A Review of the Studies on CO2–Brine–Rock Interaction in Geological Storage Process
Abstract
:1. Introduction
2. Types of Reservoirs and Their Capacities
2.1. Saline Reservoirs
2.2. Depleted Oil and Gas Fields
2.3. Mafic and Ultramafic Reservoirs
2.4. Salt Caverns
2.5. Unmineable Coal Seams
2.6. Ocean Sinks
3. Long Term Changes in CO2, Brine, and the Reservoirs
4. Effect of Different Phases of CO2–Brine on the Different Properties of Rocks
5. Trends in Geological Storage Research
6. Need for Future Study
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Piane, C.D.; Sarout, J. Effects of water and supercritical CO2 on the mechanical and elastic properties of Berea sandstone. Int. J. Greenh. Gas Control 2016, 55, 209–220. [Google Scholar] [CrossRef]
- Xiao, T.; Xu, H.; Moodie, N.; Esser, R.; Jia, W.; Zheng, L.; Rutqvist, J.; McPherson, B. Chemical-Mechanical Impacts of CO2 Intrusion Into Heterogeneous Caprock. Water Resour. Res. 2020, 56, e2020WR027193. [Google Scholar] [CrossRef]
- Valle, L.; Grima, C.; Rodríguez, R.; Llopis, C. Effect of the scCO2-brine mixture on injectivity and storage capacity in rock samples of naturally fractured carbonate formations. J. Nat. Gas Sci. Eng. 2020, 81, 103452. [Google Scholar] [CrossRef]
- Nguyen, V.A. Investigation of Reactions between Glauconite and Carbon Dioxide, with Implications for Carbon Sequestration; Mississippi State University: Starkville, MS, USA, 2020. [Google Scholar]
- Fuchs, S.J.; Espinoza, D.N.; Lopano, C.L.; Akono, A.-T.; Werth, C.J. Geochemical and geomechanical alteration of siliciclastic reservoir rock by supercritical CO2-saturated brine formed during geological carbon sequestration. Int. J. Greenh. Gas Control 2019, 88, 251–260. [Google Scholar] [CrossRef]
- IEO. International Energy Outlook 2021 (IEO2021): Independent Statistics and Analysis by Stephen Nalley and Angelina LaRose; Centre for Strategic and International Studies: Washington, DC, USA, 2021; p. 21. [Google Scholar]
- GCCSI. Geological Storage of Co2: Safe, Permanent, and Abundant; Global CCS institute: Melbourne, Australia, 2018; p. 2. [Google Scholar]
- Melara, A.J.; Singh, U.; Colosi, L.M. Is aquatic bioenergy with carbon capture and storage a sustainable negative emission technology? Insights from a spatially explicit environmental life-cycle assessment. Energy Convers. Manag. 2020, 224, 113300. [Google Scholar] [CrossRef]
- Creutzig, F.; Breyer, C.; Hilaire, J.; Minx, J.; Peters, G.P.; Socolow, R.H. The mutual dependence of negative emission technologies and energy systems. Energy Environ. Sci. 2019, 12, 1805–1817. [Google Scholar] [CrossRef]
- Lehtveer, M.; Emanuelsson, A. BECCS and DACCS as Negative Emission Providers in an Intermittent Electricity System: Why Levelized Cost of Carbon May Be a Misleading Measure for Policy Decisions. Front. Clim. 2021, 3, 15. [Google Scholar] [CrossRef]
- Klass, A.B.; Wilson, E.J. Climate change and carbon sequestration: Assessing a liability regime for long-term storage of carbon dioxide. Emory LJ 2008, 58, 103. [Google Scholar]
- Liu, L.-C.; Li, Q.; Zhang, J.-T.; Cao, D. Toward a framework of environmental risk management for CO 2 geological storage in China: Gaps and suggestions for future regulations. Mitig. Adapt. Strateg. Glob. Change 2016, 21, 191–207. [Google Scholar] [CrossRef]
- Li, X.; Wei, N.; Liu, Y.; Fang, Z.; Dahowski, R.; Davidson, C. CO2 point emission and geological storage capacity in China. Energy Procedia 2009, 1, 2793–2800. [Google Scholar] [CrossRef] [Green Version]
- Kelemen, P.; Benson, S.M.; Pilorgé, H.; Psarras, P.; Wilcox, J. An Overview of the Status and Challenges of CO2 Storage in Minerals and Geological Formations. Front. Clim. 2019, 1, 9. [Google Scholar] [CrossRef]
- Bentham, M.; Mallows, T.; Lowndes, J.; Green, A. CO2 STORage Evaluation Database (CO2 Stored). The UK’s online storage atlas. Energy Procedia 2014, 63, 5103–5113. [Google Scholar] [CrossRef] [Green Version]
- Kim, A.-R.; Cho, G.-C.; Kwon, T.-H. Site characterization and geotechnical aspects on geological storage of CO2 in Korea. Geosci. J. 2014, 18, 167–179. [Google Scholar] [CrossRef]
- Holloway, S.; Vincent, C.J.; Kirk, K. Industrial Carbon Dioxide Emissions and Carbon Dioxide Storage Potential in the UK; Keyworth, Nottingham British Geological Survey: Nottingham, UK, 2006; p. 57. [Google Scholar]
- Zulqarnain, M.; Zeidouni, M.; Hughes, R.G. Static and dynamic CO2 storage capacity estimates of a potential CO2 geological sequestration site in Louisiana chemical corridor. In Proceedings of the Carbon Management Technology Conference, Houston, TX, USA, 17–20 July 2017. [Google Scholar]
- Shukla, R.; Ranjith, P.; Haque, A.; Choi, X. A review of studies on CO2 sequestration and caprock integrity. Fuel 2010, 89, 2651–2664. [Google Scholar] [CrossRef]
- Cao, S.C.; Dai, S.; Jung, J. Supercritical CO2 and brine displacement in geological carbon sequestration: Micromodel and pore network simulation studies. Int. J. Greenh. Gas Control 2016, 44, 104–114. [Google Scholar] [CrossRef]
- Doherty, B.; Vasylkivska, V.; Huerta, N.J.; Dilmore, R. Estimating the Leakage along Wells during Geologic CO2 Storage: Application of the Well Leakage Assessment Tool to a Hypothetical Storage Scenario in Natrona County, Wyoming. Energy Procedia 2017, 114, 5151–5172. [Google Scholar] [CrossRef]
- Acevedo, L.; Chopra, A. Influence of Phase Behaviour in the Well Design of CO2 Injectors. Energy Procedia 2017, 114, 5083–5099. [Google Scholar] [CrossRef]
- Goldberg, D.S.; Takahashi, T.; Slagle, A.L. Carbon dioxide sequestration in deep-sea basalt. Proc. Natl. Acad. Sci. USA 2008, 105, 9920–9925. [Google Scholar] [CrossRef] [Green Version]
- Andreani, M.; Luquot, L.; Gouze, P.; Godard, M.; Hoisé, E.; Gibert, B. Experimental Study of Carbon Sequestration Reactions Controlled by the Percolation of CO2-Rich Brine through Peridotites. Environ. Sci. Technol. 2009, 43, 1226–1231. [Google Scholar] [CrossRef]
- Flaathen, T.K.; Gislason, S.R.; Oelkers, E.H.; Sveinbjörnsdóttir, Á.E. Chemical evolution of the Mt. Hekla, Iceland, groundwaters: A natural analogue for CO2 sequestration in basaltic rocks. Appl. Geochem. 2009, 24, 463–474. [Google Scholar] [CrossRef]
- Matter, J.M.; Kelemen, P. Permanent storage of carbon dioxide in geological reservoirs by mineral carbonation. Nat. Geosci. 2009, 2, 837–841. [Google Scholar] [CrossRef]
- Garcia, B.; Beaumont, V.; Perfetti, E.; Rouchon, V.; Blanchet, D.; Oger, P.; Dromart, G.; Huc, A.-Y.; Haeseler, F. Experiments and geochemical modelling of CO2 sequestration by olivine: Potential, quantification. Appl. Geochem. 2010, 25, 1383–1396. [Google Scholar] [CrossRef]
- Zarandi, A.E.; Larachi, F.; Beaudoin, G.; Plante, B.; Sciortino, M. Ambient mineral carbonation of different lithologies of mafic to ultramafic mining wastes/tailings—A comparative study. Int. J. Greenh. Gas Control. 2017, 63, 392–400. [Google Scholar] [CrossRef]
- Marieni, C.; Oelkers, E. Carbon sequestration potential of altered mafic reservoirs. Energy Procedia 2018, 146, 68–73. [Google Scholar] [CrossRef]
- Anthonsen, K.L.; Aagaard, P.; Bergmo, P.E.S.; Erlström, M.; Fareide, J.I.; Gislason, S.R.; Mortensen, G.M.; Snæbjörnsdottir, S.Ó. CO2 Storage Potential in the Nordic Region. Energy Procedia 2013, 37, 5080–5092. [Google Scholar] [CrossRef] [Green Version]
- Jones, K.B.; Blondes, M.S. Carbon Dioxide Storage in Unconventional Reservoirs Workshop-Summary of Recommendations; U.S Geological Survey Open-File Report 2015-1079; U.S Geological Survey: Reston, VA, USA, 2015; 10p.
