Biostratigraphy and Paleoenvironmental Reconstruction at the Gebel Nezzazat (Central Sinai, Egypt): A Paleocene Record for the Southern Tethys
Abstract
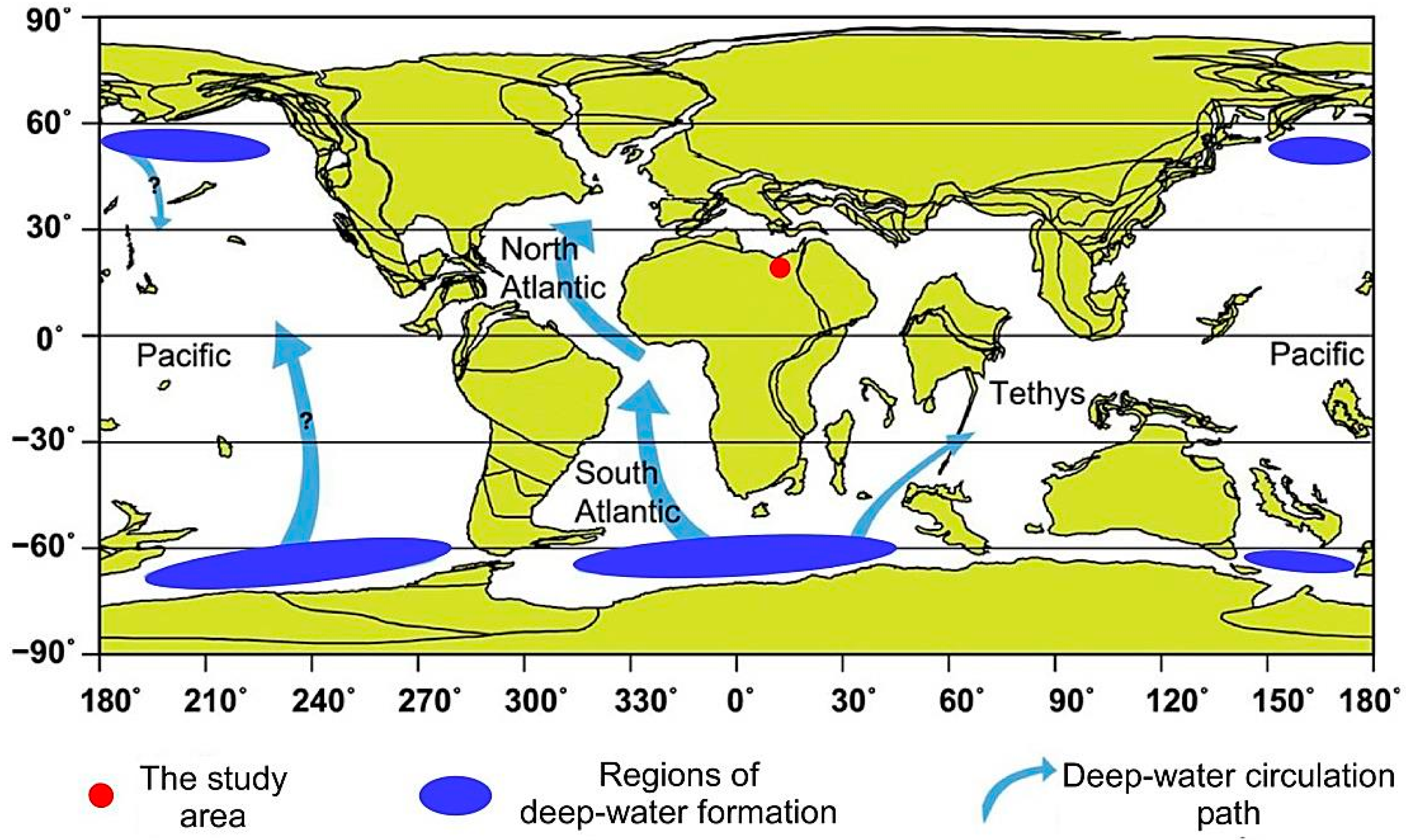
:1. Introduction
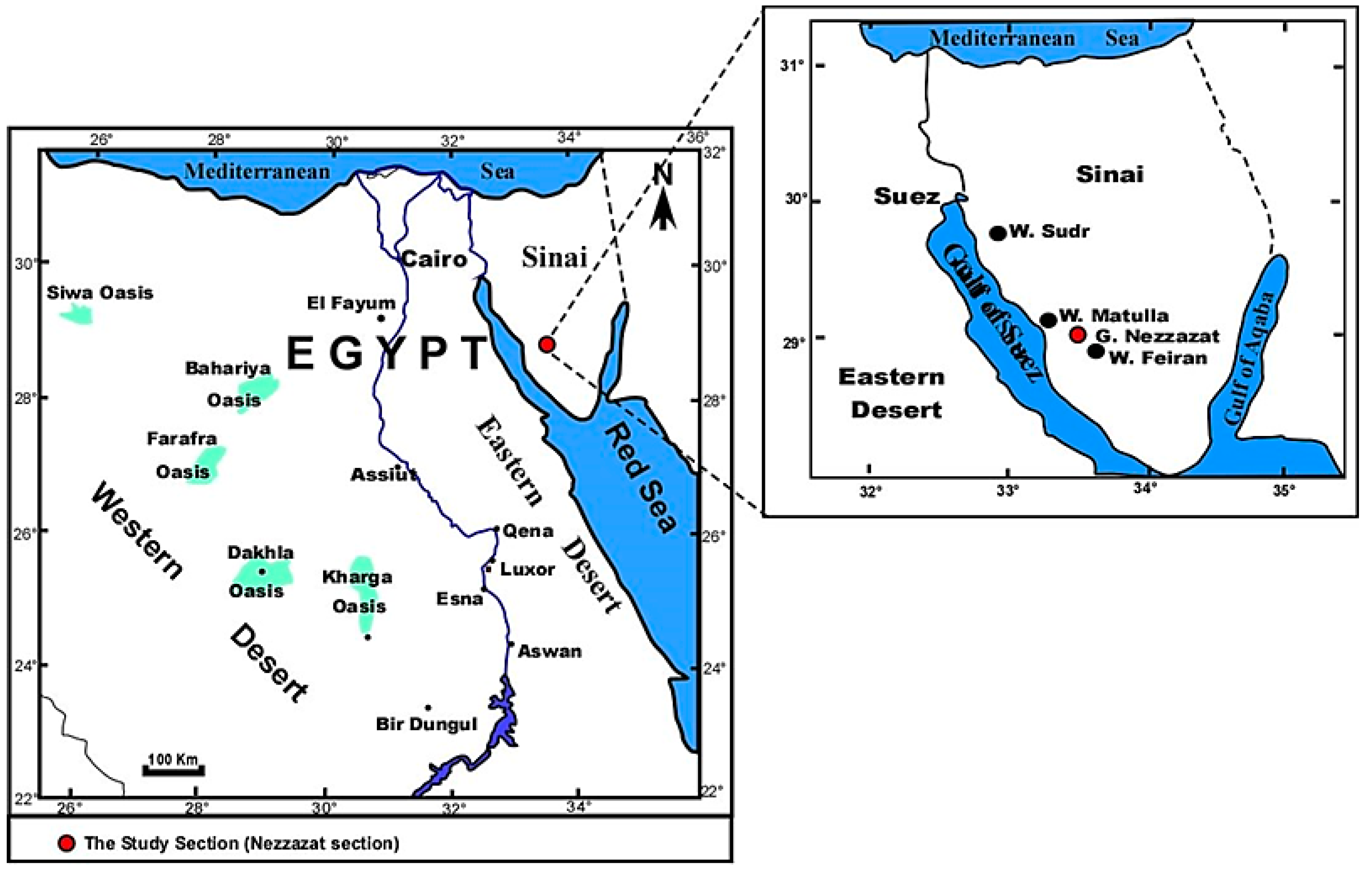
2. Geological Setting and Stratigraphical Framework
2.1. Lithostratigraphy
2.1.1. The Dakhla Formation
2.1.2. The Tarawan Formation
3. Materials and Methods
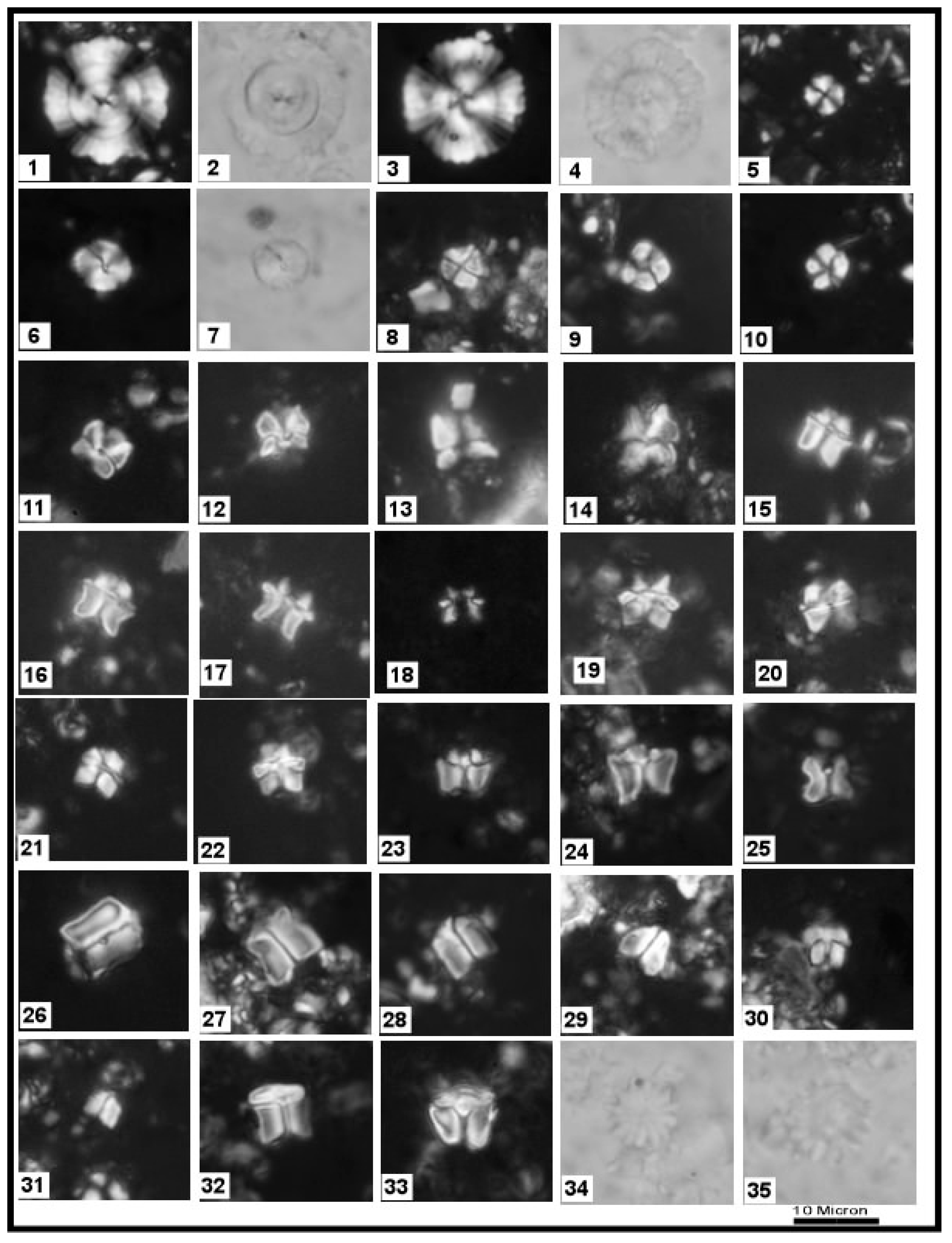
3.1. Calcareous Nannofossils
3.2. Paleoecological Inferences
4. Results
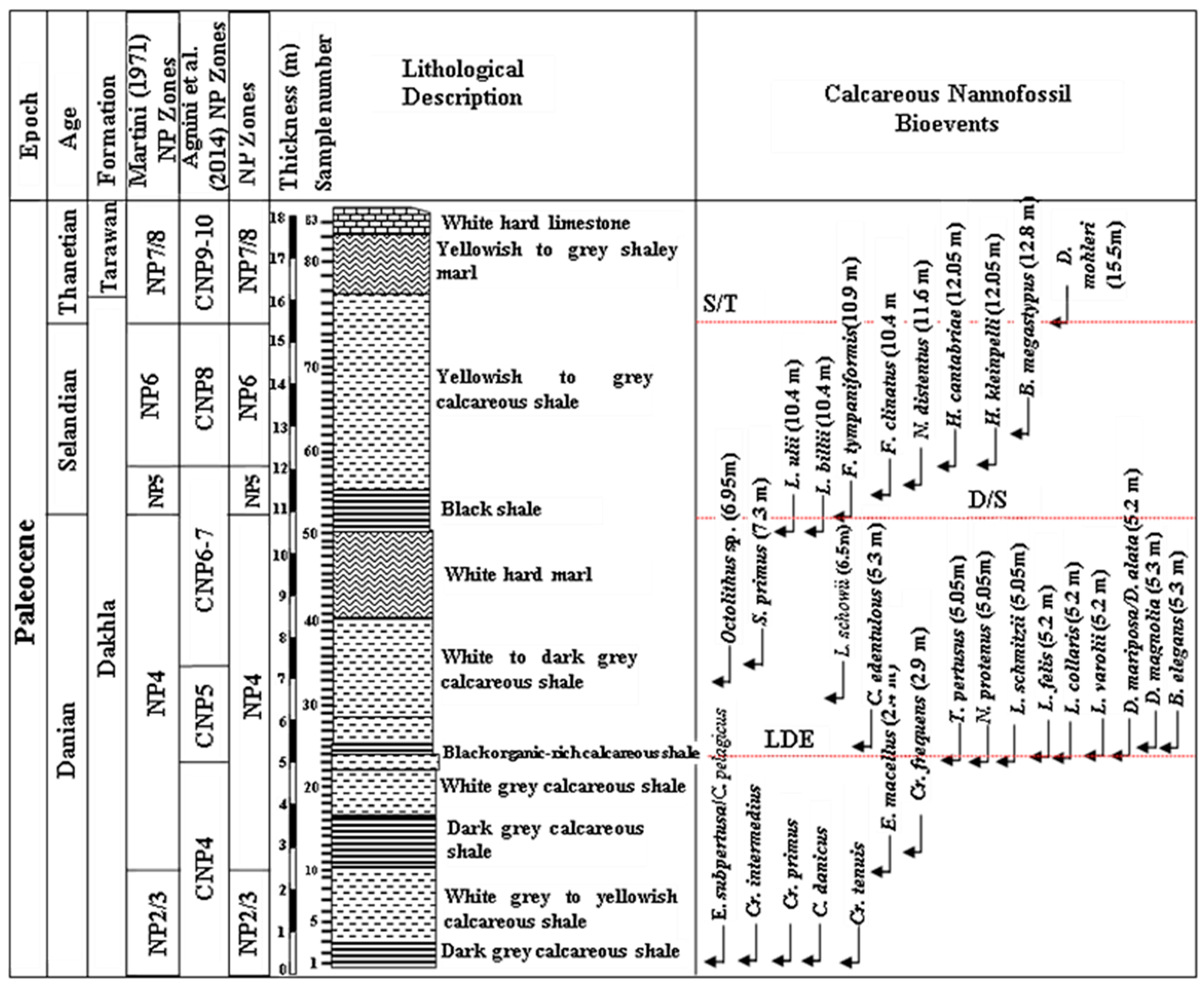
4.1. Lithostratigraphy
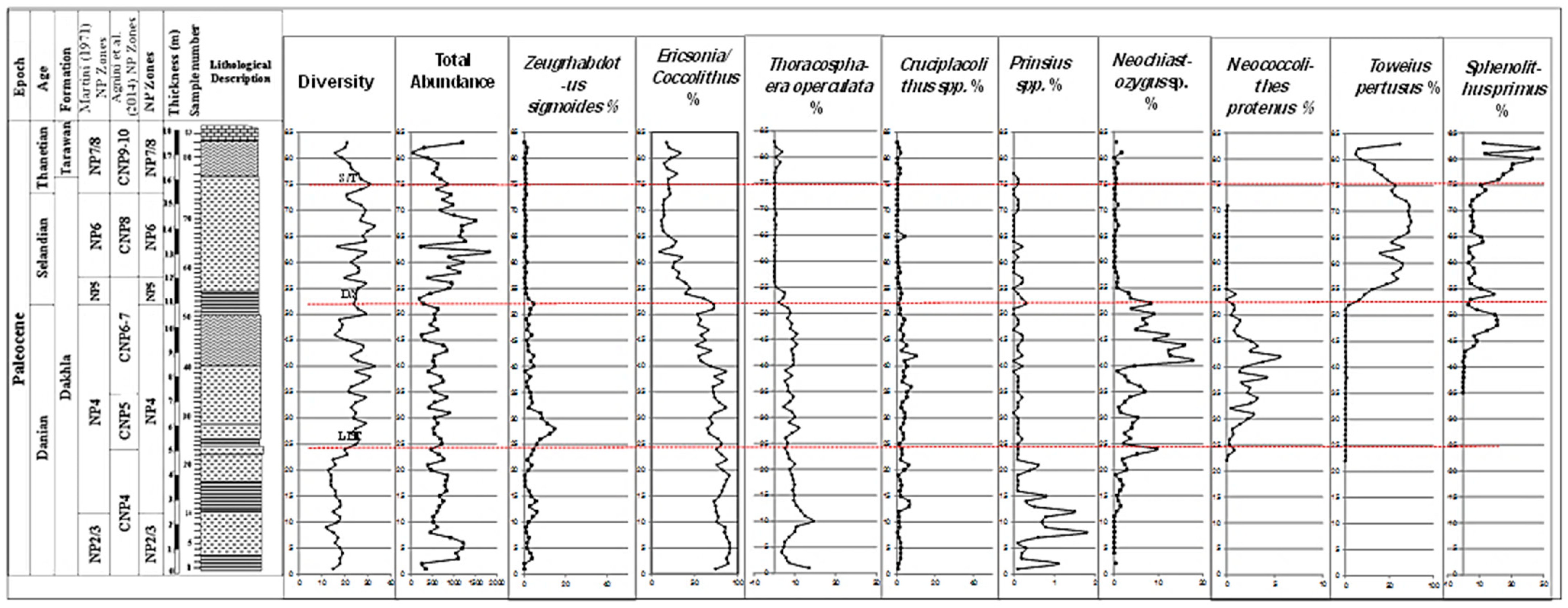
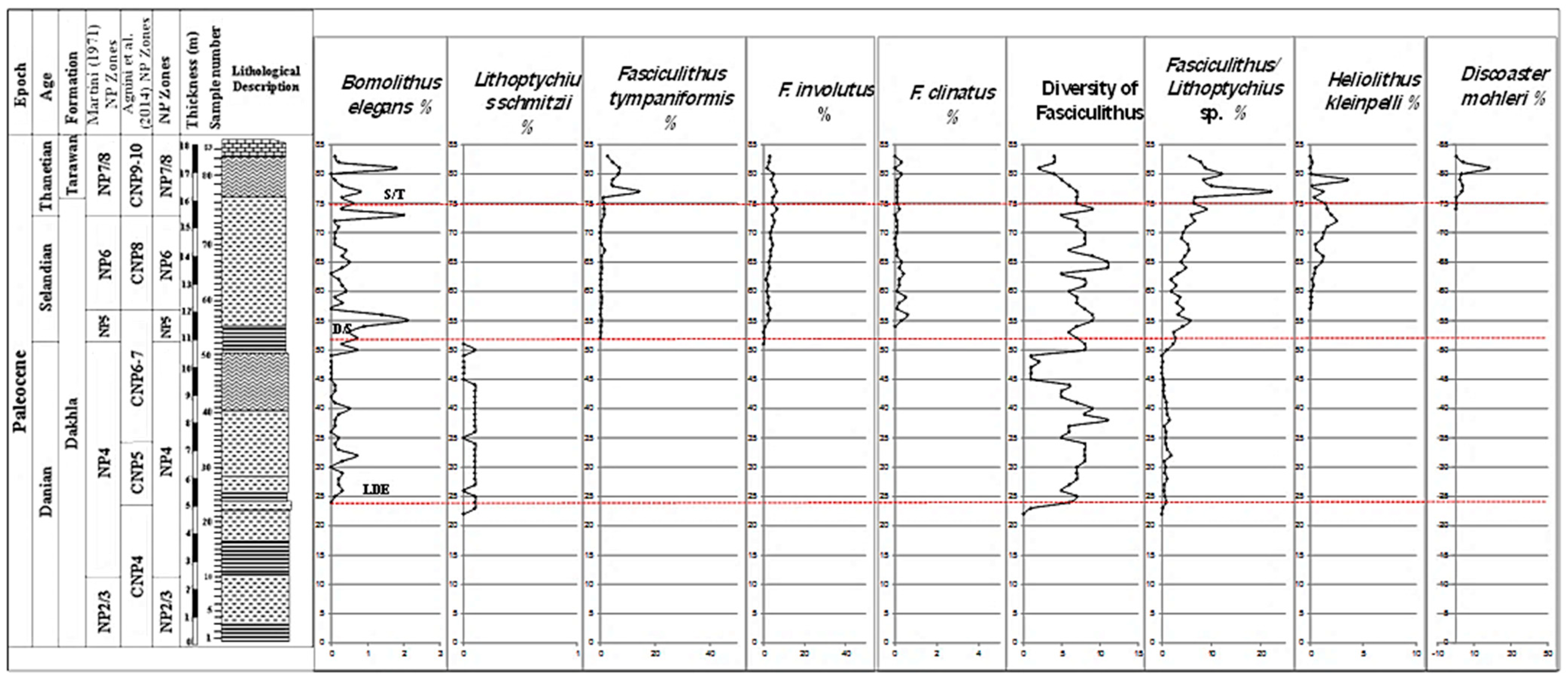
4.2. Biostratigraphy
- Cruciplacolithus tenuis Zone (NP2/3)
- Ellipsolithus macellus Zone (NP4)
- Fasciculithus tympaniformis Zone (NP5)
- Heliolithus kleinpellii Zone (NP6)
- Discoaster mohleri Zone (NP7/8)
5. Discussion
5.1. Biostratigraphical Remarks and Calcareous Nannofossil Zones
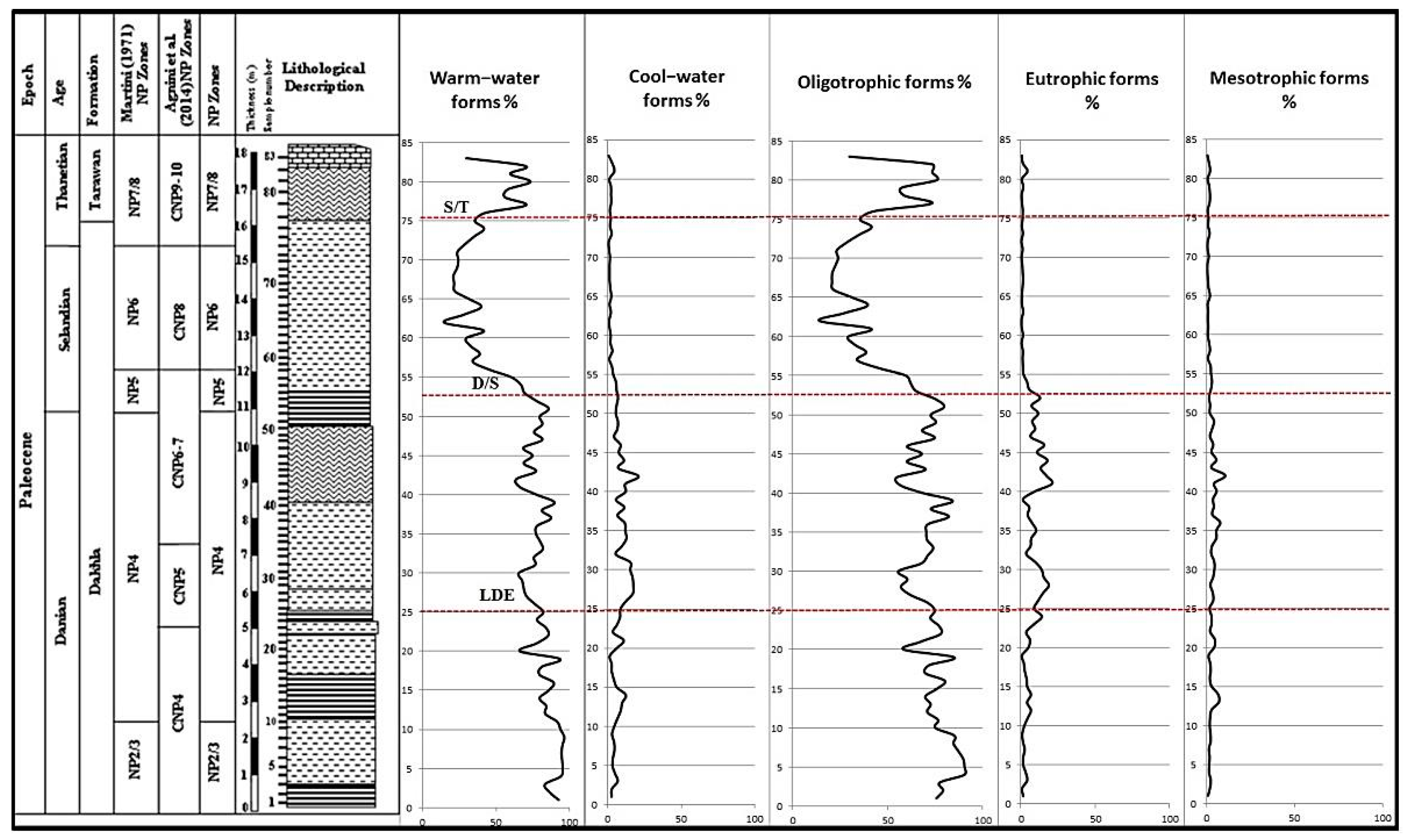
5.2. The Latest Danian Event
5.3. The Danian/Selandian Boundary
5.4. The Selandian/Thanetian Boundary
5.5. Paleoecology
5.5.1. Danian Record
5.5.2. Selandian Record
5.5.3. Thanetian Record
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jenkins, D.G.; Luterbacber, H.P. Paleogene Stages and their boundaries. Neues Jahrb. Geol. Paläontologie 1992, 186, 1–5. [Google Scholar]
- Speijer, R.P.; Pälike, H.; Hollis, C.J.; Hooker, J.J.; Ogg, J.G. The Paleogene Period. In Geologic Time Scale 2020; Elsevier: Amsterdam, The Netherlands, 2021; Volume 2, pp. 1087–1140. [Google Scholar]
- Bralower, T.J.; Premoli Silva, I.; Malone, M.J. New evidence for abrupt climate change in the Cretaceous and Paleogene: An ocean drilling program expedition to Shatsky Rise, northwest Pacific. GSA Today 2002, 12, 4–10. [Google Scholar] [CrossRef]
- Bernaola, G.; Baceta, J.I.; Orue-Extebarria, X.; Alegret, L.; Martin-Rubio, M.; Arostegui, J.; Dinarès-Turell, J. Evidence of an abrupt environmental disruption during the mid-Paleocene biotic event (Zumaia section, western Pyrenees). Geol. Soc. Am. Bull. 2007, 119, 785–795. [Google Scholar] [CrossRef]
- Frontalini, F.; Coccioni, R.; Catanzariti, R.; Jovane, L.; Savian, J.F.; Sprovieri, M. The Eocene Thermal Maximum 3: Reading the environmental perturbations at Gubbio (Italy). In The Stratigraphic Record of Gubbio: Integrated Stratigraphy of the Late Cretaceous–Paleogene Umbria-Marche Pelagic Basin; Geological Society of America: Boulder, CO, USA, 2016; Volume 524, pp. 161–326. [Google Scholar]
- Kasem, A.M.; Wise, S., Jr.; Faris, M.; Farouk, S.; Zahran, E. Calcareous nannofossil biostratigraphy of the Paleocene at the Misheiti section, East Central Sinai, Egypt. Arab. J. Geosci. 2017, 10, 455. [Google Scholar] [CrossRef]
- Faris, M.; Obaidalla, N.A.; Metwally, A.A.; Salman, A.M.; Zaky, A.S. Late Cretaceous–Early Paleogene tectonic events at Farafra-Abu Minqar Stretch, Western Desert, Egypt: Results from calcareous plankton. Arab. J. Geosci. 2018, 11, 1–18. [Google Scholar]
- Coccioni, R.; Frontalini, F.; Catanzariti, R.; Jovane, L.; Rodelli, D.; Rodrigues, I.M.; Savian, J.F.; Giorgioni, M.; Galbrun, B. Paleoenvironmental signature of the selandian-thanetian transition event (STTE) and early late paleocene event (ELPE) in the Contessa Road section (western Neo-Tethys). Palaeogeogr. Palaeoclimatol. Palaeoecol. 2019, 523, 62–77. [Google Scholar] [CrossRef]
