Hypoxia Limits the Growth of Bovine Follicles in Vitro by Inhibiting Estrogen Receptor α
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Isolation and in Vitro Growth of Bovine Follicles
2.2. Treatment of Follicles Cultured in Vitro
2.3. RNA Extraction, RT-PCR and Real-time PCR
2.4. Western Blot
2.5. Statistical Analysis
3. Results
3.1. Effects of Hypoxia on Follicular Growth in Vitro
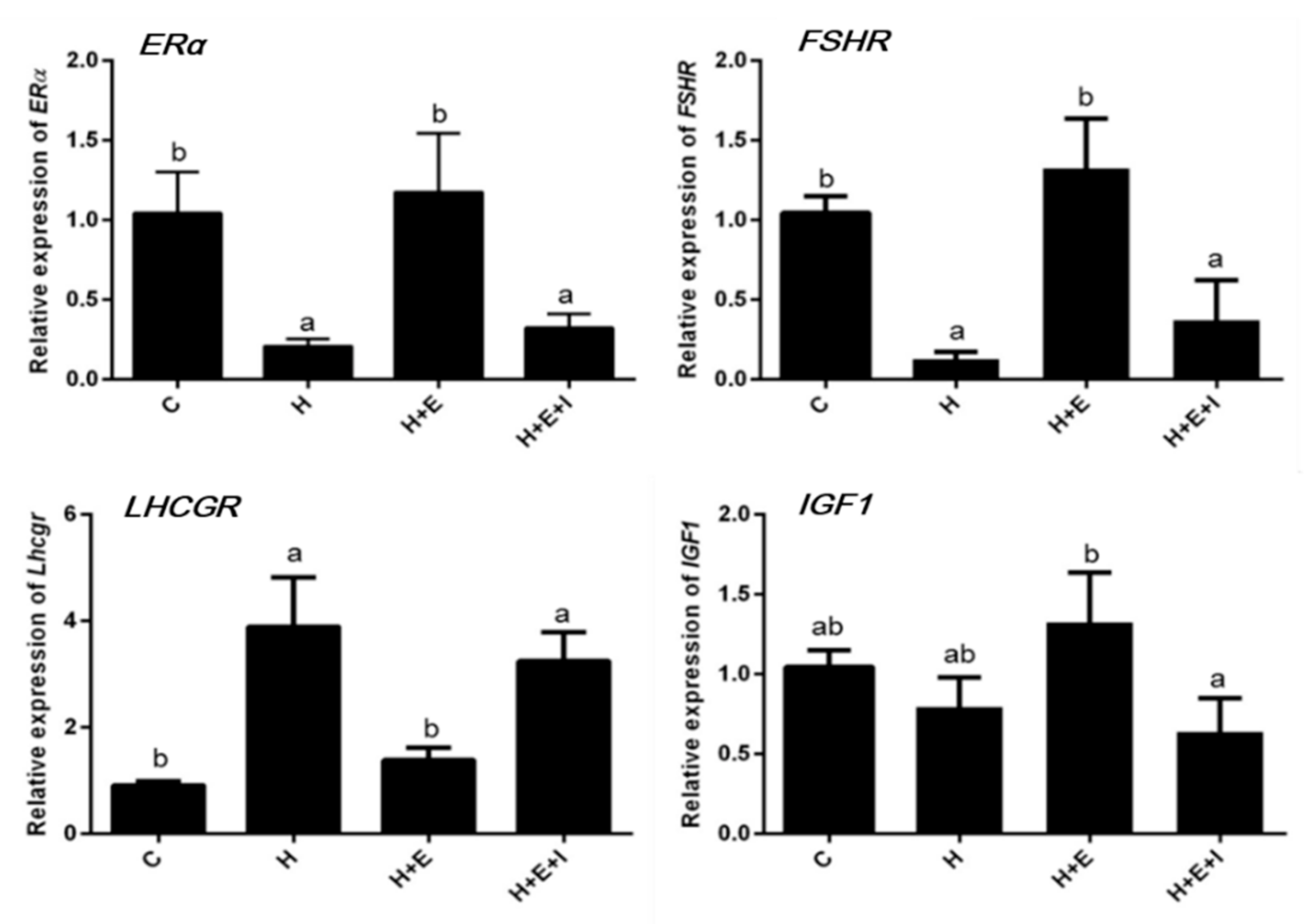
3.2. Effects of Hypoxia on Expression of Follicular Growth-Related Genes
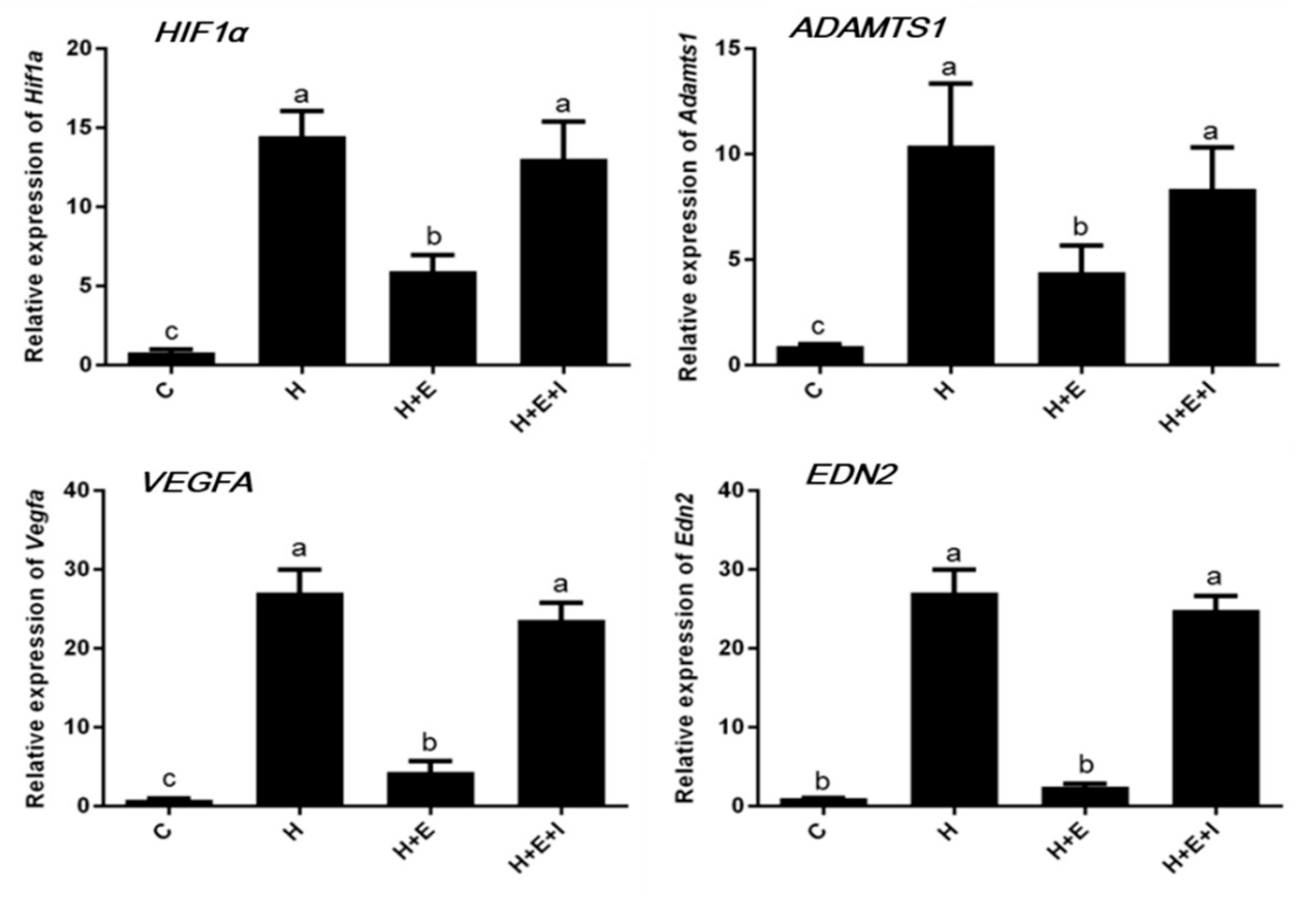
3.3. Effects of Hypoxia on Expression of Hypoxia-Inducible Factor-Related Genes
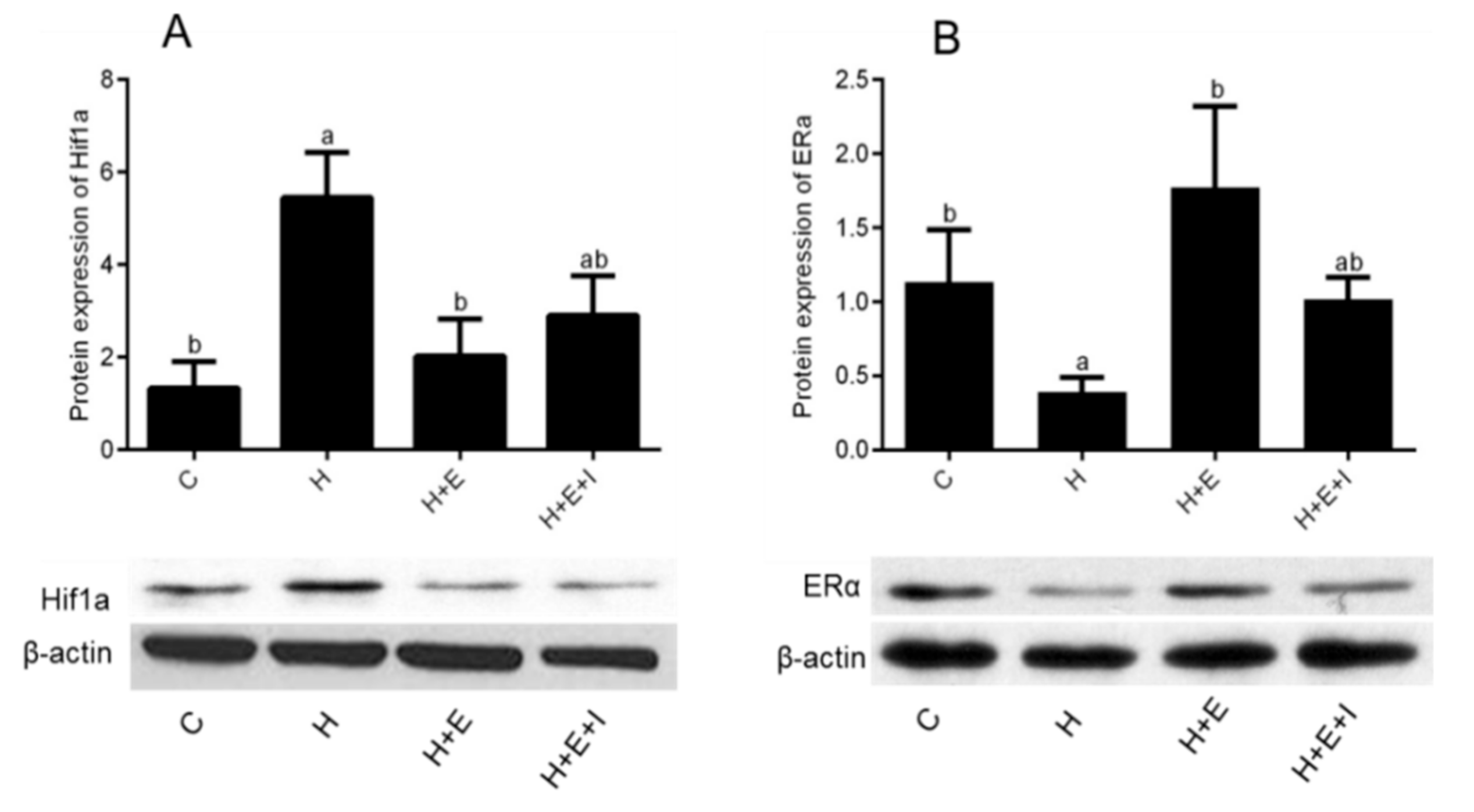
3.4. Effects of Hypoxia on Expression of HIF1A and ERα Proteins
4. Discussions
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Parraguez, V.C.; Atlagich, M.; Diaz, R.; Bruzzone, M.E.; Behn, C.; Raggi, L.A. Effect of hypobaric hypoxia on lamb intrauterine growth: Comparison between high- and low-altitude native ewes. Reprod. Fertil. Dev. 2005, 17, 497–505. [Google Scholar] [CrossRef] [PubMed]
- Parraguez, V.H.; Atlagich, M.; Díaz, R.; Cepeda, R.; González, C.; De, L.R.M.; Bruzzone, M.E.; Behn, C.; Raggi, L.A. Ovine placenta at high altitudes: Comparison of animals with different times of adaptation to hypoxic environment. Anim. Reprod. Sci. 2006, 95, 151–157. [Google Scholar] [CrossRef] [PubMed]
