Extended-Spectrum β-Lactamase-Producing Enterobacteriaceae in Hospitalized Neonatal Foals: Prevalence, Risk Factors for Shedding and Association with Infection
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Equine Study Population, Study Design and Sampling Methods
2.2. Demographic and Medical Data
2.3. ESBL-Producing Enterobacteriaceae (ESBL-E) Isolation and Species Identification
2.4. Molecular Characterization of ESBL-E
2.5. Statistical Analysis
3. Results
3.1. Characterization of Equine Study Population
3.2. Hospital Procedures, Antibiotic Therapy and Outcome
3.3. Prevalence of ESBL-E Shedding among Foals and Mares
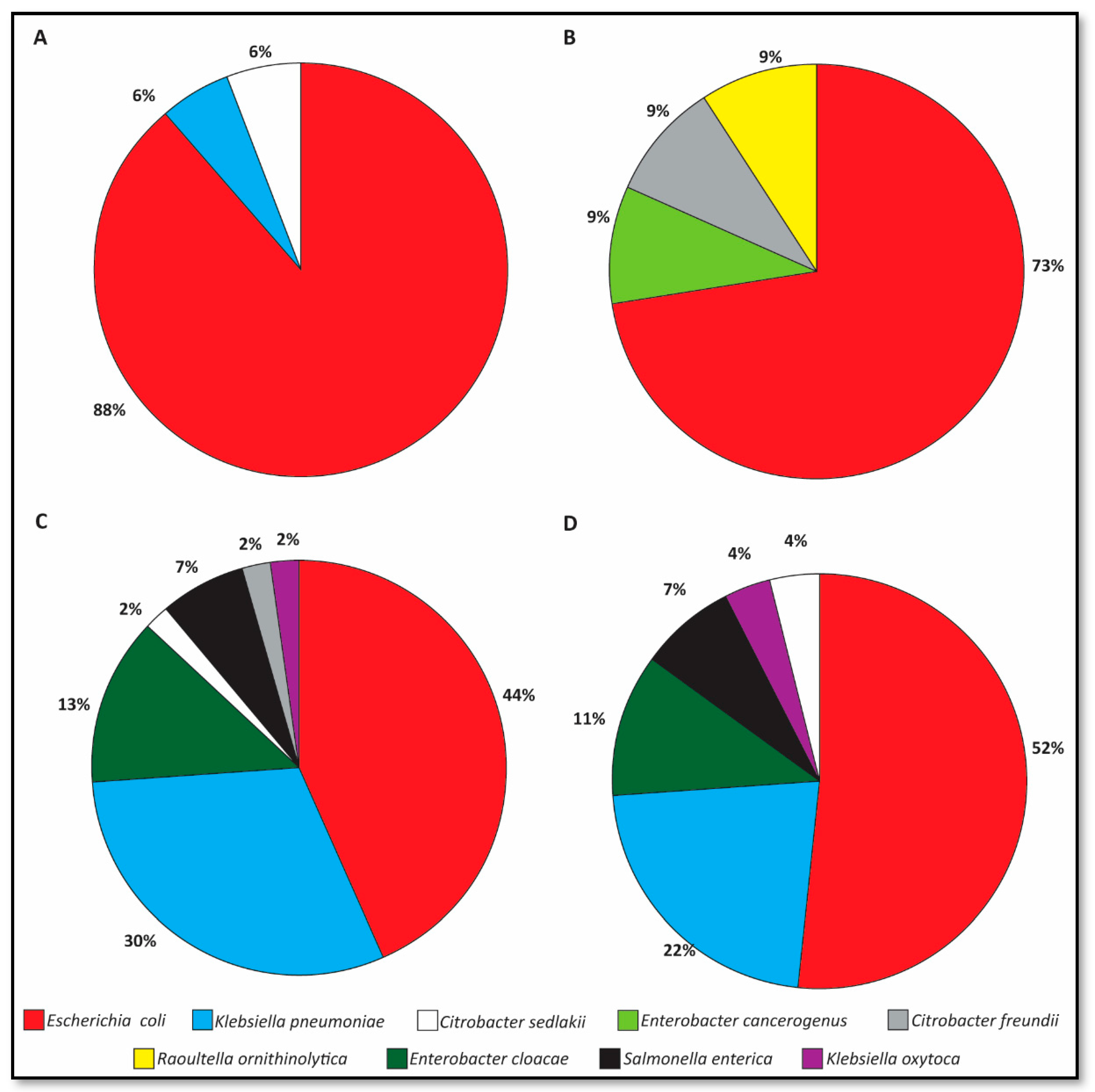
3.4. Species Distribution of ESBL-E Shedding Isolates
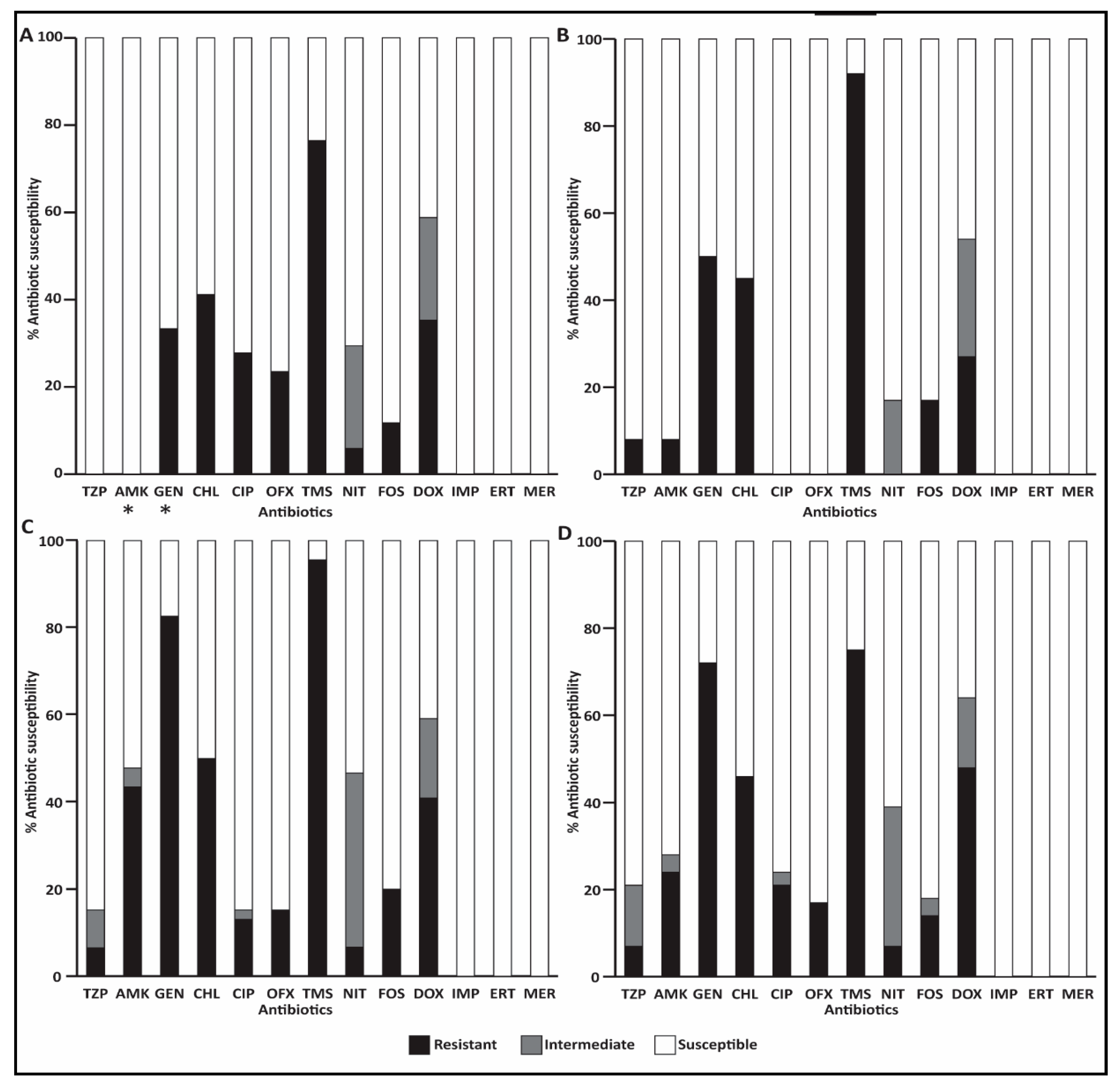
3.5. Antibiotic Susceptibility Profiles of ESBL-E Fecal Isolates
3.5.1. ESBL-E Isolates on Admission
3.5.2. ESBL-E Isolates during Hospitalization
3.5.3. ESBL-E Isolates from Clinical Samples of Infection Sites
3.6. Risk Factor Analysis for ESBL-E Shedding
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Rubin, J.E.; Pitout, J.D.D. Extended-spectrum β-lactamase, carbapenemase and AmpC producing Enterobacteriaceae in companion animals. Vet. Microbiol. 2014, 170, 10–18. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Bae, I.K.; Lee, S.H. New definitions of extended-spectrum β-lactamase conferring worldwide emerging antibiotic resistance. Med. Res. Rev. 2012, 32, 216–232. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Thungrat, K.; Boothe, D.M. Occurrence of OXA-48 Carbapenemase and Other β-Lactamase Genes in ESBL-Producing Multidrug Resistant Escherichia coli from Dogs and Cats in the United States, 2009–2013. Front. Microbiol. 2016, 7. [Google Scholar] [CrossRef] [PubMed]
- Denkel, L.A.; Gastmeier, P.; Piening, B. To screen or not to screen mothers of preterm infants for extended-spectrum beta-lactamase-producing Enterobacteriaceae (ESBL-E). J. Perinatol. 2015, 35, 893–894. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schwaber, M.J.; Navon-Venezia, S.; Kaye, K.S.; Ben-Ami, R.; Schwartz, D.; Carmeli, Y. Clinical and economic impact of bacteremia with extended- spectrum-beta-lactamase-producing Enterobacteriaceae. Antimicrob. Agents Chemother. 2006, 50, 1257–1262. [Google Scholar] [CrossRef] [PubMed]
