Abstract
Alkali-activated binders, more commonly referred to as “geopolymers”, have recently emerged as a good alternative to traditional binders (e.g., lime and cement) for soil stabilisation. Geopolymers utilise the alkaline activation of industrial waste to form cementitious products within treated soils, leading to enhanced soil properties. This paper aims to present a review of the use of fly-ash-based geopolymers for soil stabilisation, with special reference to clay. The paper provides some detailed chemical and geotechnical cross-disciplinary knowledge, which advances fly-ash geopolymer as an eco-friendly binder. The paper covers the salient features of the geopolymer treatment process, including key affecting factors, envisioned applications, potential advantages and major limitations. The paper also discusses the main challenges standing against the wide recognition of this technique for soil stabilisation by industry. The paper finally concludes that fly-ash geopolymer can be used successfully as a binder for soil stabilisation; however, further research is still needed to realise the full potential of this promising technique in the future.
1. Introduction
The nature of bonds that connect the soil particles defines the stability of soil structure and its corresponding ability to withstand the applied forces. The bonding of soil particles can be either inherent (e.g., ionic, covalent and hydrogen) or developed naturally at the soil inter-particle connections by precipitation of calcite, silica, alumina and iron oxides [1]. However, when such bonds are weak or non-existent, certain soil types (e.g., soft and expansive clays) show problematic behaviours, especially in the presence of water, due to the soil expansion tendency or excessive consolidation, even under small-superimposed loads [2]. Problematic soils are challenging for geotechnical engineers, and require some sort of ground improvement before construction, usually employed via mechanical means (e.g., soil reinforcement, densification and dewatering) or chemical treatment (e.g., stabilisation by the addition of binders) [3]. Chemical stabilisation is by far the most commonly used ground improvement technique, and seeks to achieve an enhancement in the soil stability’s characteristics by increasing soil strength and durability, while decreasing soil compressibility [2,4,5,6,7]. It involves the addition of a chemical binder to the soil to develop artificial cementation bonds between the soil particles. Ordinary Portland cement (OPC) is arguably the most popular soil stabilisation binder; however, the manufacturing industry of OPC is associated with about 8–10% of the global artificial CO2 emission per year [8,9,10]. Such an industry is also marked with other environmental limitations, including the sourcing of raw materials and the overuse of coal quarries as a source of energy [8,10,11]. Therefore, in the context of global warming, the utilisation of OPC in bulk applications such as soil stabilisation has an additional detrimental impact on the environment. This has encouraged the development of new alternative binders that possess smaller environmental footprints, without compromising the soil stabilisation capabilities.
Recently, the recycling of waste materials of aluminosilicate industrial by-products (e.g., fly-ash, slag and glass waste) through alkali-activated types of cement constitutes an attractive option for fully eliminating the use of OPC for soil stabilisation [12,13,14,15,16,17]. The alkali activation process of industrial aluminosilicate residues within treated soils usually includes the dissolution of silica and alumina using a basic (high pH) liquid phase, and the formation of artificial cementitious products, which enhances the soil properties. This process, where low calcium aluminosilicate materials are used, is broadly referred to as geopolymerisation, which differs from the hydration reaction of OPC—soil mixtures described widely in the literature (e.g., [11,18]). The use of geopolymer as a binder for soil stabilisation is relatively new, and many years of research lie ahead to commercialise its use and to meet society’s needs. In this paper, the use of a fly-ash-based geopolymer for soil stabilisation, with special attention paid to clay soils, is reviewed and discussed in some detail. The paper specifically discusses the mechanism and key factors influencing the effectiveness of this binding technique, and presents and addresses the applications, feasibility, limitations and possible future trends of this technology for soil improvement.
2. Fly-Ash Geopolymers for Soil Stabilisation
There is an increasing number of studies in the literature that seek to investigate the use of geopolymers for soil stabilisation. In general, two main geopolymer models promote soil stabilisation, depending on the nature of the cementitious components. These include the N-A-S-H model and the (N, C)-A-S-H model. Each is named for the resulting geopolymerisation products [8], as described below. Table 1 provides a summary of some selected key research on the use of the abovementioned two geopolymer models for treating different soil types, highlighting the variety of the mixtures used in each model, and the corresponding geomechanical properties of treated soils.

Table 1.
Summary of research into the use of geopolymers for soil stabilisation.
The N-A-S-H Geopolymer Model is usually derived from low-calcium, high-aluminosilicate materials such as fly-ash or metakaolin, which, when activated with a sodium-based activator, form a product with bonding characteristics of a three-dimensional framework [19,20]. This geopolymer model is represented by the chemical structure Sodium Aluminate Silicate Hydrate (N-A-S-H), and requires aggressive synthesis conditions, such as a high-alkali media and elevated temperature [8]. The literature presented in Table 1 includes some studies that utilised this model for soil stabilisation [12,13,14,15,21,22,23,24,25,26,27,28].
The (N, C)-A-S-H Geopolymer Model is formed as a result of the alkaline activation of a certain amount of aluminosilicate material (e.g., fly-ash) mixed with a calcium-based component (e.g., slag). When the activated fly-ash and slag undergo geopolymerisation, the (N, C)-A-S-H model is produced by combining two gels, i.e., Sodium Aluminate Silicate Hydrate (N-A-S-H) and Calcium Aluminate Silicate Hydrate (C-A-S-H). Contrary to the N-A-S-H model, the (N, C)-A-S-H model does not require aggressive synthesis conditions, such as a high-alkali media and elevated temperature, as it works under ambient temperature and low-alkali conditions, bringing its in-situ implementation for soil stabilisation to the economical boundary. However, the reaction products precipitated in the (N, C)-A-S-H model are complex [29], and limited studies are available in the literature on the use of this model for soil stabilisation (see Table 1 [16,17,22,30,31,32]). It should be noted that some researchers classify the formation produced from the C-A-S-H gel as a standalone geopolymer model; however, the C-A-S-H gel formation is a calcium-based product, similar to that of the OPC hydration reaction, and thus cannot be classified as geopolymer.
The following sections present detailed discussions on the use of both the N-A-S-H and (N, C)-A-S-H geopolymer models for soil stabilisation. However, given the comparative lack of literature on the (N, C)-A-S-H model compared to the N-A-S-H model, more information is provided on the N-A-S-H model, and the need for further research on the (N, C)-A-S-H model is highlighted.
2.1. The N-A-S-H Geopolymer Model for Soil Stabilisation
The N-A-S-H geopolymer model for soil stabilisation usually comprises a mix of low calcium aluminosilicate material, classified as either calcined or uncalcined, and a sodium-based activator. Examples of the calcined aluminosilicate materials include fly-ash (i.e., waste material produced from coal-fired steam power plants), metakaolin (i.e., kaolinite clay calcined at a high temperature of 500–750 °C), construction residues and pozzolanic wastes, whereas the non-calcined aluminosilicate materials include feldspars and rock-type aluminosilicate minerals [8,33]. The calcined aluminosilicate materials promote higher mechanical properties for geopolymers, such as strength and stiffness, due to their thermal treatment history during formation, compared with the uncalcined variety. This thermal phenomenon affects the structural coordination of the aluminium and oxygen ions, and transfers any stable crystalline material into an amorphous one with increased reactivity for alkaline activation [34]. The most common calcined aluminosilicate materials used for geopolymers are metakaolin and coal fly-ash [8,35]. Fly-ash is considered to be more economical and sustainable, given the fact that it has already undergone thermal treatment, and it is also widely available as a waste material, compared to metakaolin, which is produced specifically for binder applications [36]; thus, fly-ash has been the focus of research into alternative binders for soil stabilisation (e.g., [12,13,14,15,16,37]).