- Farouk, S.; Khalifa, M.A.; Hassan, M.M.A.; Papazzoni, C.A.; Frontalini, F.; Coccioni, R.; Zaky, A. Upper Paleocene to lower Eocene microfacies, biostratigraphy, and paleoenvironmental reconstruction in the northern Farafra Oasis, Western Desert (Egypt). Micropaleontology 2019, 65, 361–406. [Google Scholar]
- Bornemann, A.; Schulte, P.; Sprong, J.; Steurbaut, E.; Youssef, M.; Speijer, R.P. Latest Danian carbon isotope anomaly and associated environmental change in the southern Tethys (Nile Basin, Egypt). J. Geol. Soc. 2009, 166, 1135–1142. [Google Scholar] [CrossRef] [Green Version]
- Sprong, J.; Youssef, M.; Bornemann, A.; Schulte, P.; Stassen, P.; Steurbaut, E.; Kouwenhovens, T.J.; Speijer, R.P. A multi-proxy record of the Latest Danian Event at Gebel Qreiya, Eastern Desert, Egypt. J. Micropalaeontol. 2011, 30, 167–182. [Google Scholar] [CrossRef] [Green Version]
- Sprong, J.; Kouwenhoven, T.J.; Bornemann, A.; Schulte, P.; Stassen, P.; Steurbaut, E.; Youssef, M.; Speijer, R.P. Characterization of the Latest Danian Event by means of benthic foraminiferal assemblages along a depth transect at the southern Tethyan margin (Nile Basin, Egypt). Mar. Micropaleontol. 2012, 86–87, 15–31. [Google Scholar] [CrossRef] [Green Version]
- Sprong, J.; Kouwenhovens, T.J.; Bornemann, A.; Dupuis, C.; Speijer, R.P.; Stassen, P.; Steurbaut, E. In search of the Latest Danian Event in a paleobathymetric transect off Kasserine Island, north-central Tunisia. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2013, 379–380, 1–16. [Google Scholar] [CrossRef] [Green Version]
- Arenillas, I.; Molina, E.; Ortiz, S.; Schmitz, B. Foraminiferal and δ13C isotopic event-stratigraphy across the Danian–Selandian transition at Zumaya (northern Spain): Chronostratigraphic implications. Terra Nova 2008, 20, 38–44. [Google Scholar] [CrossRef]
- Schmitz, B.; Pujalte, V.; Molina, E.; Monechi, S.; Orue-Etxebarria, X.; Speijer, R.P.; Alegret, L.; Apellaniz, E.; Arenillas, I.; Aubry, M.-P.; et al. The Global Stratotype Sections and Points for the bases of the Selandian (Middle Paleocene) and Thanetian (Upper Paleocene) stages at Zumaia, Spain. Epis. Int. Geosci. Newsmag. 2011, 34, 220–243. [Google Scholar] [CrossRef]
- Palcu, D.V.; Muraszko, J.R.; Jaqueto, P.F.; Jovane, L. The Birth of a Connected South Atlantic Ocean: A Magnetostratigraphic Perspective. Front. Earth Sci. 2020, 8, 375. [Google Scholar] [CrossRef]
- Thomsen, E.; Heilmann-Clausen, C. The Danian–Selandian boundary at Svejstrup with remarks on the biostratigraphy of the boundary in western Denmark. B Geol. Soc. Den. 1985, 33, 341–362. [Google Scholar] [CrossRef]
- Steurbaut, E.; Sztrákos, K. Danian/Selandian boundary criteria and North Sea basin-Tethys correlation based on calcareous nannofossil and foraminiferal trends in SW France. Mar. Micropaleontol. 2008, 67, 1–29. [Google Scholar] [CrossRef]
- Bernaola, G.; Martín-Rubio, M.; Baceta, J.I. New high resolution calcareous nannofossil analysis across the Danian/Selandian transition at the Zumaia section: Comparison with South Tethys and Danish sections. Geol. Acta 2009, 7, 79–92. [Google Scholar]
- Renevier, E. Tableau des terrains sédimentaires formés pendant les époques de la phase organique du globe Terrestre. Bulletin de la Société Vaudoise des Sciences Naturelles 1873, 12, 218–252. [Google Scholar]
- Dupuis, C.; Aubry, M.-P.; Steurbaut, E.; Berggren, W.A.; Ouda, K.; Magioncalda, R.; Cramer, B.S.; Kent, D.V.; Speijer, R.P.; Heilmann-Clausen, C. The Dababiya Quarry section: Lithostratigraphy, clay mineralogy, geochemistry and paleontology. Micropaleontology 2003, 49, 41–59. [Google Scholar] [CrossRef] [Green Version]
