Modifying Effect of Soil Properties on Bio-Accessibility of As and Pb from Human Ingestion of Contaminated Soil
Abstract
1. Introduction
2. Materials and Methods
2.1. Soil Selection
2.2. Soil Chemical and Physical Properties
2.3. Preparation of Contaminated Soil
2.4. Determination of Bio-Accessibility
2.5. Statistical Analysis
3. Results
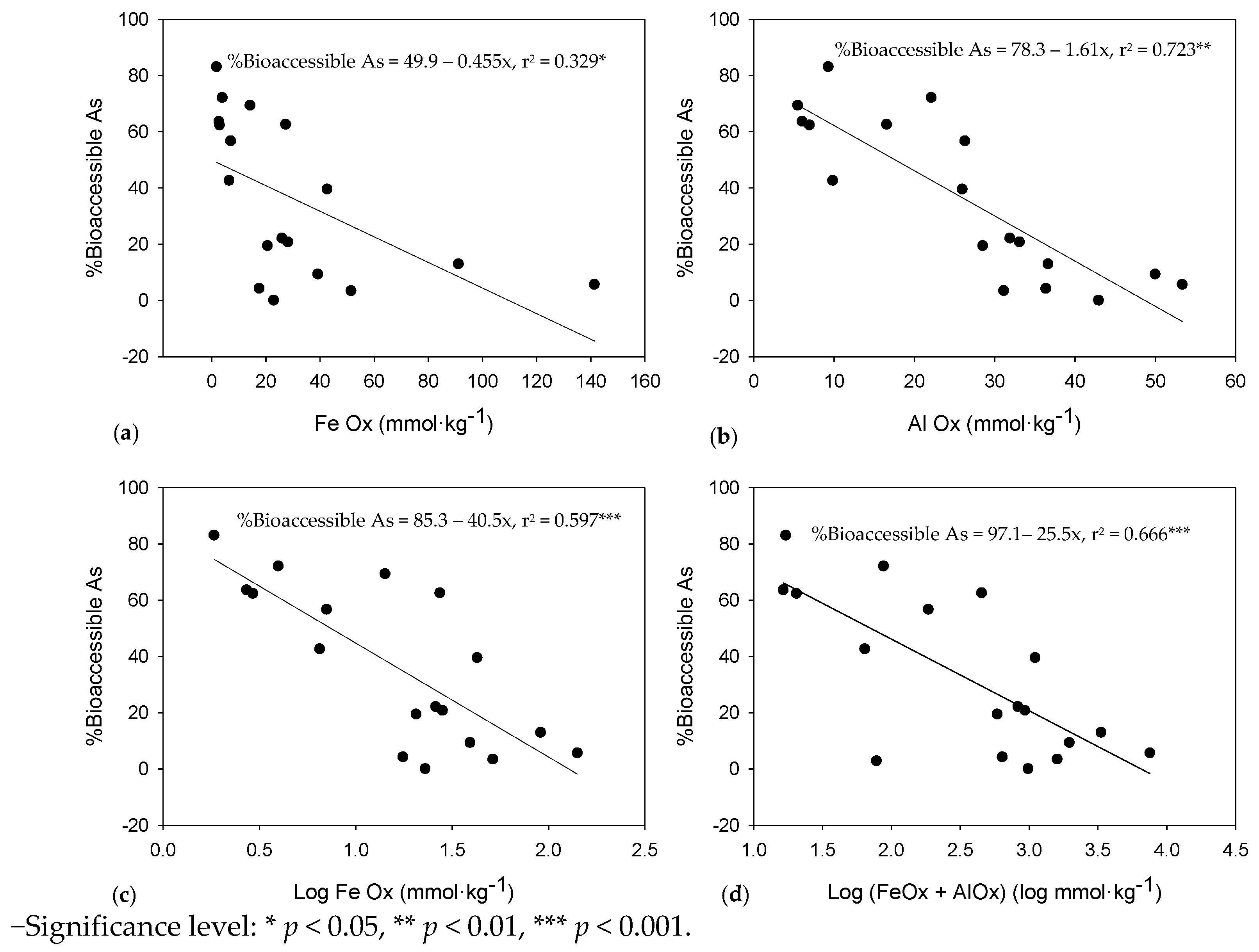
3.1. As Bio-Accessibility
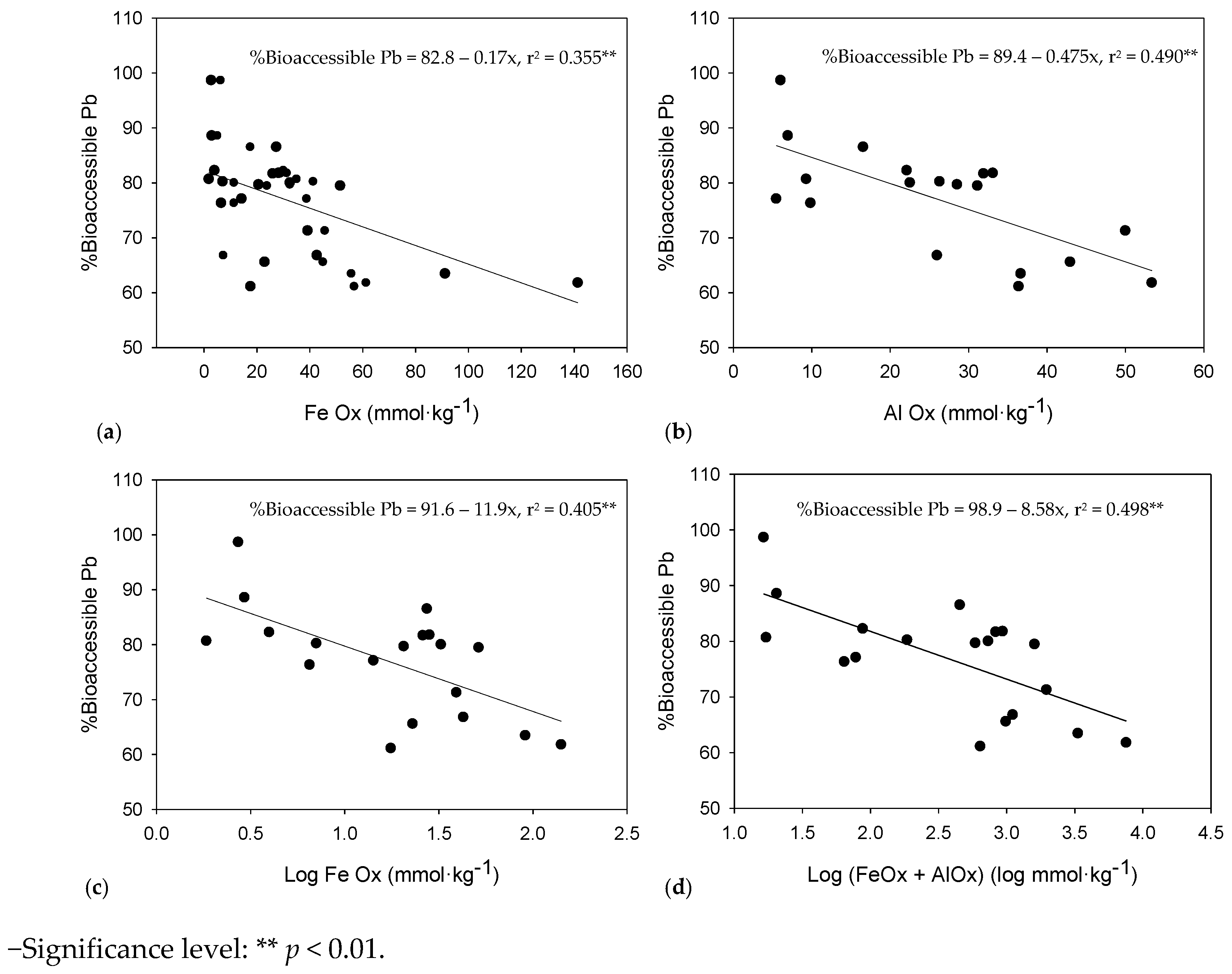
3.2. Pb Bio-Accessibility
4. Discussion
4.1. As Bio-Accessibility
4.2. Pb Bio-Accessibility
4.3. Effect of Fe and Al Oxides on Bio-Accessible As and Pb
4.4. Effect of Soil pH on Bio-Accessible As and Pb
4.5. Effect of eCEC and Clay Content on Bio-Accessible As and Pb
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Jaishankar, M.; Tseten, T.; Anbalagan, N.; Mathew, B.B.; Beeregowda, K.N. Toxicity, Mechanism and Health Effects of Some Heavy Metals. Interdiscip. Toxicol. 2014, 7, 60–72. [Google Scholar] [CrossRef]
- Adriano, D.C. Trace Elements in Terrestrial Environments, 2nd ed.; Springer: New York, NY, USA, 2001; pp. 219–261, 349–410. [Google Scholar]
- Wuana, R.A.; Okieimen, F.E. Heavy Metals in Contaminated Soils: A Review of Sources, Chemistry, Risks and Best Available Strategies for Remediation. ISRN Ecol. 2011, 2011. [Google Scholar] [CrossRef]
- Substance Priority List | ATSDR. Available online: https://www.atsdr.cdc.gov/spl/index.html (accessed on 18 May 2020).
