A New Preparation Method of Microfauna from Gypsum: Micropaleontological Association from the Middle Miocene Badenian Gypsum Deposits of Paratethys
Abstract
1. Introduction
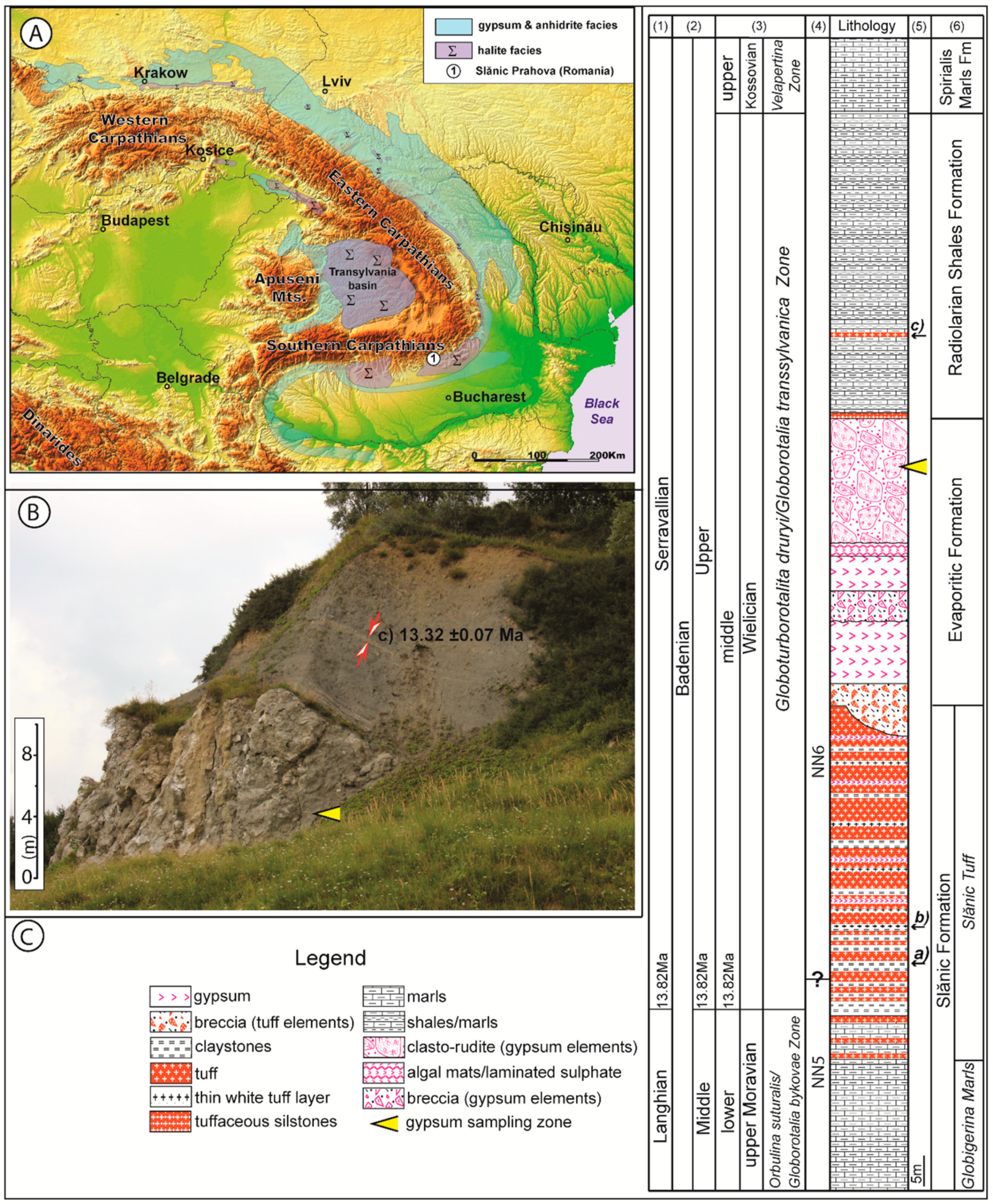
2. Geological Setting
3. Materials and Methods
3.1. Separation
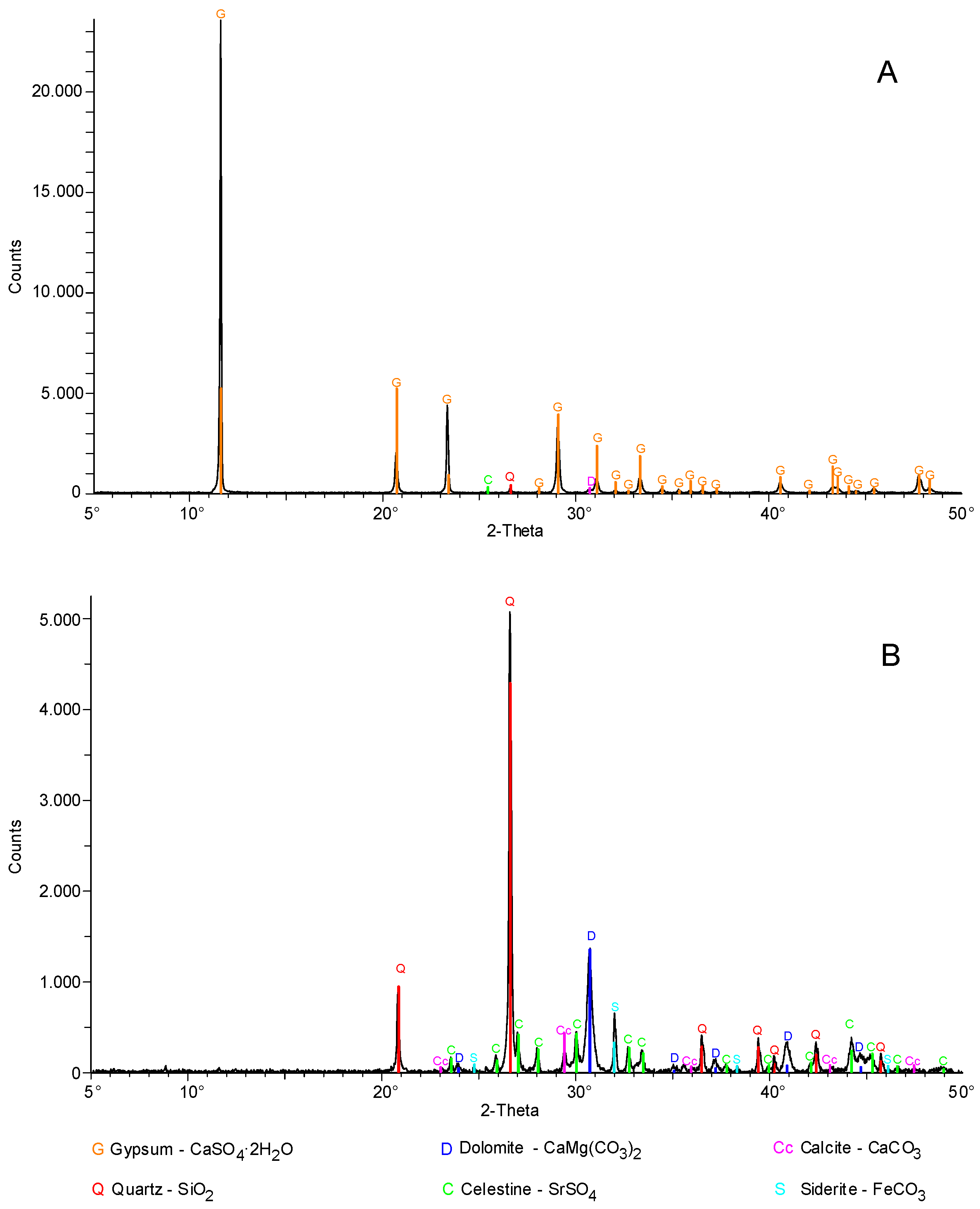
3.2. XRD Measurements
3.3. Carbonate Content
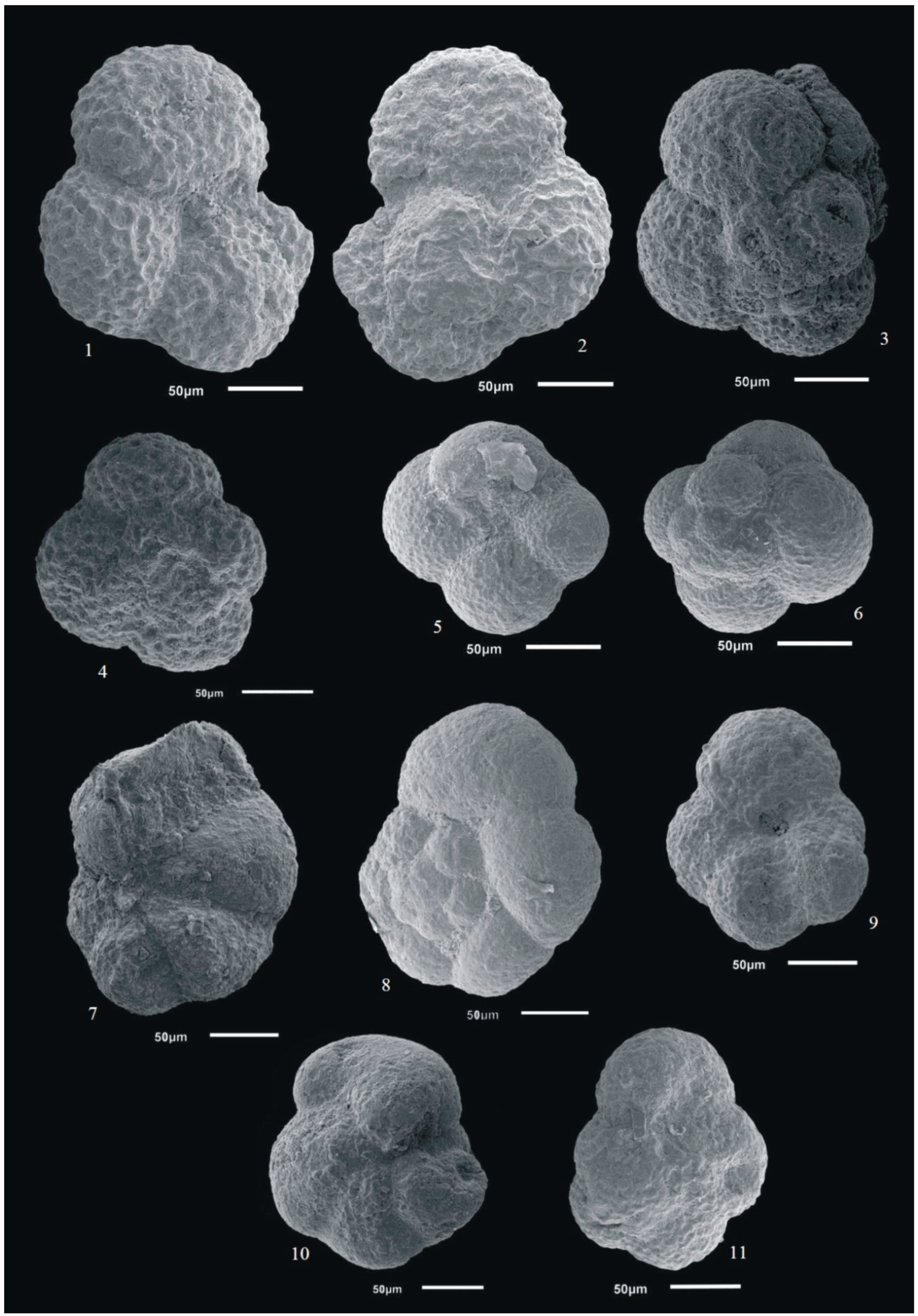
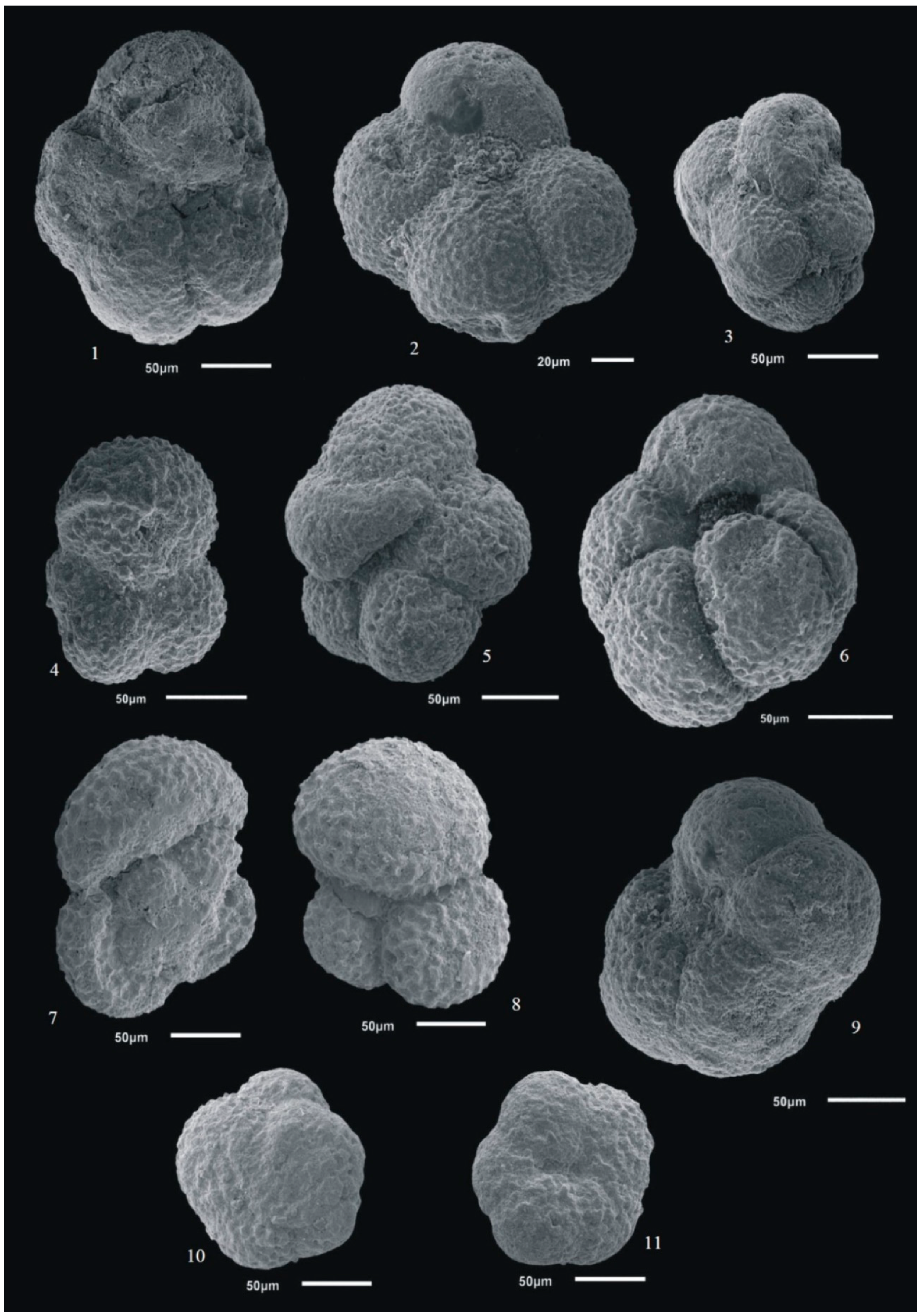
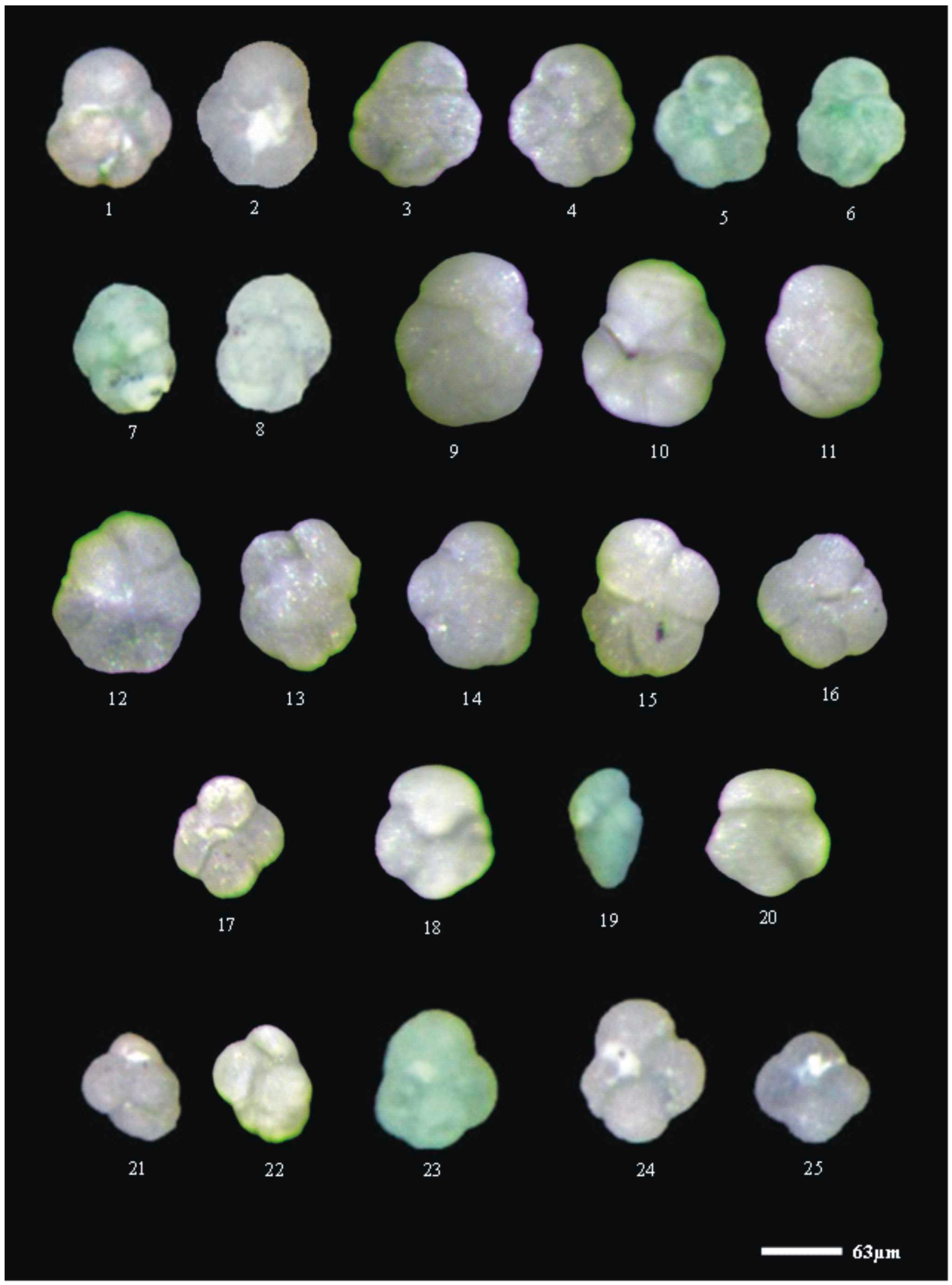
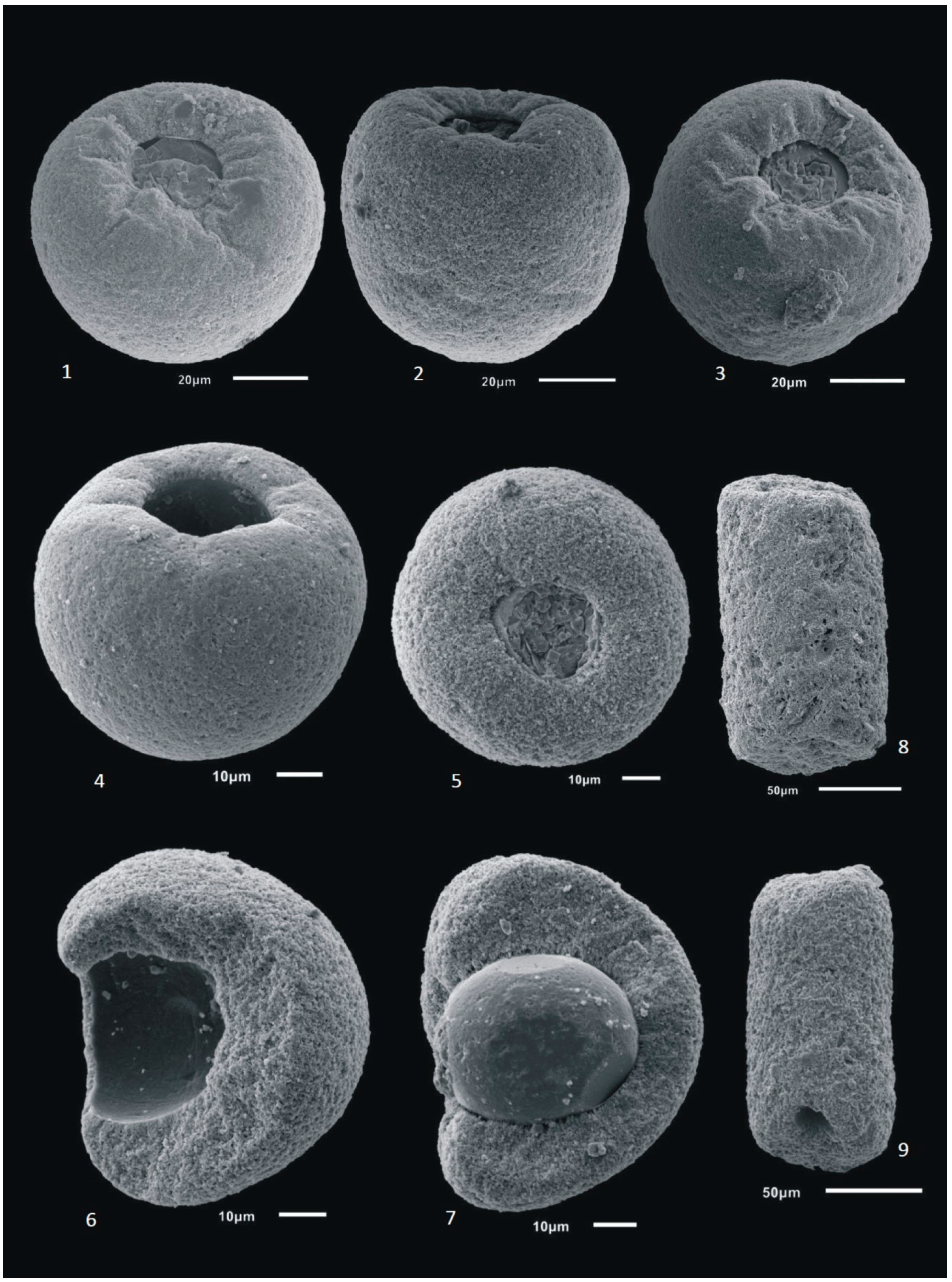
3.4. Foraminifer Images
4. Results and Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Hardie, L.A. Secular variation in seawater chemistry: An explanation for the coupled 500 secular variation in the mineralogies of marine limestones and potash evaporites over the past 600 m.y. Geology 1996, 24, 279–283. [Google Scholar] [CrossRef]
- Bojar, A.-V.; Barbu, V.; Wojtowicz, A.; Bojar, H.-P.; Hałas, S.; Deliu, O.G. Miocene Slănic Tuff, Eastern Carpathians, Romania, in the Context of Badenian Salinity Crisis. Geoscience 2018, 8, 73. [Google Scholar] [CrossRef]
- Frunzescu, D. Miocene from Southern Part of Eastern Carpathians—Sulphate Evaporitic Mega-Sequence; Editura Universităţii Petrol-Gaze din Ploieşti: Ploieşti, Romania, 2013; 344p, ISBN 978-973-719-518-0. [Google Scholar]
