Italiano, F. Hazard scenarios related to submarine volcanic-hydrothermal activity and advanced monitoring strategies: A study case from the panarea volcanic group (aeolian islands, italy). Geofluids 2019, 2019, 8728720. [Google Scholar] [CrossRef]
- Guilini, K.; Weber, M.; de Beer, D.; Schneider, M.; Molari, M.; Lott, C.; Bodnar, W.; Mascart, T.; Troch, M.D.; Vanreusel, A. Response of Posidonia oceanica seagrass and its epibiont communities to ocean acidi cation. PLoS ONE 2017, 12, e0181531. [Google Scholar] [CrossRef] [Green Version]
- Gaglioti, M.; Auriemma, R.; De Vittor, C.; Esposito, V.; Teixido, N.; Gambi, M.C. A pilot study on Posidonia oceanica features of a hydrothermal system off Panarea (aeolian islands, italy). In Proceedings of the 50° Congresso della Società Italiana di Biologia Marina, Livorno, Italy, 10–14 June 2019. [Google Scholar]
- Maugeri, T.L.; Lentini, V.; Gugliandolo, C.; Italiano, F.; Cousin, S.; Stackebrandt, E. Bacterial and archaeal populations at two shallow hydrothermal vents off Panarea Island (Eolian Islands, Italy). Extremophiles 2009, 13, 199–212. [Google Scholar] [CrossRef]
- Schönfeld, J.; Alve, E.; Geslin, E.; Jorissen, F.; Korsun, S.; Spezzaferri, S.; Members of The Fobimo. The Fobimo (FOraminiferal BIo-MOnitoring) initiative—Towards a standardized protocol for soft-bottom benthic foraminiferal monitoring studies. Mar. Micropaleontol. 2012, 94–95, 1–13. [Google Scholar] [CrossRef]
- Walton, W.R. Techniques for recognition of living foraminifera. Cont. Cushman Found. Foramin. Res. 1952, 3, 56–60. [Google Scholar]
- Lutze, G.F.; Altenbach, A. Technik und Signifikanz der Lebendfärbung benthischer Foraminiferen mit Begalrot. Geol. Jahrb. 1991, 128, 251–265. [Google Scholar]
- Bernhard, J.M. Distinguishing live from dead foraminifera: Methods review and proper applications. Micropaleontology 2000, 46, 38–46. [Google Scholar]
- Scott, D.B.; Medioli, F.S.; Schafer, C.T. Monitoring of Coastal Environments Using Foraminifera and Thecamoebian Indicators; Cambridge University Press: Cambridge, UK, 2001. [Google Scholar]
- Bernhard, J.M. Postmortem vital staining in benthic foraminifera: Duration and importance in population and distributional studies. J. Foraminifer. Res. 1988, 18, 143–146. [Google Scholar] [CrossRef]
- Hannah, F.; Rogerson, A. The temporal and spatial distribution of foraminiferans in marine benthic sediments of the Clyde Sea, Scotland. Estuar. Coast. Shelf Sci. 1997, 44, 377–383. [Google Scholar] [CrossRef]
- Murray, J.W.; Bowser, S.S. Mortality, protoplasm decay rate, and reliability of staining techniques to recognize ‘living’ foraminifera: A review. J. Foraminifer. Res. 2000, 30, 66–77. [Google Scholar] [CrossRef]
- Fontanier, C.; Jorissen, F.J.; Licari, L.; Alexandre, A.; Anschutz, P.; Carbonel, P. Live benthic foraminiferal faunas from the Bay of Biscay: Faunal density, composition, and microhabitats. Deep Sea Res. I 2002, 49, 751–785. [Google Scholar] [CrossRef]