- USEPA. Regional Screening Levels (RSLs)—Generic Tables. Available online: https://www.epa.gov/risk/regional-screening-levels-rsls-generic-tables (accessed on 18 May 2020).
- Mielke, H.W.; Gonzales, C.R.; Powell, E.T.; Laidlaw, M.A.S.; Berry, K.J.; Mielke, P.W.; Egendorf, S.P. The Concurrent Decline of Soil Lead and Children’s Blood Lead in New Orleans. Proc. Natl. Acad. Sci. USA 2019, 116, 22058–22064. [Google Scholar] [CrossRef] [PubMed]
- Basta, N.T.; Zearley, A.M.; Hattey, J.A.; Karlen, D.L. Chapter 7: A Risk-Based Soil Health Approach to Management of Soil Lead. In Soil Health: Vol. 1: Approaches to Soil Health Analysis; Soil Science Society of America (SSSA) & Wiley International, SSSA: Madison, WI, USA, 2021; Chapter 7. [Google Scholar]
- Basta, N.T.; Juhasz, A. Using in vivo bioavailability and/or in vitro gastrointestinal bioaccessibility testing to adjust human exposure to arsenic from soil ingestion. Rev. Mineral. Geochem. 2014, 79, 451–472. [Google Scholar] [CrossRef]
- Rieuwerts, J.S.; Thornton, I.; Farago, M.E.; Ashmore, M.R. Factors Influencing Metal Bioavailability in Soils: Preliminary Investigations for the Development of a Critical Loads Approach for Metals. Chem. Speciat. Bioavailab. 1998, 10, 61–75. [Google Scholar] [CrossRef]
- Scheckel, K.G.; Chaney, R.L.; Basta, N.T.; Ryan, J.A. Chapter 1 Advances in Assessing Bioavailability of Metal(Loid)s in Contaminated Soils. In Advances in Agronomy; Academic Press: Cambridge, MA, USA, 2009; Volume 104, pp. 1–52. [Google Scholar]
- Luo, X.-S.; Ding, J.; Xu, B.; Wang, Y.-J.; Li, H.-B.; Yu, S. Incorporating Bioaccessibility into Human Health Risk Assessments of Heavy Metals in Urban Park Soils. Sci. Total Environ. 2012, 424, 88–96. [Google Scholar] [CrossRef]
- USEPA. Superfund. Available online: https://www.epa.gov/superfund (accessed on 18 May 2020).
