New Biochronological Scales of Planktic Foraminifera for the Early Danian Based on High-Resolution Biostratigraphy
Abstract
:1. Introduction
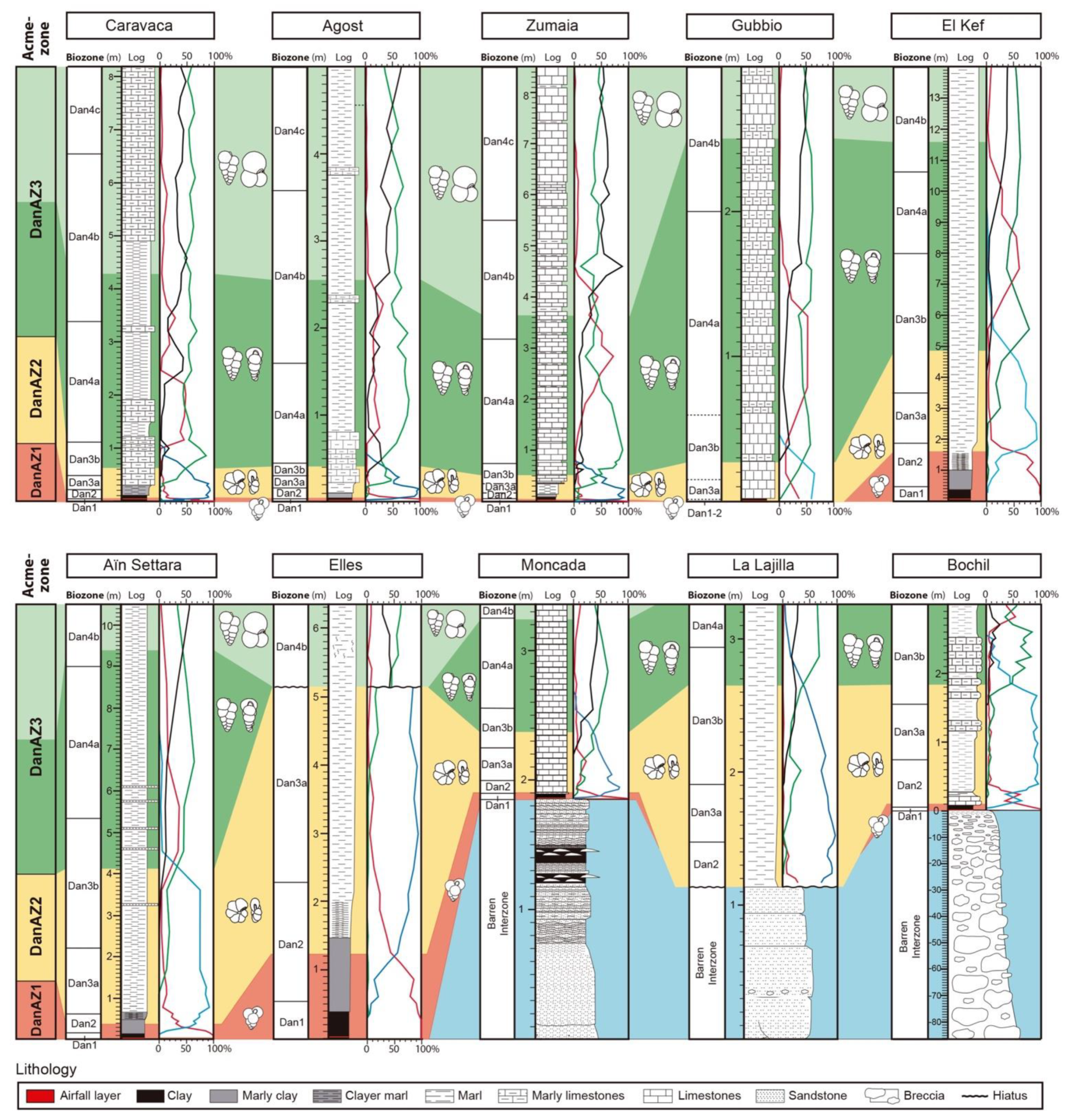
2. Reference Sections, Key Biohorizons, and Calibration Methods
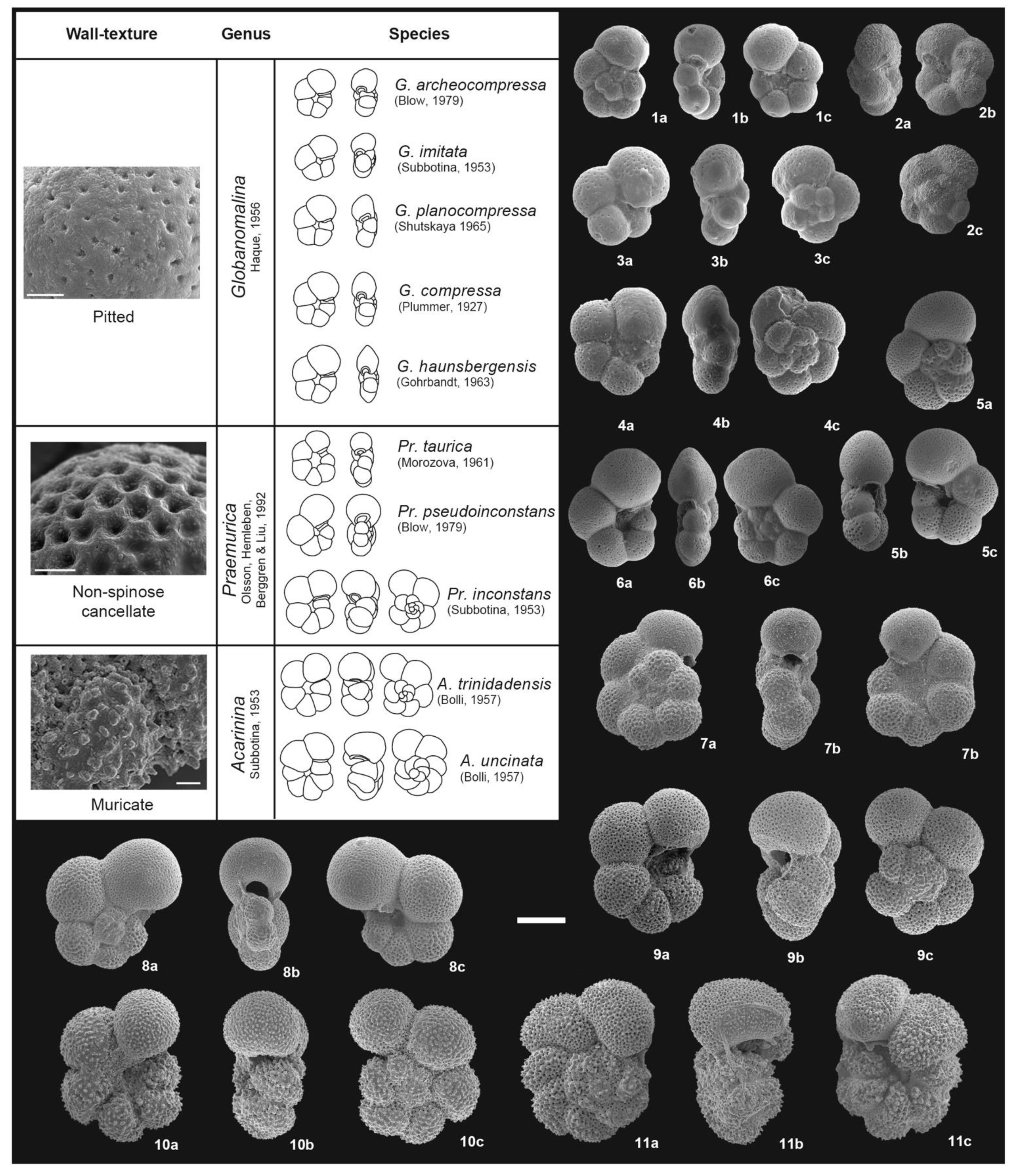
3. Taxonomic Notes
4. Some Thoughts on the Lower Danian Planktic Foraminiferal Zonations
4.1. Parallel Nomenclature in Qualitative Biozonations Based on Ranges of Index Species
4.2. Inconsistencies in Qualitative Biozonations Due to Taxonomic Discrepancies
4.3. Acme-Zonation (Quantitative Biozonation) as an Alternative
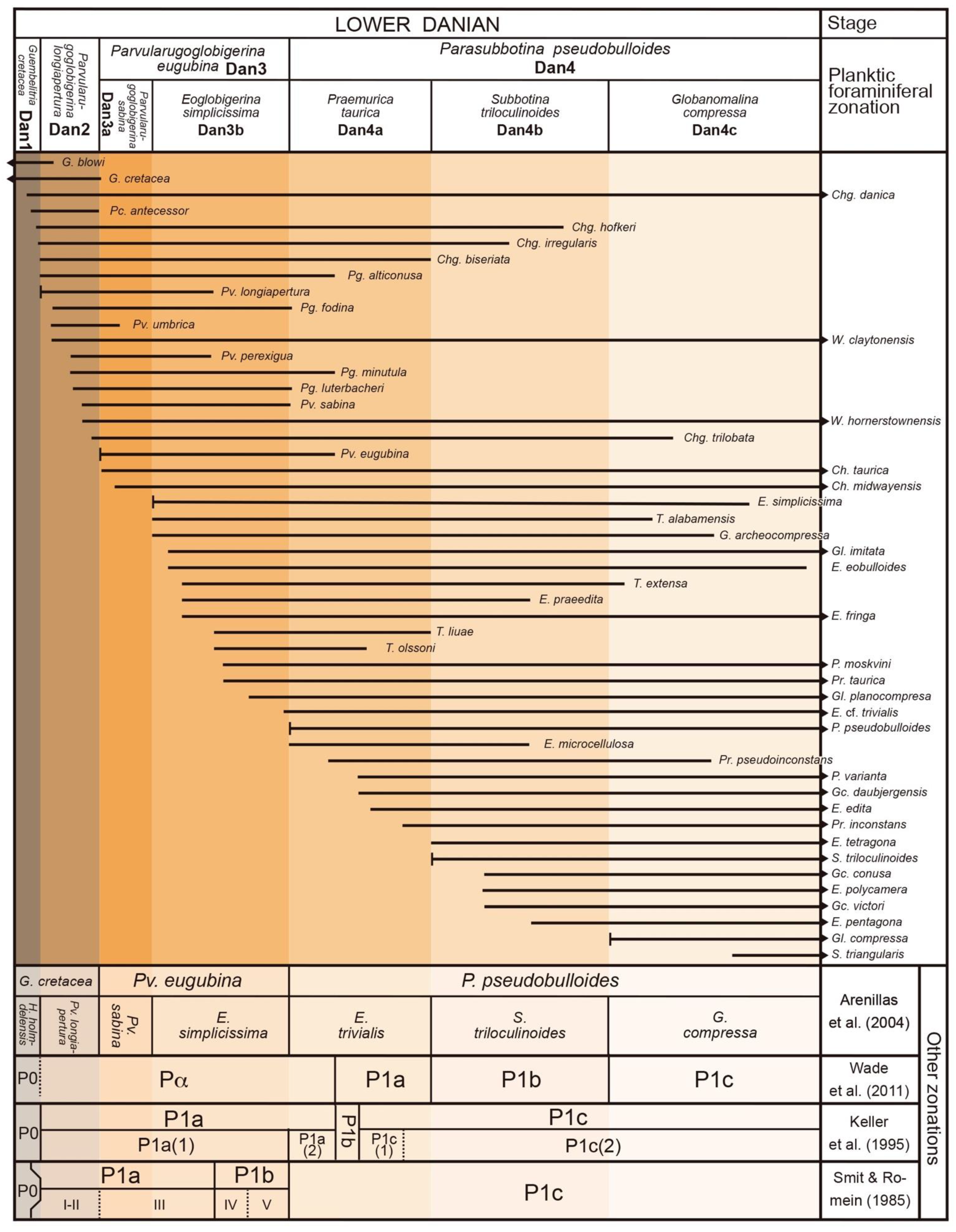
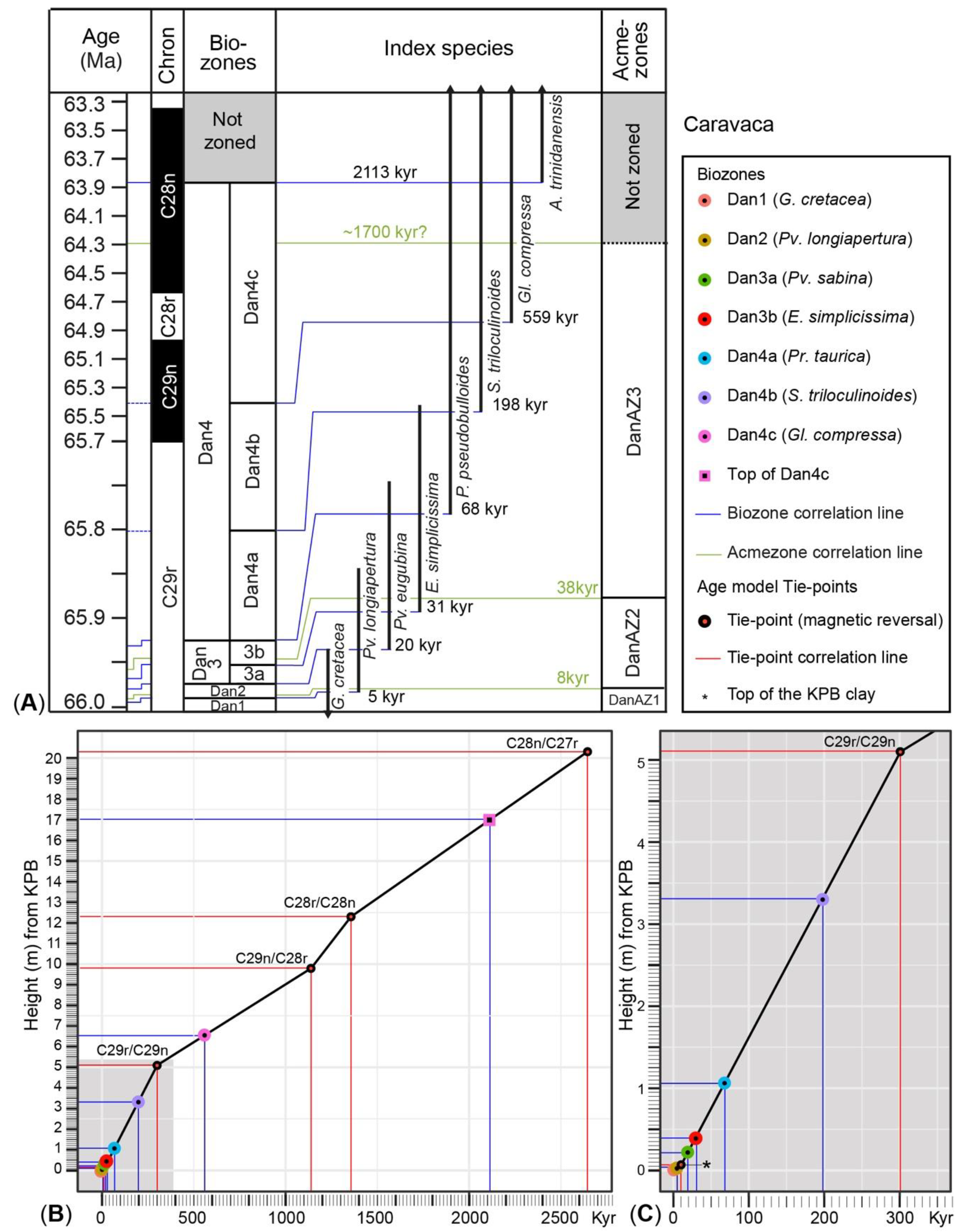
5. Calibration of Key-Biohorizons
6. Lower Danian Planktic Foraminiferal Qualitative Zonation
6.1. Guembelitria cretacea Zone (Biozone Dan1)
6.2. Parvularugoglobigerina longiapertura Zone (Biozone Dan2)
6.3. Parvularugoglobigerina eugubina Zone (Biozone Dan3)
6.3.1. Parvularugoglobigerina sabina Subzone (Sub-Biozone Dan3a)
6.3.2. Eoglobigerina simplicissima Subzone (Sub-Biozone Dan3b)
6.4. Parasubbotina pseudobulloides Zone (Biozone Dan4)
6.4.1. Praemurica taurica Subzone (Sub-Biozone Dan4a)
6.4.2. Subbotina triloculinoides Subzone (Subbiozone Dan4b)
6.4.3. Globanomalina compressa Subzone (Sub-Biozone Dan4c)
7. Lower Danian Planktic Foraminiferal Acme-Zonation
7.1. Guembelitria Abundance Zone (Acme-Zone DanAZ1)
7.2. Parvularugoglobigerina-Palaeoglobigerina Abundance Zone (Acme Zone DanAZ2)
7.3. Woodringina-Chiloguembelina Abundance Zone (Acme Zone DanAZ3)
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
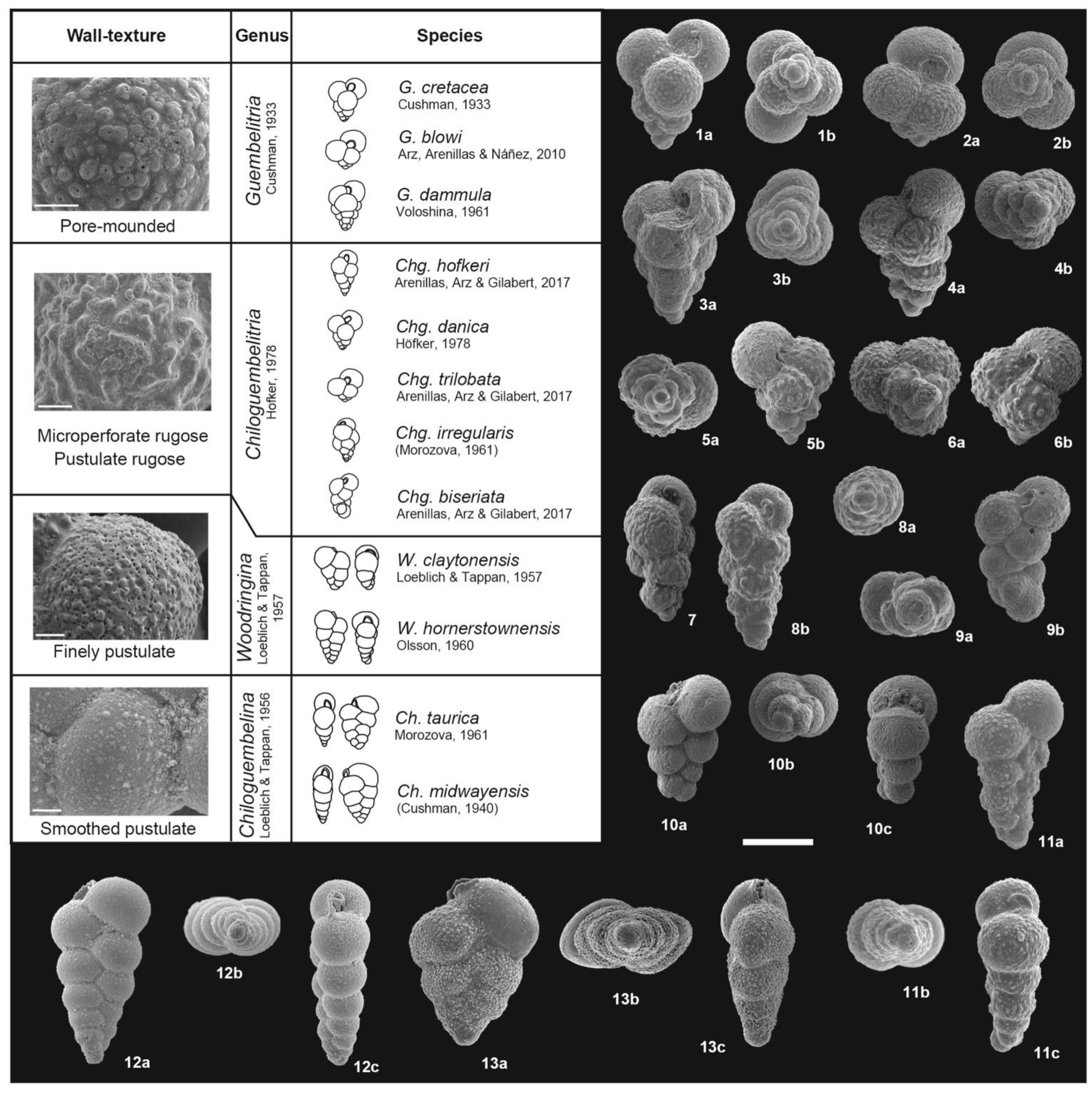
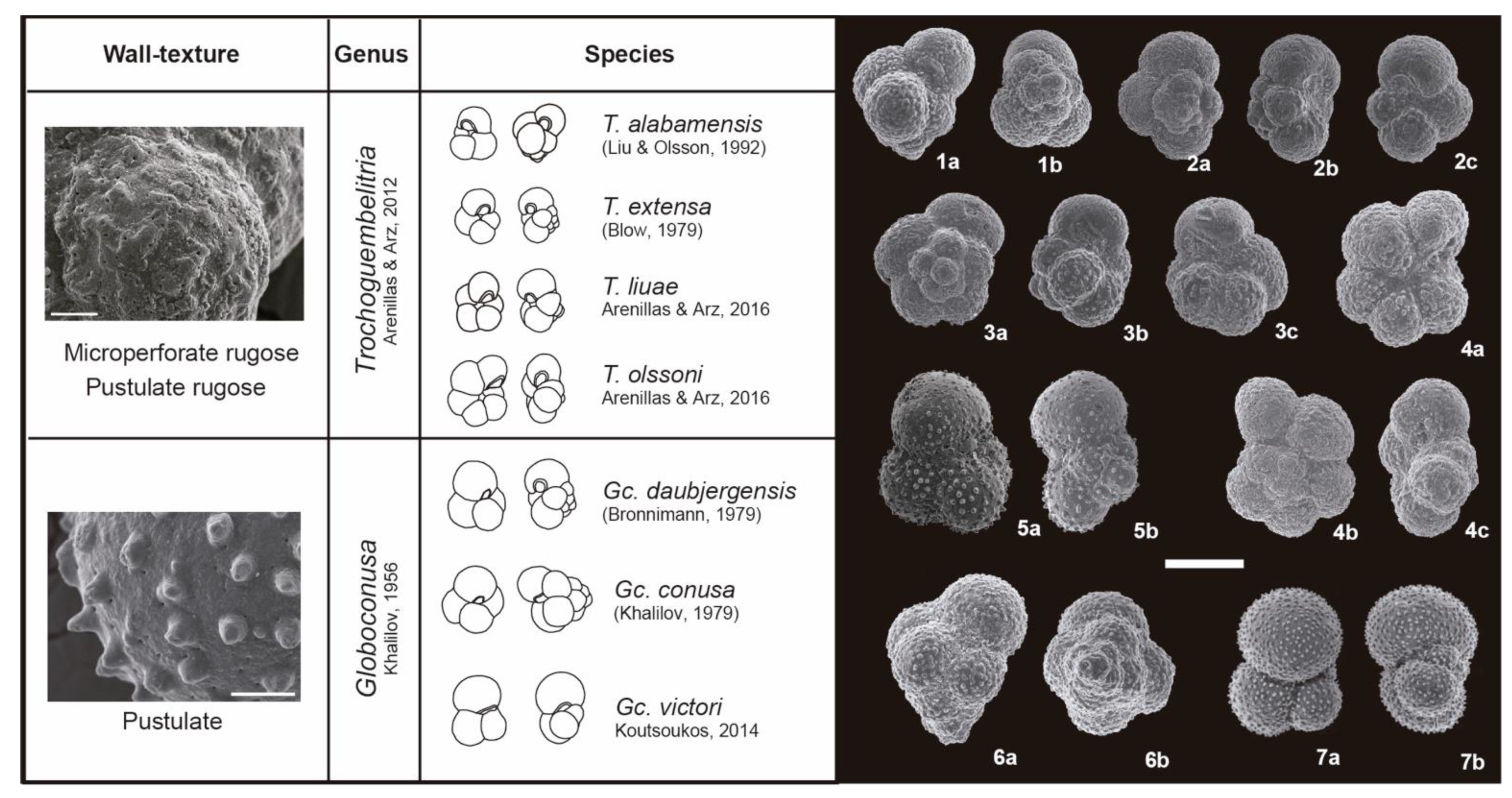
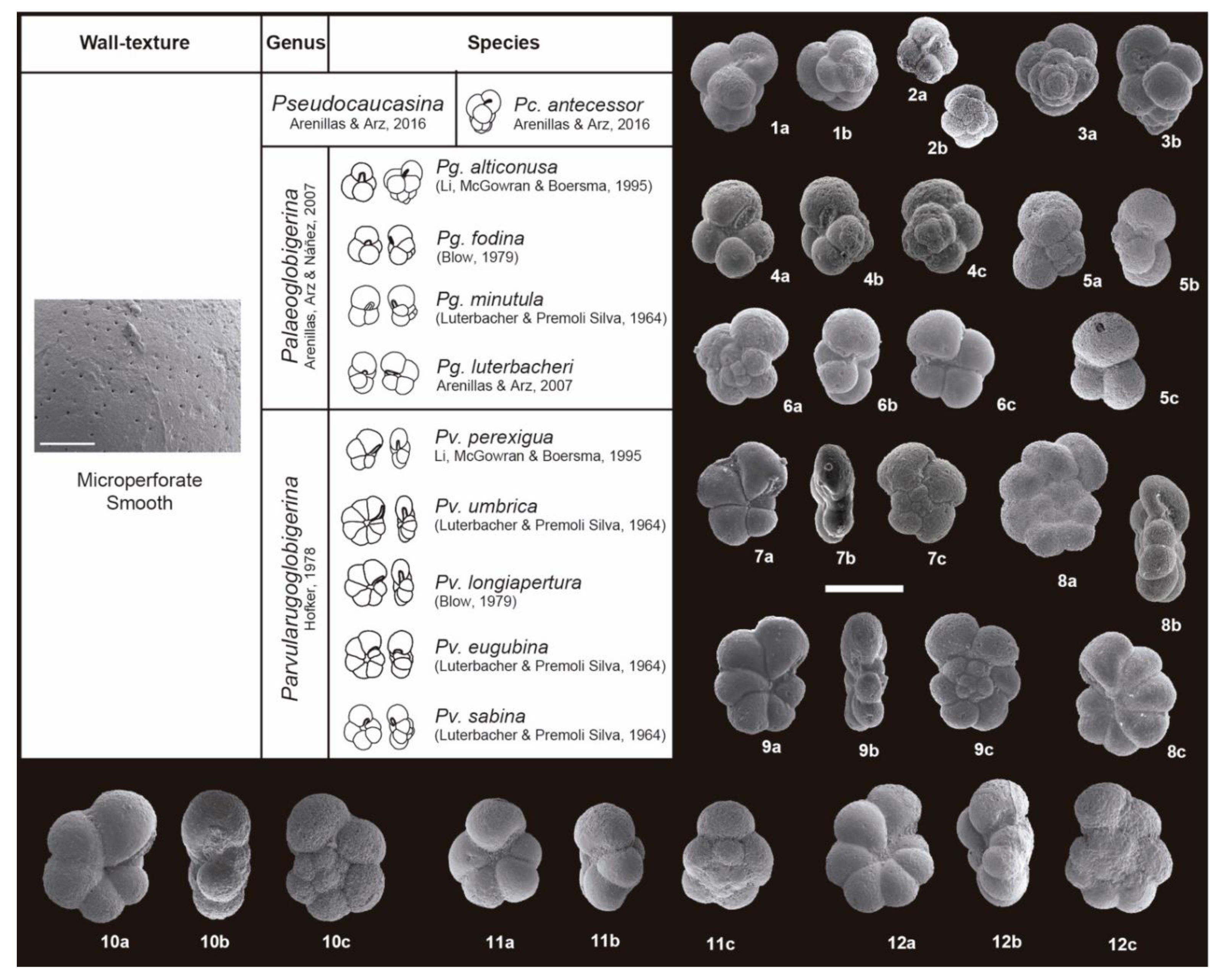
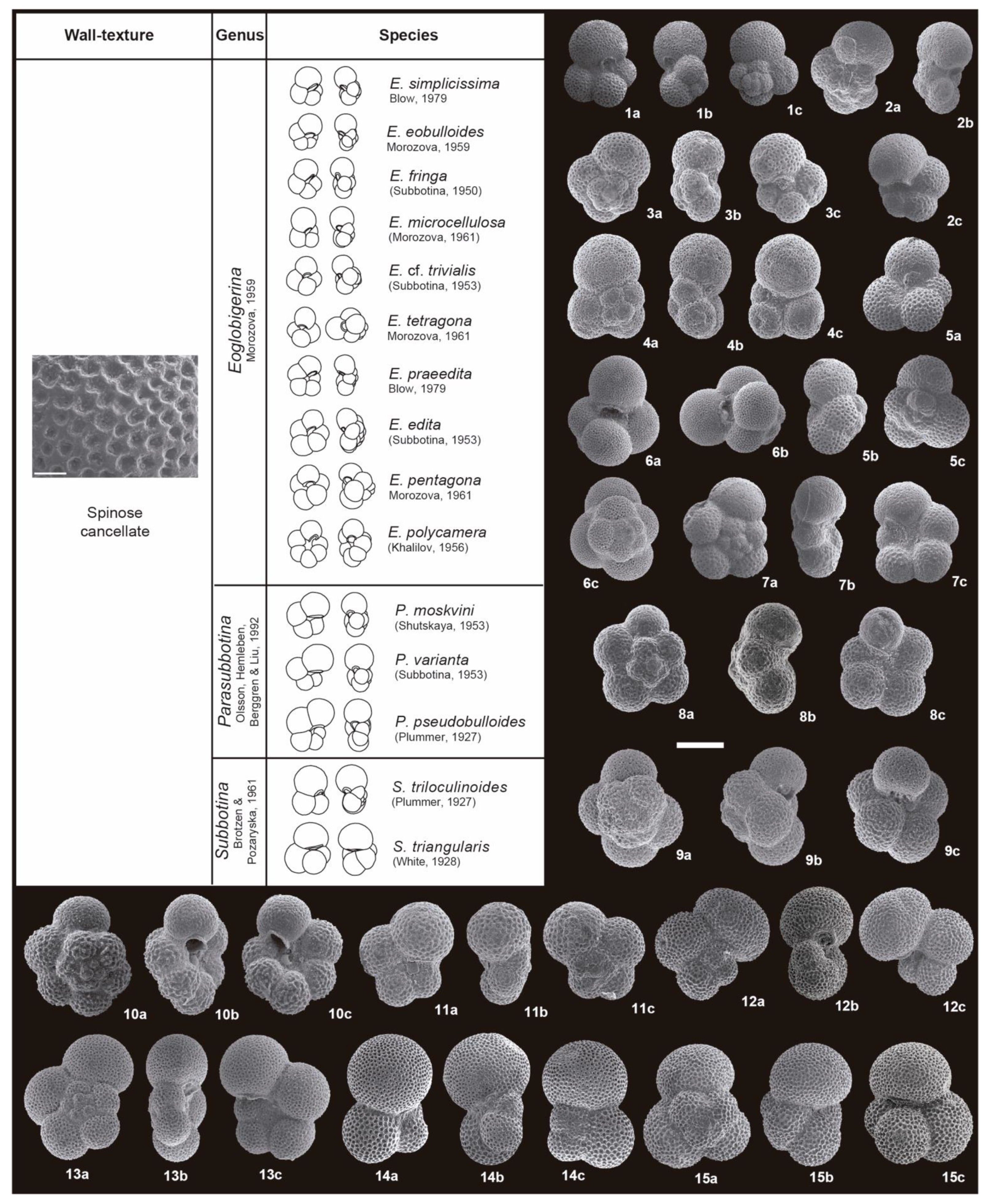
References
- Bolli, H.M. The genera Globigerina and Globorotalia in the Paleocene-lower Eocene Lizard Springs formation of Trinidad, B.W.I. US Nat. Mus. Bull. 1957, 215, 97–124. [Google Scholar]
- Wade, B.R.; Pearson, P.N.; Berggren, W.A.; Pälike, H. Review and revision of Cenozoic tropical planktonic foraminiferal biostratigraphy and calibration to the geomagnetic polarity and astronomical time scale. Earth-Sci. Rev. 2011, 104, 111–142. [Google Scholar] [CrossRef] [Green Version]
- Luterbacher, H.P.; Premoli Silva, I. Biostratigrafia del limite Cretaceo-Terziario nell’Apennino Centrale. Riv. Ital. Paleontol. Stratigr. 1964, 70, 67–128. [Google Scholar]
- Smit, J. Extinction and evolution of planktonic foraminifera after a major impact at the Cretaceous/Tertiary boundary. Geol. Soc. Am. Spec. Pap. 1982, 190, 329–352. [Google Scholar] [CrossRef]
- Koutsoukos, E.A. Phenotypic plasticity, speciations, and phylogeny in Early Danian planktic foraminifera. J. Foraminifer. Res. 2014, 44, 109–142. [Google Scholar] [CrossRef] [Green Version]
- Lowery, C.M.; Bralower, T.J.; Owens, J.D.; Rodríguez-Tovar, F.J.; Jones, H.; Smit, J.; Whalen, M.T.; Claeys, P.; Farley, K.; Gulick, S.P.S.; et al. Rapid recovery of life at ground zero of the end-Cretaceous mass extinction. Nature 2018, 558, 288–291. [Google Scholar] [CrossRef]
- Krahl, G.; Bom, M.H.H.; Kochhann, K.G.D.; Souza, L.V.; Savian, J.F.; Fauth, G. Environmental changes occurred during the Early Danian at the Rio Grande Rise, South Atlantic Ocean. Glob. Planet. Chang. 2020, 191, 103197. [Google Scholar] [CrossRef]
