Methanogenesis from Mineral Carbonates, a Potential Indicator for Life on Mars
Abstract
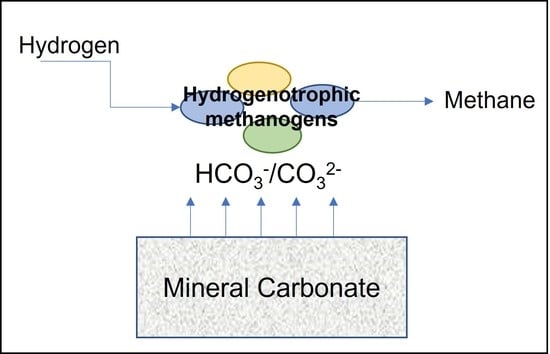
1. Introduction
2. Materials and Methods
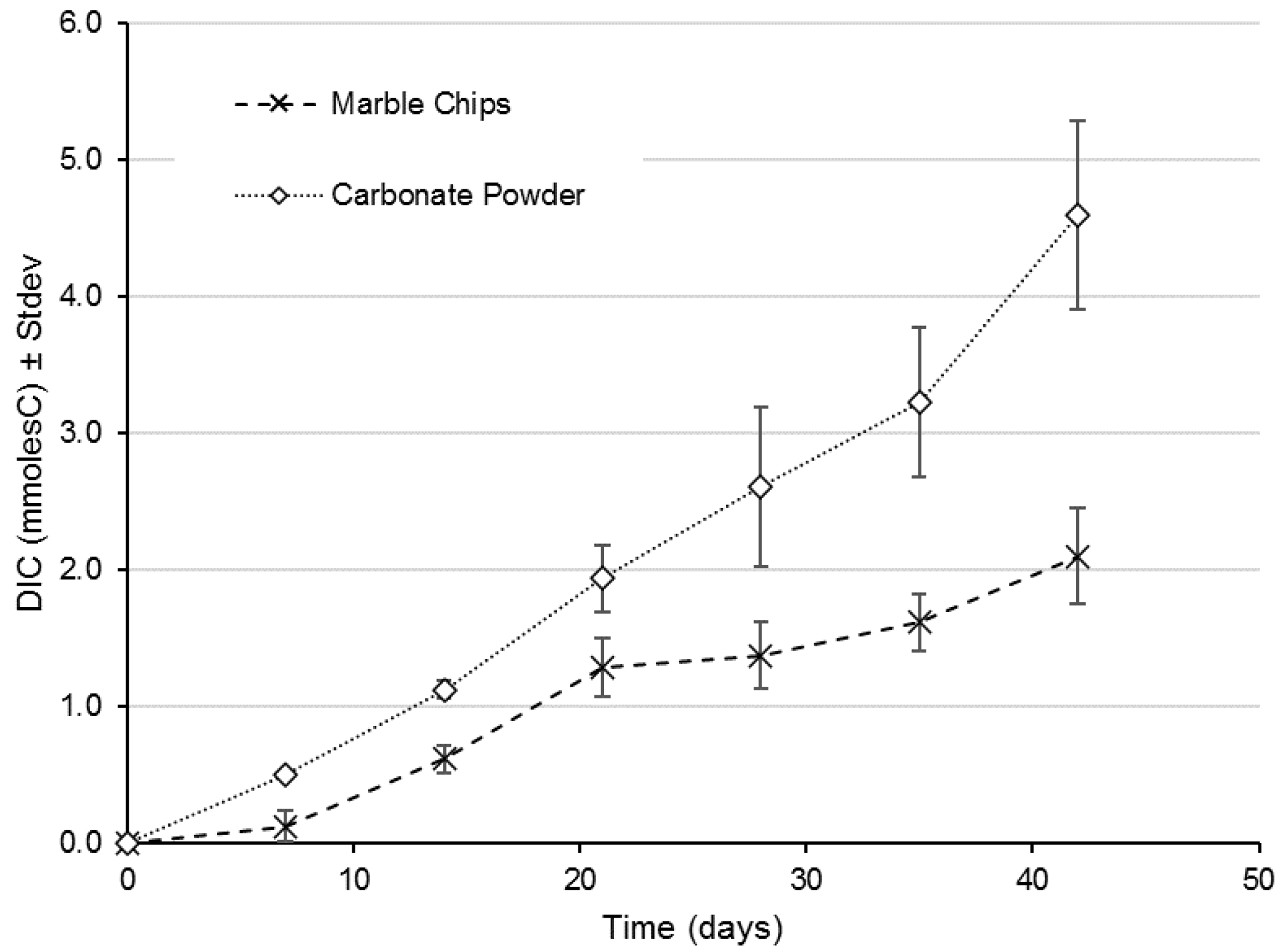
2.1. Release of Dissolved Inorganic Carbon from Carbonate Sources
2.2. Sampling Sites and Preparation of Enrichment Cultures
