Reproductive Biology of Three Important Threatened/Near-Threatened Groupers (Plectropomus leopardus, Epinephelus polyphekadion and Plectropomus areolatus) in Eastern Indonesia and Implications for Management
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
3. Results
3.1. Histological Characteristics of Gonad Developmental Stages
3.1.1. Leopard Coral Trout (Plectropomus leopardus)
3.1.2. Squaretail Coraltrout (Plectropomus areolatus)
3.1.3. Camouflage grouper (Epinephelus polyphekadion)
3.2. Mean Size at Sexual Maturity
3.3. Spawning Seasons
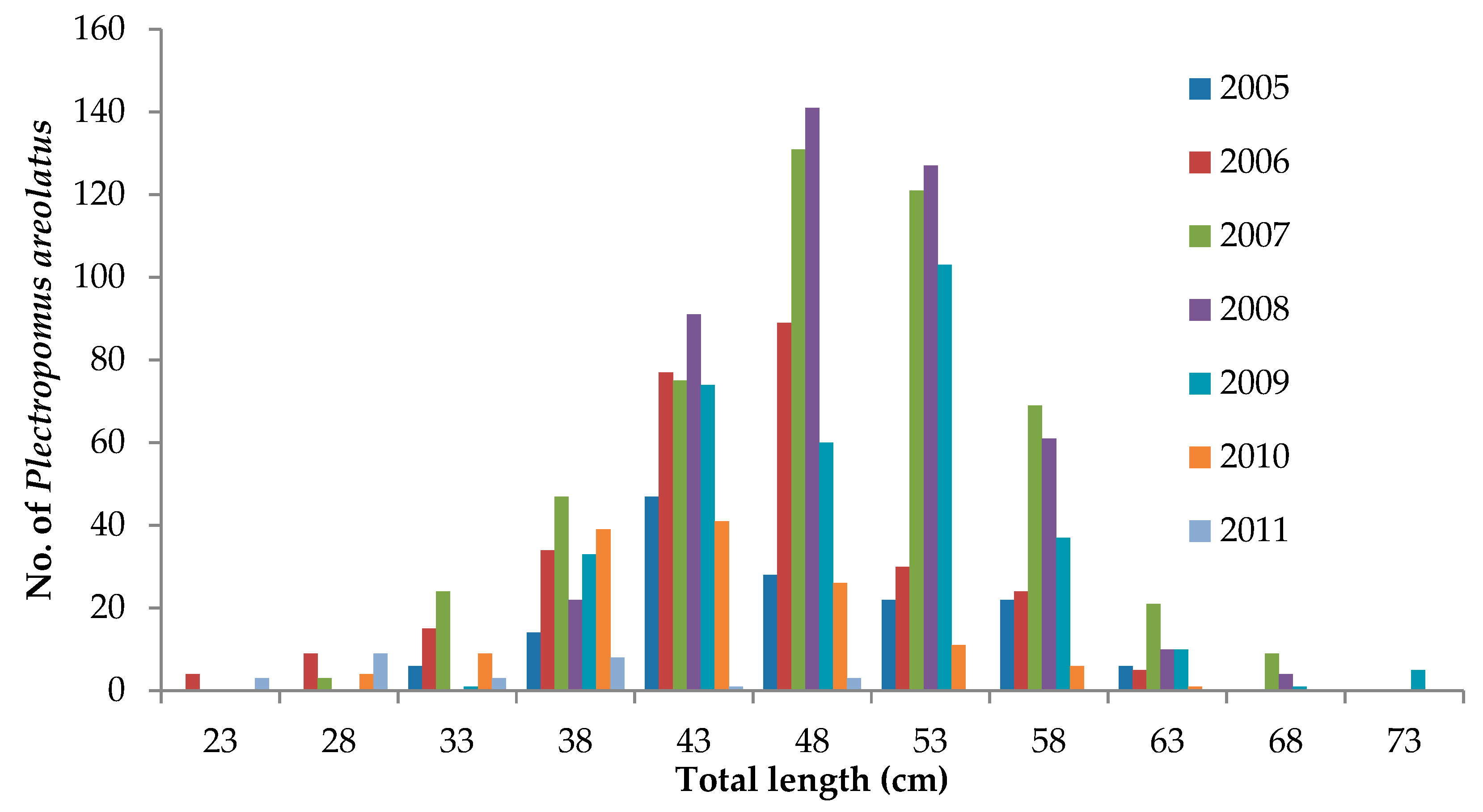
3.4. Live Grouper Market Size
4. Discussion
4.1. Sexual Patterns
4.2. Spawning Period
4.3. The Minimum Size of Sexual Maturity
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A
- When the first time you catch live reef fish?
