Abstract
A fluidized bed aerosol generator was connected to a 13-stage cascade impactor (nanoMOUDI) for the size fractionation of urban dust (<10 µm), followed by the gravimetric analysis of loaded PTFE filter samples. This method was used to characterize the PM10 (thoracic) fraction of road dust sampled from expressways, arterial roads and local roads in Toronto, Canada. The fine particle fractions (<1.8 µm) of all the studied samples accounted for 51–72% of the resuspended PM10 (by weight). Elemental analysis using ICP-MS and ICP-OES revealed an overall trend of element enrichment in the <1.8 µm fraction compared to the coarse fraction (1.8–10 µm) of the road dust. By contrast, archived house dust samples displayed the reverse trend for most elements. The lung bioaccessibility of target elements (Al, B, Ba, Co, Cr, Fe, La, Mn, Mo, Sb, Sr, Ti, V and Zn) was assessed for each road dust fraction using 0.1 M ammonium citrate (pH 4.4) to simulate intracellular fluid and Gamble solution (pH 7.2) to simulate interstitial lung fluid. The <1.8 µm fraction of local road dust displayed significantly higher bioaccessibility (p < 0.05) for Zn when using Gamble solution, and for seven out of the 14 target elements when using ammonium citrate. These results show the importance of characterizing the fine fraction of road dust.
1. Introduction
The health impacts of exposure to airborne particulate matter (PM) can include an increased risk of respiratory problems (e.g., dyspnea, asthma and chronic bronchitis), cardiovascular effects (e.g., vascular inflammation and atherosclerosis) and cancers [1,2]. It has been recognized that the mass concentration alone is not sufficient for determining the effects associated with exposures to PM, as the chemical composition of the particles contributes to their toxic potency [3,4]. Recently, there has been increased attention paid to the geochemical characterization of PM to better understand the contribution of inorganic constituents to the observed health effects [5]. A relatively new area of interest in the field of public health is the impact of resuspended road dust on human health, particularly on the respiratory and cardiovascular systems [6]. Road dust is a complex medium that is generated by the accumulation of particles derived mainly from vehicular exhaust and non-exhaust sources (particles emitted from tire and brake wear, road surface erosion and urban soil) [7]. Due to the turbulence created by road transportation and wind, resuspended road dust is recognized to be an important source of atmospheric PM, particularly in large urban centres [8,9]. As regulatory and legislative actions have helped to greatly reduce the levels of air pollutants in automotive exhaust in recent decades, the relative contribution of non-exhaust sources to atmospheric PM has increased [10]. Various studies have associated certain metals in atmospheric PM with the wear and tear of auto parts (e.g., Pb, Cu and Zn), gasoline and diesel emissions (Cr and Ni) [7], and exhaust catalysts (platinum, palladium and rhodium) [11]. Particular concerns have been raised about inhalation exposures to vehicular sources of transition metals (e.g., Zn, Mn and Cu), which generate reactive oxygen species (ROS) [12,13] and which tend to display high bioaccessibility in the respiratory tract [5].
Metal bioaccessibility plays a key role in the human health risk assessments of fugitive road dust [5,14]. The term “lung bioaccessibility” refers to the amount of a substance that is soluble in a simulated lung fluid environment and is capable of inducing pulmonary toxicity [15]. A consensus is yet to be reached regarding which bioaccessibility assays most accurately mimic the complexity of lung conditions [5,16,17,18]. The two extractions that are most commonly used to simulate the lung environment include “Gamble solution” and its various modifications (to represent neutral interstitial lung fluid), and the more acidic artificial lysosomal fluid (ALF) to represent intracellular conditions [5]. The pH of Gamble solution typically ranges from 7.3 to 7.8, while ALF is typically between pH 4.3 and 4.5 [5,15]. Mukhtar et al. [19] recommended a simple ammonium citrate buffer (pH 4.4) as an alternative to the more complex ALF formulation, as they found very good agreement between the two assays. Regardless of the extraction formulation, incubation usually proceeds at 37 °C, as this is the biologically relevant temperature for dissolution studies [5]. Caboche et al. [16] recommended an incubation time of 24 h and a solid-to-liquid ratio ranging from 1:5000 to 1:50,000.
The size fractionation of urban road dust samples is critical, as the content of bulk samples is not considered to be a reliable indicator of the concentration and bioaccessibility of metals in fine fractions [14]. Previous road dust studies have shown that metal concentrations increase as the particle size decreases, which may be partly attributable to the larger surface area and greater metal sorption capacity of smaller particles [9]. The thoracic fraction of inhalable particles (PM10), and smaller size fractions, which can penetrate further into the respiratory tract, are most commonly used for characterizing inhalation exposures to urban dust [18,20]. Several approaches have been used to isolate the <10 μm fraction of dust, soil and soil-like media, including dry sieving, ball milling and/or wet extraction [9,21,22,23,24]. Guney et al. [23] reported that efforts to obtain the <10 μm fraction of soil and tailings samples (using micromesh sieves) yielded inadequate sample mass. Rasmussen et al. [24] avoided ball milling and wet extraction, as these could be expected to alter the aerodynamic properties of dust particles, but found that sonic sieving also introduced an unacceptable analytical artefact. Their recommended method was to aerosolize the dust, with subsequent collection using a cascade impactor, which preserved the aerodynamic diameter of the resuspended particles [24].
The aim of the present study was to develop a methodology for characterizing the metal distribution within the thoracic fraction (median aerodynamic diameter < 10 µm) of PM derived from road dust. Aerosolized samples were size fractionated according to the aerodynamic diameter of the particles (from 10 µm to 10 nm) using a 13-stage micro-orifice uniform deposit impactor (nanoMOUDI). Metal bioaccessibility was assessed using a modified Gamble solution [25,26] and a 0.1 M ammonium citrate solution [27]. The developed protocol allowed for the comparison of the fine and coarse components of road dust to better understand how metals are distributed in these size fractions and to increase understanding of the influence of particle size on metal bioaccessibility. To provide additional insight, the protocol was also applied to house dust as a contrasting urban dust medium.
2. Materials and Methods
2.1. Sample Collection and Preparation
The road dust samples used in the present study were collected by the City of Toronto (ON, Canada) using regenerative-air street sweepers under their “Clean Road to Clean Air” program as described by Wiseman et al. [28]. These vehicles isolate dust particles having a median particle diameter of approximately <10 µm into an internal compartment (the “dust box”), from sweepings of local and arterial roads and municipal expressways [28]. The traffic volume and speed limit (km/h) vary from low to high on the sampled roadways (local roads < arterial roads < expressways) [28]. The road dust samples used in this study were obtained from the dust box of the Toronto street sweepers, following collection in 2015 and 2016 [28]. Although the focus of this study was to develop a method for characterizing road dust, house dust samples were included in most of the assays to gain perspective by comparing a different environmental medium. As no Toronto house dust samples were available, archived settled house dust samples (<80 µm size fraction) from the Canadian House Dust Study (CHDS) were used for comparison [29]. The term “bulk dust” is used in this paper to refer to the road dust box samples and the <80 µm house dust samples prior to aerosolization and size fractionation.
2.1.1. Aerosolization and Size Fractionation
Road dust (3 road types, 3 replicates per sample) and house dust (5 homes, 2–4 replicates per sample) were aerosolized using a Fluidized Bed Aerosol Generator (TSI model 3400A) equipped with a ½-inch cyclone, and ultra-high-purity helium as the carrier gas. The bed flow was 2 L per minute (LPM), the bead purge flow was 7 LPM, and the chain conveyor speed was set at 40. The aerosol generator is supplied with bronze beads (ACuPowder International LLC, Union, NJ, USA) in the bed chamber; as the helium enters, the beads are lifted in a boiling action to disaggregate and aerosolize the particle sample. The aerosol generator was connected with ¼-inch-diameter polyetheretherketone (PEEK) tubing to a 13-stage micro-orifice uniform deposit impactor (nanoMOUDI-II, model 125B, MSP Corp., Shoreview, MN, USA), which enabled the aerosolized particles to enter the nanoMOUDI at a flow rate of 10 LPM. Teflon filters (47 mm PTFE with polymethylpentene (PMP) support rings, Pall Corp.) were placed on each of the 13 stages of the nanoMOUDI, which had cut-point diameters ranging from 10 µm to 10 nm. The aerosol generation system was stabilized for 60 min prior to each sample collection period, which ranged from 2 to 4 h in duration, as required to obtain sufficient particle mass on each filter (for subsequent analysis). Three to four replicate aerosolization/size fractionation runs were performed for each dust sample.
The term “PM-loaded filter” is used in this paper to refer to the aerosolized dust fractions collected on the PTFE filters using the nanoMOUDI. The term PMcoarse refers to the coarse fraction of PM10, defined here as 1.8–10 µm (i.e., the three largest size fractions). The term PMfine refers to the fine fraction, defined as <1.8 µm (i.e., the remaining 10 size fractions). The <1.8 µm cut-point was selected because this was the nanoMOUDI cut-point closest to the PM2.5 fraction of dichotomous samplers, which is the most common definition of “fine particles” [30].
2.1.2. Gravimetric Measurements
Gravimetric measurements of the PM-loaded filters were performed using an Excellence Plus XP Ultra Micro balance (XP2U, MettlerToledo, Mississauga, ON, Canada) in the patented Archimedes M3TM Buoyancy-Corrected Gravimetric Analysis Facility (Health Canada, Ottawa, ON, Canada), as described previously [31,32]. The filters were conditioned inside an environmentally controlled weighing chamber for a period of 24 h before the measurements, and buoyancy corrections were applied to all the measurements. The environmental parameters were maintained at a temperature of 21 ± 0.5 °C and relative humidity of 40 ± 1% inside the weighing chamber. The system is equipped for pressure, temperature, dew point and relative humidity monitoring, as required for the application of buoyancy corrections.