2.3. Reaction Set-Up and Analysis
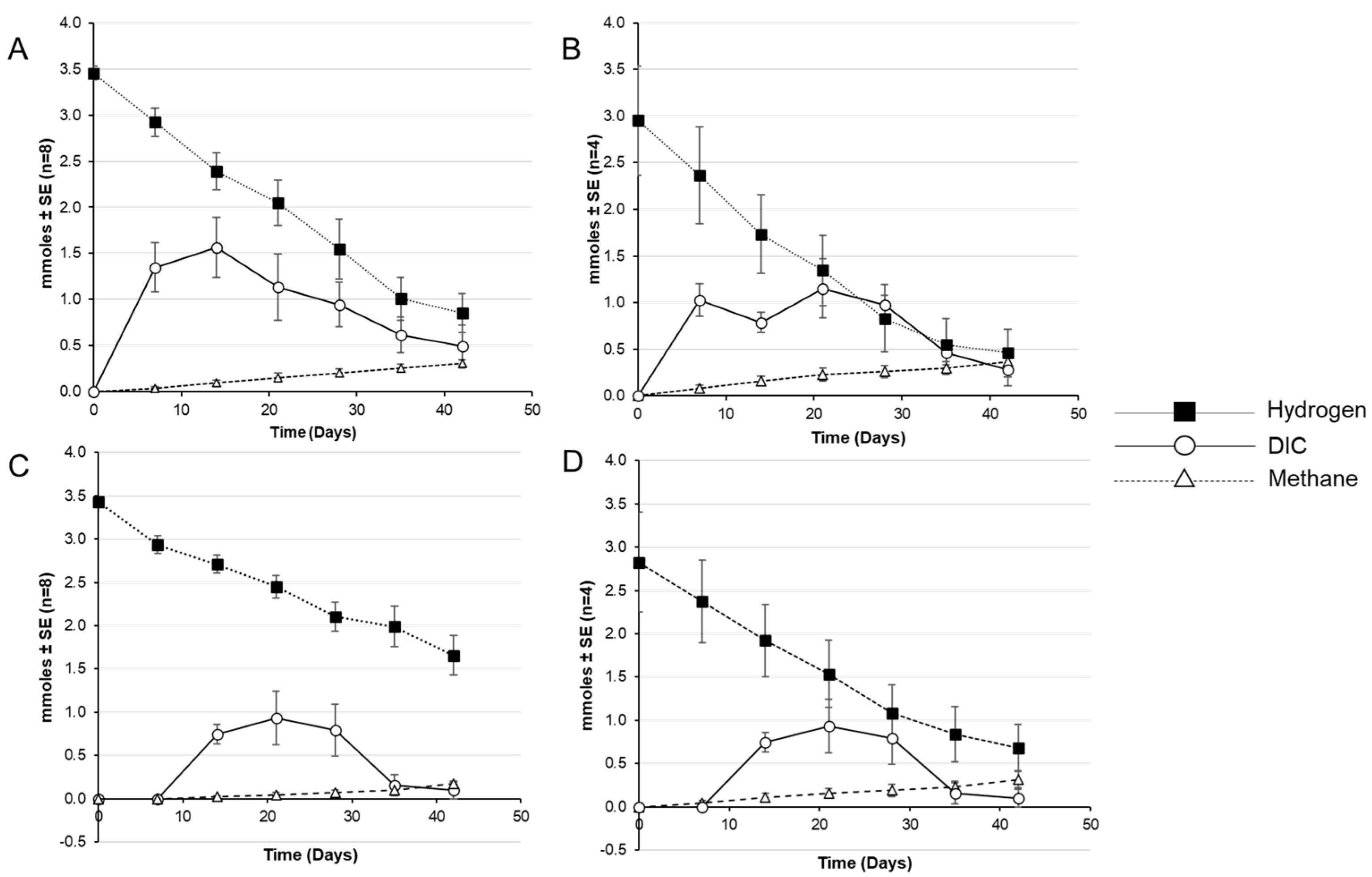
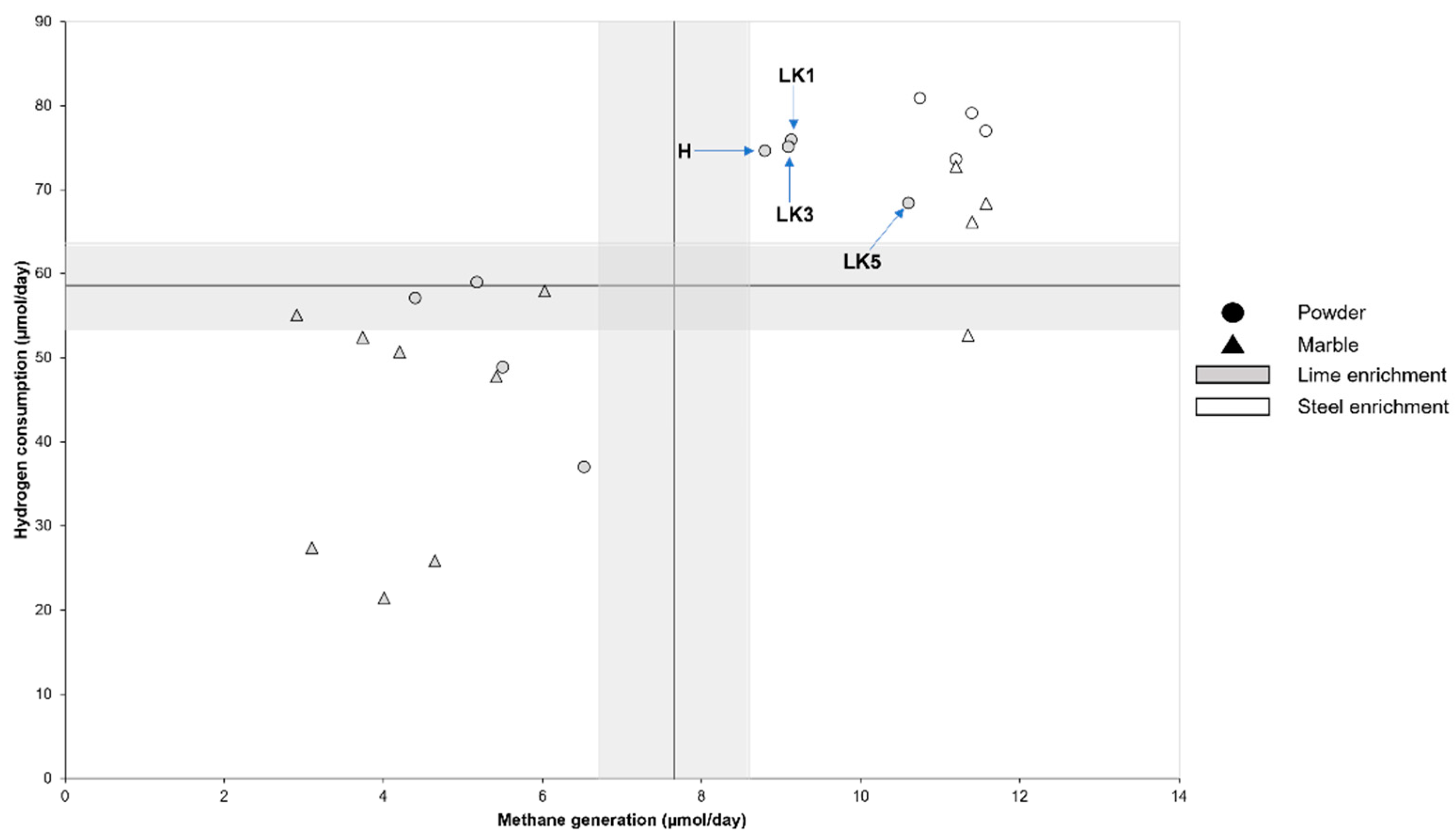
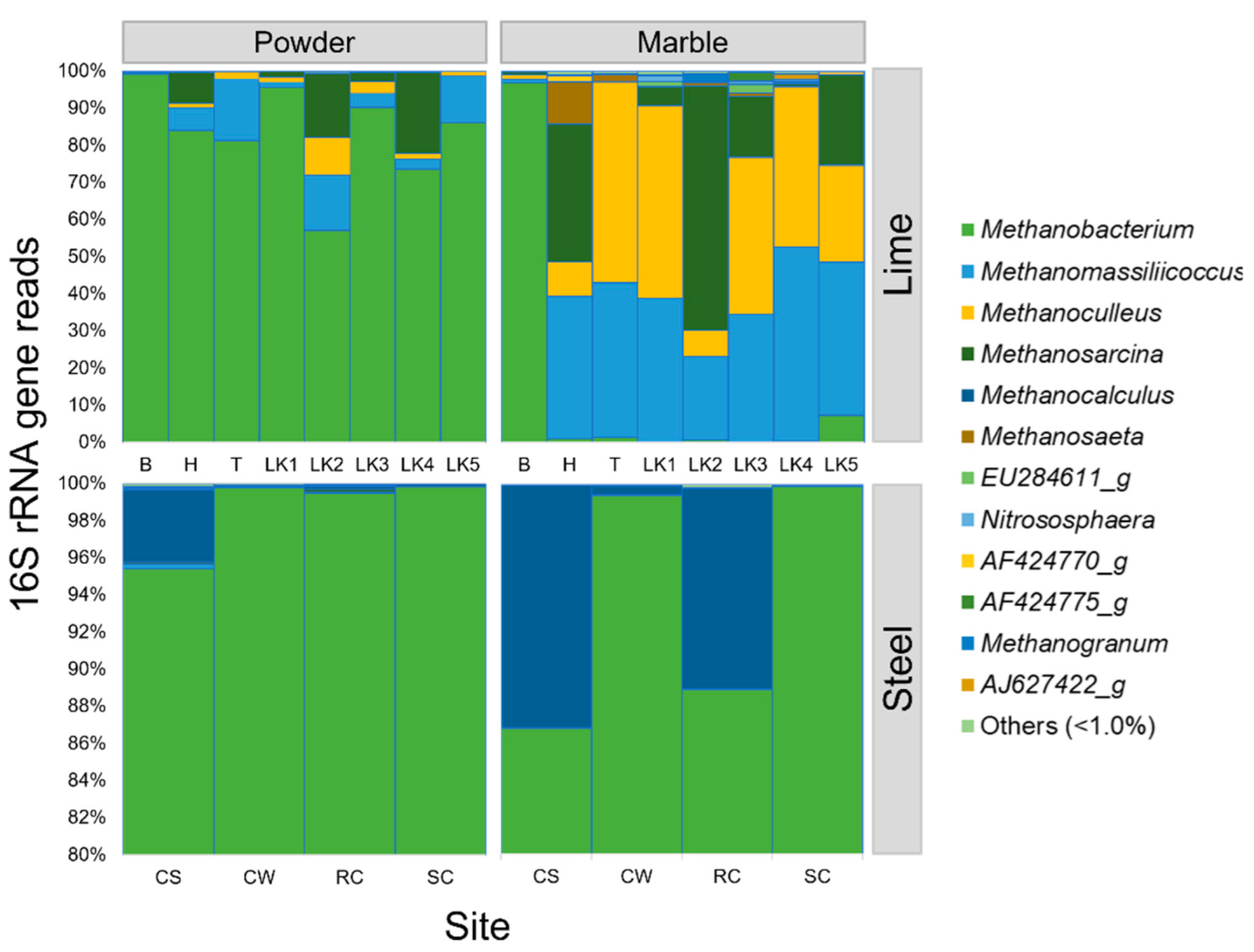
