The Molecular Weight Distribution of Occluded Hydrocarbon Gases in the Khibiny Nepheline–Syenite Massif (Kola Peninsula, NW Russia) in Relation to the Problem of Their Origin
Abstract
1. Introduction
- (1)
- (2)
- A late-magmatic (below 600 °C) origin by re-speciation of a C-O-H fluid [36,37]. The speciation of various fluids in the C-O-H system is influenced by changing temperature, pressure, oxygen fugacity, and graphite activity. A change in these parameters leads to a change in the composition of the fluid.
- (3)
- (4)
- A mixed magmatic/thermogenic origin [32]. According to this hypothesis, magmatically derived abiogenic hydrocarbons may have mixed with biogenic hydrocarbons derived from the surrounding country rocks.
- (5)
- A thermogenic origin [31]. According to this theory, hydrocarbons found throughout peralkaline complexes are the result of the migration of external thermogenically-derived hydrocarbon fluids into these complexes.
2. Geological and Occluded Gas Geochemical Backgrounds
2.1. Khibiny Massif
- khibinite is a eudialyte-bearing nepheline syenite with aegirine, alkali amphibole, and many accessory minerals, particularly those containing Ti and Zr;
- foyaite is a massive, less often weakly trachytoid, leucocratic nepheline syenite;
- rischorrite is a leucocratic nepheline syenite in which the nepheline crystals are poikilitically enclosed in microcline perthite;
- lyavochorrite is a leucocratic nepheline syenite in which only part of the feldspar crystals is poikilitic.
2.2. Occluded Gases
3. Materials and Methods
4. Results
5. Discussion
6. Conclusions
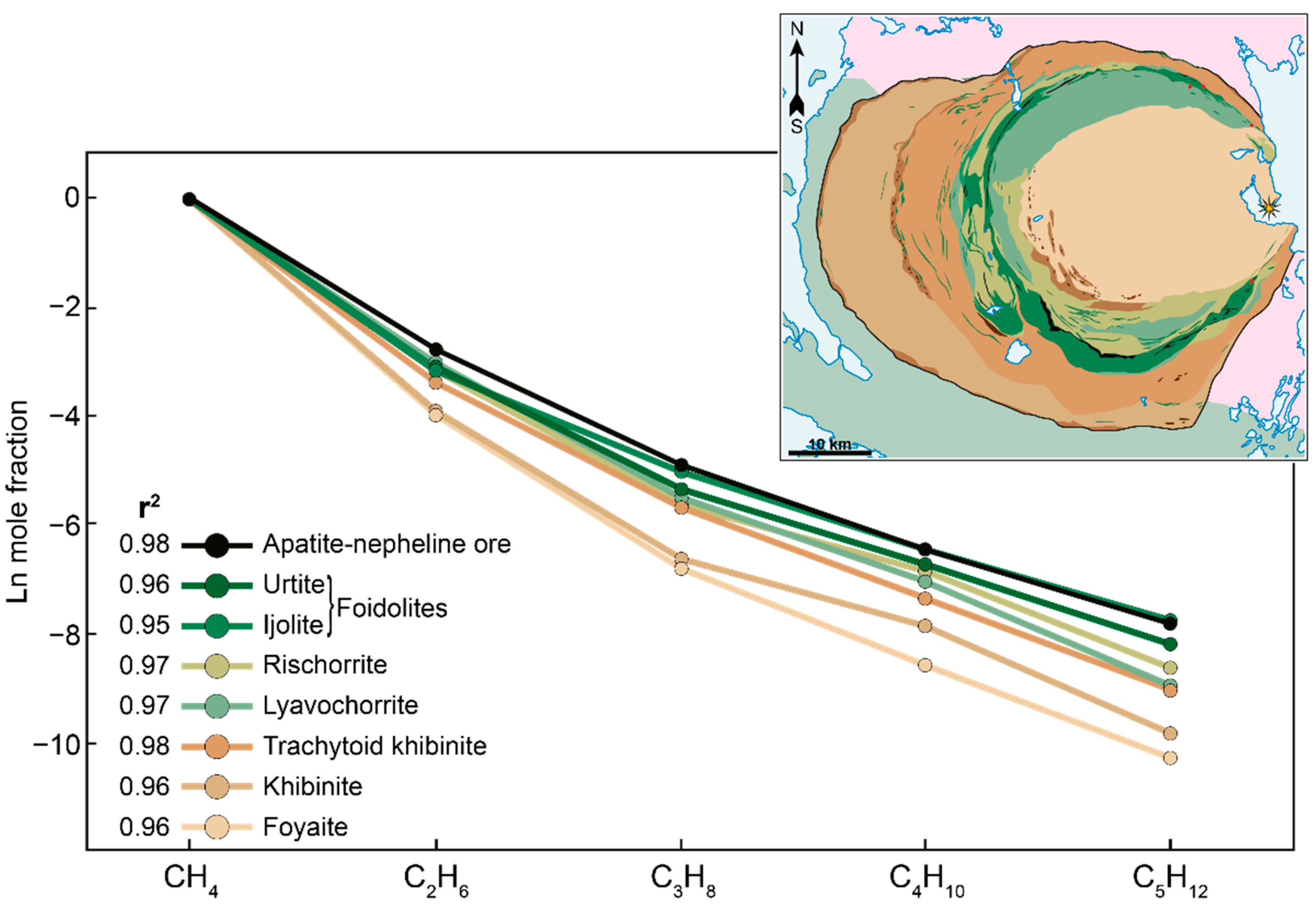
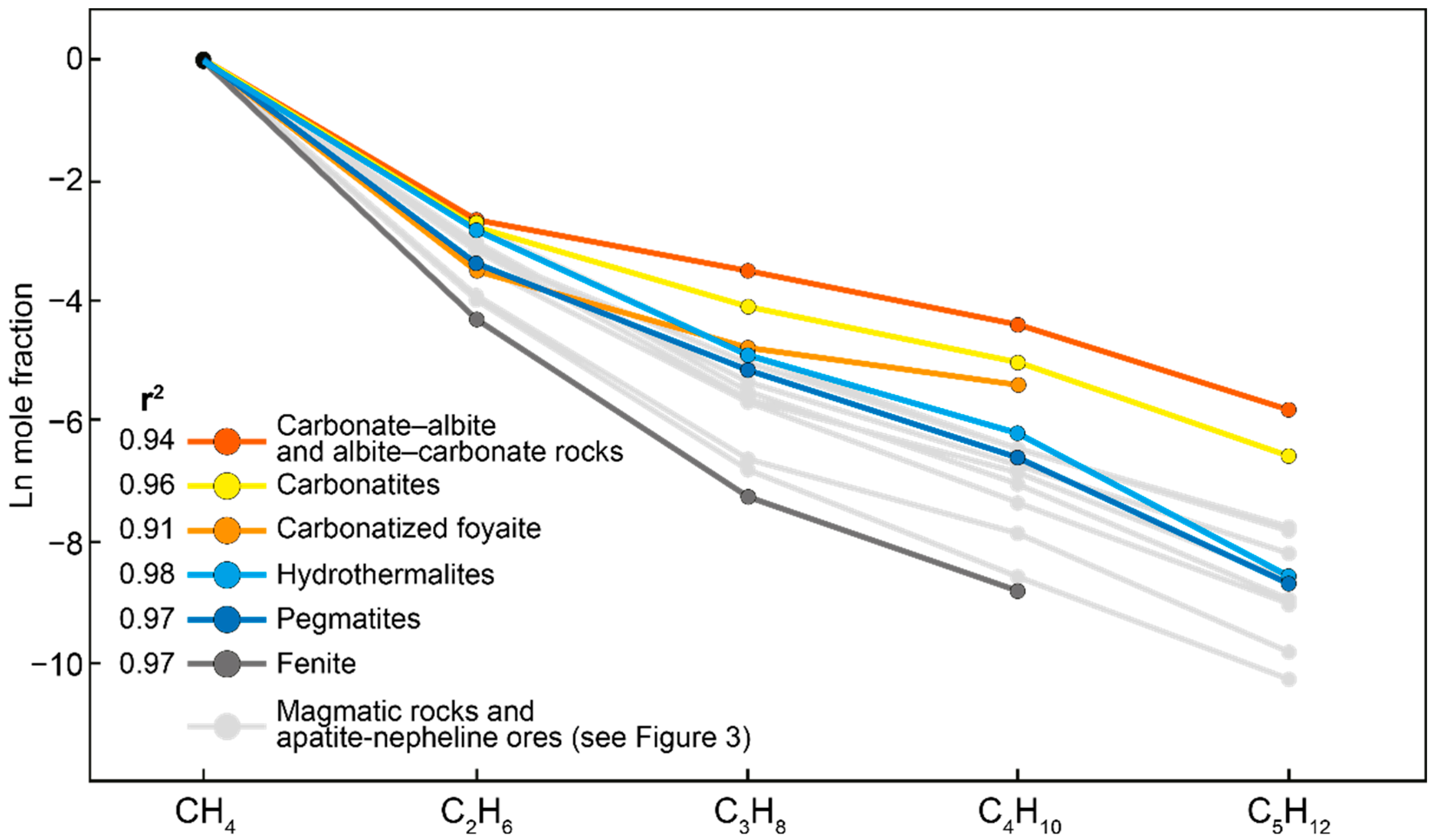
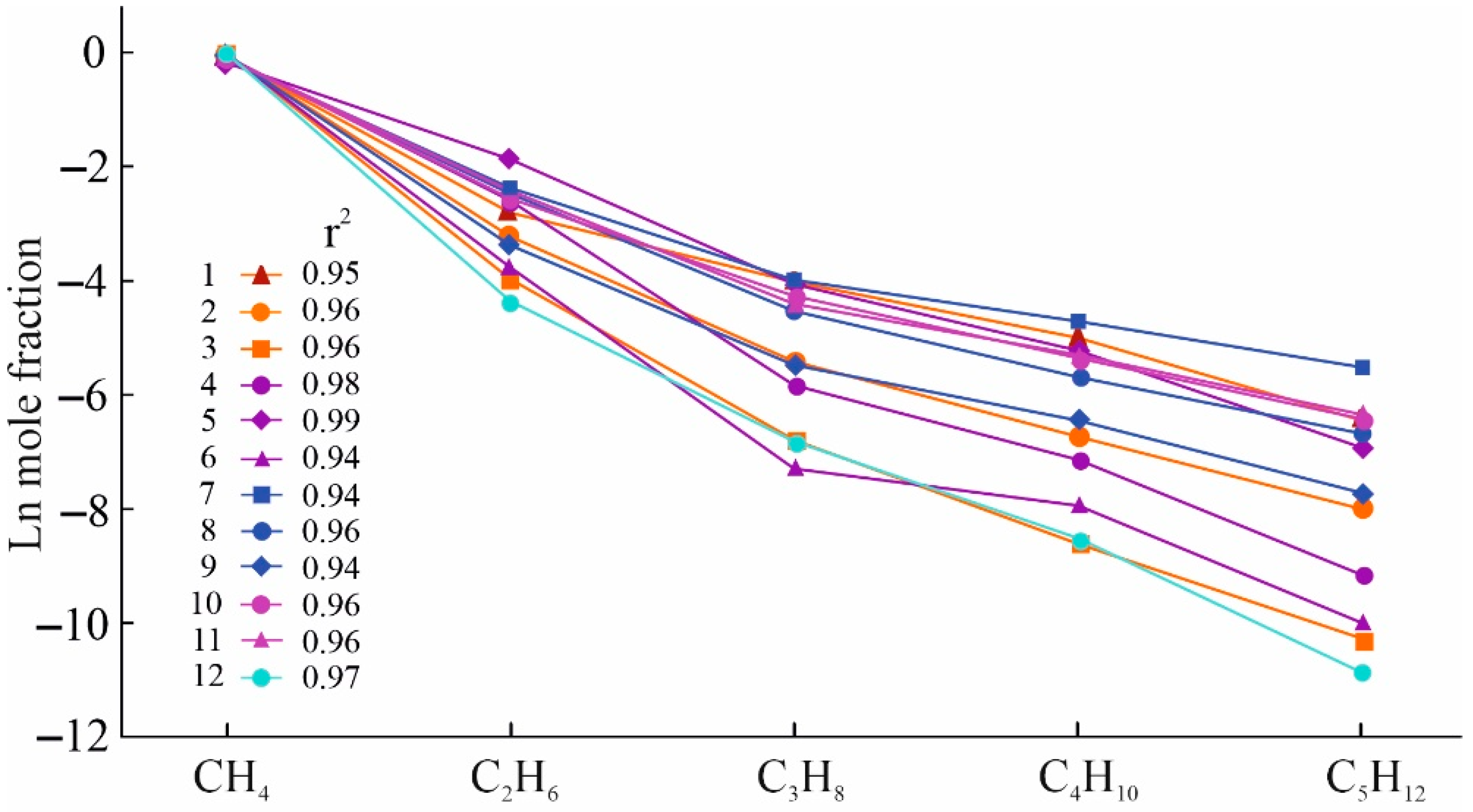
- The molecular weight distribution of occluded hydrocarbon gases in the Khibiny massif corresponds to the classical Anderson–Schulz–Flory distribution. In addition, the slopes of the linear relationships are relatively steep. This indicates a predominantly abiogenic origin of the occluded gases of the Khibiny massif. At the same time, a small proportion of biogenic hydrocarbons is present and is associated with the influence of meteoric waters.
