Abstract
Large vertebrate carcasses contain significant amounts of nutrients that upon death are transferred from the water column to the benthos, enriching the immediate environment. The organisms exploiting these ephemeral resources vary as the carcass decays, creating an ecological succession: mobile scavengers arrive first, followed by enrichment opportunists, sulfophilic taxa, and lastly reef species encrusting the exposed bones. Such communities have been postulated to subsist on the carcasses of Mesozoic marine vertebrates, but are rarely documented in the Jurassic. In particular, these communities are virtually unknown from the Early Jurassic, despite the occurrence of several productive fossil Lagerstätte that have produced thousands of vertebrate bones and skeletons. We review published occurrences and present new findings related to the development of deadfall communities in the Toarcian Posidonienschiefer Formation of southwestern Germany, focusing on the classic locality of Holzmaden. We report the presence of the mobile scavenger, enrichment opportunist, and reef stages, and found potential evidence for the poorly documented sulfophilic stage. Although rare in the Posidonienschiefer Formation, such communities do occur in association with exceptionally preserved vertebrate specimens, complementing a growing body of evidence that a temporarily oxygenated benthic environment does not preclude exceptional vertebrate fossil preservation.
1. Introduction
1.1. Background
Large vertebrate carcasses have a significant influence on the local marine demersal and benthic macrofauna. In modern oceans, pelagic deadfalls host a highly endemic invertebrate fauna and form islands of amplified abundance and diversity of nonendemic species [1,2]. The organisms exploiting these ephemeral resources vary as the carcass decays, creating an ecological succession: mobile scavengers (necrophages) arrive first, followed by enrichment opportunists, sulfophilic taxa, and lastly reef species encrusting the exposed bones [1]. The progressive changes in the fall community are directly linked to the amount of resources that the carcass offers during decay [3]: mobile scavengers (e.g., sharks, myxines, isopods, and cephalopods) rapidly remove soft tissues, enrichment opportunists (e.g., hesionid and ampharetid polychaetes, ophiuroids, and amphipods) thrive on the surrounding organically enriched sediments and microbial mats, and finally sulfide produced during the decomposition of lipids by microorganisms is oxidized by bacteria, fungi, and endosymbionts of mollusks and polychaetes [3,4,5]. The reef stage is generally thought to occur subsequent to all nutrient depletion of the deadfall; however, this phase has not been directly observed in recent deadfalls [6]. The presence or absence and degree of distinctness between these successional stages is variable, seemingly correlated with carcass taxonomy, ontogeny, size, and depositional environment [3,7,8]. Such communities have also been postulated to subsist on the carcasses of Mesozoic marine reptiles [9,10] and empirical work has provided documentary evidence thereof [11]. However, key differences between recent and early to mid-Mesozoic deadfalls appear to exist; for instance, neither sulfophilic macroinvertebrates, nor traces attributable to the bone-eating siboglinid worm Osedax, have been documented prior to the Cretaceous [12,13]. The evolution of deadfall communities in deep time is still poorly understood.
A few Jurassic deadfall communities have been described (reviewed in Table 1), but successional stages are often difficult to assess. Hexanchid shark and ichthyosaur teeth preserved in association with marine reptile carcasses have been interpreted as representing the mobile scavenger stage [14,15]. Ophiuroids were reported as associated with the enrichment opportunist stage in Lower and Upper Jurassic marine reptile carcasses [16,17]; oysters and serpulid worms have been documented as important components of the reef stage on many marine reptile carcasses spanning the Jurassic; e.g., [16,18,19]. The most complete Jurassic fall succession was reported in [11] on an Upper Jurassic ichthyosaur carcass. Bite marks on the bone by saprophagic fishes were recognized as evidence of the mobile scavenger stage; an enrichment opportunist stage was documented by echinoid grazing traces (most likely on microbial mats); and deposit-feeding gastropods and epibenthic bivalves were interpreted as profiting from increased organic matter flux in the immediate area. Pyrite framboids in the bone spongiosa, associated with microborings on the cortical bone, were reported as evidence of microbial activity, possibly involving sulfate-reducing reactions and therefore assignable to the sulfophilic stage. The reef stage was represented by serpulids, oysters, and plicatulids [11]. Although the record of such deadfall associations spans the Jurassic, most of the occurrences are concentrated in the Late Jurassic, with few documented Early Jurassic communities.

Table 1.
Review of previously published Jurassic deadfall communities.
The Lower Jurassic (Toarcian) Posidonienschiefer Formation (Posidonia Shale) of southwestern Germany (Figure 1) is known for its exceptional preservation of nektonic organisms. Despite the exceptional fossil record, deadfall communities have seldom been documented, and decomposition of the carcasses of large vertebrates has been assumed to proceed primarily via microbial action [20]. The typical absence of deadfall communities and general differences in abundance between the benthos and nekton have been used to argue for dysoxic, euxinic bottom-water conditions, inhibiting scavenging activity and bioturbation and therefore carcass disarticulation and decomposition [21]. Although extremely rare, these communities can represent environmental proxies for variations in the bottom-water conditions of the Southwest German Basin; moreover, these unique associations can bring valuable insights on the role of benthic scavenging and carcass decomposition within a Toarcian epicontinental–sea environment. Such local enrichment around a large vertebrate skeleton in fact provides a temporal snapshot into the background diversity of benthic macroinvertebrates in a given environment, and highlights trophic connections between the nektonic macrofauna and the benthos. Here, we review the association of benthic faunal components with large marine vertebrate skeletons in the Posidonienschiefer Formation to assess: (a) how uncommon such associations actually are; (b) whether the composition and frequency of such assemblages changes through the Toarcian; and (c) the relationship between such assemblages and other taphonomic parameters such as skeletal disarticulation.
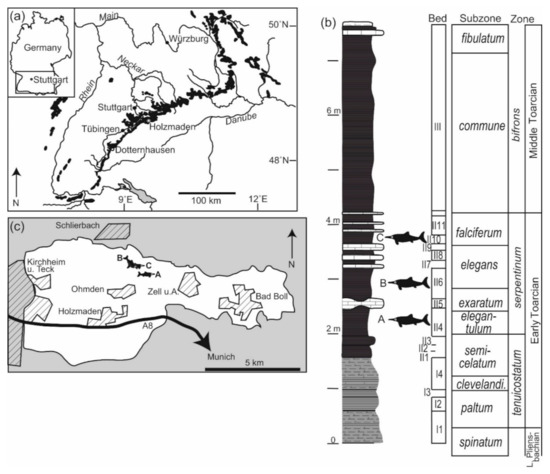
Figure 1.
Geographical and geological context. (a) Southwestern Germany, with Posidonienschiefer Formation outcrops indicated in black (modified from [33]). (b) Stratigraphic column of the Posidonienschiefer Formation at the Holzmaden locality (48°38′07.2″ N 9°31′36.4″ E) indicating the stratigraphic provenance of three of the ichthyosaur deadfall communities discussed in the text. A = SMNS 80234, B = SMNS 81841, and C = SMNS 81719. Stratigraphic column from [34], ammonite subzones following [35]. (c) Map of the Holzmaden paleontological heritage protected area showing the provenance and orientation of the same three skeletons as in part (b).
1.2. Previously Documented Associations from the Posidonienschiefer Formation
The first purported vertebrate deadfall association in the Posidonienschiefer Formation from the Holzmaden region was that of the gastropod Coelodiscus minutus, historically interpreted as a scavenger [36], with a marine reptile carcass [37,38]. However, Coelodiscus is now thought to have been holoplanktic [39], suggesting that this association was likely due to chance (as indicated by [40]).
Subsequently, [22] noted Chondrites burrows in the sediments overlying large vertebrate carcasses and not extending beyond the carcass periphery. As an example, the author figured an ichthyosaur skull in which the burrow network was restricted to the orbit. Although the trace maker of Chondrites is unknown, these burrows are related to deposit feeding by a wormlike macroinvertebrate, and can therefore be considered as part of the enrichment-opportunist stage. Alternatively, Chondrites has also been suggested to be related to sulfide mining or gardening by a chemosymbiotic animal; e.g., [41,42]; both interpretations would suggest evidence for the sulfophilic stage as well. The apparent absence of backfill structures in Chondrites in the Posidonienschiefer Formation points to open mines left by the producer and later filled passively with sediment.
The first evidence of the reef stage was provided in [35], which reported serpulid encrustation on reptile bones, and [20], which described oyster encrustation on a disarticulated ichthyosaur skull. These findings were particularly significant, as it was previously asserted that reptile bones in the Posidonienschiefer Formation never showed evidence of encrustation [21,43]. This apparent scarcity of encrusters was considered by other authors to potentially represent a bias caused by preparation of large vertebrate skeletons from the underside (i.e., the stratigraphically lower surface), rather than representing a true signal caused either by anoxic bottom waters or rapid burial [22,44]. These encrusted bones are characterized by low individual abundance and taxonomic richness; there is no evidence of community development or succession, differing from both recent deadfalls and also from other reported Jurassic communities; e.g., [11].
The oryctocoenosis described in [17] represents the sole multitaxon assemblage consisting of a large number of individuals associated with an ichthyosaur carcass from the Posidonienschiefer Formation. In addition to elements attributed to the enrichment-opportunist stage, such as an ophiuroid and ammonites, a well-developed reef stage consisting of four epifaunal bivalve genera and a crinoid was described. However, both the presence of the ammonites and the bivalves Parainoceramya dubia (formerly Pseudomytiloides dubius) and Bositra require reappraisal, as these might represent chance associations: these taxa are relatively abundant in the Posidonienschiefer Formation, and are frequently encountered without being associated with vertebrate carcasses. Dick [17] listed additional examples of marine reptile deadfall communities from the Posidonienschiefer Formation, but many of these may be spurious for similar reasons discussed for the Coelodiscus associations. One ichthyosaur specimen, SMNS 80234, represents a notable exception, being associated with two partially articulated ophiuroids potentially representing enrichment opportunists.
2. Materials and Methods
2.1. Geological Context
The Posidonienschiefer Formation (Figure 1a,b) was originally referred to as the Schwarzjura epsilon and was subsequently divided into three parts, of which the middle part (εII) is of interest here. εII comprises marly claystones and shales, biostratigraphically corresponding to the top of tenuicostatum and entire serpentinum ammonite zones. εII is further subdivided into local lithological marker beds, designated using Arabic numerals as subscript (see [35] for details, as well as for ammonite zone/subzone correlations). These beds varied in terms of both substrate consistency and bottom-water oxygenation at time of deposition [22,33,45]. The Posidonienschiefer Formation is laterally extensive within Germany, and conditions and faunas are expected to be heterogeneous, especially towards the edges of the basin. Here, we restrict our survey to the classic localities around the village of Holzmaden (Figure 1c), Baden-Württemberg, Germany. Water depth at this locality has not been estimated, but was likely between 50 and 150 m during deposition of the Posidonienschiefer Formation based on adjacent sites [33,46]. These depth estimates place the associations documented in the Posidonienschiefer Formation as occurring in a shallow-water setting (sensu [3], which considered deadfalls at <260 m depth as occurring in shallow-water settings). The sedimentation rate was generally low [20], and soft benthic sediments, in combination with low oxygenation of bottom waters, played a role in constraining benthic diversity [45].