American Society for Testing and Materials (ASTM C618) categorises fly-ash into two categories; namely, Class (C) and Class (F) [38]. Class (F) fly-ash has less than 10% calcium oxide (CaO), while Class (C) fly-ash contains more than 20% calcium [39]. Low calcium fly-ash has increased silica and alumina contents that may promote geopolymer production with superior mechanical properties [36]; thus, Class (F) fly-ash is preferable for geopolymer production, compared to Class (C) fly-ash [8,36,40,41].
The hydroxide and silicate solution used as an alkali activator in the production of geopolymers may be either potassium-based or sodium-based [33,42,43]. Alkali hydroxides are generally produced from chloride salts, where melting carbonate salts with silica and warm water produces alkali silicates in the form of a viscous, sticky solution, known as water glass [9]. Potassium-based solutions have been found to have some cost limitations [44]; therefore, sodium-based solutions are most commonly suggested as an alkali activator for geopolymers in soil stabilisation [12,13,14,15,16,21,23,24,25,26]. It should be noted that cheaper alternative solutions, for example, non-silicate solutions, such as weak acid salts (Na2CO3), strong acid salts (Na2SO4) or aluminates (M2O·nAl2O3), have been found to give unfavourable reaction behaviour, with poorly reacted and porous geopolymer products [44].
When fly-ash comes into contact with an alkaline sodium-based activator added to the soil, the geopolymerisation reaction (i.e., N-A-S-H model) begins immediately. Geopolymerisation involves the following multi-reaction steps (see Figure 1) [45]: dissolving of the aluminosilicate mineral by the effect of hydroxyl ions (OH−) presented in the high-concentrated alkaline medium; diffusion (or migration) of the dissolved Al and Si complexes; condensation (or polycondensation) with residual alkali cation (e.g., sodium Na+, gel formation, gel reorganisation and gel evolution) with curing time; and crystallisation to hardening. The geopolymerisation reaction can be presented by Equations (1) and (2) [43]. Based on these equations, geopolymer can be described as a highly connected three-dimensional chain network bond of sialite (Si-O-Al), consisting of tetrahedral silica (SiO4) and alumina (AlO4) joined by a shared oxygen (O2).
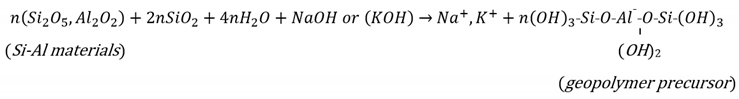
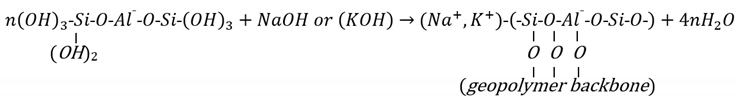
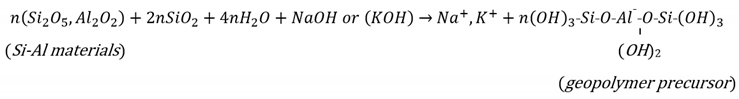
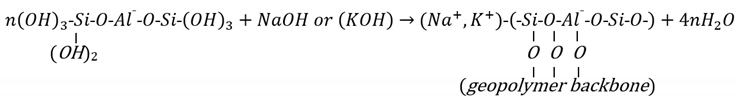
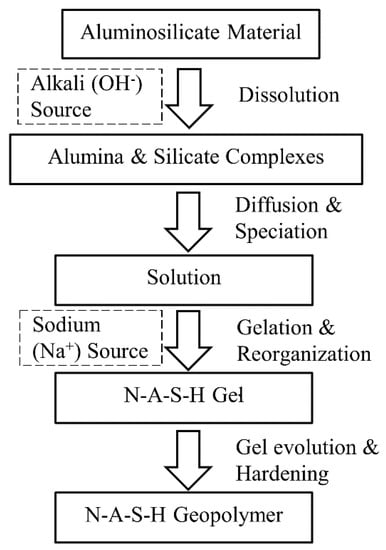
Figure 1.
Schematic diagram of the Sodium Aluminate Silicate Hydrate (N-A-S-H) geopolymer model.
The general empirical formula of the geopolymer gel can then be displayed as follows [8,33,34]:
where M is an alkali cation, such as potassium (K+) or sodium (Na+), that balances the negative charge localised on one or more of the bridging oxygens in each aluminate tetrahedron; n is the degree of polymerisation; and z is the Si/Al molar ratio, ranging from 1 to 15, or from 1 to 32 [23]. Depending on the value of z, the geopolymer can take one of several basic systems [34], as z > 3 produces a rubbery geopolymer of a linear linked two-dimensional network, and z < 3 produces a brittle cementitious product of a crossed linked three-dimensional network suitable for soil stabilisation [13,14,23,28,46,47].
It should be noted that the N-A-S-H geopolymer model products differ from the OPC reaction products, i.e., Calcium Silicate Hydrates (C-S-H) and Calcium Aluminate Hydrates (C-A-H), as their formations are calcium-based, not sodium-based [11,18]. The N-A-S-H geopolymer products bind the soil particles together after treatment, altering both the structure and mineralogy (i.e., mineral bonding) of the host soil and resulting in an overall improvement of soil behaviour [23]. However, the soil response upon mixing with geopolymer may not be limited to the main stabilisation reaction (i.e., precipitation of cementitious product). Most chemical binders involve a prior modification process (i.e., water diffusion, flocculation and agglomeration of colloid particles) when mixed with soil [48,49]. For traditional binders such as OPC, the modification process is caused by the cation exchange between the binder positive ions and those of similar charge presented in the water between the clay particles [18]. Because the ion type and concentration are essential factors that might directly affect the cation exchange process in soils, the levels of soil enhancement achieved at the pre-modification phase might be varied, compared with those of OPC. To date, no extensive studies have been found in the literature directly investigating this pre-modification phase within geopolymer-treated clay soils, and most studies are limited to investigating the main geopolymerisation reaction. Therefore, the focus of this paper will be on stabilization, not modification.
2.1.1. Engineering Properties of the N-A-S-H Geopolymer-Treated Soils
From the studies presented in Table 1, the improvement of geopolymer-stabilised soils through alkaline activation can be outlined in four primary engineering characteristics: microstructure, strength, stiffness and durability. In this section, these characteristics are discussed in some detail.