- The mechanism of formation of hydrocarbons remains debatable. The most probable ways of their formation are Fischer–Tropsch reactions (nCO2 + (3n + 1)H2→CnH2n+2 + 2nH2O), processes of polymerization of primary methane (nCH4→CnH2n+2 + (n − 1) H2), and oxidation of hydrocarbon gases (4CH4 + O2→2C2H6 + 2H2O).
- In the Khibiny massif, the proportion of relatively high-temperature gases decreases towards the Main foidolite Ring in the following sequence: foyaite and khibinite–trachytoid khibinite–rischorrite and lyavochorrite–foidolites and apatite–nepheline ores. In the same sequence, there is an increase in the proportion of heavy hydrocarbons of hydrocarbon gases, and the increasing role of oxidation and condensation reactions in the transformation of hydrocarbons occurs.
- The pattern of the molecular weight distribution of hydrocarbon gases can serve as an indicator of the conditions and mechanism of their formation, but only in combination with other signs and criteria.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Konnerup-Madsen, J. A Review of the Composition and Evolution of Hydrocarbon Gases during Solidification of the Ilímaussaq Alkaline Complex, South Greenland. Geol. Greenl. Surv. Bull. 2001, 190, 159–166. [Google Scholar] [CrossRef]
- Salvi, S.; Williams-Jones, A.E. Fischer-Tropsch Synthesis of Hydrocarbons during Sub-Solidus Alteration of the Strange Lake Peralkaline Granite, Quebec/Labrador, Canada. Geochim. Cosmochim. Acta 1997, 61, 83–99. [Google Scholar] [CrossRef]
- Ikorsky, S.V. Organic Matter in Minerals of Igneous Rocks: Case Study of the Khibiny Alkaline Massif; Nauka: Saint-Peterburg, Russia, 1967. [Google Scholar]
- Ikorsky, S.V.; Nivin, V.A.; Pripachkin, V.A. Geochemistry of Gases of Endogenous Formations; Nauka: Saint-Peterburg, Russia, 1992. [Google Scholar]
- Nivin, V.A. Occurrence Forms, Composition, Distribution, Origin and Potential Hazard of Natural Hydrogen–Hydrocarbon Gases in Ore Deposits of the Khibiny and Lovozero Massifs: A Review. Minerals 2019, 9, 535. [Google Scholar] [CrossRef]
- Nivin, V.A. Diffusively Disseminated Hydrogen-Hydrocarbon Gases in Rocks of Nepheline Syenite Complexes. Geochem. Int. 2009, 47, 672–691. [Google Scholar] [CrossRef]
- Nivin, V.A.; Treloar, P.J.; Konopleva, N.G.; Ikorsky, S.V. A Review of the Occurrence, Form and Origin of C-Bearing Species in the Khibiny Alkaline Igneous Complex, Kola Peninsula, NW Russia. Lithos 2005, 85, 93–112. [Google Scholar] [CrossRef]
- Migdisov, A.A.; Guo, X.; Xu, H.; Williams-Jones, A.E.; Sun, C.J.; Vasyukova, O.V.; Sugiyama, I.; Fuchs, S.; Pearce, K.; Roback, R. Hydrocarbons as Ore Fluids. Geochem. Perspect. Lett. 2017, 5, 47–52. [Google Scholar] [CrossRef]
- Truche, L.; Berger, G.; Destrigneville, C.; Pages, A.; Guillaume, D.; Giffaut, E.; Jacquot, E. Experimental Reduction of Aqueous Sulphate by Hydrogen under Hydrothermal Conditions: Implication for the Nuclear Waste Storage. Geochim. Cosmochim. Acta 2009, 73, 4824–4835. [Google Scholar] [CrossRef]
- Vasyukova, O.V.; Williams-Jones, A.E. Direct Measurement of Metal Concentrations in Fluid Inclusions, a Tale of Hydrothermal Alteration and REE Ore Formation from Strange Lake, Canada. Chem. Geol. 2018, 483, 385–396. [Google Scholar] [CrossRef]
- Zgonnik, V. The Occurrence and Geoscience of Natural Hydrogen: A Comprehensive Review. Earth Sci. Rev. 2020, 203, 103140. [Google Scholar] [CrossRef]
- Truche, L.; McCollom, T.M.; Martinez, I. Hydrogen and Abiotic Hydrocarbons: Molecules That Change the World. Elements 2020, 16, 13–18. [Google Scholar] [CrossRef]
- Etiope, G.; Sherwood Lollar, B. Abiotic Methane on Earth. Rev. Geophys. 2013, 51, 276–299. [Google Scholar] [CrossRef]
- Ménez, B. Abiotic Hydrogen and Methane: Fuels for Life. Elements 2020, 16, 39–46. [Google Scholar] [CrossRef]
- Nivin, V.A. Free Hydrogen-Hydrocarbon Gases from the Lovozero Loparite Deposit (Kola Peninsula, NW Russia). Appl. Geochem. 2016, 74, 44–55. [Google Scholar] [CrossRef]
- Etiope, G. Gas Seepage Classification and Global Distribution. In Natural Gas Seepage; Springer: Cham, Switzerland, 2015; pp. 17–43. [Google Scholar]
- Othman, R.; Arouri, K.R.; Ward, C.R.; McKirdy, D.M. Oil Generation by Igneous Intrusions in the Northern Gunnedah Basin, Australia. Org. Geochem. 2001, 32, 1219–1232. [Google Scholar] [CrossRef]