2.2. Survey and Documentation of Deadfall Assemblages
We independently reviewed more than 100 large vertebrate specimens from the Posidonienschiefer Formation in the collections of the Staatliches Museum für Naturkunde Stuttgart for evidence of deadfall associations. In addition, we reassessed potential associations noted during specimen examination for previous studies. While the latter approach was not systematic, it is clear that such associations are relatively uncommon and thus anecdotal findings are worthy of mention. Previous authors have considered macroinvertebrates within 10 cm of the bones to be associated with the deadfall community [11,17]. This approach is extremely conservative, as in recent whale fall communities, the area enriched by the decaying carcass can extend 1–3 m around the carcass, and this is also the case for smaller actinopterygian bait parcels [1,2]. We did not adhere to a strict 10 cm cutoff, but critically evaluated all occurrences in an attempt to eliminate associations caused by chance alone.
Invertebrates were considered to be associated with vertebrate skeletons if one or more of the following criteria were met: (a) there was direct evidence of encrustation or bioerosion of bones; (b) bioturbation was observed within the perimeter of the skeleton but not on the slab as a whole; and/or (c) one or more taxa ordinarily rare in the Posidonienschiefer Formation were present in the vicinity of the skeleton. If only a single individual of a single taxon was observed, such occurrences were noted but are treated with caution. All associations consisting of ammonites, belemnites, or the abundant bivalves Parainoceramya and Bositra are treated as coincidental. Only those associations preserving more than one invertebrate taxon are further discussed as deadfall communities.
Fossil preparation is expected to have a substantial effect on the detection of deadfall communities. Most specimens have been prepared from the underside, limiting the potential for detection of encrusting organisms; in addition, only a very narrow area around many skeletons has been exposed. The latter preparation style means that even the extremely conservative 10 cm radius around the skeleton is unlikely to be reached, limiting chances of detection still further. These biases cannot easily be quantified, but mean that the development of deadfall communities is expected to be substantially underestimated.
For those specimens preserving deadfall communities, articulation and completeness scores were calculated following [47].
Three-dimensional (3D) models of selected specimens were generated using the photogrammetry technique. Specimens were photographed with a Canon Powershot SX410 IS camera (20.0 Mpx, with a device standard lens 4.3–172 mm F3.4–F6.3) following [48,49]. In order to generate the mesh, photographs were processed in Agisoft Photoscan (standard v.1.1.4). Models were further prepared (cleaned, scaled, and aligned) using MeshLab (v.2020.07), and false-color depth maps were obtained using ParaView (v.4.1.0) following [49] (see also references therein). The 3D models were aligned to the X–Y plane to make them parallel to the stratigraphic surface, allowing us to visualize the relative stratigraphic position/height (or depth) along the Z-axis of each element. False-color depth maps are presented for those specimens for which the relative depth of particular features was important for interpretation (e.g., whether a burrow system was above, below, or within the body cavity of a vertebrate).
Cardinal directions (as indicated on the figures, and where available) were taken from data associated with the specimens at time of excavation. The compass appears mirrored in the figures due to the preparation of the specimens from the underside.
The mass of the nekton fall plays a role in the rate of biomass loss from a carcass, and therefore ultimately which successional stages are observed [1]. For the ichthyosaur skeletons, we used the regression of fork length vs. mass for Stenopterygius obtained from the model in [50]. Although critiqued elsewhere due to questionable density assumptions [51], for adult individuals of Stenopterygius, the model yielded comparable values to those observed in odontocete cetaceans of similar length [52]. The more problematic source of error was the length metric employed (fork length), which is impossible to determine with accuracy in the absence of soft-tissue preservation, as well as the influence of pregnancy on estimated mass.
2.3. Institutional Abbreviations
BCM, Buckinghamshire County Museum, UK; BRSMG, Bristol City Museum and Art Gallery, UK; GPIT, Paläontologische Sammlung der Universität Tübingen, Tübingen, Germany; MHH, Urwelt-Museum Hauff, Holzmaden, Germany; MJSN, JURASSICA Museum, Porrentruy, Switzerland; MSVG, Museo di speleo-paleontologia e archeologia di San Vittore di Genga, Ancona, Italy; NHMUK, Natural History Museum, UK; NMS, Naturmuseum Solothurn, Switzerland; OUM, Oxford University Museum, UK; PMO, Natural History Museum, University of Oslo, Norway; PMU, Paleontologiska Museet, Uppsala University, Sweden; RGCH, Riogazas-Chorro collection, Museo de Paleontología of the Universidad de Granada (Spain); SMNS, Staatliches Museum für Naturkunde Stuttgart, Germany, UMO, Urweltmuseum Oberfranken, Bayreuth, Germany; UW, University of Wyoming, USA; MCSNV, Museo Civico di Storia Naturale di Verona.
3. Results
3.1. SMNS 53363, Eurhinosaurus?
J. Fischer Quarry, Zell unter Aichelberg; εII3: this fragmentary skull consists of an associated braincase and palate, some mandibular elements, and a few teeth (Figure 2). The ventral surface of the parabasisphenoid and articulated right pterygoid is encrusted by two large oysters (Liostrea), one partially overgrowing the other [20]. These are considered to be associated with the development of the reef stage. The unusually large sizes of the two oysters (largest = 52 mm diameter) indicate a prolonged duration of the reef stage before final burial.

Figure 2.
SMNS 53363, Eurhinosaurus? (a) Photo, copyright M. Wahler/SMNS. (b) Close-up of encrusting Liostrea (indicated by arrows). Abbreviations: pbs, parabasisphenoid; pt, pterygoid. Scale bars = 50 cm (a), 50 mm (b).
3.2. SMNS 80234, Stenopterygius quadriscissus
Old K. Kromer Quarry, Ohmden; εII4 (Figure 1b,c): the specimen was prepared from the underside by R. Albersdörfer. This specimen (Figure 3) consists of a partially disarticulated female with at least four associated embryos. The adult has an articulation score of only 38%, but a completeness score of 94%. Mass = ca. 127 kg (estimate of 123 kg + 1 kg/embryo).
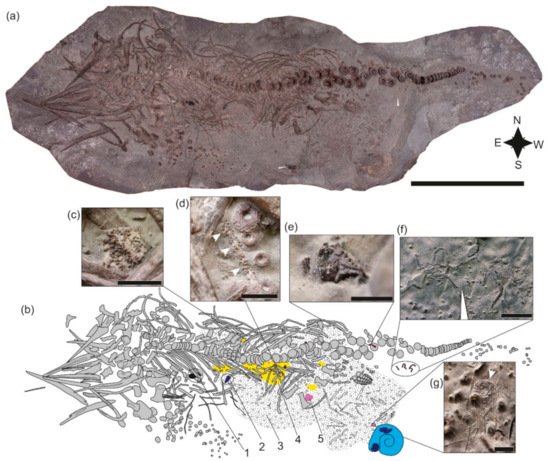
Figure 3.
SMNS 80234, Stenopterygius quadriscissus, prepared from below. (a) Photo, copyright M. Wahler/SMNS. (b) Interpretive drawing. 1, driftwood in ribcage; 2, aptychus; 3, textured area representing scattered embryonic remains; 4, yellow shaded area showing the extent of the Reophax accumulation; 5, disarticulated ophiuroid remains. (c) Reophax accumulation; (d) Reophax encrusting an embryonic rib; (e) crustacean mandibular ossicle; (f) Sinosura brodiei; (g) Diademopsis crinifera. Scale bars = 50 cm (a), 10 mm (c), 5 mm (d,e,g), 20 mm (f).
3.2.1. Taphonomic History
The skull is completely disarticulated, with the majority of elements in dorsal or left lateral view, but few bones are missing. The teeth, while for the most part displaced outside of the alveolar groove, remain in close proximity to the dentigerous elements. The ribs in the anterior dorsal part of the torso remain articulated and in alignment; gastric contents consisting of cephalopod hooklets are visible, as is a piece of driftwood (Figure 3a,b). Posteriorly, the ribs are fractured, creating a gap in the rib cage between the anterior and posterior portions. The left forelimb is roughly in position, but the distal elements have been displaced to the southwest. The ?right hind limb is articulated, but has been detached from the femur; the ?left is completely disarticulated, but is positioned close to the corresponding femur. The anterior caudal region has been disarticulated, the mid caudal region remains in perfect articulation, and the distal tail is also scattered toward the southwest. Several embryos remain within the rib cage of the female, but a large portion have been disarticulated and displaced posteroventral to the cloaca (i.e., to the southwest) (Figure 3a,b).
Based on positioning, the ichthyosaur arrived at the sea floor on its left dorsolateral side. The sediment was soft, and the anterior dorsal part of the torso and mid-caudal region became partially embedded upon landing. The ribs were broken perimortem (see [53]); the symmetrical and bilateral nature of the damage and displacement of the broken ends on the left side ventral to the articulated dorsal portion strongly suggest an injury sustained immediately prior to death, either as a result of intraspecific aggression or—more likely since the individual is a gravid female—a predation attempt [54].
3.2.2. Faunal Association
Dick [17] reported associated ammonite aptychi and two ophiuroids (Sinosura brodiei: Figure 3f). We add to these a third (completely disarticulated) ophiuroid, crustacean fragments (Figure 3e), an articulated echinoid (Diademopsis crinifera; Figure 3g), as well as isolated echinoid spines. Large accumulations of the benthic foraminifer Reophax are also present, often encrusting embryonic ichthyosaur bones (Figure 3d). The rock surrounding the specimen and associated fauna is devoid of benthic invertebrates.
3.2.3. Interpretation
This community is characterized by both low diversity and low abundance, with the exception of Reophax, which is present at high abundance. While the role of foraminifera is still not fully understood in the ecology of deadfalls, associations with vertebrate carcasses in the fossil record are known [55], including in Mesozoic marine reptile deadfalls [27,56]. Interestingly, occurrences of Reophax scorpiurus have been reported in the organic-enriched sediment below a recent whale skeleton south of Japan [57] among a rich foraminiferal assemblage. Crustaceans and ophiuroids can either be considered to be enrichment opportunists or mobile scavengers, arriving at a carcass within a few hours [2], whereas echinoids are more clearly classified as enrichment opportunists. An absence of byssate bivalves and encrusting taxa indicates that the carcass was either buried before the reef stage could develop or that long-term dysoxic conditions prevented its development. In any case, it is clear that the deadfall community was short-lived.
3.3. SMNS 81841, Stenopterygius quadriscissus
P. Kirschmann Quarry, Schlierbach State Forest; εII6 (Figure 1b,c): this specimen, which was prepared from below (inferred), and with the skull pointing south (Figure 4; Supplementary Materials File S1), was described and figured in detail in [17], and has an articulation score of 66% and a completeness score of 91%. Mass is estimated at 66 kg. The teeth show an unusual morphology for Stenopterygius, either due to congenital malformation or pathology (Figure 5j).