Microstructure
Several studies have been performed in the literature using mineralogical X-Ray Diffraction (XRD) and Scanning Electron Microscopy (SEM) techniques to provide insights into the microstructure of geopolymer-treated soils. This involves tracking the cementitious growth induced by the geopolymer to explain the enhancement mechanism of the treated soils at the micro-scale. In general, the fly-ash-based geopolymer is reported to densify the fabric of treated soils, in a manner similar to lime- or OPC-treated soils [11]. For example, it was found that the homogeneity of clay fabric was improved with the addition of the fly-ash-based geopolymer, resulting in more closely linked clay particles and fewer voids. Such enhancement is mainly attributed to the precipitation of artificial cementation products, and the corresponding development of bonds within soil particles during curing [21,28]. This finding was supported by Phummiphan et al. [15], who observed, through SEM analysis conducted on marginal lateritic soil, etched holes on the surface of partially reacted fly-ash particles within treated soil. It was claimed that these holes were formed by leaching silica and alumina from the surface of the activated fly-ash. The partially-reacted fly-ash particles and cementitious products within the treated soil are believed to serve as nucleation sites that bond clay plates into clusters, thus modifying the structure of the soil and enhancing its mechanical response [32].
Strength
The unconfined compressive strength (UCS) is the most commonly used test to characterise the strength of geopolymer-treated soils. In general, the literature indicates that the mixing of geopolymer within the soil matrix promotes enhanced unconfined compressive strength [15,21,28,32]. The addition of geopolymer increases the treated soil peak strength, and decreases the corresponding axial failure strain, both of which contribute towards a stiff response similar to that of OPC-treated soils [25,31]. As described previously, the strength enhancement of geopolymer-treated soils is attributed to the development of artificial bonding, induced by the geopolymerisation of soil particles. However, considering the literature summary presented in Table 1, a high variability in the soil’s peak UCS can be observed from one study to another, and it seems that the effect of geopolymer on UCS values varies according to the quantity and type of the reaction products of the treatment process. Curing conditions (i.e., temperature and time) also affect the volume of reaction products and the level of enhancement in strength of the treated soils [13]. Clay mineralogy also plays a major role in the level of strength enhancement using geopolymer [32].
Although the UCS testing is commonly used, due to its simplicity, to characterise the strength performance of stabilised soils, triaxial testing is recommended for the further investigation of the response of geopolymer-treated soils to the monotonic loading. Triaxial testing simulates the effects of confining pressure and pore water pressure, which are considered critical in strength evaluation. Limited literature exists on the triaxial shearing behaviour of geopolymer-treated soils, considering drained/undrained conditions, and most existing studies are mainly dedicated to treating sands rather than clays. Among the limited research, Rios et al. [25] carried out anisotropic triaxial drained tests on silty sand treated with geopolymer comprised of fly-ash (Class F) and a chemical activator based on a 50% weight ratio of sodium silicate to sodium hydroxide. A spectrum of deviator stress was documented for different stabilised mixtures. All tested mixtures showed a general stress-strain behaviour, with a brittle response similar to those of OPC-treated soils, in which high peak deviator stresses for stabilised specimens were recorded at low strains, followed by strain-softening. The volumetric strain changes indicated an initial contraction tendency before dilation. In another study, Rios et al. [12] reported the behaviour of the strength failure envelope in the q-p’ space (q = deviatory stress and p’= mean effective stress) for untreated and geopolymer-treated sand specimens. The test results showed a higher failure envelope compared to untreated soil, and increased strength parameters (i.e., friction angle, ϕ, and cohesion, c) comparable to those of lime- and OPC-treated soils. These results were related to the effects of cementation induced by geopolymer. Similar enhancement in undrained soil’s properties were also confirmed by Corrêa-Silva et al. [50] and Abdullah et al. [32] for geopolymer-treated clays. It should be noted that triaxial testing, reported in the literature, for geopolymer-treated clays is only limited to static loading conditions, and similar tests for cyclic loading conditions are yet to be investigated. Although the reliability of the cyclic triaxial test for geotechnical applications is excellent, the test has received less attention by the geopolymer–soil research, as it is relatively complex and time-consuming.
Durability Characteristics
Moisture and temperature are examples of the field-related conditions that are usually considered for a durable performance of binder–soil mixtures [5]. The destructive durability testing techniques of wetting–drying by ASTM D559-03 [51] and freezing–thawing by ASTM D560-15 [52] are usually employed to evaluate the durability characteristics of stabilised soils [18,38]. In both tests, specimens with specific dimensions are cured for seven days, and then subjected to 12 successive cycles of temperature and moisture changes (48 h each in duration), simulating potential extreme field conditions, with changes in the measured volume and residual strength.
Durability studies focusing on geopolymers are scarce, and mostly dedicated to wetting–drying tests. Rios et al. [12] confirmed a stable performance, i.e., low volumetric change and reasonable residual strength, for Class (F) fly-ash geopolymer-treated sand against wetting–drying durability cycles, promoting geopolymer as a viable competitor binder for soil stabilisation, compared to OPC. For clay treatment, Sargent et al. [22] similarly reported low volumetric changes in geopolymer-treated clay; however, low residual UCS performance was detected. According to Sargent et al. [22], the low performance of the activated fly-ash binder was attributed to the lack of clay content in the stabilised soil, which limited the cation exchange capacity and chemical reaction. However, the effects of clay mineralogy and plasticity were not considered, both of which are known to have a significant impact on the performance of clay soil stabilisation [38]. Additionally, Sargent et al. (2013) did not discuss the impact of Class (C) fly-ash used in their work, which is not recommended for alkali activation, as discussed previously, and is likely to negatively affect the expected performance of geopolymer-treated soils. In terms of the freezing–thawing of geopolymer-treated soils, only one study was carried out, by Abdullah et al. [31], which reported high volumetric changes and low residual strength for Class (F) fly-ash geopolymer-stabilised kaolin clay, suggesting that the treated clay exhibits a less stable performance in a freezing climate than tropical climate, and confirming the retardation of the geopolymerisation reaction at very low temperatures.
2.1.2. Factors Affecting Geopolymer Formation of the N-A-S-H Treated Soils
It is well defined in the literature that the level of cementation within treated soils and the corresponding enhancement of soil properties using traditional binders depends upon several factors, such as the binder content, type of soil and curing conditions [2]. It is therefore critically important to determine and understand the factors that may affect the level of formation of cementitious products, and the corresponding level of enhancement of the mechanical properties of geopolymer-treated soils, as presented below.
Aluminosilicate and Activator Requirements
The type of aluminosilicate material used in the geopolymer has been found in the literature to have an important impact on the strength enhancement of geopolymer-treated soils. For example, fly-ash Class (C) has a different chemical composition from Class (F), and this affects the reaction products. The alkaline activator attacks all types of fly-ash; however, Class (F) is more effective for strength improvement than Class (C), as indicated by Cristelo et al. [21]. This is attributed to the availability of a higher content of silica and alumina in Class (F) compared to Class (C), which contributes to producing a higher amount of cementations products, and thereby enhances the soil fabric.