- Syvorotkin, V.L. Hydrogen Degassing of the Earth: Natural Disasters and the Biosphere. In Man and the Geosphere; Florinsky, I.V., Ed.; Nova Science Publishers: New York, NY, USA, 2010; pp. 307–347. [Google Scholar]
- Ryabchikov, I.D.; Kogarko, L.N. Oxygen Fugacity in the Apatite-Bearing Intrusion of the Khibina Complex. Geochem. Int. 2009, 47, 1157–1169. [Google Scholar] [CrossRef]
- Krumrei, T.V.; Pernicka, E.; Kaliwoda, M.; Markl, G. Volatiles in a Peralkaline System: Abiogenic Hydrocarbons and F-Cl-Br Systematics in the Naujaite of the Ilímaussaq Intrusion, South Greenland. Lithos 2007, 95, 298–314. [Google Scholar] [CrossRef]
- Potter, J.; Longstaffe, F.J. A Gas-Chromatograph, Continuous Flow-Isotope Ratio Mass-Spectrometry Method for Δ13C and ΔD Measurement of Complex Fluid Inclusion Volatiles: Examples from the Khibina Alkaline Igneous Complex, Northwest Russia and the South Wales Coalfields. Chem. Geol. 2007, 244, 186–201. [Google Scholar] [CrossRef]
- Graser, G.; Potter, J.; Köhler, J.; Markl, G. Isotope, Major, Minor and Trace Element Geochemistry of Late-Magmatic Fluids in the Peralkaline Ilímaussaq Intrusion, South Greenland. Lithos 2008, 106, 207–221. [Google Scholar] [CrossRef]
- Nivin, V.A. Molecular-Mass Distribution of Saturated Hydrocarbons in Gas of the Lovozerskii Nepheline-Syenite Massif. Dokl. Earth Sci. 2009, 429, 1580–1582. [Google Scholar] [CrossRef]
- Markl, G.; Marks, M.A.W.; Ronald Frost, B. On the Controls of Oxygen Fugacity in the Generation and Crystallization of Peralkaline Melts. J. Petrol. 2010, 51, 1831–1847. [Google Scholar] [CrossRef]
- Marks, M.A.; Markl, G. A Global Review on Agpaitic Rocks. Earth Sci. Rev. 2017, 173, 229–258. [Google Scholar] [CrossRef]
- Nivin, V.A. The Origin of Hydrocarbon Gases in the Lovozero Nepheline-Syenite Massif (Kola Peninsula, NW Russia), as Revealed from He and Ar Isotope Evidence. Minerals 2020, 10, 830. [Google Scholar] [CrossRef]
- Nivin, V.A. Variations in the Composition and Origin of Hydrocarbon Gases from Inclusions in Minerals of the Khibiny and Lovozero Plutons, Kola Peninsula, Russia. Geol. Ore Depos. 2011, 53, 699–707. [Google Scholar] [CrossRef]
- Marks, M.A.W.; Markl, G. The Ilímaussaq Alkaline Complex, South Greenland. In Layered Intrusions; Springer: Dordrecht, The Netherlands, 2015; pp. 649–691. [Google Scholar]
- Potter, J.; Salvi, S.; Longstaffe, F.J. Abiogenic Hydrocarbon Isotopic Signatures in Granitic Rocks: Identifying Pathways of Formation. Lithos 2013, 182–183, 114–124. [Google Scholar] [CrossRef]
- Potter, J.; Rankin, A.H.; Treloar, P.J. Abiogenic Fischer-Tropsch Synthesis of Hydrocarbons in Alkaline Igneous Rocks; Fluid Inclusion, Textural and Isotopic Evidence from the Lovozero Complex, N.W. Russia. Lithos 2004, 75, 311–330. [Google Scholar] [CrossRef]
- Laier, T.; Nytoft, H.P. Bitumen Biomarkers in the Mid-Proterozoic Ilímaussaq Intrusion, Southwest Greenland—A Challenge to the Mantle Gas Theory. Mar. Pet. Geol. 2012, 30, 50–65. [Google Scholar] [CrossRef]
- Beeskow, B.; Treloar, P.J.; Rankin, A.H.; Vennemann, T.W.; Spangenberg, J. A Reassessment of Models for Hydrocarbon Generation in the Khibiny Nepheline Syenite Complex, Kola Peninsula, Russia. Lithos 2006, 91, 1–18. [Google Scholar] [CrossRef]
- Ryabchikov, I.D.; Kogarko, L.N. Magnetite Compositions and Oxygen Fugacities of the Khibina Magmatic System. Lithos 2006, 91, 35–45. [Google Scholar] [CrossRef]
- Ione, K.G.; Mysov, V.M.; Stepanov, V.G.; Parmon, V.N. New Data on the Possibility of Catalytic Abiogenic Synthesis of Hydrocarbons in the Earth’s Crust. Pet. Chem. 2001, 41, 159–165. [Google Scholar]
- Kryvdik, S.G.; Nivin, V.A.; Kul’chitskaya, A.A.; Voznyak, D.K.; Kalinichenko, A.M.; Zagnitko, V.N.; Dubyna, A.V. Hydrocarbons and Other Volatile Components in Alkaline Rocks from the Ukrainian Shield and Kola Peninsula. Geochem. Int. 2007, 45, 270–294. [Google Scholar] [CrossRef]
- Potter, J.; Konnerup-Madsen, J. A Review of the Occurrence and Origin of Abiogenic Hydrocarbons in Igneous Rocks. In Hydrocarbons in Crystalline Rocks; Petford, N., McCaffrey, K.J.W., Eds.; Geological Society: London, UK, 2003; Volume 214, pp. 151–173. [Google Scholar]
- Konnerup-Madsen, J.; Rose-Hansen, J. Volatiles Associated with Alkalina Igneous Rift Activity: Fluid Inclusions in the Ilimaussaq Intrusion and the Gardar Granitic Complexes (South Greenland). Chem. Geol. 1982, 37, 79–93. [Google Scholar] [CrossRef]