- Guasti, E.; Speijer, R.P.; Brinkhuis, H.; Smit, J.; Steurbaut, E. Paleoenvironmental change at the Danian–Selandian transition in Tunisia; Foraminifera, organic-walled dinoflagellate cyst and calcareous nannofossil records. Mar. Micropaleontol. 2006, 59, 210–229. [Google Scholar] [CrossRef]
- Aubry, M.-P.; Ouda, K.; Dupuis, C.; Berggren, W.A.; Van Couvering, J.A.; Members of the Working Group on the Paleocene/Eocene Boundary. The Global Standard Stratotype-Section and Point (GSSP) for the base of the Eocene Series in the Dababiya section (Egypt). Episodes 2007, 30, 271–286. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aubry, M.-P.; Rodriguez, O.; Bord, D.; Godfrey, L.; Schmitz, B.; Knox, R. The First Radiation of the Fasciculiths: Morphologic adaptations of the coccolithophores to oligotrophy. Austrian J. Earth Sc. 2012, 105, 29–38. [Google Scholar]
- Sprong, J.; Speijer, R.P.; Steurbaut, E. Biostratigraphy of the Danian/Selandian transition in the southern Tethys. Special reference to the lowest occurrence of planktic foraminifera Igorina albeari. Geol. Acta 2009, 7, 63–77. [Google Scholar]
- Aubry, M.-P.; Salem, R. The Dababiya Quarry Core: Coccolith Biostratigraphy. Stratigraphy 2013, 9, 241–259. [Google Scholar]
- Aubry, M.-P.; Salem, R. The Dababiya Core: A window into Paleocene to Early Eocene depositional history in Egypt based on coccolith stratigraphy. Stratigraphy 2013, 9, 287–346. [Google Scholar]
- Said, R. Planktonic foraminifera from the Thebes Formation, Luxor, Egypt. Micropaleontology 1960, 6, 277–286. [Google Scholar] [CrossRef]
- Awad, G.H.; Ghobrial, M.G. Zonal stratigraphy of the Kharga Oasis. Ann. Geol. Surv. Egypt 1966, 34, 1–77. [Google Scholar]
- Speijer, R. Danian-Selandian Sea-Level Change and Biotic Excursion on the Southern Tethyan Margin (Egypt); Special Paper 369; Geological Society of America: Boulder, CO, USA, 2003; pp. 275–290. [Google Scholar]
- Guasti, E.; Speijer, R.P.; Fornaciari, E.; Schmitz, B.; Kroon, D.; Gharaibeh, A. Transient Biotic Change within the Danian-Selandian Transition in Egypt and Jordan. Early Paleogene Environmental Turnover in the Southern Tethys as Recorded by Foraminiferal and Organic-Walled Dinoflagellate Cysts Assemblages. Ph.D. Thesis, University of Bremen, Bremen, Germany, 2005; pp. 75–110. [Google Scholar]
- Soliman, M.F.; Obaidalla, N.A. Danian–Selandian transition at the Gabal el-Qreiya section, Nile Valley (Egypt): Lithostratigraphy, biostratigraphy, mineralogy and geochemistry. Neues Jahrb. Geol. Paläontologie 2010, 258, 1–30. [Google Scholar] [CrossRef]
- Soliman, M.F.; Obaidalla, N.A.; Ahmed, E.A.; Kurzweil, J. Mid-Paleocene event at Gabal Nezzazat, Sinai, Egypt: Planktonic foraminiferal biostratigraphy, mineralogy and geochemistry. Arab. J. Geosci. 2014, 7, 4079–4099. [Google Scholar] [CrossRef]
- Speijer, R.P. The late Paleocene event and a potential precursor compared: First results from Egypt. GFF 2000, 122, 150–151. [Google Scholar] [CrossRef]
- Speijer, R.P. Systematics and paleoecology of the foraminifer Neoeponides duwi (Nakkady) from the Paleocene of Egypt. Micropaleontology 2003, 49, 146–150. [Google Scholar] [CrossRef]
- Obaidalla, N.A.; El-Dawy, M.H.; Kassab, A.S. Biostratigraphy and paleoenvironment of the Danian/Selandian (D/S) transition in the Southern Tethys: A case study from north Eastern Desert, Egypt. J. Afr. Earth Sci. 2009, 53, 1–15. [Google Scholar] [CrossRef]