- Maia da Costa, A.; da Costa, P.V.M.; Udebhulu, O.D.; Cabral Azevedo, R.; Ebecken, N.F.F.; Miranda, A.C.O.; de Eston, S.M.; de Tomi, G.; Meneghini, J.R.; Nishimoto, K.; et al. Potential of storing gas with high CO2 content in salt caverns built in ultra-deep water in Brazil. Greenh. Gases Sci. Technol. 2019, 9, 79–94. [Google Scholar]
- Connell, L.; Sander, R.; Pan, Z.; Camilleri, M.; Heryanto, D. History matching of enhanced coal bed methane laboratory core flood tests. Int. J. Coal Geol. 2011, 87, 128–138. [Google Scholar] [CrossRef]
- Jessen, K.; Lin, W.; Kovscek, A.R. Multicomponent sorption modeling in ECBM displacement calculations. In Proceedings of the SPE Annual Technical Conference and Exhibition, SPE 110077, Anaheim, CA, USA, 11–14 November 2007. [Google Scholar]
- Yamasaki, A. An Overview of CO2 Mitigation Options for Global Warming-Emphasizing CO2 Sequestration Options. J. Chem. Eng. Jpn. 2003, 36, 361–375. [Google Scholar] [CrossRef]
- Bickle, M.J. Geological carbon storage. Nat. Geosci. 2009, 2, 815. [Google Scholar] [CrossRef]
- Garg, A.; Shukla, P. Coal and energy security for India: Role of carbon dioxide (CO2) capture and storage (CCS). Energy 2009, 34, 1032–1041. [Google Scholar] [CrossRef]
- Viete, D.; Ranjith, P. The effect of CO2 on the geomechanical and permeability behaviour of brown coal: Implications for coal seam CO2 sequestration. Int. J. Coal Geol. 2006, 66, 204–216. [Google Scholar] [CrossRef]
- Mehic, M.; Ranjith, P.G.; Choi, S.K.; Haque, A. The Geomechanical Behavior of Australian Black Coal under the Effects of CO2 Injection: Uniaxial Testing. In Advances in Unsaturated Soil, Seepage, and Environmental Geotechnics; American Society of Civil Engineers: Reston, VA, USA, 2006; pp. 290–297. [Google Scholar]
- Turrell, W. Marine science within a net-zero emission statutory framework. ICES J. Mar. Sci. 2019, 76, 1983–1993. [Google Scholar] [CrossRef] [Green Version]
- Metz, B.; De Coninck, H. IPCC Special Report on Carbon Dioxide Capture and Storage; Cambridge University Press: Cambridge, UK, 2005. [Google Scholar]
- Adams, E.E.; Caldeira, K. Ocean Storage of CO2. Elements 2008, 4, 319–324. [Google Scholar] [CrossRef]
- Rochelle, C.; Camps, A.P.; Long, D.; Milodowski, A.; Bateman, K.; Gunn, D.; Rees, J. Can CO2 hydrate assist in the underground storage of carbon dioxide? Geol. Soc. Lond. Spec. Publ. 2009, 319, 171–183. [Google Scholar] [CrossRef] [Green Version]
- Goldthorpe, S. Potential for Very Deep Ocean Storage of CO2 Without Ocean Acidification: A Discussion Paper. Energy Procedia 2017, 114, 5417–5429. [Google Scholar] [CrossRef]
- BGS. The Geological Storage. 2012. Available online: https://iea.bgs.ac.uk/v2.4_product/introduction.html (accessed on 21 October 2021).
- Sun, Y.; Li, Q.; Yang, D.; Liu, X. Laboratory core flooding experimental systems for CO2 geosequestration: An updated review over the past decade. J. Rock Mech. Geotech. Eng. 2016, 8, 113–126. [Google Scholar] [CrossRef] [Green Version]
- Mann & Kump. Dire Predictions Understanding Climate Change: An Illustrated Guide to the Findings of the IPCC, 2nd ed.; DK Publishing: New York, NY, USA, 2015; p. 225. [Google Scholar]
- CO2CRC. Dispersion Modelling Techniques for Carbon Dioxide Pipelines In Australia; Document No. 20873-RP-001; Sherpa Consulting: Chatswood, Australia, 2015; 193p. [Google Scholar]
- CO2 Trapping Mechanisms. Available online: https://www.bigskyco2.org/node/127 (accessed on 30 December 2021).
- Jeon, P.R.; Lee, C.-H. Reaction of drilled-cores from the Janggi basin with CO2-saturated brine from subcritical to supercritical condition of CO2: Implications on sequestration of dissolved CO2. J. Nat. Gas Sci. Eng. 2021, 88, 103804. [Google Scholar] [CrossRef]
- Chabab, S.; Cruz, J.L.; Poulain, M.; Ducousso, M.; Contamine, F.; Serin, J.P.; Cézac, P. Thermodynamic Modeling of Mutual Solubilities in Gas-Laden Brines Systems Containing CO2, CH4, N2, O2, H2, H2O, NaCl, CaCl2, and KCl: Application to Degassing in Geothermal Processes. Energies 2021, 14, 5239. [Google Scholar] [CrossRef]
- Li, W.; Nan, Y.; You, Q.; Jin, Z. CO2 solubility in brine in silica nanopores in relation to geological CO2 sequestration in tight formations: Effect of salinity and pH. Chem. Eng. J. 2021, 411, 127626. [Google Scholar] [CrossRef]
- Cruz, J.L.; Neyrolles, E.; Contamine, F.; Cézac, P. Experimental Study of Carbon Dioxide Solubility in Sodium Chloride and Calcium Chloride Brines at 333.15 and 453.15 K for Pressures up to 40 MPa. J. Chem. Eng. Data 2021, 66, 249–261. [Google Scholar] [CrossRef]
- Enick, R.M.; Klara, S.M. CO2 Solubility in Water and Brine under Reservoir Conditions. Chem. Eng. Commun. 1990, 90, 23–33. [Google Scholar] [CrossRef]
- Song, Z.; Shi, H.; Zhang, X.; Zhou, T. Prediction of CO2 solubility in ionic liquids using machine learning methods. Chem. Eng. Sci. 2020, 223, 115752. [Google Scholar] [CrossRef]
- Jamshidi, T.; Zeng, F.; Tontiwachwuthikul, P.; Torabi, F. Laboratory measurements of solubility and swelling factor for CO2/Brine and CO2/heavy oil binary systems under low-medium pressure and temperature. Can. J. Chem. Eng. 2019, 97, 2137–2145. [Google Scholar] [CrossRef]
- Chabab, S.; Théveneau, P.; Corvisier, J.; Coquelet, C.; Paricaud, P.; Houriez, C.; El Ahmar, E. Thermodynamic study of the CO2 -H2O-NaCl system: Measurements of CO2 solubility and modeling of phase equilibria using Soreide and Whitson, electrolyte CPA and SIT models. Int. J. Greenh. Gas Control 2019, 91, 102825. [Google Scholar] [CrossRef]
- Hajiw, M.; Corvisier, J.; El Ahmar, E.; Coquelet, C. Impact of impurities on CO2 storage in saline aquifers: Modelling of gases solubility in water. Int. J. Greenh. Gas Control 2018, 68, 247–255. [Google Scholar] [CrossRef]
- Ahmadi, M.A.; Ahmadi, A. Applying a sophisticated approach to predict CO2 solubility in brines: Application to CO2 sequestration. Int. J. Low-Carbon Technol. 2015, 11, 325–332. [Google Scholar] [CrossRef] [Green Version]
- Jacob, R.; Saylor, B. CO2 solubility in multi-component brines containing NaCl, KCl, CaCl2 and MgCl2 at 297 K and 1–14 MPa. Chem. Geol. 2016, 424, 86–95. [Google Scholar] [CrossRef]
- Ratnakar, R.R.; Venkatraman, A.; Kalra, A.; Dindoruk, B. On the prediction of gas solubility in brine solutions with single or mixed salts: Applications to gas injection and CO2 capture/sequestration. J. Nat. Gas Sci. Eng. 2020, 81, 103450. [Google Scholar] [CrossRef]
- Mohammadian, E.; Hamidi, H.; Asadullah, M.; Azdarpour, A.; Motamedi, S.; Junin, R. Measurement of CO2 Solubility in NaCl Brine Solutions at Different Temperatures and Pressures Using the Potentiometric Titration Method. J. Chem. Eng. Data 2015, 60, 2042–2049. [Google Scholar] [CrossRef]
- Lamy-Chappuis, B. Mineral-Fluid Interactions and Their Implications for the Sequestration of CO2 in Saline Aquifers; University of Leeds: Leeds, UK, 2015. [Google Scholar]
- Yan, W.; Huang, S.; Stenby, E.H. Measurement and modeling of CO2 solubility in NaCl brine and CO2—Saturated NaCl brine density. Int. J. Greenh. Gas Control 2011, 5, 1460–1477. [Google Scholar] [CrossRef]
- Tatar, A.; Naseri, S.; Sirach, N.; Lee, M.; Bahadori, A. Prediction of reservoir brine properties using radial basis function (RBF) neural network. Petroleum 2015, 1, 349–357. [Google Scholar] [CrossRef] [Green Version]