- Dumitrică, P.; Gheta, N.; Popescu, G.H. Date noi cu privire la biostratigrafia si corelarea Miocenului mediu din aria carpatica. Dări De Seamă Ale Sedinţelor Inst. De Geol. Şi Geofiz. 1975, 61, 65–81. [Google Scholar]
- Crihan, I.M. Middle Miocene Litho- and Biostratigraphical Study between Prahova Valley and Teleajen Valley, at Southern Part of the Slănic Syncline. Ph.D. Thesis, Babeş-Bolyai University, Cluj-Napoca, Romania, 1999. [Google Scholar]
- Popescu, G.H. Lower and Middle Miocene Agglutinated Foraminifera from the Carpathian Area. Acta Palaeontol. Rom. 1999, 2, 407–425. [Google Scholar]
- Popescu, G. Marine Middle Miocene microbiostratigraphical correlation in Central Paratethys. Dări Seamă Ale Sedinţelor Inst. Geol. Şi Geofiz. 1987, 72, 149–168. [Google Scholar]
- Popescu, G.; Crihan, I.M. Middle Miocene Globigerinas of Romania. Acta Palaeontol. Rom. 2011, 7, 291–314. [Google Scholar]
- Crihan, M. Palaeoecology of the Badenian foraminifera between the Prahova Valley and Teleajen Valley (Subcarpathians of Muntenia). In Proceedings of the XVII Congress of Carpathian-Balkan Geological Association, Bratislava, Slovakia, 1–4 September 2002. [Google Scholar]
- Stoica, M.; Andrăşanu, A.; Palcu, D.; Popa, R.G. The Miocene from Buzău Area. A Geological and Geoconservation Perspective; Editura Universităţii din Bucureşti: București, Romania, 2017; 44p, ISBN 978-606-16-0913-0. [Google Scholar]