- D’Alessandro, W.; Yüce, G.; Italiano, F.; Bellomo, S.; Gülbay, A.H.; Yasin, D.U.; Gagliano, A.L. Large Compositional Differences in the Gases Released from the Kizildag Ophiolitic Body (Turkey): Evidences of Prevailingly Abiogenic Origin. Mar. Pet. Geol. 2018, 89, 174–184. [Google Scholar] [CrossRef]
- McGlynn, S.E.; Glass, J.B.; Johnson-Finn, K.; Klein, F.; Sanden, S.A.; Schrenk, M.O.; Ueno, Y.; Vitale-Brovarone, A. Hydrogenation Reactions of Carbon on Earth: Linking Methane, Margarine, and Life. Am. Mineral. 2020, 105, 599–608. [Google Scholar] [CrossRef]
- Suda, K.; Gilbert, A.; Yamada, K.; Yoshida, N.; Ueno, Y. Compound– and Position–Specific Carbon Isotopic Signatures of Abiogenic Hydrocarbons from on–Land Serpentinite–Hosted Hakuba Happo Hot Spring in Japan. Geochim. Cosmochim. Acta 2017, 206, 201–215. [Google Scholar] [CrossRef]
- Barenbaum, A.; Klimov, D. Theoretical Model Anderson-Schulz-Flory within the Framework of Studying the Mechanism of Polycondensation Synthesis. Inorg. Chem. Commun. 2020, 112, 107664. [Google Scholar] [CrossRef]
- Anderson, R.B. The Fischer–Tropsch Synthesis; Academic Press: New York, NY, USA, 1984. [Google Scholar]
- Zak, S.I.; Kamenev, E.A.; Minakov, F.V.; Armand, A.L.; Mikheichev, A.S.; Peresilie, I.A. Khibiny Alkaline Massif; Nauka: Saint-Peterburg, Russia, 1972. [Google Scholar]
- Kostyleva-Labuntsova, E.E.; Borutskii, B.E.; Sokolova, M.N.; Shlykova, Z.V. Mineralogy of the Khibiny Massif: Magmatism and Postmagmatic Transformations; Nauka: Saint-Peterburg, Russia, 1978. [Google Scholar]
- Ivanyuk, G.Y.; Yakovenchuk, V.N.; Pakhomovsky, Y.A.; Kalashnikov, A.O.; Mikhailova, J.A.; Goryainov, P.M. Self-Organization of the Khibiny Alkaline Massif (Kola Peninsula, Russia). In Earth Sciences; Dar, I.A., Ed.; InTech: Rijeka, Croatia, 2012; pp. 131–156. [Google Scholar]
- Galakhov, A.V. Petrology of the Khibiny Alkaline Massif; Nauka: Saint-Peterburg, Russia, 1975. [Google Scholar]
- Arzamastsev, A.A.; Arzamastseva, L.V.; Travin, A.V.; Belyatsky, B.V.; Shamatrina, A.M.; Antonov, A.V.; Larionov, A.N.; Rodionov, N.V.; Sergeev, S.A. Duration of Formation of Magmatic System of Polyphase Paleozoic Alkaline Complexes of the Central Kola: U–Pb, Rb–Sr, Ar–Ar Data. Dokl. Earth Sci. 2007, 413, 666–670. [Google Scholar] [CrossRef]
- Yakovenchuk, V.N.; Ivanyuk, G.Y.; Pakhomovsky, Y.A.; Men’shikov, Y.P. Khibiny; Laplandia Minerals: Apatity, Russia, 2005. [Google Scholar]
- Kalashnikov, A.O.; Konopleva, N.G.; Pakhomovsky, Y.A.; Ivanyuk, G.Y. Rare Earth Deposits of the Murmansk Region, Russia—A Review. Econ. Geol. 2016, 111, 1529–1559. [Google Scholar] [CrossRef]
- LeMaitre, R.W. Igneous Rocks. A Classification and Glossary of Terms. Recommendations of the International Union of Geological Sciences Subcommission on the Systematics of Igneous Rocks; LeMaitre, R.W., Ed.; Cambridge University Press: Cambridge, UK, 2005. [Google Scholar]
- Dudkin, O.B.; Ivanova, T.N. Khibiny Carbonatites; Kola Science Center: Apatity, Russia, 1984. [Google Scholar]
- Gorstka, V.N. Contact Zone of the Khibiny Alkaline Massif; Nauka: Saint-Peterburg, Russia, 1971. [Google Scholar]
- Korchak, Y.A.; Men’shikov, Y.P.; Pakhomovskii, Y.A.; Yakovenchuk, V.N.; Ivanyuk, G.Y. Trap Formation of the Kola Peninsula. Petrology 2011, 19, 87–101. [Google Scholar] [CrossRef]
- Yakovenchuk, V.N.; Ivanyuk, G.Y.; Pakhomovsky, Y.A.; Men’shikov, Y.P.; Konopleva, N.G.; Korchak, Y.A. Pyroxenes of the Khibiny Alkaline Pluton, Kola Peninsula. Geol. Ore Depos. 2008, 50, 732–745. [Google Scholar] [CrossRef]
- Konopleva, N.G.; Ivanyuk, G.Y.; Pakhomovsky, Y.A.; Yakovenchuk, V.N.; Men’shikov, Y.P.; Korchak, Y.A. Amphiboles of the Khibiny Alkaline Pluton, Kola Peninsula, Russia. Geol. Ore Depos. 2008, 50, 720–731. [Google Scholar] [CrossRef]
- Ivanyuk, G.Y.; Goryainov, P.M.; Pakhomovsky, Y.A.; Konopleva, N.G.; Yakovanchuk, V.N.; Bazai, A.V.; Kalashnikov, A.O. Self-Organization of Ore Complexes; GEOKART-GEOS: Moskow, Russia, 2009. [Google Scholar]
- Petersilie, I.A. Hydrocarbonic Gases and Bitumens of Igneous Massifs in the Central Part of the Kola Peninsula. Dokl. Acad. Nauk SSSR 1958, 122, 1086–1089. [Google Scholar]
- Petersilie, I.A. Geology and Geochemistry of Natural Gases and Dispersed Bitumen of Some Geological Formations of the Kola Peninsula; Nauka: Saint-Peterburg, Russia, 1964. [Google Scholar]