- Bert, F.; Larroque, B.; Paugam-Burtz, C.; Dondero, F.; Durand, F.; Marcon, E.; Belghiti, J.; Moreau, R.; Nicolas-Chanoine, M.-H. Pretransplant Fecal Carriage of Extended-Spectrum β-Lactamase–producing Enterobacteriaceae and Infection after Liver Transplant, France. Emerg. Infect. Dis. 2012, 18, 908–916. [Google Scholar] [CrossRef]
- Weese, J.S. Antimicrobial use and antimicrobial resistance in horses. Equine Vet. J. 2015, 47, 747–749. [Google Scholar] [CrossRef] [Green Version]
- Dolejska, M.; Duskova, E.; Rybarikova, J.; Janoszowska, D.; Roubalova, E.; Dibdakova, K.; Maceckova, G.; Kohoutova, L.; Literak, I.; Smola, J.; et al. Plasmids carrying blaCTX-M-1 and qnr genes in Escherichia coli isolates from an equine clinic and a horseback riding centre. J. Antimicrob. Chemother. 2011, 66, 757–764. [Google Scholar] [CrossRef] [Green Version]
- Walther, B.; Klein, K.-S.; Barton, A.-K.; Semmler, T.; Huber, C.; Wolf, S.A.; Tedin, K.; Merle, R.; Mitrach, F.; Guenther, S.; et al. Extended-spectrum beta-lactamase (ESBL)-producing Escherichia coli and Acinetobacter baumannii among horses entering a veterinary teaching hospital: The contemporary “Trojan Horse”. PLoS ONE 2018, 13, e0191873. [Google Scholar] [CrossRef]
- Wohlfender, F.D.; Barrelet, F.E.; Doherr, M.G.; Straub, R.; Meier, H.P. Diseases in neonatal foals. Part 1: the 30 day incidence of disease and the effect of prophylactic antimicrobial drug treatment during the first three days post partum. Equine Vet. J. 2009, 41, 179–185. [Google Scholar] [CrossRef]
- Theelen, M.J.P.; Wilson, W.D.; Edman, J.M.; Magdesian, K.G.; Kass, P.H. Temporal trends in prevalence of bacteria isolated from foals with sepsis: 1979-2010. Equine Vet. J. 2014, 46, 169–173. [Google Scholar] [CrossRef] [PubMed]
- Dyakova, E.; Bisnauthsing, K.N.; Querol-Rubiera, A.; Patel, A.; Ahanonu, C.; Tosas Auguet, O.; Edgeworth, J.D.; Goldenberg, S.D.; Otter, J.A. Efficacy and acceptability of rectal and perineal sampling for identifying gastrointestinal colonization with extended spectrum β-lactamase Enterobacteriaceae. Clin. Microbiol. Infect. 2017, 23, 577.e1–577.e3. [Google Scholar] [CrossRef] [PubMed]