- Gilabert, V.; Arenillas, I.; Arz, J.A.; Batenburg, S.J.; Robinson, S.A. Multiproxy analysis of paleoenvironmental, paleoclimatic and paleoceanographic changes during the early Danian in the Caravaca section (Spain). Palaeogeogr. Palaeoclimatol. Palaeoecol. 2021, 576, 110513. [Google Scholar] [CrossRef]
- Gilabert, V.; Batenburg, S.J.; Arenillas, I.; Arz, J.A. Contribution of orbital forcing and Deccan volcanism to global climatic and biotic changes across the KPB at Zumaia, Spain. Geology 2021, 49. in press. [Google Scholar] [CrossRef]
- Subbotina, N.N. lskopaemye Foraminifery SSSR Globigerinidy, Khantkeninidy i Globorotaliidy [Fossil Foraminifera of the USSR. Globigerinidae, Hantkeninidae and Globorotaliidae]. Trudy Vses. Neft. Nauchno-Issled. Geol.-Razved. Inst. (VNIGRI) 1953, 76, 1–296. [Google Scholar]
- Leonov, G.P.; Alimarina, V.P. Stratigraphy and foraminifera of Cretaceous-Paleogene “transition” beds of the central part of the North Caucasus. Mosc. Univ. Geol. Fac. Sb. Tr. 1961, 29–60. [Google Scholar]
- Bolli, H.M. Zonación de sedimentos marinos del Cretáceo hasta el Plioceno, basada en foraminíferos planctónicos. Bol. inf. Asoc. Venez. Geol. Min. Petrol. 1966, 9, 1–34. [Google Scholar]
- Berggren, W.A. Rates of evolution in some Cenozoic planktonic foraminifera. Micropaleontology 1969, 15, 351–365. [Google Scholar] [CrossRef]
- Berggren, W.A. Multiple phylogenetic zonations of the Cenozoic based on planktonic foraminifera. In Proceedings of the II Planktonic Conference, Roma, Italy, 29 September–7 October 1970; Farinacci, A., Ed.; Edizioni Tecnoscienza: Roma, Italy, 1971; pp. 41–56. [Google Scholar]
- Blow, W.H. The Cainozoic Globigerinida: A study of the morphology, taxonomy, evolutionary relationship and the stratigraphical distribution of some Globigerinidae (mainly Globigerinacea). E. J. Brill Leiden 1979, 3, 1413. [Google Scholar]
- Stainforth, R.M.; Lamb, J.L.; Luterbacher, H.P.; Beardand, J.H.; Jeffords, R.M. Cenozoic Planktonic Foraminiferal Zonation and Characteristics of Index Form; University of Kansas Library: Lawrence, KS, USA, 1975; 425p. [Google Scholar]
- Toumarkine, M.; Luterbacher, H.P. Paleocene and Eocene planktic foraminifera. In Plankton Stratigraphy; Bolli, H.M., Saunders, J.B., Perch-Nielsen, K., Eds.; Cambridge University Press: Cambridge, UK, 1985; pp. 87–154. [Google Scholar]
- Smit, J.; Romein, A.J.T. A sequence of events across the Cretaceous-Tertiary boundary. Earth Planet. Sci. Lett. 1985, 74, 155–170. [Google Scholar] [CrossRef] [Green Version]
- Berggren, W.A.; Miller, K.G. Paleogene tropical planktonic foraminiferal biostratigraphy and magnetobiochronology. Micropaleontology 1988, 34, 362–380. [Google Scholar] [CrossRef]
- Keller, G. Extinction, survivorship and evolution of planktic foraminifera across the Cretaceous/Tertiary boundary at El Kef, Tunisia. Mar. Micropaleontol. 1988, 13, 239–263. [Google Scholar] [CrossRef]
- Keller, G. The Cretaceous/Tertiary boundary transitions in the Antarctic Ocean and its global implications. Mar. Micropaleontol. 1993, 21, 1–45. [Google Scholar] [CrossRef]
- Berggren, W.A.; Kent, D.V.; Swisher, C.C., III; Aubry, M.P. A revised Paleogene Geochronology and Chronostratigraphy. In Geochronology, Time Scales and Global Stratigraphic Correlation; Berggren, W.A., Kent, D.V., Aubry, M.P., Hardenbol, J., Eds.; SEPM Sp. Pub.: Broken Arrow, OK, USA, 1995; Volume 54, pp. 129–213. [Google Scholar] [CrossRef]
- Keller, G.; Li, L.; MacLeod, N. The Cretaceous/Tertiary boundary stratotype sections at El Kef, Tunisia: How catastrophic was the mass extinction? Palaeogeogr. Palaeoclimatol. Palaeoecol. 1995, 119, 221–254. [Google Scholar] [CrossRef]