- Harter, R.D. Effect of Soil PH on Adsorption of Lead, Copper, Zinc, and Nickel. Soil Sci. Soc. Am. J. 1983, 47. [Google Scholar] [CrossRef]
- Thomas, G.W. Soil pH and Soil Acidity. In Methods of Soil Analysis; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 1996; pp. 475–490. ISBN 978-0-89118-866-7. [Google Scholar]
- Lu, Y.; Yin, W.; Huang, L.; Zhang, G.; Zhao, Y. Assessment of Bioaccessibility and Exposure Risk of Arsenic and Lead in Urban Soils of Guangzhou City, China. Environ. Geochem. Health 2011, 33, 93–102. [Google Scholar] [CrossRef]
- Palumbo-Roe, B.; Cave, M.R.; Klinck, B.A.; Wragg, J.; Taylor, H.; O’Donnell, K.E.; Shaw, R.A. Bioaccessibility of Arsenic in Soils Developed over Jurassic Ironstones in Eastern England. Environ. Geochem. Health 2005, 27, 121–130. [Google Scholar] [CrossRef]
- Roussel, H.; Waterlot, C.; Pelfrêne, A.; Pruvot, C.; Mazzuca, M.; Douay, F. Cd, Pb and Zn Oral Bioaccessibility of Urban Soils Contaminated in the Past by Atmospheric Emissions from Two Lead and Zinc Smelters. Arch. Environ. Contam. Toxicol. 2010, 58, 945–954. [Google Scholar] [CrossRef]
- Yang, J.-K.; Barnett, M.O.; Jardine, P.M.; Basta, N.T.; Casteel, S.W. Adsorption, Sequestration, and Bioaccessibility of As(V) in Soils. Environ. Sci. Technol. 2002, 36, 4562–4569. [Google Scholar] [CrossRef] [PubMed]
- Yan, K.; Dong, Z.; Wijayawardena, M.A.A.; Liu, Y.; Li, Y.; Naidu, R. The Source of Lead Determines the Relationship between Soil Properties and Lead Bioaccessibility. Environ. Pollut. 2019, 246, 53–59. [Google Scholar] [CrossRef] [PubMed]
- USEPA. EPA Method 3051A: Microwave Assisted Acid Digestion of Sediments, Sludges, and Oils. Available online: https://www.epa.gov/esam/us-epa-method-3051a-microwave-assisted-acid-digestion-sediments-sludges-and-oils (accessed on 20 May 2020).
- USGS Data Series 801: Geochemical and Mineralogical Data for Soils of the Conterminous United States. Available online: https://pubs.usgs.gov/ds/801/ (accessed on 20 May 2020).
- Basta, N.T.; Tabatabai, M.A. Effect of Cropping Systems on Adsorption of Metals by Soils: II. Effect of PH. Soil Sci. Am. J. 1992, 153. [Google Scholar] [CrossRef]
- Heanes, D.L. Determination of Total Organic-C in Soils by an Improved Chromic Acid Digestion and Spectrophotometric Procedure. Commun. Soil Sci. Plant Anal. 1984, 15, 1191–1213. [Google Scholar] [CrossRef]
- Gee, G.W.; Bauder, J.W. Particle-size Analysis. In Methods of Soil Analysis; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 1986; pp. 383–411. ISBN 978-0-89118-864-3. [Google Scholar]
- Kilmer, V.J.; Alexander, L.T. Methods of making mechanical analysis of soils. Soil Sci. 1949, 68, 15–24. [Google Scholar] [CrossRef]
- Sumner, M.E.; Miller, W.P. Cation Exchange Capacity and Exchange Coefficients. In Methods of Soil Analysis; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 1996; pp. 1201–1229. ISBN 978-0-89118-866-7. [Google Scholar]
- McKeague, J.A.; Day, J.H. Dithionite-and oxalate-extractable Fe and Al as aids in differentiating various classes of soils. Can. J. Soil Sci. 2011. [Google Scholar] [CrossRef]
- Logan, T.J.; Chaney, R.L. Utilization of municipal wastewater and sludge on land-metals. In Utilization of Municipal Wastewater and Sludge on Land; Page, A.L., Ed.; University of California: Riverside, CA, USA, 1983; pp. 235–295. [Google Scholar]
- USEPA. EPA Method 6010C (SW-846): Inductively Coupled Plasma—Atomic Emission Spectrometry. Available online: https://homeland-security-research/epa-method-6010c-sw-846-inductively-coupled-plasma-atomic-emission (accessed on 20 May 2020).
