Foraminifer and Ostracod Occurrence in a Cool-Water Carbonate Factory of the Cape Adare (Ross Sea, Antarctica): A Key Lecture for the Climatic and Oceanographic Variations in the Last 30,000 Years
Abstract
1. Introduction
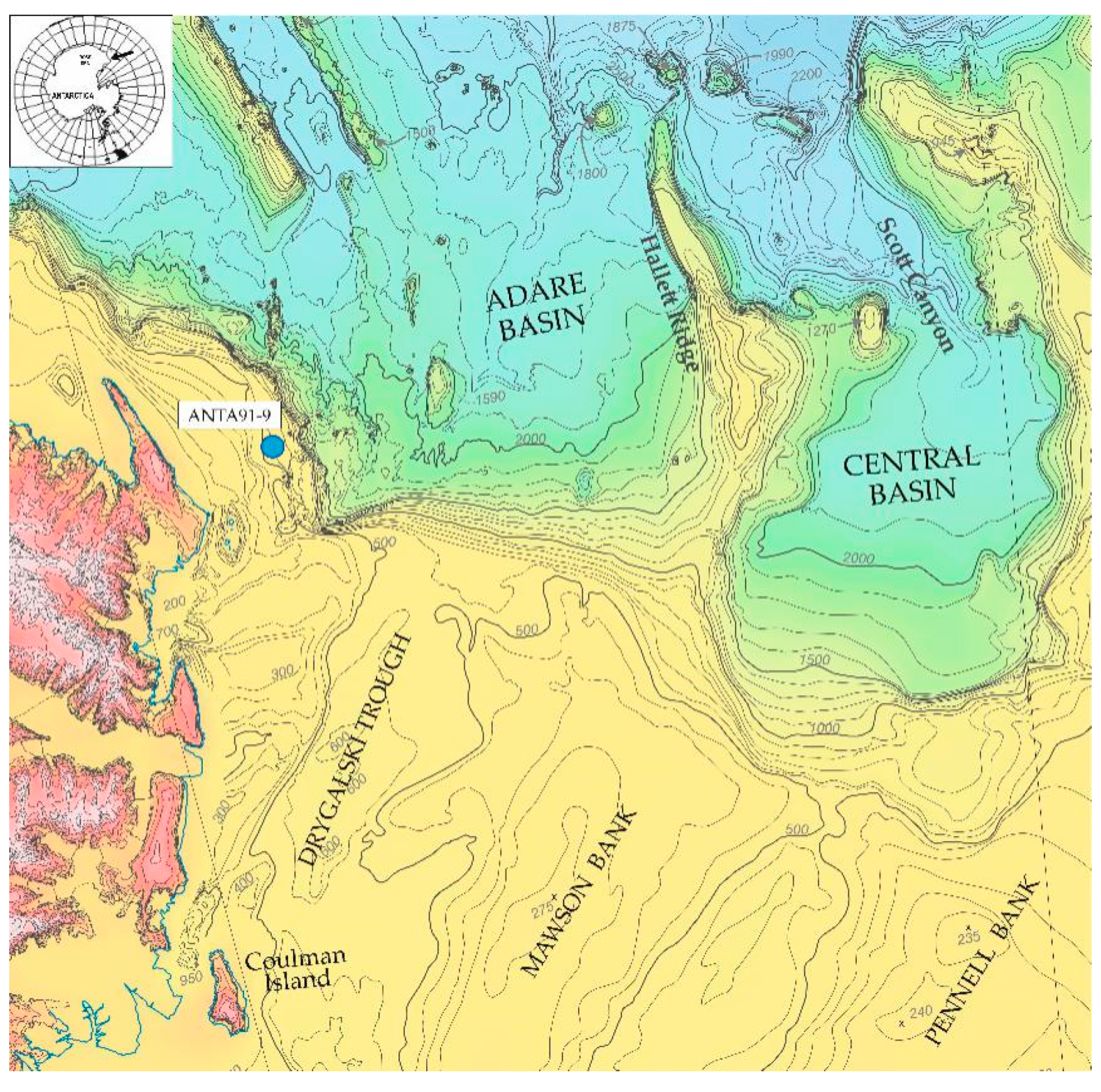
2. Materials and Methods
3. Results
3.1. ANTA91-9 Lithofacies and Biofacies Composition
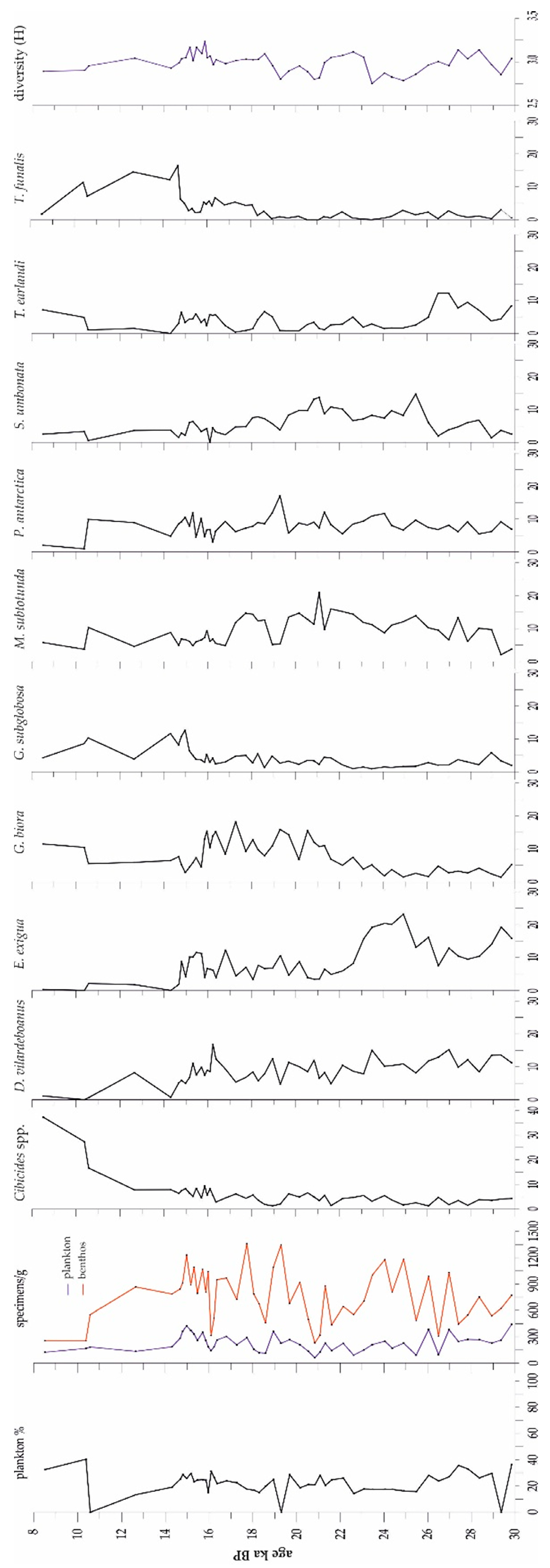
3.2. Microfossils
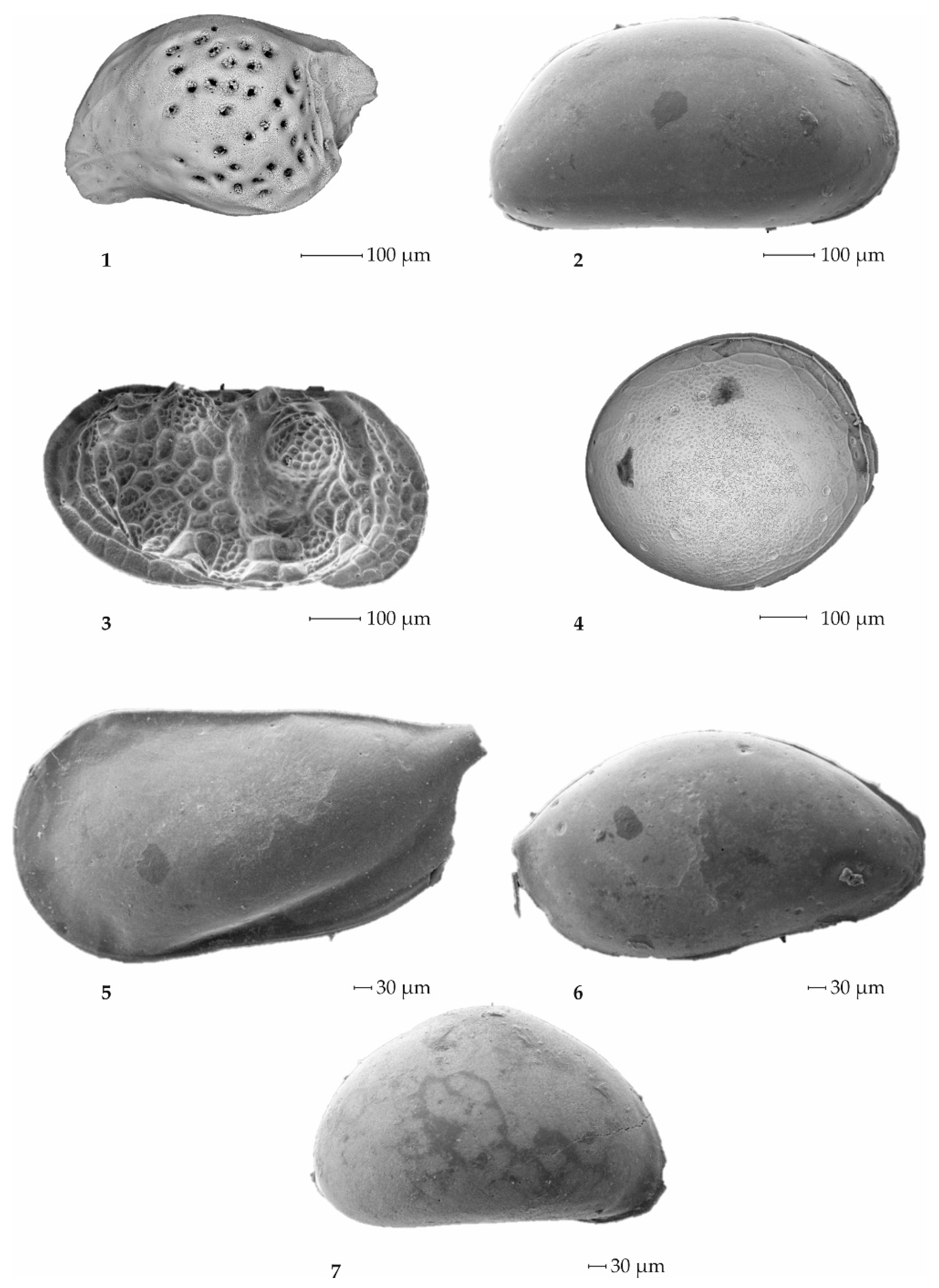
3.2.1. Foraminifers
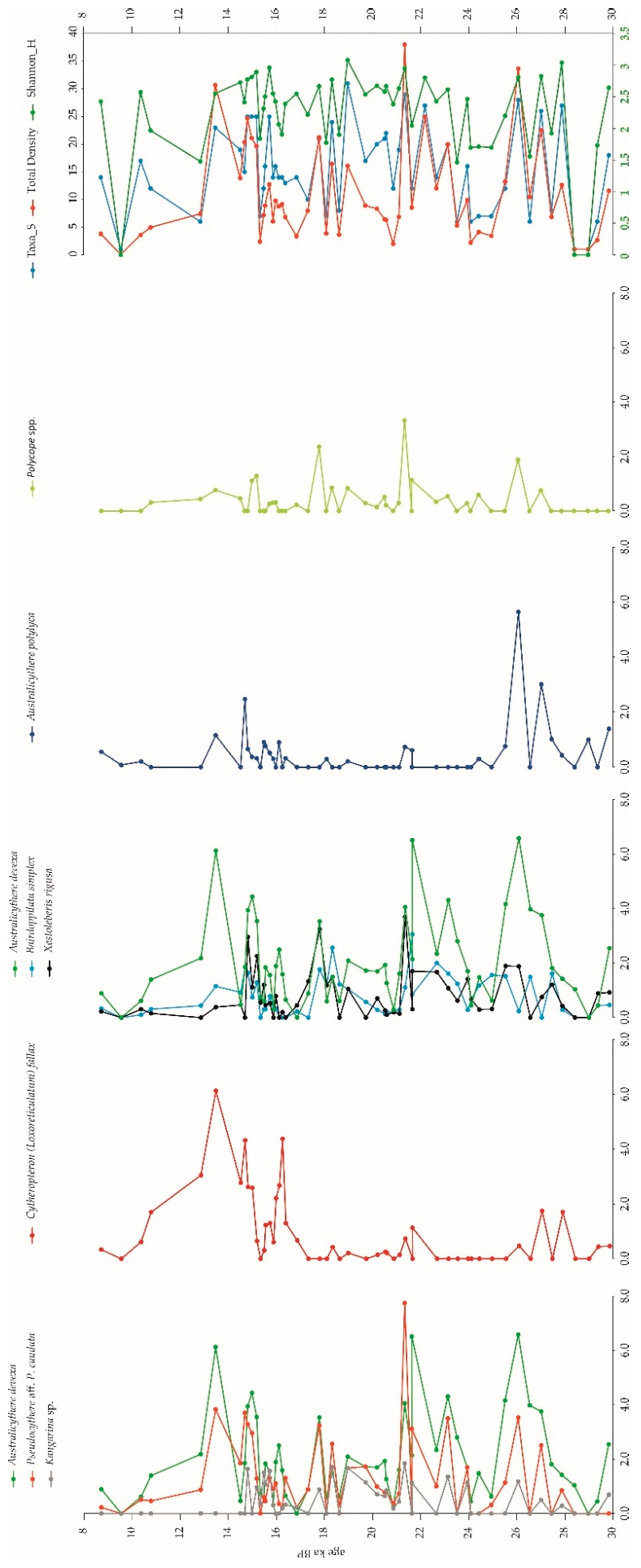
3.2.2. Ostracods
4. Discussion
4.1. The Carbonate Factory
4.2. The Microfossils
4.3. Foraminifer Associations (FAs) in the Carbonate Factory Environment
4.3.1. Epistominella Exigua FA
4.3.2. Miliolinella Subrotunda FA
4.3.3. Globocassisulina Biora FA
4.3.4. Tubinella Funalis FA
4.3.5. Cibicides spp. FA
4.4. Ostracod Associations (OAs) in the Carbonate Factory Environment
4.4.1. Pseudocythere aff. P. Caudata OA
4.4.2. Cytheropteron (Loxoreticulatum) Fallax OA
4.4.3. Bairdoppilata Simplex OA
4.4.4. Australicythere Polylyca OA
4.5. Paleoenvironmental Reconstruction Inferred by the Microorganisms
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A

References
- Kennett, J.P. Foraminiferal evidence of shallow calcium carbonate solution boundary, Ross Sea, Antarctica. Science 1966, 153, 191–193. [Google Scholar] [CrossRef] [PubMed]
- Hauck, J.; Gerdes, D.; Hillenbrand, C.-D.; Hoppema, M.; Kuhn, G.; Nehrke, G.; Völker, C.; Wolf-Gladrow, D.A. Distribution and mineralogy of carbonate sediments on Antarctic shelves. J. Mar. Syst. 2012, 90, 77–87. [Google Scholar] [CrossRef]
- Dejong, H.B.; Dunbar, R.B.; Mucciarone, D.; Koweek, D.A. Carbonate saturation state of surface waters in the Ross Sea and Southern Ocean: Controls and implications for the onset of aragonite undersaturation. Biogeosciences 2015, 12, 6881–6896. [Google Scholar] [CrossRef]
- Domack, E.W. Biogenic facies in the Antarctic glacimarine environment: Basis for a polar glacimarine summary. Palaeogeogr. Palaeoclim. Palaeoecol. 1988, 63, 357–372. [Google Scholar] [CrossRef]
- Taviani, M.; Reid, D.E.; Anderson, J.B. Skeletal and isotopic composition and paleoclimatic significance of late Pleistocene carbonates, Ross Sea, Antarctica. J. Sedim. Petrol. 1993, 63, 84–90. [Google Scholar] [CrossRef]
- Rao, C.P. Modern Carbonates: Tropical, Temperate and Polar-Introduction to Sedimentology and Geochemistry; Carbonates, Hobart: Tasmania, Australia, 1996; p. 206. [Google Scholar]
- Taviani, M.; Claps, M. Biogenic Quaternary carbonates in the CRP-1 Drillhole, Victoria Land Basin, Antarctica. Terra Antartica 1998, 5, 411–418. [Google Scholar]