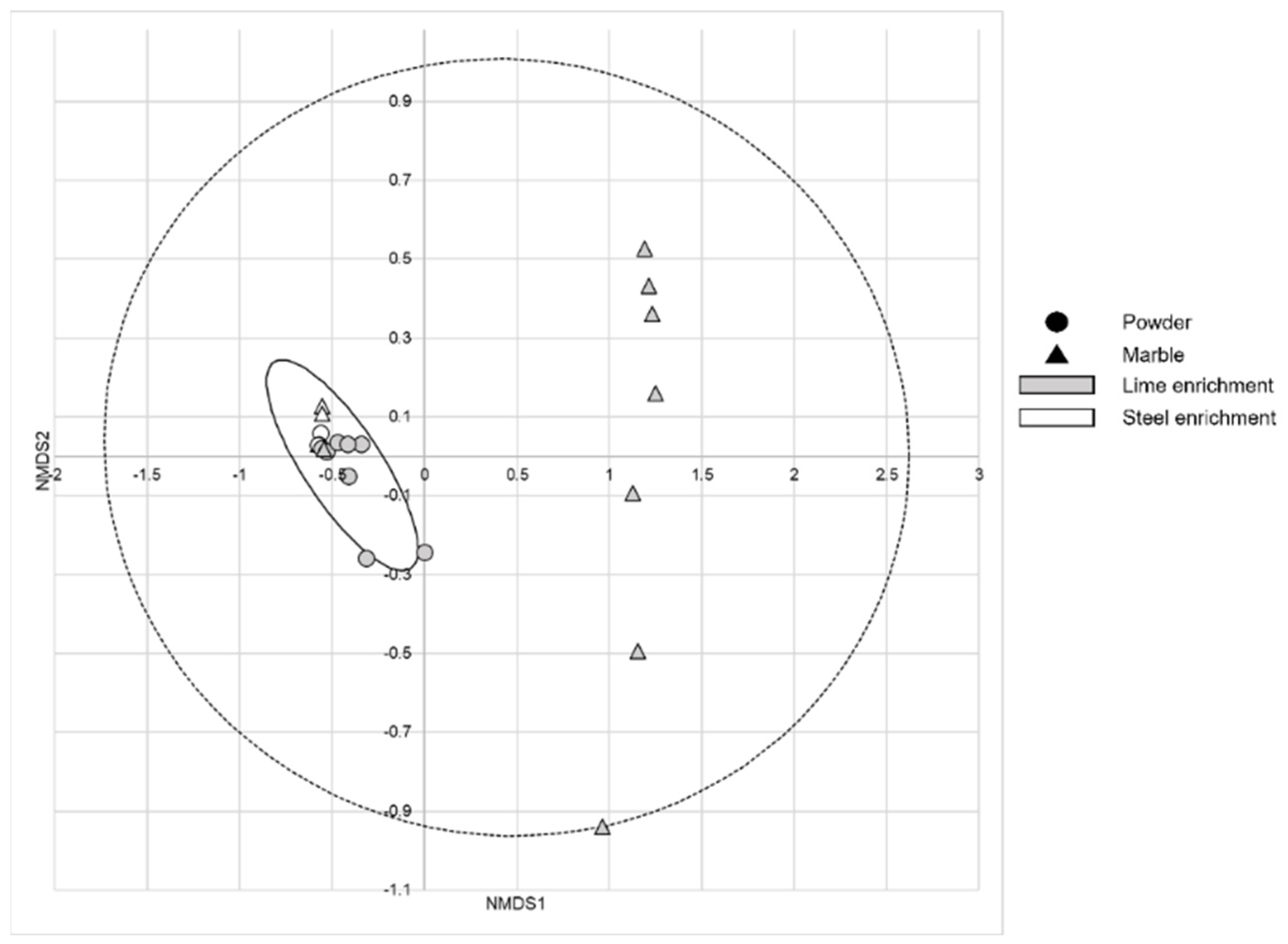
3. Results
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- NASA. NASA Astrobiology Strategy 2015. Edited by L.Hays 2015. Available online: https://nai.nasa.gov/media/medialibrary/2015/10/NASA_Astrobiology_Strategy_2015_151008.pdf (accessed on 8 November 2021).