- Monechi, S.; Reale, V.; Bernaola, G.; Balestra, B. The Danian/Selandian boundary at Site 1262 (South Atlantic) and in the Tethyan region: Biomagnetostratigraphy, evolutionary trends in fasciculiths and environmental effects of the Latest Danian Event. Mar. Micropaleontol. 2013, 98, 28–40. [Google Scholar] [CrossRef]
- Gardin, S.; Monechi, S. Palaeoecological change in middle to low latitude calcareous nannoplankton at the Cretaceous/Tertiary boundary. Br. Soc. Geol Fr. 1998, 169, 709–723. [Google Scholar]
- Martini, E. Standard Tertiary and Quaternary calcareous nannoplankton zonation. in Proc. II Planktonic Conference, Roma 1970, Roma. Tecnoscienza 1971, 2, 739–785. [Google Scholar]
- Bukry, D. Low latitude coccolith biostratigraphic zonation. Initial Rep. DSDP 1973, 15, 685–703. [Google Scholar]
- Bukry, D. Coccolith and silicoflagellate stratigraphy, northwestern Pacific Ocean, Deep Sea Drilling Project Leg 32. Init. Repts. DSDP 1975, 32, 677–701. [Google Scholar]
- Sissingh, W. Biostratigraphy of Cretaceous calcareous nannoplankton. Geologie en Mijnbouw 1977, 56, 37–65. [Google Scholar]
- Okada, H. Supplementary modification and introduction of code numbers to the low-latitude coccolith biostratigraphy (Bukry, 1973; 1975). Mar. Micropaleontol. 1980, 5, 321–325. [Google Scholar] [CrossRef]
- Perch-Nielsen, K. Cenozoic calcareous nannofossils. In Plankton Stratigraphy; Bolli, H.M., Saunders, J.B., Perch-Nielsen, K., Eds.; Cambridge University Press: Cambridge, UK, 1985; pp. 427–554. [Google Scholar]
- Varol, O. Palaeocene calcareous nannofossil biostratigraphy. In Nannofossils and Their Applications; Crux, J.A., Van Heck, S.E., Eds.; British Micropaleontological Society: Chichester, UK, 1989; Volume 12, pp. 267–310. [Google Scholar]
- Agnini, C.; Fornaciari, E.; Raffi, I.; Catanzariti, R.; Pälike, H.; Backman, J.; Rio, D. Biozonation and biochronology of Paleogene calcareous nannofossils from low and middle latitudes. Newsl. Stratigr. 2014, 47, 131–181. [Google Scholar] [CrossRef]
- Agnini, C.; Monechi, S.; Raffi, I. Calcareous nannofossil biostratigraphy: Historical background and application in Cenozoic chronostratigraphy. Lethaia 2017, 50, 447–463. [Google Scholar] [CrossRef]
- Romein, A.J.T. Lineages in early Paleogene calcareous nannoplankton. Utrecht Micropaleontol. Bull. 1979, 22, 18–22. [Google Scholar]
- Winter, A.; Jordan, R.W.; Roth, P.H. Biogeography of living coccolithophores in ocean waters. In Coccolithophores; Winter, A., Siesser, W.G., Eds.; Cambridge University Press: Cambridge, UK, 1994; pp. 161–178. [Google Scholar]
- Bralower, T.J. Evidence of surface water oligotrophy during the Paleocene-Eocene thermal maximum: Nannofossil assemblage data from ocean drilling program Site 690, Maud rise, Weddell Sea. Paleoceanography 2002, 17, 13-1–13-12. [Google Scholar] [CrossRef]
- Bornemann, A. Case Studies of Mesozoic Calcareous Nannofossils: Implications for Paleoecology, Calcareous Nannofossil Morphology and Carbonate Accumulation. Ph.D. Thesis, Ruhr-University, Bochum, Germany, 2003; 126p. [Google Scholar]
- Watkins, D.K. Nannoplankton productivity fluctuations and rhythmically-bedded pelagic carbonates of the Greenhorn Limestone (Upper Cretaceous). Palaeogeogr. Palaeoclimatol. Palaeoecol. 1989, 74, 75–86. [Google Scholar] [CrossRef]
- Fuqua, L.; Bralower, T.; Arthur, M.; Patzkowsky, M. Evolution of calcareous nannoplankton and recovery of marine food webs after the Cretaceous–Paleocene mass extinction. Palaios 2008, 23, 185–194. [Google Scholar] [CrossRef]
