Dynamic Metasomatism Experiments Investigating the Interaction between Migrating Potassic Melt and Garnet Peridotite
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Design
2.2. Starting Materials and Analytical Methods
3. Results
3.1. Phases Present in Experimental Products
3.2. Mineral Compositions
3.2.1. Phlogopite
3.2.2. Other Silicate Minerals
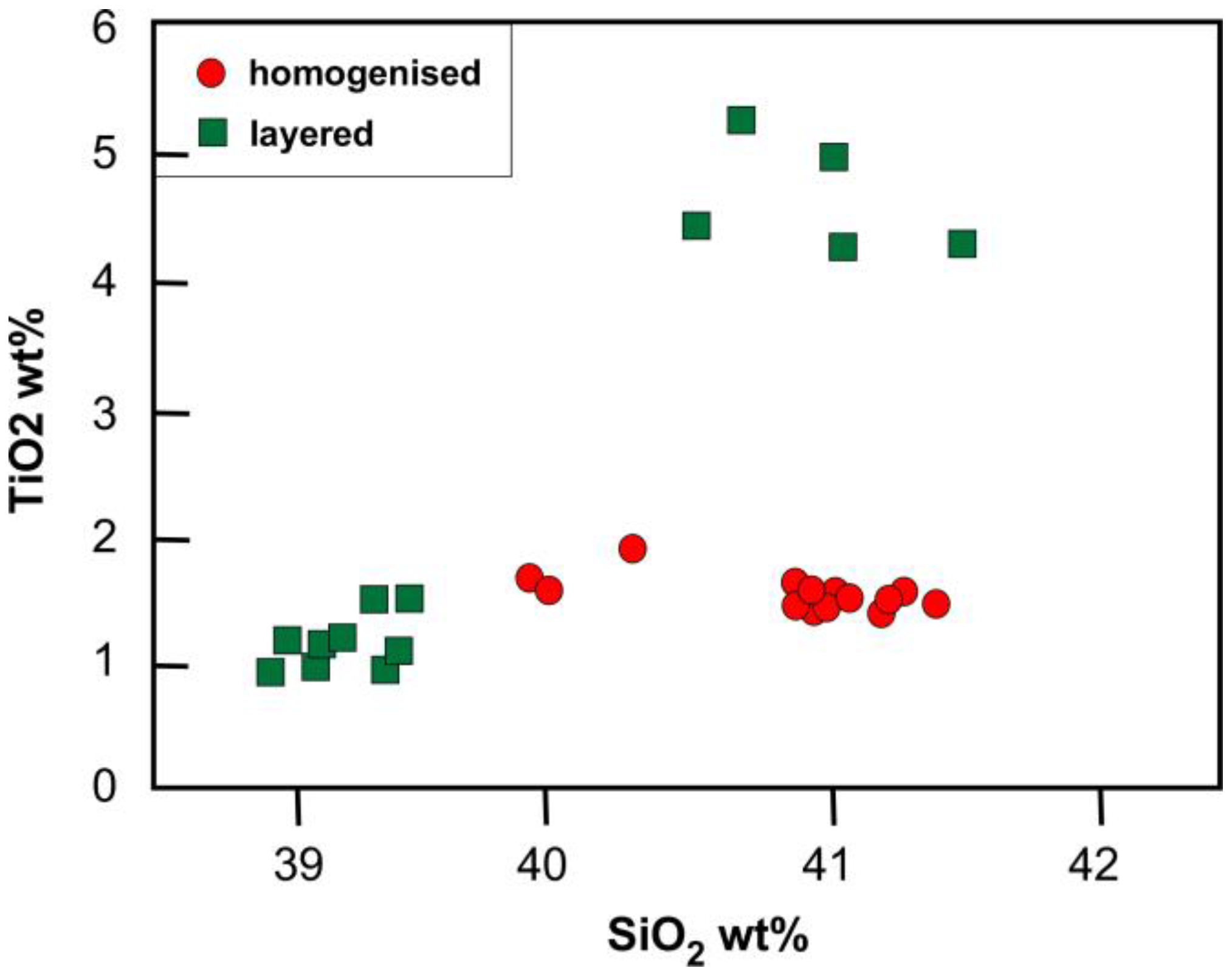
3.3. Melt Compositions
4. Discussion
4.1. Reaction Experiment Design
4.2. Melt Compositions before and after Reaction with Peridotite
4.3. Applications to Mantle Metasomatism
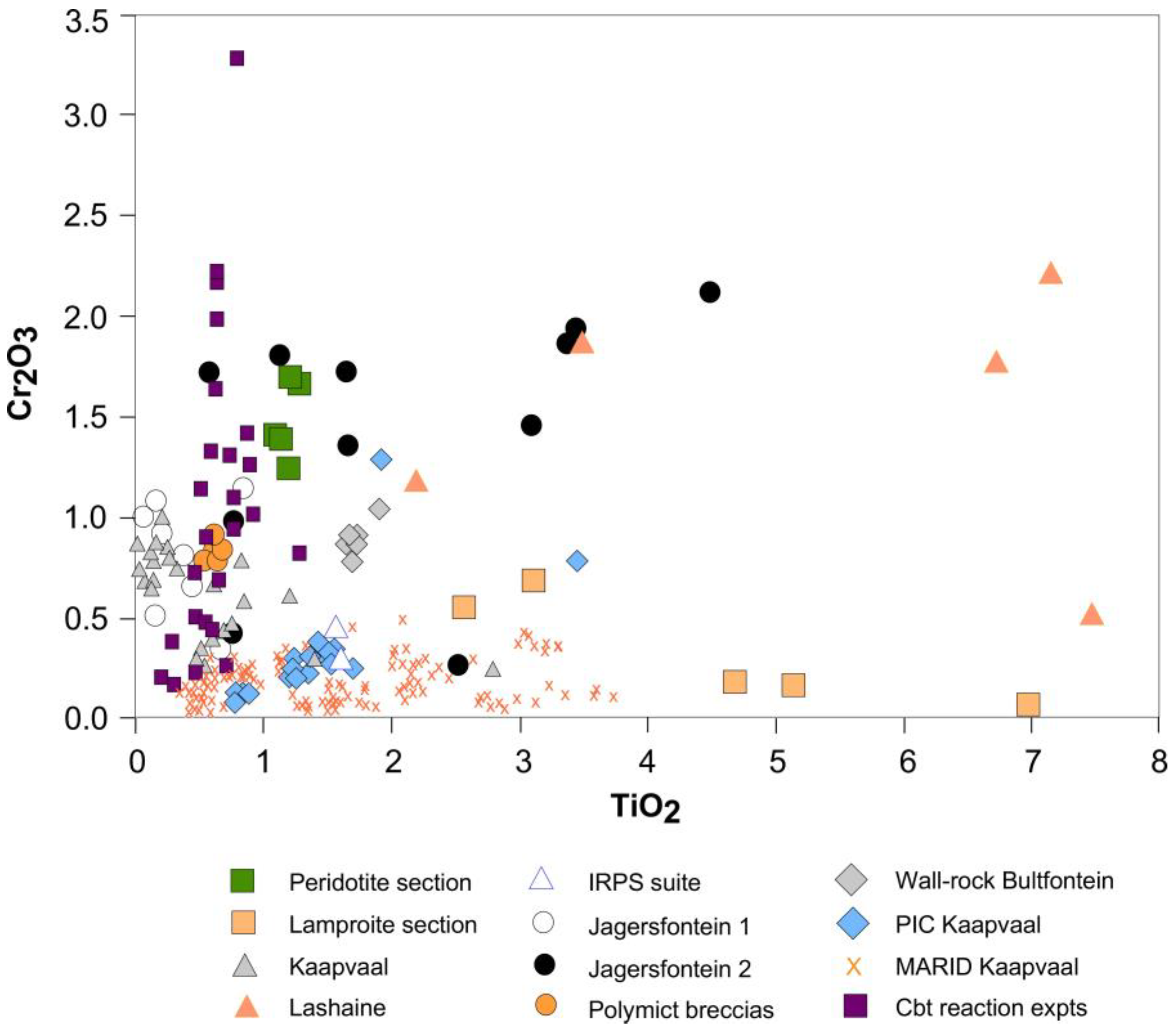
4.3.1. Composition of Phlogopites Formed by Melt/Rock Reaction
4.3.2. Reactivity of Different Alkaline Melts with Peridotite
4.3.3. Compositional Evolution of the Reacting Melt
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- O’Reilly, S.Y.; Griffin, W.L. Mantle metasomatism. In Metasomatism and the Chemical Transformation of Rock; Harlov, D.E., Austrheim, H., Eds.; Springer: Heidleberg, Germany, 2013; pp. 471–533. [Google Scholar]
- Simon, N.S.C.; Carlson, R.W.; Pearson, D.G.; Davies, G.R. The origin and evolution of the Kaapvaal cratonic lithospheric mantle. J. Petrol. 2007, 48, 589–625. [Google Scholar] [CrossRef]
- Rehfeldt, T.; Foley, S.F.; Carlson, R.W.; Lowry, D.; Jacob, D.E. Contrasting types of metasomatism in dunite, wehrlite and websterite xenoliths from Kimberley, South Africa. Geochim. Cosmochim. Acta 2008, 72, 5722–5756. [Google Scholar] [CrossRef]
- Delaney, J.S.; Smith, J.V.; Carswell, D.A.; Dawson, J.B. Chemistry of micas from kimberlites and xenoliths—II. Primary- and secondary-textured micas from peridotite xenoliths. Geochim. Cosmochim. Acta 1980, 44, 857–872. [Google Scholar] [CrossRef]
- Erlank, A.J.; Waters, F.G.; Hawkesworth, C.J.; Haggerty, S.E.; Allsopp, H.; Rickard, R.S.; Menzies, M.A. Evidence for mantle metasomatism in peridotite nodules from the Kimberley pipes, South Africa. In Mantle Metasomatism; Menzies, M.A., Hawkesworth, C.J., Eds.; Academic Press: London, UK, 1987; pp. 221–311. [Google Scholar]
- Waters, F.G.; Erlank, A.J.; Daniels, L.R.M. Contact relationships between MARID rock and metasomatised peridotite in a kimberlite xenolith. Geochem. J. 1989, 23, 11–17. [Google Scholar] [CrossRef]
- Harte, B.; Hunter, R.H.; Kinny, P.D. Melt geometry, movement and crystallization, in relation to mantle dykes, veins and metasomatism. Phil. Trans. Roy. Soc. Lond. Ser. A 1993, 342, 1–21. [Google Scholar]
- Harte, B. Mantle peridotites and processes—the kimberlite sample. In Continental Basalts and Mantle Xenoliths; Hawkesworth, C.J., Norry, M.J., Eds.; Shiva: Nantwich, UK, 1983; pp. 46–91. [Google Scholar]
- Gregoire, M.; Bell, D.R.; Le Roex, A.P. Trace element geochemistry of phlogopite-rich mafic mantle xenoliths: Their classification and their relationship to phlogopite-bearing peridotites and kimberlites revisited. Contrib. Mineral. Petrol. 2002, 142, 603–625. [Google Scholar] [CrossRef]
- Waters, F.G. A suggested origin of MARID xenoliths in kimberlites by high pressure crystallization of an ultrapotassic rock such as lamproite. Contrib. Mineral. Petrol. 1987, 95, 523–533. [Google Scholar] [CrossRef]
- Giuliani, A.; Phillips, D.; Woodhead, J.D.; Kamenetsky, V.S.; Fiorentini, M.L.; Maas, R.; Soltys, A.; Armstrong, R.A. Did diamond-bearing orangeites originate from MARID-veined peridotites in the lithospheric mantle? Nature Comm. 2015, 6, 6837. [Google Scholar] [CrossRef] [Green Version]