- Brewer, B.D.; Koterba, A.M.; Carter, R.L.; Rowe, E.D. Comparison of empirically developed sepsis score with a computer generated and weighted scoring system for the identification of sepsis in the equine neonate. Equine Vet. J. 1988, 20, 23–24. [Google Scholar] [CrossRef] [PubMed]
- Oreff, G.L.; Tatz, A.J.; Dahan, R.; Segev, G.; Berlin, D.; Kelmer, G. Surgical management and long-term outcome of umbilical infection in 65 foals (2010-2015). Vet. Surg. 2017, 46, 962–970. [Google Scholar] [CrossRef] [PubMed]
- Murk, J.-L.A.N.; Heddema, E.R.; Hess, D.L.J.; Bogaards, J.A.; Vandenbroucke-Grauls, C.M.J.E.; Debets-Ossenkopp, Y.J. Enrichment broth improved detection of extended-spectrum-beta-lactamase-producing bacteria in throat and rectal surveillance cultures of samples from patients in intensive care units. J. Clin. Microbiol. 2009, 47, 1885–1887. [Google Scholar] [CrossRef]
- Clinical and Laboratory Standards Institute (CLSI). Performance Standards for Antimicrobial Susceptibility Testing, 26th ed.; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2016. [Google Scholar]
- Falagas, M.E.; Karageorgopoulos, D.E. Pandrug Resistance (PDR), Extensive Drug Resistance (XDR), and Multidrug Resistance (MDR) among Gram-Negative Bacilli: Need for International Harmonization in Terminology. Clin. Infect. Dis. 2008, 46, 1121–1122. [Google Scholar] [CrossRef]
- Woodford, N.; Fagan, E.J.; Ellington, M.J. Multiplex PCR for rapid detection of genes encoding CTX-M extended-spectrum β-lactamases. J. Antimicrob. Chemother. 2006, 57, 154–155. [Google Scholar] [CrossRef]
- Lin, S.-P.; Liu, M.-F.; Lin, C.-F.; Shi, Z.-Y. Phenotypic detection and polymerase chain reaction screening of extended-spectrum β-lactamases produced by Pseudomonas aeruginosa isolates. J. Microbiol. Immunol. Infect. 2012, 45, 200–207. [Google Scholar] [CrossRef]
- Tofteland, S.; Haldorsen, B.; Dahl, K.H.; Simonsen, G.S.; Steinbakk, M.; Walsh, T.R.; Sundsfjord, A.; Norwegian ESBL Study Group. Effects of phenotype and genotype on methods for detection of extended-spectrum-beta-lactamase-producing clinical isolates of Escherichia coli and Klebsiella pneumoniae in Norway. J. Clin. Microbiol. 2007, 45, 199–205. [Google Scholar] [CrossRef]
- Johnson, J.R.; Clermont, O.; Johnston, B.; Clabots, C.; Tchesnokova, V.; Sokurenko, E.; Junka, A.F.; Maczynska, B.; Denamur, E. Rapid and Specific Detection, Molecular Epidemiology, and Experimental Virulence of the O16 Subgroup within Escherichia coli Sequence Type 131. J. Clin. Microbiol. 2014, 52, 1358–1365. [Google Scholar] [CrossRef] [Green Version]