Similar to the effect of the type of aluminosilicate, the type of activator also has a significant impact on soil enhancement. This was reported by Liu et al. [28], who promoted the use of a potassium hydroxide-based activator over sodium hydroxide, for greater compaction and higher artificial bonding on the micro-structural level within treated soils, resulting in substantial strength improvement. Although the potassium-based activator enhances soil structure more than the sodium hydroxide-based activator through the geopolymer, it has some cost limitations [44]. Therefore, the sodium-based activator is commonly suggested for geopolymer soil stabilisation [12,13,14,15,16,24,26].
Aluminosilicate and Activator Contents
The aluminosilicate content significantly affects the enhancement of geopolymer-treated soils. Cristelo et al. [13] reported an enhancement in strength of geopolymer-treated soils of up to 120% at 28 days of curing, when the fly-ash-to-soil ratio was increased from 20% to 50%. Similarly, Phetchuay et al. [16] reported an approximately linear increase in the unconfined strength of geopolymer-treated specimens with the increase of activated fly-ash content. In all cases, the increase in the mechanical properties of geopolymer-treated soils due to the increase in activated fly-ash content was attributed to the increased formation of the N-A-S-H cementitious products within the stabilised soils, due to the increase in the silicate and aluminate minerals leached by the chemical activator [15]. The increase in the amount of cementitious product increases the level of bonding between the soil particles, leading to soil enhancement [28,32]. However, no studies currently exist on determining the optimum amount of fly-ash to be used in soil stabilisation via geopolymers.
A strong dependency exists between the activator-to-fly-ash by weight ratio, and the level of enhancement in the mechanical properties of geopolymer-treated soils [14]. The increase in the activator concentration may increase the alkalinity (i.e., pH) of the reaction environment, which in turn increases the dissolution of the solid aluminosilicate oxides within the treated soils, and the corresponding amount of developed cementitious product. However, it was found in the literature that the optimum activator concentration is not necessarily the maximum value. This conclusion was highlighted by Cristelo et al. [13], who investigated the UCS performance of three mixtures activated with sodium silicate solution, with different NaOH concentrations of 10, 12.5 and 15 molal. The test results revealed that specimens treated with the 12.5 molal concentration gained greater long-term strength than those treated with 15 molal. This was attributed to the ratio of silica-to-sodium oxides within the activator, which was approximately one, and caused the activator solution to be unstable (crystallise), which negatively affected the geopolymer reaction. However, for economic reasons, it is necessary to minimise the amount of activator to achieve the required enhancement; the activator contributes to the highest cost, and considerably affects the total cost of the geopolymer [53]. Similar to the fly-ash content, no studies exist to quantify the optimum amount of activator for geopolymer-treated soils.
Curing Conditions
The strength gain of geopolymer-treated soils is dependent on some curing conditions, such as curing time and curing temperature. A significant strength increase was found to be achieved with a curing time of up to one year [13]. The increase in curing time allows the formation of more cementation products within treated soils, thereby enhancing the soil’s mechanical behaviour. This was particularly supported by Phummiphan et al. [15], who stated that the increase of curing time increases the leaching process and the number of developed etched holes, which in turn increases the amount of cementitious product [27]. The rate of strength gain of the geopolymer-treated soils is not constant, and gradually decreases with the increase of curing time. The gradual decrease in the rate of increase of strength can be attributed to the progressive slowing of the geopolymerisation reaction, due to the exhaustion of different components in the reaction environment [16].
In terms of curing temperature, the geopolymerisation reaction is usually favoured at elevated temperatures [34,54,55,56]. Van Jaarsveld et al. [57] reported that increasing the curing temperature to 70 ℃ causes an increase in the compressive strength of fly-ash-based, geopolymer-treated soils. Temuujin et al. [58] recommended a range between 40 ℃ and 100 ℃ for a curing time of 4 to 48 h, in order to get a high strength gain for geopolymer-treated soils. Sindhunata et al. [56] attributed the positive effect of elevated temperature to the increased solubility of silicate and aluminate, which increase the amount of cementitious product, which enhances the rate of strength gain. However, curing temperature is the most challenging issue for geotechnical bulk applications, such as in situ ground improvement [43,59,60,61]. This is because elevated temperatures are not possible for the in situ implementation of soil stabilisation, and treatment must thus be conducted at ambient temperature. However, when cured at ambient temperature, geopolymer-treated soils typically require a considerably longer period for strength development, compared to OPC-treated soils. According to Cristelo et al. [13], the UCS of geopolymer-treated soils reveal that only 20–40% of the one-year strength gain occurs at 28 days of curing time, compared to OPC-treated soils, wherein 40–60% of the one-year strength gain occurs after 3 months of curing time, which forms a significant drawback for geopolymer soil stabilisation. Therefore, to substitute the elevated temperature effects as a reaction accelerator, more aggressive alkaline media is usually required. This is a significant limitation in the practical utilisation of geopolymer soil stabilisation, which has led to the development of an enhanced geopolymer mixture through the (N, C)-A-S-H model, as presented below.
2.2. The (N, C)-A-S-H Geopolymer Model for Soil Stabilisation
Utilising calcium-based components as supplementary materials for geopolymer mixtures through the (N, C)-A-S-H model involves more complex reactions than the N-A-S-H model; the geopolymerisation products consist of multi gels (i.e., C-A-S-H and N-A-S-H, see Figure 2), each reacting at different rates [29,62]. Due to such complexity, limited studies have been conducted in the literature on the use of the (N, C)-A-S-H model for soil stabilisation. García-Lodeiro et al. [63] described the complicated activation stages of the (N, C)-A-S-H model using a mixture of fly-ash and OPC as a source of calcium. The model involves the following steps: (1) dissolution of aluminosilicate and calcium components in the alkaline solution, through rupturing of the Si-O-Si and Al-O-Al bonds in the fly-ash and the Ca-O and Si-O bonds in the calcium-based material (i.e., OPC); (2) formation of the N-A-S-H gel from the Na+ and silicon, as well as the aluminium dissolved species, and the C-S-H gel from the Ca+ and silicon dissolved species; (3) developing the N-A-S-H and C-S-H gels through the uptake of more Si into the system; (4) diffusion of the Al and Ca+ (not participating in the formation of the C-S-H gel) across the cementitious matrix, resulting in the formation of the (N, C)-A-S-H gel of three-dimensional structure and the C-S-A-H gel of two-dimensional structure; and (5) distortion of the Si-O-Si bonds by the polarising effect of the Ca+, and formation of the Si-O-Ca bonds, which induce more stress, and ultimately rupture. According to García-Lodeiro et al. [63], Steps (2) and (3) may occur rapidly, whereas Step (4) occurs over 28 days of curing. Step (5), the final stage of reaction, may take place over 1 year.