- Where you go to fishing live groupers (please mark in this map and tell me what kind of fish you will get there)?
- Is the place the same as your first time fishing live groupers? If not, please mark where the first time are you fishing live groupers?
- Did you ever see the fish aggregation? What kind of fish? How depth and when they aggregate? If any, please mark the place?
- When do you see, the fish came to aggregate? Please tell us the month and moon phase?
- Is there any missing place (a long time ago were many groupers there, and now the groupers were gone), if any, please mark the place?
References
- Sadovy de Mitcheson, Y.; Isabel, T.; Geoffrey, M.; le Clue, S.; Botsford, E.; Shea, S. The Trade in Live Reef Food Fish—Going, Going, Gone. Hong Kong Spec. Admin. Reg. 2017, 1, 1–288. [Google Scholar]
- Campbell, S.J.; Hoey, A.S.; Maynard, J.; Kartawijaya, T.; Cinner, J.; Graham, N.A.; Baird, A.H. Weak Compliance Undermines the Success of No-Take Zones in a Large Government-Controlled Marine Protected Area. PLoS ONE 2012, 7, e50074. [Google Scholar] [CrossRef] [PubMed]
- Pet-Soede, L. Options for Co-Management of an Indonesian Coastal Fishery; Wageningen Universiteit: Wageningen, The Netherlands, 2000. [Google Scholar]
- Radjawali, I. Social Networks and Live Reef Food Fish (LRFF) Trade: Examining Sustainability. J. Indones. Soc. Sci. Humanit. 2011, 4, 65–100. [Google Scholar]
- Sadovy de Mitcheson, Y.; Craig, M.T.; Bertoncini, A.A.; Carpenter, K.E.; Cheung, W.W.; Choat, J.H.; Cornish, A.S.; Fennessy, S.T.; Ferreira, B.P.; Heemstra, P.C.; et al. Fishing groupers towards extinction: A global assessment of threats and extinction risks in a billion-dollar fishery. Fish Fish. 2013, 14, 119–136. [Google Scholar] [CrossRef]
- Mcgilvray, F.; Chan, T.C. The Trade in Live Reef Food Fish a Hong Kong Perspective. Hong Kong. Aquac. Asia 2001, 7, 21–26. [Google Scholar]
- Muldoon, G.; de Mitcheson, Y.S.; Shea, S.; Tam, I.; Welford, R.; Whitford, A. Mostly Legal, But Not Sustainable How Airlines Can Support Sustainable Trade in Live Reef Food Fish; ADM Capital Foundation: Hong Kong, China, 2016. [Google Scholar]
- Erdmann, M.V.; Pet-Soede, L. How fresh is too fresh? The live reef food fish trade in Eastern Indonesia. SPC Live Reef Fish Inf. Bull. 1997, 3, 41–45. [Google Scholar]
- Frisch, A.J.; Cameron, D.S.; Pratchett, M.S.; Williamson, D.H.; Williams, A.J.; Reynolds, A.D.; Hoey, A.S.; Rizzari, J.R.; Evans, L.; Kerrigan, B.; et al. Key aspects of the biology, fisheries, and management of Coral grouper. Rev. Fish Biol. Fish. 2016, 26, 303–325. [Google Scholar] [CrossRef]
- Rhodes, K.L.; Taylor, B.M.; Wichilmel, C.B.; Joseph, E.; Hamilton, R.J.; Almany, G.R. Reproductive biology of squaretail coral grouper Plectropomus areolatus using age-based techniques. J. Fish Biol. 2013, 82, 1333–1350. [Google Scholar] [CrossRef]
- Russell, M.W.; de Mitcheson, Y.S.; Erisman, B.E.; Hamilton, R.J.; Luckhurst, B.E.; Nemeth, R.S. Status Report World’s Fish Aggregations 2014; Science and Conservation of Fish Aggregations: Fallbrook, CA, USA, 2014; pp. 2–4. [Google Scholar]
- Sadovy de Mitcheson, Y. Troubled times for trysting trio: Three aggregating groupers in the live reef food-fish trade. SPC Live Reef Fish Bull. 2005, 13, 3–6. [Google Scholar]
- Pet, J.S.; Mous, P.J.; Muljadi, A.H.; Sadovy, Y.J.; Squire, L. Aggregations of Plectropomus areolatus and Epinephelus fuscoguttatus (groupers, Serranidae) in the Komodo National Park, Indonesia: Monitoring and implications for management. Environ. Biol. Fishes 2005, 74, 209–218. [Google Scholar] [CrossRef]