- Melinte-Dobrinescu, M.C.; Stoica, M. Badenian calcareous nannofossil fluctuation in the Eastern Carpathians: Palaeoenvironmental significance. Acta Palaeontol. Rom. 2014, 9, 47–57. [Google Scholar]
- Popescu, G. Observaţii asupra breciei sării şi a unor masive de sare din regiunea paleogen-miocenă a judeţului Prahova. Dări Seamă Ale Inst. Geol. 1951, 32, 3–13. [Google Scholar]
- De Leeuw, A.; Bukowski, K.; Krijgsman, W.; Kuiper, K.F.; Stoica, M.; Tulbure, M. Chronology of the Badenian Salinity Crisis of the Central Paratethys. In Proceedings of the Paratethys-Mediterranean Interactions: Environmental Crises during the Neogene, Abstract volume RCMNS Colloqium, Bucharest, Romania, 27–28 September 2012. [Google Scholar]
- De Leeuw, A.; Tulbure, M.; Kuiper, K.F.; Melinte-Dobrinescu, M.C.; Stoica, M.; Krijsman, W. New 40Ar/39 Ar, magnetostratigraphic and biostratigraphic constrains on the termination on the Badenian Salinity Crisis: Indications for tectonic improvement of basin interconnectivity in Southern Europe. Glob. Planet. Chang. 2018, 169, 1–55. [Google Scholar] [CrossRef]
- Mărunţeanu, M. Litho- and biostratigraphy (calcareous nannoplankton) of the Miocene deposits from the Outer Moldavides. Geol. Carpath. 1999, 50, 313–324. [Google Scholar]