- Mitchell, R.H. Kimberlites, Orangeites and Related Rocks; Plenum Press: New York, NY, USA; London, UK, 1995; p. 410. [Google Scholar]
- Pearson, D.G.; Woodhead, J.; Janney, P.E. Kimberlites as geochemical probes of Earth’s mantle. Elements 2019, 15, 387–392. [Google Scholar] [CrossRef]
- Borghini, G.; Rampone, E.; Zanetti, A.; Class, C.; Fumagalli, P.; Godard, M. Ligurian pyroxenite-peridotite sequences (Italt) and the role of melt-rock reaction in creating enriched-MORB mantle sources. Chem. Geol. 2020, 532, 119252. [Google Scholar] [CrossRef]
- Odling, N.W.A. An experimental simulation of upper mantle metasomatism. Amer. Mineral. 1994, 79, 148–153. [Google Scholar]
- Odling, N.W.A. An experimental replication of upper mantle metasomatism. Nature 1995, 373, 58–60. [Google Scholar] [CrossRef]
- Jaques, A.L.; Lewis, J.D.; Smith, C.B. The kimberlites and lamroites of Western Australia. Geol. Surv. Western Aus. Bull. 1986, 132, 268. [Google Scholar]
- Foley, S.F.; Ezad, I.S.; van der Laan, S.R.; Pertermann, M. Melting of hydrous pyroxenite assemblages with alkali amphiboles in the continental mantle lithosphere. Part 1: Melting relations and major element compositions of melts. Unpublished work. 2021. [Google Scholar]
- Falloon, T.J.; Green, D.H.; Danyushevsky, L.V.; Faul, U.H. Peridotite melting at 1.0 and 1.5 GPa: An experimental evaluation of techniques using diamond aggregates and mineral mixes for determination of near-solidus melts. J. Petrol. 1999, 40, 1343–1375. [Google Scholar] [CrossRef]
- Baker, M.B.; Stolper, E.M. Determining the composition of high-pressure mantle melts using diamond aggregates. Geochim Cosmochim Acta 1994, 58, 2811–2827. [Google Scholar] [CrossRef]
- Hirose, K.; Kushiro, I. Partial melting of dry peridotites at high pressures: Determination of compositions of melts segregated from peridotite using aggregates of diamond. Earth Planet. Sci. Lett. 1997, 114, 477–489. [Google Scholar] [CrossRef]
- Van den Bleeken, G.; Müntener, O.; Ulmer, P. Reaction processes between tholeiitic melt and residual peridotite in the uppermost mantle: An experimental study at 0.8 GPa. J. Petrol. 2010, 51, 153–183. [Google Scholar] [CrossRef]
- Van den Bleeken, G.; Müntener, O.; Ulmer, P. Melt variability in percolated peridotite: An experimental study applied to reactive migration of tholeiitic basalt in the upper mantle. Contrib. Mineral. Petrol. 2011, 161, 921–945. [Google Scholar] [CrossRef] [Green Version]
- Foley, S.F. The genesis of lamproitic magmas in a reduced, fluorine-rich mantle. In Kimberlites and Related Rocks; Ross, J., Ed.; Blackwell: Melbourne, Australia, 1989; Volume 1, pp. 616–632. [Google Scholar]
- Mallik, A.; Dasgupta, R. Effect of variable CO2 on eclogite-derived andesite and lherzolite reaction at 3 GPa—implications for mantle source characteristics of alkali ocean island basalts. Geochem. Geophys. Geosys. 2014, 15, 1533–1557. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Prelevic, D.; Buhre, S.; Foley, S.F. Constraints on the sources of post-collisional K-rich magmatism: The roles of continental clastic sediments and terrigenous blueschists. Chem. Geol. 2017, 455, 192–207. [Google Scholar] [CrossRef]
- Pertermann, M. High-Pressure Experiments on the Origin of Metasomatic Melts in the Mantle. An Experimental Investigation in Two Parts. Diploma Thesis, Mineralogisch-Petrologisches Institute, Universität Göttingen, Göttingen, Germany, 1997; p. 76. [Google Scholar]