- Okada, H.; Honjo, S. The distribution of oceanic coccolithophorids in the Pacific. Deep Sea Res. Oceanogr. Abstr. 1973, 20, 355–374. [Google Scholar] [CrossRef]
- Thibault, N.; Gardin, S. The late Maastrichtian nannofossil record of climate change in the South Atlantic DSDP Hole 525A. Mar. Micropaleontol. 2007, 65, 163–184. [Google Scholar] [CrossRef]
- Haq, B.U.; Lohmann, G.P. Early Cenozoic calcareous nannoplankton biogeography of the Atlantic Ocean. Mar. Micropaleontol. 1976, 1, 119–194. [Google Scholar] [CrossRef]
- Bramlette, M.N.; Martini, E. The great change in calcareous nannoplankton fossils between the Maestrichtian and Danian. Micropaleontology 1964, 10, 291–322. [Google Scholar] [CrossRef]
- Gartner, S. Calcareous nannofossils from the JOIDES Blake Plateau cores and revision of Paleogene nannofossil zonation. Tulane Stud. Geol. Paleontol. 1971, 8, 101–121. [Google Scholar]
- Okada, H.; McIntyre, A. Seasonal distribution of modern coccolithophores in the western North Atlantic Ocean. Mar. Biol. 1979, 54, 319–328. [Google Scholar] [CrossRef]
- Wei, W.; Wise, S.W., Jr. Biogeographic gradients of middle Eocene-Oligocene calcareous nannoplankton in the South Atlantic Ocean. Palaeogeogr. Palaeoclimatol. Palaeoecol. 1990, 79, 29–61. [Google Scholar] [CrossRef]
- Fisher, C.G.; Hay, W.W. Calcareous Nannofossils as Indicators of Mid-Cretaceous Paleofertility along an Ocean Front, US Western Interior; Special Paper 332; Geological Society of America: Boulder, CO, USA, 1999; pp. 161–180. [Google Scholar]
- Tremolada, F.; Bralower, T.J. Nannofossil assemblage fluctuations during the Paleocene–Eocene thermal maximum at Sites 213 (Indian Ocean) and 401 (North Atlantic Ocean): Palaeoceanographic implications. Mar. Micropaleontol. 2004, 52, 107–116. [Google Scholar] [CrossRef]
- Gibbs, S.J.; Bown, P.R.; Bralower, T.J. Ocean acidification and calcareous nannoplankton at the Paleocene-Eocene Thermal Maximum. Clim. Biota Early Paleogene 2006, 51. [Google Scholar]
- Bernaola, G.; Monechi, S. Calcareous nannofossil extinction and survivorship across the Cretaceous−Paleogene boundary at Walvis Ridge (ODP Hole 1262C, South Atlantic Ocean). Palaeogeogr. Palaeoclimatol. Palaeoecol. 2007, 255, 132–156. [Google Scholar] [CrossRef]
- Raffi, I.; Backman, J.; Zachos, J.C.; Sluijs, A. The response of calcareous nannofossil assemblages to the Paleocene Eocene Thermal Maximum at the Walvis Ridge in the South Atlantic. Mar. Micropaleontol. 2009, 70, 201–212. [Google Scholar] [CrossRef] [Green Version]
- Hay, W.W.; Mohler, H.P. Calcareous nannoplankton from early Tertiary rocks at Pont Labau, France, and Paleocene–early Eocene correlations. J. Paleontol. 1967, 41, 1505–1541. [Google Scholar]
- Hay, W.W.; Mohler, H.P.; Roth, P.H.; Schmidt, R.R.; Boudreaux, J.E. Calcareous nannoplankton zonation of the Cenozoic of the Gulf Coast and Caribbean-Antillean area and transoceanic correlation. Gulf Coast Assoc. Geol. Soc. J. 1967, 17, 428–480. [Google Scholar]
- Hay, W.W. Utilisation stratigraphique des Discoasterides pour la zonation du Paleocene et de L’Eocene inferieur. Memoire Bur. Rech. Geol. Minières 1964, 28, 885–889. [Google Scholar]
- Stradner, H. Vorkommen von Nannofossilien im Mesozoikum und Alttertiär. Erdöl-Zeitschrift für Bohr-und Fördertechnik 1961, 77, 77–88. [Google Scholar]