- Brambati, A.; Fanzutti, G.P.; Finocchiaro, F.; Melis, R.; Pugliese, N.; Salvi, G.; Faranda, C. Some paleoecological remarks on the Ross Sea Shelf, Antarctica. In Ross Sea Ecology, Itali Antartide Expeditions (1987); Faranda, F., Guglielmo, E., Ianora, A., Eds.; Springer: Berlin/Heidelberg, Germany; New York, NY, USA, 1999; pp. 51–61. [Google Scholar]
- Melis, R.; Colizza, E.; Pizzolato, F.; Rosso, A. Late Quaternary glacial marine sequences in Ross Sea (Antarctica): Paleoenvironmental inferences throughout calcareous taxa. Geobios 2002, 35, 207–218. [Google Scholar] [CrossRef]
- Frank, T.D.; James, N.P.; Bone, Y.; Malcom, I.; Brenizer, L. Late Quaternary carbonate deposition at the bottom of the world. Sedim. Geol. 2014, 305, 1–16. [Google Scholar] [CrossRef]
- Frank, T.D.; James, N.P.; Shultis, A.I. Lack of sinsedimentary chemical alteration in polar carbonates (Ross Sea, Antarctica): Resolution of a conundrum. J. Sedim. Res. 2020, 90, 449–467. [Google Scholar] [CrossRef]
- Domack, E.W.; Taviani, M.; Rodriguez, A. Recent remoulding on a deep shelf, Ross Sea: Implications for radiocarbon dating of Antarctic marine sediments. Quat. Sci. Rev. 1999, 18, 1445–1451. [Google Scholar] [CrossRef]
- Anderson, J.B.; Conway, H.; Bart, P.J.; Witus, A.E.; Greenwood, S.L.; McKay, R.M.; Hall, B.L.; Ackert, R.P.; Licht, K.; Jakobsson, M.; et al. Ross sea paleo-ice sheet drainage and deglacial history during and since the LGM. Quat. Sci. Rev. 2014, 100, 31–54. [Google Scholar] [CrossRef]
- Halberstadt, A.R.W.; Simkins, L.M.; Greenwood, S.L.; Anderson, J.B. Past ice-sheet behaviour: Retreat scenarios and changing controls in the Ross Sea, Antarctica. Cryosphere 2016, 10, 1003–1020. [Google Scholar] [CrossRef]
- James, N.P. The cool-water carbonate depositional realm. In Cool-Water Carbonates; James, N.P., Clarke, J.A.D., Eds.; SEPM Special Publication: Tulsa, OK, USA, 1997; Volume 56, pp. 1–20. [Google Scholar]
- Webb, P.N.; Strong, C.P. Occurrence, stratigraphic distribution and palaeoecology. of quaternary foraminifera from CRP-1. Terra Antartica 1998, 5, 455–472. [Google Scholar]
- Kidwell, S.M.; Fursich, F.T.; Aigner, T. Conceptual framework for the analysis and classification of fossil concentrations. Palaios 1986, 1, 228–238. [Google Scholar] [CrossRef]
- Bart, P.J.; Coquereau, L.; Warny, S.; Majewski, W. In situ foraminifera in grounding zone diamict: A working hypothesis. Antarct. Sci. 2016, 28, 313–321. [Google Scholar] [CrossRef]