- Nivin, V.A. Gas Components in Magmatic Rocks: Geochemical, Mineragenic and Environmental Aspects and Results. Ph.D. Thesis, Vernadsky Institute of Geochemistry and Analytical Chemistry of Russian Academy of Sciences, Moscow, Russia, 2013. [Google Scholar]
- Potter, J.; Rankin, A.H.; Treloar, P.J.; Nivin, V.A.; Ting, W.; Ni, P. A Preliminary Study of Methane Inclusions in Alkaline Igneous Rocks of the Kola Igneous Province, Russia; Implications for the Origin of Methane in Igneous Rocks. Eur. J. Mineral. 1998, 10, 1167–1180. [Google Scholar] [CrossRef]
- Kogarko, L.N. Problems of Genesis of Agpaitic Magmas; Nauka: Saint-Peterburg, Russia, 1977. [Google Scholar]
- Beeskow, B. The Occurrence, Distribution and Origin of Hydrocarbons in the Khibiny Nepheline Syenite Complex, Kola Peninsula, Russia. Ph.D. Thesis, Kingston University, Kingston upon Thames, UK, 2007. [Google Scholar]
- Potter, J. The Characterisation and Origin of Hydrocarbons in Alkaline Rocks of the Kola Alkaline Province. Ph.D. Thesis, Kingston University, Kingston upon Thames, UK, 2000. [Google Scholar]
- Kogarko, L.N.; Kosztolanyi, C.; Ryabchikov, I.D. Geochemistry of the Reduced Fluid in Alkali Magmas. Geochem. Int. 1987, 12, 1688–1695. [Google Scholar]
- Kul’chitskaya, A.A.; Nivin, V.A.; Avedisyan, A.A.; Voznyak, D.K.; Vasyuta, Y.V. Comparison of the Results of Extracting Methane from Minerals by Mechanical and Thermal Methods. Mineral. J. 2009, 31, 84–94. [Google Scholar]
- Mironova, O.F.; Savelyeva, N.I.; Ikorsky, S.V.; Vasyuta, Y.V. Comparison of the Results of the Bulk Analysis of Fluid Inclusions with Different Methods of Extracting the Gas Phase. Geochemistry 1985, 1, 111–117. [Google Scholar]
- Szatmri, P. Petroleum Formation by Fischer-Tropsch Synthesis in Plate Tectonics. Am. Assoc. Pet. Geol. Bull. 1989, 7, 989–998. [Google Scholar]
- Glebov, L.S.; Kliger, G.A. Molecular-Weight Distribution of Fischer-Tropsch Synthesis Products. Fuel Energy Abstr. 1995, 5, 328. [Google Scholar] [CrossRef]
- Vasyukova, O.V.; Williams-Jones, A.E.; Blamey, N.J.F. Fluid Evolution in the Strange Lake Granitic Pluton, Canada: Implications for HFSE Mobilisation. Chem. Geol. 2016, 444, 83–100. [Google Scholar] [CrossRef]
- Nivin, V.A. Gas Concentrations in Minerals with Reference to the Problem of the Genesis of Hydrocarbon Gases in Rocks of the Khibiny and Lovozero Massifs. Geochem. Int. 2002, 40, 883–898. [Google Scholar]
- Huber, M.V.; Mokrushin, A.V. Sulfur Isotope Signatures of Sulfides from the Khibina and Lovozero Massifs (Kola Alkaline Province, Fennoscandian Shield). Vestn. MGTU 2021, 24, 80–87. [Google Scholar] [CrossRef]
- Darling, W.G. Hydrothermal Hydrocarbon Gases: 1, Genesis and Geothermometry. Appl. Geochem. 1998, 13, 815–824. [Google Scholar] [CrossRef]
- Elliott, H.A.L.; Wall, F.; Chakhmouradian, A.R.; Siegfried, P.R.; Dahlgren, S.; Weatherley, S.; Finch, A.A.; Marks, M.A.W.; Dowman, E.; Deady, E. Fenites Associated with Carbonatite Complexes: A Review. Ore Geol. Rev. 2018, 93, 38–59. [Google Scholar] [CrossRef]
- Kramm, U.; Sindern, S. Volume Characteristics and Element Transfer of Fenite Aureoles: A Case Study from the Iivaara Alkaline Complex, Finland. Lithos 2000, 51, 75–93. [Google Scholar]
- Hosgormez, H.; Etiope, G.; Yalçin, M.N. New Evidence for a Mixed Inorganic and Organic Origin of the Olympic Chimaera Fire (Turkey): A Large Onshore Seepage of Abiogenic Gas. Geofluids 2008, 8, 263–273. [Google Scholar] [CrossRef]
- Zhang, S.; Mi, J.; He, K. Synthesis of Hydrocarbon Gases from Four Different Carbon Sources and Hydrogen Gas Using a Gold-Tube System by Fischer-Tropsch Method. Chem. Geol. 2013, 349–350, 27–35. [Google Scholar] [CrossRef]
- Yunyan, N.; Jinxing, D.; Qinghua, Z.; Xia, L.; Anping, H.; Chun, Y. Geochemical Characteristics of Abiogenic Gas and Its Percentage in Xujiaweizi Fault Depression, Songliao Basin, NE China. Pet. Explor. Dev. 2009, 36, 35–45. [Google Scholar] [CrossRef]
- Prinzhofer, A.A.; Huc, A.Y. Genetic and Post-Genetic Molecular and Isotopic Fractionations in Natural Gases. Chem. Geol. 1995, 126, 281–290. [Google Scholar] [CrossRef]
- Eremenko, N.A. (Ed.) Handbook of Oil and Gas Geology; Nedra: Moskow, Russia, 1984. [Google Scholar]
- Volkov, M.M.; Mikheev, A.L.; Konev, K.A. Reference Book of Workers in the Gas Industry; Nedra: Moskow, Russia, 1989. [Google Scholar]
