Evidence of Predation on Early Pleistocene Freshwater Ostracods (Umbria, Central Italy)
Abstract
1. Introduction
Microborings in Ostracods
2. Geological Setting
2.1. The Cava Nuova Section
- Interval 1 (from the base of the section up to meter 23): silty clay, massive to slightly parallel-laminated. Fossil record presents rare gastropods, ostracods, bivalves, plants (leaves and wood fragments). Thin horizons of lignite and lignite-bearing clay also occur, often marked by black or deep brown color. In the interval between meters 6 and 19, iron-enriched reddish horizons, FeCO3 (siderite) crusts and nodules are common within the clay [36]. Deposits are related to a moderately deep lacustrine environment below the wave base. Inside this interval, the range between meters 4 and 22 is the only one containing ostracod valves affected by microboring.
- Interval 2 (from 23 m to 38 m): silty clay, alternating with fine sand and/or silty beds. Sands are usually ripple-laminated. Thin lignite horizons are commonly documented within the clay, as well as less frequent iron-enriched reddish horizons. Deposits are still referrable to a lacustrine environment, showing clear shallowing-upward trend, intermittent interaction with wave motion and resedimentation processes.
- Interval 3 (from 38 m to 45 m): alternation of sand and silt, from parallel-laminated to cross-laminated. Plant remains, frequently including leaves, are common, as well as lignite horizons. Deposits are associated with a lacustrine margin subject to wave motion.
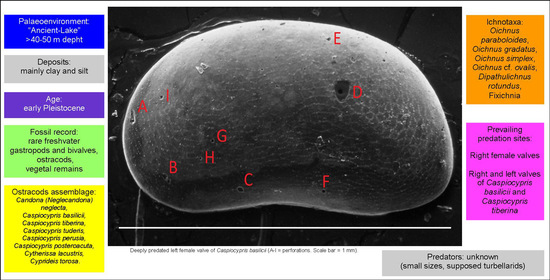
2.2. Fossils Content and Palaeoenvironmental Restoration
3. Materials and Methods
4. Results
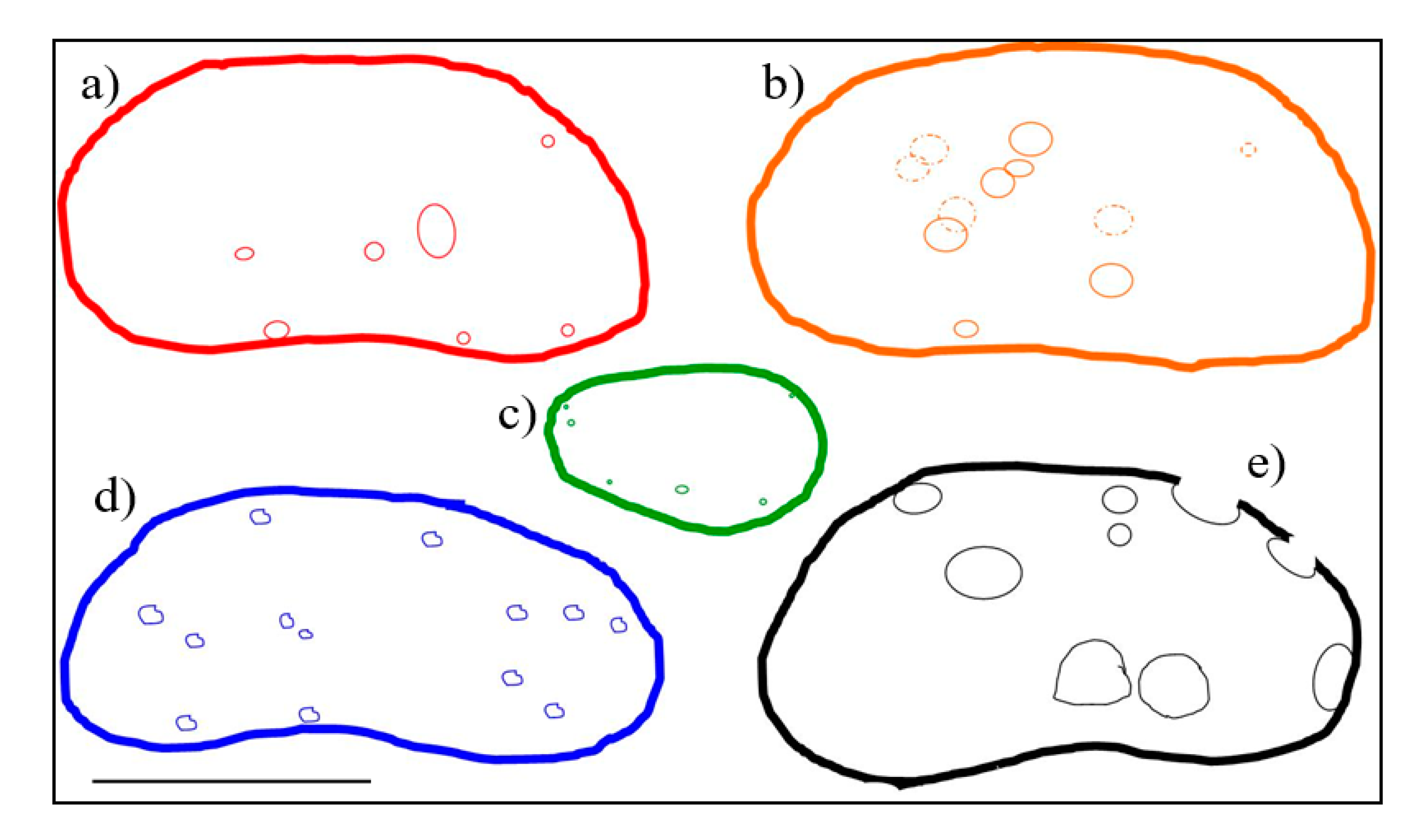
4.1. Total Abundance of Ostracods and Distribution of Microborings
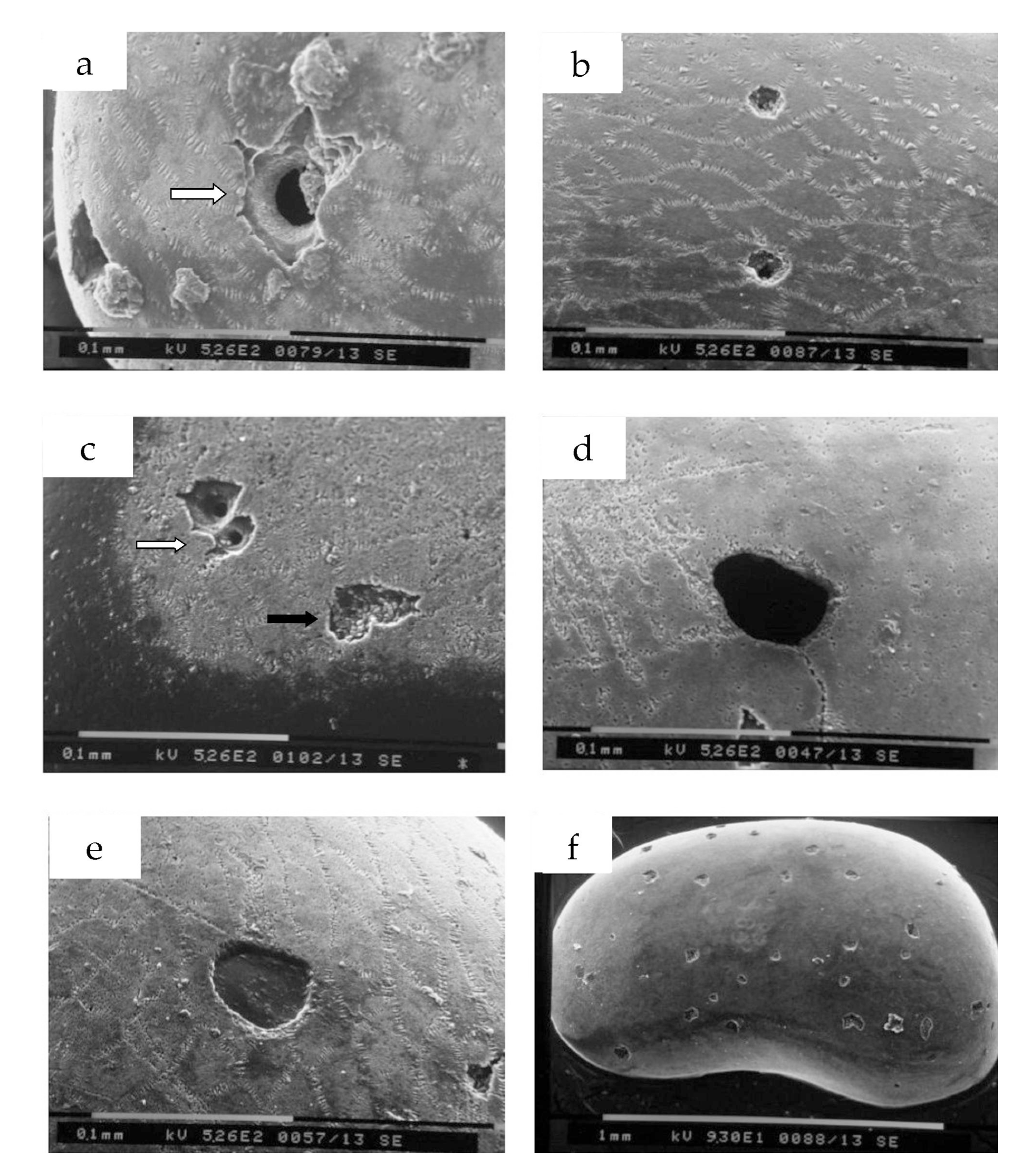
4.2. Shape and Morphology of Predation Holes
- Type I (Figure 5a). Single hole with dimensions over 60 μm; truncated conical hole (symmetric paraboloid) with well outlined walls; evident traces of excavation, with special imprints (teeth or radula scratching) on the outer chitinous layer to reach and perforate the central calcitic layer. Referrable to ichnospecies Oichnus paraboloides Bromley, 1981.
- Type II (Figure 5b). Single perforation with circular to subcircular contour, dimension less than 50 μm; inner walls not well defined and hole eccentric with respect to the perforation of the outer chitinous layer. Referable to ichnospecies Oichnus gradatus Nielsen and Nielsen, 2001.
- Type III (Figure 5c). Perforation formed by two adjacent holes, comparable with ichnospecies Dipatulichnus rotundus Nielsen and Nielsen, 2001 (white arrow) and two irregular, partly overlapping holes (black arrow), which affected the outer chitinous layer and the central calcitic layer (not penetrative), referrable to a failed attack.
- Type IV (Figure 5d). Single large hole, over 80 μm, with vertical walls, associated with multiple and single traces of scratching. The external chitinous layer appears to be less thick, and traces of scratching seem to have affected only this part. Referrable to ichnospecies Oichnus gradatus Nielsen and Nielsen, 2001.
- Type V (Figure 5e). Large single hole, higher than 80 μm, with little marked vertical walls; the perforation crossed the chitinous outer layer and the central calcitic layer, while the chitinous inner layer was not perforated. Referrable to ichnospecies Oichnus simplex Bromley, 1981 (not penetrative).