- Maruyama, G.M. Basics of Structural Equation Modeling; Sage Publications, Inc.: Thousand Oaks, CA, USA, 1998; ISBN 978-0-8039-7408-1. [Google Scholar]
- Pedhazur, E.J. Multiple Regression in Behavioral Research, 3rd ed.; Harcourt Brace: Orlando, FL, USA, 1997. [Google Scholar]
- Smith, E.; Naidu, R.; Weber, J.; Juhasz, A.L. The Impact of Sequestration on the Bioaccessibility of Arsenic in Long-Term Contaminated Soils. Chemosphere 2008, 71, 773–780. [Google Scholar] [CrossRef]
- Bradham Karen, D.; Scheckel Kirk, G.; Nelson Clay, M.; Seales Paul, E.; Lee Grace, E.; Hughes Michael, F.; Miller Bradley, W.; Yeow, A.; Gilmore, T.; Serda Sophia, M.; et al. Relative Bioavailability and Bioaccessibility and Speciation of Arsenic in Contaminated Soils. Environ. Health Perspect. 2011, 119, 1629–1634. [Google Scholar] [CrossRef]
- Whitacre, S.; Basta, N.; Stevens, B.; Hanley, V.; Anderson, R.; Scheckel, K. Modification of an Existing in Vitro Method to Predict Relative Bioavailable Arsenic in Soils. Chemosphere 2017, 180, 545–552. [Google Scholar] [CrossRef]
- Zheng, S.; Wang, F.; Li, X.; Wang, H.; Wan, X. Application of in vitro digestion approach for estimating lead bioaccessibility in contaminated. Res. Environ. Sci. 2013, 26, 851–857. [Google Scholar]
- Liu, Y.; Bello, O.; Rahman, M.M.; Dong, Z.; Islam, S.; Naidu, R. Investigating the Relationship between Lead Speciation and Bioaccessibility of Mining Impacted Soils and Dusts. Environ. Sci. Pollut. Res. 2017, 24, 17056–17067. [Google Scholar] [CrossRef]
- Poggio, L.; Vrščaj, B.; Schulin, R.; Hepperle, E.; Ajmone Marsan, F. Metals Pollution and Human Bioaccessibility of Topsoils in Grugliasco (Italy). Environ. Pollut. 2009, 157, 680–689. [Google Scholar] [CrossRef]
- Badawy, S.H.; Helal, M.I.D.; Chaudri, A.M.; Lawlor, K.; McGrath, S.P. Soil Solid-Phase Controls Lead Activity in Soil Solution. J. Environ. Qual. 2002, 31, 162–167. [Google Scholar] [CrossRef]
- Hettiarachchi, G.M.; Pierzynski, G.M. Soil Lead Bioavailability and in Situ Remediation of Lead-Contaminated Soils: A Review. Environ. Prog. 2004, 23, 78–93. [Google Scholar] [CrossRef]
- Violante, A.; Cozzolino, V.; Perelomov, L.; Caporale, A.G.; Pigna, M. Mobility and bioavailability of heavy metals and metalloids in soil environments. J. Soil Sci Plant Nutr. 2010, 10, 268–292. [Google Scholar] [CrossRef]
- Li, J.; Li, K.; Cave, M.; Li, H.-B.; Ma, L.Q. Lead Bioaccessibility in 12 Contaminated Soils from China: Correlation to Lead Relative Bioavailability and Lead in Different Fractions. J. Hazard. Mater. 2015, 295, 55–62. [Google Scholar] [CrossRef] [PubMed]
- Pelfrêne, A.; Waterlot, C.; Mazzuca, M.; Nisse, C.; Cuny, D.; Richard, A.; Denys, S.; Heyman, C.; Roussel, H.; Bidar, G.; et al. Bioaccessibility of Trace Elements as Affected by Soil Parameters in Smelter-Contaminated Agricultural Soils: A Statistical Modeling Approach. Environ. Pollut. 2012, 160, 130–138. [Google Scholar] [CrossRef] [PubMed]
- Perez, J.P.H.; Tobler, D.J.; Thomas, A.N.; Freeman, H.M.; Dideriksen, K.; Radnik, J.; Benning, L.G. Adsorption and Reduction of Arsenate during the Fe2+-Induced Transformation of Ferrihydrite. ACS Earth Space Chem. 2019, 3, 884–894. [Google Scholar] [CrossRef]
- Beak, D.G.; Basta, N.T.; Scheckel, K.G.; Traina, S.J. Bioaccessibility of Arsenic(V) Bound to Ferrihydrite Using a Simulated Gastrointestinal System. Environ. Sci. Technol. 2006, 40, 1364–1370. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.-K.; Barnett, M.O.; Jardine, P.M.; Brooks, S.C. Factors Controlling the Bioaccessibility of Arsenic(V) and Lead(II) in Soil. Soil Sediment Contam. Int. J. 2003, 12, 165–179. [Google Scholar] [CrossRef]


| Title of Paper | Authors | Number of Soils Studied | Properties Studied | IVBA Method a | Soil Type | Significant Properties on IVBA |
|---|---|---|---|---|---|---|