- Mao, S.; Duan, Z. The P,V,T,x properties of binary aqueous chloride solutions up to T = 573K and 100MPa. J. Chem. Thermodyn. 2008, 40, 1046–1063. [Google Scholar] [CrossRef]
- Teng, Y.; Wang, P.; Jiang, L.; Liu, Y.; Song, Y.; Wei, Y. An experimental study of density-driven convection of fluid pairs with viscosity contrast in porous media. Int. J. Heat Mass Transf. 2020, 152, 119514. [Google Scholar] [CrossRef]
- Mahmoodpour, S.; Rostami, B.; Soltanian, M.R.; Amooie, M.A. Effect of brine composition on the onset of convection during CO2 dissolution in brine. Comput. Geosci. 2019, 124, 1–13. [Google Scholar] [CrossRef]
- Islam, A.; Meckel, T.; Sun, A.; Krishnamurthy, P. Numerical experiments of density driven CO2 saturated brine migration in heterogeneous two-dimensional geologic fabric materials. Int. Commun. Heat Mass Transf. 2016, 71, 148–156. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Hou, M.; Yang, G.; Han, B. Solubility of CO2 in aqueous solutions of NaCl, KCl, CaCl2 and their mixed salts at different temperatures and pressures. J. Supercrit. Fluids 2011, 56, 125–129. [Google Scholar] [CrossRef]
- Mosavat, N.; Torabi, F. Application of CO2-Saturated Water Flooding as a Prospective Safe CO2 Storage Strategy. Energy Procedia 2014, 63, 5408–5419. [Google Scholar] [CrossRef] [Green Version]
- Duan, Z.; Hu, J.; Li, D.; Mao, S. Densities of the CO2–H2O and CO2–H2O–NaCl systems up to 647 K and 100 MPa. Energy Fuels 2008, 22, 1666–1674. [Google Scholar] [CrossRef]
- Mao, S.; Duan, Z.; Hu, J.; Zhang, D. A model for single-phase PVTx properties of CO2–CH4–C2H6–N2–H2O–NaCl fluid mixtures from 273 to 1273 K and from 1 to 5000 bar. Chem. Geol. 2010, 275, 148–160. [Google Scholar] [CrossRef]
- Ahmad, N.; Wörman, A.; Sanchez-Vila, X.; Jarsjö, J.; Bottacin-Busolin, A.; Hellevang, H. Injection of CO2 -saturated brine in geological reservoir: A way to enhanced storage safety. Int. J. Greenh. Gas Control 2016, 54, 129–144. [Google Scholar] [CrossRef] [Green Version]
- Jeong, G.S.; Ki, S.; Lee, D.S.; Jang, I. Effect of the Flow Rate on the Relative Permeability Curve in the CO2 and Brine System for CO2 Sequestration. Sustainability 2021, 13, 1543. [Google Scholar] [CrossRef]
- Abdoulghafour, H.; Sarmadivaleh, M.; Hauge, L.P.; Fernø, M.; Iglauer, S. Capillary pressure characteristics of CO2-brine-sandstone systems. Int. J. Greenh. Gas Control 2020, 94, 102876. [Google Scholar] [CrossRef]
- Basirat, F.; Yang, Z.; Niemi, A. Pore-scale modeling of wettability effects on CO2—Brine displacement during geological storage. Adv. Water Resour. 2017, 109, 181–195. [Google Scholar] [CrossRef]
- Sidiq, H.; Amin, R.; Kennaird, T. The study of relative permeability and residual gas saturation at high pressures and high temperatures. Adv. Geo-Energy Res. 2017, 1, 64–68. [Google Scholar] [CrossRef]
- Jeong, G.S.; Lee, J.; Ki, S.; Huh, D.G.; Park, C.H. Effects of viscosity ratio, interfacial tension and flow rate on hysteric relative permeability of CO2/brine systems. Energy 2017, 133, 62–69. [Google Scholar] [CrossRef]
- Ajibola, J.; Adam, A.; Muggeridge, A. Gravity Driven Fingering and Mixing During CO2 Sequestration. In Proceedings of the SPE Asia Pacific Oil & Gas Conference and Exhibition, Society of Petroleum Engineers, Richardson, TX, USA, 25–27 October 2016. [Google Scholar]
- Shukla, P.; De Wit, A. Fingering dynamics driven by a precipitation reaction: Nonlinear simulations. Phys. Rev. E 2016, 93, 023103. [Google Scholar] [CrossRef] [Green Version]
- Al-Menhali, A.; Niu, B.; Krevor, S. Capillarity and wetting of carbon dioxide and brine during drainage in b erea sandstone at reservoir conditions. Water Resour. Res. 2015, 51, 7895–7914. [Google Scholar] [CrossRef] [Green Version]
- Jung, J.; Hu, J.W. Impact of pressure and brine salinity on capillary pressure-water saturation relations in geological CO2 sequestration. Adv. Condens. Matter Phys. 2016, 2016, 5603739. [Google Scholar] [CrossRef] [Green Version]
- Reynolds, C.A.; Krevor, S. Characterizing flow behavior for gas injection: Relative permeability of CO2-brine and N2-water in heterogeneous rocks. Water Resour. Res. 2015, 51, 9464–9489. [Google Scholar] [CrossRef] [Green Version]
- Krevor, S.; Reynolds, C.; Al-Menhali, A.; Niu, B. The impact of reservoir conditions and rock heterogeneity on CO2-brine multiphase flow in permeable sandstone. Petrophysics 2016, 57, 12–18. [Google Scholar]
- Saeedi, A.; Rezaee, R.; Evans, B.; Clennell, B. Multiphase flow behaviour during CO2 geo-sequestration: Emphasis on the effect of cyclic CO2—Brine flooding. J. Pet. Sci. Eng. 2011, 79, 65–85. [Google Scholar] [CrossRef]
- Kuo, C.-W.; Perrin, J.-C.; Benson, S.M. Simulation studies of effect of flow rate and small scale heterogeneity on multiphase flow of CO2 and brine. Energy Procedia 2011, 4, 4516–4523. [Google Scholar] [CrossRef]
- Vilarrasa, V.; Rinaldi, A.P.; Rutqvist, J. Long-term thermal effects on injectivity evolution during CO2 storage. Int. J. Greenh. Gas Control 2017, 64, 314–322. [Google Scholar] [CrossRef]
- Pruess, K.; Nordbotten, J. Numerical Simulation Studies of the Long-term Evolution of a CO2 Plume in a Saline Aquifer with a Sloping Caprock. Transp. Porous Media 2011, 90, 135–151. [Google Scholar] [CrossRef] [Green Version]
- Xu, T.; Kharaka, Y.K.; Doughty, C.; Freifeld, B.; Daley, T.M. Reactive transport modeling to study changes in water chemistry induced by CO2 injection at the Frio-I Brine Pilot. Chem. Geol. 2010, 271, 153–164. [Google Scholar] [CrossRef] [Green Version]
- Whittaker, S.; Rostron, B.; Hawkes, C.; Gardner, C.; White, D.; Johnson, J.; Chalaturnyk, R.; Seeburger, D. A decade of CO2 injection into depleting oil fields: Monitoring and research activities of the IEA GHG Weyburn-Midale CO2 Monitoring and Storage Project. Energy Procedia 2011, 4, 6069–6076. [Google Scholar] [CrossRef] [Green Version]
- Ilgen, A.G.; Newell, P.; Hueckel, T.; Espinoza, D.N.; Hu, M. Coupled chemical-mechanical Processes Associated With the Injection of CO2 into Subsurface. In Science of Carbon Storage in Deep Saline Formations; Elsevier: Amsterdam, The Netherlands, 2019; pp. 337–359. [Google Scholar]
- Rutqvist, J. The Geomechanics of CO2 Storage in Deep Sedimentary Formations. Geotech. Geol. Eng. 2012, 30, 525–551. [Google Scholar] [CrossRef] [Green Version]
- Peter, A.; Jin, X.; Fan, X.; Eshiet, K.I.-I.; Sheng, Y.; Yang, D. Microscopy and image analysis of the micro-fabric and composition of saline rocks under different phaseCO2-Brine states. J. Pet. Sci. Eng. 2021, 208, 109411. [Google Scholar] [CrossRef]
- Pimienta, L.; Esteban, L.; Sarout, J.; Liu, K.; Dautriat, J.; Piane, C.D.; Clennell, M.B. Supercritical CO2 injection and residence time in fluid-saturated rocks: Evidence for calcite dissolution and effects on rock integrity. Int. J. Greenh. Gas Control 2017, 67, 31–48. [Google Scholar] [CrossRef]
- Dávila, G.; Dalton, L.; Crandall, D.M.; Garing, C.; Werth, C.J.; Druhan, J.L. Reactive alteration of a Mt. Simon Sandstone due to CO2-rich brine displacement. Geochim. Cosmochim. Acta 2020, 271, 227–247. [Google Scholar] [CrossRef]