2.2. Sample Digestion and Analysis
2.2.1. Reagents, Standards and Certified Reference Materials
All the standards and digestion solutions were prepared using deionized water (DIW, Milli-Q 18.2 MΩ). The total digestion solution was prepared using ultrapure concentrated nitric acid (HNO3, 67–70%, Seastar Chemical Inc., Sidney, BC, Canada) and hydrofluoric acid (HF, 47–51%, Seastar Chemical Inc., Sidney, BC, Canada). Matrix-matched calibration standards for instrumental analysis were prepared using 10 and 100 µg/mL multi-element standards (plasmaCal, SCP Science, Quebec, QC, Canada) and 10,000 µg/mL single-element standards (IsoSpec (Delta Scientific), Ontario, ON, Canada, and SCP Science, Quebec, QC, Canada), as required. Table 1 lists the ingredients of the Gamble solution (mean pH, 7.18 ± 0.04) modified according to Stopford et al. [25] and Midander et al. [26]. The compounds were obtained from Sigma-Aldrich (St. Louis, MO, USA) or Fluka (Fisher Scientific: Waltham, MA, USA) as indicated in Table 1, and were added in the specified order to avoid salt precipitation. The preparation of the 0.1 M ammonium citrate solution (citric acid diammonium salt, 99+%, Alpha Aesar, Tewksbury, MA, USA) was based on the method used by Thomassen et al. [27]. The pH was adjusted to pH 4.4 (mean pH, 4.42 ± 0.01) using 2N HNO3. Both extractions were performed at a 1:10,000 solid-to-liquid ratio, as recommended by Caboche et al. [16]. For the analysis of the Gamble extracts, the inductively coupled plasma–mass spectrometry (ICP-MS) calibration standards ranged from 0 to 100 ng/mL, while a range of 0 to 500 ng/mL was used for the analysis of the ammonium citrate extracts and total digests. The inductively coupled plasma–optical emission spectrometry (ICP-OES) calibration standards ranged from 0 to 10,000 ng/mL. Leachates and digests were diluted 10 times when needed to ensure that the analytical results were within the calibration range. NIST 2584 Trace Elements in Indoor Dust, Nominal 1% Lead Certified Reference Material (CRM) (National Institute of Standards and Technology, Gaithersburg, MD, USA) was used to evaluate the recovery of the targeted elements.

Table 1.
Composition (g/L) of Gamble solution (modified according to Stopford et al., 2003, and Midander et al., 2007).
2.2.2. Total Element Digestion
The method for the total element digestion of the PM-loaded filters and bulk dust samples was based on the 1 h ultrasonic dissolution procedure described previously [33]. The PM-loaded filters and blank filters were carefully inserted into 15 mL disposable centrifuge tubes (Corning Inc., Corning, NY, USA). For the bulk dust and NIST CRM digestion, 2.0 mg subsamples were quantitatively transferred into centrifuge tubes, and 4 mL of HNO3, 0.1 mL of HF and 1.9 mL of DIW were added. Each tube was vortexed (Fisher Scientific, Waltham, MA, USA) for 60 s and incubated at 90 °C in an ultrasonic bath (Branson 8510R-DTH, Branson Ultrasonics, North Billerica, MA, USA) for 60 min. After the first 30 min of incubation, the tubes were inverted to mix the digests. Once cooled, the digests were diluted to 10 mL with DIW, mixed and centrifuged (Allegra X-22, Beckman coulter, Brea, CA, USA) at 3500 rpm for 10 min. The supernatant was decanted to a test tube for elemental determination.
2.2.3. Gamble Extraction
The approach used for the application of the Gamble extraction was based on recommendations by Caboche et al. [16] and was applied to 6 bulk road dust samples (including all road types), 5 house dust samples, and PM-loaded filters from three road dust samples (local road, arterial and expressway dust). PM-loaded filters, blank filters, and 2.0 mg of bulk dust or 2.0 mg of NIST 2584 were inserted into 15 mL disposable centrifuge tubes. A 6 mL volume of Gamble solution (Table 1) was added to the tubes. After mixing, the extraction proceeded at 37 °C for 24 h in an orbital shaker (MaxQ 4000, Thermo Scientific, Waltham, MA, USA) at a speed of 40 rpm. After 24 h, 4 mL of DIW was added, and the extracts were immediately centrifuged at 14,600 rpm for 20 min at 6 °C (Allegra 64R, Beakman Coulter, Brea, CA, USA). The supernatant was carefully transferred into a test tube, acidified using 100 µL of HNO3 and stored at 4 °C until ICP-MS analysis.
2.2.4. Ammonium Citrate (AC) Extraction
The 0.1 M ammonium citrate extraction was based on the first step of the inhalation bioaccessibility method of Thomassen et al. [27], which was developed to represent intra-cellular fluid conditions. It was applied to 6 bulk road dust samples (including all road types), 5 house dust samples, and PM-loaded filters from one road dust sample (local road dust only), with the same experimental approach used for the Gamble extraction, to facilitate comparisons. That is, PM-loaded filters, blank filters, and 2.0 mg of bulk dust or NIST 2584 were inserted into 15 mL centrifuge tubes. A 6 mL volume of extraction fluid (0.1 M ammonium citrate) was added to the tubes, which were placed in the orbital shaker for 24 h of incubation at an agitation rate of 40 rpm and temperature of 37 °C. After incubation, 4 mL of DIW was added to the tubes. The extracts were mixed and immediately centrifuged at 14,600 rpm for 20 min at 6 °C. A 9 mL volume of supernatant was transferred and acidified with 100 µL of HNO3 for sample stabilization.
2.3. Elemental Determination
ICP-MS or ICP-OES was used for elemental determination, depending on the concentration levels present in the extracts. Elements present at low concentrations were determined using the NexION 300s Dual-channel Universal Cell ICP-MS (PerkinElmer, Woodbridge, ON, Canada) equipped with an apex-ST PFA MicroFlow nebulizer, an SC-Fast autosampler (Elemental Scientific, Omaha, NE, USA), a cyclonic spray chamber cooled at 2 °C using a PC3x Peltier cooler and a triple-cone interface. The instrument was controlled using the Syngistix version 1.1 software (PerkinElmer, Woodbridge, ON, Canada) and was operated using an RF power of 1600 W and the following argon flow rates: 94–97, 1.2 and 18 L/min for the nebulizer, auxiliary and plasma, respectively. Standard mode was used to report all the elemental concentrations with the exception of vanadium and chromium in the ammonium citrate extracts, for which kinetic energy discrimination (KED) mode was used with helium (with a flow rate of 3.9 L/min) to attenuate polyatomic interferences. Daily optimization was performed using a tuning solution consisting of Be, Ce, Fe, In, Li, Mg, Pb and U (1 mg/mL). An in-line 25 ng/mL Ge, In and Re internal standard solution was used during the analysis. Elements present at higher concentrations (e.g., Al, Cu, Fe, Mn and Zn) were quantified using the 5100 SVDV ICP-OES (Agilent Technologies, Santa Clara, CA, USA) in the axial viewing mode (viewing height set to 8 mm). The ICP-OES was equipped with a oneNeb Nebulizer and an SPS-2 autosampler (Agilent Technologies, Santa Clara, CA, USA) and was controlled using the ICP Expert software version 7.4.1.10449. The RF power was set to 1200 W, and the flow rates for the nebulizer, plasma and auxiliary were 0.7, 12 and 1 L/min, respectively. The ICP-OES performance check solution (10 mg/L Mn in 2% HNO3) was measured daily. Bronze beads were dissolved using aqua regia (a 1:3 mixture of HNO3 and HCl) to investigate their potential to contaminate the dust during aerosolization. Triplicate samples (0.5 g) of bronze beads were digested in 5 mL of 1:1 aqua regia:DIW until completely dissolved, and the volume was brought to 25 mL with DIW. The extracts were diluted as necessary for elemental determination by ICP-MS and ICP-OES.
2.4. Calculations and Data Analysis
The PM mass distribution across the 13 nanoMOUDI size fractions is expressed in the units of weight percent (wt%), calculated by dividing the weight of PM recovered for each size fraction (i.e., on each PM-loaded filter) by the total weight of PM recovered on all the size fractions in a given aerosolization run, multiplied by 100. To calculate the percent bioaccessibility, the bioaccessible metal concentration (e.g., AlS) was divided by the total metal concentration for each size fraction (e.g., AlT) and multiplied by 100 (e.g., AlS/AlT × 100). The total concentrations obtained using the ultrasonic dissolution method were used in the bioaccessibility calculation for all the PM-loaded filters. In the case of the bulk dust samples, the same method was used to calculate the bioaccessibility for all elements except Cd, Ce and Sn. The results for these three elements in the bulk dust were obtained using a four-acid digestion (HF, HClO4, HNO3 and HCl), followed by ICP-MS or ICP-OES analysis by ActLabs Inc. (Ancaster, ON, Canada). The results from the elemental analysis of the PM-loaded filters from the top three nanoMOUDI stages (<10 to >1.8 µm) were combined to report the coarse fraction (PMcoarse), and the results from the PM-loaded filters from the remaining ten stages (<1.8 µm) were combined to report the fine fraction (PMfine). Three to four replicate aerosolization–fractionation runs were conducted for each sample, which yielded multiple PM-loaded filters for characterizing the PMcoarse and PMfine. Student’s t-tests were used to compare means. The Wilcoxon signed rank test was used to compare medians, and statistical significance was established at a probability level of p < 0.05, unless indicated otherwise.