- Majewski, W.; Prothro, L.O.; Simkins, L.M.; Demianiuk, E.J.; Anderson, J.B. Foraminiferal patterns in deglacial sediment in the Western Ross Sea, Antarctica: Life near grounding lines. Paleoceanogr. Paleoclim. 2020, 35, e2019PA003716. [Google Scholar] [CrossRef]
- Majewski, W.; Anderson, J.B. Holocene foraminiferal assemblages from Firth of Tay, Antarctic Peninsula: Paleoclimate implications. Mar. Micropal. 2009, 73, 135–147. [Google Scholar] [CrossRef]
- Jacobs, S.S.; Giulivi, C.F. Thermohaline data and ocean circulation on the Ross Sea continental shelf. In Oceanography of the Ross Sea, Antarctica; Spezie, G., Manzella, G., Eds.; Springer: Milano, Italy, 1999; pp. 3–16. [Google Scholar]
- Bergamasco, A.; Defendi, V.; Budillon, G.; Spezie, G. Downslope flow observations near Cape Adare shelf-break. Antarct. Sci. 2004, 16, 199–204. [Google Scholar] [CrossRef][Green Version]
- Gordon, A.L.; Zambianchi, E.; Orsi, A.; Visbeck, M.; Giulivi, C.F.; Whitworth, T., III; Spezie, G. Energetic plumes over the western Ross Sea continental slope. Geoph. Res. Lett. 2004, 31, L21302. [Google Scholar] [CrossRef]
- Gordon, A.L.; Orsi, A.H.; Muench, R.; Huber, B.A.; Zambianchi, E.; Visbeck, M. Western Ross Sea continental slope gravity currents. Deep Sea Res. II 2009, 56, 796–817. [Google Scholar] [CrossRef]
- Muench, R.D.; Wåhlin, A.K.; Özgökmen, T.M.; Hallberg, R.; Padman, L. Impacts of bottom corrugations on a dense Antarctic outflow: NW Ross Sea. Geophys. Res. Lett. 2009, 36, L23607. [Google Scholar] [CrossRef]
- Davey, F.J. (Ed.) Ross Sea Bathymetry 1:2,000,000, Version 1.0; Institute of Geological & Nuclear Sciences Limited: Lower Hutt, New Zealand, 2004. [Google Scholar]
- Violanti, D. Taxonomy and distribution of recent benthic foraminifers from Terra Nova Bay (Ross Sea, Antarctica), Oceanographic Campaign 1987/1988. Palaeontogr. Ital. 1996, 83, 25–71. [Google Scholar]
- Murray, J.W.; Pusdey, C.J. Living (stained) and dead foraminifera from the newly ice-free Larsen Ice Shelf, Weddell Sea, Antarctica: Ecology and taphonomy. Mar. Micropaleontol. 2004, 53, 67–81. [Google Scholar] [CrossRef]
- Majewski, W. Benthic foraminiferal distribution and ecology in Admiralty Bay, King George Island, West Antarctica. Pol. Polar Res. 2005, 26, 159–214. [Google Scholar]
- Majewski, W.; Bart, P.J.; McGlannan, A.J. Foraminiferal assemblages from ice-proximal paleo-settings in the Whales Deep Basin, Eastern Ross Sea, Antarctica. Palaeogeogr. Palaeoclim. Palaeoecol. 2018, 493, 64–81. [Google Scholar] [CrossRef]
- Ellis and Messina Catalogues. Available online: http://www.micropress.org (accessed on 30 June 2020).