- Frunzescu, D. Miocene evaporites from the southern part of Eastern Carpathians—Sedimentological approach. Rom. J. Miner. Depos. 2010, 85, 23–30. [Google Scholar]
- Hilgen, F.; Lourens, L.J.; van Dam, J.A. The Neogene period. In The Time Geologic Scale 2012; Gradstein, F.M., Ogg, J.G., Schmitz, M.A., Ogg, G.M., Eds.; Elsevier: Amsterdam, The Netherlands, 2012; pp. 1083–1127. ISBN 978-0-444-59425-9. [Google Scholar]
- Hohenegger, J.; Ćorić, S.; Wagreich, M. Timing of the Middle Miocene Badenian Stage of the Central Paratethys. Geol. Carpath. 2014, 65, 55–66. [Google Scholar] [CrossRef]
- Martini, E. Standard Tertiary and Quaternary calcareous nannoplankton zonation. In Proceedings of the 2nd Planktonic Conference 1970, Roma, Italy, 1970. [Google Scholar]
- Liu, J. Ammonium Acetate. Encyclopedia of Reagents for Organic Synthesis; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2017; pp. 1–2. [Google Scholar]
- Barthakur, M.G. Ammonium Acetate. Synlett 2007, 9, 1475–1476. [Google Scholar] [CrossRef][Green Version]
- Falbe, J.; Regitz, M. Ammoniumacetat, Römmp Lexikon der Chemie Band 1: A-Cl; Thieme: Stuttgart, Germany, 1996; 780p. [Google Scholar]
- Williams, R.J.; Lyman, C.M. A Neutral Buffered Standard for Hydrogen Ion Work and Accurate Titrations Which Can be Prepared in One Minute. J. Am. Chem. Soc. 1932, 54, 1911–1912. [Google Scholar] [CrossRef]