- Foley, S. An experimental study of olivine lamproite—first results from the diamond stability field. Geochim. Cosmochim. Acta 1993, 57, 483–489. [Google Scholar] [CrossRef]
- Sheraton, J.W.; Cundari, A. Leucitites from Gaussberg, Antarctica. Contrib. Mineral. Petrol. 1980, 71, 417–427. [Google Scholar] [CrossRef]
- Wang, Y.; Foley, S.F. Hybridization melting between continent-derived sediment and depleted peridotite in subduction zones. J. Geophys. Res. 2018, 123, 3414–3429. [Google Scholar] [CrossRef]
- Sekine, T.; Wyllie, P.J. Experimental simulation of mantle hybridization in subduction zones. J. Geol. 1983, 91, 511–528. [Google Scholar] [CrossRef]
- Bulatov, V.K.; Brey, G.P.; Girnis, A.V.; Gerdes, A.; Höfer, H. Carbonated sediment-peridotite interaction and melting at 7.5-12 GPa. Lithos 2014, 200–201, 368–385. [Google Scholar] [CrossRef]
- Castro, A.; Gerya, T.V. Magmatic implications of mantle wedge plumes: Experimental study. Lithos 2008, 103, 138–148. [Google Scholar] [CrossRef]
- Förster, M.W.; Prelevic, D.; Schmück, H.R.; Buhre, S.; Veter, M.; Mertz-Kraus, R.; Foley, S.F.; Jacob, D.E. Melting and dynamic metasomatism of mixed harzburgite+glimmerite mantle source: Implications for the origin of orogenic potassic magmas. Chem. Geol. 2017, 455, 182–191. [Google Scholar] [CrossRef]
- Förster, M.W.; Prelevic, D.; Buhre, S.; Mertz-Kraus, R.; Foley, S.F. An experimental study of the role of partial melts of sediments versus mantle melts as sources of potassic magmatism. J. Asian Earth Sci. 2019, 177, 76–88. [Google Scholar] [CrossRef]
- Förster, M.W.; Bussweiler, Y.; Prelevic, D.; Daczko, N.R.; Buhre, S.; Mertz-Kraus, R.; Foley, S.F. Sediment-peridotite reaction controls fore-arc metasomatism and arc-magma geochemical signatures. Geosciences 2021, in press. [Google Scholar]
- Gervasoni, F.; Klemme, S.; Rohrbach, A.; Grützner, T.; Berndt, J. Experimental constraints on mantle metsomatism caused by silicate and carbonate melts. Lithos 2017, 282–283, 173–186. [Google Scholar] [CrossRef]
- Johnston, A.D.; Wyllie, P.J. The system tonalite-peridotite-H2O at 30 kbar, with applications to hybridization in subduction zone magmatism. Contrib. Mineral. Petrol. 1989, 102, 257–264. [Google Scholar] [CrossRef]
- Mallik, A.; Dasgupta, R. Reaction between MORB-eclogite derived melts and fertile peridotite and generation of ocean island basalts. Earth Planet. Sci. Lett. 2012, 329–330, 97–108. [Google Scholar] [CrossRef]
- Mallik, A.; Dasgupta, R. Reactive infiltration of MORB-eclogite-derived carbonated silicate melt into fertile peridotite at 3GPa and genesis of alkali magmas. J. Petrol. 2013, 54, 2267–2300. [Google Scholar] [CrossRef]
- Gao, M.D.; Xu, H.; Zhang, J.F.; Foley, S.F. Experimental interaction of granitic melt and peridotite at 1.5 GPa: Implications for the origin of post-collisional K-rich magmatism in continental subduction zones. Lithos 2019, 350–351, 105241. [Google Scholar] [CrossRef]
- Ryabchikov, I.D.; Orlova, G.P.; Kalenchuk, G.Y.; Ganeyev, I.I.; Udovkina, N.G.; Nosik, L.P. Reactions of spinel lherzolite with H2O-CO2 fluids at 20 kbar and 900 °C. Geochem. Internat. 1989, 56–62. [Google Scholar]
- Kushiro, I.; Hirose, K. Experimental determination of composition of melt formed by equilibrium partial melting of peridotite at high pressures using aggregates of diamond grains. Proc. Japan Acad. Ser. B 1992, 68, 63–68. [Google Scholar] [CrossRef] [Green Version]