- Grenfell, J.L. A review of exoplanetary biosignatures. Phys. Rep. 2017, 713, 1–17. [Google Scholar] [CrossRef]
- Williford, K.H.; Farley, K.A.; Stack, K.M.; Allwood, A.C.; Beaty, D.; Beegle, L.W.; Bhartia, R.; Brown, A.J.; de la Torre Juarez, M.; Hamran, S.-E. The NASA Mars 2020 rover mission and the search for extraterrestrial life. In From Habitability to Life on Mars; Elsevier: Amsterdam, The Netherlands, 2018; pp. 275–308. [Google Scholar]
- Banerdt, W.B.; Smrekar, S.E.; Banfield, D.; Giardini, D.; Golombek, M.; Johnson, C.L.; Lognonné, P.; Spiga, A.; Spohn, T.; Perrin, C. Initial results from the InSight mission on Mars. Nat. Geosci. 2020, 13, 183–189. [Google Scholar] [CrossRef]
- Vago, J.L.; Westall, F.; Coates, A.J.; Jaumann, R.; Korablev, O.; Ciarletti, V.; Mitrofanov, I.; Josset, J.-L.; De Sanctis, M.C.; Bibring, J.-P. Habitability on early Mars and the search for biosignatures with the ExoMars Rover. Astrobiology 2017, 17, 471–510. [Google Scholar] [CrossRef] [PubMed]
- Webster, C.R.; Mahaffy, P.R.; Atreya, S.K.; Moores, J.E.; Flesch, G.J.; Malespin, C.; McKay, C.P.; Martinez, G.; Smith, C.L.; Martin-Torres, J.; et al. Background levels of methane in Mars’ atmosphere show strong seasonal variations. Science 2018, 360, 1093–1096. [Google Scholar] [CrossRef]
- Oehler, D.Z.; Etiope, G. Methane Seepage on Mars: Where to Look and Why. Astrobiology 2017, 17, 1233–1264. [Google Scholar] [CrossRef] [PubMed]
- Ferry, J.G. How to Make a Living by Exhaling Methane. Annu. Rev. Biochem. 2010, 64, 453–473. [Google Scholar] [CrossRef]
- Nixon, S.L.; Cousins, C.R.; Cockell, C.S. Plausible microbial metabolisms on Mars: Microbial metabolisms on Mars. Astron. Geophys. 2013, 54, 1.13–1.16. [Google Scholar] [CrossRef]
- Holm, N.G.; Oze, C.; Mousis, O.; Waite, J.H.; Guilbert-Lepoutre, A. Serpentinization and the Formation of H2 and CH4 on Celestial Bodies (Planets, Moons, Comets). Astrobiology 2015, 15, 587–600. [Google Scholar] [CrossRef]
- McMahon, S.; Parnell, J.; Blamey, N.J.F. Evidence for Seismogenic Hydrogen Gas, a Potential Microbial Energy Source on Earth and Mars. Astrobiology 2016, 16, 690–702. [Google Scholar] [CrossRef]
- Blank, J.G.; Green, S.J.; Blake, D.; Valley, J.W.; Kita, N.T.; Treiman, A.; Dobson, P.F. An alkaline spring system within the Del Puerto Ophiolite (California, USA): A Mars analog site. Planet. Space Sci. 2009, 57, 533–540. [Google Scholar] [CrossRef]
- Etiope, G.; Ehlmann, B.L.; Schoell, M. Low temperature production and exhalation of methane from serpentinized rocks on Earth: A potential analog for methane production on Mars. Icarus 2013, 224, 276–285. [Google Scholar] [CrossRef]
- Alexander, W.R.; Milodowski, A.E. Cyprus Natural Analogue Project (CNAP) Phase IV Final Report; Nuclear Decommissioning Agency: Harwell, UK, 2015. [Google Scholar]