- Arenillas, I.; Arz, J.A.; Molina, E. A new high-resolution planktic foraminiferal zonation and subzonation for the lower Danian. Lethaia 2004, 37, 79–95. [Google Scholar] [CrossRef]
- Rasmussen, J.A.; Heinberg, C.; Håkansson, E. Planktonic foraminifers, biostratigraphy and the diachronous nature of the lowermost Danian Cerithium Limestone at Stevns Klint, Denmark. Bull. Geol. Soc. Den. 2005, 52, 113–131. [Google Scholar] [CrossRef]
- Berggren, W.A.; Pearson, P.N. A revised tropical to subtropical Paleogene planktonic foraminiferal zonation. J. Foraminifer. Res. 2005, 35, 279–298. [Google Scholar] [CrossRef] [Green Version]
- Berggren, W.A. Atlas of Paleogene Planktonic Foraminifera. Some species of the genera Subbotina, Planorotalites, Morozovella, Acarinina and Truncorotaloides. In Oceanic Micropaleontology; Ramsay, A.T.S., Ed.; Academic Press: London, UK, 1977; Volume 1, pp. 250–299. [Google Scholar]
- Berggren, W.A.; Norris, R.D. Biostratigraphy, phylogeny and systematics of Paleocene trochospiral planktic foraminifera. Micropaleontology 1997, 43, 1–116. [Google Scholar] [CrossRef]
- Olsson, R.K.; Hemleben, C.; Berggren, W.A.; Huber, B.T. Atlas of Paleocene Planktonic Foraminifera. Smithson. Contrib. Paleobiol. 1999, 85, 1–252. [Google Scholar] [CrossRef]
- Molina, E.; Alegret, L.; Arenillas, I.; Arz, J.A.; Gallala, N.; Grajales-Nishimura, M.; Murillo-Muñetón, G.; Zaghbib-Turki, D. The Global Boundary Stratotype Section and Point for the base of the Danian Stage (Paleocene, Paleogene, “Tertiary”, Cenozoic): Auxiliary sections and correlation. Episodes 2009, 32, 84–95. [Google Scholar] [CrossRef] [Green Version]
- Arz, J.A.; Arenillas, I.; Molina, E.; Sepúlveda, R. La estabilidad faunística de foraminíferos planctónicos en el Maastrichtiense superior y su extinción en masa catastrófica en el límite K/T de Caravaca, España. Rev. Geol. Chile 2000, 27, 27–47. [Google Scholar] [CrossRef]
- Arenillas, I.; Molina, E. Análisis cuantitativo de los foraminíferos planctónicos del Paleoceno de Caravaca (Cordilleras Béticas): Cronoestratigrafía, bioestratigrafía y evolución de las asociaciones. Rev. Esp. Paleontol. 1997, 12, 207–232. [Google Scholar]
- Arenillas, I.; Arz, J.A.; Molina, E. El límite Cretácico/Terciario de Zumaya, Osinaga y Músquiz (Pirineos): Control bioestratigráfico y cuantitativo de hiatos con foraminíferos planctónicos. Rev. Soc. Geol. Esp. 1998, 11, 127–138. [Google Scholar]
- Molina, E.; Arenillas, I.; Arz, J.A. The Cretaceous/Tertiary boundary mass extinction in planktic foraminifera at Agost (Spain). Rev. Micropaléontol. 1996, 39, 225–243. [Google Scholar] [CrossRef]
- Metsana-Oussaid, F.; Belhai, D.; Arenillas, I.; Arz, J.A.; Gilabert, V. New sections of the Cretaceous-Paleogene transition in the southwestern Tethys (Médéa, northern Algeria): Planktic foraminiferal biostratigraphy and biochronology. Arabian J. Geosciences 2019, 12, 217. [Google Scholar] [CrossRef]
- Arenillas, I.; Arz, J.A. Parvularugoglobigerina eugubina type-sample at Ceselli (Italy): Planktic foraminiferal assemblage and lowermost Danian biostratigraphic implications. Riv. Ital. Paleontol. Stratigr. 2000, 106, 379–390. [Google Scholar]
- Molina, E.; Alegret, L.; Arenillas, I.; Arz, J.A.; Gallala, N.; Hardenbol, J.; von Salis, K.; Steurbaut, E.; Vandenberghe, N.; Zaghbib-Turki, D. The Global Stratotype Section and Point of the Danian Stage (Paleocene, Paleogene, “Tertiary”, Cenozoic) at El Kef, Tunisia: Original definition and revision. Episodes 2006, 29, 263–278. [Google Scholar] [CrossRef] [Green Version]