- Frontalini, F.; Semprucci, F.; Di Bella, L.; Caruso, A.; Cosentino, C.; Maccotta, A.; Scopelliti, G.; Sbrocca, C.; Bucci, C.; Balsamo, M.; et al. The Response of Cultured Meiofaunal and Benthic Foraminiferal Communities to Lead Exposure: Results from Mesocosm Experiments. Environ. Toxicol. Chem. 2018, 37, 2439–2447. [Google Scholar] [CrossRef]
- Figueira, B.O.; Grenfell, H.R.; Hayward, B.W.; Alfaro, A.C. Comparison of Rose Bengal and Cell Tracker Green staining for identification of live salt-marsh foraminifera. J. Foraminifer. Res. 2012, 42, 206–215. [Google Scholar] [CrossRef]
- Fisher, R.A.; Corbet, A.S.; Williams, C.B. The relationship between the number of species and the number of individuals in random samples of an animal population. J. Anim. Ecol. 1943, 12, 42–58. [Google Scholar] [CrossRef]
- Shannon, C.E. A mathematical theory of communication. Bell Syst. Tech. J. 1948, 27, 379–423. [Google Scholar] [CrossRef] [Green Version]
- Hammer, Ø.; Harper, D.A.T.; Ryan, P.D. PAST: Paleontological statistic software package for education and data analysis. Palaeontol. Electron. 2001, 4, 9. [Google Scholar]
- Loeblich, A.R., Jr.; Tappan, H. Foraminiferal Genera and Their Classification; Van Nostrand Reinhold: New York, NY, USA, 1987; Volume 2, 1182p. [Google Scholar]
- Cimerman, F.; Langer, M.R. Mediterranean Foraminifera; Academia Scientiarum et Artium Slovenica: Ljubljana, Slovenia, 1991; Volume 30, pp. 1–11. [Google Scholar]
- Sgarrella, F.; Moncharmont Zei, M. Benthic Foraminifera of the Gulf of Naples (Italy): Systematics and autoecology. Boll. Soc. Paleont. Ital. 1993, 32, 145–264. [Google Scholar]
- SIBM. Notiziario Società Italiana Di Biologia Marina; Società Italiana Di Biologia Marina: Genova, Italy, 2019. [Google Scholar]
- Gerovasileiou, V.; Bailly, N. Brachiopoda of Greece: An annotated checklist. Biodivers. Data J. 2016, 4, e8169. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Russell, B.D.; Harley, C.D.G.; Wernberg, T.; Mieszkowska, N.; Widdicombe, S.; Hall-Spencer, J.M.; Connell, S.D. Predicting ecosystem shifts requires new ap- proaches that integrate the e ects of climate change across entire systems. Biol. Lett. 2012, 8, 164–166. [Google Scholar] [CrossRef] [Green Version]
- Garrard, S.L.; Gambi, M.C.; Scipione, M.B.; Patti, F.P.; Lorenti, M.; Zupo, V.; Paterson, D.M.; Buia, M.C. Indirect effects may buffer negative responses of seagrass invertebrate communities to ocean acidification. J. Exp. Mar. Biol. Ecol. 2014, 461, 31–38. [Google Scholar] [CrossRef]
- Jeffrey, S.R. Structurally-Controlled Hydrothermal Diagenesis of Mississippian Reservoir Rocks Exposed in the Big Snowy Arch, Central Montana. Master’s Thesis, Montana State University (MSU), Bozeman, MT, USA, 2014. Available online: https://scholarworks.montana.edu/xmlui/handle/1/3352 (accessed on 20 April 2021).