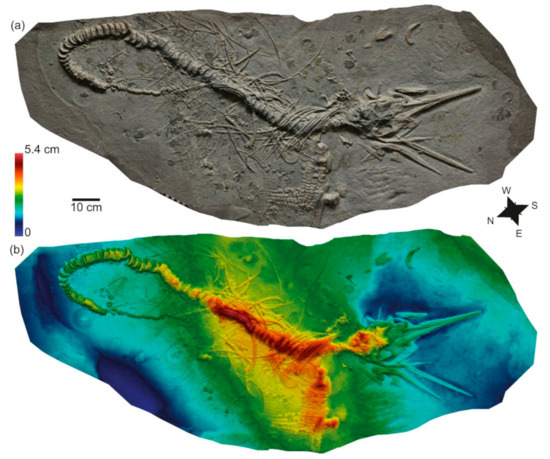
Figure 4.
SMNS 81841, Stenopterygius quadriscissus, with preparation from below inferred. (a) Orthophoto; (b) false-color depth map showing relative topography of the specimen. Warmer colors (reds) indicate deeper penetration of the bones into the sediment; cooler colors (blues) indicate elements closer to the sediment surface.

Figure 5.
Macroinvertebrates associated with SMNS 81841, Stenopterygius quadriscissus; hatching indicates the extent of the gastric contents. Bivalves are indicated in yellow, aptychi in dark blue, and echinoderms in pink. (a) Propeamussium pumilus; (b) Plagiostoma sp.; (c) indeterminate partial echinoid test; (d) indeterminate serpulid encrusting a dorsal rib; (e) posterior maxilla in dorsal view showing borings, indicated by arrows; (f) Eopecten strionatis; (g) Oxytoma inaequivalvis with articulated right and left valves and encrusted with small Liostrea (arrow); (h) Meleagrinella with ichthyosaurian pedal phalanx; (i) lateral ischiopubis showing accumulation of ophiuroid ossicles in the surrounding sediment; (j) ichthyosaur tooth with distinctive malformation of the crown; (k) fauna encrusting the upper surface of a dissolved ammonite shell: L, Liostrea, S, Serpulidae, C, “Cucullaea” muensteri. Scale bars = 10 mm (a,b,e–i,k), 5 mm (c,d,j).
3.3.1. Taphonomic History
The skull and pectoral girdle are preserved in dorsal view (Figure 4). The mandible and ventral cranial elements are disarticulated, and the teeth have been transported away from the skull (mostly toward the north-northwest). The vertebral column is predominantly in right ventral view, and is exceptionally well articulated, whereas the ribs are only well articulated in the anterior dorsal region, posterior to the skull. Some ribs are aligned to and beneath the midportion of the vertebral column. Gastric contents, consisting predominantly of cephalopod hooklets, form a well-defined mass over the vertebral column (Figure 5). The pectoral girdle was moved slightly posteriorly as well as rotated to the west, and the left forelimb has been detached. The hindlimbs have been completely disarticulated, and many of the bones appear to have been lost. The tail curves 180° to the south, forming a loop. Inside this loop, an accumulation of small elements was deposited (mostly echinoderm fragments, and a substantial number of ichthyosaur teeth); this accumulation is most dense anterior to the tail, on the northeast side of the carcass.
The orientation of the skull, pectoral girdle, and vertebral column suggest a dorsal landing, followed by a slump of the postcranium to the left while the skull roof remained in place (see, e.g., [50]: Pl. 8, Figure 3 showing a similar orientation of the torso and pectoral girdle). The skull was not completely buried on impact; and decomposition resulted in the dissociation of the mandible, ventral cranial elements, and teeth. The pectoral girdle was detached prior to the slump, and remained in dorsal view. The ribs aligned to and beneath the midportion of the vertebral column are interpreted as having been dragged by the vertebral column when it turned. The curvature of the tail and deposition of small fragments inside the loop could be viewed as the carcass serving as a baffle, resulting in coarse-grained bioclasts being deposited on the leeward side (see [58]) and suggesting currents predominantly from the southwest, but the teeth have clearly been moved in a south to north-northwest direction, and ribs and cranial elements have been transported towards the southwest.
3.3.2. Macroinvertebrates
A partial arm of an ophiuroid was mentioned in [17], but is here reinterpreted as a serpulid; however, the skeleton is surrounded by a mass of disarticulated ophiuroid remains (Figure 5i). We also identified two indeterminate echinoid tests (Figure 5c), as well as some isolated spines. The crinoid fragments labeled in [17] were not detected, although an isolated crinoid ossicle was preserved posterior to the gastric contents. The byssate bivalve Oxytoma inaequivalvis (Figure 5g) is abundant and is present in several generations; the pectinid Propeamussium pumilus (Figure 5a) is only slightly less abundant. Eopecten strionatis (Figure 5f), Plagiostoma sp. (Figure 5b), Meleagrinella sp. (Figure 5h), and “Cucullaea” muensteri (Figure 5k) are rarer. Parainoceramya dubia and Liostrea are directly associated with the carcass, and are also abundant in the vicinity of the carcass. Many of the bivalves show evidence of overgrowth (Figure 5g), indicating that the community persisted for some time. At least one serpulid is directly encrusting a rib (Figure 5d); others are distributed around the carcass. Several of the latter are encrusting the upper surface of dissolved ammonites (Figure 5k), as are the bivalves “Cucullaea” muensteri and Liostrea (Figure 5k). Whether this encrustation is related to local enrichment around the ichthyosaur carcass or simply utilization of all hard substrates may be impossible to disentangle and is a topic for further discussion, but this association was tentatively treated as carcass-associated. Whether the aptychi are related to the carcass fall community is unclear. The macroinvertebrate fauna does not include any infaunal or semi-infaunal taxa.
3.3.3. Trace Fossils
Dick [17] did not note any trace fossils; however, some bones show signs of bioerosion. The left maxilla shows several borings on its posterior end (Figure 5e); some fragmentary sclerotic ossicles preserved near the pectoral girdle display similar borings. The maxilla is close to its anatomical position and is stratigraphically shallow (Figure 4b), whereas the sclerotic ring fragment has been extensively displaced and is deeper than most of the skull (Figure 4b). The borings have a round shape with smoothed and curved walls, reminiscent of Gastrochaenolites and attributed to mechanical bivalve borers (e.g., Pholadidae) [59,60,61]. Nevertheless, a narrower neck with a widened chamber typical of boring bivalves [59] was not confidently identified in these traces. This, together with the low number of trace fossils observed and their poor development, preclude a confident ichnotaxonomic identification, thus these traces are referred to Gastrochaenolites-like.
3.3.4. Pyritization
None of the ichthyosaurian remains show evidence of pyritization, but pyrite is present on both the bivalves (Figure 5g,h) and echinoid spines. As noted previously [36], pyrite has been deposited on the inner and outer surfaces of the shells, rather than replacing the calcite, and thus is diagenetic in nature.
3.3.5. Interpretation
This deadfall community is characterized by both moderate diversity and abundance. Ophiuroids can either be considered to be enrichment opportunists or mobile scavengers; echinoids represent the enrichment-opportunist stage. All recovered byssate bivalves are characteristic of ammonite shell-surface communities [22,43], and are therefore interpreted as part of the reef phase, along with the encrusters. Gastrochaenolites is commonly found in lithified marine substrates, but has also been reported on shark [62], cetacean [59] and washed-out dinosaur bones [63]. If the borings on the maxilla correspond to Gastrochaenolites (or Gastrochaenolites-like), produced by boring bivalves [64], these occur in relatively shallow waters [59,65]. This is in accordance with the environmental interpretations of the Posidonienschiefer Formation, as well as with the presence of encrusted oysters on some ribs. The presence of Gastrochaenolites-like borings implies prolonged exposure of the skeleton on the seafloor, with erosive bivalves growing on bones devoid of organics, typical of the reef stage [66]. Based on multiple phases of colonization and overgrowth, it appears that the deadfall community persisted for some time.
3.4. SMNS 81719, Stenopterygius uniter
J. Fischer Quarry, Ohmden State Forest; εII10 (Figure 1b,c): this specimen was prepared from the underside by R. Albersdörfer. The skull points south. The specimen has articulation and completeness scores of 92%. The mass is estimated at 221 kg.
3.4.1. Taphonomic History
The skull is projecting from the slab. The anterior premaxilla and dentary are splayed and twisted, and the rostrum has been fractured. The teeth have been posteriorly displaced. The posterior exposed portion of the skull is unevenly compacted and has suffered extensive breakage. The anterior vertebral column has been disrupted with the anteriormost vertebrae being forced into the cranial cavity, and more posterior centra distributed around the right side of the skull. The torso is ventrolaterally oriented, and the right pectoral fin is posteriorly situated beneath the body. The spine is fractured and offset around the posterior third of the presacral region, but both the proximal and distal caudal regions are beautifully preserved; some disarticulation has occurred around the tail bend. No bioerosion or encrustation was observed in the posterior half of the body. The pectoral girdle is preserved in ventral view, and there is evidence of considerable bioerosion on its dorsal surface, surrounding the bones with a halo of tiny fragments. The original part of the right forelimb (the lower forelimb) is largely articulated, but selected elements from the proximal posterior limb have been displaced, (e.g., the ulnare was observed anterior to the humerus). The ribcage and vertebrae in the vicinity of the right forelimb are also missing or damaged. The left humerus appears to be exposed in anterior view, but distally the leading edge elements are in ventral view; more distally the fin has been further twisted. The ulna has been disarticulated and rotated. The dorsal and anterior surfaces of the carpals show evidence of encrustation (Figure 6l). Both hind limbs are preserved. The right limb is complete and pinned under the vertebral column; that of the left is slightly disarticulated distally. Buff-colored phosphatic matter coats the lower surface of the left limb (Figure 6h), and anterior to the proximal femur is a patch of a black-to-brownish substance.
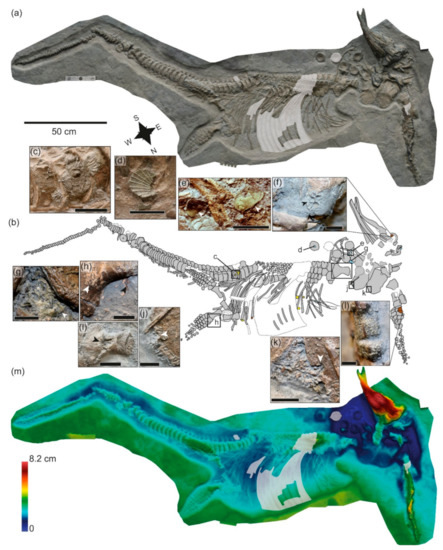
Figure 6.