- Arenillas, I.; Arz, J.A. Benthic origin and earliest evolution of the first planktonic foraminifera after the Cretaceous/Paleogene boundary mass extinction. Hist. Biol. 2017, 29, 17–24. [Google Scholar] [CrossRef]
- Arenillas, I.; Arz, J.A.; Gilabert, V. Blooms of aberrant planktic foraminifera across the KPB in the Western Tethys: Causes and evolutionary implications. Paleobiology 2018, 44, 460–489. [Google Scholar] [CrossRef] [Green Version]
- Arenillas, I.; Arz, J.A.; Molina, E.; Dupuis, C. An independent test of planktonic foraminiferal turnover across the Cretaceous/Paleogene (K/P) boundary at El Kef, Tunisia: Catastrophic mass extinction and possible survivorship. Micropaleontology 2000, 46, 31–49. [Google Scholar]
- Arenillas, I.; Arz, J.A.; Molina, E.; Dupuis, C. The Cretaceous/Paleogene (K/P) boundary at Aïn Settara, Tunisia: Sudden catastrophic mass extinction in planktic foraminifera. J. Foraminifer. Res. 2000, 30, 202–218. [Google Scholar] [CrossRef] [Green Version]
- Alegret, L.; Arenillas, I.; Arz, J.A.; Molina, E. Foraminiferal event-stratigraphy across the Cretaceous/Tertiary boundary. Neues Jahrb. Geol. Paläontol. Abh. 2004, 234, 25–50. [Google Scholar] [CrossRef]
- Arz, J.A.; Arenillas, I.; Molina, E.; Dupuis, C. Los efectos tafonómico y “Signor-Lipps” sobre la extinción en masa de foraminíferos planctónicos en el límite Cretácico/Terciario de Elles (Tunicia). Rev. Soc. Geol. Esp. 1999, 12, 251–268. [Google Scholar]
- Arz, J.A.; Arenillas, I.; Grajales-Nishimura, J.M.; Liesa, C.; Soria, A.R.; Rojas-Consuegra, R.; Calmus, T.; Gilabert, V. No evidence of multiple impact scenario across the K/Pg boundary based on planktic foraminiferal biochronology. In From the Guajira Desert to the Apennines, and from Mediterranean Microplates to the Mexican Killer Asteroid: Honoring the Career of Walter; Geological Society of America Special Paper 557; Koeberl, C., Claeys, P., Montanari, A., Eds.; Geological Society of America: Boulder, CO, USA, 2022; in press. [Google Scholar]
- Arenillas, I.; Arz, J.A.; Grajales-Nishimura, J.M.; Murillo-Muñetón, G.; Alvarez, W.; Camargo-Zanoguera, A.; Molina, E.; Rosales-Domínguez, C. Chicxulub impact event is Cretaceous/Paleogene boundary in age: New micropaleontological evidence. Earth Planet. Sci. Lett. 2006, 249, 241–257. [Google Scholar] [CrossRef]
- Arenillas, I.; Arz, J.A.; Grajales-Nishimura, J.M.; Rojas-Consuegra, R. The Chicxulub impact is synchronous with the planktonic foraminifera mass extinction at the Cretaceous/Paleogene boundary: New evidence from the Moncada section, Cuba. Geol. Acta 2016, 14, 35–51. [Google Scholar]
- Schulte, P.; Alegret, L.; Arenillas, I.; Arz, J.A.; Barton, P.J.; Bown, P.R.; Bralower, T.J.; Christeson, G.L.; Claeys, P.; Cockell, C.S.; et al. Response-Cretaceous Extinctions. Science 2010, 328, 975–976. [Google Scholar] [CrossRef]
- Chacón, B.; Martín-Chivelet, J. Subdivisión litoestratigráfica de las series hemipelágicas de edad Coniaciense-Thanetiense en el Prebético oriental (SE de España). Rev. Soc. Geol. Esp. 2005, 18, 3–20. [Google Scholar]
- Smit, J. The section of the Barranco del Gredero (Caravaca, SE Spain): A crucial section for the Cretaceous/Tertiary boundary impact extinction hypothesis. J. Iberian Geol. 2004, 31, 179–191. [Google Scholar]
- Martínez-Ruiz, F.; Ortega-Huertas, M.; Palomo-Delgado, I.; Barbieri, M. The geochemistry and mineralogy of the Cretaceous-Tertiary boundary at Agost (southeast Spain). Chem. Geol. 1992, 95, 265–281. [Google Scholar] [CrossRef]