- Versalovic, J.; Koeuth, T.; Lupski, J.R. Distribution of repetitive DNA sequences in eubacteria and application to fingerprinting of bacterial genomes. Nucleic Acids Res. 1991, 19, 6823–6831. [Google Scholar] [CrossRef] [PubMed]
- Heras, J.; Domínguez, C.; Mata, E.; Pascual, V.; Lozano, C.; Torres, C.; Zarazaga, M. GelJ – a tool for analyzing DNA fingerprint gel images. BMC Bioinforma. 2015, 16, 270. [Google Scholar] [CrossRef] [PubMed]
- Herzog, K.A.T.; Schneditz, G.; Leitner, E.; Feierl, G.; Hoffmann, K.M.; Zollner-Schwetz, I.; Krause, R.; Gorkiewicz, G.; Zechner, E.L.; Högenauer, C. Genotypes of Klebsiella oxytoca isolates from patients with nosocomial pneumonia are distinct from those of isolates from patients with antibiotic-associated hemorrhagic colitis. J. Clin. Microbiol. 2014, 52, 1607–1616. [Google Scholar] [CrossRef] [PubMed]
- Wirth, T.; Falush, D.; Lan, R.; Colles, F.; Mensa, P.; Wieler, L.H.; Karch, H.; Reeves, P.R.; Maiden, M.C.J.; Ochman, H.; et al. Sex and virulence in Escherichia coli: an evolutionary perspective. Mol. Microbiol. 2006, 60, 1136–1151. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Diancourt, L.; Passet, V.; Verhoef, J.; Grimont, P.A.D.; Brisse, S. Multilocus Sequence Typing of Klebsiella pneumoniae Nosocomial Isolates. J. Clin. Microbiol. 2005, 43, 4178–4182. [Google Scholar] [CrossRef]
- Palmer, J. Update on the management of neonatal sepsis in horses. Vet. Clin. North Am. Equine Pract. 2014, 30, 317–336. [Google Scholar] [CrossRef]
- McHugh, M.L. Interrater reliability: the kappa statistic. Biochem. Med. 2012, 22, 276–282. [Google Scholar] [CrossRef]
- Maddox, T.W.; Clegg, P.D.; Diggle, P.J.; Wedley, A.L.; Dawson, S.; Pinchbeck, G.L.; Williams, N.J. Cross-sectional study of antimicrobial-resistant bacteria in horses. Part 1: Prevalence of antimicrobial-resistant Escherichia coli and methicillin-resistant Staphylococcus aureus. Equine Vet. J. 2012, 44, 289–296. [Google Scholar] [CrossRef]
- Maddox, T.W.; Williams, N.J.; Clegg, P.D.; O’Donnell, A.J.; Dawson, S.; Pinchbeck, G.L. Longitudinal study of antimicrobial-resistant commensal Escherichia coli in the faeces of horses in an equine hospital. Prev. Vet. Med. 2011, 100, 134–145. [Google Scholar] [CrossRef]