- Pascarelli, S. Shallow-Water Geothermal Activity Offshore Panarea, Aeolian Island Arc, Italy. Master’s Thesis, Colorado School of Mines, Golden, CO, USA, 2021. [Google Scholar]
- Linke, P.; Lutze, G.F. Microhabitat preferences of benthic foraminifera—a static concept or a dynamic adaptation to optimize food acquisition? Mar. Micropaleontol. 1993, 20, 215–234. [Google Scholar] [CrossRef] [Green Version]
- Schönfeld, J. The impact of the Mediterranean Outflow Water (MOW) on benthic foraminiferal assemblages and surface sediments at the southern Portuguese continental margin. Mar. Micropaleontol. 1997, 29, 211–236. [Google Scholar] [CrossRef]
- Schönfeld, J. Recent benthic foraminiferal assemblages in deep high-energy environments from the Gulf of Cadiz (Spain). Mar. Micropaleontol. 2002, 44, 141–162. [Google Scholar] [CrossRef]
- Baruffo, A.; Ciaralli, L.; Ardizzone, G.; Gambi, M.C.; Casoli, E. Ocean acidification and mollusc settlement in Posidonia oceanica meadows: Does the seagrass buffer lower pH effects at CO2 vents? Diversity 2021, 13, 311. [Google Scholar] [CrossRef]
- Buosi, C.; du ChaTelet, E.A.; Cherchi, A. Benthic foraminiferal assemblages in the current-dominated Strait of Bonifacio (Mediterranean Sea). J. Foraminifer. Res. 2012, 42, 39–55. [Google Scholar] [CrossRef]
- Hendriks, I.E.; Olsen, Y.S.; Ramajo, L.; Basso, L.; Steckbauer, A.; Moore, T.S.; Howard, J.; Duarte, C.M. Photosynthetic activity buffers ocean acidification in seagrass meadows. Biogeosciences 2014, 11, 333–346. [Google Scholar] [CrossRef] [Green Version]
- Ricart, A.M.; Ward, M.; Hill, T.M.; Sanford, E.; Kroeker, K.J.; Takeshita, Y.; Merolla, S.; Shukla, P.; Ninokawa, A.T.; Elsmore, K.; et al. Coast-wide evidence of low pH amelioration by seagrass ecosystems. Glob. Chang. Biol. 2021, 27, 2580–2591. [Google Scholar] [CrossRef] [PubMed]
- Larkum, A.W.; Orth, R.J.; Duarte, C.M. Seagrasses: Biology, ecology and conservation. Phycologia 2006, 45, 5. [Google Scholar]
- Piazzi, L.; De Biasi, A.M.; Balata, D.; Pardi, G.; Boddi, S.; Acunto, S.; Sartoni, G. Species composition and spatial variability patterns of morphological forms in macroalgal epiphytic assemblages of the seagrass Posidonia oceanica. Vie Milieu 2007, 57, 171. [Google Scholar]
- Uku, J.; Björk, M.; Bergman, B.; Díez, B. Characterization and Comparison of Prokaryotic Epiphytes Associated with Three East African Seagrasses 1. J. Phycol. 2007, 43, 768–779. [Google Scholar] [CrossRef]
- Wilson, B. Guilds among epiphytal foraminifera on fibrous substrates, Nevis, West Indies. Mar. Micropaleontol. 2007, 63, 1–18. [Google Scholar] [CrossRef]
- Bandy, O.L.; Ingle, J.C., Jr.; Resig, J.M. Foraminifera, Los Angeles County Outfall Area, California 1. Limnol. Oceanogr. 1964, 9, 124–137. [Google Scholar] [CrossRef]
- Sliter, W.V. Laboratory experiments on the life cycle and ecologic controls of Rosalina globularis d’Orbigny. J. Protozool. 1965, 12, 210–215. [Google Scholar] [CrossRef]
- Frankel, L. Subsurface reproduction in foraminifera. J. Paleontol. 1972, 46, 62–65. [Google Scholar]
- Frankel, L. Observations and speculations on the habitat and habits of Trochammina ochracea (Williamson) in subsurface sediments. J. Paleontol. 1974, 48, 143–148. [Google Scholar]
- Kitazato, H. Observations and behaviour and mode of life of benthic foraminifers in the laboratory. Geosci. Rep. Shizuoka Univ. 1981, 6, 61–71. [Google Scholar]