- Martínez-Ruiz, F.; Ortega-Huertas, M.; Palomo-Delgado, I.; Acquafredda, P. Quench textures in altered spherules from the Cretaceous-Tertiary boundary layer at Agost and Caravaca, SE Spain. Sediment. Geol. 1997, 113, 137–147. [Google Scholar] [CrossRef]
- Kaiho, K.; Kajiwara, Y.; Tazaki, K.; Ueshima, M.; Takeda, N.; Kawahata, H.; Arinobu, T.; Ishiwatari, R.; Hirai, A.; Lamolda, M.A. Oceanic primary productivity and dissolved oxygen levels at the Cretaceous/Tertiary Boundary: Their decrease, subsequent warming, and recovery. Paleoceanography 1999, 14, 511–524. [Google Scholar] [CrossRef] [Green Version]
- Canudo, J.I.; Keller, G.; Molina, E. Cretaceous/Tertiary boundary extinction pattern and faunal turnover at Agost and Caravaca, SE Spain. Mar. Micropaleontol. 1991, 17, 319–341. [Google Scholar] [CrossRef]
- Gradstein, F.M.; Ogg, J.G.; Schmitz, M.D.; Ogg, G.M. The Geologic Time Scale 2020; Elsevier: Amsterdam, The Netherlands, 2020; p. 1357. [Google Scholar]
- Dinarès-Turell, J.; Westerhold, T.; Pujalte, V.; Röhl, U.; Kroon, D. Astronomical calibration of the Danian stage (Early Paleocene) revisited: Settling chronologies of sedimentary records across the Atlantic and Pacific Oceans. Earth Planet. Sci. Lett. 2014, 405, 119–131. [Google Scholar] [CrossRef]
- Mukhopadhyay, S.; Farley, K.A.; Montanari, A. A short duration of the Cretaceous-Tertiary boundary event: Evidence from extraterrestrial Helium-3. Science 2001, 291, 1952–1955. [Google Scholar] [CrossRef] [Green Version]
- Groot, J.J.; de Jonge, R.B.G.; Langereis, C.G.; Ten Kate, W.G.H.Z.; Smit, J. Magnetostratigraphy of the Cretaceous-Tertiary boundary at Agost (Spain). Earth Planet. Sci. Lett. 1989, 94, 385–397. [Google Scholar] [CrossRef] [Green Version]
- Margolis, S.V.; Mount, J.F.; Doehne, E.; Showers, W.J. The Cretaceous/Tertiary Boundary Carbon and Oxygen Isotope Stratigraphy, Diagenesis, and Paleoceanography at Zumaya, Spain. Paleoceanogr. Paleoclimatol. 1987, 2, 361–377. [Google Scholar] [CrossRef]
- Smit, J. Meteorite impact, extinctions and the Cretaceous-Tertiary boundary. Geol. Mijnbouw 1990, 69, 187–204. [Google Scholar]
- Ortega-Huertas, M.; Martínez-Ruiz, F.; Palomo, I.; Chamley, H. Comparative mineralogical and geochemical clay sedimentation in the Betic Cordilleras and Basque-Cantabrian Basin areas at the Cretaceous-Tertiary boundary. Sediment. Geol. 1995, 94, 209–227. [Google Scholar] [CrossRef]
- Ten Kate, W.G.H.Z.; Sprenger, A. Orbital cyclicities above and below the Cretaceous/Paleogene boundary at Zumaya (N Spain), Agost and Relleu (SE Spain). Sediment. Geol. 1993, 87, 69–101. [Google Scholar] [CrossRef]
- Dinarès-Turell, J.; Baceta, J.I.; Pujalte, V.; Orue-Etxebarria, X.; Bernaola, G.; Lorito, S. Untangling the Palaeocene climatic rhythm: An astronomically calibrated Early Palaeocene magnetostratigraphy and biostratigraphy at Zumaia (Basque basin, northern Spain). Earth Planet. Sci. Lett. 2003, 216, 483–500. [Google Scholar] [CrossRef]
- Kuipier, K.F.; Deino, A.; Hilgen, F.J.; Krijgsman, W.; Renne, P.R.; Wijbrans, J.R. Synchronizing rock clocks of earth history. Science 2008, 320, 500–504. [Google Scholar] [CrossRef] [Green Version]
- Westerhold, T.; Röhl, U.; Raffi, I.; Fornaciari, E.; Monechi, S.; Reale, V.; Bowles, J.; Evans, H.F. Astronomical calibration of the Paleocene time. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2008, 257, 377–403. [Google Scholar] [CrossRef]