2.5. Quality Control
The limits of detection (LODs) for each type of extraction were calculated on the basis of three times the standard deviation of procedural blanks (n= 30 for total digestion; n = 6 for Gamble; n = 6 for ammonium citrate; Table 2). The median values for the filter blanks were subtracted from the PM-loaded filter values on a batch basis (n = 3 per batch). NIST 2584 was included in all the batches to monitor the recovery and reproducibility. The median values for the procedural blanks were subtracted from the NIST CRM and bulk sample results on a batch basis (n = 3 per batch). Analysis of the bronze beads used for aerosolization indicated they were a potential source of contamination for Ag, As, Bi, Cu, Ni, Pb and Sn; therefore, these elements are not reported for the PM-loaded filters. Other elements are not reported for the PM-loaded filters because they were below the LODs, including Ag, As, Be, Bi, Cr, Ti, Tl and U in the Gamble extracts and Ag, Be, Se, Tl and U in the ammonium citrate extracts. Ripped nanoMOUDI filters and filters with particle loading insufficient for gravimetric analysis were discarded and are reported as not available (NA).

Table 2.
Quality Assurance/Quality Control (QA/QC) for inductively coupled plasma (ICP) measurements of NIST 2584 following Gamble (* n = 6) and ammonium citrate (* n = 6) extractions and total digestion (¥ n = 11); limits of detection (LODs) were calculated based on 3 times the standard deviation of procedural blanks (* n = 6; ‡ n = 30); RSD = Relative Standard Deviation. (%RSD = Standard Deviation/mean expressed as percent).
Table 2 includes the recovery and reproducibility for the total digestion method evaluated using NIST 2584, and shows that the total element recoveries fell within the 80–120% range, with the exception of those for cadmium (77%) and lanthanum (74%). The bioaccessibility and reproducibility results obtained using NIST 2584 for the Gamble and ammonium citrate extractions are also included in Table 2. The higher relative standard deviation (RSD) of the Gamble results compared to that of the ammonium citrate (AC) extraction is attributed to the relatively low soluble metal concentrations yielded by Gamble, often approaching the LODs. The bioaccessibility results in Table 2 demonstrate the important role of pH, with the more acidic AC extraction yielding greater bioaccessibilities (up to 81.8%) than the neutral Gamble extraction (<32%), consistent with other studies that have used NIST 2584 to test bioaccessibility assays [16,34,35,36]. To the authors’ knowledge, these are the first reported results for NIST 2584 using 0.1 M ammonium citrate extraction.
3. Results and Discussion
3.1. Road Dust and House Dust PM Mass Distribution Profiles
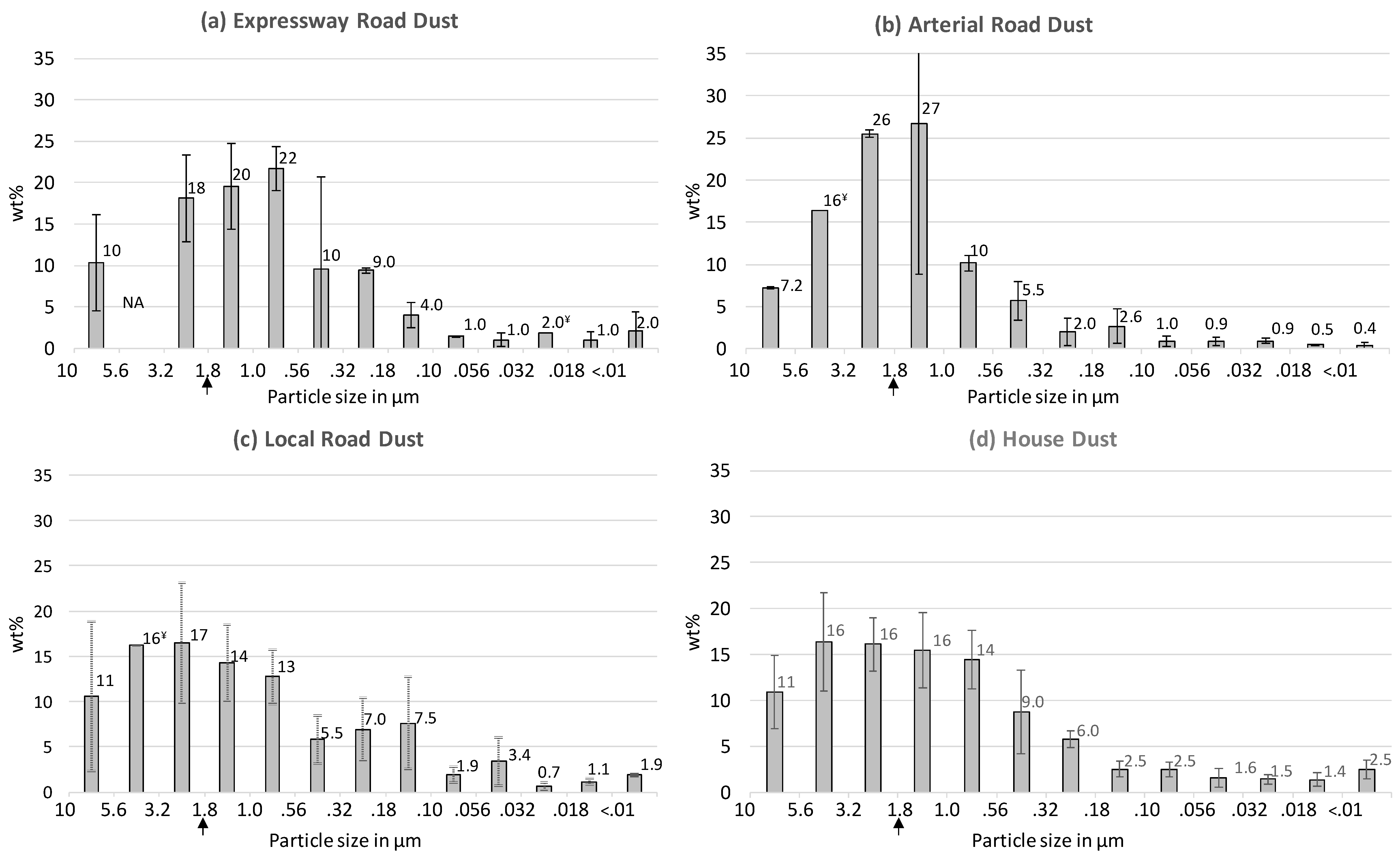
Figure 1a–c display the PM mass distribution profiles obtained by aerosolizing three types of urban road dust followed by size fractionation into 13 nanoMOUDI stages and the gravimetric analysis of the PM-loaded filters. The particle size modes of these road dust profiles display a decreasing trend from local roads (mode = 1.8–3.2 µm) to arterial roads (mode = 1.0–1.8 µm) to expressways (mode = 0.56–1.0 µm). In other words, as the speed limit and traffic volume increase, the particle size mode decreases in the following order: local roads > arterial roads > expressways. A comparison of the relative proportions of fine particles (PMfine) versus coarse particles (PMcoarse) can be derived from Figure 1, by combining the wt% values for the ten fine fractions (<1.8 µm) and the three coarse fractions (>1.8 to <10 µm). This comparison shows that the PM10 generated from expressway dust is dominated by PMfine (72 wt% fine; 28 wt% coarse), whereas the relative proportions are almost equal in the PM10 generated from the other types of road dust (PMfine = 51 wt% for arterial road and 56 wt% for local road; Figure 1).
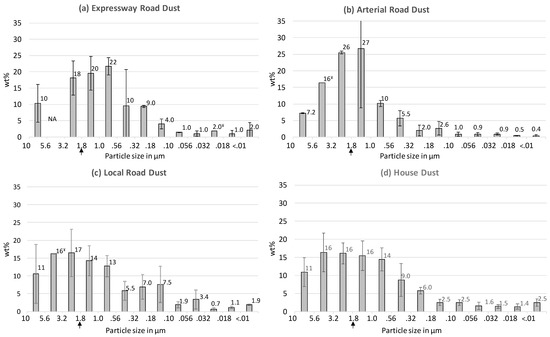
Figure 1.
Particulate matter (PM) mass distribution profiles for (a) expressway dust, (b) arterial road dust, (c) local road dust and (d) house dust. Weight of each fraction was divided by total weight of all fractions, expressed as % by weight (= wt%). Error bars for road dust profiles = standard deviations of triplicate runs unless indicated otherwise (NA = no filters passed QC/QC criteria, ¥ = only one filter passed); error bars for house dust = standard deviations of four different homes; fine particles defined as <1.8 µm in this study (indicated by the vertical arrow). See supplementary material (Figure S1) for details of individual homes.
3.2. Total Element Concentrations in Fine versus Coarse Dust Fractions
Typical urban house dust samples were included in the present study for the purpose of comparison with an environmental medium of differing composition and physical–chemical characteristics (Figure 1d). In general, road dust tends to be slightly more alkaline, typically pH 6–8 [14,37], and has a lower organic carbon content, typically ≤10% [28,38], compared to house dust (16–37% organic carbon; pH 6) [39]. The house dust PM mass distribution profile (Figure 1d) indicates that the PM10 generated from indoor settled dust has a broad particle size mode, between 1.8 and 5.6 µm, and consists of 57 wt% PMfine (43 wt% PMcoarse). Previous size fractionation of CHDS samples, using a sonic sieve, indicated that the <10 µm fraction constituted 50% of the bulk dust (<80 µm) by weight [24]. Based on the nanoMOUDI results in Figure 1d showing that fine particles (<1.8 µm) constitute approximately half of the <10 µm fraction by weight, which in turn constitutes approximately half of the bulk dust by weight [24], it can be estimated that indoor settled dust contains about 25 wt% fine particles. Such estimates are helpful for assessing potential inhalation exposures to metals and other contaminants via resuspended house dust.