- Gürgey, K.; Philp, R.P.; Clayton, C.; Emiroǧlu, H.; Siyako, M. Geochemical and Isotopic Approach to Maturity/Source/Mixing Estimations for Natural Gas and Associated Condensates in the Thrace Basin, NW Turkey. Appl. Geochem. 2005, 20, 2017–2037. [Google Scholar] [CrossRef]
- Wang, K.; Pang, X.; Zhang, H.; Zhao, Z.; Su, S.; Hui, S. Characteristics and Genetic Types of Natural Gas in the Northern Dongpu Depression, Bohai Bay Basin, China. J. Pet. Sci. Eng. 2018, 170, 453–466. [Google Scholar] [CrossRef]
- Lollar, B.S.; Westgate, T.D.; Ward, J.A.; Slater, G.F.; Lacrampe-Couloume, G. Abiogenic Formation of Alkanes in the Earth’s Crust as a Minor Source for Global Hydrocarbon Reservoirs. Nature 2002, 416, 522–524. [Google Scholar] [CrossRef]
- Nivin, V.A.; Devirts, A.L.; Lagutina, Y.P. The Origin of the Gas Phase in the Lovozero Massif Based on Hydrogen-Isotope Data. Geochem. Int. 1995, 32, 65–71. [Google Scholar]
- McCollom, T.M.; Seewald, J.S. Abiotic Synthesis of Organic Compounds in Deep-Sea Hydrothermal Environments. Chem. Rev. 2007, 107, 382–401. [Google Scholar] [CrossRef] [PubMed]
- McDermott, J.M.; Seewald, J.S.; German, C.R.; Sylva, S.P. Pathways for Abiotic Organic Synthesis at Submarine Hydrothermal Fields. Proc. Natl. Acad. Sci. USA 2015, 112, 7668–7672. [Google Scholar] [CrossRef] [PubMed]
- Sinev, M.Y.; Fattakhova, Z.T.; Lomonosov, V.I.; Gordienko, Y.A. Kinetics of Oxidative Coupling of Methane: Bridging the Gap between Comprehension and Description. J. Nat. Gas Chem. 2009, 18, 273–287. [Google Scholar] [CrossRef]
- Lollar, B.S.; Lacrampe-Couloume, G.; Voglesonger, K.; Onstott, T.C.; Pratt, L.M.; Slater, G.F. Isotopic Signatures of CH4 and Higher Hydrocarbon Gases from Precambrian Shield Sites: A Model for Abiogenic Polymerization of Hydrocarbons. Geochim. Cosmochim. Acta 2008, 72, 4778–4795. [Google Scholar] [CrossRef]
- Nivin, V.A.; Mel’nik, N.A. Effects of Radioactivity on Gas-Component Contents in Alkali Igneous Rocks. Geochem. Int. 1990, 27, 94–97. [Google Scholar]
- Mavrogenes, J.A.; Bodnar, R.J. Hydrogen Movement into and out of Fluid Inclusions in Quartz: Experimental Evidence and Geologic Implications. Geochim. Cosmochim. Acta 1994, 58, 141–148. [Google Scholar] [CrossRef]
- Ermolaeva, V.N.; Chukanov, N.V.; Pekov, I.V.; Kogarko, L.N. The Geochemical and Genetic Role of Organic Substances in Postmagmatic Derivatives of Alkaline Plutons. Geol. Ore Depos. 2009, 51, 513–524. [Google Scholar] [CrossRef]
- Chukanov, N.V.; Pekov, I.V.; Sokolov, S.V.; Nekrasov, A.N.; Ermolaeva, V.N.; Naumova, I.S. On the Problem of the Formation and Geochemical Role of Bituminous Matter in Pegmatites of the Khibiny and Lovozero Alkaline Massifs, Kola Peninsula, Russia. Geochem. Int. 2006, 44, 715–728. [Google Scholar] [CrossRef]
- Pripachkin, V.A.; Pavlova, M.A.; Galakhova, T.N.; Volokhova, T.S. Bitumens of Khibiny Carbonatites. Dokl. Akad. Nauk Ukr. SSR 1985, 281, 24–29. [Google Scholar]
- Pokrovsky, B.G. Crustal Contamination of Mantle Magmas According to Isotope Geochemistry; Nauka: Saint-Petersburg, Russia, 2000. [Google Scholar]



| Rock | CH4 | C2H6 | C3H8 | iC4H10 | nC4H10 | iC5H12 | nC5H12 |
|---|---|---|---|---|---|---|---|
| Khibinite | 4.29–77.0 25.8 (19) | 0.09–3.10 0.51 (19) | 0.005–0.312 0.051 (19) | 0.0002–0.0230 0.0028 (19) | 0.0007–0.0620 0.0083 (19) | 0.0001–0.0167 0.0012 (19) | 0.00005–0.0028 0.00043 (19) |
| Trachytoid khibinite | 1.04–66.6 18.8 (27) | 0.03–3.20 0.62 (27) | 0.002–0.358 0.049 (26) | 0.0001–0.0320 0.0023 (27) | 0.0003–0.0670 0.0091 (27) | 0.0–0.010 0.0012 (27) | 0.00002–0.0043 0.00052 (27) |
| Rischorrite | 2.9–116.5 9.6 (15) | 0.12–6.92 0.52 (15) | 0.008–0.770 0.049 (15) | 0.0005–0.060 0.0025 (15) | 0.0013–0.1450 0.0088 (15) | 0.0003–0.0470 0.0014 (15) | 0.00010–0.0180 0.00069 (15) |
| Ijolite | 1.0–74.60 12.4 (27) | 0.04–2.52 0.55 (27) | 0.006–0.540 0.071 (27) | 0.0002–0.10 0.0077 (27) | 0.0009–0.120 0.0110 (27) | 0.0001–0.0470 0.0030 (27) | 0.00002–0.0480 0.00130 (27) |
| Urtite | 4.77–86.0 23.4 (60) | 0.12–5.12 0.98 (60) | 0.012–0.520 0.10 (60) | 0.0009–0.0690 0.0083 (60) | 0.0023–0.0930 0.0209 (60) | 0.0003–0.0330 0.0043 (60) | 0.00015–0.0210 0.00190 (60) |
| Apatite–nepheline ore | 0.05–10.8 2.4 (9) | 0.01–1.04 0.12 (9) | 0.001–0.075 0.011 (9) | 0.0001–0.0076 0.0007 (9) | 0.0001–0.0150 0.0015 (9) | 0.0–0.0041 0.0006 (9) | 0.00001–0.0014 0.00016 (9) |