- Foley, S.F.; Musselwhite, D.S.; van der Laan, S.R. Melt compositions from ultramafic vein assemblages in the lithospheric mantle: A comparison of cratonic and non-cratonic settings. In J.B. Dawson Volume, Proceedings of the Cape Town Kimberlite Conference; Red Roof Publishers: Cape Town, South Africa, 1999; pp. 238–246. [Google Scholar]
- Walter, M.J. Melt extraction and compositional variability in mantle lithosphere. Treatise Geochem. 2003, 2, 363–394. [Google Scholar]
- Carswell, D.A. Primary and secondary phlogopites and clinopyroxenes in garnet lherzolite xenoliths. Phys. Chem. Earth 1975, 9, 417–430. [Google Scholar] [CrossRef]
- Dawson, J.B. Metasomatism and partial melting in upper-mantle peridotite xenoliths from the Lshaine volcano, northern Tanzania. J. Petrol. 2002, 43, 1749–1777. [Google Scholar] [CrossRef] [Green Version]
- Giuliani, A.; Phlillips, D.; Kamenetsky, V.S.; Kendrick, M.A.; Wyatt, B.A.; Goemann, K.; Hutchinson, G. Petrogenesis of mantle polymict breccias: Insights into mantle processes coeval with kimberlite magmatism. J. Petrol. 2014, 55, 831–858. [Google Scholar] [CrossRef]
- Field, S.W.; Haggerty, S.E.; Erlank, A.J. Subcontinental metasomatism in the region of Jagersfontein, South Africa. In kimBerlites and Related Rocks 2; Ross, J., Ed.; Blackwell: Melbourne, Australia, 1989; pp. 771–783. [Google Scholar]
- Jones, A.P.; Smith, J.V.; Dawson, J.B. Mantle metasomatism in 14 veined peridotites from Bultfontein Mine, South Africa. J. Geol. 1982, 90, 435–453. [Google Scholar] [CrossRef]
- Harte, B.; Winterburn, P.A.; Gurney, J.J. Metasomatic and enrichment phenomena in garnet peridotite facies mantle xenoliths from the Matsoku kimberlite pipe Lesotho. In Mantle Metasomatism; Menzies, M.A., Hawkesworth, C.J., Eds.; Academic Press: London, UK, 1987; pp. 145–220. [Google Scholar]
- Fitzpayne, A.; Giuliani, A.; Hergt, J.; Phillips, D.; Janney, P. New geochemical constraints on the origins of MARID and PIC rocks: Implications for mantle metasomatism and mantle-derived potassic magmatism. Lithos 2018, 318–319, 478–493. [Google Scholar] [CrossRef]
- Shatskiy, A.; Bekhtenova, A.; Arefiev, A.V.; Podborodnikov, I.V.; Vinogradova, Y.G.; Rezvukhin, D.I.; Litasov, K.D. Solidus and melting of carbonated phlogopite peridotite at 3—6.5 GPa: Implications for mantle metasomatism. Gondwana Res. 2022, 101, 156–174. [Google Scholar] [CrossRef]
- Ziberna, L.; Klemme, S.; Nimis, P. Garnet and sinel in fertile and depleted mantle: Insights from thermodynamic modelling. Contrib. Mineral. Petrol. 2013, 166, 411–421. [Google Scholar] [CrossRef]
- Ezad, I.S.; Dobson, D.P.; Thompson, A.R.; Jennings, E.S.; Hunt, S.A.; Brodholt, J.P. Kelyphite textures experimentally reproduced through garnet breakdown in the presence of a transient melt phase. J. Petrol. 2021, in press. [Google Scholar]
- Dasgupta, R.; Hirschmann, M.M. Effect of variable carbonate concentration on the solidus of mantle peridotite. Amer. Mineral. 2007, 92, 370–379. [Google Scholar] [CrossRef]
- Pintér, Z.; Foley, S.F.; Yaxley, G.M.; Rosenthal, A.; Rapp, R.P.; Lanati, A.W.; Rushmer, T. Experimental investigation of the composition of incipient melts in upper mantle peridotites in the presence of CO2 and H2O. Lithos 2021, 396–397, 106224. [Google Scholar] [CrossRef]
- Mitchell, R.H. Igneous rock associations 26. Lamproites, exotic potassic alkaline rocks: A review of their nomenclature, characterization and origins. Geosci. Can. 2020, 47, 119–142. [Google Scholar] [CrossRef]