- Han, J.; Han, S.; Kang, D.H.; Kim, Y.; Lee, J.; Lee, Y. Application of digital rock physics using X-ray CT for study on alteration of macropore properties by CO2 EOR in a carbonate oil reservoir. J. Pet. Sci. Eng. 2020, 189, 107009. [Google Scholar] [CrossRef]
- Lamy-Chappuis, B.; Angus, D.; Fisher, Q.J.; Yardley, B.W. The effect of CO2 -enriched brine injection on the mechanical properties of calcite-bearing sandstone. Int. J. Greenh. Gas Control 2016, 52, 84–95. [Google Scholar] [CrossRef] [Green Version]
- Garcia-Rios, M.; Luquot, L.; Soler, J.M.; Cama, J. Influence of the flow rate on dissolution and precipitation features during percolation of CO2-rich sulfate solutions through fractured limestone samples. Chem. Geol. 2015, 414, 95–108. [Google Scholar] [CrossRef]
- Grombacher, D.; Vanorio, T.; Ebert, Y. Time-lapse acoustic, transport, and NMR measurements to characterize microstructural changes of carbonate rocks during injection of CO2-rich water. Geophysics 2012, 77, WA169–WA179. [Google Scholar] [CrossRef]
- Vialle, S.; Vanorio, T. Laboratory measurements of elastic properties of carbonate rocks during injection of reactive CO2-saturated water. Geophys. Res. Lett. 2011, 38, 1–5. [Google Scholar] [CrossRef]
- Lei, X.; Xue, Z. Ultrasonic velocity and attenuation during CO2 injection into water-saturated porous sandstone: Measurements using difference seismic tomography. Phys. Earth Planet. Inter. 2009, 176, 224–234. [Google Scholar] [CrossRef]
- Espinoza, D.N.; Jung, H.; Major, J.R.; Sun, Z.; Ramos, M.J.; Eichhubl, P.; Balhoff, M.T.; Choens, R.C.; Dewers, T.A. CO2 charged brines changed rock strength and stiffness at Crystal Geyser, Utah: Implications for leaking subsurface CO2 storage reservoirs. Int. J. Greenh. Gas Control 2018, 73, 16–28. [Google Scholar] [CrossRef] [Green Version]
- Huang, Y.-H.; Yang, S.-Q.; Li, W.-P.; Hall, M.R. Influence of super-critical CO 2 on the strength and fracture behavior of brine-saturated sandstone specimens. Rock Mech. Rock Eng. 2020, 53, 653–670. [Google Scholar] [CrossRef]
- Zheng, H.; Feng, X.-T.; Pan, P.-Z. Experimental investigation of sandstone properties under CO2–NaCl solution-rock interactions. Int. J. Greenh. Gas Control 2015, 37, 451–470. [Google Scholar] [CrossRef]
- Rinehart, A.J.; Dewers, T.A.; Broome, S.T.; Eichhubl, P. Effects of CO2 on mechanical variability and constitutive behavior of the Lower Tuscaloosa Formation, Cranfield Injection Site, USA. Int. J. Greenh. Gas Control 2016, 53, 305–318. [Google Scholar] [CrossRef] [Green Version]
- Marbler, H.; Erickson, K.P.; Schmidt, M.; Lempp, C.; Pöllmann, H. Geomechanical and geochemical effects on sandstones caused by the reaction with supercritical CO2: An experimental approach to in situ conditions in deep geological reservoirs. Environ. Earth Sci. 2013, 69, 1981–1998. [Google Scholar] [CrossRef]
- Hangx, S.; van der Linden, A.; Marcelis, F.; Bauer, A. The effect of CO2 on the mechanical properties of the Captain Sandstone: Geological storage of CO2 at the Goldeneye field (UK). Int. J. Greenh. Gas Control 2013, 19, 609–619. [Google Scholar] [CrossRef]
- Peter, A.; Jin, X.; Sheng, Y.; Fan, X.; Yang, D. Static fatigue of saline rocks under different CO2 phase conditions. J. Pet. Sci. Eng. 2020, 195, 107940. [Google Scholar] [CrossRef]
- Hangx, S.; Bakker, E.; Bertier, P.; Nover, G.; Busch, A. Chemical-mechanical coupling observed for depleted oil reservoirs subjected to long-term CO2-exposure—A case study of the Werkendam natural CO2 analogue field. Earth Planet. Sci. Lett. 2015, 428, 230–242. [Google Scholar] [CrossRef]
- Zhang, Q.; Hu, S.; Li, X.; Shi, L. Preliminary Experimental Study of Effect of CO2-H2O Biphasic Fluid on Mechanical Behavior of Sandstone under True Triaxial Compression. Int. J. Geomech. 2019, 19, 06018036. [Google Scholar] [CrossRef]
- Liteanu, E.; Niemeijer, A.; Spiers, C.J.; Peach, C.J.; De Bresser, J.H.P. The effect of CO2 on creep of wet calcite aggregates. J. Geophys. Res. Earth Surf. 2012, 117, 1–20. [Google Scholar] [CrossRef]
- Grgic, D. Influence of CO2 on the long-term chemomechanical behavior of an oolitic limestone. J. Geophys. Res. Earth Surf. 2011, 116, 1–22. [Google Scholar] [CrossRef]
- Vanorio, T.; Nur, A.; Ebert, Y. Rock physics analysis and time-lapse rock imaging of geochemical effects due to the injection of CO2 into reservoir rocks. Geophysics 2011, 76, O23–O33. [Google Scholar] [CrossRef]
- Zhang, G.; Zhou, D.; Wang, P.; Zhang, K.; Tang, M. Influence of supercritical CO2-water on the micromechanical properties of sandstone. Int. J. Greenh. Gas Control 2020, 97, 103040. [Google Scholar] [CrossRef]
- Zhang, K.; Jia, N.; Liu, L. CO2 storage in fractured nanopores underground: Phase behavior study. Appl. Energy 2019, 238, 911–928. [Google Scholar] [CrossRef]
- Bemer, E.; Lombard, J. From injectivity to integrity studies of CO2 geological storage-chemical alteration effects on carbonates petrophysical and geomechanical properties. Oil Gas Sci. Technol. Rev. Inst. Fr. Pét. 2010, 65, 445–459. [Google Scholar] [CrossRef] [Green Version]
- Sun, L.; Jessen, K.; Tsotsis, T.T. Impact of exposure to brine/CO2 on the mechanical and transport properties of the Mt. Simon Sandstone. Greenh. Gases Sci. Technol. 2021, 11, 1043–1055. [Google Scholar] [CrossRef]
- Muñoz-Ibáñez, A.; Falcon-Suarez, I.H.; Marin-Moreno, H.; Martin, J.D.; Mackin, P. Transport properties of saline CO2 storage reservoirs with unconnected fractures from brine-CO2 flow-through tests. J. Pet. Sci. Eng. 2020, 184, 106551. [Google Scholar] [CrossRef]
- Wang, H.; Alvarado, V.; Bagdonas, D.A.; McLaughlin, J.F.; Kaszuba, J.P.; Grana, D.; Campbell, E.; Ng, K. Effect of CO2-brine-rock reactions on pore architecture and permeability in dolostone: Implications for CO2 storage and EOR. Int. J. Greenh. Gas Control 2021, 107, 103283. [Google Scholar] [CrossRef]
- Olabode, A.; Radonjic, M. Experimental Investigations of Caprock Integrity in CO2 Sequestration. Energy Procedia 2013, 37, 5014–5025. [Google Scholar] [CrossRef] [Green Version]
- Alemu, B.L.; Aagaard, P.; Munz, I.A.; Skurtveit, E. Caprock interaction with CO2: A laboratory study of reactivity of shale with supercritical CO2 and brine. Appl. Geochem. 2011, 26, 1975–1989. [Google Scholar] [CrossRef]
- Makhnenko, R.; Vilarrasa, V.; Mylnikov, D.; Laloui, L. Hydromechanical Aspects of CO2 Breakthrough into Clay-rich Caprock. Energy Procedia 2017, 114, 3219–3228. [Google Scholar] [CrossRef] [Green Version]
- Dávila, G.; Luquot, L.; Soler, J.M.; Cama, J. Interaction between a fractured marl caprock and CO2 -rich sulfate solution under supercritical CO2 conditions. Int. J. Greenh. Gas Control 2016, 48, 105–119. [Google Scholar] [CrossRef] [Green Version]
- Orlic, B. Geomechanical effects of CO 2 storage in depleted gas reservoirs in the Netherlands: Inferences from feasibility studies and comparison with aquifer storage. J. Rock Mech. Geotech. Eng. 2016, 8, 846–859. [Google Scholar] [CrossRef]
- Vilarrasa, V.; Makhnenko, R.Y.; Laloui, L. Potential for fault reactivation due to CO2 injection in a semi-closed saline aquifer. Energy Procedia 2017, 114, 3282–3290. [Google Scholar] [CrossRef] [Green Version]
- Olabode, A.; Radonjic, M. Geochemical Markers in Shale-CO2 Experiment at Core Scale. Energy Procedia 2017, 114, 3840–3854. [Google Scholar] [CrossRef]