- Hulett, G.A.; Allen, L.E. The solubility of Gypsum. J. Am. Chem. Soc. 1902, 24, 667–679. [Google Scholar] [CrossRef]
- Marden, J.W. Solubilities of the Sulfates of Barium, Strontium, Calcium, and Lead in Ammonium Acetate Solutions at 25° and a Criticism of the Present Methods for the Separation of These Substances by Means of Ammonium Acetate Solution. J. Am. Chem. Soc. 1916, 38, 310–316. [Google Scholar] [CrossRef]
- Popescu, G. Etudes des foraminiferes du Miocene inferieur et moyen du Nord-Ouest de la Transylvanie. Memoires 1975, 23, 121. [Google Scholar]
- Filipescu, R.; Filipescu, S. New data on the Early-Middle Badenian transition in the NW Transylvanian Basin (Romania) reveald by planktonic foraminifera assemblages. Studia Ubb Geol. 2014, 59, 39–44. [Google Scholar] [CrossRef][Green Version]
- Iaccarino, S.M.; Premoli, S.; Biolzi, M.; Foresi, L.M.; Lirer, F.; Turco, E.; Petrizzo, M.R. Practical Manual of Neogene Planktonic Foraminifera; International School of Planktonic Foraminifera, Universita degli Studi di Milano, Dipartimento di Scienze della Terra “Ardito Desio”: Milano, Italy, 2007; 143p. [Google Scholar]
- Cicha, I.; Rögl, F.; Rupp, C.; Ctyroká, J. Oligocene–Miocene foraminifera of the Central Paratethys. Abh. Der Senckenbergischen Nat. Ges. 1998, 549, 1–325. [Google Scholar]