The predominance of PMfine observed in the expressway dust is consistent with previous conclusions that higher traffic volumes are associated with increased grinding of road dust by vehicles [40]. Otherwise, it is difficult to compare the profiles in Figure 1 with literature studies due to the paucity of data on the fine particle size distribution of road dust. The high proportions of PMfine observed in all the studied samples (Figure 1) have particular significance for inhalation exposure assessments, due to the increase in particle number concentrations for a given mass of fine particles compared to coarse particles [20].
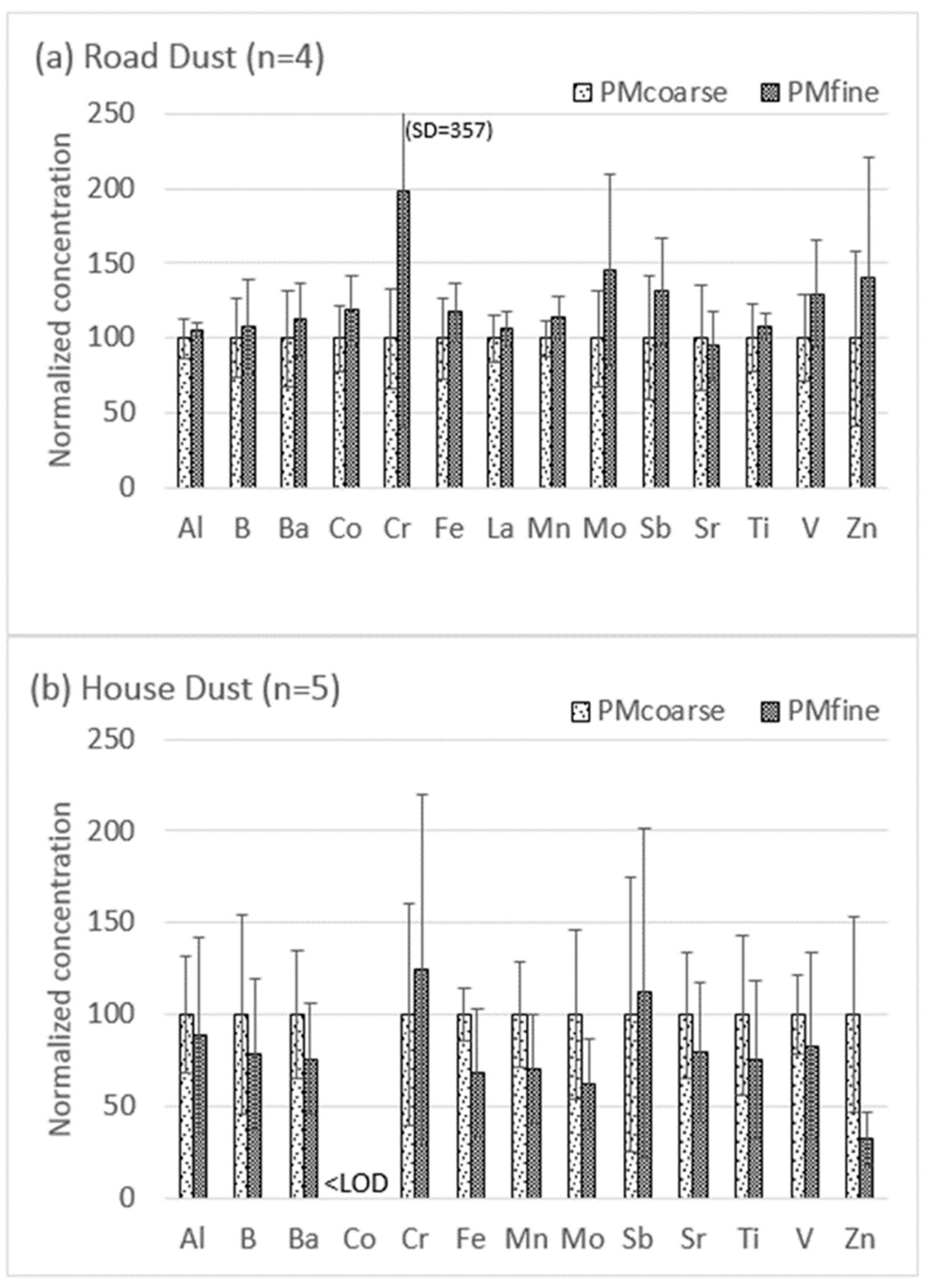
Figure 2 compares the total element concentrations in PMfine versus PMcoarse for the two studied media, normalized to enable multi-element presentation in a single graph. A key feature of Figure 2 is the reversal in metal distribution trends between the two media: in the road dust, PMfine ≥ PMcoarse for all the studied elements, whereas in the house dust, PMfine ≤ PMcoarse for most elements. The original data presented for the individual samples in the supplementary material (Tables S1 and S2) show that the differences between the size fractions are statistically significant (p <0.05) in some samples and not in others, but it is the overall difference (reversal in trend) between the two media that is relevant and worth further investigation. Metal enrichment in smaller size fractions is often attributed to the larger surface area and greater metal sorption capacity of small particles [9], but the difference in trend between the two media in Figure 2 indicates an overriding influence of other factors, such as different metal species and sources. In the case of road dust, the observed enrichment of metals in PMfine compared to PMcoarse (Figure 2a) is consistent with Kong et al. [40], who also reported higher metal concentrations (Cr, Co, Ni, Cu, Zn, As, Cd and Pb) in the PM2.5 fraction of road dust compared to PM10. Padoan et al. [14] suggested that the metal enrichment in the fine fractions of road dust might be attributable to the greater proportion of combustion products (vs. non-exhaust traffic emissions) present in PM2.5 compared to PM10.
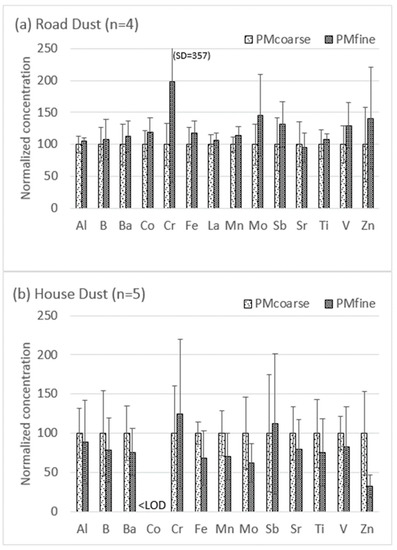
Figure 2.
Normalized total metal concentrations in fine versus coarse fractions of (a) road dust and (b) house dust. For each element, the coarse fraction was normalized (to 100), with the fine fraction converted proportionately. Fine = <1.8 µm and coarse = 1.8–10 µm; error bars show normalized standard deviations (see supplementary material Tables S1 and S2 for all original data).
By contrast, most of the element concentrations in the house dust displayed the reverse trend (PMfine ≤ PMcoarse) with two exceptions: Sb and Cr in the house dust both displayed PMfine ≥ PMcoarse (Figure 3b). This was consistent with the indoor PM2.5/PM10−2.5 trends for Sb and Cr observed in living room air samples from Windsor Ontario, which displayed median values of 25.5/11.0 µg/g for Sb and 1214/267 µg/g for Cr [24]. A study of Edmonton homes [41] concluded that Sb in ultrafine PM in indoor air could arise from both indoor sources (the resuspension of flame-retardant-treated carpet fibers) and outdoor sources (traffic-related brake/tire wear). Resuspended house dust was also identified as a source of Cr [41], which has both indoor sources (e.g., stainless steel debris) and outdoor sources (traffic and industry) [41,42,43].
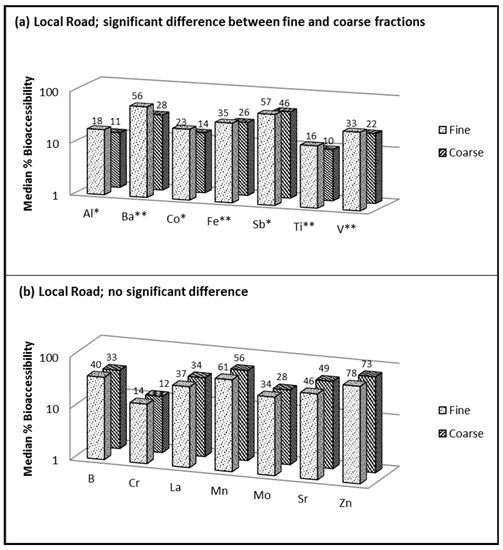
Figure 3.