- Blunt, M.J.; Bijeljic, B.; Dong, H.; Gharbi, O.; Iglauer, S.; Mostaghimi, P.; Pentland, C. Pore-scale imaging and modelling. Adv. Water Resour. 2013, 51, 197–216. [Google Scholar] [CrossRef] [Green Version]
- Zou, Y.; Li, S.; Ma, X.; Zhang, S.; Li, N.; Chen, M. Effects of CO2-brine-rock interaction on porosity/permeability and mechanical properties during supercritical-CO2 fracturing in shale reservoirs. J. Nat. Gas Sci. Eng. 2018, 49, 157–168. [Google Scholar] [CrossRef]
- Luquot, L.; Gouze, P. Experimental determination of porosity and permeability changes induced by injection of CO2 into carbonate rocks. Chem. Geol. 2009, 265, 148–159. [Google Scholar] [CrossRef]
- Yu, Z.; Yang, S.; Liu, K.; Zhuo, Q.; Yang, L. An Experimental and Numerical Study of CO2-Brine-Synthetic Sandstone Interactions under High-Pressure (P)—Temperature (T) Reservoir Conditions. Appl. Sci. 2019, 9, 3354. [Google Scholar] [CrossRef] [Green Version]
- Zhu, H.; Xu, T.; Tian, H.; Feng, G.; Yang, Z.; Zhou, B. Understanding of Long-Term CO2-Brine-Rock Geochemical Reactions Using Numerical Modeling and Natural Analogue Study. Geofluids 2019, 2019, 1426061. [Google Scholar] [CrossRef] [Green Version]
- Bemer, E.; Nguyen, M.T.; Dautriat, J.; Adelinet, M.; Fleury, M.; Youssef, S. Impact of chemical alteration on the poromechanical properties of carbonate rocks. Geophys. Prospect. 2016, 64, 810–827. [Google Scholar] [CrossRef]
- Luquot, L.; Gouze, P.; Niemi, A.; Bensabat, J.; Carrera, J. CO2-Rich brine percolation experiments through Heletz reservoir rock samples (Israel): Role of the flow rate and brine composition. Int. J. Greenh. Gas Control 2016, 48, 44–58. [Google Scholar] [CrossRef]
- Chen, G.; Gao, H.; Fu, K.; Zhang, H.; Liang, Z.; Tontiwachwuthikul, P. An improved correlation to determine minimum miscibility pressure of CO2—Oil system. Green Energy Environ. 2020, 5, 97–104. [Google Scholar] [CrossRef]
- Yusof, M.A.M.; Neuyam, Y.A.S.; Ibrahim, M.A.; Saaid, I.M.; Idris, A.K.; Mohamed, M.A. Experimental study of CO2 injectivity impairment in sandstone due to salt precipitation and fines migration. J. Pet. Explor. Prod. Technol. 2022, 1–12. [Google Scholar] [CrossRef]
- Goral, J.; Andrew, M.; Olson, T.; Deo, M. Correlative core- to pore-scale imaging of shales. Mar. Pet. Geol. 2020, 111, 886–904. [Google Scholar] [CrossRef]
- Mehmani, A.; Verma, R.; Prodanović, M. Pore-scale modeling of carbonates. Mar. Pet. Geol. 2020, 114, 104141. [Google Scholar] [CrossRef]
- Iassonov, P.; Gebrenegus, T.; Tuller, M. Segmentation of X-ray computed tomography images of porous materials: A crucial step for characterization and quantitative analysis of pore structures. Water Resour. Res. 2009, 45, 1–12. [Google Scholar] [CrossRef]
- Plug, W.-J.; Bruining, J. Capillary pressure for the sand-CO2-water system under various pressure conditions. Application to CO2 sequestration. Adv. Water Resour. 2007, 30, 2339–2353. [Google Scholar] [CrossRef]
- Bachu, S. Sequestration of CO2 in geological media: Criteria and approach for site selection in response to climate change. Energy Convers. Manag. 2000, 41, 953–970. [Google Scholar] [CrossRef]
- Holloway, S.; Vincent, C.J.; Bentham, M.S.; Kirk, K.L. Top-down and bottom-up estimates of CO2 storage capacity in the United Kingdom sector of the southern North Sea basin. Environ. Geosci. 2006, 13, 71–84. [Google Scholar] [CrossRef]
- Buscheck, T.A.; Mansoor, K.; Yang, X.; Wainwright, H.M.; Carroll, S.A. Downhole pressure and chemical monitoring for CO2 and brine leak detection in aquifers above a CO2 storage reservoir. Int. J. Greenh. Gas Control 2019, 91, 102812. [Google Scholar] [CrossRef]
- Ennis-King, J.; Paterson, L. Rate of dissolution due to convective mixing in the underground storage of carbon dioxide. In Proceedings of the Greenhouse Gas Control Technologies-6th International Conference, 1–4 October 2002; Kyoto, Japan; Elsevier: Amsterdam, The Netherlands, 2002. [Google Scholar]
- Chadwick, A.; Arts, R.; Bernstone, C.; May, F.; Thibeau, S.; Zweigel, P. Best Practice for the Storage of CO2 in Saline Aquifers-Observations and Guidelines from the SACS and CO2STORE Projects; British Geological Survey: Nottingham, UK, 2008; Volume 14.

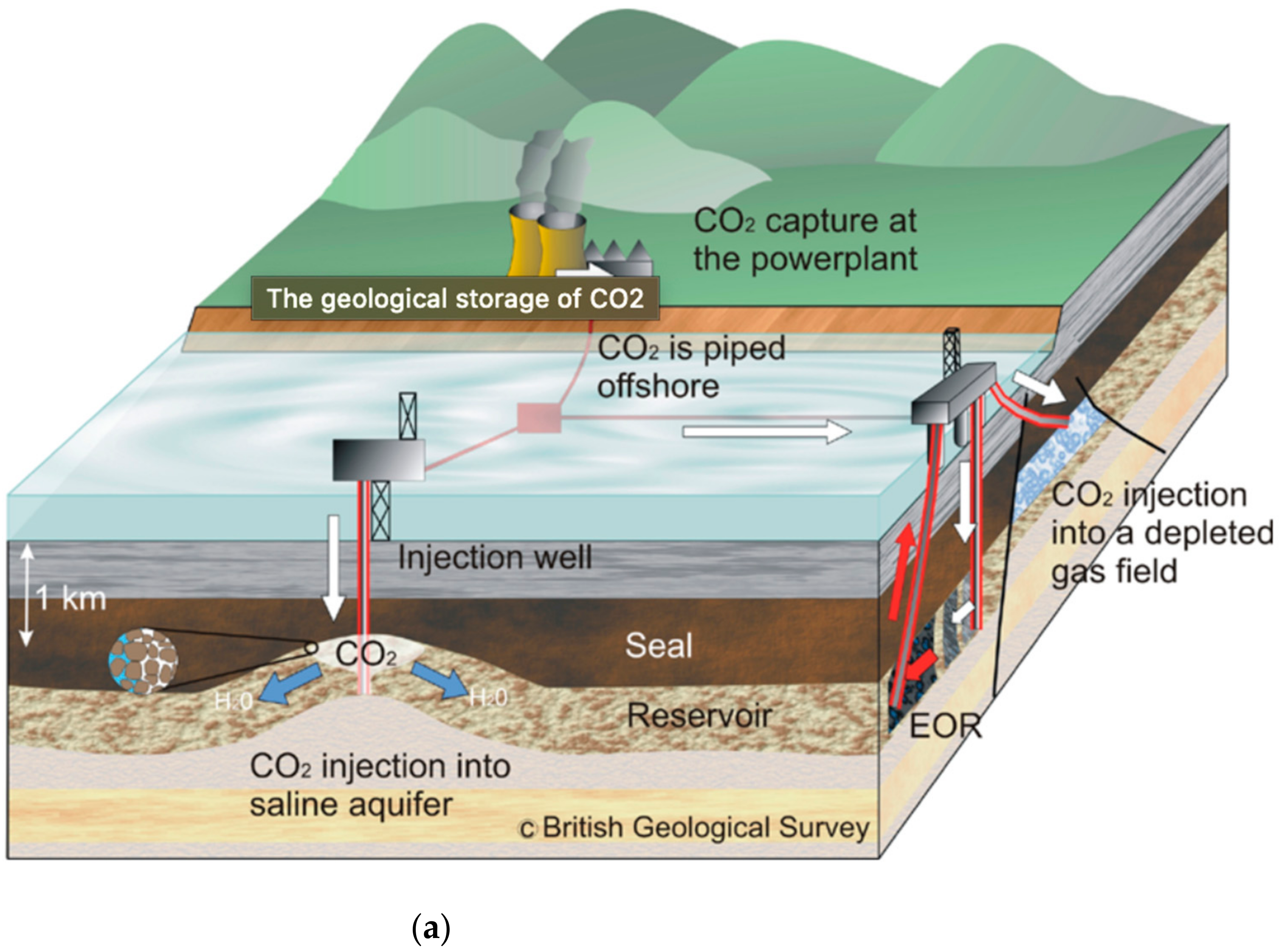

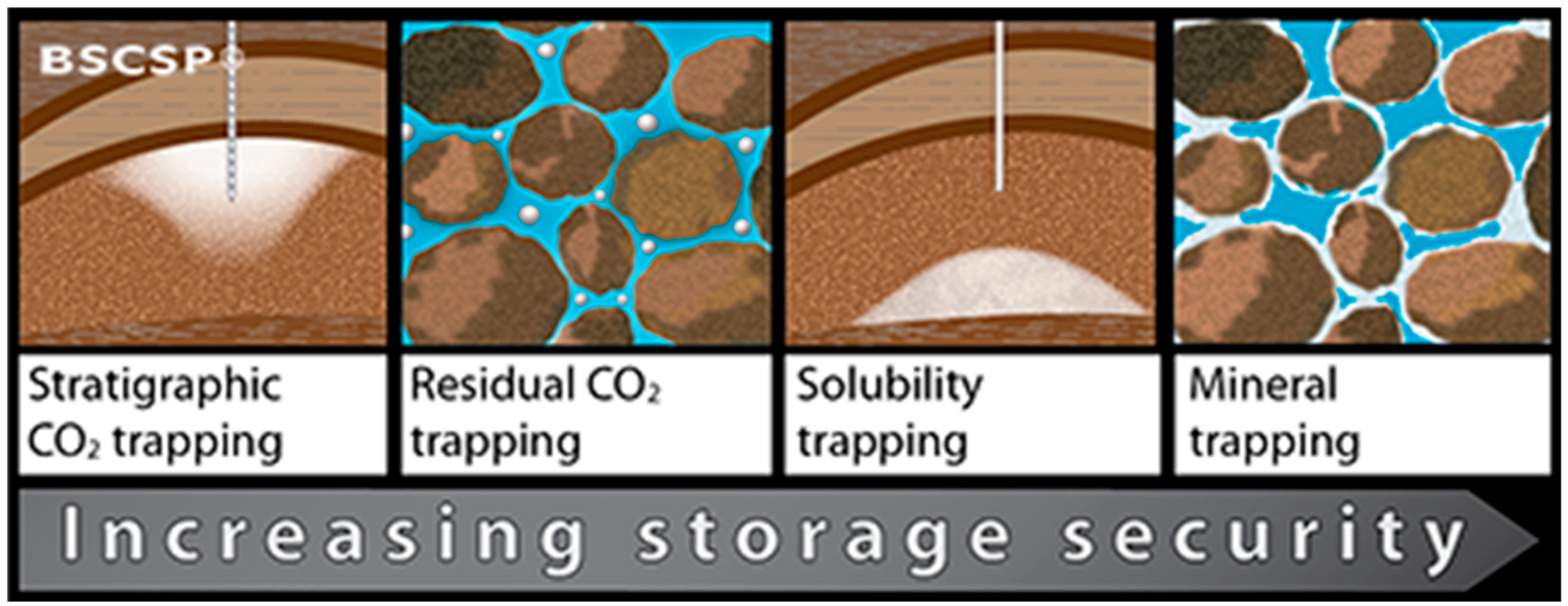
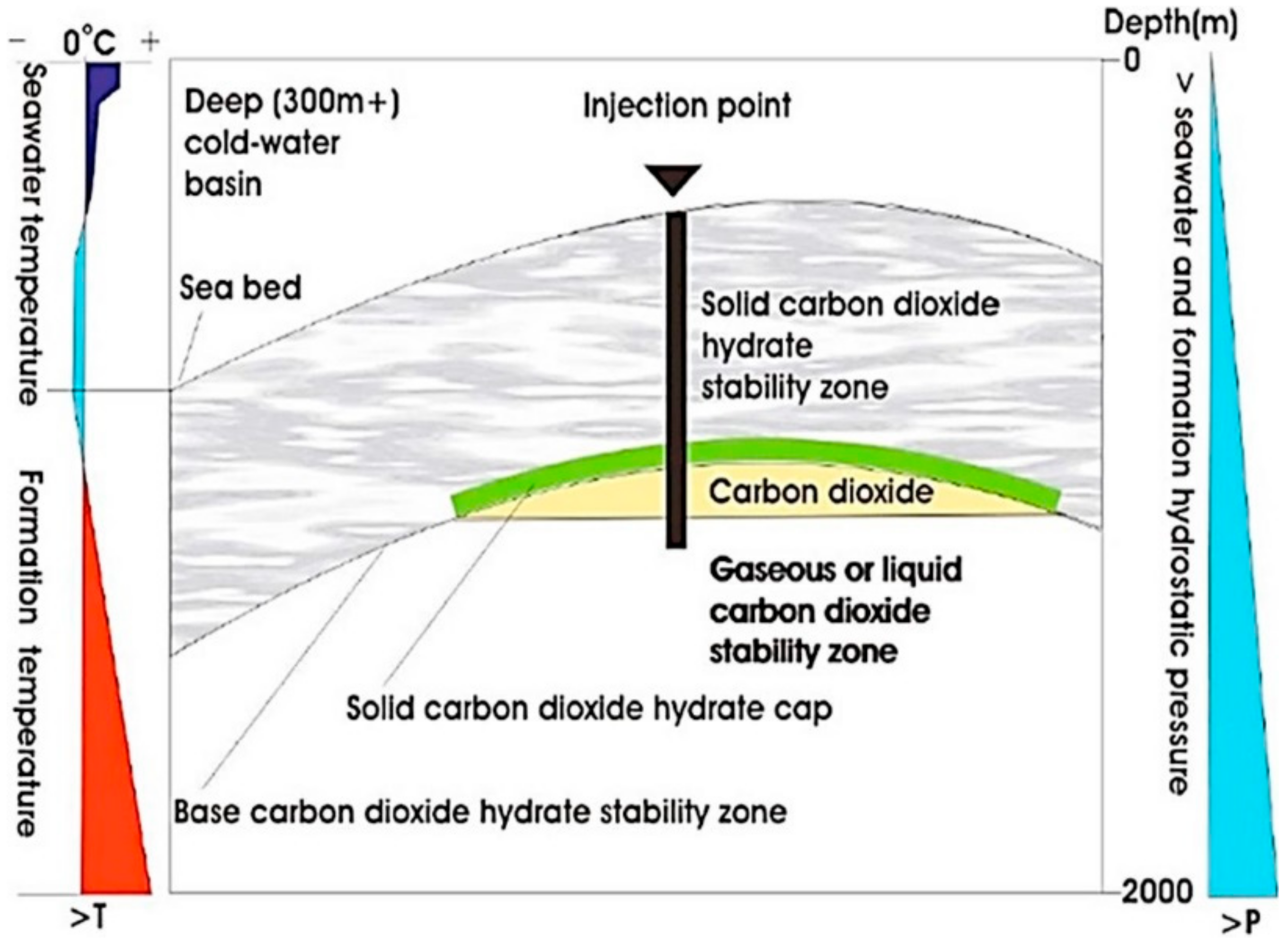
| Storage Option | Capacity | Source |
|---|---|---|
| Oil and gas fields | 675–900 Gt-C | [11,21] |
| Unmineable coal seams (This has been abandoned) | 3–200 Gt-C | [11,35] |
| Deep saline fields | >1000 Gt-C | [11] |
| Mafic and ultramafic rocks | 60,000,000 Gt-C | [14] |
| Cavern storage | - | - |
| a. Density, Solubility, and Viscosity of CO2 | ||
| Authors | CO2-brine state | Findings |
| Jeon and Lee [50] | gCO2, ScCO2 and brine | Studied the effect of viscosity ratio and interfacial tension using unsteady-state relative permeability experiments. Viscosity ratio is the ratio of the viscosity of the solution to the solvent, whereas interfacial tension is the force of attraction between molecules at the interface of two fluids. A dual high-pressure separator was used to measure the fluid saturation. They found a high residual brine saturation of the two-phase CO2–brine system and showed that relative permeability depends on the viscosity ratio and interfacial tension of CO2–brine. |
| Chabab et al. [51] | gCO2 and brine | Extended the Soriede and Whitson model to develop a model that predicts the water content and solubilities of CO2 and other gases in different types of brine over a range of temperature and pressure. This developed model has been successful in predicting bubble point pressure and gas emissions by comparing its result with data from geothermal power plants. |
| Li et al. [52] | gCO2 and brine | Found that an increase in salinity reduces the solubility of CO2 in brine in nanopores |
| Lara Cruz et al. [53] | Aqueous CO2 and mixture of CaCl2 and NaCl | Showed the solubility of CO2 in NaCl and CaCl2 brines at pressures up to 40 Mpa and a temperature range of 333.15–453.15 K. Their results showed that the solubility of CO2 decreases as the aqueous phase salinity increases. |
| Enick and Klara [54], Song et al. [55] | gCO2 and and Water or brine | Showed that the solubility of CO2 varies with temperature, pressure, and composition of the brine, the correlations show a wide scatter in each case but there was a decrease in solubility of CO2 in brine at a temperature range of 298 to 523 K and pressure range of 3.0 to 85.0 MPa. |
| Jamshidi et al. [56] | gCO2 and brine, and gCO 2 and heavy oil | Showed that the solubility of CO2 in heavy oil increased as pressure increased and as temperature decreased. |
| Chebab et al. [57] | - | The static analytic method was used to measure the solubility of CO2 and model phase equilibria. The Peng–Robinson Cubic Plus Association (PR-CPA) model which uses cubic equations of state (EOS) for determining the properties of fluid was extended to electrolyte-CPA and the Soriede and Whitson model for determining the properties of petroleum fraction was improved. Duan model in software was tested. The improved models were tested with a wide range of temperature, pressure, and molality. The new models were validated against data in the literature and performed well. |