- Hammer, O.; Harper, D.A.T.; Ryan, P.D. PAST: Paleonto-logical statistics software package for education and data analysis. Palaeontol. Electron. 2001, 4, 1–9. [Google Scholar]
- Yasuhara, M.; Kato, M.; Ikeya, N.; Seto, K. Modern benthic ostracodes from Lützow-Holm Bay, East Antarctica: Paleoceanographic, paleobiogeographic, and evolutionary significance. Micropaleontology 2007, 53, 469–496. [Google Scholar] [CrossRef]
- Brandão, S.N.; Horne, D.V. The platycopid signal of oxygen depletion in the ocean: A critical evaluation of the evidence from modern ostracod biology, ecology and depth distribution. Palaeogeogr. Palaeoclim. Palaeoecol. 2009, 283, 126–133. [Google Scholar] [CrossRef]
- Benson, R.H. Recent Cytheracean Ostracodes from McMurdo Sound and the Ross Sea, Antarctica. Arthropoda 1964, 6, 1–36. [Google Scholar]
- Neale, J.W. An Ostracod Fauna Halley Bay, Coast Land, British Antarctic Territory; British Antarctic Survey: Cambridge, UK, 1967; Volume 68, pp. 1–50. [Google Scholar]
- Hartmann, G. Antarktische benthische Ostracoden IX. Ostracoden von der Antarktischen Halbinsel und von der Isla de los Estados (Feuerland/Argentinien). Auswertung der “Polarstern” Reise PS ANT/X/1b. Mitteilungen aus dem Hamburgischen zoologischen Museum und Institut 1993, 90, 227–237. [Google Scholar]
- Hartmann, G. Antarktische benthische Ostracoden X. Bemerkungen zur Gattung Krithe mit Beschreibung einer neuen Untergattung Austrokrithe. Mitteilungen aus dem Hamburgischen zoologischen Museum und Institut 1994, 91, 77–79. [Google Scholar]
- Hartmann, G. Antarktische und Subantarktische Podocopa (Ostracoda). In Synopses of the Antarctic Benthos; Wagele, J.W., Sieg, J., Eds.; Koeltz Scientific Books, Koenigstein: Oberreifenberg, Germany, 1997; Volume 7, pp. 1–355. [Google Scholar]
- Whatley, R.C.; Moguilevsky, A.; Ramos, M.I.F.; Coxill, D.J. Recent deep and shallow water Ostracoda from the Antarctic Peninsula and the Scotia Sea. Rev. Esp. Micropal. 1998, 30, 111–135. [Google Scholar]
- Dingle, R.V. Insular endemism in recent Southern Ocean benthic Ostracoda from Marion Island: Palaeozoogeographical and evolutionary implications. Rev. Esp. Micropal. 2002, 34, 215–233. [Google Scholar]
- Dingle, R.V. Recent subantarctic benthic ostracod faunas from the Marion and Prince Edward Islands archipelago, Southern Ocean. Rev. Esp. Micropal. 2003, 35, 119–155. [Google Scholar]
- Majewski, W.; Olempska, E. Recent ostracods from Admiralty Bay, King George Island, West Antarctica. Pol. Polar Res. 2005, 26, 13–36. [Google Scholar] [CrossRef]
- Hedges, J.I.; Stern, J.H. Carbon and nitrogen determinations of carbonate-containing solids. Limnol. Oceanogr. 1984, 29, 657–663. [Google Scholar] [CrossRef]
- Hillenbrand, C.D.; Smith, J.; Hodell, D.A.; Greaves, M.; Poole, C.R.; Kender, S.; Williams, M.; Andersen, T.J.; Jernas, P.E.; Elderfield, H.; et al. West Antarctic Ice Sheet retreat driven by Holocene warm water incursions. Nature 2017, 547, 43–48. [Google Scholar] [CrossRef]
- Reimer, P.J.; Bard, E.; Bayliss, A.; Beck, J.W.; Blackwell, P.G.; Bronk Ramsey, C.; Buck, C.E.; Edwards, R.L.; Friedrich, M.; Grootes, P.M.; et al. IntCal13 and Marine13 radiocarbon age calibration curves, 0–50,000 years cal BP. Radiocarbon 2013, 55, 1869–1887. [Google Scholar] [CrossRef]
- Blaauw, M. Methods and code for ’classical’ age-modelling of radiocarbon sequences. Quat. Geochr. 2010, 5, 512–518. [Google Scholar] [CrossRef]
- Malmgren, B.A.; Haq, B.U. Assessment of quantitative techniques in paleobiogeography. Mar. Micropal. 1982, 7, 213–236. [Google Scholar] [CrossRef]
- Majewski, W.; Wellner, J.S.; Anderson, J.B. Environmental connotations of benthic foraminiferal assemblages from coastal West Antarctica. Mar. Micropal. 2016, 124, 1–15. [Google Scholar] [CrossRef]
- Licht, K.J.; Andrews, J.T. The 14 C record of Late Pleistocene ice advance and retreat in the central Ross Sea, Antarctica. Arct. Antarct. Alp. Res. 2002, 34, 324–333. [Google Scholar] [CrossRef]
- Mosola, A.B.; Anderson, J.B. Expansion and rapid retreat of the West Antarctic Ice Sheet in eastern Ross Sea: Possible consequence of over-extended ice streams? Quat. Sci. Rev. 2006, 25, 2177–2196. [Google Scholar] [CrossRef]
- Prothro, L.O.; Majewski, W.; Yokoyama, Y.; Simkins, L.M.; Anderson, J.B.; Yamane, M.; Miyairi, Y.; Ohkouchi, N. Timing and pathways of East Antarctic Ice Sheet retreat. Quat. Sci. Rev. 2020, 230, 106166. [Google Scholar] [CrossRef]
- Martin, S.; Drucker, R.; Aster, R.; Davey, F.; Okal, E.; Scambos, T.; MacAyeal, D. Kinematic and seismic analysis of giant tabular iceberg breakup at Cape Adare, Antarctica. J. Geophys. Res. 2010, 115, B06311. [Google Scholar] [CrossRef]
- Clark, P.U.; Dyke, A.S.; Shakun, J.D.; Carlson, A.E.; Clark, J.; Wohlfarth, B.; Mitrovica, J.X.; Hostetler, S.W.; McCabe, A.M. The Last Glacial Maximum. Science 2009, 325, 710–714. [Google Scholar] [CrossRef]
- Bullivant, J.S. Ecology of the Ross Sea benthos. In The Fauna of the Ross Sea, Part 5. General Accounts, Station Lists and Benthic Ecology; Bullivant, J.S., Deaborn, J.H., Eds.; New Zealand Department of Scientific and Industrial Research Bulletin: Washington, DC, USA, 1967; Volume 176, pp. 49–77. [Google Scholar]
- Nota, D.J.G. Sediments of the Western Guiana Shelf. Ph.D. Thesis, Mededel, Landbouwhogaschool, Wageningen, The Netherlands, 1958. [Google Scholar]