- Paterson, D.L.; Bonomo, R.A. Extended-Spectrum β-Lactamases: a Clinical Update. Clin. Microbiol. Rev. 2005, 18, 657–686. [Google Scholar] [CrossRef]
- Adler, A.; Sturlesi, N.A.; Fallach, N.; Zilberman-Barzilai, D.; Hussein, O.; Blum, S.E.; Klement, E.; Schwaber, M.J.; Carmeli, Y. Prevalence, Risk Factors, and Transmission Dynamics of Extended-Spectrum-β-Lactamase-Producing Enterobacteriaceae: a National Survey of Cattle Farms in Israel in 2013. J. Clin. Microbiol. 2015, 53, 3515–3521. [Google Scholar] [PubMed]
- Damborg, P.; Marskar, P.; Baptiste, K.E.; Guardabassi, L. Faecal shedding of CTX-M-producing Escherichia coli in horses receiving broad-spectrum antimicrobial prophylaxis after hospital admission. Vet. Microbiol. 2012, 154, 298–304. [Google Scholar] [CrossRef] [PubMed]
- Olivo, G.; Lucas, T.M.; Borges, A.S.; Silva, R.O.S.; Lobato, F.C.F.; Siqueira, A.K.; da Silva Leite, D.; Brandão, P.E.; Gregori, F.; de Oliveira-Filho, J.P.; et al. Enteric Pathogens and Coinfections in Foals with and without Diarrhea. BioMed Res. Int. 2016, 2016, 1512690. [Google Scholar] [CrossRef] [PubMed]
- Barceló Oliver, F.; Russell, T.M.; Uprichard, K.L.; Neil, K.M.; Pollock, P.J. Treatment of septic arthritis of the coxofemoral joint in 12 foals. Vet. Surg. 2017, 46, 530–538. [Google Scholar] [CrossRef] [PubMed]
- Ernst, N.S.; Hernandez, J.A.; MacKay, R.J.; Brown, M.P.; Gaskin, J.M.; Nguyen, A.D.; Giguere, S.; Colahan, P.T.; Troedsson, M.R.; Haines, G.R.; et al. Risk factors associated with fecal Salmonella shedding among hospitalized horses with signs of gastrointestinal tract disease. J. Am. Vet. Med. Assoc. 2004, 225, 275–281. [Google Scholar] [CrossRef] [PubMed]
- Denkel, L.A.; Schwab, F.; Kola, A.; Leistner, R.; Garten, L.; von Weizsäcker, K.; Geffers, C.; Gastmeier, P.; Piening, B. The mother as most important risk factor for colonization of very low birth weight (VLBW) infants with extended-spectrum β-lactamase-producing Enterobacteriaceae (ESBL-E). J. Antimicrob. Chemother. 2014, 69, 2230–2237. [Google Scholar] [CrossRef] [PubMed]