- Type VI (Figure 5f, Figure 6m). Surface valve (left male of C. tiberina) covered by many sub-circular perforations and polyhedral scars. The subcircular type shows evidence of holes that reach through to the interior (referrable to Oichnus simplex), while the polyhedral etching scars do not seem to go over the central calcitic layer. Some of these scars could be attributable to fixichnia, i.e., to traces left by sessile organisms when they are anchored to a rigid substrate [18,43].
5. Discussion
5.1. Frequency and Type of the Predated Valves
5.2. Position of Microborings
5.3. Size Comparison of Ichnotaxa
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Neumann, A.C. Observations on coastal erosion in Bermuda and measurements of the boring rate of the sponge Cliona Lampa. Limnol. Oceanogr. 1966, 11, 92–108. [Google Scholar] [CrossRef]
- Bromley, R.G. Bioerosion: Eating Rocks for Fun and Profit. Short courses Paleontol. 1992, 5, 121–129. [Google Scholar] [CrossRef]
- Bromley, R.G. The palaeoecology of bioerosion. In The Palaeobiology of Trace Fossils; Donovan, S.K., Ed.; Wiley: London, UK, 1994; pp. 134–154. [Google Scholar]
- Bromley, R.G. Concepts in ichnotaxonomy illustrated by small round holes in shells. Acta Geol. Hispanica 1981, 16, 55–64. [Google Scholar]
- Kelley, P.H.; Hansen, T.A. The fossil record of drilling predation on bivalves and gastropods. In Predator–Prey Interactions in the Fossil Record; Kelley, P.H., Kowalewsky, M., Hansen, T.A., Eds.; Kluwer Academic Plenum Publishers: New York, NY, USA, 2003; pp. 112–139. [Google Scholar]
- Kelley, P.H. Predation by Miocene gastropods of the Chesapeake Group: Stereotyped and predictable. Palaios 1988, 3, 436–448. [Google Scholar] [CrossRef]
- Ziegelmeier, E. Beobachtungen uber den Nahrungserwerb bei der Naticidae Lunatia nitida Donovan (Gastropoda, prosobranchia). Helgoland. Wiss. Meeresunters. 1954, 5, 1–33. [Google Scholar] [CrossRef]
- Thomas, R.D.K. Gastropod predation on sympatric Neogene species of Glycymeris (Bivalvia) from the eastern United States. J. Paleontol. 1976, 50, 488–499. [Google Scholar]
- Carriker, M.R. Shell penetration and feeding by naticacean and muricacean predatory gastropods: A synthesis. Malacologia 1981, 20, 403–422. [Google Scholar]
- Huebner, J.D.; Edwards, D.C. Energy budget of the predatory marine gastropod Polinices duplicatus. Mar. Biol. 1981, 61, 221–226. [Google Scholar] [CrossRef]
- Kitchell, J.A.; Boggs, C.H.; Kitchell, J.F.; Rice, J.A. Prey selection by naticid gastropods: Experimental tests and application to the fossil record. Paleobiology 1981, 7, 533–552. [Google Scholar] [CrossRef]
- Kabat, A.R. Predatory ecology of naticid gastropods with a review of shell boring. Malacologia 1990, 32, 155–193. [Google Scholar]