- Hartinger, S.; Tapia, V.; Carrillo, C.; Bejarano, L.; Gonzales, G.F. Birth weight at high altitudes in Peru. Int. J. Gynecol. Obstet. 2006, 93, 275–281. [Google Scholar] [CrossRef] [PubMed]
- Parraguez, V.H.; Diaz, F.; Cofre, E.; Urquieta, B.; De Los, R.M.; Astiz, S.; Gonzalez-Bulnes, A. Fertility of a high-altitude sheep model is compromised by deficiencies in both preovulatory follicle development and plasma LH availability. Reprod. Domest. Anim. 2014, 49, 977–984. [Google Scholar] [CrossRef] [PubMed]
- Connolly, J.M.; Kane, M.T.; Quinlan, L.R.; Dockery, P.; Hynes, A.C. Corrigendum to: Hypoxia limits mouse follicle growth in vitro. Reprod. Fertil. Dev. 2017, 29, 431. [Google Scholar] [CrossRef] [PubMed]
- Korach, K.S. Insights from the study of animals lacking functional estrogen receptor. Science 1994, 266, 1524–1527. [Google Scholar] [CrossRef]
- Krege, J.H.; Hodgin, J.B.; Couse, J.F.; Enmark, E.; Warner, M.; Mahler, J.F.; Sar, M.; Korach, K.S.; Gustafsson, J.A.; Smithies, O. Generation and reproductive phenotypes of mice lacking estrogen receptor beta. Proc. Natl. Acad. Sci. USA 1998, 95, 15677–15682. [Google Scholar] [CrossRef]