- Wade, B.S.; Pearson, P.N.; Berggren, W.A.; Pälike, H. Review and revision of Cenozoic tropical planktonic foraminiferal biostratigraphy and calibration to the geomagnetic polarity and astronomical time scale. Earth-Sci. Rev. 2011, 104, 111–142. [Google Scholar] [CrossRef]
- Pearson, P.N.; Bridget, S.; Wade, B.S.; Huber, B.T. Taxonomy, biostratigraphy, and phylogeny of Oligocene Globigerinitidae (Dipsidripella, Globigerinita, and Tenuitella). In Atlas of Oligocene Planktonic Foraminifera; Wade, B.S., Olsson, R.K., Pearson, P.N., Huber, B.T., Berggren, W.A., Eds.; Cushman Foundation for Foraminiferal Research: Lawrence, KS, USA, 2018; Chapter 16; pp. 429–458. [Google Scholar]
- Spezzaferri, S.; Coxall, H.K.; Olsson, R.K.; Hemleben, C. Taxonomy, biostratigraphy, and phylogeny of Oligocene Globigerina, Globigerinella, and Quiltyella n. gen. In Atlas of Oligocene Planktonic Foraminifera; Wade, B.S., Olsson, R.K., Pearson, P.N., Huber, B.T., Berggren, W.A., Eds.; Cushman Foundation for Foraminiferal Research: Lawrence, KS, USA, 2018; Chapter 6; pp. 179–214. [Google Scholar]
- Violanti, C.; Donata, V.; Lozar, F.; Natalicchio, M.; Dela Pierre, F.; Bernardi, E.; Clari, P.; Cavagna, S. Stress-tolerant microfossils of a Messinian succession from the Northern Mediterranean basin (Pollenzo section, Piedmont, northwestern Italy). Boll. Della Soc. Paleontol. Ital. 2013, 52, 45–54. [Google Scholar]