- D’Alessandro, W.; Daskalopoulou, K.; Calabrese, S.; Bellomo, S. Water chemistry and abiogenic methane content of a hyperalkaline spring related to serpentinization in the Argolida ophiolite (Ermioni, Greece). Mar. Pet. Geol. 2018, 89, 185–193. [Google Scholar] [CrossRef]
- Ramirez, R.M.; Craddock, R.A. The geological and climatological case for a warmer and wetter early Mars. Nat. Geosci. 2018, 11, 230–237. [Google Scholar] [CrossRef]
- Hu, R.; Kass, D.M.; Ehlmann, B.L.; Yung, Y.L. Tracing the fate of carbon and the atmospheric evolution of Mars. Nat. Commun. 2015, 6, 10003. [Google Scholar] [CrossRef] [PubMed]
- Horgan, B.H.N.; Anderson, R.B.; Dromart, G.; Amador, E.S.; Rice, M.S. The mineral diversity of Jezero crater: Evidence for possible lacustrine carbonates on Mars. Icarus 2020, 339, 113526. [Google Scholar] [CrossRef]
- Burke, I.; Mortimer, R.; Palani, S.; Whittleston, R.; Lockwood, C.; Ashley, D.; Stewart, D. Biogeochemical reduction processes in a hyper-alkaline affected leachate soil profile. Geomicrobiol. J. 2012, 29, 769–779. [Google Scholar] [CrossRef]
- Wormald, R.M.; Rout, S.P.; Mayes, W.; Gomes, H.; Humphreys, P.N. Hydrogenotrophic Methanogenesis under Alkaline Conditions. Front. Microbiol. 2020, 11, 614227. [Google Scholar] [CrossRef]
- Rout, S.P.; Radford, J.; Laws, A.P.; Sweeney, F.; Elmekawy, A.; Gillie, L.J.; Humphreys, P.N. Biodegradation of the Alkaline Cellulose Degradation Products Generated during Radioactive Waste Disposal. PLoS ONE 2014, 9, e107433. [Google Scholar] [CrossRef]
- Griffiths, R.I.; Whiteley, A.S.; O’Donnell, A.G.; Bailey, M.J. Rapid Method for Coextraction of DNA and RNA from Natural Environments for Analysis of Ribosomal DNA- and rRNA-Based Microbial Community Composition. Appl. Environ. Microbiol. 2000, 66, 5488–5491. [Google Scholar] [CrossRef]
- Kral, T.A.; Birch, W.; Lavender, L.E.; Virden, B.T. Potential use of highly insoluble carbonates as carbon sources by methanogens in the subsurface of Mars. Planet. Space Sci. 2014, 101, 181–185. [Google Scholar] [CrossRef]
- Phillips-Lander, C.M.; Parnell, S.R.; McGraw, L.E.; Madden, M.E.E. Carbonate dissolution rates in high salinity brines: Implications for post-Noachian chemical weathering on Mars. Icarus 2018, 307, 281–293. [Google Scholar] [CrossRef]
- Zastro, A.M.; Glotch, T.D. Distinct carbonate lithologies in Jezero crater, Mars. Geophys. Res. Lett. 2021, 48, 9. [Google Scholar] [CrossRef] [PubMed]
- Siegert, M.; Li, X.-F.; Yates, M.D.; Logan, B.E. The presence of hydrogenotrophic methanogens in the inoculum improves methane gas production in microbial electrolysis cells. Front. Microbiol. 2015, 5, 778. [Google Scholar] [CrossRef] [PubMed]