- Van Heck, S.E.; Prins, B. A refined nannoplankton zonation for the Danian of the Central North Sea. Abhandlungen der Geologischen Bundesanstalt 1987, 39, 285–303. [Google Scholar]
- Brotzen, F. On Tylocidaris species (Echinoidea) and the stratigraphy of the Danian of Sweden: With a bibliography of the Danian and Paleocene. In Sveriges Geologiska Undersokning, Arsbok; Norstedt & Söner: Stockholm, Sweden, 1959; Volume 54, pp. 1–81. [Google Scholar]
- Monechi, S.; Bleil, U.; Backman, J. Magnetobiochronology of Late Cretaceous–Paleogene and Late Cenozoic pelagic sedimentary sequences from the Northwest Pacific (Deep Sea drilling Project). In Initial Reports of the Deep Sea Drilling Project Leg, 86U.S.; Heath, G.R., Burckle, L.H., Eds.; Government Printing Office: Washington, DC, USA, 1985; pp. 787–797. [Google Scholar]
- Fornaciari, E.; Giusberti, L.; Luciani, V.; Tateo, F.; Agnini, C.; Backman, J.; Oddone, M.; Rio, D. An expanded Cretaceous–Tertiary transition in a pelagic setting of the Southern Alps (central-western Tethys). Palaeogeogr. Palaeoclimatol. Palaeoecol. 2007, 255, 98–131. [Google Scholar] [CrossRef]
- Agnini, C.; Fornaciari, E.; Raffi, I.; Rio, D.; Röhl, U.; Westerhold, T. High-resolution nannofossil biochronology of middle Paleocene to early Eocene at ODP Site 1262: Implications for calcareous nannoplankton evolution. Mar. Micropaleontol. 2007, 64, 215–248. [Google Scholar] [CrossRef]
- Martini, E. Cretaceous to Recent Calcareous Nannoplankton from the Central Pacific Ocean (DSDP Leg 33). Initial. Rep. DSDP 1976, 33, 383–423. [Google Scholar]
- Steurbaut, E.; Dupuis, C.; Arenillas, I.; Molina, E.; Matmati, M.F. The Kalaat Senan section in central Tunisia: A potential reference section for the Danian/Selandian boundary. GFF 2000, 122, 158–160. [Google Scholar] [CrossRef]
- Quillévéré, F.; Aubry, M.-P.; Norris, R.D.; Berggren, W.A. Paleocene oceanography of the eastern subtropical Indian Ocean: An integrated magnetobiostratigraphic and stable isotope study of ODP Hole 761B (Wombat Plateau). Palaeogeogr. Palaeoclimatol. Palaeoecol. 2002, 184, 371–405. [Google Scholar] [CrossRef]
- Faris, M.; Salem, R. Paleocene-Eocene calcareous nannofossil biostratigraphy in west central Sinai, Egypt. In Proceedings of the 8th Conference on Geology of Sinai for Development, Ismailia, Egypt, 3 December 2007; pp. 1–14. [Google Scholar]
- Dinarès-Turell, J.; Stoykova, K.; Baceta, J.I.; Ivanov, M.; Pujalte, V. High-resolution intra- and interbasinal correlation of the Danian–Selandian transition (Early Paleocene): The Bjala section (Bulgaria) and the Selandian GSSP at Zumaia (Spain). Palaeogeogr. Palaeoclimatol. Palaeoecol. 2010, 297, 511–533. [Google Scholar] [CrossRef]
- Arney, J.; Wise, S.W. Paleocene-Eocene Nannofossil Biostratigraphy of ODP Leg 183, Kerguelen Plateau. In Proceedings of the Ocean Drilling Program, Scientific Results; Frey, F.A., Coffin, M.F., Wallace, P.J., Quilty, P.G., Eds.; Ocean Drilling Program: College Station, TX, USA, 2003; Volume 183, pp. 1–59. [Google Scholar]
- Faris, M.; Farouk, S. Integrated biostratigraphy of two Upper Maastrichtian-Paleocene successions in north-central Sinai, Egypt. Geol. Croat. 2012, 65/2, 139–160. [Google Scholar] [CrossRef]
- Dinarès-Turell, J.; Baceta, J.I.; Bernaola, G.; Orue-Etxebarria, X.; Pujalte, V. Closing the Mid-Palaeocene gap: Toward a complete astronomically tuned Palaeocene Epoch and Selandian and Thanetian GSSPs at Zumaia (Basque Basin, W Pyrenees). Earth Planet Sci. Lett. 2007, 262, 450–467. [Google Scholar] [CrossRef]