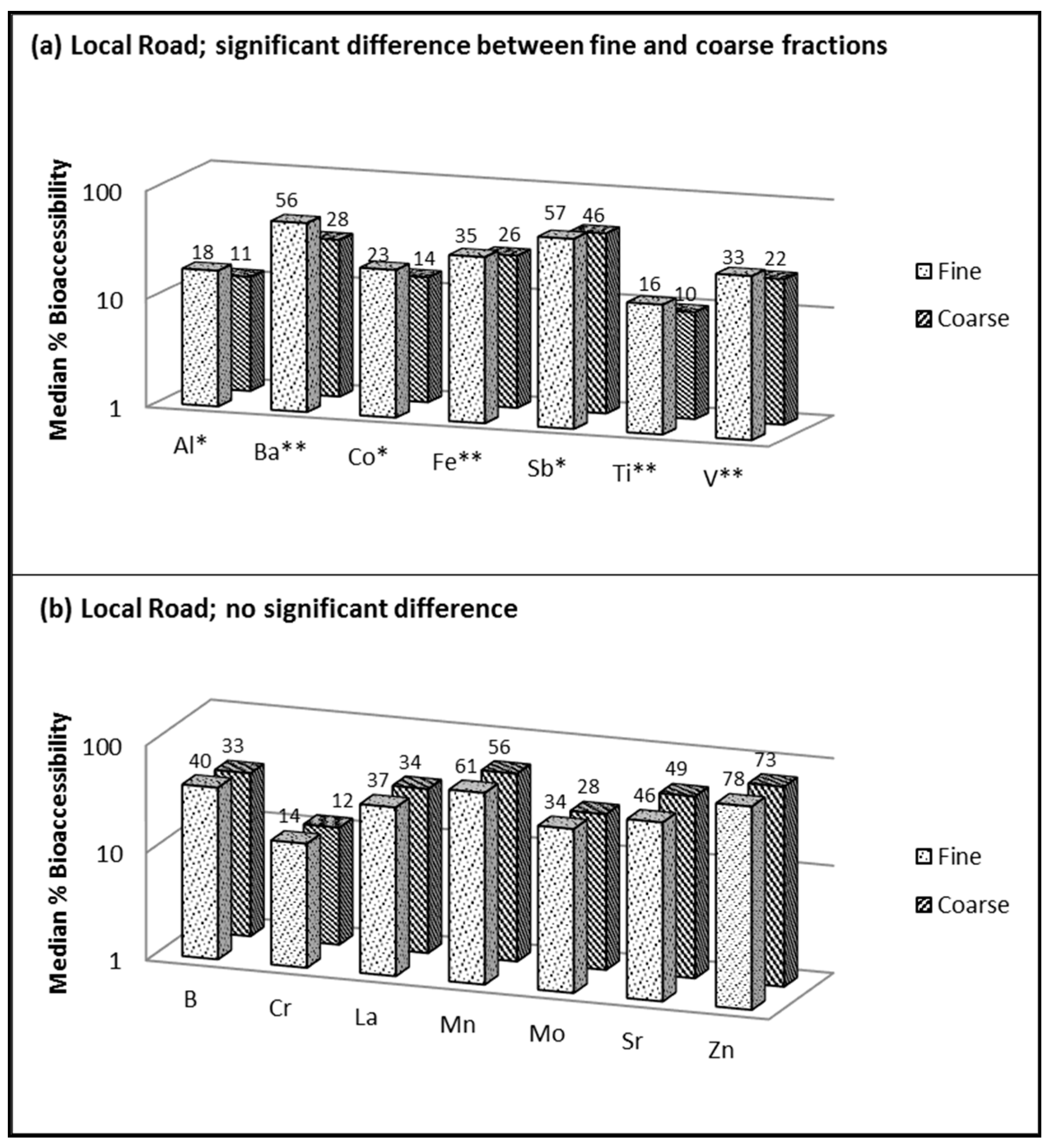
Bioaccessibility of fine versus coarse fractions of resuspended road dust when using 0.1 M ammonium citrate (pH 4.4) showing (a) significant difference between fractions and (b) no significant difference; fine = <1.8 µm and coarse = 1.8–10 µm; * significant at p < 0.05; ** significant at p < 0.01.
3.3. Metal Bioaccessibility in Fine versus Coarse Dust Fractions
Figure 3 compares the median bioaccessibility results obtained for PMfine versus PMcoarse using ammonium citrate extraction (AC; pH 4.4), which was applied only to the local road dust sample (insufficient sample was available for the arterial road and expressway dust). Seven (out of the 14) targeted elements displayed a statistically significant influence of particle size on bioaccessibility (Al, Ba, Co, Fe, Sb, Ti and V), and in each case, the bioaccessibility was higher in PMfine than in PMcoarse (Figure 3a). The simple 0.1 M AC solution used in this study does not include reagents commonly used in ALF such as glycine, citrate and tartrate, which may enhance extraction efficiency [44]. However, the key benefit of using a simple AC solution (especially for determining trace elements in lightly loaded filter samples) is minimizing matrix interferences. More complex extraction fluids can introduce analytical problems such as salt build up, matrix effects and high blanks (depending on the purity of the reagents) [19].
Of all the target elements, the bioaccessibility was the highest for Zn (Figure 3b) in both size fractions extracted with AC (78% in PMfine and 73% in PMcoarse), consistent with previous studies [7,14]. Hong et al. [7] concluded that tire wear and diesel exhaust were the two greatest contributors to bioaccessible Zn in road dust. The bioaccessibility was the second highest for Mn (Figure 3b), which may reflect weathered soil minerals and/or Mn-based gasoline additives. The tendency for the total concentrations of Zn, Mn and other metals to be enriched in PMfine compared to PMcoarse (Figure 2; Table S1), in combination with their high bioaccessibility (Figure 3), highlights the importance of characterizing inhalation exposures to metals in the fine fraction of resuspended road dust in health risk assessments.
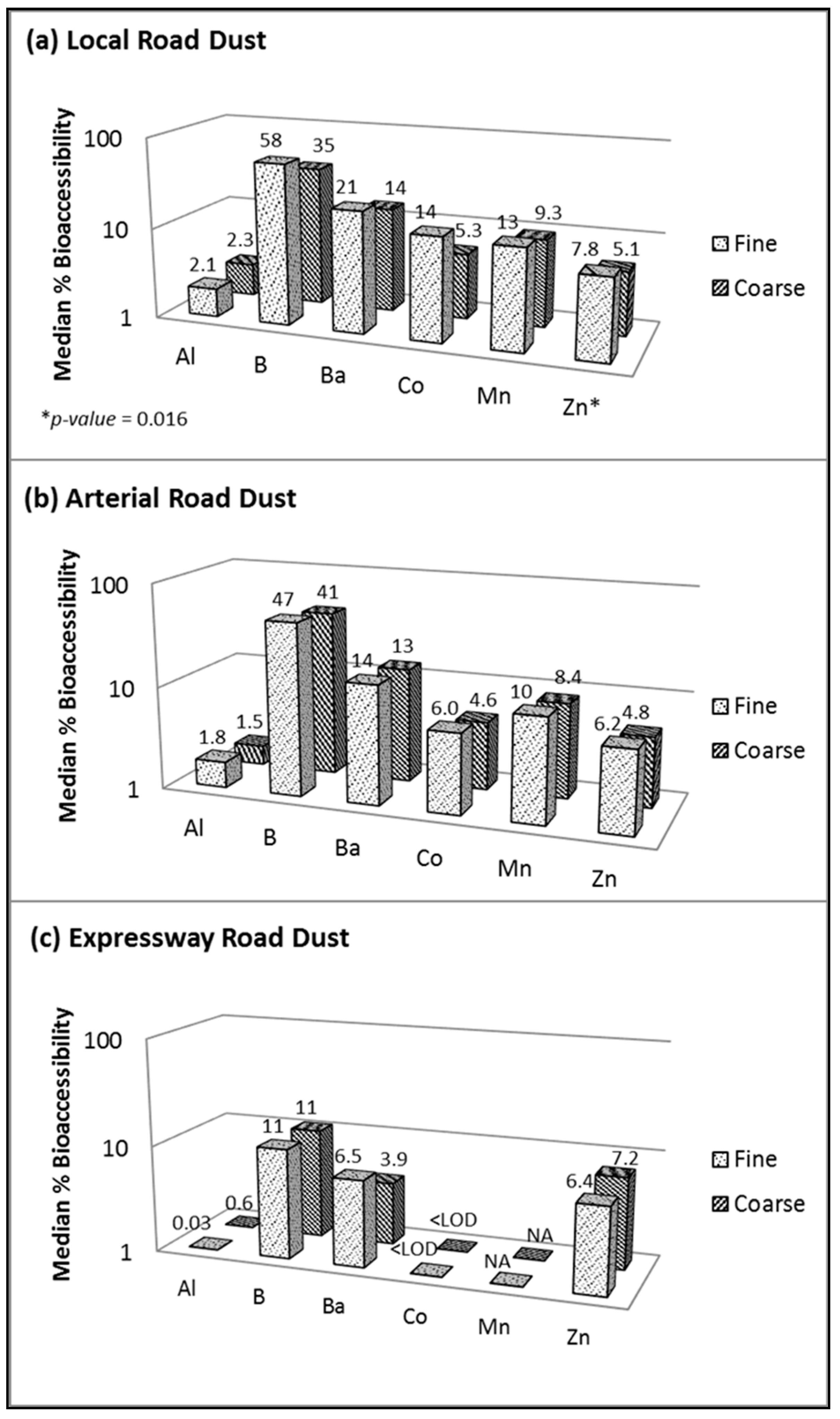
Figure 4 displays the median elemental bioaccessibilities in the PMfine and PMcoarse fractions of three types of road dust obtained using Gamble extraction. Due to the neutral pH of the Gamble extraction (pH 7.2), only six elements exceeded the LOD for local road dust (Figure 4a), compared to 14 when using the more acidic AC extraction (Figure 3). The boron (B) bioaccessibility values in the local road dust were similar regardless of which assay was used (40–58% in PMfine and 33–35% in PMcoarse), but the bioaccessibility values for the other elements (Al, Ba, Co, Mn and Zn) were two to ten times lower when using Gamble (Figure 3) compared to AC (Figure 4a). The high RSD values yielded by Gamble (observed using NIST CRM, Table 2) introduced another challenge: the greater the variability in the results, the greater the number of replicates needed to establish a significant difference between the size fractions. Thus, while Figure 4 shows that the overall trend when using Gamble was toward higher bioaccessibility in PMfine versus PMcoarse, the difference between the size fractions was significant only in the case of Zn in local road dust (Figure 4a). Specifically, the Zn in local road dust exhibited significantly higher bioaccessibility in PMfine (7.8%) than in PMcoarse (5.1%). Similarly, Pelfrêne et al. [15] reported that bioaccessibility was critically dependent on the pH of the simulated lung fluid and observed that ALF (pH 4.5) yielded more stable results across different solid:liquid ratios and greater extraction ability than Gamble (pH 7.3) for assessing the bioaccessibility of fugitive smelter dusts.