| Hajiw et al. [58] | gCO2 and pure water | Evaluated the impact of impurities from flue gases on the solubility of CO2 in water by vapor-liquid equilibria (VLE) calculations using a geochemical model and thermodynamic models such as the Group Contribution-Peng–Robinson-Cubic Plus Association (GC-PR-CPA) and the Enhanced Predictive Peng–Robinson (E-PPR78) equation of state models. These impurities were noted to increase the density, viscosity and alter the behavior of CO2. The GC-PR-CPA and geochemical model give results agreeable with literature data, but, this was dependent on the availability and quality of data |
| Ali Ahmadi and Ahmadi [59] | CO2 and brine | Used the least square support vector machine (LS-SVM) to predict the solubility of CO2 in brine and showed that solubility of CO2 increases with decreasing temperature. The result from the LS-SVM method proved to be more reliable robust and compatible than other conventional methods such as Whiteson and modified Whitson methods under certain conditions. |
| Jacob and Saylor [60], Ratnakar et al. [61] | gCO2 and NaCl, CaCl2, KCl and a mixture of all the brine | Showed that the ionic composition of brine affects the solubility of CO2. Solubility was shown to decrease with an increase in salt content in single component brines, whereas solubility in multi-component brines was shown to depend on the salt present. |
| Mohammadian et al. [62] | gCO2 and NaCl brine | Presented solubility data of CO2 in brine for a salinity range of 0–15,000 ppm, temperature range of 60–100 °C, and pressure up to 25 MPa. Measurement was carried out using the potentiometric titration method. An increase in pressure caused increased solubility of CO2 in distilled water and brine and vice versa for an increase in temperature. An increase in salinity reduces solubility. The reduction in the solubility is by about 13% as the salinity increases by 1.5% from an initial state of 0. The solubility obtained was consistent with those obtained from other methods. |
| Lamy-Chappuis [63] | gCO2 and brine | Provided estimates of the changes in density, and viscosity of CO2 at depths >800 m. Density and viscosity of brine are controlled by temperature, pressure, and salinity of the brine, and affect the convection current and rate of dissolution. CO2 density was found to be about 20% higher at injection pressure than at hydrostatic pressure below 800 m. 1 M and 5 M NaCl-brine were used, the addition of salt to water was seen to result in a 10% increase in density and an 80% increase in viscosity. |
| Yan et al. [64] | gCO2 and NH4Cl or NaHPO4 brine | Presented solubility data of CO2 in brine and water under different temperatures, pressure, and salinity. The result showed that the dissolution of CO2 increases the brine density if the mass density of CO2 in brine is higher than the density under the same conditions. At high salinity and temperature, the dissolution of CO2 decreases the brine density |
| b. Density and Viscosity of Brine | ||
| Tatar et al. [65] | - | Used radial basis function neural networks and genetic algorithm to predict the density of brine |
| Mao and Duan [66] | - | Developed a temperature, pressure, and salt content (P, V, T, x) model for calculating the density and viscosity of brine under varying temperature, pressure, and salinity conditions. This model compares well with previous experimental data with an average deviation of only 0.020% to 0.066% in density |
| c. Solubility, Density, and Viscosity of CO2-Saturated Brine | ||
| Li et al. [52]. | gCO2–brine | Investigated the impact of pH on solubility of CO2 in brine and found that the solubility of CO2 in brine reduces as the pH increases |
| Teng et al. [67]. | CO2 saturated brine and only brine | Showed that an increase in viscosity contrast between CO2 and brine hinders density-driven convection and slows down the rate of solubility |
| Mahmoodpour et al. [68] | gCO2 and NaCl or mixture of NaCL and CaCl2 | Studied the effect of brine composition on the onset of convection. They showed the onset of convection for a brine solution containing NaCl occurs earlier and with a higher wavenumber, whereas a mixture of NaCl and CaCl2 results in a late-onset of convection and a higher CO2 diffusion coefficient. This implies that the onset of instabilities and fingering in a multi-ion brine is delayed compared with a single ion brine |
| Islam et al. [69] | gCO2 and brine | Showed that the convection current is a hydrodynamic process that promotes the mixing and dissolution of CO2 in brine |
| Liu et al. [70]; Mosavat and Torabi [71]. | CO2 and NaCl, CaCl2, KCl and a mixture of all the brine | Shows that an increase in pressure leads to an increase in the solubility of CO2, whereas an increase in temperature leads to a decrease in solubility of CO2 in brine |
| Duan et al. [72], Mao et al. [73]; Ahmad et al. [74]. | gCO2 and water or CO2-H2O-NaCl | Reported an increase in density and a decrease in the buoyancy of CO2 when it dissolves in brine |
| d. Relative Permeability, Capillary Pressure, and Fingering | ||
| Jeong et al. [75] | gCO2 and brine | Found that the endpoint permeability of CO2 increases as the residual brine saturation decreases and as the flow rate increases |
| Abdoulghafour et al. [76] | Liquid CO2–brine | Reported that low capillary pressure promotes high residual CO2 saturation and improved capillary trapping |
| Basirat et al. [77] | scCO2 and gCO2 with N2 and CH4 impurities. | Posited that the wetting condition of the reservoir affects the relative permeability, CO2 breakthrough, and saturation. The wetting condition is the fluid that surrounds the grains and fills the pores, saline rocks are water wet. Strongly water-wet rock was seen to significantly reduce the relative permeability of CO2 This provides a suitable condition for dissolution trapping due to an increase in the interfacial angle between CO2 and brine. Water wet conditions also enhanced the capillary effect, which is helpful for residual trapping. A decrease in water-wet conditions increased the saturation of the wetting phase and interfacial area. The results showed no clear relationship between breakthrough, saturation, and wetting condition |
| Sidiq et al. [78]. | gCO2 | Showed that the capillarity end effect in measuring relative permeability can be minimized by using longer cores |
| Jeong et al. [79] | gCO2 and brine | Reported that relative permeability is a function of viscous force and injection rates |
| Ajibola et al. [80]. | gCO2 and water | Showed that difference in density and vertical permeability had great control on fingering |
| Shukla and De Wit [81] | - | Showed that fingering can also be caused by a change in mobility due to precipitation reaction decreasing the permeability of the medium |