- Bucur, I.I.; Nicorici, E.; Surar, N. Sarmatian Calcareous Algae from Rumania; Mucchi: Mondena, Italy, 1993; pp. 81–91. [Google Scholar]
- Barattolo, F.; Ionesi, V.; Tibulea, P. A new polyshysacean alga from the Miocene of Romania and its biomineralization. Acta Palaeontol. Pol. 2019, 64, 85–100. [Google Scholar] [CrossRef]
- Rado, G. Dasycladacee in Tortonian deposits from R.S. Romania. Anal. Univ. Buc. Geol. Geogr. 1966, 15, 61–66. [Google Scholar]
- Szczechura, J. Middle Miocene (Badenian) ostracods and green algae (Chlorophyta) from Kamienica Nawojowska, Nowy Sacz Basin (Western Carpathians, Poland). Geol. Carpath. 2006, 57, 103–122. [Google Scholar]
- Paruch-Kulczycha, J. Algae in the Sarmatian deposits from the Machow outcrop and from the boreholes Jamnica M-83 and S-119 (Carpathian Foredeep). Geol. Q. 1994, 38, 571–576. [Google Scholar]
| Foraminifera Species | Badenian | ||
|---|---|---|---|
| Moravian | Wielician | Kossovian | |
| Globorotalia bykovae Aisenstat |  | ||
| Globorotalia praescitula Blow |  | ||
| Globorotalia transsylvanica Popescu |  | ||
| Globoturborotalita aff. woodi (Jenkins) |  | ||
| Globigerinita uvula (Ehrenberg) |  | ||
| Globigerina bulloides d’Orbigny |  | ||
| Globigerina bollii Cita & Premoli-Silva | 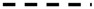 | ||
| Globigerina praebulloides Blow |  | ||