- Boreen, T.D.; James, N.P. Holocene sediment dynamics on cool-water carbonate shelf: Otway, Southeaster Australia. J. Sedim. Petrol. 1993, 63, 574–588. [Google Scholar] [CrossRef]
- Passlow, V. Slope sedimentation and shelf to basin sediment transfer: A cool-water carbonate example from the Otway Margin, Southeastern Australia. In Cool-Water Carbonates; James, N.P., Clarke, A.D., Eds.; SEPM Special Publication: Tulsa, OK, USA, 1997; Volume 56, pp. 107–125. [Google Scholar]
- Bé, A.W.H. Some observations on arctic planktonic Foraminifera. Contr. Cush. Found. Foram. Res. 1960, 11, 64. [Google Scholar]
- Kohfeld, K.E.; Fairbanks, R.G.; Smith, S.L.; Walsh, I.D. Neogloboquadrina pachyderma sinistral coiling as paleoceanographic tracers in polar oceans: Evidence from Northeast Water Polynya plankton tow, sediment traps, and surface sediments. Paleoceanography 1996, 11, 679. [Google Scholar] [CrossRef]
- Greenwood, S.L.; Simkins, L.M.; Halberstadt, A.R.W.; Prothro, L.O.; Anderson, J.B. Holocene reconfiguration and readvance of the East Antarctic Ice Sheet. Nat. Commun. 2018, 9, 3176. [Google Scholar] [CrossRef]
- Prothro, L.O.; Simkins, L.M.; Majewski, W.; Anderson, J.B. Glacial retreat patterns and processes determined from integrated sedimentology and geomorphology records. Mar. Geol. 2018, 395, 104–119. [Google Scholar] [CrossRef]
- Anderson, J.B.; Brake, C.F.; Myers, N.C. Sedimentation on the Ross Sea continental shelf, Antarctica. Mar. Geol. 2018, 57, 295–333. [Google Scholar] [CrossRef]
- Simkins, L.M.; Anderson, J.B.; Greenwood, S.L.; Gonnermann, H.M.; Prothro, L.O.; Halberstadt, A.R.W.; Stearns, L.A.; Pollard, D.; DeConto, R.M. Anatomy of a meltwater drainage system beneath the ancestral East Antarctic ice sheet. Nat. Geosci. 2017, 10, 691–697. [Google Scholar] [CrossRef]
- Yokoyama, Y.; Purcell, A.; Ishiwa, T. Gauging quaternary sea level changes through scientific ocean drilling. Oceanography 2019, 32, 64–71. [Google Scholar] [CrossRef]
- Licht, K.J.; Dunbar, N.W.; Andrews, J.T.; Jennings, A.E. Distinguishing subglacial till and glacial marine diamictons in the western Ross Sea, Antarctica: Implications for a last glacial maximum grounding line. Geol. Soc. Am. Bull. 1999, 111, 91–103. [Google Scholar] [CrossRef]
- Cairns, S.D. Global Diversity of the Stylasteridae (Cnidaria: Hydrozoa: Athecatae). PLoS ONE 2011, 6, e21670. [Google Scholar] [CrossRef]
- King, T.M.; Rosenheim, B.E.; Post, A.L.; Gabris, T.; Burt, T.; Domack, E.W. Large-scale intrusion of Circumpolar Deep Water on Antarctic margin recorded by stylasterid corals. Paleoceanogr. Paleoclim. 2018, 33, 1306–1321. [Google Scholar] [CrossRef]
- Smith, W.O., Jr.; Marra, J.; Hiscock, M.R.; Barber, R.T. The seasonal cycle of phytoplankton biomass and primary productivity in the Ross Sea, Antarctica. Deep Sea Res. Part II 2000, 47, 119–140. [Google Scholar] [CrossRef]
- Jouzel, J.; Masson, V.; Cattani, O.; Falourd, S.; Stievenard, M.; Stenni, B.; Longinelli, A.; Johnsen, S.J.; Steffenssen, J.P.; Petit, J.R.; et al. A new 27 ky high resolution East Antarctic climate record. Geoph. Res. Lett. 2001, 28, 3199–3202. [Google Scholar] [CrossRef]
- Grootes, P.M.; Steig, E.J.; Stuiver, M. The oxygen isotope record from Taylor Dome, Antarctica. EOS Trans. 1994, 76, S176. [Google Scholar] [CrossRef]
- Fairbanks, R.A. 17,000-year glacio-eustatic sea level record: Influence of glacial melting rates on the Younger Dryas event and deep-ocean circulation. Nature 1989, 342, 637–642. [Google Scholar] [CrossRef]
- EPICA members. One-to-one coupling of glacial climate variability in Greenland and Antarctica. Nature 2006, 444, 195–198. [Google Scholar]
- Xiao, W.; Esper, O.; Gersonde, R. Last Glacial—Holocene climate variability in the Atlantic sector of the Southern Ocean. Quat. Sci. Rev. 2016, 135, 115–137. [Google Scholar] [CrossRef]
- Golledge, N.R.; Menviel, L.; Carter, L.; Fogwill, C.J.; England, M.H.; Levy, R.H. Antarctic contribution to meltwater pulse 1A from reduced Southern Ocean overturning. Nat. Commun. 2014, 5, 5107. [Google Scholar] [CrossRef]
- Lowry, D.P.; Golledge, N.R.; Bertler, N.A.N.; Jones, R.S.; McKay, R. Deglacial grounding-line retreat in the Ross Embayment, Antarctica, controlled by ocean and atmosphere forcing. Sci. Adv. 2019, 5, eaav8754. [Google Scholar] [CrossRef] [PubMed]
- Osterman, L.E.; Kellogg, T.B. Recent benthic foraminiferal distributions from the Ross Sea, Antarctica: Relation to ecologic and oceanographic conditions. J. For. Res. 1979, 9, 250–269. [Google Scholar] [CrossRef]
- Milam, R.W.; Anderson, J.B. Distribution and ecology of recent benthonic foraminifera of the Adelie-George V continental shelf and slope, Antarctica. Mar. Micropal. 1981, 6, 297–325. [Google Scholar] [CrossRef]
- Mackensen, A.; Grobe, H.; Kuhn, G.; Fütterer, D.K. Benthic foraminiferal assemblages from the eastern Weddell Sea between 68° and 73° S: Distribution, ecology and fossilization potential. Mar. Micropal. 1990, 16, 241–283. [Google Scholar] [CrossRef]
- Ishman, S.E.; Domack, E.W. Oceanographic controls on benthic foraminifers from the Bellingshausen margin of the Antarctic Peninsula. Mar. Micropal. 1994, 24, 119–155. [Google Scholar] [CrossRef]
- Mikhalevich, V.I. The general aspects of the distribution of Antarctic foraminifera. Micropaleontology 2004, 50, 179–194. [Google Scholar] [CrossRef]
- Rodrigues, A.R.; Cattini Maluf, J.C.; De Santis Braga, E.; Eichler, B.B. Recent benthic foraminiferal distribution and related environmental factors in Ezcurra Inlet, King George Island, Antarctica. Antarct. Sci. 2010, 22, 343–360. [Google Scholar] [CrossRef]