- Ali, M.Y. High resolution calcareous nannofossil biostratigraphy and paleoecology across the Latest Danian Event (LDE) in central Eastern Desert, Egypt. Mar. Micropaleontol. 2009, 72, 111–128. [Google Scholar]
- Wei, W.; Wise, S.W., Jr. Paleogene calcareous nannofossil magnetobiochronology: Results from South Atlantic DSDP site 616. Mar. Micropaleontol. 1989, 14, 119–152. [Google Scholar] [CrossRef]
- Tantawy, A.A. Stratigraphical and Paleoecological Studies on Some Paleocene–Eocene Successions in Egypt. Unpub. Ph.D. Thesis, South Valley University, Quena, Egypt, 1998; 273p. [Google Scholar]
- Faris, M.; Abdel Hameed, A.T.; Shaaban, M. Calcareous nannofossil events at the Cretaceous/Paleocene boundary in central Egypt. In Proceedings of the 1st International Conference on the Geology of Africa, Assiut, Egypt, 23 November 1999; Volume 2, pp. 21–42. [Google Scholar]
- Bukry, D.; Percival, S.F. New Tertiary calcareous nannofossils. Tulane Stud. Geol. Paleontol. 1971, 8, 123–146. [Google Scholar]
- Marzouk, A.M.; Luning, S. Comparative biostratigraphy of calcareous nannofossils and planktonic foraminifera in the Paleocene of the Eastern Sinai, Egypt. Neues Jahrb. Geol. Paläontologie 1998, 207, 77–105. [Google Scholar] [CrossRef]
- Clemmensen, A.; Thomsen, E. Palaeoenvironmental changes across the Danian–Selandian boundary in the North Sea Basin. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2005, 219, 351–394. [Google Scholar] [CrossRef]
- Westerhold, T.; Röhl, U.; Donner, B.; McCarren, H.K.; Zachos, J. A complete high-resolution Paleocene benthic stable isotope record for the central Pacific (ODP Site 1209). Paleoceanography 2011, 26, PA2216. [Google Scholar] [CrossRef] [Green Version]
- Aubry, M.-P.; Bord, D.; Rodriguez, O. New taxa of the Order Discoasterales Hay 1977. Micropaleontology 2011, 57, 269–287. [Google Scholar]
- Faris, M.; Abu Shama, A.M. Nannofossil biostratigraphy of the Paleocene lower Eocene succession in the Thamad area, east central Sinai, Egypt. Micropaleontology 2007, 53, 127–144. [Google Scholar] [CrossRef]
- Faris, M.; Ayyad, S.N.; El Nahass, H.A.; Al Wosabi, K. Early Palaeogene stages and their boundaries in Sinai, Egypt. In Proceedings of the Fourth International Conference on the Geology of Africa, Assiut, Egypt, 15–16 November 2005; Volume 2, pp. 753–768. [Google Scholar]
- Berggren, W.A.; Aubry, M.-P.; van Fossen, M.; Kent, D.V.; Norris, R.D.; Quillevere, F. Integrated Paleocene calcareous plankton magnetobiochronology and stable isotope stratigraphy: DSDP Site 384 (NW Atlantic Ocean). Palaeogeogr. Palaeoclimatol. Palaeoecol. 2000, 159, 1–51. [Google Scholar] [CrossRef] [Green Version]
- Tantawy, A.A.; Ouda, K.; Von Salis, K.; El-Din, M.S. Biostratigraphy of Paleocene sections in Egypt. GFF 2000, 122, 163–165. [Google Scholar] [CrossRef]
- Zachos, J.C.; Kroon, D.; Blum, P.; Bowles, J.; Gaillot, P.; Hasegawa, T.; Hathorne, E.C.; Hodell, D.A.; Kelly, D.C.; Jung, J.H.; et al. Early Cenozoic extreme Climates: The Walvis Ridge transect, Proceedings of the Ocean Drilling Program, Initial reports Leg 208. Proc. Ocean Drill. Program Initial Rep. 2004, 208, 1–117. [Google Scholar]
- Bernaola, G.; Nuño-Arana, Y. Calcareous nannofossil assemblages across the mid-Paleocene. In The Paleocene and Lower Eocene of the Zumaia Section (Basque Basin). Climate and Biota of the Early Paleogene 2006; Post conference Field Trip Guidebook; University of the Basque Country: Bilbao, Spain; pp. 44–46.