| Tenuitella angustiumbilicata (Bolii) | 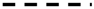 | ||
| Globigerinita aff. glutinata (Egger) |  | ||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bojar, H.-P.; Antoniade, C.; Barbu, V.; Bojar, A.-V. A New Preparation Method of Microfauna from Gypsum: Micropaleontological Association from the Middle Miocene Badenian Gypsum Deposits of Paratethys. Geosciences 2020, 10, 158. https://doi.org/10.3390/geosciences10050158
Bojar H-P, Antoniade C, Barbu V, Bojar A-V. A New Preparation Method of Microfauna from Gypsum: Micropaleontological Association from the Middle Miocene Badenian Gypsum Deposits of Paratethys. Geosciences. 2020; 10(5):158. https://doi.org/10.3390/geosciences10050158
Chicago/Turabian StyleBojar, Hans-Peter, Claudia Antoniade, Victor Barbu, and Ana-Voica Bojar. 2020. "A New Preparation Method of Microfauna from Gypsum: Micropaleontological Association from the Middle Miocene Badenian Gypsum Deposits of Paratethys" Geosciences 10, no. 5: 158. https://doi.org/10.3390/geosciences10050158
APA StyleBojar, H.-P., Antoniade, C., Barbu, V., & Bojar, A.-V. (2020). A New Preparation Method of Microfauna from Gypsum: Micropaleontological Association from the Middle Miocene Badenian Gypsum Deposits of Paratethys. Geosciences, 10(5), 158. https://doi.org/10.3390/geosciences10050158