- Capotondi, L.; Bergami, C.; Giglio, F.; Langone, L.; Ravaioli, M. Benthic foraminifera distribution in the Ross Sea (Antarctica) and its relationship to oceanography. Boll. Della Soc. Paleontol. Ital. 2018, 57, 187–202. [Google Scholar] [CrossRef]
- McGlannan, A.J.; Bart, P.J.; Chow, J.M.; DeCesare, M. On the influence of post-LGM ice shelf loss and grounding zone sedimentation on West Antarctic ice sheet stability. Mar. Geol. 2017, 392, 151–169. [Google Scholar] [CrossRef]
- Wollenburg, J.E.; Mackenesen, A. Living benthic foraminifers from the central Arctic Ocean: Faunal composition, standing stock and diversity. Mar. Micropal. 1998, 34, 153–185. [Google Scholar] [CrossRef]
- Murray, J.W. Ecology and Applications of Benthic Foraminifera; Cambridge University Press: New York, NY, USA, 2006; p. 426. [Google Scholar]
- Capotondi, L.; Bonomo, S.; Budillon, G.; Giordano, P.; Langone, L. Living and dead benthic foraminiferal distribution in two areas of the Ross Sea (Antarctica). Rendiconti Lincei. Scienze Fisiche e Naturali 2020, 1–17. [Google Scholar] [CrossRef]
- Cai, H.M. Holocene Ostracoda and sedimentary environment implication in the core NG931-1 from the Great Wall Bay, Antarctica. Antarct. Res. 1996, 7, 141–149. [Google Scholar]
- Dingle, R.V. Ostracoda from CRP-1 and CRP-2/2A, Victoria Land Basin, Antarctica. Terra Antarctica 2000, 7, 479–492. [Google Scholar]
- Rathburn, A.E.; Pichon, J.J.; Ayress, M.A.; De Deckker, P. Microfossil and stable-isotope evidence for changes in Late Holocene palaeoproductivity and palaeoceanographic conditions in the Prydz Bay region of Antarctica. Palaeogeogr. Palaeoclim. Palaeoecol. 1997, 131, 485–510. [Google Scholar] [CrossRef]
- Whatley, R.C.; Roberts, R. Late Quaternary Ostracoda from a core in the Weddell Sea, Antarctica. Pesquisas em Geociências 1999, 26, 11–19. [Google Scholar] [CrossRef][Green Version]
- Whatley, R.C.; Staunton, M.; Kaesler, R.L.; Moguilevsky, A. The taxonomy of Recent Ostracoda from the southern part of the Strait of Magellan. Revista Española de Micropaleontología 1996, 28, 51–76. [Google Scholar]
- Whatley, R.C.; Staunton, M.; Kaesler, R.L. The depth distribution of recent marine Ostracoda from the southern Strait of Magellan. J. Micropal. 1997, 16, 121–130. [Google Scholar] [CrossRef]
- Ayress, M.A.; De Deckker, P.; Coles, G.P. A taxonomic and distributional survey of marine benthonic Ostracoda off Kerguelen and Heard Islands, South Indian Ocean. J. Micropal. 2004, 23, 15–38. [Google Scholar] [CrossRef][Green Version]
- Mackensen, A.; Fütterer, D.K.; Groge, H.; Schmield, G. Benthic foraminiferal assemblages from the eastern South Atlantic Polar Front region between 35° and 57° S: Distribution, ecology and fossilization potential. Mar. Micropal. 1993, 22, 33–69. [Google Scholar] [CrossRef]
- Gooday, A.J. Deep sea benthic foraminiferal species which exploit phytodetritus: Characteristic features and controls on distribution. Mar. Micropal. 1993, 22, 187–205. [Google Scholar] [CrossRef]
- Schmiedl, G.; Mackensen, A.; Muller, P.J. Recent benthic foraminifera from eastern South Atlantic Ocean: Dependence on food supply and water masses. Mar. Micropal. 1997, 32, 249–287. [Google Scholar] [CrossRef]
- Echols, R.J. Distribution of Foraminifera in Sediments of the Scotia Sea Area, Antarctic Waters; Reid, J.L., Ed.; Antarctic Oceanology I, Antarctic Research Series; American Geophysical Union: Washington, DC, USA, 1971; Volume 15, pp. 93–168. [Google Scholar]
- Melis, R.; Salvi, G. Late Quaternary foraminiferal assemblages from western Ross Sea (Antarctica) in relation to the main glacial and marine lithofacies. Mar. Micropal. 2009, 70, 39–53. [Google Scholar] [CrossRef]
- Majewski, W.; Pawlowski, J. Morphologic and molecular diversity of the foraminiferal genus globocassidulina in Admiralty Bay, West Antarctica. Antarct. Sci. 2010, 22, 271–281. [Google Scholar] [CrossRef]
- Ishman, S.E.; Szymcek, P. Foraminiferal distributions in the former Laresen-A ice shelf and Prince Gustav channel region, eastern Antarctic Peninsula margin: A baseline for Holocene paleoenvironmental change. Antar. Res. Ser. 2003, 79, 239–260. [Google Scholar] [CrossRef]
- Kennett, J.P. The Fauna of the Ross Sea: Part 6: Ecology and Distribution of Foraminifera; New Zealand Oceanographic Institute: Auckland, New Zealand, 1968; Volume 186, pp. 1–47.
- Berggren, W.W.; Miller, K.G. Cenozoic bathyal and abyssal calcareous benthic foraminiferal zonation. Micropaleontology 1989, 35, 308–320. [Google Scholar] [CrossRef]
- Sicinski, J.; Jazdzewski, K.; De Broyer, C.; Presier, P.; Ligowski, R.; Nonato, E.F.; Corbisier, T.N.; Petti, M.A.V.; Brito, T.A.S.; Lavrado, H.P.; et al. Admiralty bay benthos diversity—A census of a complex polar ecosystem. Deep Sea Res. II 2011, 58, 30–48. [Google Scholar] [CrossRef]
- Brandt, A.; Gooday, A.J.; Brandaõ, S.N.; Brix, S.; Brökeland, W.; Cedhagen, T.; Choudhury, M.; Cornelius, N.; Danis, B.; De Mesel, I.; et al. First insights into the biodiversity and biogeography of the Southern Ocean deep sea. Nature 2007, 447, 307–311. [Google Scholar] [CrossRef] [PubMed]
- Cronin, T.M.; Holtz, T.R., Jr.; Stein, R.; Spielhagen, R.; Fütterer, D.; Wollenberg, J. Late quaternary paleoceanography of the Eurasian Basin, Arctic Ocean. Paleoceanography 1995, 10, 259–281. [Google Scholar] [CrossRef]