- Peitso, E.; Hui, E.; Hartwick, B.; Bourne, N. Predation by the naticid gastropod Polinices lewisii (Gould) on littleneck clams Protothaca staminea (Conrad) in British Columbia. Can. J. Zool. 1994, 72, 319–325. [Google Scholar] [CrossRef]
- Huntley, J.W.; Kowalewsky, M. Strong coupling of predation intensity and diversity in the Phanerozoic fossil record. Proc. Natl. Acad. Sci. USA 2007, 104, 15006–15010. [Google Scholar] [CrossRef] [PubMed]
- Zonneveld, J.P.; Gingras, M.K. Sedilichnus, Oichnus, Fossichnus and Tremichnus: ‘Small round holes in shells’ revisited. J. Paleontol. 2014, 88, 895–905. [Google Scholar]
- Wisshak, M.; Kroh, A.; Bertling, M.; Knaust, D.; Nielsen, J.K.; Jagt, J.W.M.; Neumann, C.; Nielsen, K.S.S. In defence of an iconic ichnogenus—Oichnus Bromley, 1981. Ann. Soc. Geol. Pol. 2015, 85, 445–451. [Google Scholar] [CrossRef][Green Version]
- Wisshak, M.; Knaust, D.; Bertling, M. Bioerosion ichnotaxa: Review and annotated list. Facies 2019, 65, 1–39. [Google Scholar] [CrossRef]
- De Gibert, J.M.; Domenech, R.; Martinell, J.C. Bioerosion in shell beds from the Pliocene Roussillon Basin, France: Implications for the (macro)bioerosion ichnofacies model. Acta Palaeontol. Pol. 2007, 52, 783–798. [Google Scholar]
- Yanes, Y.; Kowalewsky, M.; Romanek, S.C. Seasonal variation in ecological and taphonomic processes recorded in shelly death assemblages. Palaios 2012, 27, 373–385. [Google Scholar] [CrossRef]
- Reyement, R.A.; Reyement, E.R.; Honigstein, A. Predation by boring gastropods on Late Cretaceus and Early Palaeocene ostracods. Cretac. Res. 1987, 8, 189–209. [Google Scholar] [CrossRef]
- Maddocks, R.F. One hundred million years of predation on ostracods. In Evolutionary Biology of Ostracoda; Hanai, T., Ikeya, N., Ishizaki, K., Eds.; Elsevier: Amsterdam, The Netherlands, 1988; pp. 637–657. [Google Scholar]
- Reyment, R.; Elewa, A.M.T. Predation by Drills on Ostracoda. In Predator-Prey Interactions in the Fossil Record; Kelley, P.H., Kowalewski, M., Hansen, T.H., Eds.; Kluwer Academic Plenum Publishers: New York, NY, USA, 2003; pp. 93–111. [Google Scholar]
- Ruiz, F.; Abad, M.; González-Regalado, M.L.; Civis, J.; González-Delgado, J.A.; García, E.X.; Toscano, A. Predation on Neogene ostracods of southwestern Spain. Riv. It. Paleontol. Strat. 2010, 116, 253–260. [Google Scholar]
- Jonkers, H.A. Gastropod predation patterns in Pliocene and Recent pectinid bivalves from Antarctica and New Zealand. N. Zeal. J. Geol. Geop. 2000, 43, 247–254. [Google Scholar] [CrossRef]
- Martinell, J.; Domenech, R.; Aymar, J.; Kowalewski, M. Confamilial predation in Pliocene naticid gastropods from southern France: Utility of preexisting collections in quantitative paleoecology. Palaios 2010, 25, 221–228. [Google Scholar] [CrossRef]
- Blissett, D.J.; Pickerill, R.K. Soft-sediment ichnotaxa from the Cenozoic White Limestone Group, Jamaica, West Indies. Scr. Geol. 2007, 127, 341–378. [Google Scholar]