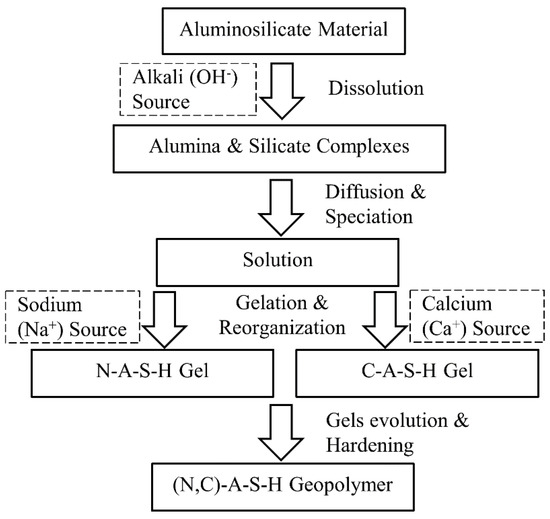
Figure 2.
Schematic diagram of the (N, C)-A-S-H geopolymer model.
Phetchuay et al. [16] suggested the use of calcium carbide residue, referred to as CCR, as a source of calcium oxide to enhance the performance of the fly-ash-based geopolymer in the stabilisation of soft marine clay (see Table 1). CCR is a waste material from acetylene gas factories, which has a high calcium hydroxide Ca(OH)2 content. The study revealed that the addition of CCR by 12% can enhance the strength of clay stabilised with fly-ash-based geopolymer by up to 1.5 times, using the same activator content. The significance of this calcium oxide addition for enhancing the mechanical properties of silty sand soils treated with a fly-ash-based geopolymer was also observed by Sargent et al. [22], wherein an increase of approximately 600% in the UCS values after 28 days of curing was observed when slag was blended, as a calcium oxide source, with fly-ash, in a 1:1 ratio. Singhi et al. [30] also indicated that a similar increasing trend in the UCS was observed when partial replacement of fly-ash by slag was used for treating the clay; UCS of 2.5 MPa at 28 days of curing was achieved when only 20% fly-ash was replaced by slag, compared to 0.2 MPa for activated fly-ash at zero slag content. This was confirmed by Abdullah et al. [31], who indicated that introducing the partial replacement of Class (F) fly-ash by slag assists, when synthesised in certain ratios, in achieving strength properties of geopolymer-stabilised clay comparable to OPC-stabilised clay. This is particularly interesting when considering the use of low activator content (i.e., 40% of fly-ash and slag added by dry weight) within the treated clay. The enhancement in the mechanical properties of clay was attributed to the complex reactions and role of C-A-S-H within slag in filling the voids within the geopolymer binder, which helps to bridge the gaps between alkaline cement products (i.e., N-A-S-H) and unreacted soil particles, and in turn contributes to the development of enhanced soil mechanical properties [29]. By comparing the N-A-S-H and the (N,C)-A-S-H geopolymer models, the latter appears to offer the best option for practical clay stabilisation; however, the utilisation of the (N,C)-A-S-H model for soil stabilisation is relatively new, and further research is required to commercialise its use and meet the society’s needs.
3. Utilisation of Geopolymer for Clay Stabilisation
Different studies have highlighted the feasibility of geopolymers in treating clay soils (e.g., [13,17,21,23,28,30,31,32]). However, the effect of clay mineralogy on geopolymer treatment is scarce. Among limited research, Abdullah et al. [17,31,32] reported the strength (unconfined, triaxial drained and undrained) behaviour and wetting–drying durability performance of nine engineered and natural clay types, treated with fly-ash-based geopolymer incorporating slag. The results showed variable strength and durability performances for tested clays treated with certain geopolymer contents. Based on the initial plasticity/activity of the clay used, it was found that the most adequate clays for successful geopolymer stabilisation, in terms of both strength and durability performance, are those of low-to-moderate plasticity/activity. For highly plastic/reactive clays, the integrity of the geopolymer matrix surrounding the agglomerates of treated clay plates was found to be compromised, resulting in significant performance degradation. The high plasticity nature of any clay might influence the workability during mixing with the geopolymer, as in the case of OPC [64], which in turn affects the homogeneity of the mixture and the uniform distribution of the binder within the soil. Moreover, the inherent nature of natural clay colloids (organic and inorganic) and their buffering capacity, i.e., absorbing, holding and releasing ions, including the (OH-) ions, was found to affect the pH response and post-treatment geopolymerisation reaction. Because the addition of the geopolymer may alter the gradation and mineralogy of the host clay, the response to treatment may likely be different from clay to clay.
Adequate binders used for soil stabilisation are generally required to cover a broad spectrum of soil types. However, not all soils are suitable for stabilisation using specific binders, due to the complexity in materials and composition [11]. Over a wide variety of soil types and the effects of extreme field conditions (i.e., temperature and moisture changes), the possibility exists for a premature failure within treated soils. For clays, this is due to both the clay content and related mineralogy/plasticity, which may affect the level of formation of artificial cementations bonds within the soil matrix, and the overall stabilisation effectiveness. For geopolymer-treated clays, Abdullah et al. [17] reported that the effectiveness of geopolymer decreases with the increase in clay content and plasticity within the host soil, due to mixing difficulties and varied pH responses. Therefore, fly-ash-based geopolymers should not be considered as a universal binder for stabilisation of all types of clay, and practical procedures are required to guide the effective use of geopolymers for the treatment of clays, similar to those exist for traditional binders.
4. Limitations to Broad Utilisation of Geopolymer for Soil Stabilisation
The utilisation of geopolymers for soil stabilisation is still unrecognised widely by the geotechnical engineering industry. This is partly due to the position of the well-defined traditional binders (e.g., OPC and lime,) and the conservative attitudes of the geotechnical community towards replacing existing products [33]. Underutilisation of geopolymers for soil stabilisation is also related to the cost limitations, caused by the need for high activator contents to allow for curing at ambient temperature. It is also due to the uncertainty of treating all soil types, as explained earlier, and the absence of practical design procedures compared to those existing for traditional binders. These limitations are discussed in some detail below.
4.1. Curing at Ambient Temperature
Although the information presented in Table 1 suggests that soil treatment with a fly-ash-based geopolymer through the N-A-S-H model of alkaline activation is competitive with OCP treatment, in terms of UCS values, the high consumption of activator content is required for geopolymer treatment. The use of high activator content in most geopolymer–soil studies is necessary to facilitate an initial dissolution and condensation reaction (i.e., geopolymerisation) within treated soils at ambient temperature curing; otherwise, an elevated temperature is required, which is considered non-applicable for in situ implementation of soil stabilisation. In Australia, fly-ash-based geopolymer, as a binder used for concrete applications cured at elevated temperature, is currently 10–15% more expensive than OPC, due to the cost of sodium silicate activator [53]; the fly-ash within the geopolymer contributes to minimising cost, as it is utilised from industrial waste. Increasing activator content for facilitating ambient curing, as in in situ soil stabilisation, increases the stabilisation cost, which in turn restricts the wider utilisation of geopolymers for soil stabilisation. This prompted the development of an enhanced geopolymer mixture suitable for ambient temperature curing, through the (N, C)-A-S-H model, as discussed previously. However, the literature regarding enhanced geopolymer mixtures for clay treatment is still scarce.