- Gemery, L.; Cronin, T.M.; Poirier, R.K.; Pearce, C.; Barrientos, N.; O’Regan, M.; Johansson, C.; Koshurnikov, A.; Jakobsson, M. Central Arctic Ocean paleoceanography from~50 ka to present, on the basis of ostracode faunal assemblages from the SWERUS 2014 expedition. Clim. Past 2017, 13, 1473–1489. [Google Scholar] [CrossRef]
- Kornicker, L.S. Distribution of the ostracod suborder Cladopoda, a new species from the Bahamas. Micropaleontology 1959, 5, 69–75. [Google Scholar] [CrossRef]
- Neale, J.W. Geological history of the Cladocopina. In Applications on Ostracoda, Proceedings of the Eighth International Symposium on Ostracoda, Houston, TX, USA, 26–29 July 1982; Maddocks, R.F., Ed.; Department of Geosciences, University of Oslo: Oslo, Norway, 1983; pp. 612–626. [Google Scholar]
- Cronin, T.M.; Holtz, T.R.; Whatley, R.C. Quaternary paleoceanography of the deep Arctic Ocean based on quantitative analysis of Ostracoda. Mar. Geol. 1994, 119, 305–332. [Google Scholar] [CrossRef]
- Gersonde, R.; Zielinski, U. The reconstruction of late Quaternary Antarctic sea-ice distribution—The use of diatoms as a proxy for sea ice. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2000, 162, 263–286. [Google Scholar] [CrossRef]
- Hendry, K.R.; Rickaby, R.E.; Meredith, M.P.; Elderfield, H. Controls on stable isotope and trace metal uptake in Neogloboquadrina pachyderma (sinistral) from an Antarctic sea-ice environment. Earth Planet Sci. Lett. 2009, 278, 67–77. [Google Scholar] [CrossRef]
- Mikis, A.; Hendry, K.R.; Pike, J.; Schmidt, D.N.; Edgar, K.M.; Peck, V.; Peeters, F.J.C.; Leng, M.J.; Meredith, M.P.; Todd, C.L.; et al. Temporal variability in foraminiferal morphology and geochemistry at the West Antarctic Peninsula: A sediment trap study. Biogeosciences 2019, 16, 3267–3282. [Google Scholar] [CrossRef]
- Dieckmann, G.S.; Spindler, M.; Lange, M.A.; Ackley, S.F.; Eicken, H. Antarctic sea ice: A habitat for the foraminifer Neogloboquadrina pachyderma. J. Foram. Res. 1991, 21, 182–189. [Google Scholar] [CrossRef]
- Tinto, K.J.; Padman, L.; Siddoway, C.S.; Springer, S.R.; Fricker, H.A.; Das, I.; Tontini, F.C.; Porter, D.F.; Frearson, N.P.; Howard, S.L.; et al. Ross Ice Shelf response to climate driven by the tectonic imprint on seafloor bathymetry. Nat. Geosci. 2019, 12, 441–449. [Google Scholar] [CrossRef]
- Bonaccorsi, R.; Quaia, T.; Burckle, L.H.; Anderson, R.F.; Melis, R.; Brambati, A. C-14 age control of pre- and post-LGM events using N. pachyderma preserved in deep-sea sediments (Ross Sea, Antarctica). In Antarctica: A Keystone in a Changing World; Cooper, A.K., Raymond, C.R., Eds.; Department of Geological, Environmental, and Marine Sciences, University of Trieste: Trieste, Italy, 2007; p. 4, USGS Open-File Report 2007-1047, Extended Abstract 098. [Google Scholar] [CrossRef]
- Weber, M.E.; Clark, P.U.; Kuhn, G.; Timmermann, A.; Sprenk, D.; Gladstone, R.; Zhang, X.; Lohmann, G.; Menviel, L.; Chikamoto, M.O.; et al. Millennial-scale variability in Antarctic ice-sheet discharge during the last. Nature 2014, 510, 134–138. [Google Scholar] [CrossRef] [PubMed]
- Stenni, B.; Masson, V.; Johnsen, S.J.; Jouzel, J.; Longinelli, A.; Monnin, E.; Selmo, E. An oceanic cold reversal during the last deglaciation. Science 2001, 293, 2074–2077. [Google Scholar] [CrossRef]
- Pedro, J.B.; Bostock, H.C.; Bitz, C.M.; He, F.; Vandergoes, M.J.; Steig, E.J.; Chase, B.M.; Krause, C.E.; Rasmussen, S.O.; Markle, B.R.; et al. The spatial extent and dynamics of the Antarctic Cold Reversal. Nat. Geosci. 2015, 9, 51–55. [Google Scholar] [CrossRef]
- Salvi, C.; Salvi, G.; Stenni, B.; Brambati, A. Palaeoproductivity in the Ross Sea, Antarctica, during the last 15 kyr B.P. and its link with ice-core temperature proxies. Ann. Glaciol. 2004, 39, 445–451. [Google Scholar] [CrossRef][Green Version]
- Yokoyama, Y.; Anderson, J.B.; Yamane, Y.; Simkins, L.M.; Miyairi, Y.; Yamazaki, T.; Koizumi, M.; Suga, H.; Kusahara, K.; Prothro, L.; et al. Widespread collapse of the Ross Ice Shelf during the late Holocene. Proc. Natl. Acad. Sci. USA 2016, 113, 2354–2359. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
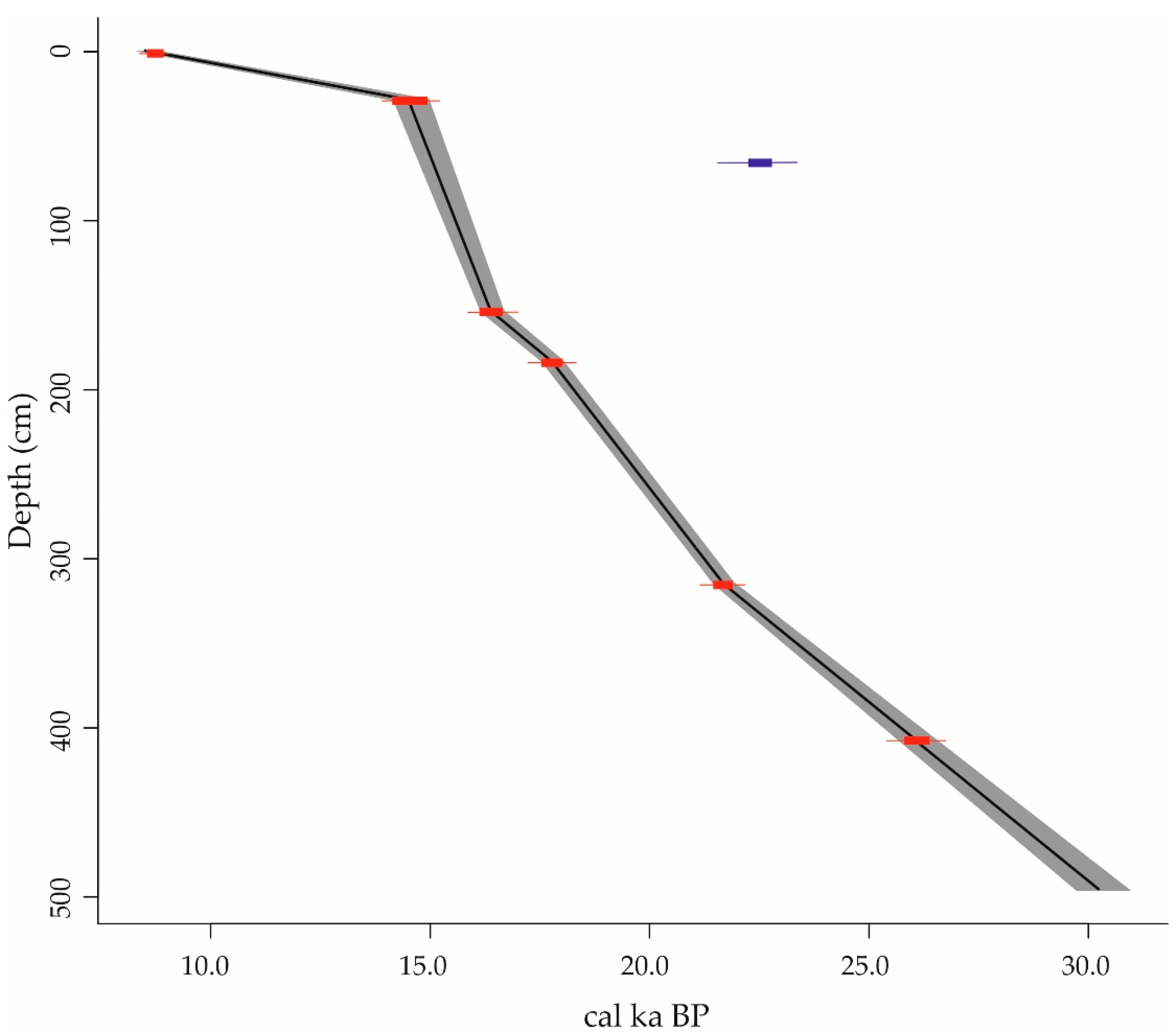


| Core Number | Sample Depth (cm) | Laboratory Code | Carbon Source | Conventional 14C Age (yr BP) | δ13C | Calibrated 14C Age (yr (BP) | Min 95% | Max 95% |
|---|---|---|---|---|---|---|---|---|
| ANTA91-9 | 0–2 | GX-23025 | foraminifers | 8900 ± 60 | −0.5 | 8736 | 8546 | 8927 |
| ANTA91-9 | 28–30 | GX-23026 | foraminifers | 13,510 ± 80 | −0.6 | 14,538 | 14,137 | 14,940 |
| ANTA91-9 | 73–75 | GX-23403 | foraminifers | 19,680 ± 160 | −3.6 | 22,439 | 21,844 | 23,019 |
| ANTA91-9 | 153–155 | GX-23027 | foraminifers | 14,690 ± 80 | 0.9 | 16,390 | 16,120 | 16,662 |
| ANTA91-9 | 183–185 | GX-23404 | foraminifers | 15,710 ± 100 | −1.3 | 17,776 | 17,531 | 18,022 |
| ANTA91-9 | 315–316 | OS-78316 | foraminifers | 19,000 ± 70 | n.d. | 21,672 | 21,451 | 21,894 |
| ANTA91-9 | 407–408 | Poz-122918 | benthic forams | 22,920 ± 150 | n.d. | 26,089 | 25,792 | 26,386 |
| E. exigua FA | M. subrotunda FA | G. biora FA | T. funalis FA | Cibicides spp. FA | |
|---|---|---|---|---|---|
| Total variance explained (%) | 31.4 | 27.8 | 10.8 | 10.5 | 6.0 |
| Astrononion spp. | −0.45 | −0.13 | −0.10 | −0.58 | −0.25 |
| Cibicides spp. | 0.11 | −0.19 | −0.25 | 0.92 | 4.28 |
| Cornuspira involvens | −0.57 | −0.08 | −0.27 | −0.04 | −0.86 |