- Sadovy, Y.J.; Donaldson, T.J.; Graham, T.R.; McGilvray, F.; Muldoon, G.J.; Phillips, M.J.; Rimmer, M.A.; Smith, A.; Yeeting, B. While Stocks Last: The Live Reef Food Fish Trade; Asian Development Bank: Mandaluyong, Philippines, 2003; pp. 1–169. [Google Scholar]
- Bergenius, M.A.J.; Begg, G.A.; Mapstone, B.D. The use of otolith morphology to indicate the stock structure of common coral trout (Plectropomus leopordus) on the Great Barrier Reef, Australia. Fish. Bull. 2006, 104, 498–511. [Google Scholar]
- Bawole, R.; Rembet, U.N.; Amir, A.; Runtuboi, F.; Sala, R. Exploitation rate of Plectropomus leopardus (Pisces: Serranidae) taken from Rumberpon island water, Cenderawasih Bay National Park, Indonesia. AACL Bioflux 2018, 11, 19–28. [Google Scholar]
- Lau, P.; Jones, R. The Hong Kong trade in live reef fish for food. Live Reef Fish 1999, 38, 27. [Google Scholar]
- Sadovy de Mitcheson, Y. Mainstreaming Fish Spawning Aggregations into Fishery Management Calls for a Precautionary Approach. Bioscience 2016, 66, 295–306. [Google Scholar] [CrossRef] [Green Version]
- Hamilton, R.J.; Giningele, M.; Aswani, S.; Ecochard, J.L. Fishing in the dark-local knowledge, night spearfishing and spawning aggregations in the Western Solomon Islands. Biol. Conserv. 2012, 145, 246–257. [Google Scholar] [CrossRef]
- Rhodes, K.L. “Plectropomus areolatus,” The IUCN Red List of Threatened Species. 2018. Available online: http://dx.doi.org/10.2305/IUCN.UK.2018-2.RLTS.T64411A100466794.en (accessed on 25 June 2019).
- Rhodes, K.; Choat, J.H.; Sadovy, Y.; Myers, R.; To, A.; Ma, K.; Samoilys, M.; Suharti, S.; Law, C.; Amorim, P. “Epinephelus polyphekadion,” The IUCN Red List of Threatened Species. 2018. Available online: http://dx.doi.org/10.2305/IUCN.UK.2018-2.RLTS.T61339A100553967.en (accessed on 25 June 2019).
- Choat, J.H.; Samoilys, M.A. “Plectropomus leopardus,” The IUCN Red List of Threatened Species. 2018. Available online: http://www.iucnredlist.org/details/44684/0 (accessed on 25 June 2019).
- Rhodes, K.L.; Tupper, M.H. A preliminary market-based analysis of the Pohnpei, Micronesia, grouper (Serranidae: epinephelinae) fishery reveals unsustainable fishing practices. Coral Reefs 2007, 26, 335–344. [Google Scholar] [CrossRef]
- Rhodes, K.L.; de Mitcheson, Y.S. Reproduction in the Camouflage Grouper Federated States of Micronesia (Pisces: Serranidae) in Pohnpei. Bull. Mar. Sci. 2002, 70, 851–869. [Google Scholar]
- Froese, R. Keep it simple: Three indicators to deal with overfishing. Fish Fish. 2004, 5, 86–91. [Google Scholar] [CrossRef]
- Sadovy de Mitcheson, Y.; Liu, M. Report on the Current Status and Exploitation History of Reef Fish Spawning Aggregations in Eastern Indonesia; The University of Hong Kong: Hong Kong, China, 2004. [Google Scholar]
- Sadovy, Y.; Colin, P.L. Sexual development and sexuality in the Nassau grouper. J. Fish Biol. 1995, 46, 961–976. [Google Scholar] [CrossRef]
- Sadovy de Mitcheson, Y.; Shapiro, D.Y. Criteria for the Diagnosis of Hermaphroditism in Fishes. Copeia 1987, 1987, 136–156. [Google Scholar] [CrossRef]
- Sadovy de Mitcheson, Y.; Liu, M. Functional hermaphroditism in teleosts. Fish Fish. 2008, 9, 1–43. [Google Scholar] [CrossRef]
- King, M. Fisheries Biology, Assessment, and Management, 2nd ed.; Blackwell Publishing: Victoria, Australia, 2013. [Google Scholar]
- Colin, P.L.; Sadovy, Y.J.; Domeier, M.L. Section VII. Spawning Aggregation Conservation Methods and Long Term Monitoring. In Manual for the Study and Conservation of Reef Fish Spawning Aggregations; Special Pu.; Science and Conservation of Fish Aggregations: Fallbrook, CA, USA, 2003; pp. 83–92. [Google Scholar]