- Callens, B.; Faes, C.; Maes, D.; Catry, B.; Boyen, F.; Francoys, D.; de Jong, E.; Haesebrouck, F.; Dewulf, J. Presence of antimicrobial resistance and antimicrobial use in sows are risk factors for antimicrobial resistance in their offspring. Microb. Drug Resist. 2015, 21, 50–58. [Google Scholar] [CrossRef] [PubMed]
- Simonsen, K.A.; Anderson-Berry, A.L.; Delair, S.F.; Davies, H.D. Early-Onset Neonatal Sepsis. Clin. Microbiol. Rev. 2014, 27, 21–47. [Google Scholar] [CrossRef] [Green Version]
- Li, X.; Xu, X.; Yang, X.; Luo, M.; Liu, P.; Su, K.; Qing, Y.; Chen, S.; Qiu, J.; Li, Y. Risk factors for infection and/or colonisation with extended-spectrum β-lactamase-producing bacteria in the neonatal intensive care unit: a meta-analysis. Int. J. Antimicrob. Agents 2017, 50, 622–628. [Google Scholar] [CrossRef]
| Pathology | No. of Horses (%) | |
|---|---|---|
| On Admission | Developed during Hospitalization | |
| Foals | n = 55 | n = 33 |
| Diarrhea | 13 (24) | 4 (12) |
| Umbilical infection | 13 (24) | 0 |
| Sepsis | 12 (22) | 0 |
| Prematurity | 10 (17) | 0 |
| Septic polyarthritis | 9 (16) | 0 |
| Orthopedic problems (other than septic polyarthritis) | 9 (15) | 1 (3) |
| Perinatal Asphyxia Syndrome (PAS) | 8 (13) | 0 |
| Respiratory problems | 6 (11) | 0 |
| Colic | 6 (10) | 1 (3) |
| Injury | 3 (5) | 0 |
| Neurological signs (other than PAS) | 1 (2) | 4 (12) |
| Uroperitoneum | 1 (2) | 1 (3) |
| Phlebitis | 0 | 1 (3) |
| Uveitis | 0 | 2 (6) |
| Peritonitis | 0 | 1 (3) |
| Other (hernia, guttural pouch tympany and piroplasmosis) | 3 (5) | 0 |
| Mares | n = 55 | n = 33 |
| Colic | 2 (4) | 2 (6) |
| Retained placenta | 2 (4) | 0 |
| Injury | 1 (2) | 0 |
| Orthopedic syndromes | 1 (2) | 0 |
| Placentitis | 1 (2) | 0 |
| Colitis | 0 | 1 (3) |
| on Admission 1 | ≥ 72 h of Hospitalization 2 | |||||
|---|---|---|---|---|---|---|
| Horses | Shedding (%) | Total No. of ESBL-E Isolates | blaESBL Genes (%) 3 | (%)Shedding | Total No. of ESBL-E Isolates | blaESBL Genes (%) 3 |
| Foals | 18/55 (33) (95% CI 21–47) | 18 | BlaCTXM-1: 14/19 (74) BlaCTXM-9: 2/19 (11) | 28/33 (85) 7 (95% CI 70–94) | 46 4 | CTX-M-1: 31/46 (67) CTX-M-2: 1/46 (2) OXA-1: 3/46 (7) |
| Mares | 9/55 (16) (95% CI 8–29) | 115 | BlaCTXM-1: 6/11 (55) | 19/33 (58) 8 (95% CI 40–73) | 27 6 | CTX-M-1: 16/27 (59) CTX-M-2: 1/27 (4) CTX-M-9: 2/27 (7) TEM-163: 2/27 (7) |
| Foal | Age on Admission | ESBL-E Shedding Status | Clinical ESBL-E Infection | |||||