4.2. Availability of Practical Procedures
Practices vary among professional practitioners, regarding the selection of binders, testing procedures, and the evaluation criteria for what constitutes an “effective” soil stabilisation. Consequently, procedures have been established by several agencies for traditional binders, including USACE [65], CSIRO [64], Portland Cement Association, National Lime Association and Texas Department of Transportation (TxDOT), to facilitate appropriate selection of the binder type, effective dosage, and recommended mixture evaluation tests. These diverse practical procedures for traditional binders are based on extensive research; each considering specific geological landscapes and certain weather conditions. At present, there are no such practical procedures for soil stabilisation using geopolymers, which represents a significant barrier to the wider use of geopolymers for soil stabilisation by industry.
An attempt at developing practical guidelines was made by Abdullah et al. [17], in order to quantify the use of fly-ash-based geopolymer, incorporating slag, in the stabilisation of clays; while the range of clay types was not extensive, it did cover a range of plasticities and mineralogies. The developed guidelines require an initial assessment of the suitability of a certain type of clay for geopolymer treatment, using criteria based on plasticity, similar to those widely used for traditional binders. Clay soils with PI ≤ 26% were found to achieve durable mechanical performance, while clays with PI > 26% were observed to provide only temporary strength enhancement. If the clay is suitable for geopolymer treatment, the next step is performing pH testing, which provides a guide to the necessary geopolymer dosage required for an effective treatment. The basic concept of the pH criterion is to ensure that sufficient geopolymer is added to the clay to ensure an adequately high pH (12.4) that will sustain the geopolymerisation reaction, and the associated strength development, during the curing period. It should be noted that the geopolymer content derived from the pH test only satisfies the minimum requirement of geopolymer content, and does not guarantee an adequate strength and durability performance. Therefore, additional strength and durability testing for trial mixtures is required, using the pH recommended content as a starting point. While Abdullah et al. [17] have proposed a quantification method, for the practical use of an enhanced mixture of geopolymer in clay stabilisation at ambient temperature, their study only provides insights into the effects of clay mineralogy and plasticity using limited clay types. To gain economic confidence and to support the broader use of geopolymers for clay stabilisation, more research is required on additional clay types.
5. Conclusions
This paper provided a review of the available literature on using geopolymer as an eco-friendly alternative binder for soil stabilisation. It is evident from the review that fly-ash-based geopolymers can be used successfully as binders for soil stabilisation, replacing the need for OPC and allowing the recycling of industrial by-products, ultimately reducing the carbon footprint associated with traditional chemical soil stabilisation techniques. When mixed with soil, fly-ash-based geopolymer creates an artificial bonding, along with the interface or contact between soil particles, similar to that of OPC, which increases the integrity and stability of the soil.
Two geopolymer models, i.e., the (N, C)-A-S-H and N-A-S-H models, were discussed in the current review, with the former improving the strength and durability performance of treated clay at an ambient temperature. However, thus far, the available research has failed to address the broader mechanical properties of treated clay under the full range of expected loading conditions. As such, further studies are required to assess the impact of geopolymer treatment on the consolidation performance of stabilised clay, as well as the undrained shear performance under cyclic loading conditions. Further undrained triaxial testing, and detailed interpretation of the results within the critical state framework, are highly recommended.
To date, no constitutive models exist for the mechanical behaviour and structural characteristics of geopolymer-stabilised soils. Since the nature of the cementation products in geopolymer–soil mixtures is chemically different from that of those in OPC–soil mixtures, the nature of the geopolymer–soil structure and the degradation upon shearing may significantly differ. It is thus anticipated that the currently available constitutive models for the OPC–soil mixture may not be sufficient to capture the key characteristics of geopolymer-treated soils. In summary, the paucity of experimental data and the lack of experimentally-validated theoretical models need to be overcome to realise the full potential of the use of the (N, C)-A-S-H geopolymer model, as a highly promising binder, in soil stabilisation.
Despite its advantages, the use of fly-ash-based geopolymer through the (N, C)-A-S-H model for soil stabilisation at ambient temperature is still not widely recognised by the geotechnical industry. This underutilisation is generally attributed to the entrenched status of the OPC as a popular binder for soil stabilisation. Further research to confirm and demonstrate the engineering benefits of geopolymers for soil stabilisation are required from the geopolymer–soil research community in order to overcome this barrier. To gain commercial confidence and support for the broader use of geopolymers in soil stabilisation, practical procedures are required; these should consider the specimen preparation, curing conditions, testing procedures and specific mix design criteria. To provide an adequate enhancement of soil properties, the practical procedures should enable the classification of soil, concerning soil suitability for geopolymer treatment, and determining appropriate binder contents for effective treatment. To date, such practical procedures are scarce and limited to the stabilisation of clay, so future work will need to generalise similar procedures for other soil types.
Author Contributions
Conceptualization, H.H.A., M.A.S. and M.L.W.; methodology, H.H.A., M.A.S. and M.L.W.; formal analysis, H.H.A., M.A.S. and M.L.W.; investigation, H.H.A. and M.A.S.; writing—original draft preparation, H.H.A., M.A.S. and M.L.W.; writing—review and editing, H.H.A. and M.A.S.; supervision, M.A.S. and M.L.W.; project administration, H.H.A. and M.A.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Acknowledgments