| Al-Menhali et al. [82]; Jung and Hu [83]. | Liquid and supercritical CO2 | Showed the impact of reservoir conditions such as pressure, temperature, and salinity on the capillary strength and interfacial tension. At a given salinity, increasing the temperature and the transition from liquid to supercritical CO2, there was a small weakening of the capillary strength and a small increase in interfacial tension. With an increase in pressure, and pressure range within the gaseous, low-density supercritical, and high-density supercritical phase, the interfacial tension between the fluids decreased. With an increase in temperature and the temperature range within the liquid or supercritical phase, the interfacial tension increases. With an increase in salinity, and at constant temperature and pressure, the interfacial tension increased |
| Reynolds and Krevor [84] | gCO2, brine and N2-water | Showed that reservoir conditions have little impact on relative permeability and residual trapping. They further showed that relative permeability is sensitive to capillary heterogeneity in the rock. With capillary heterogeneity in the rock, capillary-driven flow redistributed fluid. The effective relative permeability curves were seen to be sensitive to pressure, temperature and brine salinity, and flow rate. At a constant flow rate, the relative permeability minimized the capillary end effects |
| e. Multiphase Flow of CO2–brine | ||
| Krevor et al. [85] | gCO2 and NaCl | Showed that reservoir heterogeneity has little impact on the multiphase flow of CO2–brine in the reservoir |
| Saeedi et al. [86] | - | Found that flooding cycles affect the multiphase flow characteristics in the CO2–brine system. The flooding cycle is the alternating CO2–brine injection or periodic CO2 injection. The effect of cyclic flooding on saturation is minimal but strongly influences differential pressures across the medium. These effects are due to capillary hysteresis, the reaction between the solute and host rock, stress, and changes to the reservoir due to CO2 or alternating CO2–brine injection |
| Kuo et al. [87] | gCO2 and brine | Displayed the effect of viscous, capillary, and gravity forces on displacement efficiency. They showed that when injection rates are large enough, the flow is dominated by viscous forces, but when the injection rate is low, the flow is dominated by capillary forces. Gravity forces are negligible |
| f. CO2 Injection and Long Term Evolution | ||
| Vilarrasa et al. [88] | gCO2 | Observed that CO2 reaches the bottom of injection wells at a colder temperature. This cooling and overpressure tend to enhance injectivity |
| Pruess and Nordbotten [89] | gCO2 | Reported that the process of long-term CO2 plume advancement differs from that of forced immiscible displacement. Instead of the fluid being pushed forward, the fluid collapses ahead of the plume tip. This is because the vertical pressure gradient in the plume is smaller than the hydrostatic pressure |
| Xu et al. [90]; Whittaker et al. [91] | gCO2 | Found a significant drop in the pH of brine over time; observed that CO2 plume expands gradually due to capillary forces and that gas saturation gradually decreases due to its dissolution and the precipitation of Carbonates. The gas-phase was predicted to disappear after 500 years |
| Author | State of CO2 | Pore Fluid | Rock Type | Contributions |
|---|---|---|---|---|
| a. Effect of CO2–brine on the chemical composition of reservoir rocks | ||||
| Peter et al. [94] | scCO2, gCO2 | NaCl brine | Sandstone | These researches show that CO2–brine–rock processes affect the geochemical and geomechanical properties of the rocks through dissolution, precipitation, or stress corrosion. In sandstone, porosity increased significantly and there was a reduction in fracture toughness by clay-cement weakening. There was a reduction in bulk modulus and strength and an increase in the rate of deformation, there was a loss of clays. In Carbonates, there was dissoution of calcite. Precipitation of clays was also reported. In shales, porosity decreased due to the precipitation of minerals and there was a reduced risk of induced fractures |
| Xiao et al. [2] | CO2 (aq) | Samples of limestone and shale from Farnsworth unit CO2-EOR and GCS demonstration site | Limestone | |
| Valle et al. [3] | scCO2 | - | Carbonate rock | |
| Xiao et al. [2], Valle et al. [3], Nguyen [4], Fuchs et al. [5] | scCO2 | For 5, a Solution of NaCl, CaCl2, KCl, KBr, LiCl, SrCl2, and Borax | Siliciclastic rock | |
| Pimienta et al. [95] | scCO2 | - | Carbonate rock | Found that dissolution of minerals in CO2–brine increased with residence time |
| Davila et al. [96] | scCO2 | A synthetic mixture of different salts referred to as IBDP-1 and IBDP-2 | Sandstone | Reported a fast consumption of silicates that indicates the immediate influence of geochemical alteration on the transmissivity and structure of the rock, they observed that the alteration of the core occurred mostly along the inlet. |
| b. Effect of CO2–brine on the petro-physical properties of reservoir rocks | ||||
| Han et al. [97] | - | Decane and distilled H2O | Carbonate rocks | Concluded that CO2 flooding in Carbonate reservoirs can significantly alter pore network, causing an increase in non-connected pores and a reduction in permeability |
| Lamy-Chappuis et al. [98] | scCO2 | NaCl brine | Calcite-rich sandstone | Reported that a 10% increase in porosity resulted in a 10% decrease in sonic velocity in calcite-rich Cayton bay sandstone saturated with gaseous CO2–brine |
| Garcia Rios et al. [99] | scCO2 | Solution of CaCl2·2H2O, MgCl2·6H2O, NaCl, KCl, Na2SO4, and NaBr with and without sulfate | Fractured Limestone | Opined that CO2–brine reaction occurs mostly in the fracture which serves as flow paths and they observed that fracture permeability increased depending on the dissolution pattern |
| Grombacher et al. [100] | CO2(aq) | H2O | Carbonate rocks | Showed change in microstructure due to change in the pore space and dissolution in grain coating cement and formation of cracks around larger grains, these resulted in a reduction in acoustic velocity in Carbonate rocks exposed to CO2-rich brine |
| Vialle and Vanorio [101] | CO2(aq) | H2O | Carbonate rock | Observed the damping of S and P-wave velocities due to the effect of reactive CO2–brine, and concluded that the reduction in velocities was connected to an increase in porosity and permeability of the rock and deformation of micro-fabric |
| Lei and Xue [102] | gCO2, lCO2, scCO2 | Distilled H2O | Sandstones | Reported that P-velocity and rock strength reduced more in sandstone saturated with supercritical CO2 in comparison to those saturated with gaseous and liquid CO2 |
| c. Effect of CO2–brine on the geomechanical properties of reservoir rocks | ||||
| Ilgen et al. [92], Rutqvist [93] | - | - | Sandstone | An increase in pore pressure and decrease in temperature due to CO2–brine led to an increase in stress, and the new stress regime triggered changes in shear, bulk, and elastic moduli, and a reduction in strength, scratch toughness, hardness, permeability, and porosity. There was an enlargement of macropores, increase in porosity, and dissolution of smaller particles. Removal of the mineral mass led to microcracking and compaction that subsequently affected the properties of the rock. Dissolution of intergranular cement and mineral precipitation led to a coupled chemical-mechanical response |