- Wu, M.H.; Lu, C.W.; Chang, F.M.; Tsai, S.J. Estrogen receptor expression affected by hypoxia inducible factor-1alpha in stromal cells from patients with endometriosis. Taiwan J. Obstet. Gynecol. 2012, 51, 50–54. [Google Scholar] [CrossRef]
- Kim, J.; Bagchi, I.C.; Bagchi, M.K. Signaling by hypoxia-inducible factors is critical for ovulation in mice. Endocrinology 2009, 150, 3392–3400. [Google Scholar] [CrossRef]
- Kim, M.; Neinast, M.D.; Frank, A.P.; Sun, K.; Park, J.; Zehr, J.A.; Vishvanath, L.; Morselli, E.; Amelotte, M.; Palmer, B.F.; et al. ERα upregulates Phd3 to ameliorate HIF-1 induced fibrosis and inflammation in adipose tissue. Mol. Metab. 2014, 3, 642–651. [Google Scholar] [CrossRef]
- Xu, M.; Barrett, S.L.; West-Farrell, E.; Kondapalli, L.A.; Kiesewetter, S.E.; Shea, L.D.; Woodruff, T.K. In vitro grown human ovarian follicles from cancer patients support oocyte growth. Hum. Reprod. 2009, 24, 2531–2540. [Google Scholar] [CrossRef] [PubMed]
- West, E.R.; Zelinski, M.B.; Kondapalli, L.A.; Gracia, C.; Chang, J.; Coutifaris, C.; Critser, J.; Stouffer, R.L.; Shea, L.D.; Woodruff, T.K. Preserving female fertility following cancer treatment: Current options and future possibilities. Pediatr. Blood Cancer 2009, 53, 289–295. [Google Scholar] [CrossRef] [PubMed]