| Lyavochorrite | 6.15–74.5 19.5 (18) | 0.23–2.96 1.19 (18) | 0.011–0.310 0.108 (18) | 0.0007–0.0260 0.0074 (18) | 0.0020–0.0571 0.0195 (18) | 0.0003–0.0174 0.0027 (18) | 0.00003–0.0053 0.00120 (17) |
| Foyaite | 2.97–33.2 9.3 (13) | 0.05–1.12 0.20 (13) | 0.002–0.078 0.010 (13) | 0.0001–0.0054 0.0005 (13) | 0.0002–0.0110 0.0021 (13) | 0.0–0.0021 0.0003 (13) | 0.00002–0.0009 0.00018 (13) |
| Carbonatized foyaite | 0.04–0.30 0.1 (4) | 0.0–0.01 0.0 (4) | 0.0–0.002 0.001 (4) | 0.0–0.0012 0.0001 (4) | 0.0001–0.0005 0.0003 (4) | b.d.l. | b.d.l. |
| Carbonatites | 0.17–4.08 0.8 (19) | 0.01–0.36 0.06 (19) | 0.002–0.081 0.014 (19) | 0.0004–0.0120 0.0024 (19) | 0.0003–0.0170 0.0026 (19) | 0.0001–0.0032 0.0008 (19) | 0.00003–0.0021 0.00050 (19) |
| Carbonate-albite and albite-carbonate rocks | 0.13–2.74 1.1 (13) | 0.01–0.25 0.09 (13) | 0.002–0.090 0.045 (13) | 0.0003–0.0220 0.0130 (13) | 0.0003–0.0180 0.0064 (13) | 0.0001–0.0050 0.0032 (13) | 0.00009–0.0043 0.00160 (13) |
| Pegmatites | 9.29–31.6 23.1 (3) | 0.32–2.31 0.84 (3) | 0.089–0.170 0.092 (3) | 0.0039–0.0130 0.0060 (3) | 0.0072–0.0280 0.0140 (3) | 0.0022–0.0046 0.0030 (3) | 0.00078–0.0014 0.0010 (3) |
| Hydrothermalites | 6.23–11.0 8.6 (2) | 0.29–0.98 0.64 (2) | 0.022–0.170 0.096 (2) | 0.0014–0.0420 0.0217 (2) | 0.0026–0.0360 0.0193 (2) | 0.0001–0.0086 0.0043 (2) | 0.00011–0.0053 0.00271 (2) |
| Fenite | 0.01–13.4 0.5 (7) | 0.0–0.12 0.01 (7) | 0.0–0.005 0.0 (7) | 0.0–0.0002 0.0 (7) | 0.0–0.0006 0.0 (7) | b.d.l. | b.d.l. |
| Rock | H2 | CO2 | N2 | O2 |
|---|---|---|---|---|
| Khibinite | 0.37–2.30 1.27 (19) | 0.04–0.31 0.17 (3) | 0.19–1.70 0.53 (18) | 0.013–0.46 0.07 (18) |
| Trachytoid khibinite | 0.61–1.89 1.04 (27) | 0.21–0.21 0.21 (1) | 0.07–2.60 0.38 (27) | 0.025–0.45 0.05 (19) |
| Rischorrite | 0.38–5.10 1.39 (15) | 0.34–3.26 0.70 (5) | 0.35–1.55 0.79 (13) | 0.030–0.41 0.07 (13) |
| Ijolite | 0.17–26.4 0.67 (26) | 0.07–1.24 0.68 (7) | 0.25–11.3 0.99 (17) | 0.020–0.55 0.12 (17) |
| Urtite | 0.19–5.10 0.95 (60) | 0.01–7.46 0.55 (9) | 0.49–9.53 1.26 (50) | 0.025–0.84 0.15 (50) |
| Apatite–nepheline ore | 0.12–2.83 0.41 (9) | b.d.l. | 0.25–2.28 0.75 (9) | 0.041–0.41 0.14 (9) |
| Lyavochorrite | 0.53–9.43 1.24 (18) | 0.01–6.6 1.94 (4) | 0.29–2.31 0.98 (14) | 0.024–0.32 0.14 (14) |
| Foyaite | 0.58–3.80 0.88 (13) | 0.17–1.75 0.96 (2) | 0.16–1.98 0.29 (10) | 0.007–0.21 0.02 (10) |
| Carbonatized foyaite | 0.58–2.51 1.20 (4) | 1.49–20.0 9.19 (4) | 0.30–1.0 0.47 (4) | 0.021–0.06 0.03 (4) |
| Carbonatite | 0.04–14.4 2.22 (19) | 0.33–16.20 3.61 (19) | 0.19–1.62 0.43 (19) | 0.003–0.21 0.04 (19) |
| Carbonate-albite and albite-carbonate rocks | 0.92–8.33 4.07 (13) | 0.99–14.20 4.35 (13) | 0.24–2.64 0.57 (13) | 0.015–0.32 0.05 (13) |
| Pegmatite | 0.54–1.19 1.19 (3) | b.d.l. | 0.57–2.65 1.49 (3) | 0.079–0.19 0.08 (3) |
| Hydrothermalite | 0.24–0.87 0.56 (2) | b.d.l. | 0.87–1.05 0.96 (2) | 0.083–0.35 0.22 (2) |
| Fenite | 0.18–4.59 0.59 (7) | 0.03–15.85 0.11 (4) | 0.33–2.21 0.93 (5) | 0.030–0.36 0.17 (4) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nivin, V.A.; Pukha, V.V.; Mokrushina, O.D.; Mikhailova, J.A. The Molecular Weight Distribution of Occluded Hydrocarbon Gases in the Khibiny Nepheline–Syenite Massif (Kola Peninsula, NW Russia) in Relation to the Problem of Their Origin. Geosciences 2022, 12, 416. https://doi.org/10.3390/geosciences12110416
Nivin VA, Pukha VV, Mokrushina OD, Mikhailova JA. The Molecular Weight Distribution of Occluded Hydrocarbon Gases in the Khibiny Nepheline–Syenite Massif (Kola Peninsula, NW Russia) in Relation to the Problem of Their Origin. Geosciences. 2022; 12(11):416. https://doi.org/10.3390/geosciences12110416
Chicago/Turabian StyleNivin, Valentin A., Vyacheslav V. Pukha, Olga D. Mokrushina, and Julia A. Mikhailova. 2022. "The Molecular Weight Distribution of Occluded Hydrocarbon Gases in the Khibiny Nepheline–Syenite Massif (Kola Peninsula, NW Russia) in Relation to the Problem of Their Origin" Geosciences 12, no. 11: 416. https://doi.org/10.3390/geosciences12110416
APA StyleNivin, V. A., Pukha, V. V., Mokrushina, O. D., & Mikhailova, J. A. (2022). The Molecular Weight Distribution of Occluded Hydrocarbon Gases in the Khibiny Nepheline–Syenite Massif (Kola Peninsula, NW Russia) in Relation to the Problem of Their Origin. Geosciences, 12(11), 416. https://doi.org/10.3390/geosciences12110416