| Adsorption, Sequestration, and Bio-accessibility of As (V) in Soils | Yang et al., 2002 | 36 | soil pH, cation exchange capacity (CEC), total inorganic carbon TOC, particle size, and Fe and Mn oxides | PBET | Spiked soils | Inverse relationship: FeOx IVBA As (%) = 11.3 pH − 30.5 log Fe Positive relationship: soil pH |
| Adsorption, Oxidation, and Bio-accessibility of As(III) in Soils | Yang et al., 2005 | 36 | soil pH, CEC, total inorganic carbon, TOC, particle size, and Fe and Mn oxides | PBET | Spiked soils | Inverse relationship: FeOx Positive relationship: soil pH |
| Bio-accessible and non-bio-accessible fractions of soil As | Whitacre et al., 2013 | 19 | determination of sorptive phases in IVBA and non-IVBA As soil fractions | OSU-IVG | Spiked soils | Inverse relationship: FeOx and Al Ox Reduction in IVBA As greater for FeOx than AlOx |
| Bio-accessibility of arsenic and cadmium assessed for in vitro bio-accessibility in spiked soils and their interaction during the Unified BARGE Method (UBM) extraction | Xia et al., 2016 | 7 | TOC, CEC, reactive Fe, Mn, and Al oxides, soil pH, particle size | Unified BARGE Method (UBM) | Spiked soils | Inverse relationship: FeOx, AlOx, %TOC Positive relationship: soil pH |
| Modifying Effect of Soil Properties on Bioaccessibility of As and Pb from Human Ingestion of Contaminated Soil | Lake et al., 2021 | soil pH, eCEC, Fe and Al oxides, clay content, organic carbon content | USEPA Method 1340 | Spiked soils | Inverse relationship: FeOx, AlOx, %clay Positive relationship: soil pH | |
| Bio-accessibility of arsenic in soils developed over Jurassic ironstones in eastern England | Palumbo-Roe et al., 2005 | 73 | arsenic fractionation in soil, source of IVBA arsenic in soil | PBET | Naturally contaminated | Inverse relationship: less reactive iron oxide phases Positive relationship: carbonate and iron aluminosilicate/oxyhydroxide phases |
| The impact of sequestration on the bio-accessibility of arsenic in long-term contaminated soils | Smith et al., 2008 | 12 | sequential fractionation of soils, soil pH, total and Free fe, total as, total, Al, total P | SBET/USEPA Method 1340 | Polluted soils | Inverse: FeOx and AlOx but varies with crystallinity of the fractions |
| Relative bioavailability and bio-accessibility and speciation of arsenic in contaminated soils | Bradham et al., 2011 | 9 | Soil pH, total As, Al, Fe, and Mn | SBRC in vitro assay/USEPA Method 1340 | Polluted soils | Inverse relationship: Fe and Al concentration IVBA As (%) = 50.1–67.5 logFeAl |
| Assessment of bio-accessibility and exposure risk of arsenic and lead in urban soils of Guangzhou City, China | Lu et al., 2011 | 25 | GI tract phases, soil pH, OM, particle size, soil metal content (Pb, As, Mn, Fe) | OSU-IVG | Polluted Soils | Stomach phase positive relationship: organic matter content inverse relationship: silt and clay Intestinal phase positive relationship: organic matter and total As content inverse relationship: silt and cla |
| Title of Paper | Authors | Number of Soils Studied | Properties Studied | IVBA Method a | Soil Type | Significant Properties on IVBA |
|---|---|---|---|---|---|---|
| Metals pollution and human bio-accessibility of topsoils in Grugliasco (Italy) | Poggio et al., 2009 | 66 | pH, organic matter content, cation exchange capacity, and particle size distribution | PBET | Polluted soils | Inverse relationship: silt and clay contents Positive relationship: SOM, total Pb content, and sand content |
| Cd, Pb, and Zn oral bio-accessibility of urban soils contaminated in the past by atmospheric emissions from two lead and zinc smelters | Roussel et al., 2010 | 27 | total metal trace element content, total nitrogen, total carbonates, clay contents, soil pH, particle size distribution, organic matter (OM), cation exchange capacity (CEC), assimilated P, free Mn, Al, and Fe | UBM | Polluted soils | Inverse relationship: Fe and Mn oxides; carbonate and iron content (gastric phase); total N and pH (gastrointestinal phase) Gastric IVBA Pb (%) = 171.7 − 1.09 CaCO3 − 225.9 Fe + 0.68 total Pb Gastrointestinal (GI) IVBA Pb (%) = 1020.6 − 32.6 Ntot − 131.1 + 0.39 total Pb Positive relationship: total metal concentration |