- Foley, S.F. Experimental constraints on phlogopite chemistry in lamproites: 1. The effect of water activity and oxygen fugacity. Eur. J. Mineral. 1989, 1, 411–426. [Google Scholar] [CrossRef]
- Ngwenya, N.S.; Tappe, S. Diamondiferous lamproites of the Luangwa Rift in central Africa and links to remobilized cratonic lithosphere. Chem. Geol. 2021, 568, 120019. [Google Scholar] [CrossRef]
- Foley, S. Vein-plus-wall-rock melting mechanisms in the lithosphere and the origin of potassic alkaline magmas. Lithos 1992, 28, 435–453. [Google Scholar] [CrossRef]
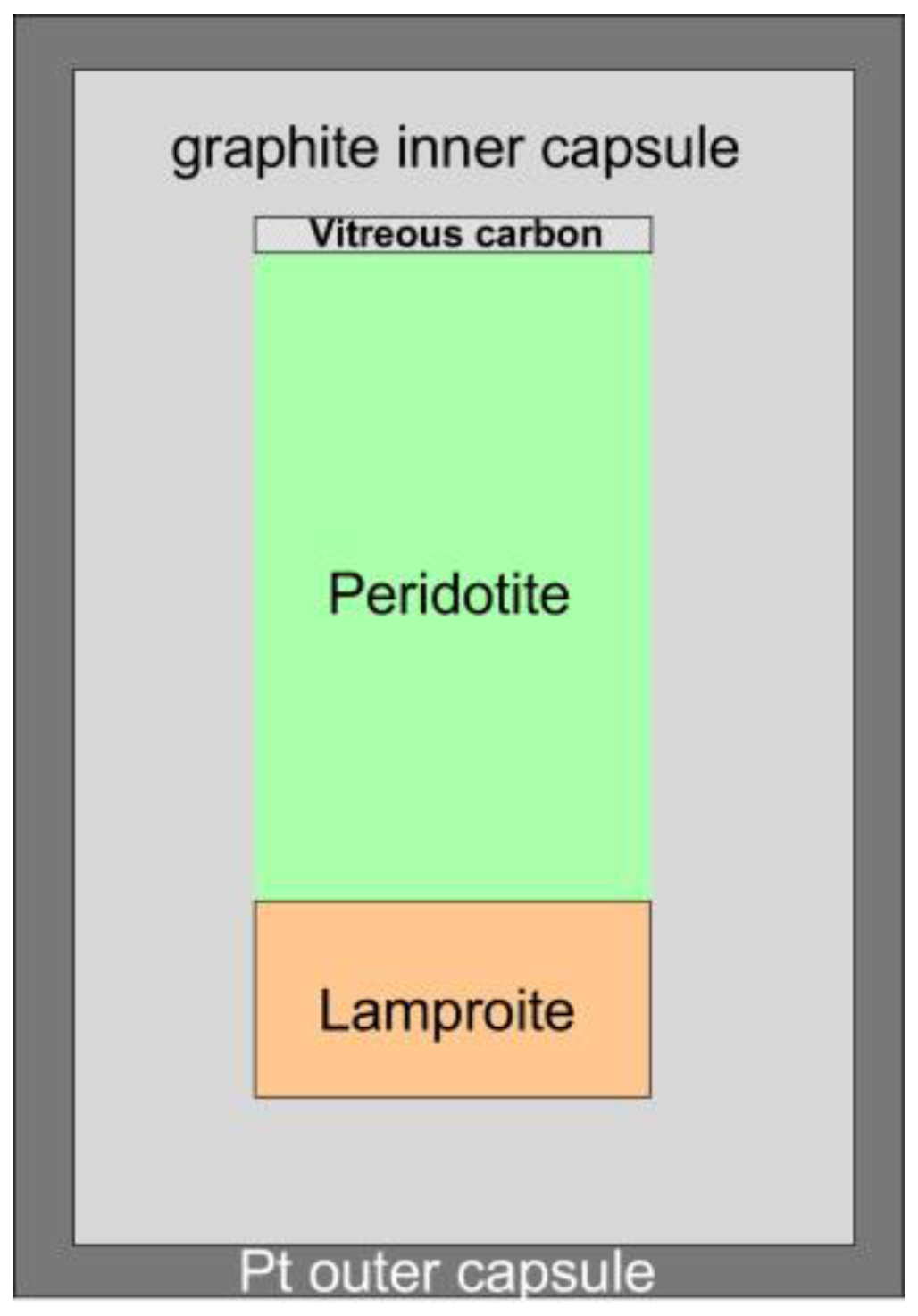
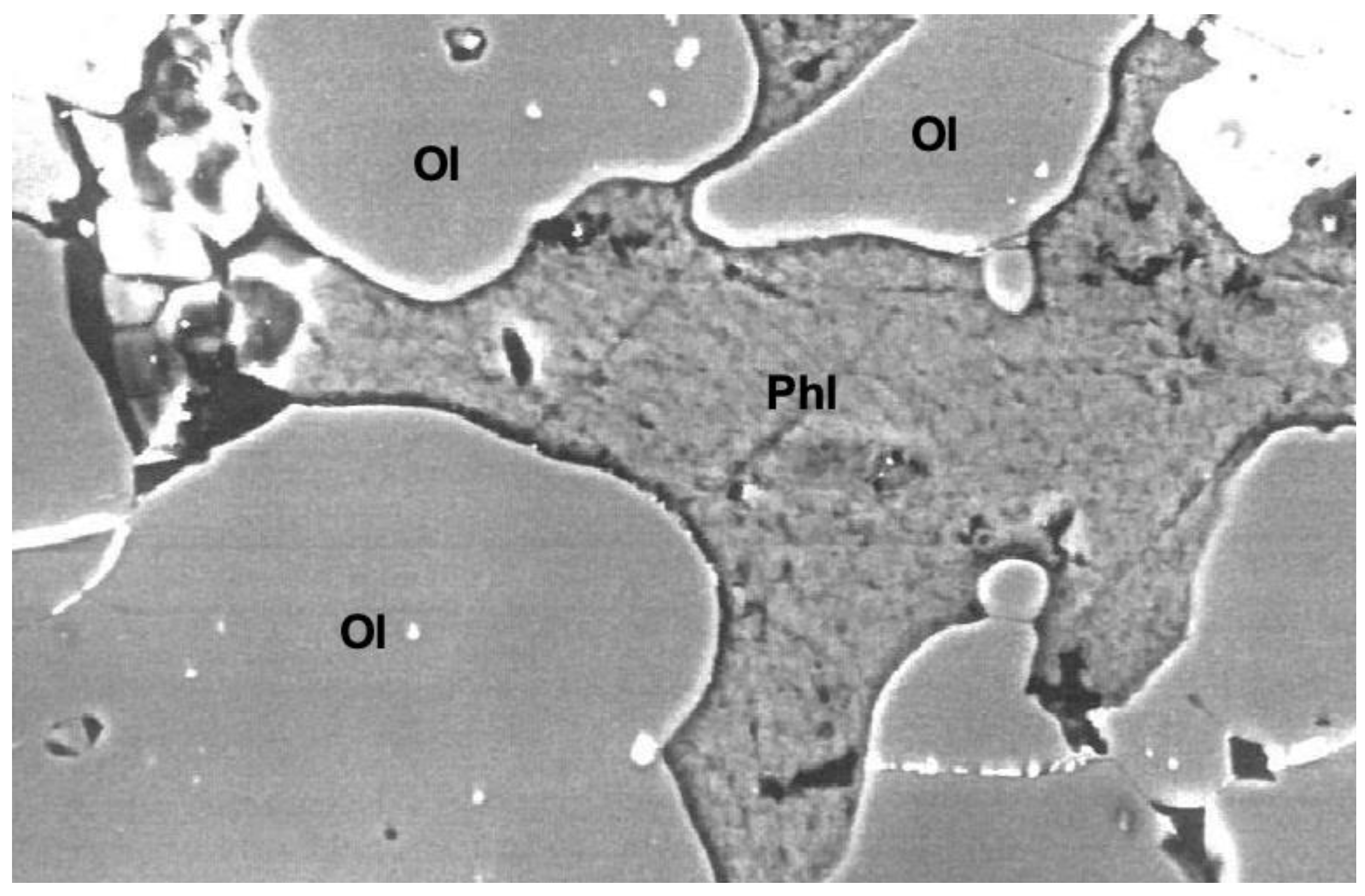

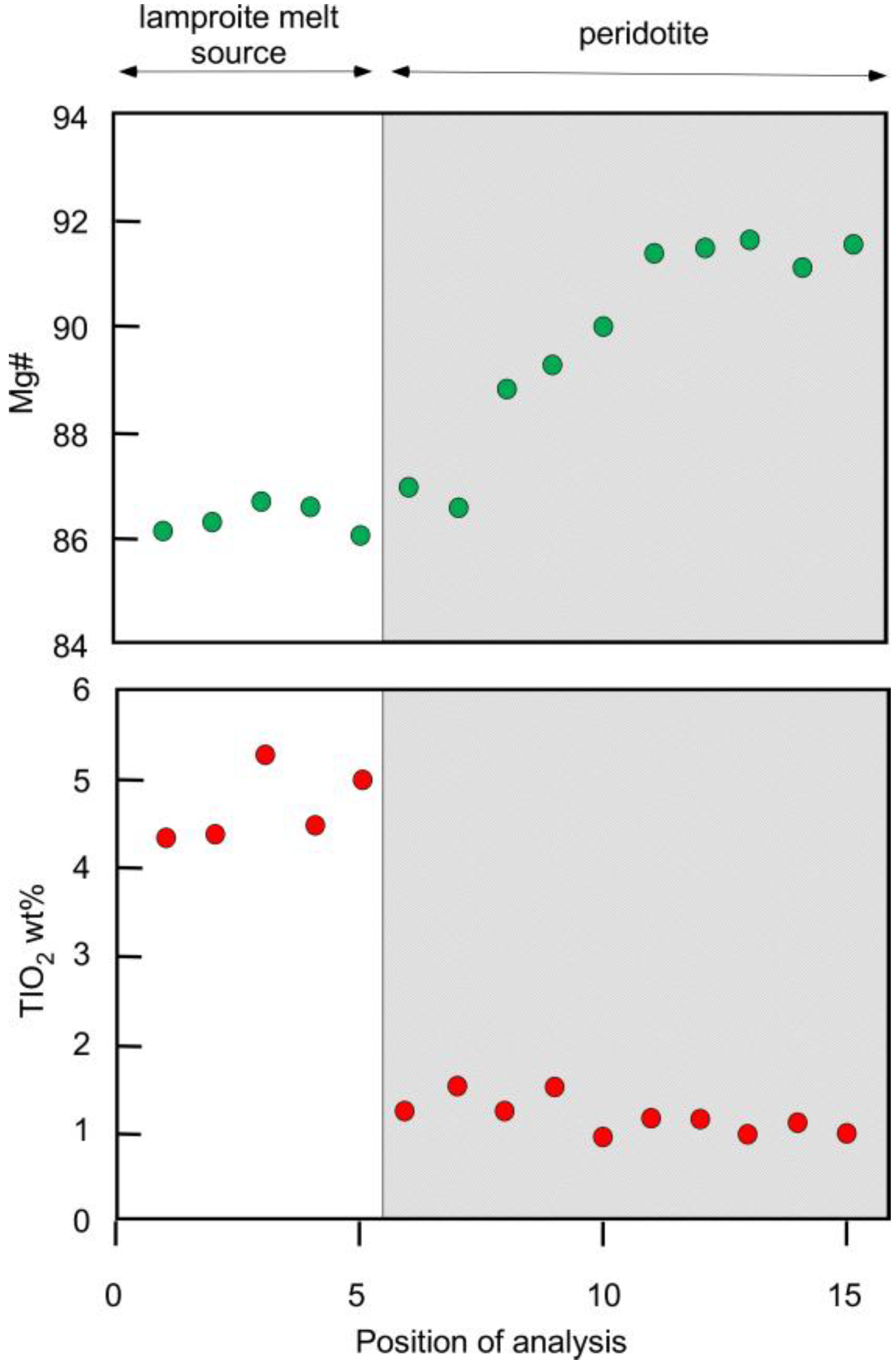

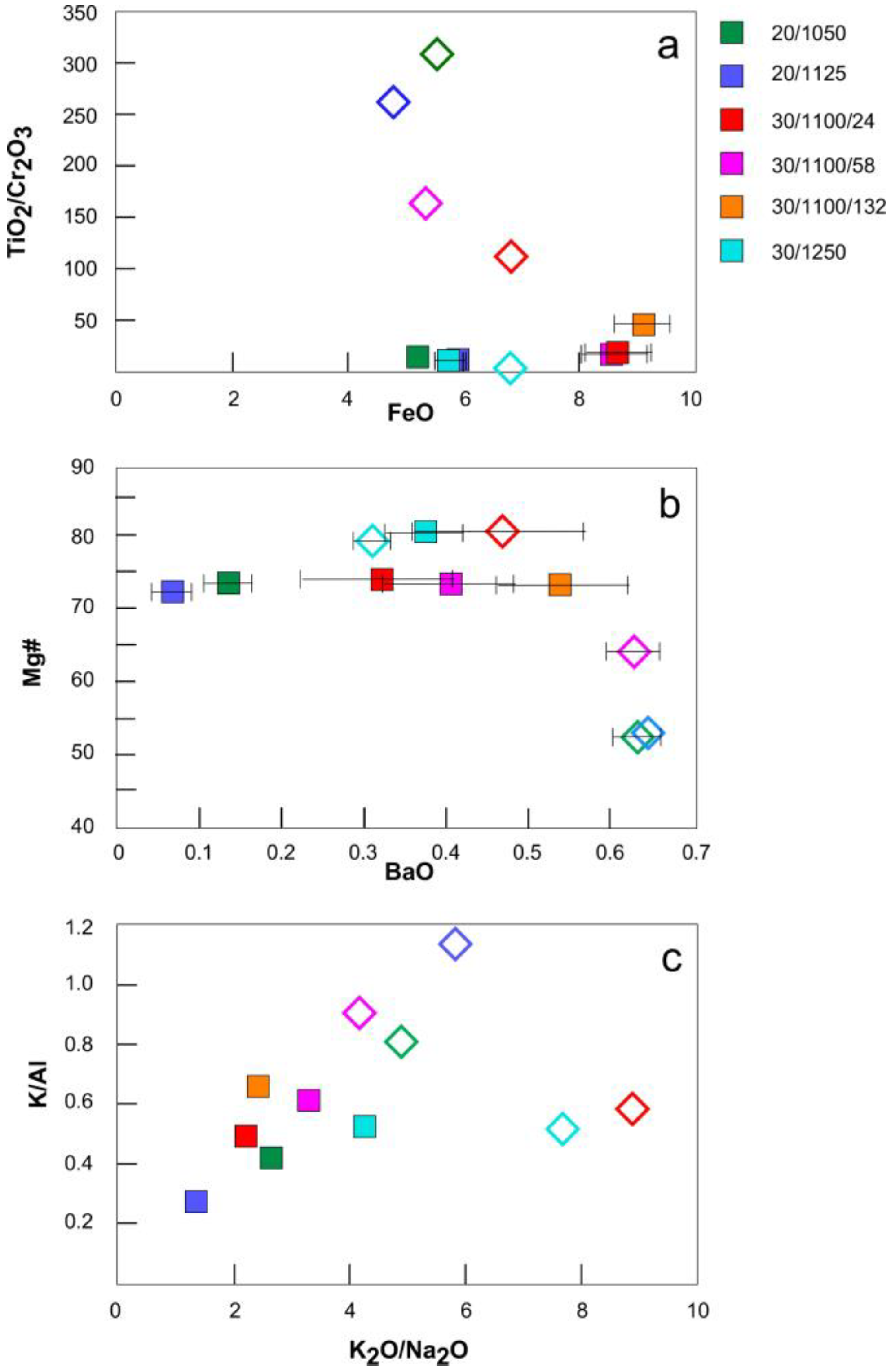
| Experiment Label | Pressure GPa | Temperature °C | Duration Hours | Ratio Peridotite/ Lamproite a | Ratio Trap/ Silicate Charge b | Phases Present c |
|---|---|---|---|---|---|---|
| G132 | 2 | 1050 | 72 | 3.29 | 0.043 | OL + CPX + PHL + L |
| G128 | 2 | 1125 | 72 | 2.52 | 0.039 | OL + CPX + PHL + L |
| G138 | 3 | 1100 | 24 | 3.15 | 0.046 | OL + CPX + OPX + PHL + L |
| G131 | 3 | 1100 | 58 | 2.57 | 0.043 | OL + CPX + OPX + PHL + L |
| G137 | 3 | 1100 | 132 | 3.18 | 0.044 | OL + CPX + OPX + PHL + L |
| G139 | 3 | 1250 | 24 | 3.19 | 0.041 | OL + (SP) + L |
| G141 * | 3 | 1100 | 72 | 3.19 | 0.036 | OL + (CPX) + OPX + PHL + L |
| Starting Material | 65% | 15% | 10% | 10% | Average | ||
|---|---|---|---|---|---|---|---|
| Gaussberg | J4 | J4 | J4 | J4 | J4 | Garnet | |
| Lamproite | Peridotite | Olivine | Opx | Cpx | Grt | Lherzolite | |
| SiO2 | 51.1 | 44.88 | 40.8 | 57.58 | 55.14 | 42.1 | 45.98 |
| TiO2 | 3.3 | 0.18 | 0.03 | 0.23 | 0.49 | 0.78 | 0.09 |
| Al2O3 | 10.28 | 2.56 | 0.04 | 0.98 | 2.89 | 20.93 | 1.57 |
| MgO | 7.08 | 40.89 | 48.98 | 33.94 | 18.11 | 21.51 | 43.46 |
| FeO | 6.08 | 8.56 | 9.94 | 6.08 | 4.05 | 7.81 | 6.91 |
| CaO | 4.03 | 2.19 | 0.07 | 1.02 | 15.65 | 4.25 | 1.16 |
| Na2O | 1.8 | 0.3 | 0.03 | 0.32 | 2.25 | 0.11 | 0.16 |
| K2O | 12.46 | 0.01 | 0.01 | 0.01 | 0.03 | 0.01 | 0.12 |
| Cr2O3 | 0.03 | 0.34 | 0.03 | 0.21 | 0.82 | 2.02 | 0.32 |
| MnO | 0.08 | 0.13 | 0.11 | 0.12 | 0.13 | 0.28 | 0.11 |
| NiO | 0.01 | 0.23 | 0.36 | 0.29 | |||
| BaO | 0.55 | ||||||
| P2O5 | 1.37 | ||||||
| H2O | 1.17 | ||||||
| Sum | 99.34 | 100.27 | 100.4 | 100.49 | 99.56 | 99.8 | 100.17 |
| Mg# | 67.49 | 89.49 | 89.78 | 90.87 | 88.85 | 83.08 | 91.81 |
| Expt | G132 | G132 | G128 | G128 | ||||
| P (GPa) | 2 | 2 | 2 | 2 | ||||
| T (°C) | 1050 | 1050 | 1125 | 1125 | ||||
| Duration (h) | 72 | 72 | 72 | 72 | ||||
| Location | Residual | interstitial in | Residual | interstitial in | ||||
| melt | s.d. | peridotite | s.d. | melt | s.d. | peridotite | s.d. | |
| SiO2 | 40.29 | 0.12 | 39.7 | 0.27 | 39.73 | 0.24 | 38.72 | 0.51 |
| TiO2 | 5.14 | 0.43 | 1.2 | 0.08 | 6.98 | 0.37 | 1.26 | 0.11 |
| Al2O3 | 11.86 | 0.1 | 14.7 | 0.28 | 11.49 | 0.27 | 15.99 | 0.43 |
| MgO | 20.12 | 0.36 | 23.2 | 0.17 | 17.98 | 0.22 | 22.4 | 0.25 |
| FeO | 6.19 | 0.22 | 3.7 | 0.14 | 7.42 | 0.2 | 3.68 | 0.09 |
| CaO | 0.03 | 0.05 | 0.0 | 0.02 | 0.05 | 0.03 | 0.03 | 0.02 |
| Na2O | 0.12 | 0.02 | 0.6 | 0.03 | 0.16 | 0.03 | 0.74 | 0.04 |
| K2O | 9.98 | 0.11 | 9.4 | 0.16 | 9.81 | 0.16 | 9.32 | 0.09 |
| Cr2O3 | 0.17 | 0.07 | 1.7 | 0.21 | 0.07 | 0.02 | 1.67 | 0.2 |
| MnO | 0.05 | 0.03 | 0.0 | 0.02 | 0.05 | 0.03 | 0.05 | 0.03 |
| NiO | 0.08 | 0.03 | 0.2 | 0.04 | 0.04 | 0.02 | 0.14 | 0.07 |