SMNS 81719, Stenopterygius uniter, prepared from below. (a) Orthophoto; grey shaded areas indicate reconstructed parts. (b) Interpretive drawing; dashed lines represent reconstructed areas of the skeleton. Yellow = invertebrates not associated with the skeleton; light blue = associated macroinvertebrates; brown = invertebrates directly encrusting the bones. (c) Ammonite (Dactylioceras) with lower surface encrusted with Liostrea showing xenomorphic sculpture; (d) Propeamussium pumilus; (e) Parainoceramya dubia; (f) Liostrea encrusting skull; (g) Plagiostoma sp.; (h) hind limb showing buff-colored crust and dark-colored film; (i) potential bioerosion on the right humerus; (j) right and (k) left coracoids, showing damaged area surrounded by bone fragments; (l) Liostrea encrusting forelimb elements; (m) false-color depth map showing relative topography of the specimen. Warmer colors (reds) indicate deeper penetration of the bones into the sediment; cooler colors (blues) indicate elements closer to the sediment surface. Grey shaded areas indicate reconstructed parts. Scale bars = 50 cm (a), 10 mm (d,f,g,l); 20 mm (e,h,j,k); 40 mm (c); 50 mm (i).
The specimen shows numerous features consistent with head-first arrival at the sea floor (Figure 6; Supplementary Materials File S2). These include the projecting skull, ventrolateral carcass orientation, and posterior placement of the pectoral fin beneath the body [53], as well as splaying and torsion of the anterior premaxilla and dentary, breakage of the rostrum, differential compaction of the posterior exposed portion of the skull [26], and posterior displacement of the teeth [16]. We propose that perimortem breakage of the posterior skull and disruption of the anterior vertebral column, with the anteriormost vertebrae forced into the cranial cavity, are also diagnostic of a head-first seafloor arrival. The skull penetrated the sediment at an angle, and remained embedded, with the left ventral surface directed downward, and the torso slumped to the seafloor with the right ventral surface directed downward, with the opposite direction expected based on gravity. This suggests that the animal arrived at the sea floor with a great deal of momentum, and that the sea floor was soft enough to allow penetration of the rostrum into the sediment over many centimeters, but not soft enough to accommodate the entire length of the skull nor to allow the weight of the torso to pull the skull into a less steep angle after impact. Currents are hypothesized to have played a role in the unexpected orientation of the torso in a southwesterly direction; disarticulated bones from the anterior vertebral column are also shifted towards the southwest. The spinal fracture and offset around the posterior third of the presacral region likely occurred as the carcass collapsed following decomposition, with the fracture attributed to the stress caused by the initial headfirst seafloor impact. Bioerosion of the dorsal surface of the pectoral girdle suggests that it remained exposed on the sea floor for a long time. The displacement of posterior elements from the right forelimb suggests that the proximal limb was also not immediately embedded at time of death. Loss and damage to the ribs and vertebral column in this region is consistent with this interpretation. The left limb has a more complicated interpretation (Figure 6l). Based on twisting and bioencrustation, the forelimb presumably landed on the sea floor in ventral orientation, but flipped dorsally due to currents, where it remained for some time. Scour may have created a relatively deep and narrow excavation into which the limb collapsed via an anterior rotation.
The buff-colored material associated with the left hind limb is difficult to interpret. While the color is consistent with preservation of ichthyosaurian soft tissues, the texture and morphology suggest phosphatized microbial mats might represent a better interpretation. The dark substance anterior to the proximal femur is reminiscent of the type of soft tissue outline preserved in other ichthyosaur specimens. This dark-colored film may represent soft tissue, but a biofilm is also possible. The high degree of articulation, as well as the lack of bioerosion or encrustation in the posterior half of the body, suggest that it was buried much more rapidly than the anterior half.
3.4.2. Faunal Association
This specimen was not well prepared for taphonomic analysis. Liostrea is observed directly encrusting the skull, some distal ends of ribs from the left side of the body (i.e., the side exposed with the corpse lying on the substrate), and phalanges of the left pectoral fin (Figure 6f,l); additional examples are also associated with the skeleton. The byssate bivalves Propeamussium pumilus (Figure 6d), Plagiostoma sp. (Figure 6g), and Parainoceramya dubia (Figure 6e) are present, but represented by only a single occurrence each, with the exception of the latter taxon. Some of the invertebrates present on the slab include individuals clearly not part of the deadfall community (Figure 6c). There are several ammonites and Parainoceramya valves stratigraphically below the specimen that had colonized the benthos prior to the arrival of the carcass on the seafloor. The specimen was apparently deposited during an interval when bottom waters were sufficiently oxygenated to permit colonization by the bivalves [46], although it has also been proposed that inoceramid bivalves harbored chemosymbionts that would have facilitated colonization of low-oxygen habitats [67]. However, aside from Parainoceramya and the ammonite conchs, there is no evidence for additional background benthos or nekton benthos, respectively, indicating that the associated faunal elements listed above are specifically related to the presence of the carcass.
3.4.3. Trace Fossils
Bioerosion around the edges of the coracoids (Figure 6j,k) clearly points to scavenging activity, possibly by crustaceans; e.g., [68], or nautiloids. Traces seem to start from the edge of the bone and penetrate it while widening the starting entry point, generating a V-shaped form with the vertex oriented opposite the edge of the bone. The right humerus shows extensive damage on its ventral surface, especially on the mid-distal shaft, where the whole cortex and part of the spongiosa are removed (Figure 6i). Tentatively, the removed bone could be due to bioerosion as in the case of the coracoids, but much more extensively developed.
3.4.4. Pyritization
The invertebrates situated below the carcass show no trace of pyritization. Pyrite has been deposited on the inner and outer surfaces of the bivalve shells associated with the deadfall (Figure 6d,e,g,l); this is likely of diagenetic origin [36].
3.4.5. Interpretation
The bioerosion of the coracoids, also potentially present on the humerus, is clearly indicative of the enrichment-opportunist stage. These trace fossils were likely produced by crustaceans feeding directly from the bone and from the remaining flesh still covering it. The associated bivalves and the encrusted oysters on the distal end of some ribs evince the reef stage. As a whole, evidence of different carcass decay stages, including the last one (the reef stage), indicates a relatively prolonged time of exposure of the carcass before being completely buried, although the small size of the associated bivalves and encrusting Liostrea specimens points to a short period of time between encrustation and final burial.
3.5. SMNS 80113, Stenopterygius triscissus
K. Kromer Quarry, Ohmden; εII4.
3.5.1. Taphonomic History
The specimen is preserved in a dark-colored shale populated by rare Parainoceramya shells (Figure 7). The skeleton is exposed in left lateral view, the vertebrae in dorsal view, and the skull in right dorsal view. Regarding the gastric contents, a coleoid cephalopod preserving soft tissues, as well as numerous hooklets, remain in the abdominal cavity. The skull and pectoral girdle are detached from and embedded on a slightly deeper layer than the postcranium, and the skull shows slight telescoping of the nasals. The side of the anterior dorsal region that would have been exposed to the water column is disarticulated. The limbs are largely disarticulated, as is the distal half of the tail. The vertebral centra are detached from the neural arches and ribs in the mid-dorsal region.

Figure 7.
SMNS 80113, Stenopterygius triscissus, prepared from below. (a) Photo, copyright M. Wahler/SMNS; (b) Eopecten strionatis; (c) Mesomiltha pumila. Abbreviation: cr, caudal ribs. Scale bars = 50 cm (a), 20 mm (b), 5 mm (c).
The embedding of the skull on a slightly deeper layer, as well as the telescoping of the nasals, support a head-first sea floor arrival [26]. In addition, the disarticulation of the stratigraphically upper side of the anterior dorsal region is consistent with this interpretation. However, in general, the angle and force of impact appear to be much lower than in SMNS 81719. The interpretation is that the postcranium detached from the embedded skull and landed on its dorsal left side; slight decay prior to burial resulted in detachment of the vertebral centra from the neural arches and ribs.
3.5.2. Macroinvertebrates
Dick [17] mentioned this specimen in association with two specimens of Parainoceramya, which was a chance association, as these are associated with the same stratigraphic level as the skull; i.e., were deposited before the carcass. However, there is a specimen of Eopecten strionatis in the caudal region immediately above the skeleton (Figure 7b) and, more intriguingly, a specimen of the small infaunal lucinid Mesomiltha pumila (Figure 7c).
3.5.3. Interpretation
The low number of individuals involved make this association difficult to interpret. Isolated valves of Eopecten are usually viewed as being of pseudoplanktic provenance [34]. Extant lucinids are chemosymbiotic; thus, the presence of Mesomiltha provides equivocal evidence for the sulfophilic stage. However, this occurrence might also be attributed to chance.
3.6. Chondrites Associations
3.6.1. SMNS 51144, Saurostomus esocinus
G. Fischer Quarry, Holzmaden. εII4, prepared from the underside (inferred). Chondrites burrows are situated above and within the abdominal cavity of the fish [69] (Figure 8); bioturbation of the surrounding sediment is absent. The skeleton is perfectly articulated, demonstrating rapid or immediate burial upon arrival at the sea floor; wherein only infaunal benthos (in this case Chondrites) could successfully utilize the carcass. The restriction of the burrows to within the carcass suggest a true association, representing either the enrichment opportunistic or sulfophilic stage.
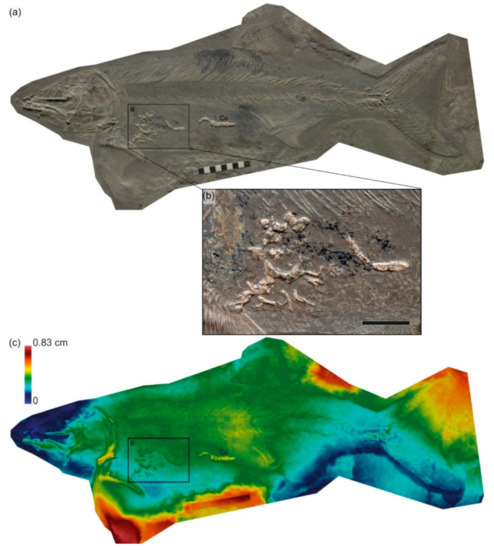
Figure 8.
SMNS 51144, Saurostomus esocinus, prepared from below. (a) Orthophoto; (b) inset showing Chondrites burrows; (c) false-color depth map showing the topographical relationship between the carcass and the burrows. Warmer colors (reds) indicate deeper penetration of the bones into the sediment; cooler colors (blues) indicate elements closer to the sediment surface. Co = cololite. Scale bar in cm (a), 20 mm (b).
3.6.2. SMNS 17500 and MHH 1981/25, Stenopterygius uniter; Holzmaden, εII8
Chondrites occurs in the sediments overlying and surrounding the carcasses, extending well beyond the edges of the specimen. Whereas the Chondrites system associated with SMNS 51144 is within the carcass in a nonbioturbated layer and thus is likely associated with the carcass itself [22], the association of Chondrites with the two large S. uniter specimens is much more tenuous. These two specimens originate from the same bed, and the type of preservation and distribution of trace fossils surrounding the skeletons is very similar. Einsele and Mosebach [36] noted the presence of Chondrites in bed εII8 at Holzmaden; the authors of [35] also noted a distinct weak bioturbation horizon in εII8, but did not report this trace from Holzmaden specifically. Thus, it is most plausible that these ichthyosaurs were preserved immediately below one such bioturbation horizon, rather than representing deadfall associations.