- El Kateb, A.; Beccari, V.; Stainbank, S.; Spezzaferri, S.; Coletti, G. Living (stained) foraminifera in the Lesser Syrtis (Tunisia): Influence of pollution and substratum. PeerJ 2020, 8, e8839. [Google Scholar] [CrossRef] [PubMed]
- Vénec-Peyré, M. Etude de la distribution des foraminifères vivant dans la Baie de Banyuls-Sur-Mer. In Ecologie des Microorganismes en Méditerranée Occidentale: Ecomed; Bizon, J.J., Burollet, P.F., Eds.; Association Française des Techniciens du Pétrole: Paris, France, 1984; pp. 60–80. [Google Scholar]
- López-Belzunce, M.; Blázquez, A.M.; Pretus, J.L. Recent benthic foraminiferal assemblages and their relationship to environmental variables on the shoreface and inner shelf off Valencia (Western Mediterranean). Mar. Environ. Res. 2014, 101, 169–183. [Google Scholar] [CrossRef] [PubMed]
- Frezza, V.; Mateu-Vicens, G.; Gaglianone, G.; Baldassarre, A.; Brandano, M. Mixed carbonate-siliclastic sediments and benthic foraminiferal assemblages from Posidonia oceanica seagrass meadows of the central Tyrrhenian continental shelf (Latium, Italy). Ital. J. Geosci. 2011, 130, 352–369. [Google Scholar]
- Bergamin, L.; Di Bella, L.; Ferraro, L.; Frezza, V.; Pierfranceschi, G.; Romano, E. Benthic foraminifera in a coastal marine area of the eastern Ligurian Sea (Italy): Response to environmental stress. Ecol. Indic. 2019, 96, 16–31. [Google Scholar] [CrossRef]
- De Nooijer, L.J.; Toyofuku, T.; Kitazato, H. Foraminifera promote calcification by elevating their intracellular pH. Proc. Natl. Acad. Sci. USA 2009, 106, 15374–15378. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Andersson, A.J.; Mackenzie, F.T.; Bates, N.R. Life on the margin: Implications of ocean acidification on Mg-calcite, high latitude and cold-water marine calcifiers. Mar. Ecol. Prog. Ser. 2008, 373, 265–273. [Google Scholar] [CrossRef]
- Arieli, R.N.; Almogi-Labin, A.; Abramovich, S.; Herut, B. The effect of thermal pollution on benthic foraminiferal assemblages in the Mediterranean shoreface adjacent to Hadera power plant (Israel). Mar. Pollut. Bull. 2011, 62, 1002–1012. [Google Scholar] [CrossRef]
- Bradshaw, J.S. Laboratory experiment on the ecology of foraminifera. Contr. Cushman Found Res. 1961, 12, 87–106. [Google Scholar]
- Manda, S.; Titelboim, D.; Ashckenazi-Polivoda, S.; Almogi-Labin, A.; Herutd, B.; Abramovich, S. Epiphytic benthic foraminiferal preferences for macroalgal habitats: Implications for coastal warming. Mar. Environ. Res. 2020, 161, 105084. [Google Scholar] [CrossRef]
- Damak, M.; Fourati, R.; Elleuch, B.; Kallel, M. Environmental quality assessment of the fish farms’ impact in the Monastir Bay (eastern of Tunisia, Central Mediterranean): A benthic foraminiferal perspective. Environ. Sci. Pollut. Res. 2020, 27, 9059–9074. [Google Scholar] [CrossRef] [PubMed]
- Romano, E.; Bergamin, L.; Di Bella, L.; Frezza, V.; Pierfranceschi, G.; Marassich, A.; Provenzani, C. Benthic foraminifera as environmental indicators in extreme environments: The marine cave of Bue Marino (Sardinia, Italy). Ecol. Indic. 2021, 120, 106977. [Google Scholar] [CrossRef]
- Lei, Y.L.; Li, T.G.; Bi, H.; Cui, W.L.; Song, W.P.; Li, J.Y.; Li, C.C. Responses of benthic foraminifera to the 2011 oil spill in the Bohai Sea, PR China. Mar. Pollut. Bull. 2015, 96, 245–260. [Google Scholar] [CrossRef] [PubMed]
- Gupta, B.K.S. (Ed.) Modern Foraminifera; Kluwer Academic Publishers: Dordrecht, The Netherlands, 1999; pp. 7–36. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).