| BaO | 0.29 | 0.04 | 0.2 | 0.06 | 0.35 | 0.03 | 0.22 | 0.06 |
| Sum | 94.32 | 94.58 | 94.13 | 94.22 | ||||
| Mg# | 85.3 | 91.8 | 81.2 | 91.6 | ||||
| n | 5 | 14 | 5 | 8 | ||||
| Expt | G138 | G138 | G131 | G131 | ||||
| P (GPa) | 3 | 3 | 3 | 3 | ||||
| T (°C) | 1100 | 1100 | 1100 | 1100 | ||||
| Duration (h) | 24 | 24 | 58 | 58 | ||||
| Location | Residual | interstitial in | Residual | interstitial in | ||||
| melt | s.d. | peridotite | s.d. | melt | s.d. | peridotite | s.d. | |
| SiO2 | 40.97 | 0.84 | 39.31 | 0.53 | 40.97 | 0.38 | 39.22 | 0.19 |
| TiO2 | 2.56 | 0.62 | 1.12 | 0.24 | 4.67 | 0.43 | 1.19 | 0.22 |
| Al2O3 | 13.97 | 0.58 | 15.27 | 0.34 | 11.84 | 0.15 | 14.3 | 0.26 |
| MgO | 23.15 | 0.47 | 23.52 | 0.57 | 20.08 | 0.33 | 22.53 | 0.3 |
| FeO | 5.09 | 0.2 | 4.47 | 0.22 | 5.7 | 0.21 | 4.28 | 0.56 |
| CaO | 0.04 | 0.02 | 0.03 | 0.03 | 0.19 | 0.25 | 0.02 | 0.02 |
| Na2O | 0.28 | 0.07 | 0.42 | 0.07 | 0.11 | 0.02 | 0.28 | 0.11 |
| K2O | 8.78 | 0.23 | 8.54 | 0.26 | 10.06 | 0.22 | 9.88 | 0.09 |
| Cr2O3 | 0.56 | 0.38 | 1.39 | 0.26 | 0.18 | 0.03 | 1.25 | 0.23 |
| MnO | 0.03 | 0.01 | 0.03 | 0.02 | ||||
| NiO | 0.1 | 0.06 | 0.18 | 0.04 | 0.04 | 0.03 | 0.13 | 0.07 |
| BaO | 0.36 | 0.09 | 0.4 | 0.08 | 0.31 | 0.06 | 0.39 | 0.09 |
| Sum | 95.86 | 94.65 | 94.18 | 93.5 | ||||
| Mg# | 89.0 | 90.4 | 86.3 | 90.4 | ||||
| n | 8 | 11 | 5 | 12 | ||||
| Expt | G137 | G137 | ||||||
| P (GPa) | 3 | 3 | ||||||
| T (°C) | 1100 | 1100 | ||||||
| Duration (h) | 132 | 132 | ||||||
| Location | Residual | interstitial in | ||||||
| melt | s.d. | peridotite | s.d. | |||||
| SiO2 | 39.36 | 1.09 | 39.85 | 0.93 | ||||
| TiO2 | 3.1 | 0.49 | 1.07 | 0.56 | ||||
| Al2O3 | 13.76 | 0.43 | 15.1 | 0.53 | ||||
| MgO | 23.03 | 0.34 | 23.89 | 0.42 | ||||
| FeO | 4.41 | 0.21 | 4.21 | 0.14 | ||||
| CaO | 0.02 | 0.01 | 0.02 | 0.02 | ||||
| Na2O | 0.14 | 0.02 | 0.24 | 0.04 | ||||
| K2O | 9.09 | 0.22 | 8.91 | 0.2 | ||||
| Cr2O3 | 0.68 | 0.13 | 1.41 | 0.42 | ||||
| MnO | ||||||||
| NiO | 0.08 | 0.03 | 0.16 | 0.03 | ||||
| BaO | 0.28 | 0.08 | 0.3 | 0.06 | ||||
| Sum | 93.95 | 95.16 | ||||||
| Mg# | 90.3 | 91.0 | ||||||
| n | 6 | 16 |
| Expt | G132 | G128 | G138 | G131 | G137 | |||||
| P (GPa) | 2 | 2 | 2 | 3 | 3 | |||||
| T (°C) | 1050 | 1125 | 1100 | 1100 | 1100 | |||||
| Duration (h) | 72 | 72 | 24 | 58 | 132 | |||||
| Mineral | CPX | s.d. | CPX | s.d. | CPX | s.d. | CPX | s.d. | CPX | s.d. |
| SiO2 | 53.06 | 0.53 | 53.9 | 1.11 | 54.32 | 0.48 | 53.86 | 0.26 | 53.93 | 0.65 |
| TiO2 | 0.28 | 0.03 | 0.5 | 0.12 | 0.15 | 0.05 | 0.17 | 0.03 | 0.33 | 0.18 |
| Al2O3 | 3.15 | 0.52 | 3.5 | 0.7 | 2.61 | 0.18 | 3.06 | 0.23 | 2.67 | 0.45 |
| MgO | 17.74 | 0.28 | 18.0 | 0.56 | 18.83 | 0.31 | 18.38 | 0.4 | 17.72 | 1.01 |
| FeO | 3.08 | 0.16 | 3.8 | 0.39 | 3.44 | 0.37 | 3.37 | 0.09 | 4.18 | 1.09 |
| CaO | 20.48 | 0.27 | 17.3 | 1.7 | 18.57 | 0.3 | 18.33 | 0.35 | 19.05 | 0.45 |
| Na2O | 0.62 | 0.04 | 1.6 | 0.64 | 0.98 | 0.12 | 1.11 | 0.14 | 1.28 | 0.27 |
| K2O | 0.01 | 0.01 | 0.0 | 0.02 | 0.02 | 0.01 | 0.01 | 0.01 | 0.03 | 0.01 |
| Cr2O3 | 1.29 | 0.15 | 1.0 | 0.16 | 1.33 | 0.46 | 1.37 | 0.15 | 0.85 | 0.65 |
| MnO | 0.12 | 0.02 | 0.1 | 0.03 | 0.12 | 0.03 | ||||
| NiO | 0.04 | 0.02 | 0.1 | 0.01 | 0.05 | 0.03 | 0.04 | 0.02 | 0.04 | 0.05 |
| Sum | 99.87 | 99.72 | 100.3 | 99.82 | 100.08 | |||||
| Mg# | 91.1 | 89.4 | 90.7 | 90.7 | 88.3 | |||||
| n | 9 | 28 | 18 | 12 | 16 | |||||
| Expt | G132 | G128 | G138 | G131 | G137 | |||||
| P (GPa) | 2 | 2 | 3 | 3 | 3 | |||||