- Picton, H.M.; Harris, S.E.; Muruvi, W.; Chambers, E.L. The in vitro growth and maturation of follicles. Reproduction 2008, 136, 703–715. [Google Scholar] [CrossRef] [PubMed]
- Smitz, J.E.; Cortvrindt, R.G. The earliest stages of folliculogenesis in vitro. Reproduction 2002, 123, 185–202. [Google Scholar] [CrossRef] [PubMed]
- Telfer, E.E.; Zelinski, M.B. Ovarian follicle culture: Advances and challenges for human and nonhuman primates. Fertil. Steril. 2013, 99, 1523–1533. [Google Scholar] [CrossRef] [PubMed]
- Thompson, J.G.; Brown, H.M.; Kind, K.L.; Russell, D.L. The Ovarian Antral Follicle: Living on the Edge of Hypoxia or Not? Biol. Reprod. 2015, 92, 153. [Google Scholar] [CrossRef] [PubMed]
- Nishimura, R.; Okuda, K. Hypoxia is important for establishing vascularization during corpus luteum formation in cattle. J. Reprod. Dev. 2010, 56, 110–116. [Google Scholar] [CrossRef]
- Wang, G.L.; Jiang, B.H.; Rue, E.A.; Semenza, G.L. Hypoxia-inducible factor 1 is a basic-helix-loop-helix-PAS heterodimer regulated by cellular O2 tension. Proc. Natl. Acad. Sci. USA 1995, 92, 5510–5514. [Google Scholar] [CrossRef]
- Spears, N.; Boland, N.I.; Murray, A.A.; Gosden, R.G. Mouse oocytes derived from in vitro grown primary ovarian follicles are fertile. Hum. Reprod. 1994, 9, 527–532. [Google Scholar] [CrossRef]