- Ruiz, F.; Abad, M.; González-Regalado, M.L.; Tosquella, J.; García, E.X.; Toscano, A.; Muñoz, A.; Pendón, J.G. Predation on recent marine ostracod populations of southwestern Spain. Ameghiniana 2011, 48, 113–121. [Google Scholar] [CrossRef]
- Ruiz, F.; Abad, M.; García, E.X.; Toscano, A.; Prudencio, M.I.; Dias, M.I.; Galán, E. Predation on ostracod populations of two North African lagoons. Crustaceana 2011, 84, 1537–1545. [Google Scholar]
- Kihn, R.G.; Martinez, D.E.; Gómez, E.A. Depredación de ostrácodos del Cuaternario del sur de la provincia de Buenos Aires, Argentina. Rev. Mus. Argent. Cienc. Nat. 2011, 13, 175–182. [Google Scholar] [CrossRef][Green Version]
- Barchi, M.; Brozzetti, F.; Lavecchia, G. Analisi strutturale e geometrica dei bacini della media Valle del Tevere e della Valle Umbra. Boll. Soc. Geol. It. 1991, 110, 65–76. [Google Scholar]
- Martini, I.P.; Sagri, M. Tectono-sedimentary characteristics of late Miocene—Quaternary extensional basins of the North Apennines, Italy. Earth Sci. Rev. 1993, 34, 197–223. [Google Scholar] [CrossRef]
- Basilici, G. Sedimentary facies in an extensional and deep-lacustrine depositional system: The Pliocene Tiberino Basin, Central Italy. Sediment. Geol. 1997, 109, 73–94. [Google Scholar] [CrossRef]
- Martinetto, E.; Bertini, A.; Basilici, G.; Baldanza, A.; Bizzarri, R.; Cherin, M.; Gentili, S.; Pontini, M.R. The plant record of the Dunarobba and Pietrafitta sites in the Plio-Pleistocene palaeoenvironmental context of central Italy. AMQ 2014, 27, 29–72. [Google Scholar]
- Martinetto, E.; Momohara, A.; Bizzarri, R.; Baldanza, A.; Delfino, M.; Esu, D.; Sardella, R. Late persistence and deterministic extinction of “humid thermophilous plant taxa of East Asian affinity” (HUTEA) in southern Europe. Palaeogeog. Palaeoclim. Palaeoecol. 2017, 46, 211–231. [Google Scholar] [CrossRef]
- Baldanza, A.; Bizzarri, R.; Di Matteo, L.; Lezzerini, M.; Mencaroni, L.; Pagnotta, S.; Raneri, S.; Vinti, G. New integrated data from clay lacustrine deposits of the Dunarobba area (Umbria, central Italy). AMQ 2018, 31, 87–104. [Google Scholar] [CrossRef]
- Gallo, G.; Baldanza, A.; Bizzarri, R.; Cavalazzi, B.; Comodi, P.; Frondini, F.; Agangi, A. The relevance of biosignatures in siderite-nodules: An example from Quaternary continental deposits (Tiberino Basin, Italy). In Proceedings of the EANA 2014, Edimburgh, Scotland, 13–16 October 2014. [Google Scholar]
- Spadi, M.; Gliozzi, E.; Medici, M.C. A Plio–Pleistocene Caspiocypris species flock (Candoninae, Ostracoda) from the Palaeolake Tiberino (Umbria, central Italy). J. Syst. Palaeontol. 2018, 16, 417–434. [Google Scholar] [CrossRef]
- Medici, M.C.; Gliozzi, E. Preliminary data on the freshwater ostracods from the Middle-Late Pliocene Tiberino “Palaeo-ancient lake” (Umbria, Central Italy). Atti. Mus. Civ. St. Nat. Trieste 2008, 53, 39–48. [Google Scholar]