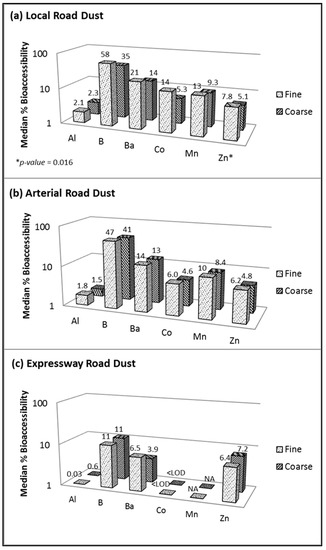
Figure 4.
Element bioaccessibility in fine versus coarse fractions of resuspended road dust when using Gamble solution (pH 7.2) for (a) local road, (b) arterial road and (c) expressway dust. Fine = <1.8 µm and coarse = 1.8–10 µm; Mn not reportable for (c) expressway.
Previous observations of higher metal bioaccessibilities in the fine versus coarse fraction of road dust have been attributed to differences in speciation [14], suggesting that different metal sources may influence each size fraction. It follows that differences in speciation may also influence the bioaccessibility of metals in dust from different road types. Indeed, Figure 4 does display a general trend for the three road types: as the traffic volume and speed limits increased, the metal bioaccessibility in PMfine decreased (local road > arterial road > expressway), although this trend was statistically significant only for Co (p < 0.05). A similar trend was observed in PMcoarse, with the exception of Zn bioaccessibility, which was relatively stable regardless of the road type (ranging from 4.8 to 7.8%; Figure 4). These preliminary results suggest that the differences in metal bioaccessibility between different road dust types may be attributable to differences in speciation.
3.4. Metal Bioaccessibility in Bulk Samples of House Dust and Road Dust
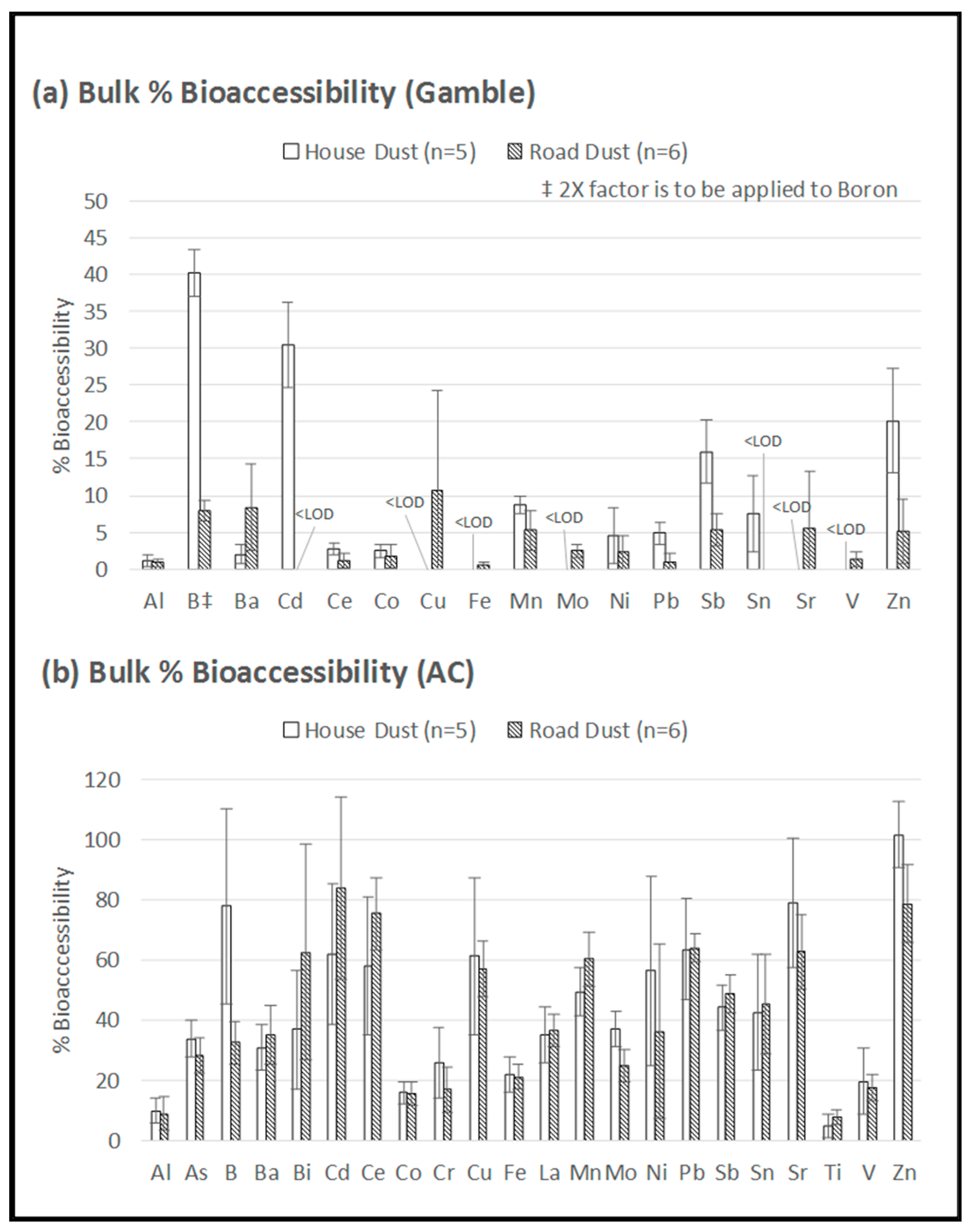
Figure 5 displays the data from the bulk dust samples (i.e., not aerosolized), contrasting the results obtained with the two different bioaccessibility assays, which highlight the role of pH having a controlling influence on metal solubility, independently of the matrix. Because the bulk dust samples shown in Figure 5 were not aerosolized, there was no risk of contamination by bronze beads, thereby permitting Ag, As, Bi, Cu, Ni, Pb and Sn to be reported. Furthermore, the small sample masses of the PM-loaded filters were inadequate to exceed the LODs for many elements, and therefore, the bulk samples were useful for comparing the bioaccessibility of a broader range of elements in the road dust versus house dust.
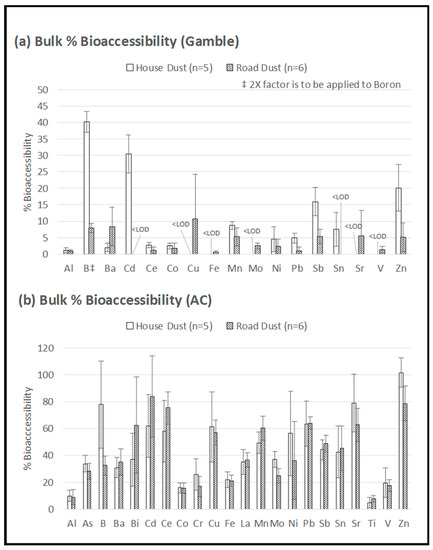
Figure 5.
Bioaccessibility of elements in bulk house dust and bulk road dust using (a) Gamble extraction at pH 7.2, and (b) 0.1 M ammonium citrate (AC) extraction at pH 4.4. ‡ Boron concentration was halved in 5a to fit the graph. Error bars = standard deviations.
The more neutral Gamble extraction (Figure 5a) permitted the detection of only 17 elements (above the LOD) compared to the 22 elements detected using the more acidic AC extraction (Figure 5b). This observation that the more acidic extraction (AC) yielded higher bioaccessibility was consistent with Pelfrêne et al. [15], who reported higher bioaccessibility when using ALF compared to Gamble solution for three CRM matrices: NIST 1648a (urban PM), NIST 2710a (soil), and BCR 723 (road dust). In addition to the role of pH, the higher bioaccessibility obtained with AC in the present study could also be related to the role of citrate as a chelating agent [19], as the citrate concentration was higher in AC than in the Gamble solution (Table 1).
There was one exception to the above-noted pH influence: Figure 5 shows that the bioaccessibility of B in the house dust appeared to be unaffected by the difference in pH between the two extractions (Gamble: 80%; AC: 78%). In the road dust, B bioaccessibility when using AC (32%) was double that when using Gamble solution (16%), which is similar to the trend shown by other elements. The observed behaviour of B suggests that B speciation in house dust is different from B speciation in road dust, possibly due to the different sources of B in the indoor environment (such as household cleaning products). Overall, the metal bioaccessibility tended to be higher in house dust than in road dust (Figure 5). When using the Gamble extraction, 11 out of the 17 targeted elements (Al, B, Cd, Ce, Co, Mn, Ni, Pb, Sb, Sn and Zn) showed higher bioaccessibility in house dust compared to road dust (Figure 5). When using AC, 12 out of the 22 targeted elements (Al, As, B, Co, Cr, Cu, Fe, Mo, Ni, Sr, V and Zn) showed higher bioaccessibility in house dust than in road dust (Figure 5). These results are consistent with Rasmussen et al. [24,39], who attributed differences between outdoor soil and indoor dust metal bioaccessibility to differences in matrix composition (especially organic carbon content) and metal sources/speciation. Further physical–chemical characterization of the samples would be required to fully understand the observed differences in metal dissolution behavior between house dust and road dust.