| Espinoza et al. [103] | Natural CO2 field | Natural rock as sampled, no synthetic brine | Sandstone | Studied the effect of CO2–brine on the strength of rocks and reported a reduction in the strength |
| Pimienta et al. [95] | scCO2 | Sodium Iodide (NaI) solution | Calcite-rich rocks | |
| Delle and Sarout [104] | scCO2 | H2O and dry | Berea sandstone | |
| Zheng et al. [105] | CO2 (aq) | NaCl brine | Sandstone | |
| Rinehart et al. [106] | scCO2 | Solution of Ca(NO3)·2.4H2O, NaNO3, MgCO3, and deionized H2O | Sandstone | Showed that degradation in the elastic moduli, strength, and porosity of the rocks depends on the mineral composition of the rocks |
| Marbler et al. [107] | scCO2 | Formation of H2O of the North German Basin | Sandstone | |
| Hangx et al. [108] | scCO2 | Solution of NaCl, Mg2Cl, KCl, CaCl2, CaCO3, and distilled H2O | Sandstone | |
| Peter et al. [109] | scCO2 and gCO2 | NaCl brine | Sandstone | They agreed that the effect of CO2–brine on the bulk modulus and rock deformation is affected by the pore fluid, mineralogy, phase of CO2, and effective pressure. scCO2 induced a greater change in bulk modulus and strength compared with the gaseous CO2. Carbonate rocks had a greater change in bulk modulus, strength, and porosity compared with siliciclastic rocks |
| Hangx et al. [110] | Natural CO2 field | Natural rock as sampled, with no synthetic brine | Sandstone | |
| Hangx et al. [111] | scCO2 | Solution of NaCl, Mg2Cl, KCl, CaCl2, CaCO3, and distilled H2O | Sandstone | |
| Liteanu et al. [112] | scCO2 | Boiled distilled H2O | Calcite-rich rock | |
| Grgic [113] | scCO2 and gCO2 | Solution of deionized H2O and limestone powder | Carbonate rocks | |
| Han et al. [97] | - | Decane and distilled H2O | Carbonate rock | Showed that injecting CO2 into brine-rock system-induced chemo-mechanical processes that reduce the strength of the rock and the induced precipitation led to the closing of pores and micro-fracture, whereas dissolution and pore fluid pressure expands the pores |
| Delle and Sarout [1] | scCO2 | H2O and Dry | Berea sandstone | |
| Rutqvist [93] | - | - | Sandstone | |
| Vanorio et al. [114]. | scCO2, CO2 (aq) | H2O and Brine | Sandstone | |
| Zhang et al. [115] | scCO2 | H2O | Sandstone | These researchers reported that CO2–brine reaction led to a reduction in the strength, bulk, elastic modulus, and permeability of rocks. There was a difference in the number of changes in these properties and these differences are due to differences in the mineral composition of the rocks, phase of CO2, and the Physico-chemical conditions |
| Zhang et al. [116] | CO2 (aq) | N2 or C1–10 | Sandstone | |
| Lamy-Chappuis et al. [98] | scCO2 | NaCl | Calcite-rich sandstone | |
| Grombacher et al. [100] | CO2 (aq) | H2O | Carbonate rocks | |
| Bemer and Lombard [117] | - | - | Carbonate rocks | |
| d. Effect of CO2–brine on the transport properties of reservoir rocks | ||||
| Sun and Jessen [118] | - | - | Sandstone | Permeability and porosity of sandstones increased due to brine/CO2 |
| Munoz-Ibanez et al. [119] | LCO2 | NaCl brine | Synthetic Sandstone | There was enhanced trapping of CO2 in poorly connected fractured reservoirs. |
| Wang et al. [120] | - | Multi-ion brine containing SO4, Cl, Na, K, Mg, Ca, | Limestone | Flow and transport properties in rocks with carbonic acid differ from those without carbonic acid. Permeability is seen to increase in the former. This is due to changes in pore body and throat sizes. The viscosity of the brine increases due to dissolution and precipitation. |
| Jeon and Lee [50] | ScCO2 | NaCl, deionized H2O, and AOS | Sandstone | They found a high residual brine saturation of the two-phase CO2–brine system and showed that relative permeability depends on the viscosity ratio and interfacial tension of CO2–brine. |
| Teng et al. [67] | - | MnCl2 in deionized H2O and D2O in deionized H2O | Porous media | Showed that an increase in viscosity contrast between CO2 and brine hinders density-driven convection and slows down the rate of solubility |
| Author | State of CO2 | Pore Fluid | Rock Type | Contributions |
|---|---|---|---|---|
| Xiao et al. [2] | CO2 (aq) | Sample from a CO2-EOR and GCS demonstration site | Shale | CO2–brine–rock processes affect the geochemical and geomechanical properties of the rocks through dissolution, precipitation, or stress corrosion. Precipitation of clays was also reported. In shales, porosity decreased due to the precipitation of minerals and there was a reduced risk of induced fractures |
| Olabode and Radonjic [121] | CO2 (aq) | - | Shale | The geochemical reactivity of acidic fluid contained in an interconnected pore network of shale triggers a slow reactive process that alters the properties of the rock. They linked the changes in the properties to changes in pore surface area and pore distribution. Induced mineral dissolution or precipitation led to the closing of pores and micro-fracture networks. |
| Alemu et al. [122] | scCO2 | NaCl | Clay-rich and Carbonate-rich shale | Observed higher dissolution of metals into CO2–brine compared with the brine. More metals are dissolved in the Carbonate rich shale |
| Ilgen et al. [92], Rutqvist [93]) | - | - | Mudrock | An increase in pore pressure and a decrease in temperature due to CO2–brine led to an increase in stress, and the new stress regime triggered changes in shear, bulk, and elastic moduli, reduction in strength, scratch toughness, and hardness as well as permeability and porosity. |
| Espinoza et al. [103] | Natural CO2 field | Natural rock sample, with no synthetic brine | Shale | CO2–brine caused a reduction in the strength of rocks |
| Makhnenko et al. [123] | LCO2, scCO2 | - | Shale | Reported that CO2–brine reaction led to a reduction in the strength, bulk, elastic modulus, and permeability of rocks |
| Davila et al. [124] | scCO2 | NaCl with sulfate-rich H2O, calcite, and gypsum | Marl | Observing that the composition of the brine affects the fracture permeability of fractured caprock, they reported a dissolution of calcite and precipitation of gypsum forming a framework. |
| Jeon and Lee [50] | ScCO2 | NaCl, deionized H2O, and AOS | Mudstone | Found a high residual brine saturation of the two-phase CO2–brine system and showed that relative permeability depends on the viscosity ratio and interfacial tension of CO2–brine. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Peter, A.; Yang, D.; Eshiet, K.I.-I.I.; Sheng, Y. A Review of the Studies on CO2–Brine–Rock Interaction in Geological Storage Process. Geosciences 2022, 12, 168. https://doi.org/10.3390/geosciences12040168
Peter A, Yang D, Eshiet KI-II, Sheng Y. A Review of the Studies on CO2–Brine–Rock Interaction in Geological Storage Process. Geosciences. 2022; 12(4):168. https://doi.org/10.3390/geosciences12040168
Chicago/Turabian StylePeter, Ameh, Dongmin Yang, Kenneth Imo-Imo Israel Eshiet, and Yong Sheng. 2022. "A Review of the Studies on CO2–Brine–Rock Interaction in Geological Storage Process" Geosciences 12, no. 4: 168. https://doi.org/10.3390/geosciences12040168
APA StylePeter, A., Yang, D., Eshiet, K. I.-I. I., & Sheng, Y. (2022). A Review of the Studies on CO2–Brine–Rock Interaction in Geological Storage Process. Geosciences, 12(4), 168. https://doi.org/10.3390/geosciences12040168