The authors would like to acknowledge the Higher Committee for Education Development in Iraq for their PhD financial sponsorship provided to the first author.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Mitchell, J.K.; Soga, K. Fundamentals of Soil Behavior, 3rd ed.; John Wiley & Sons: New York, NY, USA, 2005. [Google Scholar]
- Bergado, D.; Anderson, L.; Miura, N.; Balasubramaniam, A. Soft Ground Improvement in Lowland and Other Environments; American Society of Civil Engineers: New York, NY, USA, 1996. [Google Scholar]
- Han, J. Principles and Practice of Ground Improvement; John Wiley & Sons: Hoboken, NJ, USA, 2015. [Google Scholar]
- Moseley, M.P.; Kirsch, K. Ground improvement; CRC Press: Boca Raton, FL, USA, 2004. [Google Scholar]
- Nicholson, P.G. Soil Improvement and Ground Modification Methods; Butterworth-Heinemann: Waltham, MA, USA, 2014. [Google Scholar]
- Kirsch, K. Ground improvement by deep vibratory methods. Noise Control. Eng. J. 2010, 58. [Google Scholar] [CrossRef]
- Karol, R. Chemical Grouting and Soil Stabilization, Revised and Expanded; Informa UK Limited: London, UK, 2003; Volume 12. [Google Scholar]
- Lodeiro, I.G.; Palomo, A.; Fernández-Jiménez, A. An overview of the chemistry of alkali-activated cement-based binders. In Handbook of Alkali-Activated Cements, Mortars and Concretes; Elsevier BV: Amsterdam, The Netherlands, 2015; pp. 19–47. [Google Scholar]
- Provis, J.L.; Bernal, S.A. Geopolymers and related alkali-activated materials. Annu. Rev. Mater. Res. 2014, 44, 299–327. [Google Scholar] [CrossRef]
- Tayibi, H.; Choura, M.; López, F.A.; Alguacil, F.J.; López-Delgado, A. Environmental impact and management of phosphogypsum. J. Environ. Manag. 2009, 90, 2377–2386. [Google Scholar] [CrossRef] [PubMed]
- Sargent, P. The development of alkali-activated mixtures for soil stabilisation. In Handbook of Alkali-Activated Cements, Mortars and Concretes; Elsevier BV: Amsterdam, The Netherlands, 2015; pp. 555–604. [Google Scholar]
- Rios, S.; Ramos, C.; Da Fonseca, A.V.; Cruz, N.; Rodrigues, C. Mechanical and durability properties of a soil stabilised with an alkali-activated cement. Eur. J. Environ. Civ. Eng. 2017, 23, 245–267. [Google Scholar] [CrossRef]
- Cristelo, N.; Glendinning, S.; Pinto, A.T. Deep soft soil improvement by alkaline activation. Proc. Inst. Civil Eng.-Ground Improv. 2011, 164, 73–82. [Google Scholar] [CrossRef]
- Cristelo, N.; Glendinning, S.; Fernandes, L.S.G.; Pinto, A.T. Effects of alkaline-activated fly ash and Portland cement on soft soil stabilisation. Acta Geotech. 2013, 8, 395–405. [Google Scholar] [CrossRef]
- Phummiphan, I.; Horpibulsuk, S.; Sukmak, P.; Chinkulkijniwat, A.; Arulrajah, A.; Shen, S.-L. Stabilisation of marginal lateritic soil using high calcium fly ash-based geopolymer. Road Mater. Pavement Des. 2016, 17, 877–891. [Google Scholar] [CrossRef]
- Phetchuay, C.; Horpibulsuk, S.; Arulrajah, A.; Suksiripattanapong, C.; Udomchai, A. Strength development in soft marine clay stabilized by fly ash and calcium carbide residue based geopolymer. Appl. Clay Sci. 2016, 127, 134–142. [Google Scholar] [CrossRef]
- Abdullah, H.H.; Shahin, M.A.; Walske, M.L.; Karrech, A. Systematic approach to assessing the applicability of fly-ash-based geopolymer for clay stabilization. Can. Geotech. J. 2019, 1–37. [Google Scholar] [CrossRef]
- Das, B.M. Ground Improvement. In Geotechnical Engineering Handbook; Petry, T., Ed.; J. Ross Publishing: Plantation, FL, USA, 2010; p. 9 (1ߝ37). [Google Scholar]
- Pacheco-Torgal, F.; Castro-Gomes, J.; Jalali, S. Alkali-activated binders: A review. Part 2. About materials and binders manufacture. Constr. Build. Mater. 2008, 22, 1315–1322. [Google Scholar] [CrossRef]
- Shi, C.; Fernandez-Jiménez, A.; Palomo, A. New cements for the 21st century: The pursuit of an alternative to Portland cement. Cem. Concr. Res. 2011, 41, 750–763. [Google Scholar] [CrossRef]
- Cristelo, N.; Glendinning, S.; Fernandes, L.S.G.; Pinto, A.T. Effect of calcium content on soil stabilisation with alkaline activation. Constr. Build. Mater. 2012, 29, 167–174. [Google Scholar] [CrossRef]
- Sargent, P.; Hughes, P.N.; Rouainia, M.; White, M.L. The use of alkali activated waste binders in enhancing the mechanical properties and durability of soft alluvial soils. Eng. Geol. 2013, 152, 96–108. [Google Scholar] [CrossRef]
- Zhang, M.; Guo, H.; El-Korchi, T.; Zhang, G.; Tao, M. Experimental feasibility study of geopolymer as the next-generation soil stabilizer. Constr. Build. Mater. 2013, 47, 1468–1478. [Google Scholar] [CrossRef]
- Zhang, M.; Zhao, M.; Zhang, G.; Nowak, P.; Coen, A.; Tao, M. Calcium-free geopolymer as a stabilizer for sulfate-rich soils. Appl. Clay Sci. 2015, 108, 199–207. [Google Scholar] [CrossRef]
- Rios, S.; Cristelo, N.; Da Fonseca, A.V.; Ferreira, C. Structural performance of alkali-activated soil ash versus soil cement. J. Mater. Civ. Eng. 2016, 28, 04015125. [Google Scholar] [CrossRef]
- Rios, S.; Cristelo, N.; Da Fonseca, A.V.; Ferreira, C. Stiffness behavior of soil stabilized with alkali-activated fly ash from small to large strains. Int. J. Géoméch. 2017, 17, 04016087. [Google Scholar] [CrossRef]
- Phummiphan, I.; Horpibulsuk, S.; Rachan, R.; Arulrajah, A.; Shen, S.-L.; Chindaprasirt, P. High calcium fly ash geopolymer stabilized lateritic soil and granulated blast furnace slag blends as a pavement base material. J. Hazard. Mater. 2018, 341, 257–267. [Google Scholar] [CrossRef]
- Liu, Z.; Cai, C.; Liu, F.; Fan, F. Feasibility Study of Loess Stabilization with Fly Ash–Based Geopolymer. J. Mater. Civ. Eng. 2016, 28, 04016003. [Google Scholar] [CrossRef]
- Yip, C.; Lukey, G.; Van Deventer, J. The coexistence of geopolymeric gel and calcium silicate hydrate at the early stage of alkaline activation. Cem. Concr. Res. 2005, 35, 1688–1697. [Google Scholar] [CrossRef]
- Singhi, B.; Laskar, A.I.; Ahmed, M.A. Investigation on soil–geopolymer with slag, fly ash and their blending. Arab. J. Sci. Eng. 2015, 41, 393–400. [Google Scholar] [CrossRef]
- Abdullah, H.; Shahin, M.A.; Sarker, P. Use of fly-ash geopolymer incorporating ground granulated slag for stabilisation of kaolin clay cured at ambient temperature. Geotech. Geol. Eng. 2018, 37, 721–740. [Google Scholar] [CrossRef]
- Abdullah, H.H.; Shahin, M.A.; Walske, M.L. Geo-mechanical behavior of clay soils stabilized at ambient temperature with fly-ash geopolymer-incorporated granulated slag. Soils Found. 2019, 59, 1906–1920. [Google Scholar] [CrossRef]