3.7. Crustacean Associations
In addition to the trace fossils potentially generated by crustaceans in SMNS 81719, several vertebrate specimens show associations with crustacean exoskeletal remains. These include GPIT-PV-31586, a large, articulated specimen of Pachycormus macropterus from Holzmaden with an indeterminate crustacean in the anal region surrounded by buff-colored material (Figure 9). Likely necrophagous interaction by the crustacean is indicative of the mobile scavenging stage. Additional crustacean–vertebrate co-occurrences are present in SMNS 55934, a juvenile of Stenopterygius quadriscissus from εII3 preserving a complete body outline with an isolated claw above the caudal skeleton and additional fragments in the dorsal region; SMNS 95401, a specimen of Metopacanthus, also from εII3, with a claw preserved well below the skeleton and additional fragments on a similar bedding plane to the skeleton; and SMNS 58389, a specimen of Pachycormus macropterus from εII4 with fragmentary crustacean remains on the slab, but on a different plane than the skeleton. The latter three occurrences are almost certainly spurious, given the fragmentary nature of the crustacean remains in combination with the exceptional preservation of the vertebrate remains, and the observation that the claw in SMNS 95401 was not associated with the overlying skeleton, suggesting an abundance of fragmentary crustacean remains in this horizon.
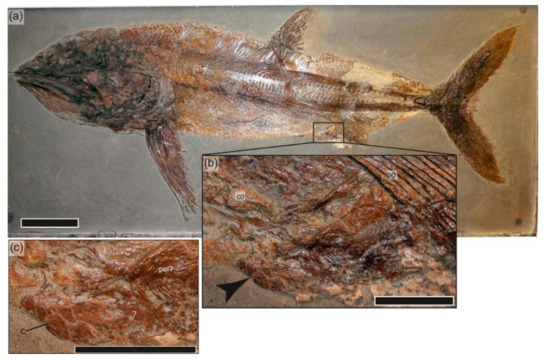
Figure 9.
GPIT-PV-31586, Pachycormus macropterus. (a) Overview photo; (b) inset showing the crustacean remains anterior to the anal fin, indicated by arrow. (c) Close-up of (b). Abbreviations: c, crustacean; co, cololite; pel? pelvic plate; pg, anal pterygiophores. Scale bars = 10 cm (a); 20 mm (b,c).
3.8. Microborings
Danise, Twitchett, and Matts [11] reported microborings oriented perpendicular to the bone surface in Ophthalmosaurus from the Upper Jurassic Sandsfoot Formation (Dorset, UK), attributed to the action of cyanobacteria. These microborings were associated with localized pyrite framboids, considered a marker of bacterial sulfate reduction. Vertebrae from recent and fossil cetacean carcass falls show a similar pattern, in which microbial cell density, as well as pyrite, is densest at the periphery of a bone and declines towards the center, indicative of a centripetal pattern of microbial degradation [3]. Both microborings and a centripetal gradient in pyrite (or interstitial pyrite more generally) are notably absent from most histological sections of vertebrate bone from the Posidonienschiefer Formation [36,70]. However, no histological sections from a deadfall community specimen are available for examination.
4. Discussion
4.1. Carcass Disarticulation and Taphonomy
Ichthyosaur carcasses have been hypothesized to have sunk to the seafloor immediately after death, and water depths in the Posidonienschiefer Formation were likely sufficient to prevent the carcass from refloating [51]. This means that the bulk of soft tissue removal and decomposition in the specimens under consideration is likely to have taken place when the carcass was already lying on the seafloor. The three ichthyosaur skeletons showing unambiguous development of a deadfall association (SMNS 80234, SMNS 81719, and SMNS 81841; Figure 3, Figure 4, Figure 5 and Figure 6) are all characterized by a high degree of completeness (all over 90%) and, depending on definition, exceptional preservation (embryos: SMNS 80234; in situ gastric contents: SMNS 81841; a high degree of articulation: SMNS 81719) despite seafloor exposure in at least a periodically oxygenated water column. This is consistent with recent work suggesting that exceptional fossil preservation occurs during short-term pulses of oxygenation in anoxic basins [71]. Burial of the three skeletons clearly occurred following at least partial skeletonization, based on direct encrustation and bioerosion of bones (Figure 5e and Figure 6f,i–l). The role of metazoan scavengers in carcass disarticulation is inferred to have been minimal, following the results in [72]. The observed disarticulation is most likely related to the action of currents and the degree to which the skeleton was embedded in the sediment [51,53].
Based on partial embedding of the skeletons (SMNS 80234, SMNS 81719) and distribution of invertebrates densely clustered on available hard substrates (SMNS 81841), sediments were soft at the time of carcass settling. Soft to soupy sediments remain the classic model for exceptional preservation in the Posidonienschiefer Formation [20,51,53]; however, other mechanisms may also have played a minor role. All three skeletons show evidence of displacement of elements by currents flowing in a more or less southwesterly direction (see [51] for detailed argumentation); this roughly agrees with the inferred north-to-south paleocurrent direction during deposition of εII [43]. SMNS 81719 shows limited evidence of current-induced scour affecting the forelimb exposed to the water column and the skull, based on a cluster of encrusting Liostrea (Figure 6f) at a stratigraphically lower level than the postcranium (Figure 6m), which was presumably lying on the sediment surface. Current-induced scour has been hypothesized to accelerate burial in other marine Lagerstätten [73], but was previously dismissed as an agent facilitating rapid burial in the Posidonienschiefer Formation [74]. Its relative importance as a burial mechanism remains to be critically evaluated. Retention of the gastric contents as an intact mass in SMNS 81841, despite disarticulation of the rib cage and evident physical displacement of the carcass following skeletonization (Figure 5), suggests a potential role for adipocere stabilization, also proposed as a mechanism of exceptional preservation in Lagerstätten [75]. The development of adipocere in Posidonienschiefer ichthyosaurs has been demonstrated [76], but remains somewhat controversial as a mechanism facilitating soft-tissue preservation [77]. Multiple factors appear to be influencing rapid burial and exceptional preservation in the Posidonienschiefer Formation, independent of the oxygenation levels in the bottom waters.
4.2. Invertebrate Associations
Despite being the most disarticulated skeleton (score = 38%), SMNS 80234 preserves the least diverse deadfall association, both in terms of represented successional stages and recorded taxa. This specimen originates from εII4 (elegantulum–exaratum subzone; Figure 1b), a horizon characterized by well-developed lamination and high organic content [35], and viewed as representing prolonged periods of benthic anoxia and photic zone euxinia [33,46,78]. The macroinvertebrate association is composed exclusively of mobile taxa. We interpreted this association as forming during an extremely brief period of bottom-water oxygenation.
SMNS 81841, from εII6 (upper exaratum–lower elegans subzone; Figure 1b) is also partially disarticulated (score = 66%), but less so than SMNS 80234. This specimen preserves the most diverse benthic association, and one that clearly persisted over a reasonably long time frame, allowing for several generations of colonization and overgrowth by byssate bivalves and oysters. This horizon is still characterized by periodic prolonged benthic anoxia/dysoxia and photic zone euxinia, but with longer periods of bottom-water oxygenation [33,78]. SMNS 81841 must have been deposited during such an oxic interval, which persisted long enough to allow the development of a deadfall community to reach the reef stage.
SMNS 81719, from εII10 (upper elegans–lower falciferum subzone; Figure 1b) is the most articulated specimen preserving a benthic association (score = 92%). This specimen was deposited against a background of prevailing benthic oxygenation (“Pseudomytiloides dubius” association in [33]). The carcass was skeletonized and encrusted under oxygenated conditions, but favorable conditions did not persist: all associated fauna are rather small in size, and only a single generation is present.
In the Posidonienschiefer Formation sample, the mobile scavenger stage is potentially represented by crustacean remains (SMNS 80234; Figure 3e); however, in the majority of the associations, this stage was not detected. Whether this reflects a true absence of scavenging is unclear. A relatively minor role for metazoan scavengers in the Posidonienschiefer Formation has long been assumed due to a combination of long-term lower-water-column anoxia and rapid burial [79,80], but a detection bias cannot be ruled out.
In contrast, the enrichment opportunist stage is well documented (Table 2). Echinoderms are the most visible components of this stage. Ophiuroids were well represented (Figure 3f and Figure 5i); this group is relatively abundant as microfossils in the serpentinum zone of the Posidonienschiefer Formation [81], so their association with the carcasses likely represents only a slight enrichment above background levels. Unlike other Jurassic deadfall associations [11,25,32] (see Table 1), echinoid grazing traces were not detected, with echinoids represented exclusively by body fossils and spines (Figure 3g and Figure 5c). Some of the observed bioerosion (Figure 6j,k) was attributed to crustaceans or nautiloids in the enrichment-opportunistic stage. Chondrites (Figure 8) may also be related to this stage.

Table 2.
Summary of ichthyosaur deadfalls from the Posidonienschiefer Formation associated with macroinvertebrates. Taxa in bold were recovered from more than one association. B = byssate; C = cemented.
Metazoan components of the sulfophilic stage appear to be absent, with the possible exception of the producers of the Chondrites burrows, and indeed, this stage has never been conclusively demonstrated in the Jurassic [11] (see also Table 1). SMNS 80113, preserved in association with a lucinid bivalve (Figure 7c), provides tantalizing, if equivocal, evidence that this stage, while rare, may have occasionally been present.
The best-represented stage is the reef stage (Table 2). Since this stage is made up of the best-mineralized and least motile taxa of all four stages, the quality of its record is not a surprise. Kauffmann [22] devised a successional scheme based on the colonization of ammonite shells by benthic encrusters in the Posidonienschiefer Formation. Initial colonization was by (1) serpulid worms and Parainoceramya dubia, replaced by (2) oysters; then (3) diverse byssate bivalves (Oxytoma, pectinids); and lastly (4) the development of an infaunal component. This succession documented from relatively nutrient-poor ammonite shells indicates that reef development in such a hostile benthic environment can create enriched pockets of localized abundance and diversity independent of the influx of organic matter brought in by a large vertebrate carcass, and that the deadfall communities discussed here must be interpreted in this light. However, the reef stage can still be viewed as related to the deadfall community, since these hardground habitats would not exist without the arrival of a large skeleton. SMNS 80234 exemplifies an early stage distinct from an ammonite shell-surface community, consisting of several mobile benthic taxa including echinoids, ophiuroids, and crustaceans, and an apparent absence of encrusters (Figure 3). SMNS 81719 and SMNS 81841 represent later-stage development of the reef phase, and the existence of the early phases can only be indirectly identified by damage to and remains surrounding the skeleton. Both of these skeletons have been later encrusted, with SMNS 81719 (Figure 6) reaching Kauffman stage (3) and SMNS 81841 (Figure 4 and Figure 5) representing an end phase in the reef community. SMNS 81841 differs from the ammonite shell-surface assemblages in that the bone itself has been bored during development of the infaunal component (Figure 5e), a habitat not available on the thinner ammonite shells.