| Discorbis vilardeboanus | 1.91 | 0.28 | 2.34 | −0.84 | −0.41 |
| Ehrembergina glabra | −0.24 | −0.34 | −0.61 | −0.86 | 0.48 |
| Entosolenia sp. | 0.13 | −0.33 | −0.05 | −0.87 | −0.02 |
| Epistominella exigua | 3.61 | −0.24 | −0.84 | −0.18 | −0.32 |
| Fissurina spp. | 0.00 | −0.26 | −0.59 | 0.96 | −0.10 |
| Globocassidulina biora | −1.39 | 1.84 | 3.41 | −0.02 | 0.46 |
| Globocassidulina subglobosa | −0.12 | −0.40 | −0.62 | 2.20 | 0.32 |
| Lagena spp. | −0.43 | −0.27 | −0.44 | −0.87 | −0.13 |
| Miliolinella subrotundata | 0.32 | 3.44 | −1.17 | 0.19 | 0.50 |
| Nonionella spp. | −0.04 | −0.54 | 0.62 | 0.87 | −1.19 |
| Oolina spp. | −0.27 | −0.16 | −0.62 | −0.70 | 0.02 |
| Patellina antarctica | 1.04 | 0.79 | 0.09 | 1.91 | −1.43 |
| Planispirinoides bucculentus | −0.76 | −0.39 | −0.56 | −0.85 | −0.07 |
| Pseudobulimina chapmani | −0.49 | −0.49 | −0.48 | −0.67 | −0.07 |
| Pullenia subcarinata | −0.51 | −0.54 | −0.46 | −0.72 | 0.12 |
| Pyrgo spp. | −0.70 | −0.61 | −0.42 | −0.94 | −0.26 |
| Rosalina globularis | 0.84 | −1.05 | 1.01 | 0.38 | 0.41 |
| Sigmoilina umbonata | 0.02 | 2.09 | −1.01 | −0.70 | −0.22 |
| Spirillina spp. | −0.83 | 0.35 | −0.14 | −0.12 | −0.81 |
| Trifarina earlandi costate | 0.91 | −0.98 | 1.07 | −1.14 | 0.87 |
| Triloculina trigonula | −0.53 | −0.05 | −0.51 | 0.44 | −0.44 |
| Trochammina multiloculata | −0.79 | −0.85 | 0.21 | −0.28 | −0.57 |
| Tubinella funalis | −0.76 | −0.87 | 0.38 | 2.52 | −0.32 |
| P. caudata OA | C. fallax OA | B. simplex OA | A. polylyca OA | |
|---|---|---|---|---|
| Total variance explained (%) | 27.5 | 22.4 | 20.7 | 7.8 |
| Aglaiella setigera | 0.08 | −0.20 | 0.16 | −0.25 |
| Antarcticythere laevior | 0.81 | −0.32 | −0.19 | 0.07 |
| Antarctiloxoconcha frigida | −0.37 | −0.37 | −0.23 | 0.13 |
| Argilloecia antarctica | −0.21 | −0.49 | 0.09 | −0.30 |
| Argilloecia spp. | −0.30 | −0.47 | 0.05 | −0.22 |
| Australicythere devexa | 2.85 | 1.59 | 3.16 | 2.40 |
| Australicythere polylyca | −0.85 | 0.19 | −0.09 | 5.41 |
| Austrocythere reticulotuberculata | −0.46 | −0.40 | −0.19 | −0.03 |
| Austrotrachyleberis antarctica | −0.44 | −0.43 | −0.21 | 0.17 |
| Aversovalva antarctica | −0.52 | −0.30 | −0.18 | −0.08 |
| Bairdia sp. | −0.43 | −0.34 | 0.23 | −0.43 |
| Bairdoppilata simplex | −0.52 | −0.05 | 4.35 | −1.71 |
| Bythoceratina dubia | −0.50 | −0.33 | −0.11 | −0.04 |
| Cativella bensoni | −0.44 | −0.30 | −0.25 | −0.02 |
| Convexochilus meridionalis | −0.47 | −0.19 | −0.25 | 0.08 |
| Cytherois sp. | −0.43 | −0.37 | −0.20 | −0.09 |
| Cytheropteron (Loxoreticulatum) fallax | −1.71 | 5.89 | −0.65 | −0.87 |
| Cytheropteron abyssorum | −0.53 | −0.21 | −0.21 | −0.10 |
| Cytheropteron antarcticum | −0.45 | −0.44 | −0.05 | −0.15 |
| Cytheropteron gaussi | −0.42 | 0.36 | −0.33 | 0.17 |
| Glacioloxoconcha suedshetlandensis | −0.22 | −0.18 | −0.40 | −0.10 |
| Hemicytherura anomala | −0.29 | −0.19 | −0.15 | 0.09 |
| Hemicytherura irregularis | 1.00 | 0.52 | −0.81 | 0.16 |
| Kangarinasp. | 2.54 | −0.73 | −1.15 | 0.03 |
| Krithe (Austrokrithe) magna | −0.55 | −0.26 | −0.11 | −0.07 |
| Macropyxis similis | −0.02 | −0.36 | −0.05 | −0.32 |
| Microcythere frigida | −0.32 | −0.35 | −0.25 | −0.05 |
| Microcythere scaphoides | −0.29 | −0.34 | −0.20 | −0.24 |
| Monoceratina sp. | −0.51 | −0.36 | −0.17 | −0.07 |
| Orthopolycope antarctica | 0.00 | −0.49 | −0.32 | −0.09 |
| Paracytheridea antarctica | −0.20 | −0.22 | −0.32 | −0.11 |
| Paracytherois sp. | 0.55 | −0.19 | −0.61 | −0.42 |
| Paradoxostoma hypselum | −0.30 | 0.08 | −0.33 | −0.35 |
| Paradoxostoma spp. | 0.05 | 0.03 | −0.49 | −0.36 |
| Patagonacythere longiducta | −0.70 | 0.13 | 1.12 | 0.13 |
| Polycope spp. | 0.71 | −0.16 | −0.31 | −0.41 |
| Pseudocythereaff.P. caudata | 4.00 | 1.25 | −1.65 | −1.07 |
| Sclerochilus (Praesclerochilus) antarcticus | −0.36 | −0.33 | −0.15 | 0.07 |
| Sclerochilus (Praesclerochilus) reniformis | 0.45 | 0.19 | −0.30 | 0.69 |
| Semicytherura costellata | −0.44 | −0.15 | −0.24 | −0.17 |
| Semicytherura sp. | −0.50 | −0.25 | −0.14 | −0.05 |
| Xestoleberis rigusa | 1.00 | −0.27 | 2.33 | −1.08 |
| Xiphichilus gracilis | −0.30 | −0.22 | −0.20 | −0.32 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Melis, R.; Salvi, G. Foraminifer and Ostracod Occurrence in a Cool-Water Carbonate Factory of the Cape Adare (Ross Sea, Antarctica): A Key Lecture for the Climatic and Oceanographic Variations in the Last 30,000 Years. Geosciences 2020, 10, 413. https://doi.org/10.3390/geosciences10100413
Melis R, Salvi G. Foraminifer and Ostracod Occurrence in a Cool-Water Carbonate Factory of the Cape Adare (Ross Sea, Antarctica): A Key Lecture for the Climatic and Oceanographic Variations in the Last 30,000 Years. Geosciences. 2020; 10(10):413. https://doi.org/10.3390/geosciences10100413
Chicago/Turabian StyleMelis, Romana, and Gianguido Salvi. 2020. "Foraminifer and Ostracod Occurrence in a Cool-Water Carbonate Factory of the Cape Adare (Ross Sea, Antarctica): A Key Lecture for the Climatic and Oceanographic Variations in the Last 30,000 Years" Geosciences 10, no. 10: 413. https://doi.org/10.3390/geosciences10100413
APA StyleMelis, R., & Salvi, G. (2020). Foraminifer and Ostracod Occurrence in a Cool-Water Carbonate Factory of the Cape Adare (Ross Sea, Antarctica): A Key Lecture for the Climatic and Oceanographic Variations in the Last 30,000 Years. Geosciences, 10(10), 413. https://doi.org/10.3390/geosciences10100413