|---|---|---|---|---|---|---|---|---|
| 1st Admission | 1st Hospitalization | 2nd Admission | 2nd Hospitalization | ESBL-E Species | Source | Outcome | ||
| 1 | <12 h | Negative | E. coli ST88 | K. pneumoniae ST1552 | Not sampled | K. pneumoniae ST1552 | abscess 1 | Discharged |
| 2 | <12 h | Negative | E. coli ST38 | E. coli ST86 K. oxytoca ST194 S. enterica | Enterobacter cloacae | E. coli ST746 | umbilicus 2 | Euthanized |
| E. coli ST746 | wound | |||||||
| 3 | <12 h | Negative | E. coli ST746 K. pneumoniae ST37 | No second hospitalization | E. coli ST746 K. pneumoniae ST585 | umbilicus 3 | Euthanized | |
| S. enterica | ||||||||
| 4 | 17 d | E. coli4 | Discharged | No second hospitalization | E. coli ST69 | wound 5 | Discharged | |
| E. coli ST69 | umbilicus | |||||||
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shnaiderman-Torban, A.; Paitan, Y.; Arielly, H.; Kondratyeva, K.; Tirosh-Levy, S.; Abells-Sutton, G.; Navon-Venezia, S.; Steinman, A. Extended-Spectrum β-Lactamase-Producing Enterobacteriaceae in Hospitalized Neonatal Foals: Prevalence, Risk Factors for Shedding and Association with Infection. Animals 2019, 9, 600. https://doi.org/10.3390/ani9090600
Shnaiderman-Torban A, Paitan Y, Arielly H, Kondratyeva K, Tirosh-Levy S, Abells-Sutton G, Navon-Venezia S, Steinman A. Extended-Spectrum β-Lactamase-Producing Enterobacteriaceae in Hospitalized Neonatal Foals: Prevalence, Risk Factors for Shedding and Association with Infection. Animals. 2019; 9(9):600. https://doi.org/10.3390/ani9090600
Chicago/Turabian StyleShnaiderman-Torban, Anat, Yossi Paitan, Haia Arielly, Kira Kondratyeva, Sharon Tirosh-Levy, Gila Abells-Sutton, Shiri Navon-Venezia, and Amir Steinman. 2019. "Extended-Spectrum β-Lactamase-Producing Enterobacteriaceae in Hospitalized Neonatal Foals: Prevalence, Risk Factors for Shedding and Association with Infection" Animals 9, no. 9: 600. https://doi.org/10.3390/ani9090600
APA StyleShnaiderman-Torban, A., Paitan, Y., Arielly, H., Kondratyeva, K., Tirosh-Levy, S., Abells-Sutton, G., Navon-Venezia, S., & Steinman, A. (2019). Extended-Spectrum β-Lactamase-Producing Enterobacteriaceae in Hospitalized Neonatal Foals: Prevalence, Risk Factors for Shedding and Association with Infection. Animals, 9(9), 600. https://doi.org/10.3390/ani9090600