4.3. Comparison with Other Jurassic Deadfall Communities
As outlined in Table 1, the published record of Jurassic deadfall communities is limited. From a temporal perspective, data from the Early Jurassic is sparse: only two studies have mentioned the development of deadfall communities in geological units other than the Posidonienschiefer Formation, and in both of these studies, almost all associated faunal evidence had been removed during preparation, resulting in only superficial mentions in the resulting publications [19,23]. The published record remains poor in the Middle Jurassic, focusing almost exclusively on the Oxford Clay of the UK [18]. In contrast, the Late Jurassic record is much better documented, with deadfall communities reported from a much broader geographical, latitudinal, and depositional range than in earlier time intervals. However, it should be noted that no marine Jurassic deadfall communities have been documented either from outside of Europe or from deep-water paleoenvironments. All of these factors mean that extreme caution must be used when extrapolating patterns from the deadfall communities documented in the present study.
One of the few generalities to be extracted from the comparative data is the predominance of oysters, serpulids, and diverse byssate bivalves encrusting bone in many of the Jurassic deadfall assemblages described to date (Table 1). Bryozoans have not been reported in this role during the Early Jurassic, being first mentioned from the Kimmeridgian [27].
4.4. Jurassic Deadfall Assemblages: Limitations of Recent Analogues
Recent deadfall stages are dominated by soft bodied metazoans [3] whose taxonomic groups originated well prior to the Jurassic [82]. The apparent absence of these groups from Mesozoic marine vertebrate deadfall assemblages could be attributed to their poor fossilization potential rather than major differences in ecological succession. In the same way, traces by many extant scavengers or opportunistic feeders of carcass exploitation on bone are still poorly known (e.g., hagfish scrape marks), making recognition in the fossil record problematic.
In Recent cetacean deadfalls, the presence or absence and degree of distinctness between the deadfall successional stages is variable, driven by intrinsic factors (e.g., carcass size and composition) and extrinsic factors (e.g., depositional environment) [3,7]. In this contribution, we have focused primarily on extrinsic factors affecting Jurassic deadfall assemblages; however, some potential intrinsic factors limiting the use of recent analogues for the interpretation of Jurassic deadfall communities should also be discussed.
Taxonomic differences in the overall amount of blubber, as well as the lipid content and size of bones, affect development of the sulfophilic stage in recent cetacean carcasses [1,3,8]. Ichthyosaurs are likewise thought to have had high carcass lipid content: a thick subcutaneous layer of blubber has been reported in a specimen preserving soft tissues [76], and bones show a spongious inner organization shared with cetaceans [83], an adaptation associated with high bone lipid content in active-swimming pelagic vertebrates [84,85]. The latter inference has received additional support in the form of a centripetal gradient of pyrite within the bone cortex of an ichthyosaur attributed to microbial lipid decomposition similar to that seen in recent cetacean deadfalls [3,11]. Thus, there is no reason to exclude potential development of a sulfophilic stage in Jurassic ichthyosaurs a priori. However, carcass lipid content—and thus the potential to support a well-developed sulfophilic stage—varies considerably among cetacean species [84], and where ichthyosaurs would fall on this continuum is uncertain.
The apparent absence of Osedax prior to the Cretaceous [12] is thought to have had an effect on carcass persistence, and thus on the potential development of the reef stage [11]. Osedax rapidly degrades bone, resulting in the complete decomposition of the skeleton before a reef stage can develop [7,86]. This may explain the well-developed reef stage in the Jurassic deadfall communities documented here and in Table 1 relative to recent deadfall associations. The magnitude of this effect appears to be correlated with depth, such that this carcass persistence bias between Jurassic and recent assemblages is less severe for shallow water deadfalls [86,87]. No Jurassic deep-water deadfalls have been described (Table 1). Osedax is not the only bone-eating macroinvertebrate acting to accelerate carcass breakdown [88], but evidence for any invertebrate occupying an analogous niche is unknown from the Jurassic record.
Microbial action, although slower-acting than bone-eating macroinvertebrates, can also decompose skeletal tissue prior to burial [84]. High bone lipid content has been postulated to slow this process [3,84]; thus, microbial skeletal decomposition should show a pattern inversely correlated with bone porosity. Bone porosity varies across the skeleton in ichthyosaurs [83], but is influenced by taxon [89] and is in general poorly documented for most elements. However, clear differences between ichthyosaurs and cetaceans in the distribution of bone porosity are in evidence; for instance, the jaws are particularly lipid-rich in cetaceans [84], but are unlikely to have been so in ichthyosaurs [70]. Regional differences in preservation and completeness should be expected between marine reptile and cetacean skeletons.
5. Conclusions
Deadfall community associations from the Lower Jurassic Posidonienschiefer Formation of Germany are seldom reported, and their absence has been used to argue for a combination of soupy sediments and anoxic bottom waters during emplacement of the spectacularly preserved marine reptile skeletons. Here, we reviewed associations and found the following:
- Deadfall community assemblages are indeed exceptionally rare, making up a small fraction of all vertebrate specimens (<3%), but can be identified even in specimens prepared from the underside.
- These communities are found in several horizons within the middle part of the Posidonienschiefer Formation (εII), but overall rarity precludes a detailed understanding of changes in their composition and frequency through the Toarcian.
- Seafloor exposure under oxygenated conditions does not preclude exceptional preservation, including high skeletal articulation, gastric contents, and embryonic remains.
- Articulation and community diversity are unrelated, with the latter being hypothesized to be controlled by duration of benthic oxygenation, and the former related to exposure time on the seafloor prior to burial.
In addition:
- The macroinvertebrate communities associated with the Posidonienschiefer Formation deadfalls are not especially diverse, and reflect amplification of background diversity rather than a specialist deadfall fauna.
- The mobile scavenger, enrichment opportunist, and reef stages are all detected in the Posidonienschiefer deadfall assemblages; the presence of the sulfophilic stage is equivocal.
- The published record of Jurassic deadfall communities is sparse, with strong geographical and paleoenvironmental biases. The deadfall communities reported from the Posidonienschiefer Formation complement the published record, but the uneven quality of comparative data masks potential trends in faunal composition and diversity.
In summary, a relatively complete story of the decomposition of large vertebrate carcasses in the Posidonienschiefer Formation is preserved, providing critical data on the evolution of these ecosystems during the Early Jurassic. This detailed knowledge of Early Jurassic deadfall communities complements more detailed Late Jurassic records; e.g., [11]. Such data are essential to understand how the faunal composition of these ecosystems may have evolved over the course of the Mesozoic, and how they resemble or differ from younger (including recent) deadfall communities.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/geosciences12040158/s1, Supplementary File S1: 3D photogrammetric model of SMNS 81841, in .ply format; Supplementary File S2: 3D photogrammetric model of SMNS 81719, in .ply format.
Author Contributions
Conceptualization, E.E.M., E.M., G.S. (Giovanni Serafini), and S.L.A.C.; Methodology, E.E.M., E.M., G.S. (Giovanni Serafini), and S.L.A.C.; Investigation, E.E.M., E.M., G.S. (Günter Schweigert), G.S. (Giovanni Serafini), F.M., and S.L.A.C.; Writing—Original Draft Preparation, E.E.M., E.M., G.S. (Günter Schweigert), G.S. (Giovanni Serafini), and S.L.A.C.; Writing—Review and Editing, E.E.M., E.M., G.S. (Günter Schweigert), G.S. (Giovanni Serafini), F.M., and S.L.A.C.; Visualization, E.E.M., E.M., and F.M.; Supervision, E.E.M.; Project Administration, E.E.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
Not applicable.
Acknowledgments
Thanks to A. Helmold for preliminary documentation of SMNS 80234, to R. B. Hauff (MHH) and I. Werneburg (GPIT) for access to comparative collections, and to A. Serrano (ICP) for assistance in the generation of the 3D photogrammetric models, with the support of the CERCA program (ICP) from Generalitat de Catalunya. Thanks also to the conveners of the Special Issue, as well as the three anonymous reviewers and the editor, who provided comments that improved the manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Smith, C.R.; Baco, A.R. Ecology of Whale Falls at the Deep-Sea Floor. Oceanogr. Mar.Biol. Annu. Rev. 2003, 41, 311–354. [Google Scholar]