| Assessment of bio-accessibility and exposure risk of arsenic and lead in urban soils of Guangzhou City, China | Lu et al., 2011 | 25 | GI tract phases, soil pH, OM, particle size, soil metal content (Pb, As, Mn, Fe) | OSU-IVG | Polluted soils | Intestinal phase No observed relationships Gastric phase Inverse relationship: soil Fe and Mn content Positive relationship: soil organic matter and total Pb content |
| Bio-accessibility of trace elements as affected by soil parameters in smelter-contaminated agricultural soils: A statistical modeling approach | Pelfrêne et al., 2012 | 280 to build the model and 110 to test (390 total) | particle size distribution, soil pH, OM, total carbonate, assimilated P, and free Mn, Fe and Al oxides | UBM | Polluted soils | Negative relationship: total carbonate, OM, and pseudo-total Al and Pb contents Positive relationship: assimilated P |
| Application of in vitro digestion approach for estimating lead bio-accessibility in contaminated soils: influence of soil properties | ShunAn et al., 2013 | 22 | in vitro extraction methods, soil pH | SBET/US EPA Method 1340 and PBET | Polluted soils | SBET results in greater IVBA Pb than PBET Inverse relationship: clay content and soil pH |
| Lead bio-accessibility in 12 contaminated soils from China: Correlation to lead relative bioavailability and lead in different fractions | Li et al., 2015 | 12 | relationship between IVBA Pb and Pb sorbent pools | UBM, SBRC, OSU-IVG, PBET | Polluted soils | Exchangeable and carbonate soil fractions contributed most to IVBA Pb |
| Investigating the relationship between lead speciation and bio-accessibility of mining impacted soils and dusts | Liu et al., 2017 | 36 (18 top soils and 18 house dusts) | relationship between IVBA Pb and speciation of sorbed Pb | USEPA Method 1340 | Polluted soils | Inverse: Mn oxyhydroxides and amorphous Fe and Al oxyhydroxides Positive: carbonates |
| The source of lead determines the relationship between soil properties and lead bio-accessibility | Yan et al., 2019 | 31 | distribution of soil properties based on size fractions, CEC, TOC, soil pH, total metal content, particle size | USEPA Method 1340 | Polluted Soils | Inverse relationship: EC IVBA Pb (%) = 1.79 CEC − 4.165 EC + 1.666 Clay + 0.007Total Pb + 38.71 Positive relationship: CEC, total Pb content |
| Modifying Effect of Soil Properties on Bio-accessibility of As and Pb from Human Ingestion of Contaminated Soil | Lake et al., 2021 | soil pH, eCEC, Fe and Al oxides, clay content, organic carbon content, | USEPA Method 1340 | Spiked soils | Inverse relationship: FeOx, AlOx, %clay |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lake, L.M.; Basta, N.T.; Barker, D.J. Modifying Effect of Soil Properties on Bio-Accessibility of As and Pb from Human Ingestion of Contaminated Soil. Geosciences 2021, 11, 126. https://doi.org/10.3390/geosciences11030126
Lake LM, Basta NT, Barker DJ. Modifying Effect of Soil Properties on Bio-Accessibility of As and Pb from Human Ingestion of Contaminated Soil. Geosciences. 2021; 11(3):126. https://doi.org/10.3390/geosciences11030126
Chicago/Turabian StyleLake, Loryssa M., Nicholas T. Basta, and David J. Barker. 2021. "Modifying Effect of Soil Properties on Bio-Accessibility of As and Pb from Human Ingestion of Contaminated Soil" Geosciences 11, no. 3: 126. https://doi.org/10.3390/geosciences11030126
APA StyleLake, L. M., Basta, N. T., & Barker, D. J. (2021). Modifying Effect of Soil Properties on Bio-Accessibility of As and Pb from Human Ingestion of Contaminated Soil. Geosciences, 11(3), 126. https://doi.org/10.3390/geosciences11030126