4. Conclusions
This study addressed the need for a relevant methodology for isolating and size-fractionating urban dust particles within the thoracic fraction (<10 µm) for subsequent elemental analysis. The resuspension of dust using a fluidized bed aerosol generator connected to a nanoMOUDI to load particles onto filters enabled (a) the characterization of the PM mass distribution across 13 aerodynamic size fractions ranging from 10 µm to <10 nm in aerodynamic diameter, and (b) the subsequent characterization of the total and bioaccessible metal composition of each size fraction. The method proved suitable for both the environmental media studied: road dust and house dust. However, one limitation of the method arose from the use of bronze beads to disaggregate and fluidize the dust particles, which were found to be a potential source of metal contamination. As a result, Ag, As, Bi, Cu, Ni, Pb and Sn could not be reported for the PM-loaded filters. Therefore, future studies will evaluate alternative bead materials. Another limitation was that the method was quite labour-intensive, particularly when running multiple replicates of each sample. Time and effort could be saved in future studies by installing fewer stages in the nanoMOUDI, which is an option described by the manufacturer. A further advantage of using fewer stages would be the increased PM mass loading that would be obtained on each filter, which would improve the likelihood of exceeding the ICP limits of detection. The analytical challenge presented by the lightly loaded filters in the present study, particularly in the ultrafine size range (<100 nm), resulted in a limited number of elements exceeding the limits of detection.
As this study focused on method development, emphasis was placed on obtaining multiple aerosolization–nanoMOUDI replicates of a small number of samples to characterize the within-sample reproducibility. In future studies, more representative sampling across different types of roads will be required to further investigate the preliminary results presented here. Specifically, it was observed that the expressway dust sample contained a greater proportion of fine particles (PMfine defined as <1.8 µm) compared to dust from other road types (local and arterial) and urban house dust. It was also observed that, in general, road dust showed enriched total metal concentrations in PMfine compared to PMcoarse (1.8–10 µm). Conversely, house dust showed equal or higher total metal concentrations in PMcoarse compared to PMfine, with only two exceptions (Sb and Cr). The observed higher proportion of fine particles in the expressway dust and the observed tendency for metals to be more concentrated in the fine fraction of road dust (but not house dust) are consistent with previous studies that concluded that combustion and non-combustion products generated by vehicular traffic are a key source of PM-bound metals in the fine size range (commonly defined as PM2.5).
In addition, the fine fraction of local road dust displayed significantly higher bioaccessibility than the coarse fraction (p < 0.05) for Zn when using Gamble extraction (pH 7.2; to simulate neutral interstitial lung fluid) and for seven out of the 14 targeted elements when using ammonium citrate (AC) extraction (pH 4.4; to simulate intracellular fluid). The simple AC solution showed promise by reducing the analytical challenges often associated with the more complex ALF matrices used to simulate intracellular dissolution. In general, the high bioaccessibility of many metals in the thoracic fraction of road dust, in combination with the observed enrichment of total metal concentrations in the fine fraction, emphasizes the importance of rigorous and well-established methodologies for refining assessments of inhalation exposures to resuspended road dust.
Supplementary Materials
The following are available online at https://www.mdpi.com/2076-3263/11/2/87/s1. Figure S1: PM mass distribution profiles of four individual homes from the Canadian House Dust Study, Table S1: Fine and coarse metal concentrations (µg/g) in individual samples of road dust, Table S2: Fine and coarse metal concentrations (µg/g) in individual samples of house dust.
Author Contributions
Conceptualization, P.E.R. and C.L.S.W.; methodology, P.E.R. and C.L.; analytical analysis, C.L.; data analysis, C.L. with assistance from P.E.R. and S.B.; data interpretation, C.L. and P.E.R.; writing—original draft preparation, C.L.; writing—revised draft preparation, P.E.R. with review and editing by S.B. and C.L.S.W.; supervision, P.E.R.; funding acquisition, P.E.R. and C.L.S.W. All authors have read and agreed to the published version of the manuscript.
Funding
This project was funded by Health Canada’s Chemical Management Plan and the Natural Sciences and Engineering Research Council of Canada (NSERC Discovery Grants Program, RGPIN-2018-05966).
Acknowledgments
The authors thank Marc Chénier and Mary-Luyza Avramescu for their advice throughout the project, and Jianjun Niu for technical assistance in the laboratory. The authors are grateful to Shabana Siddique and Julie Buick for valuable comments on an earlier draft. Thanks go to the participants of the Canadian House Dust Study, which was approved by Health Canada’s Research Ethics Board, and to Vesna Stefanovic-Briatico from the City of Toronto for her role as a coordinator for the collection of the road dust sweepings.
Conflicts of Interest
The authors declare no conflict of interest.
References
- World Health Organization. Health Effects of Particulate Matter; World Health Organization: Geneva, Switzerland, 2013; 15p, ISBN 9789289000017. [Google Scholar]
- Kim, K.-H.; Kabir, E.; Kabir, S. A review on the human health impact of airborne particulate matter. Environ. Int. 2015, 74, 136–143. [Google Scholar] [CrossRef] [PubMed]
- Franklin, M.; Koutrakis, P.; Schwartz, P. The Role of Particle Composition on the Association between PM2.5 and Mortality. Epidemiology 2008, 19, 680–689. [Google Scholar] [CrossRef] [PubMed]
- Jin, L.; Xie, J.; Wong, C.K.C.; Chan, S.K.Y.; Abbaszade, G.; Schnelle-Kreis, J.; Zimmermann, R.; Li, J.; Zhang, G.; Fu, P.; et al. Contributions of City-Specific Fine Particulate Matter (PM2.5) to Differential In Vitro Oxidative Stress and Toxicity Implications between Beijing and Guangzhou of China. Environ. Sci. Technol. 2019, 53, 2881–2891. [Google Scholar] [CrossRef] [PubMed]
- Wiseman, C.L. Analytical methods for assessing metal bioaccessibility in airborne particulate matter: A scoping review. Anal. Chim. Acta 2015, 877, 9–18. [Google Scholar] [CrossRef] [PubMed]
- Khan, K.R.; Strand, A.M. Road dust and its effect on human health: A literature review. Epidemiol. Health 2018, 40, e2018013. [Google Scholar] [CrossRef] [PubMed]
- Hong, N.; Guan, Y.; Yang, B.; Zhong, J.; Zhu, P.; Ok, Y.S.; Hou, D.; Tsang, D.C.; Guan, Y.; Liu, A. Quantitative source tracking of heavy metals contained in urban road deposited sediments. J. Hazard. Mater. 2020, 393, 122362. [Google Scholar] [CrossRef]
- Pant, P.; Harrison, R.M. Estimation of the contribution of road traffic emissions to particulate matter concentrations from field measurements: A review. Atmos. Environ. 2013, 77, 78–97. [Google Scholar] [CrossRef]
- Logiewa, A.; Miazgowicz, A.; Krennhuber, K.; Lanzerstorfer, C. Variation in the Concentration of Metals in Road Dust Size Fractions Between 2 μm and 2 mm: Results from Three Metallurgical Centres in Poland. Arch. Environ. Contam. Toxicol. 2020, 78, 46–59. [Google Scholar] [CrossRef]
- Van Der Gon, H.A.D.; Gerlofs-Nijland, M.E.; Gehrig, R.; Gustafsson, M.; Janssen, N.; Harrison, R.M.; Hulskotte, J.; Johansson, C.; Jozwicka, M.; Keuken, M.; et al. The Policy Relevance of Wear Emissions from Road Transport, Now and in the Future—An International Workshop Report and Consensus Statement. J. Air Waste Manag. Assoc. 2013, 63, 136–149. [Google Scholar] [CrossRef]
- Zereini, F.; Alsenz, H.; Wiseman, C.L.; Püttmann, W.; Reimer, E.; Schleyer, R.; Bieber, E.; Wallasch, M. Platinum group elements (Pt, Pd, Rh) in airborne particulate matter in rural vs. urban areas of Germany: Concentrations and spatial patterns of distribution. Sci. Total Environ. 2012, 416, 261–268. [Google Scholar] [CrossRef] [PubMed]