- Tricas, T.C.; Hiramoto, J.T. Sexual differentiation, gonad development, and spawning seasonality of the Hawaiian butterflyfish, Chaetodon multicinctus. Environ. Biol. Fishes 1989, 25, 111–124. [Google Scholar] [CrossRef]
- Adams, S.; Mapstone, B.D.; Russ, G.R.; Davies, C.R. Geographic variation in the sex ratio, sex-specific size, and the age structure of Plectropomus leopardus (Serranidae) between reefs open and closed to fishing on the Great Barrier Reef. Can. J. Fish. Aquat. Sci. 2000, 57, 1448–1458. [Google Scholar] [CrossRef]
- Hamilton, R.J.; Matawai, M. Live reef food fish trade cause rapid declines in abundance of squaretail coral grouper (Plectropomus areolatus) at a spawning aggregation site in Manus, Papua New Guinea Richard. SPC Live Reef Fish Bull. 2006, 16, 13–18. [Google Scholar]
- Kindsvater, H.K.; Reynolds, J.D.; de Mitcheson, Y.S.; Mangel, M. Selectivity matters: Rules of thumb for management of plate-sized, sex-changing fish in the live reef food fish trade. Fish Fish. 2017, 18, 821–836. [Google Scholar] [CrossRef]
- Edrus, I.N.; Suman, A. Ikan Napoleon (Cheilinus Undulatus Rüppell 1835) Status Stok dan Pengelolaannya di Indonesia; IPB Press: Bogor, Indonesia, 2013. [Google Scholar]
- Booth, L. Identifying Conservation Strategies for Group-Spawning Coral Reef Fish in the Indo-Pacific, Using a Case Study of a Protogynous Giant Wrasse. Consilience 2017, 17, 33–45. [Google Scholar]
- Frisch, A.J.; McCormick, M.I.; Pankhurst, N.W. Reproductive periodicity and steroid hormone profiles in the sex-changing coral-reef fish, Plectropomus leopardus. Coral Reefs 2007, 26, 189–197. [Google Scholar] [CrossRef]









| Gonad Development | Histological Description |
|---|---|
| Immature female | The whole gonad was small and compact; the gonads dominated with primary growth stage 1/2 oocytes (Figure 2A). |
| Mature resting female | The oocytes stage 1/2 and three presented with occasional early stage 4 oocytes (Figure 2B). This stage was mostly found between spawning seasons, but it was not possible to determine whether this stage of ovary had previously ovulated or was maturing for the first time. In this research, the mature resting female was dominated by large species. |
| Mature ripe female | All gonads dominated with oocytes stage 1/2, 3, 4, and vitellogenic oocytes present in various proportions, the ovarian wall may become thin (Figure 2C). There are only a few (if any) post-ovulatory follicles visible. At ovulation stage, hydrated eggs released from their follicular developed into the ovary lumen forming post-ovulatory follicles (that were only found around 24 h after ovulation) [32]. |
| Post spawning female | Gonad contains degeneration of vitellogenic oocytes and 1/2 stages oocytes and lamella wall becomes thick (Figure 2D). |
| Bisexual | Gonad contained stages 1/2 oocytes and1/2 spermatocytes (immature bisexual) (Figure 3E); 3/4 oocytes and 3/4 spermatocytes (mature bisexual) appear together in similar amount (Figure 4F). |
| Immature male | All gonads were small and compact, contained a central lumen, and were dominated by seminiferous tubules. Scattered cysts of spermatocyte stage 1/2 were evident, but not common (Figure 3A). An occasional stage 1/2 oocyte was present. |
| Mature resting male | Gonad was dominated by early stages of spermatogenesis (i.e., spermatocyte stages 1 and 2) with scattered cysts of sperm increasingly evident as the spawning season approached. Occasional dispersed phase 1/2 oocytes were present. The testis lumen became smaller than in immature phase (Figure 3B). |