- Komnitsas, K.; Zaharaki, D. Geopolymerisation: A review and prospects for the minerals industry. Miner. Eng. 2007, 20, 1261–1277. [Google Scholar] [CrossRef]
- Davidovits, J. Geopolymer Chemistry and Applications, 2nd ed.; Institut Géopolymère: Saint-Quentin, France, 2008; pp. 1–585. [Google Scholar]
- Provis, J.L.; van Deventer, J.S.J. Introduction to Geopolymers. In Geopolymers: Structure, Processing, Properties and Industrial Applications; Provis, J., Deventer, J., Eds.; Woodhead Publishing: Abingdon, UK, 2009. [Google Scholar]
- Hardjito, D. Studies on Fly Ash-based Geopolymer Concrete. Ph.D. Thesis, Curtin University of Technology, Perth, Australia, August 2005. [Google Scholar]
- Rios, S.; Cristelo, N.; Miranda, T.; Araújo, N.; Oliveira, J.; Lucas, E. Increasing the reaction kinetics of alkali-activated fly ash binders for stabilisation of a silty sand pavement sub-base. Road Mater. Pavement Des. 2016, 19, 201–222. [Google Scholar] [CrossRef]
- Little, D.; Nair, S. Recommended Practice for Stabilization of Subgqrade Soils and Base Materials; National Cooperative Highway Research Program, Transportation Research Board of the National Academies: Washington, DC, USA, 2009. [Google Scholar]
- Khale, D.; Chaudhary, R. Mechanism of geopolymerization and factors influencing its development: A review. J. Mater. Sci. 2007, 42, 729–746. [Google Scholar] [CrossRef]
- Duxson, P. Geopolymer precursor design. In Geopolymers; Elsevier BV: Amsterdam, The Netherlands, 2009; pp. 37–49. [Google Scholar]
- Tenepalli, J.S.; Neeraja, D.; Sai, T.J. Properties of class F fly ash based Geopolymer mortar produced with alkaline water. J. Build. Eng. 2018, 19, 42–48. [Google Scholar] [CrossRef]
- Van Jaarsveld, J.G.S.; Van Deventer, J.S.J. Effect of the alkali metal activator on the properties of fly ash-based Geopolymers. Ind. Eng. Chem. Res. 1999, 38, 3932–3941. [Google Scholar] [CrossRef]
- Xu, H.; Van Deventer, J. The geopolymerisation of alumino-silicate minerals. Int. J. Miner. Process. 2000, 59, 247–266. [Google Scholar] [CrossRef]
- Provis, J.L. Activating solution chemistry for Geopolymers. In Geopolymers: Structure, Processing, Properties and Industrial Applications; Provis, J., Deventer, J., Eds.; Woodhead Publishing: Abingdon, UK, 2009; pp. 50–71. [Google Scholar]
- Duxson, P.; Fernandez-Jiménez, A.; Provis, J.L.; Lukey, G.C.; Palomo, A.; Van Deventer, J.S.J. Geopolymer technology: the current state of the art. J. Mater. Sci. 2006, 42, 2917–2933. [Google Scholar] [CrossRef]
- Wilkinson, A.; Haque, A.; Kodikara, J. Stabilisation of clayey soils with industrial by-products: Part B. Proc. Inst. Civil Eng.-Ground Improv. 2010, 163, 165–172. [Google Scholar] [CrossRef]
- Markou, I.; Atmatzidis, D.K. Development of a pulverized fly ash suspension grout. Geotech. Geol. Eng. 2002, 20, 123–147. [Google Scholar] [CrossRef]
- Mechanisms of soil-lime stabilization. Available online: http://onlinepubs.trb.org/onlinepubs/hrr/1965/92/92-006.pdf (accessed on 29 May 2020).
- Reactions accompanying stabilization of clay with cement. Available online: http://onlinepubs.trb.org/Onlinepubs/hrr/1963/36/36-008.pdf (accessed on 29 May 2020).
- Corrêa-Silva, M.; Araujo, N.; Cristelo, N.; Miranda, T.; Gomes, A.T.; Coelho, J. Improvement of a clayey soil with alkali activated low-calcium fly ash for transport infrastructures applications. Road Mater. Pavement Des. 2018, 20, 1912–1926. [Google Scholar] [CrossRef]
- Standard Test Methods for Wetting and Drying Compacted Soil-Cement Mixtures. Available online: https://www.astm.org/Standards/D559 (accessed on 29 May 2020).
- Standard Test Methods for Freezing and Thawing Compacted Soil-Cement Mixtures. Available online: https://www.astm.org/Standards/D560 (accessed on 29 May 2020).
- Rethinking Cement. Available online: https://bze.org.au/research/manufacturing-industrial-processes/rethinking-cement/ (accessed on 29 May 2020).
- Palomo, A.; Grutzeck, M.; Blanco, M. Alkali-activated fly ashes. Cem. Concr. Res. 1999, 29, 1323–1329. [Google Scholar] [CrossRef]
- Rovnaník, P. Effect of curing temperature on the development of hard structure of metakaolin-based geopolymer. Constr. Build. Mater. 2010, 24, 1176–1183. [Google Scholar] [CrossRef]
- Sindhunata; Van Deventer, J.S.J.; Lukey, G.C.; Xu, H. Effect of Curing Temperature and Silicate Concentration on Fly-Ash-Based Geopolymerization. Ind. Eng. Chem. Res. 2006, 45, 3559–3568. [Google Scholar] [CrossRef]
- Van Jaarsveld, J.; Van Deventer, J.; Lukey, G. The effect of composition and temperature on the properties of fly ash- and kaolinite-based geopolymers. Chem. Eng. J. 2002, 89, 63–73. [Google Scholar] [CrossRef]
- Temuujin, J.; Williams, R.; Van Riessen, A. Effect of mechanical activation of fly ash on the properties of geopolymer cured at ambient temperature. J. Mater. Process. Technol. 2009, 209, 5276–5280. [Google Scholar] [CrossRef]
- Gianoncelli, A.; Zacco, A.; Struis, R.P.W.J.; Borgese, L.; Depero, L.E.; Bontempi, E. Fly Ash Pollutants, Treatment and Recycling. In Advanced Nanostructured Materials for Environmental Remediation; Springer Science and Business Media LLC: Berlin, Germany, 2013; Volume 4, pp. 103–213. [Google Scholar]
- Deb, P.S.; Nath, P.; Sarker, P. The effects of ground granulated blast-furnace slag blending with fly ash and activator content on the workability and strength properties of geopolymer concrete cured at ambient temperature. Mater. Des. 2014, 62, 32–39. [Google Scholar] [CrossRef]
- Deb, P.S.; Nath, P.; Sarker, P. Properties of fly ash and slag blended geopolymer concrete cured at ambient temperature. In Proceedings of the Seventh International Structural Engineering and Construction Conference, Honolulu, HI, USA, 18–23 June 2013; pp. 1–6. [Google Scholar]
- Granizo, M.L.; Alonso, S.; Palomo, A.; Blanco-Varela, M.T. Alkaline Activation of Metakaolin: Effect of Calcium Hydroxide in the Products of Reaction. J. Am. Ceram. Soc. 2004, 85, 225–231. [Google Scholar] [CrossRef]
- Lodeiro, I.G.; Fernandez-Jiménez, A.; Palomo, A. Variation in hybrid cements over time. Alkaline activation of fly ash–portland cement blends. Cem. Concr. Res. 2013, 52, 112–122. [Google Scholar] [CrossRef]
- Middleton, G.F.; Schneider, L.M. Earth-wall Construction; Commonwealth Scientific and Industrial Research Organisation (Division of Building Construction and Engineering): Canberra, Australia, 1987. [Google Scholar]
- USACE. Engineering and Design: Soil Stabilization for Pavements Mobilization Construction; Engineer Manual No. 1110-3-137; Department of the US Army-Corps of Engineers: Washington, DC, USA, 1984.
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).