- Hilgen, F.J.; Kuiper, K.F.; Lourens, L.J. Evaluation of the astronomical time scale for the Paleocene and earliest Eocene. Earth Planet. Sci. Lett. 2010, 300, 139–151. [Google Scholar] [CrossRef]
- Hilgen, F.J.; Abels, H.A.; Kuiper, K.F.; Lourens, L.J.; Wolthers, M. Towards a stable astronomical time scale for the Paleocene: Aligning Shatsky Rise with the Zumaia–Walvis Ridge ODP site 1262 composite. Newsl. Stratigr. 2015, 48, 91–110. [Google Scholar] [CrossRef]
- Batenburg, S.J.; Sprovieri, M.; Gale, A.; Hilgen, F.J.; Hüsing, S.; Laskar, J.; Liebrand, D.; Lirer, F.; Orue-Etxebarria, X.; Pelosi, N.; et al. Cyclostratigraphy and astronomical tuning of the Late Maastrichtian at Zumaia (Basque country, Northern Spain). Earth Planet. Sci. Lett. 2012, 359–360, 264–278. [Google Scholar] [CrossRef]
- Batenburg, S.J.; Gale, A.; Sprovieri, M.; Hilgen, F.J.; Thibault, N.; Boussaha, M.; Orue-Etxebarria, X. An astronomical time scale for the Maastrichtian based on the Zumaia and Sopelana sections (Basque country, northern Spain). J. Geol. Soc. Lond. 2014, 171, 165–180. [Google Scholar] [CrossRef] [Green Version]
- Laskar, J.; Gastineau, M.; Delisle, J.-B.; Farrés, A.; Fienga, A. Strong chaos induced by close encounters with Ceres and Vesta. Astron. Astrophys. 2011, 532, 4. [Google Scholar] [CrossRef]
- Schoene, B.; Eddy, M.P.; Samperton, K.M.; Keller, C.B.; Keller, G.; Adatte, T.; Khadri, S.F.R. U-Pb constraints on pulsed eruption of the Deccan Traps across the end-Cretaceous mass extinction. Science 2019, 363, 862–866. [Google Scholar] [CrossRef]
- Sprain, C.J.; Renne, P.R.; Clemens, W.A.; Wilson, G.P. Calibration of chron C29r: New high-precision geochronologic and paleomagnetic constraints from the Hell Creek region, Montana. Bull. Geol. Soc. Am. 2018, 130, 1615–1644. [Google Scholar] [CrossRef]
- Asgharian Rostami, M.; Leckie, R.M.; Font, E.; Frontalini, F.; Finkelstein, D.; Koeberl, C. The Cretaceous-Paleogene transition at Galanderud (northern Alborz, Iran): A multidisciplinary approach. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2018, 493, 82–101. [Google Scholar] [CrossRef]
- Coccioni, R.; Frontalini, F.; Bancalà, G.; Fornaciari, E.; Jovane, L.; Sprovieri, M. The Dan-C2 hyperthermal event at Gubbio (Italy): Global implications, environmental effects, and cause(s). Earth Planet. Sci. Lett. 2010, 297, 298–305. [Google Scholar] [CrossRef]
- Lowery, C.M.; Jones, H.L.; Bralower, T.; Pérez-Cruz, L.; Gebhardt, C.; Whalen, M.T.; Chenot, E.; Smit, J.; Phillips, M.P.; Choumiline, K.; et al. Early Paleocene paleoceanography and export productivity in the Chicxulub Crater. Paleoceanogr. Paleoclimatol. 2021, 36, e2021PA004241. [Google Scholar] [CrossRef]
- Gallala, N.; Zaghbib-Turki, D.; Molina, E.; Arenillas, I.; Arz, J.A. Catastrophic mass extinction and assemblage evolution in planktic foraminiferal across the Cretaceous/Paleogene (K/Pg) boundary at Bidart (SW France). Mar. Micropaleontol. 2009, 72, 196–209. [Google Scholar] [CrossRef]
- D’Hondt, S.; Keller, G. Some patterns of planktic foraminiferal assemblage turnover at the Cretaceous-Tertiary boundary. Mar. Micropaleontol. 1991, 17, 77–118. [Google Scholar] [CrossRef]
- Apellaniz, E.; Baceta, J.I.; Bernaola-Bilbao, G.; Núñez-Betelu, K.; Orue-Etxebarria, X.; Payros, A.; Pujalte, V.; Robin, E.; Rocchia, R. Analysis of uppermost Cretaceous-lowermost Tertiary hemipelagic successions in the Basque Country (Western Pyrenees): Evidence for a sudden extinction of more than half planktic foraminifer species at the K/T boundary. Bull. Soc. Géol. France 1997, 168, 783–793. [Google Scholar]