| T (°C) | 1050 | 1125 | 1100 | 1100 | 1100 | |||||
| Duration (h) | 72 | 72 | 24 | 58 | 132 | |||||
| Mineral | OL | s.d. | OL | s.d. | OL | s.d. | OL | s.d. | OL | s.d. |
| SiO2 | 40.45 | 0.17 | 40.32 | 0.19 | 41.61 | 0.58 | 40.64 | 0.12 | 41.38 | 0.23 |
| TiO2 | 0.02 | 0.01 | 0.03 | 0.02 | 0.03 | 0.02 | 0.01 | 0.01 | 0.03 | 0.02 |
| Al2O3 | 0.02 | 0.01 | 0.03 | 0.01 | 0.06 | 0.06 | 0.04 | 0.01 | 0.02 | 0.02 |
| MgO | 48.85 | 0.22 | 48.84 | 0.19 | 48.74 | 0.36 | 48.8 | 0.22 | 49.02 | 0.28 |
| FeO | 10.38 | 0.27 | 10.31 | 0.2 | 10.77 | 0.45 | 10.43 | 0.29 | 11.31 | 0.37 |
| CaO | 0.09 | 0.01 | 0.11 | 0.03 | 0.11 | 0.02 | 0.08 | 0.01 | 0.08 | 0.02 |
| Na2O | 0.01 | 0.01 | 0.01 | 0.01 | 0.02 | 0.02 | 0.01 | 0.01 | 0.02 | 0.02 |
| K2O | 0 | 0 | 0 | 0 | 0.03 | 0.05 | 0 | 0.01 | 0 | 0 |
| Cr2O3 | 0.04 | 0.01 | 0.06 | 0.01 | 0.09 | 0.08 | 0.06 | 0.01 | 0.07 | 0.04 |
| MnO | 0.16 | 0.04 | 0.14 | 0.03 | 0.15 | 0.02 | ||||
| NiO | 0.35 | 0.05 | 0.36 | 0.04 | 0.26 | 0.04 | 0.38 | 0.03 | 0.29 | 0.05 |
| Sum | 100.37 | 100.21 | 101.72 | 100.6 | 102.22 | |||||
| Mg# | 89.3 | 89.4 | 89.0 | 89.3 | 88.5 | |||||
| n | 12 | 19 | 14 | 9 | 6 | |||||
| Expt | G139 | G138 | G131 | G137 | ||||||
| P (GPa) | 3 | 3 | 3 | 3 | ||||||
| T (°C) | 1250 | 1100 | 1100 | 1100 | ||||||
| Duration (h) | 24 | 24 | 58 | 132 | ||||||
| Mineral | OL | s.d. | OPX | s.d. | OPX | s.d. | OPX | s.d. | ||
| SiO2 | 40.63 | 0.35 | 57.58 | 0.52 | 56.4 | 0.58 | 57.14 | 0.93 | ||
| TiO2 | 0.02 | 0.02 | 0.19 | 0.06 | 0.13 | 0.03 | 0.18 | 0.06 | ||
| Al2O3 | 0.03 | 0.01 | 1.41 | 0.57 | 2.01 | 0.53 | 1.22 | 0.37 | ||
| MgO | 50.43 | 0.56 | 32.93 | 95 | 33.13 | 0.39 | 33.48 | 0.75 | ||
| FeO | 8.81 | 0.41 | 7.54 | 1.24 | 6.39 | 0.42 | 7.35 | 0.87 | ||
| CaO | 0.12 | 0.02 | 1.36 | 0.23 | 1.21 | 0.06 | 1.13 | 0.08 | ||
| Na2O | 0.01 | 0.01 | 0.19 | 0.08 | 0.17 | 0.05 | 0.17 | 0.07 | ||
| K2O | 0.01 | 0.02 | 0.01 | 0.04 | 0.01 | 0.01 | 1 | 0.07 | ||
| Cr2O3 | 0.21 | 0.07 | 0.3 | 0.36 | 0.59 | 0.28 | 0.24 | 0.16 | ||
| MnO | 0.15 | 0.03 | ||||||||
| NiO | 0.17 | 0.09 | 0.06 | 0.03 | 0.12 | 0.02 | 0.07 | 0.04 | ||
| Sum | 100.44 | 101.57 | 100.31 | 101.984 | ||||||
| Mg# | 91.1 | 88.6 | 90.2 | 89.0 | ||||||
| n | 21 | 12 | 10 | 23 |
| Expt | G132 | G132 | G128 | G128 | ||||
| P (GPa) | 2 | 2 | 2 | 2 | ||||
| T (°C) | 1050 | 1050 | 1125 | 1125 | ||||
| Duration (h) | 72 | 72 | 72 | 72 | ||||
| Residual | Melt | Residual | Melt | |||||
| melt | s.d. | trap | s.d. | melt | s.d. | trap | s.d. | |
| SiO2 | 56.33 | 2.14 | 52.49 | 2.92 | 55.54 | 0.30 | 48.63 | 2.42 |
| TiO2 | 2.98 | 0.26 | 1.24 | 0.25 | 2.41 | 0.13 | 1.32 | 0.21 |
| Al2O3 | 10.10 | 0.15 | 14.96 | 1.20 | 10.62 | 0.09 | 15.44 | 1.04 |
| MgO | 3.61 | 2.40 | 8.21 | 2.18 | 3.14 | 0.76 | 8.57 | 1.44 |
| FeO | 5.58 | 0.64 | 5.21 | 1.01 | 4.79 | 0.17 | 5.89 | 0.59 |
| CaO | 4.18 | 0.39 | 4.96 | 2.05 | 2.09 | 0.11 | 8.52 | 2.99 |
| Na2O | 1.57 | 0.28 | 2.22 | 0.46 | 1.93 | 0.15 | 3.00 | 0.67 |
| K2O | 7.59 | 0.26 | 5.82 | 1.04 | 11.20 | 0.18 | 3.93 | 0.84 |
| Cr2O3 | 0.01 | 0.02 | 0.09 | 0.04 | 0.01 | 0.01 | 0.09 | 0.04 |
| MnO | 0.10 | 0.03 | 0.12 | 0.06 | 0.09 | 0.02 | 0.15 | 0.07 |
| NiO | 0.01 | 0.02 | 0.03 | 0.04 | 0.01 | 0.01 | 0.02 | 0.03 |
| BaO | 0.65 | 0.06 | 0.13 | 0.06 | 0.66 | 0.05 | 0.07 | 0.04 |
| P2O5 | 2.28 | 0.30 | 0.39 | 0.25 | 1.66 | 0.08 | 0.39 | 0.09 |
| Sum | 94.98 | 95.87 | 94.16 | 96.01 | ||||
| Mg# | 53.5 | 73.7 | 53.8 | 72.2 | ||||
| n | 8 | 23 | 7 | 23 | ||||