- Smith, C.R. Food for the Deep Sea: Utilization, Dispersal, and Flux of Nekton Falls at the Santa Catalina Basin Floor. Deep.-Sea Res. 1985, 32, 417–442. [Google Scholar] [CrossRef]
- Smith, C.R.; Glover, A.G.; Treude, T.; Higgs, N.D.; Amon, D.J. Whale-Fall Ecosystems: Recent Insights into Ecology, Paleoecology, and Evolution. Annu. Rev. Mar. Sci. 2015, 7, 571–596. [Google Scholar] [CrossRef] [PubMed]
- Smith, C.R. Bigger is better: The role of whales as detritus in marine ecosystems. In Whales, Whaling and Ocean Ecosystems, Estes, J.A., DeMaster, D.P., Doak, D.F., Williams, T.M., Brownell, R.L., Eds.; University of California Press: Berkeley, CA, USA, 2006; pp. 286–302. [Google Scholar]
- Glover, A.G.; Källström, B.; Smith, C.R.; Dahlgren, T.G. World-Wide Whale Worms? A New Species of Osedax from the Shallow North Atlantic. Proc. R. Soc. B 2005, 272, 2587–2592. [Google Scholar] [CrossRef]
- Hilario, A.; Cunha, M.R.; Génio, L.; Marçal, A.R.; Ravara, A.; Rodrigues, C.F.; Wiklund, H. First Clues on the Ecology of Whale Falls in the Deep Atlantic Ocean: Results from an Experiment Using Cow Carcasses. Mar. Ecol. 2015, 36, 82–90. [Google Scholar] [CrossRef]
- Lundsten, L.; Schlining, K.L.; Frasier, K.; Johnson, S.B.; Kuhnz, L.A.; Harvey, J.B.J.; Clague, G.; Vrijenhoek, R.C. Time-Series Analysis of Six Whale-Fall Communities in Monterey Canyon, California, USA. Deep.-Sea Res. I 2010, 57, 1573–1584. [Google Scholar] [CrossRef]
- Higgs, N.D.; Gates, A.R.; Jones, D.O.B. Fish Food in the Deep Sea: Revisiting the Role of Large Food-Falls. PLoS ONE 2014, 9, e96016. [Google Scholar] [CrossRef]
- Hogler, J.A. Speculations on the Role of Marine Reptile Deadfalls in Mesozoic Deep-Sea Paleoecology. Palaios 1994, 9, 42–47. [Google Scholar] [CrossRef]
- Martill, D.M.; Cruickshank, A.; Taylor, M. Dispersal via Whale Bones. Nature 1991, 351, 193. [Google Scholar] [CrossRef]
- Danise, S.; Twitchett, R.J.; Matts, K. Ecological Succession of a Jurassic Shallow-Water Ichthyosaur Fall. Nat. Commun. 2014, 5, 4789. [Google Scholar] [CrossRef]
- Danise, S.; Higgs, N.D. Bone-Eating Osedax Worms Lived on Mesozoic Marine Reptile Deadfalls. Biol. Lett. 2015, 11, 20150072. [Google Scholar] [CrossRef] [PubMed]
- Kaim, A.; Kobayashi, Y.; Echizenya, H.; Jenkins, R.G.; Tanabe, K. Chemosynthesis-Based Associations on Cretaceous Plesiosaurid Carcasses. Acta Palaeontol. Polon. 2008, 53, 97–104. [Google Scholar] [CrossRef]
- Paparella, I.; Maxwell, E.E.; Cipriani, A.; Roncacè, S.; Caldwell, M.W. The First Ophthalmosaurid Ichthyosaur from the Upper Jurassic of the Umbrian–Marchean Apennines (Marche, Central Italy). Geol. Mag. 2017, 154, 837–858. [Google Scholar] [CrossRef]
- Serafini, G.; Amalfitano, J.; Cobianchi, M.; Fornaciari, B.; Maxwell, E.E.; Papazzoni, C.A. Evidence of Opportunistic Feeding between Ichthyosaurs and the Oldest Occurrence of the Hexanchid Shark Notidanodon from the Upper Jurassic of Northern Italy. Riv. Ital. Paleontol. Stratigr. 2020, 126, 629–655. [Google Scholar]
- Delsett, L.L.; Novis, L.K.; Roberts, A.J.; Koevoets, M.J.; Hammer, Ø.; Druckenmiller, P.S.; Hurum, J.H. The Slottsmøya Marine Reptile Lagerstätte: Depositional Environments, Taphonomy and Diagenesis. Geol. Soc. Lond. Spec. Publ. 2016, 434, 165–188. [Google Scholar] [CrossRef]
- Dick, D.G. An Ichthyosaur Carcass-Fall Community from the Posidonia Shale (Toarcian) of Germany. Palaios 2015, 30, 353–361. [Google Scholar] [CrossRef]
- Martill, D.M. A Taphonomic and Diagenetic Case Study of a Partially Articulated Ichthyosaur. Palaeontology 1987, 30, 543–555. [Google Scholar]
- Cruickshank, A.R.I. A Juvenile Plesiosaur (Plesiosauria: Reptilia) from the Lower Lias (Hettangian: Lower Jurassic) of Lyme Regis, England: A Pliosauroid-Plesiosauroid Intermediate? Zool. J. Linn. Soc. 1994, 112, 151–178. [Google Scholar] [CrossRef]
- Martill, D.M. Soupy Substrates: A Medium for the Exceptional Preservation of Ichthyosaurs of the Posidonia Shale (Lower Jurassic) of Germany. Kaupia 1993, 2, 77–97. [Google Scholar]
- Seilacher, A. Posidonia Shales (Toarcian, S. Germany)-Stagnant basin model revalidated. In Paleontology, Essential of Historical Geology; Montanaro Gallitelli, E., Ed.; S.T.E.M. Mucchi: Modena, Italy, 1982; pp. 25–55. [Google Scholar]
- Kauffmann, E.G. Ecological Reappraisal of the German Posidonienschiefer (Toarcian) and the stagnant basin model. In Communities of the Past, Gray, J., Boucot, A.J., Berry, W.B.N., Eds.; Hutchinson Ross Publishing: Stroudsburg, PA, USA, 1981; pp. 311–381. [Google Scholar]
- Pardo-Pérez, J.; Kear, B.P.; Mallison, H.; Gómez, M.; Moroni, M.; Maxwell, E.E. Pathological Survey on Temnodontosaurus from the Early Jurassic of Southern Germany. PLoS ONE 2018, 13, e0204951. [Google Scholar]
- Martill, D.M. The Stratigraphic Distribution and Preservation of Fossil Vertebrates in the Oxford Clay of England. Mercian Geol. 1986, 10, 161–186. [Google Scholar]
- Reolid, M.; Santos, A.; Mayoral, E. Grazing Activity as Taphonomic Record of Necrobiotic Interaction: A Case Study of a Sea Turtle Carapace from the Upper Jurassic of the Prebetic (South Spain). Rev. Mex. Cienc. Geol. 2015, 32, 21–28. [Google Scholar]
- Wahl, W.R. Taphonomy of a Nose Dive: Bone and Tooth Displacement and Mineral Accretion in an Ichthyosaur Skull. Paludicola 2009, 7, 107–116. [Google Scholar]
- Grange, D.R.; Benton, M.J. Kimmeridgian Metriorhynchid Crocodiles from England. Palaeontology 1996, 39, 497–514. [Google Scholar]
- Wilkinson, L.E.; Young, M.T.; Benton, M.J. A New Metriorhynchid Crocodilian (Mesoeucrocodylia: Thalattosuchia) from the Kimmeridgian (Upper Jurassic) of Wiltshire, UK. Palaeontology 2008, 51, 1307–1333. [Google Scholar] [CrossRef]
- Palmer, C.P. The Kimmeridge Fauna Associated with the Portland Plesiosaur. Proc. Dorset Nat. Hist. Archaeol. Soc. 1987, 109, 109–112. [Google Scholar]
- Gale, A.; Smith, A.B.; Thuy, B. Echinoderms. In Fossils of the Kimmeridge Clay Formation Volume 1, Introduction, Geology and Invertebrate Palaeontology; Palaeontological Association Filed Guide to Fossils: Number 16; Martill, D.M., Etches, S., Eds.; The Palaeontological Association: London, UK, 2020; p. 327. [Google Scholar]
- Leuzinger, L.; Cuny, G.; Popov, E.; Billon-Bruyat, J.-P. A New Chondrichthyan Fauna from the Late Jurassic of the Swiss Jura (Kimmeridgian) Dominated by Hybodonts, Chimaeroids and Guitarfishes. Pap. Palaeontol. 2017, 3, 471–511. [Google Scholar] [CrossRef]
- Meyer, C.A. Amazing Graze–Grazing Traces of Sea Urchins on Turtles–An Example from the Late Jurassic of Switzerland. Ann. Naturhist. Mus. Wien. Ser. A 2011, 113, 555–565. [Google Scholar]
- Röhl, H.-J.; Schmid-Röhl, A.; Oschmann, W.; Frimmel, A.; Schwark, L. Erratum to “The Posidonia Shale (Lower Toarcian) of SW-Germany: An Oxygen-Depleted Ecosystem Controlled by Sea Level and Palaeoclimate”. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2001, 165, 27–52. [Google Scholar] [CrossRef]
- Urlichs, M.; Wild, R.; Ziegler, B. Der Posidonien-Schiefer und Seine Fossilien. Stuttgarter Beitr. Naturk. Ser. C 1994, 36, 1–95. [Google Scholar]
- Riegraf, W.; Werner, G.; Lörcher, F. Der Posidonienschiefer. Biostratigraphie, Fauna und Fazies des südwestdeutschen Untertoarciums (Lias ε); Ferdinand Enke: Stuttgart, Germany, 1984; p. 195. [Google Scholar]
- Einsele, G.; Mosebach, R. Zur Petrographie, Fossilerhaltung und Entstehung des Posidonienschiefers im Schwäbischen Jura. N. Jb. Geol. Paläontol. Abh. 1955, 101, 319–430. [Google Scholar]
- Hauff, B. Untersuchung der Fossilfundstätten von Holzmaden im Posidonienschiefer des Oberen Lias Württembergs. Palaeontographica 1921, 64, 1–42. [Google Scholar]
- Wild, R. Holzmaden. In Palaeobiology: A Synthesis; Briggs, D.E., Crowther, P.R., Eds.; Blackwell Scientific Publications: Oxford, UK, 1990; pp. 282–285. [Google Scholar]
- Teichert, S.; Nützel, A. Early Jurassic Anoxia Triggered the Evolution of the Oldest Holoplanktonic Gastropod Coelodiscus minutus by Means of Heterochrony. Acta Palaeontol. Pol. 2015, 60, 269–276. [Google Scholar] [CrossRef]
- Seilacher, A. Die Holzmadener Posidonienschiefer Entstehung der Fossillagerstätte und eines Erdölmuttergesteins. In Klassische Fundstellen der Paläontologie; Weidert, W.K., Ed.; Goldschneck-Verlag: Korb, Germany, 1990; Volume 2, pp. 107–131. [Google Scholar]
- Mángano, M.G.; Buatois, L.A.; West, R.R.; Maples, C.G. Ichnology of a Pennsylvanian Equatorial Tidal Flat—The Stull Shale Member at Waverly, Eastern Kansas. Bull. Kans. Geol. Surv. 2002, 245, 1–133. [Google Scholar]
- Seilacher, A. Trace Fossil Analysis; Springer: Berlin, Germany, 2007; p. 226. [Google Scholar]
- Brenner, K.; Seilacher, A. New Aspects about the Origin of the Toarcian Posidonia Shales. N. Jb. Geol. Paläontol. Abh. 1978, 157, 11–18. [Google Scholar]
- Kauffmann, E.G. Benthic Environments and Paleoecology of the Posidonienschiefer (Toarcian). N. Jb. Geol. Paläontol. Abh. 1978, 157, 18–36. [Google Scholar]
- Schmid-Röhl, A.; Röhl, H.-J. Overgrowth on Ammonite Conchs: Environmental Implications for the lower Toarcian Posidonia Shale. Palaeontology 2003, 46, 339–352. [Google Scholar] [CrossRef]
- Röhl, H.-J.; Schmid-Röhl, A. Lower Toarcian (Upper Liassic) Black Shales of the Central European Basin: A Sequence Stratigraphic Case Study from the SW German Posidonia Shale. SEPM Spec. Publ. 2005, 82, 165–189. [Google Scholar]
- Beardmore, S.R.; Furrer, H. Evidence of a Preservational Gradient in the Skeletal Taphonomy of Ichthyopterygia (Reptilia) from Europe. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2016, 443, 131–144. [Google Scholar] [CrossRef]