- Birch, H.S.; Coxall, H.C.; Pearson, P.N.; Kroon, D.; Schmidt, D.N. Partial collapse of the marine carbon pump after the Cretaceous–Paleogene boundary. Geology 2016, 44, 287–290. [Google Scholar] [CrossRef] [Green Version]
- Aze, T.; Ezard, T.H.G.; Purvis, A.; Coxall, H.; Stewart, D.R.M.; Wade, B.S.; Pearson, P.N. A phylogeny of Cenozoic macroperforate planktonic foraminifera from fossil data. Biol. Rev. 2011, 86, 900–927. [Google Scholar] [CrossRef]
- Huber, B.T. Evidence for planktonic foraminifer reworking versus survivorship across the Cretaceous–Tertiary boundary at high latitudes. Geol. Soc. Am. S. Pap. 1996, 307, 319–334. [Google Scholar] [CrossRef]
- Smit, J.; Nederbragt, A.J. Analysis of the El Kef blind test II. Mar. Micropaleontol. 1997, 29, 95–100. [Google Scholar] [CrossRef]
- Kaiho, K.; Lamolda, M.A. Catastrophic extinction of planktonic foraminifera at the Cretaceous-Tertiary boundary evidenced by stable isotopes and foraminiferal abundance at Caravaca, Spain. Geology 1999, 27, 355–358. [Google Scholar] [CrossRef]
- Huber, B.T.; MacLeod, K.G.; Norris, R. Abrupt extinction and subsequent reworking of Cretaceous planktonic foraminifera across the Cretaceous/Tertiary boundary: Evidence for the subtropical North Atlantic. Geol. Soc. Am. 2002, 356, 277–290. [Google Scholar] [CrossRef] [Green Version]
- Herm, D.; Von Hillebrandt, A.; Perch-Nielsen, K. Die Kreide/Tertiär-Grenze im Lattengebirge (Nördliche Kalkalpen) in mikropaläontologischer Sicht. Geol. Bavarica 1981, 82, 319–344. [Google Scholar]
- Premoli Silva, I.; Bolli, H.M. Late Cretaceous to Eocene planktonic foraminifera and stratigraphy of Leg 15 sites in the Caribbean Sea. Initial Rep. Deep Sea Drill. Proj. 1973, 15, 449–547. [Google Scholar] [CrossRef]
- Canudo, J.I.; Molina, E. Bioestratigrafía con foraminíferos planctónicos del Paleógeno del Pirineo. Neues Jahrb. Geol. Paläontol. Abh. 1992, 186, 97–135. [Google Scholar]
- Luciani, V. Planktonic foraminiferal turnover across the Cretaceous-Tertiary boundary in the Vajont valley (Southern Alps, northern Italy). Cretac. Res. 1997, 18, 799–821. [Google Scholar] [CrossRef]
- Morozova, V.G. Stratigrafiya Datsko-Montskikh otlozhenii Kryma po foraminiferam Stratigraphy of the Danian-Montian deposits of the Krimea according to the foraminifera. Dokl. Akad. Nauk SSSR 1959, 124, 1113–1116. [Google Scholar]
- Morozova, V.G. Zonal’naya stratigrafiya Datsko-Monstkikh otlozhenii CCCR i granitsa mela c paleogenom Stratigraphical zonation of the Danish-Montian deposits of the USSR and the Cretaceous-Paleogene boundary. In Problema V: Granitsa Melovoi i Paleogenovoi Sistem. Mezhdunarodnyi Geologicheskii Kongress; XXI Sessiya, Doklady Sovetskikh Geologov, Izdatelstvo Akademiya Nauk SSR; Academy of Science of the Union of Soviet Socialist Republics: Moscow, Union of Soviet Socialist Republics, 1960; pp. 83–100. [Google Scholar]
- Keller, G.; Adatte, T.; Stinnesbeck, W.; Luciani, V.; Karoui-Yaakoub, N.; Zaghbib-Turkie, D. Paleoecology of the Cretaceous–Tertiary mass extinction in planktonic foraminifera. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2002, 178, 257–297. [Google Scholar] [CrossRef]
- Luciani, V. High-resolution planktonic foraminiferal analysis from the Cretaceous–Tertiary boundary at Ain Settara (Tunisia): Evidence of an extended mass extinction. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2002, 178, 299–319. [Google Scholar] [CrossRef]