| Mature ripe male | Late stages of spermatogenesis were dominant and spread to fill almost all parts of the testis. Sperm occurred in expanded lobules, and spermatocyte stages 1 and 2 were relatively less abundant (Figure 3C). |
| Post spawning male | Most of the late stages of spermatogenesis have released. Spermatocyte stages 1 and 2 were relatively less abundant than the mature male phase. The gonad often had a thickened wall; there was space between the muscular wall and sperm (Figure 3D). |
| Species | Sexual Differentiation | 2017 | 2018 | Total | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| May | Jun | Oct | Nov | Dec | Jan | Feb | Mar | Apr | |||
| Plectropomus leopardus | F | 0 | 4 | 4 | 21 | 25 | 7 | 9 | 0 | 0 | 70 |
| M | 1 | 0 | 3 | 2 | 2 | 1 | 1 | 0 | 0 | 10 | |
| T | 1 | 0 | 0 | 2 | 1 | 1 | 0 | 0 | 0 | 5 | |
| Plectropomus areolatus | F | 0 | 0 | 0 | 7 | 11 | 5 | 0 | 0 | 1 | 24 |
| M | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 2 | |
| T | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Epinephelus polyphekadion | F | 0 | 0 | 3 | 6 | 1 | 7 | 0 | 0 | 1 | 18 |
| M | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 2 | 4 | |
| T | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Total | 133 | ||||||||||
| Species Name | Sampling Area | Lm50 (TL, cm) | Estimated Weight (g) | No. of Samples |
|---|---|---|---|---|
| Plectropomus leopardus | Kapoposang MTP | 31.56 | 404.4 | 34 |
| Takabonerate NP | 37.18 | 670.3 | 14 | |
| Karas Islands | 40.35 | 723.9 | 31 | |
| Kei Island | 47.78 | 1150.3 | 5 | |
| Plectropomus areolatus | Takabonerate NP | 37.80 | 826.9 | 9 |
| Wakatobi NP | 40.20 | 948.4 | 15 | |
| Epinephelus polyphekadion | Takabonerate NP | 37.48 | 885.9 | 15 |
| Wakatobi NP | 40.90 | 1058.4 | 5 |
| Spawning Month/Location | Spawning Month | No. of Respondents | ||
|---|---|---|---|---|
| P. leopardus | P. areolatus | E. polyphekadion | ||
| Kapoposang MTP | Nov–Jan | - | - | 30 |
| Takabonerate NP | - | Sept–Feb | Jan–Apr | 100 |
| Wakatobi NP | - | Nov–Dec, Feb–May | Oct–Jan | 48 |
| Kei Islands | Oct–Dec | - | - | 24 |
| Karas Islands | - | Oct–Feb | Nov–Dec | 26 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khasanah, M.; Nurdin Kadir, N.; Jompa, J. Reproductive Biology of Three Important Threatened/Near-Threatened Groupers (Plectropomus leopardus, Epinephelus polyphekadion and Plectropomus areolatus) in Eastern Indonesia and Implications for Management. Animals 2019, 9, 643. https://doi.org/10.3390/ani9090643
Khasanah M, Nurdin Kadir N, Jompa J. Reproductive Biology of Three Important Threatened/Near-Threatened Groupers (Plectropomus leopardus, Epinephelus polyphekadion and Plectropomus areolatus) in Eastern Indonesia and Implications for Management. Animals. 2019; 9(9):643. https://doi.org/10.3390/ani9090643
Chicago/Turabian StyleKhasanah, Miftakhul, Nadiarti Nurdin Kadir, and Jamaluddin Jompa. 2019. "Reproductive Biology of Three Important Threatened/Near-Threatened Groupers (Plectropomus leopardus, Epinephelus polyphekadion and Plectropomus areolatus) in Eastern Indonesia and Implications for Management" Animals 9, no. 9: 643. https://doi.org/10.3390/ani9090643
APA StyleKhasanah, M., Nurdin Kadir, N., & Jompa, J. (2019). Reproductive Biology of Three Important Threatened/Near-Threatened Groupers (Plectropomus leopardus, Epinephelus polyphekadion and Plectropomus areolatus) in Eastern Indonesia and Implications for Management. Animals, 9(9), 643. https://doi.org/10.3390/ani9090643