- Mallison, H.; Wings, O. Photogrammetry in Paleontology, a Practical Guide. J. Paleontol. Tech. 2014, 12, 1–31. [Google Scholar]
- Mujal, E.; Marchetti, L.; Schoch, R.R.; Fortuny, J. Upper Paleozoic to Lower Mesozoic Tetrapod Ichnology Revisited: Photogrammetry and Relative Depth Pattern Inferences on Functional Prevalence of Autopodia. Front. Earth Sci. 2020, 8, 248. [Google Scholar] [CrossRef]
- Motani, R. Estimating Body Mass from Silhouettes: Teting the Assumption of Elliptical Body Cross-Sections. Paleobiology 2001, 27, 735–750. [Google Scholar] [CrossRef]
- Reisdorf, A.G.; Bux, R.; Wyler, D.; Benecke, M.; Klug, C.; Maisch, M.W.; Fornaro, P.; Wetzel, A. Float, Explode or Sink: Postmortem Fate of Lung-Breathing Marine Vertebrates. Palaeobiodivers. Palaeoenviron. 2012, 92, 67–81. [Google Scholar] [CrossRef]
- Reisdorf, A.G. No Joke Movement: Mehr über den Hauensteiner Ichthyosaurier und rezente marine Lungenatmer. Textnoten zur Physiologie, Pathologie und Taphonomie; weiterführende Literatur. Mitt. Naturk. Ges. Kanton Soloth. 2007, 40, 23–49. [Google Scholar]
- Hofmann, J. Einbettung und Zerfall der Ichthyosaurier im Lias von Holzmaden. Meyniana 1958, 6, 10–55. [Google Scholar]
- Pardo-Pérez, J.; Kear, B.P.; Maxwell, E.E. Palaeoepidemiology in Extinct Vertebrate Populations: Factors Influencing Skeletal Health in Jurassic Marine Reptiles. R. Soc. Open Sci. 2019, 6, 190264. [Google Scholar] [CrossRef]
- Baldanza, A.; Bizzarri, R.; Famiani, F.; Garassino, A.; Pasini, G.; Cherin, M.; Rosatini, F. The Early Pleistocene Whale-Fall Community of Bargiano (Umbria, Central Italy): Paleoecological Insights from Benthic Foraminifera and Brachyuran Crabs. Palaeontol. Electron. 2018, 21, 1–27. [Google Scholar] [CrossRef]
- Jagt, J.W.M.; Deckers, M.M.L.; De Leebeeck, M.; Donovan, S.K.; Nieuwenhuis, E. Episkeletozoans and Bioerosional Ichnotaxa on Isolated Bones of Late Cretaceous Mosasaurs and Cheloniid Turtles from the Maastricht Area, the Netherlands. Geologos 2020, 26, 39–49. [Google Scholar] [CrossRef]
- Wada, H.; Naganuma, T.; Fujioka, K.; Kitazato, H.; Kawamura, K.; Akazawa, Y. The Discovery of the Torishima Whale Bone Animal Community and Its Meaning. J. Deep.-Sea Res. 1994, 10, 37–47. [Google Scholar]
- Massare, J.A.; Wahl, W.R.; Ross, M.; Connely, M.V. Palaeoecology of the Marine Reptiles of the Redwater Shale Member of the Sundance Formation (Jurassic) of central Wyoming, USA. Geol. Mag. 2014, 151, 167–182. [Google Scholar] [CrossRef]
- Belaústegui, Z.; de Gibert, J.M.; Domnèch, R.; Muñez, F.; Martinell, J. Clavate Borings in a Miocene Cetacean Skeleton from Tarragona (NE Spain) and the Fossil Record of Marine Bone Bioerosion. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2012, 323–325, 68–74. [Google Scholar] [CrossRef]
- Kelly, S.R.; Bromley, R.G. Ichnological Nomenclature of Clavate Borings. Palaeontology 1984, 27, 793–807. [Google Scholar]
- Bertling, M.; Braddy, S.J.; Bromley, R.G.; Demathieu, G.R.; Genise, J.; Mikuláš, R.; Nielsen, J.K.; Nielsen, K.S.S.; Rindsberg, A.K.; Schlirf, M.; et al. Names for Tracefossils: A Uniform Approach. Lethaia 2006, 39, 265–286. [Google Scholar] [CrossRef]
- Amalfitano, J.; Giusberti, L.; Fornaciari, E.; Dalla Vecchia, F.M.; Luciani, V.; Kriwet, J.; Carnevale, G. Large Deadfalls of the ‘Ginsu’ Shark Cretoxyrhina mantelli (Agassiz, 1835) (Neoselachii, Lamniformes) from the Upper Cretaceous of Northeastern Italy. Cretac. Res. 2019, 98, 250–275. [Google Scholar] [CrossRef]
- Serrano-Brañas, C.I.; Espinosa-Chávez, B.; Maccracken, S.A. Gastrochaenolites Leymerie in Dinosaur Bones from the Upper Cretaceous of Coahuila, North-Central Mexico: Taphonomic Implications for Isolated Bone Fragments. Cretac. Res. 2018, 92, 18–25. [Google Scholar] [CrossRef]
- Boreske, J.R.; Goldberg, L.; Cameron, B. A Reworked Cetacean with Clam Borings: Miocene of North Carolina. J. Paleontol. 1972, 46, 130–139. [Google Scholar]
- Bromley, R.G. Bioerosion: Eating rocks for fun and profit. In Trace Fossils; Maples, C.G., West, R.R., Eds.; Paleontological Society: Knoxville, TN, USA, 1992; pp. 121–129. [Google Scholar]
- Höpner, S.; Bertling, M. Holes in Bones: Ichnotaxonomy of Bone Borings. Ichnos 2017, 24, 259–282. [Google Scholar] [CrossRef]
- MacLeod, K.; Hoppe, K. Evidence that Inoceramid Bivalves Were Benthic and Harbored Chemosynthetic Symbionts. Geology 1992, 20, 117–120. [Google Scholar] [CrossRef]
- Esperante, R.; Muñiz Guinea, F.; Nick, K.E. Taphonomy of a Mysticeti Whale in the Lower Pliocene Huelva Sands Formation (Southern Spain). Geol. Acta 2009, 7, 489–505. [Google Scholar]
- Cooper, S.L.; Maxwell, E.E. Revision of the Pachycormid Fish Saurostomus Esocinus AGASSIZ from the Early Jurassic (Toarcian) of Europe, with New Insight into the Origins of Suspension Feeding in Pachycormidae. Pap. Palaeontol. in review.
- Anderson, K.L.; Druckenmiller, P.S.; Erickson, G.M.; Maxwell, E.E. Skeletal microstructure of Stenopterygius quadriscissus (Reptilia, Ichthyosauria) from the Posidonienschiefer (Posidonia Shale, Lower Jurassic) of Germany. Palaeontology 2019, 62, 433–449. [Google Scholar] [CrossRef]
- Sinha, S.; Muscente, A.D.; Schiffbauer, J.D.; Williams, M.; Schweigert, G.; Martindale, R.C. Global Controls on Phosphatization of Fossils during the Toarcian Oceanic Anoxic Event. Sci. Rep. 2021, 11, 24087. [Google Scholar] [CrossRef] [PubMed]
- Danise, S.; Dominici, S. A Record of Fossil Shallow-Water Whale Falls from Italy. Lethaia 2014, 47, 229–243. [Google Scholar] [CrossRef]
- Bosio, G.; Collareta, A.; Di Celma, C.; Lambert, O.; Marx, F.G.; de Muizon, C.; Gioncada, A.; Gariboldi, K.; Malinverno, E.; Varas Malca, R.; et al. Taphonomy of Marine Vertebrates of the Pisco Formation (Miocene, Peru): Insights into the Origin of an Outstanding Fossil-Lagerstätte. PLoS ONE 2021, 16, e0254395. [Google Scholar] [CrossRef] [PubMed]
- van Loon, A.J. Ichthyosaur Embryos Outside the Mother Body: Not due to Carcass Explosion but to Carcass Implosion. Palaeobiodivers. Palaeoenviron. 2013, 93, 103–109. [Google Scholar] [CrossRef][Green Version]
- Smith, K.T.; Wuttke, M. From Tree to Shining Sea: Taphonomy of the Arboreal Lizard Geiseltaliellus maarius from Messel, Germany. Palaeobiodivers. Palaeoenviron. 2012, 92, 45–65. [Google Scholar] [CrossRef]
- Lindgren, J.; Sjövall, P.; Thiel, V.; Yheng, W.; Ito, S.; Wakamatsu, K.; Hauff, R.; Kear, B.P.; Engdahl, A.; Alwmark, C.; et al. Soft-Tissue Evidence for Homeothermy and Crypsis in a Jurassic Ichthyosaur. Nature 2018, 564, 359–365. [Google Scholar] [CrossRef]
- Keller, T. “Weichteil-Erhaltung” bei Großen Vertebraten (Ichthyosauriern) des Posidonienschiefers Holzmadens (Oberer Lias, Mesozoikum Süddeutschlands). Kaupia Darmstädter Beitr. Naturk. 1992, 1, 23–62. [Google Scholar]
- Schwark, L.; Frimmel, A. Chemostratigraphy of the Posidonia Black Shale, SW-Germany II. Assessment of Extent and Persistence of Photic-Zone Anoxia Using Aryl Isoprenoid Distributions. Chem. Geol. 2004, 206, 231–248. [Google Scholar] [CrossRef]
- Etter, W.; Tang, C.M. Posidonia shale: Germany’s Jurassic marine park. In Exceptional Fossil Preservation: A Unique View on the Evolution of Marine Life; Bottjer, D.J., Etter, W., Hagadorn, J.W., Tang, C.M., Eds.; Columbia University Press: New York, NY, USA, 2002; pp. 265–292. [Google Scholar]
- Seilacher, A.; Reif, W.-E.; Westphal, F. Sedimentological, Ecological and Temporal Patterns of Fossil Lagerstätten. Phil. Trans. R. Soc. Lond. Ser. B 1985, 311, 5–23. [Google Scholar]
- Riegraf, W. Mikrofauna, Biostratigraphie und Fazies im Unteren Toarcium Südwestdeutschlands und Vergleiche mit Benachbarten Gebieten. Tübinger Mikropaläontol. Mitt. 1985, 3, 1–232. [Google Scholar]
- Bardack, D. First Fossil Hagfish (Myxinoidea): A Record from the Pennsylvanian of Illinois. Science 1991, 254, 701–703. [Google Scholar] [CrossRef] [PubMed]
- Houssaye, A.; Sander, P.M.; Klein, N. Adaptive Patterns in Aquatic Amniote Bone Microanatomy—More Complex than Previously Thought. Integr. Comp. Biol. 2016, 56, 1349–1369. [Google Scholar] [CrossRef] [PubMed]
- Higgs, N.D.; Little, C.T.S.; Glover, A.G. Bones as Biofuel: A Review of Whale Bone Composition with Implications for Deep-Sea Biology and Palaeoanthropology. Proc. R. Soc. B 2011, 278, 9–17. [Google Scholar] [CrossRef] [PubMed]
- Shadwick, R.E.; Goldbogen, J.A.; Pyenson, N.D.; Whale, J.C.A. Structure and Function in the Lunge Feeding Apparatus: Mechanical Properties of the Fin Whale Mandible. Anat. Rec. 2017, 300, 1953–1962. [Google Scholar] [CrossRef] [PubMed]
- Braby, C.E.; Rouse, G.W.; Jouhnson, S.B.; Jones, W.J.; Vrijenhoek, R.C. Bathymetric and Temporal Variation among Osedax Boneworms and Associated Megafauna on Whale-Falls in Monterey Bay, California. Deep. Sea Res. Part I Oceanogr. Res. Pap. 2007, 54, 1773–1791. [Google Scholar] [CrossRef]
- Dominici, S.; Danise, S.; Cau, S.; Freschi, A. The Awkward Rcord of Fossil Whales. Earth-Sci. Rev. 2020, 205, 103057. [Google Scholar] [CrossRef]
- Johnson, S.B.; Warén, A.; Lee, R.W.; Kano, Y.; Kaim, A.; Davis, A.; Strong, E.E.; Vrijenhoek, R.C. Rubyspira, New Genus and Two New Species of Bone-Eating Deep-Sea Snails with Ancient Habits. Biol. Bull. 2010, 219, 166–177. [Google Scholar] [CrossRef]
- Talevi, M.; Fernández, M.S. Unexpected Skeletal Histology of an Ichthyosaur from the Middle Jurassic of Patagonia: Implications for Evolution of Bone Microstructure among Secondary Qquatic Tetrapods. Naturwissenschaften 2012, 99, 241–244. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).