- Coccioni, R.; Luciani, V. Guembelitria irregularis bloom at the K-T boundary: Morphological abnormalities induced by impact-related extreme environmental stress? In Biological Processes Associated with Impact Events; Cockell, C., Gilmour, I., Koeberl, C., Eds.; Springer: Berlin/Heidelberg, Germany, 2006; pp. 179–196. [Google Scholar]
- Brinkhuis, H.; Zachariasse, W.J. Dinoflagellate cysts, sea level changes and planktonic foraminifers across the Cretaceous-Tertiary boundary at El Haria, Northwest Tunisia. Mar. Micropaleontol. 1988, 13, 153–191. [Google Scholar] [CrossRef]

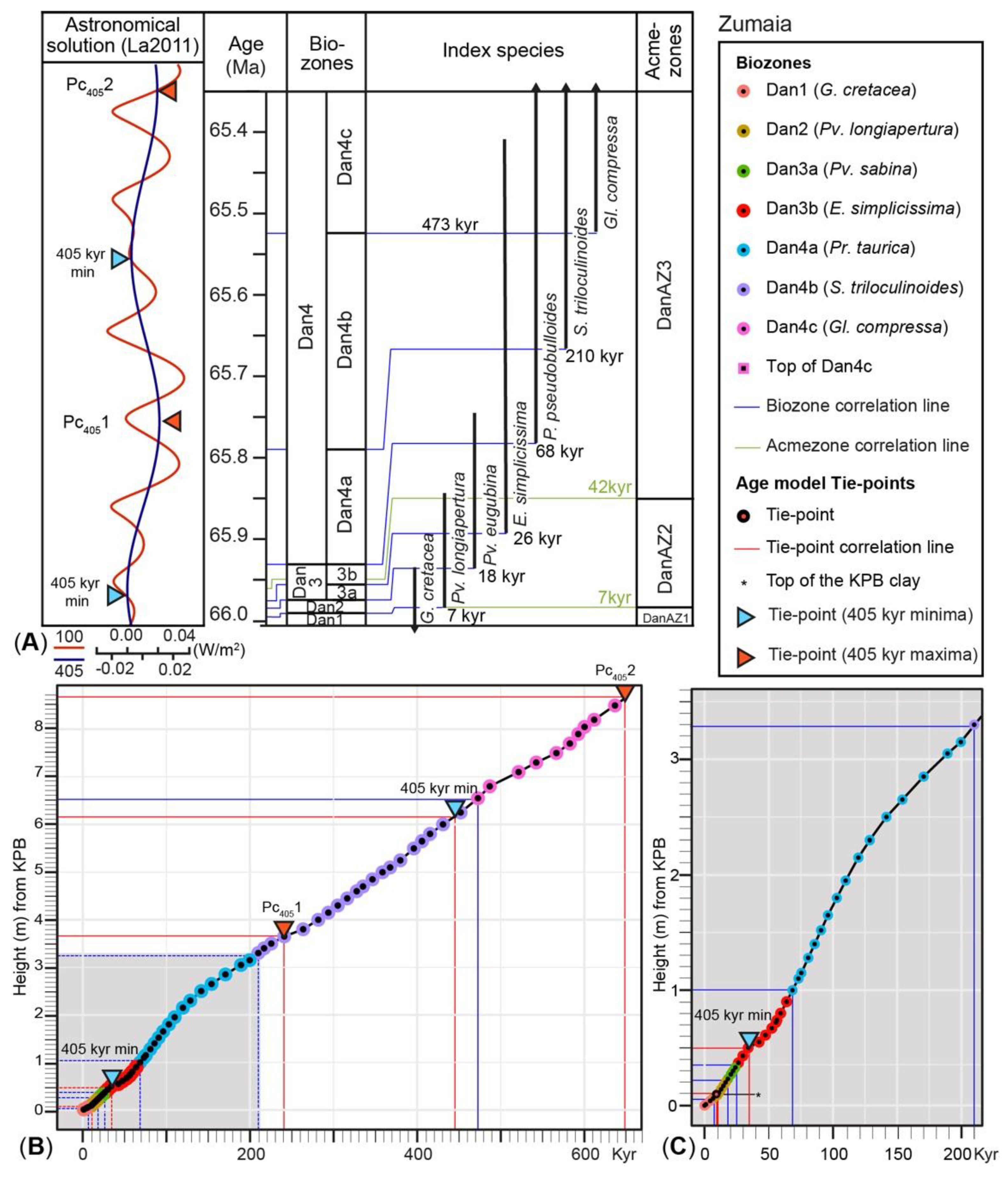
| Tie Points (Key-Horizons) | Section | Height cm from KPB | GTS2020 Ma | kyr after KPB |
|---|---|---|---|---|
| KPB | Caravaca | 0 | 66.001 | 0 |
| KPB dark clay bed top | Caravaca | 6 | 65.991 | 10 |
| C29r/C29n reversal | Caravaca | 510 | 65.700 | 301 |
| C29n/C28r reversal | Caravaca | 980 | 64.862 | 1139 |
| C28r/C28n reversal | Caravaca | 1230 | 64.645 | 1356 |
| C28n/C27r reversal | Caravaca | 2030 | 63.537 | 2464 |
| KPB | Zumaia | 0 | 66.001 | 0 |
| KPB dark clay bed top | Zumaia | 9 | 65.991 | 10 |
| 405-kyr min. | Zumaia | 50 | 65.967 | 34 |
| 405-kyr max. Pc4051 | Zumaia | 365 | 65.760 | 241 |
| 405-kyr min. | Zumaia | 615 | 65.555 | 446 |
| 405-kyr max. Pc4052 | Zumaia | 865 | 65.353 | 648 |
| Caravaca | Zumaia | |||||
|---|---|---|---|---|---|---|
| Key-Biohorizons | Height cm from KPB | GTS2020 Ma | kyr after KPB | Height cm from KPB | GTS2020 Ma | kyr after KPB |
| KPB mass extinction horizon (Dan1 base) | 0 | 66.001 | 0 | 0 | 66.001 | 0 |
| LOD G. acme (DanAZ-1 base) | 0 | 66.001 | 0 | 0 | 66.001 | 0 |
| LOD Pv. longiapertura (Dan2 base) | 3 | 65.996 | 5 | 6 | 65.994 | 7 |
| LOD Pv-Pg acme (DanAZ-2 base) | 5 | 65.993 | 8 | 6 | 65.994 | 7 |
| LOD Pv. eugubina (Dan3a base) | 22 | 65.982 | 19 | 23 | 65.983 | 18 |
| LOD E. simplicissima (Dan3b base) | 42 | 65.970 | 31 | 37 | 65.975 | 26 |
| LOD W-Ch acme (DanAZ-3 base) | 55 | 65.963 | 38 | 55 | 65.959 | 42 |
| LOD P. pseudobulloides (Dan4a base) | 107 | 65.933 | 68 | 100 | 65.933 | 68 |
| LOD S. triloculinoides (Dan4b base) | 332 | 65.803 | 198 | 330 | 65.791 | 210 |
| lower/upper DanAZ-3 | 430 | 65.746 | 255 | 365 | 65.760 | 241 |
| LOD G. compressa (Dan4c base) | 655 | 65.441 | 560 | 655 | 65.528 | 473 |
| LOD E-S-P-Gl-Pr dominance (DanAZ-3 top) | ? | ? | ? | – | – | – |
| LOD A. trinidadensis (Dan4c top) | 1700 | 63.888 | 2113 | – | – | – |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Arenillas, I.; Gilabert, V.; Arz, J.A. New Biochronological Scales of Planktic Foraminifera for the Early Danian Based on High-Resolution Biostratigraphy. Geosciences 2021, 11, 479. https://doi.org/10.3390/geosciences11110479
Arenillas I, Gilabert V, Arz JA. New Biochronological Scales of Planktic Foraminifera for the Early Danian Based on High-Resolution Biostratigraphy. Geosciences. 2021; 11(11):479. https://doi.org/10.3390/geosciences11110479
Chicago/Turabian StyleArenillas, Ignacio, Vicente Gilabert, and José A. Arz. 2021. "New Biochronological Scales of Planktic Foraminifera for the Early Danian Based on High-Resolution Biostratigraphy" Geosciences 11, no. 11: 479. https://doi.org/10.3390/geosciences11110479
APA StyleArenillas, I., Gilabert, V., & Arz, J. A. (2021). New Biochronological Scales of Planktic Foraminifera for the Early Danian Based on High-Resolution Biostratigraphy. Geosciences, 11(11), 479. https://doi.org/10.3390/geosciences11110479