- Frogley, M.R.; Griffiths, H.I.; Martens, K. Modern and fossil Ostracods from ancient lakes. In The Ostracoda-Application in Quaternary Research; Holmes, J.A., Chivas, A.R., Eds.; American Geophysical Union: Washington, DC, USA, 2002; pp. 167–184. [Google Scholar]
- Meisch, C. Freshwater Ostracoda of Western and Central Europe; Spektrum Academischer: Heidelberg, Germany; Berlin, Germany, 2000. [Google Scholar]
- Ruiz, F.; Abad, M.; Bodergat, A.M.; Carbonel, P.; Rodriguez-Lazaro, J.; Gonzalez-Regalado, M.L.; Toscano, A.; Garcia, E.X.; Prenda, J. Freshwater ostracods as environmental tracers. Int. J. Environ. Sci. Technol. 2013, 10, 1115–1128. [Google Scholar] [CrossRef]
- Spadi, M.; Gliozzi, E.; Medici, M.C. Early Pleistocene (Gelasian) non marine ostracods from the Dunarobba fossil forest (Tiberino Basin, Umbria, central Italy). Palaeontology 2018, 61, 1–44. [Google Scholar]
- De Gibert, J.M.; Domenech, R.; Martinell, J. An ethological framework for animal bioerosion trace fossils upon mineral substrates with proposal of a new class, fixichnia. Lethaia 2004, 37, 429–437. [Google Scholar] [CrossRef]
- Reyment, R.A. Preliminary observations on gastropod predation in the western Niger Delta. Palaeogeogr. Palaeodim. Palaeoecol. 1966, 2, 81–102. [Google Scholar] [CrossRef]
- Bizzarri, R.; Corrado, P.; Magri, D.; Martinetto, E.; Esu, D.; Caprai, V.; Colacicchi, R.; Napoleone, G.; Albianelli, A.; Baldanza, A. Palaeoenvironmental and climatic inferences from the late early Pleistocene lacustrine deposits in the eastern Tiberino Basin (central Italy). Quat. Res. 2018, 90, 201–221. [Google Scholar] [CrossRef]







| Species | Total Per Sample | % Predated Per Sample | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sample | Number of Valves/Predated Valves | Candona (N.) neglecta | Caspiocypris basilicii | Caspiocypris tiberina | Caspiocipris perusia | Caspiocypris tuderis | Caspiocypris posteroacuta | Cytherissa lacustris | Cyprideis torosa | Juveniles Not Identifiable | ||
| 4 | valves | 5 | 33 | 10 | 9 | 0 | 0 | 0 | 0 | 57 | 1.8% | |
| predated | 1LVm | 1 | ||||||||||
| 5 | valves | 3 | 25 | 17 | 8 | 6 | 0 | 0 | 0 | 59 | 5.1% | |
| predated | 1LVm; 1RVm | 1LVm | 3 | |||||||||
| 6 | valves | 2 | 38 | 16 | 8 | 18 | 0 | 0 | 0 | 82 | 4.9% | |
| predated | 2RVf | 1RVf | 1RVf | 4 | ||||||||
| 7 | valves | 2 | 35 | 14 | 9 | 14 | 0 | 0 | 0 | 74 | 5.4% | |
| predated | 1RVf | 2RVf | 1LVm | 4 | ||||||||
| 8 | valves | 3 | 31 | 8 | 8 | 9 | 0 | 0 | 0 | 59 | 3.4% | |
| predated | 1RVf; 1LVm | 2 | ||||||||||
| 9 | valves | 2 | 27 | 12 | 12 | 10 | 0 | 0 | 0 | 63 | 4.8% | |
| predated | 1RVf | 1RVf; 1LVf | 3 | |||||||||
| 10 | valves | 2 | 26 | 6 | 6 | 4 | 0 | 0 | 0 | 44 | 9.1% | |
| predated | 1LVm1LVf | 1RVf; 1LVm | 4 | |||||||||