- Aubry, M.-P. Paleogene calcareous nannofossils from the Kerguelen Plateau, Leg 120. In Proceedings of the Ocean Drilling Program, Scientific Results; Ocean Drilling Program: College Station, TX, USA, 1992; Volume 120, pp. 471–491. [Google Scholar]
- Agnini, C.; Fornaciari, E.; Rio, D.; Tateo, F.; Backman, J.; Giusberti, L. Responses of calcareous nannofossil assemblages, mineralogy and geochemistry to the environmental perturbations across the Paleocene/Eocene boundary in the Venetian Pre-Alps. Mar. Micropaleontol. 2007, 63, 19–38. [Google Scholar] [CrossRef]
- Bown, P.R.; Pearson, P. Calcareous plankton evolution and the Paleocene/Eocene thermal maximum event: New evidence from Tanzania. Mar. Micropaleontol. 2009, 71, 60–70. [Google Scholar] [CrossRef]
- Aubry, M.-P. Early Paleogene calcareous nannoplankton evolution: A tale of climatic amelioration. In Late Paleocene–Early Eocene Biotic and Climatic Events in the Marine and Terrestrial Record; Aubry, M.-P., Ouda, K., Eds.; Columbia University Press: New York, NY, USA, 1998; pp. 158–201. [Google Scholar]
- Kahn, A.; Aubry, M.-P. Provincialism associated with the Paleocene/Eocene Thermal Maximum: Temporal constraint. Mar. Micropaleontol. 2004, 52, 117–132. [Google Scholar] [CrossRef]
- Haq, B.U.; Lohmann, G.P.; Wise, S.W. Calcareous nannoplankton biogeography and its paleoclimatic implications: Cenozoic of Falkland Plateau (DSDP Leg 36) and Miocene of the Atlantic Ocean. Initial Rep. Deep Sea Drill. Proj. 1976, 36, 745–760. [Google Scholar]
- Wise, S.W., Jr.; Wind, F.H. Mesozoic and Cenozoic calcareous nannofossils recovered by DSDP Leg 36 drilling on the Falkland Plateau, southwest Atlantic sector of the Southern Ocean. Proc. Deep Sea Proj. Initial Rep. 1977, 36, 269–491. [Google Scholar]
- Pospichal, J.; Wise, S.W., Jr. Paleocene to middle Eocene calcareous nannofossils of ODP Sites 689 and 690, Maud Rise, Weddell Sea. In Ocean Drilling Program, Scientific Results, 113Ocean Drilling Program; Barker, P.R., Kennett, J.P., Eds.; Ocean Drilling Program: College Station, TX, USA, 1990; pp. 613–638. [Google Scholar]
- Firth, J.V.; Wise, S.W., Jr. A preliminary study of the evolution of Chiasmolithus in the Middle Eocene to Oligocene of Sites 647 and 748, ODP Leg 120. In Ocean Drilling Program Scientific Results; Ocean Drilling Program: College Station, TX, USA, 1992; Volume 120, pp. 493–508. [Google Scholar]
- Mutterlose, J.; Linnert, C.; Norris, R. Calcareous nannofossils from the Paleocene–Eocene thermal maximum of the equatorial Atlantic (ODP Site 1260B): Evidence for tropical warming. Mar. Micropaleontol. 2007, 65, 13–31. [Google Scholar] [CrossRef]
- Worsley, T.R. The Cretaceous–Tertiary boundary event. In Studies in Paleoceanography; SEPM Special Publication; Hay, W.W., Ed.; SEPM: Broken Arrow, OK, USA, 1974; Volume 20, pp. 94–125. [Google Scholar]
- Jiang, M.J.; Gartner, S. Calcareous nannofossil succession across the Cretaceous–Tertiary boundary in east-central Texas. Micropaleontology 1986, 32, 232–255. [Google Scholar] [CrossRef]
- Eshet, Y.; Moshkovitz, S.; Habib, D.; Benjamini, C.; Margaritz, M. Calcareous nannofossil and dinoflagellate stratigraphy across the Cretaceous/Tertiary boundary at Hor Hahar, Israel. Mar. Micropaleontol. 1992, 18, 199–228. [Google Scholar] [CrossRef]
- Pospichal, J.J. Cretaceous/Tertiary Boundary Calcareous Nannofossils from Agost, Spain. In Proceedings of the 5th INA Conference in Salamanca, Salmanaca, Spain, September 1993; Flores, J.A., Sierro, F.J., Eds.; Universidad de SalamancaPress: Salamanca, Spain, 1995; pp. 185–217. [Google Scholar]
- Lamolda, M.A.; Mihaela, C.; Melinte, M.C.; Kaiho, K. Nannofloral extinction and survivorship across the K/T boundary at Caravaca, southeastern Spain. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2005, 224, 27–52. [Google Scholar] [CrossRef]
- Tantawy, A.A. Calcareous Nannofossils across the Cretaceous–Tertiary Boundary at Brazos, Texas, U.S.A.: Extinction and Survivorship, Biostratigraphy, and Paleoecology. In The End-Cretaceous Mass Extinction and the Chicxulub Impact in Texas; SEPM Special Publication 100, Keller, G., Adatte, T., Eds.; SEPM: Tulsa, OK, USA, 2011; pp. 157–178. [Google Scholar]
- Gardin, S. Late Maastrichtian to early Danian calcareous nannofossils at Elles (Northwest Tunisia). A tale of one million years across the K–T boundary. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2002, 178, 211–231. [Google Scholar] [CrossRef]
- Abdel-Hameed, A.T.; Faris, M. Changes of relative surface water temperature throughout the Maastrichtian-Ypresian in Central Egypt. MERC Ain Shams Uni. Earth Sci. 1984, 1–16. [Google Scholar]
- Tremolada, F.; Erba, E.; Bralower, T.J. A review of calcareous nannofossil changes during the early Aptian oceanic anoxic event 1a and the Paleocene–Eocene thermal maximum: The influence of fertility, temperature, and pCO2. In Large Ecosystem Perturbations: Causes and Consequences; Monechi, S., Coccioni, R., Rampino, M.R., Eds.; Geolical Society of America: Boulder, CO, USA, 2007; Volume 424, pp. 87–96. [Google Scholar]
- Agnini, C.; Muttoni, G.; Kent, D.V.; Rio, D. Eocene biostratigraphy and magnetic stratigraphy from Possagno, Italy: The calcareous nannofossil response to climate variability, Earth. Planet. Sc. Lett. 2006, 241, 815–830. [Google Scholar] [CrossRef] [Green Version]
- Gibbs, S.J.; Bown, P.R.; Sessa, J.A.; Bralower, T.J.; Wilson, P.A. Nannoplankton extinction and origination across the Paleocene–Eocene Thermal Maximum. Science 2006, 314, 1770–1773. [Google Scholar] [CrossRef] [PubMed]
- Erba, E. Nannofossils and superplumes: The early Aptian “nannoconid crisis”. Paleoceanography 1994, 9, 483–501. [Google Scholar] [CrossRef]
| Warm-Water Taxa | Cold-Water Taxa | Eutrophic Taxa | Mesotrophic Taxa | Meso-Oligotrophic Taxa | Oligotrophic Taxa |
|---|---|---|---|---|---|
| Bomolithus spp. | Arkhangelskiella cymbiformis | Arkhangelskiella cymbiformis | Cruciplacolithus spp. | Heliolithus spp. | Bomolithus spp. |
| Coccolithus pelagicus | Blackites spp. | Blackites spp. | Prinsius spp. | - | Coccolithus pelagicus |
| Cylindralithus sculptus | Chiasmolithus spp. | Chiasmolithus spp. | T. tovae | - | Cylindralithus sculptus |
| Discoaster spp. | Cruciplacolithus spp. | Cruciplacolithus spp. | Toweius eminens | - | Discoaster spp. |
| Discoasteroides | Eiffellithus spp. | Neochiastozygus spp. | - | - | Discoasteroides |
| Ericsonia subpertusa | Markalius inversus | Prinsius spp. | - | - | Eiffellithus spp. |
| Fasciculithus spp. | Neococolithus protens | T. saxea | - | - | Ericsonia subpertusa |
| Heliolithus kleinpellii | Prinsius spp. | Zeugrhabdotus sigmoides | - | - | Fasciculithus spp. |
| Lithraphidites carniolensis | Toweius tovae | - | - | - | Heliolithus kleinpellii |
| M. prinsii | Toweius eminens | - | - | - | Lithraphidites carniolensis |
| Micula murus | Zeugrhabdotus sigmoides | - | - | - | Octolithus spp. |
| Pontosphaera spp. | - | - | - | - | Prediscosphaera cretacea |
| Rhomboaster spp. | - | - | - | - | Rhomboaster spp. |
| Sphenolithus spp. | - | - | - | - | Sphenolithus spp. |
| Thoracosphaera saxea | - | - | - | - | Tribrachiatus spp. |
| Thoracosphaera operculata | - | - | - | - | Watznaueria barnesae |
| Tribrachiatus spp. | - | - | - | - | - |
| Watznaueria barnesae | - | - | - | - | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kasem, A.M.; Faris, M.; Jovane, L.; Ads, T.A.; Frontalini, F.; Zaky, A.S. Biostratigraphy and Paleoenvironmental Reconstruction at the Gebel Nezzazat (Central Sinai, Egypt): A Paleocene Record for the Southern Tethys. Geosciences 2022, 12, 96. https://doi.org/10.3390/geosciences12020096
Kasem AM, Faris M, Jovane L, Ads TA, Frontalini F, Zaky AS. Biostratigraphy and Paleoenvironmental Reconstruction at the Gebel Nezzazat (Central Sinai, Egypt): A Paleocene Record for the Southern Tethys. Geosciences. 2022; 12(2):96. https://doi.org/10.3390/geosciences12020096
Chicago/Turabian StyleKasem, Atef M., Mahmoud Faris, Luigi Jovane, Taysir Abdelhamid Ads, Fabrizio Frontalini, and Amr S. Zaky. 2022. "Biostratigraphy and Paleoenvironmental Reconstruction at the Gebel Nezzazat (Central Sinai, Egypt): A Paleocene Record for the Southern Tethys" Geosciences 12, no. 2: 96. https://doi.org/10.3390/geosciences12020096
APA StyleKasem, A. M., Faris, M., Jovane, L., Ads, T. A., Frontalini, F., & Zaky, A. S. (2022). Biostratigraphy and Paleoenvironmental Reconstruction at the Gebel Nezzazat (Central Sinai, Egypt): A Paleocene Record for the Southern Tethys. Geosciences, 12(2), 96. https://doi.org/10.3390/geosciences12020096