- Maus, I.; Wibberg, D.; Stantscheff, R.; Cibis, K.; Eikmeyer, F.-G.; König, H.; Pühler, A.; Schlüter, A. Complete genome sequence of the hydrogenotrophic archaeon Methanobacterium sp. Mb1 isolated from a production-scale biogas plant. J. Biotechnol. 2013, 168, 734–736. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Chen, H.; Yu, B.; He, J.; Zheng, P.; Mao, X.; Tian, G.; Yu, J.; Huang, Z.; Luo, J. Dietary pea fiber increases diversity of colonic methanogens of pigs with a shift from Methanobrevibacter to Methanomassiliicoccus-like genus and change in numbers of three hydrogenotrophs. BMC Microbiol. 2017, 17, 17. [Google Scholar] [CrossRef]
- Maus, I.; Wibberg, D.; Stantscheff, R.; Eikmeyer, F.-G.; Seffner, A.; Boelter, J.; Szczepanowski, R.; Blom, J.; Jaenicke, S.; König, H. Complete genome sequence of the hydrogenotrophic, methanogenic archaeon Methanoculleus bourgensis strain MS2T, isolated from a sewage sludge digester. Am. Soc. Microbiol. 2012, 194, 19. [Google Scholar]
- Lambie, S.C.; Kelly, W.J.; Leahy, S.C.; Li, D.; Reilly, K.; McAllister, T.A.; Valle, E.R.; Attwood, G.T.; Altermann, E. The complete genome sequence of the rumen methanogen Methanosarcina barkeri CM1. Stand. Genom. Sci. 2015, 10, 57. [Google Scholar] [CrossRef]
- Ragsdale, S.W.; Pierce, E. Acetogenesis and the Wood–Ljungdahl pathway of CO2 fixation. Biochim. Biophys. Acta BBA Proteins Proteom. 2008, 1784, 1873–1898. [Google Scholar] [CrossRef]
- Borrel, G.; Adam, P.S.; Gribaldo, S. Methanogenesis and the Wood–Ljungdahl Pathway: An Ancient, Versatile, and Fragile Association. Genome Biol. Evol. 2016, 8, 1706–1711. [Google Scholar] [CrossRef]
- Orange, F.; Westall, F.; Disnar, J.R.; Prieur, D.; Bienvenu, N.; Le Romancer, M.; Défarge, C. Experimental silicification of the extremophilic Archaea Pyrococcus abyssi and Methanocaldococcus jannaschii: Applications in the search for evidence of life in early Earth and extraterrestrial rocks. Geobiology 2009, 7, 403–418. [Google Scholar] [CrossRef]
- Joseph, R.G.; Planchon, O.; Duxbury, N.S.; Latif, K.; Kidron, G.J.; Consorti, L.; Armstrong, R.A.; Gibson, C.; Schild, R. Oceans, Lakes, and Stromatolites on Mars. Adv. Astron. 2020, 2020, 6959532. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wormald, R.M.; Hopwood, J.; Humphreys, P.N.; Mayes, W.; Gomes, H.I.; Rout, S.P. Methanogenesis from Mineral Carbonates, a Potential Indicator for Life on Mars. Geosciences 2022, 12, 138. https://doi.org/10.3390/geosciences12030138
Wormald RM, Hopwood J, Humphreys PN, Mayes W, Gomes HI, Rout SP. Methanogenesis from Mineral Carbonates, a Potential Indicator for Life on Mars. Geosciences. 2022; 12(3):138. https://doi.org/10.3390/geosciences12030138
Chicago/Turabian StyleWormald, Richard M., Jeremy Hopwood, Paul N. Humphreys, William Mayes, Helena I. Gomes, and Simon P. Rout. 2022. "Methanogenesis from Mineral Carbonates, a Potential Indicator for Life on Mars" Geosciences 12, no. 3: 138. https://doi.org/10.3390/geosciences12030138
APA StyleWormald, R. M., Hopwood, J., Humphreys, P. N., Mayes, W., Gomes, H. I., & Rout, S. P. (2022). Methanogenesis from Mineral Carbonates, a Potential Indicator for Life on Mars. Geosciences, 12(3), 138. https://doi.org/10.3390/geosciences12030138