| 11 | valves | 3 | 19 | 11 | 6 | 5 | 0 | 0 | 0 | 44 | 9.1% | |
| predated | 1RVf | 1RVf | 1LVf | 1RVf | 4 | |||||||
| 12 | valves | 2 | 21 | 5 | 3 | 7 | 0 | 0 | 0 | 38 | 7.9% | |
| predated | 2RVf; 1LVf | 3 | ||||||||||
| 13 | valves | 0 | 25 | 17 | 0 | 29 | 0 | 0 | 0 | 29 | 100 | 10.0% |
| predated | 1RVm; 1RVf 2LVf; 1LVj | 2RVf | 1RVf; 2RVj | 10 | ||||||||
| 14 | valves | 0 | 28 | 30 | 3 | 20 | 0 | 0 | 0 | 81 | 2.5% | |
| predated | 1RVf; 1LVf | 2 | ||||||||||
| 15 | valves | 0 | 6 | 3 | 0 | 4 | 0 | 4 | 0 | 17 | 17.6% | |
| predated | 2RVf | 1LV | 3 | |||||||||
| 16 | valves | 1 | 12 | 1 | 2 | 18 | 0 | 0 | 0 | 34 | 17.6% | |
| predated | 1RVf | 1RVf; 1LVf 1LVm | 2RVf | 6 | ||||||||
| 17 | valves | 1 | 24 | 15 | 1 | 6 | 0 | 0 | 0 | 47 | 17.0% | |
| predated | 1RVf | 1LVf; 3RVf | 2LVm | 1RVf | 8 | |||||||
| 18 | valves | 0 | 23 | 14 | 5 | 8 | 0 | 0 | 0 | 50 | 16.0% | |
| predated | 3RVf; 2LVm | 2LVf | 1LVm | 8 | ||||||||
| 19 | valves | 0 | 9 | 11 | 3 | 3 | 1 | 0 | 0 | 27 | 7.4% | |
| predated | 1LVf | 1LVf | 2 | |||||||||
| 20 | valves | 0 | 18 | 10 | 4 | 7 | 11 | 0 | 0 | 50 | 20.0% | |
| predated | 2LVm; 3LVf1RVf | 1RVf | 3LVm | 10 | ||||||||
| 21 | valves | 0 | 10 | 14 | 3 | 3 | 4 | 0 | 0 | 34 | 2.9% | |
| predated | 1LVf | 1 | ||||||||||
| 22 | valves | 0 | 24 | 25 | 16 | 7 | 3 | 0 | 1 | 76 | 7.9% | |
| predated | 1RVf; 1LVm 3LVF | 1RVf | 6 | |||||||||
| 25 | valves | 0 | 53 | 15 | 25 | 4 | 28 | 0 | 6 | 131 | 3.1% | |
| predated | 1RVf | 1RVf; 2LVf | 4 | |||||||||
| Total per species | 26 | 487 | 254 | 131 | 182 | 47 | 4 | 7 | 29 | 1167 | 7.6% | |
| 8 | 50 | 15 | 3 | 8 | 3 | 1 | 0 | 0 | 88 | |||
| % predated per species | 31% | 10% | 6% | 2% | 4% | 6% | 25% | 0% | 0% | |||
| Distribution of predated valves | 6RV,2LV 1m,7f | 27RV, 23LV 12m,37f,1j | 6RV, 9LV 3m,12f | 1RV, 2LV 1m,2f | 7RV, 1LV 1m,5f,2j | 3LV 3m | 1LV | 47RV,41LV 21m, 63f,3j | ||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baldanza, A.; Bizzarri, R.; Posati, F.; Ravoni, M. Evidence of Predation on Early Pleistocene Freshwater Ostracods (Umbria, Central Italy). Geosciences 2020, 10, 416. https://doi.org/10.3390/geosciences10100416
Baldanza A, Bizzarri R, Posati F, Ravoni M. Evidence of Predation on Early Pleistocene Freshwater Ostracods (Umbria, Central Italy). Geosciences. 2020; 10(10):416. https://doi.org/10.3390/geosciences10100416
Chicago/Turabian StyleBaldanza, Angela, Roberto Bizzarri, Francesco Posati, and Manuel Ravoni. 2020. "Evidence of Predation on Early Pleistocene Freshwater Ostracods (Umbria, Central Italy)" Geosciences 10, no. 10: 416. https://doi.org/10.3390/geosciences10100416
APA StyleBaldanza, A., Bizzarri, R., Posati, F., & Ravoni, M. (2020). Evidence of Predation on Early Pleistocene Freshwater Ostracods (Umbria, Central Italy). Geosciences, 10(10), 416. https://doi.org/10.3390/geosciences10100416