| Expt | G138 | G138 | G131 | G131 | ||||
| P (GPa) | 3 | 3 | 3 | 3 | ||||
| T (°C) | 1100 | 1100 | 1100 | 1100 | ||||
| Duration (h) | 24 | 24 | 58 | 58 | ||||
| Residual | Melt | Residual | Melt | |||||
| melt | s.d. | trap | s.d. | melt | s.d. | trap | s.d. | |
| SiO2 | 41.75 | 1.89 | 44.58 | 1.92 | 52.01 | 1.53 | 43.68 | 2.74 |
| TiO2 | 3.24 | 0.93 | 2.08 | 0.32 | 3.02 | 0.21 | 2.09 | 0.48 |
| Al2O3 | 12.23 | 1.44 | 10.34 | 1.25 | 10.21 | 0.40 | 10.20 | 1.88 |
| MgO | 16.36 | 5.61 | 13.92 | 2.83 | 5.45 | 2.37 | 13.28 | 2.96 |
| FeO | 6.85 | 2.25 | 8.67 | 1.39 | 5.39 | 0.59 | 8.59 | 1.41 |
| CaO | 5.41 | 2.91 | 7.37 | 1.55 | 3.73 | 0.61 | 7.16 | 3.12 |
| Na2O | 0.75 | 0.35 | 2.15 | 0.60 | 2.08 | 0.33 | 1.74 | 0.47 |
| K2O | 6.67 | 2.23 | 4.72 | 0.97 | 8.61 | 0.28 | 5.71 | 1.74 |
| Cr2O3 | 0.03 | 0.04 | 0.11 | 0.06 | 0.02 | 0.02 | 0.12 | 0.14 |
| MnO | 0.00 | 0.00 | 0.00 | 0.12 | 0.01 | 0.18 | 0.06 | |
| NiO | 0.07 | 0.05 | 0.02 | 0.03 | 0.02 | 0.02 | 0.04 | 0.04 |
| BaO | 0.47 | 0.21 | 0.32 | 0.18 | 0.63 | 0.07 | 0.41 | 0.16 |
| P2O5 | 0.09 | 0.10 | 0.60 | 0.30 | 2.01 | 0.33 | 1.54 | 0.69 |
| Sum | 93.90 | 94.89 | 93.30 | 94.76 | ||||
| Mg# | 81.0 | 74.1 | 64.3 | 73.4 | ||||
| n | 11 | 11 | 9 | 23 | ||||
| Expt | G137 | G139 | G139 | G139 | ||||
| P (GPa) | 3 | 3 | 3 | 3 | ||||
| T (°C) | 1100 | 1250 | 1250 | 1250 | ||||
| Duration (h) | 132 | 24 | 24 | 24 | ||||
| Melt | Residual | interstital in | Melt | |||||
| trap | s.d. | melt | s.d. | peridotite | s.d. | trap | s.d. | |
| SiO2 | 42.70 | 1.89 | 49.06 | 0.81 | 51.03 | 0.40 | 50.46 | 2.28 |
| TiO2 | 2.63 | 0.23 | 1.87 | 0.07 | 1.83 | 0.10 | 1.47 | 0.22 |
| Al2O3 | 10.01 | 0.84 | 9.60 | 0.13 | 9.80 | 0.21 | 10.82 | 0.89 |
| MgO | 14.07 | 2.27 | 14.76 | 2.02 | 11.96 | 1.76 | 13.23 | 3.71 |
| FeO | 9.11 | 1.19 | 6.80 | 0.18 | 6.83 | 0.38 | 5.65 | 1.09 |
| CaO | 5.82 | 1.85 | 5.88 | 1.38 | 7.51 | 1.32 | 6.46 | 1.17 |
| Na2O | 2.64 | 0.87 | 0.60 | 0.08 | 0.53 | 0.09 | 1.25 | 0.39 |
| K2O | 6.10 | 1.32 | 4.62 | 0.43 | 4.32 | 0.34 | 5.27 | 1.13 |
| Cr2O3 | 0.06 | 0.06 | 0.41 | 0.09 | 0.38 | 0.08 | 0.39 | 0.13 |
| MnO | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| NiO | 0.03 | 0.04 | 0.01 | 0.02 | 0.01 | 0.02 | 0.03 | 0.05 |
| BaO | 0.54 | 0.17 | 0.31 | 0.05 | 0.33 | 0.06 | 0.37 | 0.10 |
| P2O5 | 0.56 | 0.70 | 0.57 | 0.06 | 0.54 | 0.08 | 0.48 | 0.15 |
| Sum | 94.27 | 94.50 | 95.07 | 95.88 | ||||
| Mg# | 73.4 | 79.5 | 75.7 | 80.7 | ||||
| n | 14 | 10 | 12 | 11 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Foley, S.F.; Pertermann, M. Dynamic Metasomatism Experiments Investigating the Interaction between Migrating Potassic Melt and Garnet Peridotite. Geosciences 2021, 11, 432. https://doi.org/10.3390/geosciences11100432
Foley SF, Pertermann M. Dynamic Metasomatism Experiments Investigating the Interaction between Migrating Potassic Melt and Garnet Peridotite. Geosciences. 2021; 11(10):432. https://doi.org/10.3390/geosciences11100432
Chicago/Turabian StyleFoley, Stephen F., and Maik Pertermann. 2021. "Dynamic Metasomatism Experiments Investigating the Interaction between Migrating Potassic Melt and Garnet Peridotite" Geosciences 11, no. 10: 432. https://doi.org/10.3390/geosciences11100432
APA StyleFoley, S. F., & Pertermann, M. (2021). Dynamic Metasomatism Experiments Investigating the Interaction between Migrating Potassic Melt and Garnet Peridotite. Geosciences, 11(10), 432. https://doi.org/10.3390/geosciences11100432