- Angelé-Martínez, C.; Goodman, C.; Brumaghim, J. Metal-mediated DNA damage and cell death: Mechanisms, detection methods, and cellular consequences. Metallomics 2014, 6, 1358–1381. [Google Scholar] [CrossRef] [PubMed]
- Abbasi, S.; Keshavarzi, B.; Moore, F.; Hopke, P.K.; Kelly, F.J.; Dominguez, A.O. Elemental and magnetic analyses, source identification, and oxidative potential of airborne, passive, and street dust particles in Asaluyeh County, Iran. Sci. Total Environ. 2020, 707, 136132. [Google Scholar] [CrossRef] [PubMed]
- Padoan, E.; Romè, C.; Ajmone-Marsan, F. Bioaccessibility and size distribution of metals in road dust and roadside soils along a peri-urban transect. Sci. Total Environ. 2017, 89–98. [Google Scholar] [CrossRef] [PubMed]
- Pelfrêne, A.; Cave, M.R.; Wragg, J.; Douay, F. In Vitro Investigations of Human Bioaccessibility from Reference Materials Using Simulated Lung Fluids. Int. J. Environ. Res. Public Health 2017, 14, 112. [Google Scholar] [CrossRef] [PubMed]
- Caboche, J.; Perdrix, E.; Malet, B.; Laurent, Y.A. Development of an in vitro method to estimate lung bioaccessibility of metals from atmospheric particles. J. Environ. Monit. 2011, 13, 621–630. [Google Scholar]
- Wragg, J.; Cave, M. Assessment of a geochemical extraction procedure to determine the solid phase fractionation and bioaccessibility of potentially harmful elements in soils: A case study using the NIST 2710 reference soil. Anal. Chim. Acta 2012, 722, 43–54. [Google Scholar] [CrossRef] [PubMed]
- Kastury, F.; Smith, E.; Juhasz, A.L. A critical review of approaches and limitations of inhalation bioavailability and bioaccessibility of metal(loid)s from ambient particulate matter or dust. Sci. Total Environ. 2017, 574, 1054–1074. [Google Scholar] [CrossRef] [PubMed]
- Mukhtar, A.; Mohr, V.; Limbeck, A. The suitability of extraction solutions to assess bioaccessible trace metal fractions in airborne particulate matter: A comparison of common leaching agents. Environ. Sci. Pollut. Res. 2015, 22, 16620–16630. [Google Scholar] [CrossRef] [PubMed]
- Terzano, C.; Di Stefano, F.; Conti, V.; Graziani, E.; Petroianni, A. Air pollution ultrafine particles: Toxicity beyond the lung. Eur. Rev. Med. Pharmacol. Sci. 2010, 14, 809–821. [Google Scholar] [PubMed]
- Broadway, A.; Cave, M.R.; Wragg, J.; Fordyce, F.M.; Bewley, R.J.; Graham, M.C.; Ngwenya, B.T.; Farmer, J.G. Determination of the bioaccessibility of chromium in Glasgow soil and the implications for human health risk assessment. Sci. Total Environ. 2010, 409, 267–277. [Google Scholar] [CrossRef]
- Ljung, K.; Siah, W.S.; Devine, B.; Maley, F.; Wensinger, A.; Cook, A.; Smirk, M. Extracting dust from soil: Improved efficiency of a previously published process. Sci. Total Environ. 2011, 410411, 269–270. [Google Scholar] [CrossRef]
- Guney, M.; Chapuis, R.P.; Zagury, G.J. Lung bioaccessibility of contaminants in particulate matter of geological origin. Environ. Sci. Pollut. Res. 2016, 23, 24422–24434. [Google Scholar] [CrossRef] [PubMed]
- Rasmussen, P.E.; Levesque, C.; Chénier, M.; Gardner, H.D. Contribution of metals in resuspended dust to indoor and personal inhalation exposures: Relationships between PM10 and settled dust. Build. Environ. 2018, 143, 513–522. [Google Scholar] [CrossRef]
- Stopford, W.; Turner, J.; Cappellini, D.; Brock, T. Bioaccessibility testing of cobalt compounds. J. Environ. Monit. 2003, 5, 675–680. [Google Scholar] [CrossRef] [PubMed]
- Midander, K.; Pan, J.; Wallinder, I.O.; Leygraf, C. Metal release from stainless steel particles in vitro—Influence of particle size. J. Environ. Monit. 2007, 9, 74–81. [Google Scholar] [CrossRef] [PubMed]
- Thomassen, Y.; Nieboer, E.; Romanova, N.; Nikanov, A.; Hetland, S.; VanSpronsen, E.P.; Odland, J.Ø.; Chashchine, V. Multi-component assessment of worker exposures in a copper refinery Part 1. Environmental monitoring. J. Environ. Monit. 2004, 6, 985–991. [Google Scholar] [CrossRef] [PubMed]
- Wiseman, C.L.; Niu, J.; Levesque, C.; Chénier, M.; Rasmussen, P.E. An assessment of the inhalation bioaccessibility of platinum group elements in road dust using a simulated lung fluid. Environ. Pollut. 2018, 241, 1009–1017. [Google Scholar] [CrossRef] [PubMed]
- Rasmussen, P.E.; Levesque, C.; Chénier, M.; Gardner, H.D.; Jones-Otazo, H.; Petrovic, S. Canadian House Dust Study: Population-based concentrations, loads and loading rates of arsenic, cadmium, chromium, copper, nickel, lead, and zinc inside urban homes. Sci. Total Environ. 2013, 443, 520–529. [Google Scholar] [CrossRef] [PubMed]
- Health Canada. Human Health Risk Assessment for Coarse Particulate Matter; Cat.: H144-30/2016E-PDF, Pub.: 150213; Water and Air Quality Bureau, Health Canada, Government of Canada: Ottawa, ON, Canada, 2016; 289p, ISBN 978-0-660-04440-8.
- Rasmussen, P.E.; MacIntyre, D.J.; Guenette, J. Buoyancy-Corrected Gravimetric Analysis System. U.S. Patent US 7,357,045 B2, 15 April 2008. [Google Scholar]
- Rasmussen, P.E.; Gardner, H.D.; Niu, J. Buoyancy-Corrected Gravimetric Analysis of Lightly Loaded Filters. J. Air Waste Manag. Assoc. 2010, 60, 1065–1077. [Google Scholar] [CrossRef]
- Niu, J.; Rasmussen, P.E.; Chénier, M. Ultrasonic dissolution for ICP-MS determination of trace elements in lightly loaded airborne PM filters. Int. J. Environ. Anal. Chem. 2013, 93, 661–678. [Google Scholar] [CrossRef]
- Dodd, M.; Rasmussen, P.E.; Chénier, M. Comparison of Two In Vitro Extraction Protocols for Assessing Metals’ Bioaccessibility Using Dust and Soil Reference Materials. Hum. Ecol. Risk Assess. Int. J. 2013, 19, 1014–1027. [Google Scholar] [CrossRef]
- Boros, K.; Fortin, D.; Jayawardene, I.; Chénier, M.; Levesque, C.; Rasmussen, P.E. Comparison of Gastric versus Gastrointestinal PBET Extractions for Estimating Oral Bioaccessibility of Metals in House Dust. Int. J. Environ. Res. Public Health 2017, 14, 92. [Google Scholar] [CrossRef]
- Avramescu, M.-L.; Rasmussen, P.E.; Chénier, M.; Gardner, H.D. Influence of pH, particle size and crystal form on dissolution behaviour of engineered nanomaterials. Environ. Sci. Pollut. Res. 2017, 24, 1553–1564. [Google Scholar] [CrossRef]
- Kasimov, N.S.; Kosheleva, N.E.; Vlasov, D.V.; Nabelkina, K.S.; Ryzhov, A.V. Physicochemical Properties of Road Dust in Moscow. Geogr. Environ. Sustain. 2019, 12, 96–113. [Google Scholar] [CrossRef]
- Gunawardana, C.; Goonetilleke, A.; Egodawatta, P.; Dawes, L.A.; Kokot, S. Source characterisation of road dust based on chemical and mineralogical composition. Chemosphere 2012, 87, 163–170. [Google Scholar] [CrossRef] [PubMed]
- Rasmussen, P.E.; Beauchemin, S.; Nugent, M.; Dugandzic, R.; Lanouette, M.; Chénier, M. Influence of Matrix Composition on the Bioaccessibility of Copper, Zinc, and Nickel in Urban Residential Dust and Soil. Hum. Ecol. Risk Assess. Int. J. 2008, 14, 351–371. [Google Scholar] [CrossRef]
- Kong, S.; Lu, B.; Ji, Y.; Zhao, X.; Bai, Z.; Xu, Y.; Liu, Y.; Jiang, H. Risk assessment of heavy metals in road and soil dusts within PM2.5, PM10 and PM100 fractions in Dongying city, Shandong Province, China. J. Environ. Monit. 2012, 14, 791–803. [Google Scholar] [CrossRef] [PubMed]
- Bari, A.; Kindzierski, W.B.; Wallace, L.A.; Wheeler, A.J.; MacNeill, M.; Héroux, M.-È. Indoor and Outdoor Levels and Sources of Submicron Particles (PM1) at Homes in Edmonton, Canada. Environ. Sci. Technol. 2015, 49, 6419–6429. [Google Scholar] [CrossRef] [PubMed]
- Yoshinaga, J.; Yamasaki, K.; Yonemura, A.; Ishibashi, Y.; Kaido, T.; Mizuno, K.; Takagi, M.; Tanaka, A. Lead and other elements in house dust of Japanese residences—Source of lead and health risks due to metal exposure. Environ. Pollut. 2014, 189, 223–228. [Google Scholar] [CrossRef]
- Brehmer, C.; Norris, C.; Barkjohn, K.K.; Bergin, M.H.; Zhang, J.; Cui, X.; Zhang, Y.; Black, M.; Li, Z.; Shafer, M.; et al. The impact of household air cleaners on the chemical composition and children’s exposure to PM2.5 metal sources in suburban Shanghai. Environ. Pollut. 2019, 253, 190–198. [Google Scholar] [CrossRef]
- Meza-Figueroa, D.; Barboza-Flores, M.; Romero, F.M.; Acosta-Elias, M.; Hernández-Mendiola, E.; Maldonado-Escalante, F.; Pérez-Segura, E.; González-Grijalva, B.; Meza-Montenegro, M.; García-Rico, L.; et al. Metal bioaccessibility, particle size distribution and polydispersity of playground dust in synthetic lysosomal fluids. Sci. Total Environ. 2020, 713, 136481. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).