- Wycherley, G.; Downey, D.; Kane, M.T.; Hynes, A.C. A novel follicle culture system markedly increases follicle volume, cell number and oestradiol secretion. Reproduction 2004, 127, 669–677. [Google Scholar] [CrossRef][Green Version]
- Arao, Y.; Hamilton, K.J.; Ray, M.K.; Scott, G.; Mishina, Y.; Korach, K.S. Estrogen receptor alpha AF-2 mutation results in antagonist reversal and reveals tissue selective function of estrogen receptor modulators. Proc. Natl. Acad. Sci. USA 2011, 108, 14986–14991. [Google Scholar] [CrossRef] [PubMed]
- Jungyoon, C.; Dukkyung, K.; Seungki, L.; Youngjoo, L. Cobalt chloride-induced estrogen receptor alpha down-regulation involves hypoxia-inducible factor-1alpha in MCF-7 human breast cancer cells. Mol. Endocrinol. 2005, 19, 1191–1199. [Google Scholar]
- Yi, J.M.; Kwon, H.Y.; Cho, J.Y.; Lee, Y.J. Estrogen and hypoxia regulate estrogen receptor alpha in a synergistic manner. Biochem. Biophys. Res. Commun. 2009, 378, 842–846. [Google Scholar] [CrossRef] [PubMed]
- Kind, K.L.; Banwell, K.M.; Gebhardt, K.M.; Macpherson, A.; Gauld, A.; Russell, D.L.; Thompson, J.G. Microarray analysis of mRNA from cumulus cells following in vivo or in vitro maturation of mouse cumulus-oocyte complexes. Reprod. Fertil. Dev. 2013, 25, 426–438. [Google Scholar] [CrossRef] [PubMed]
- Brown, H.M.; Anastasi, M.R.; Frank, L.A.; Kind, K.L.; Richani, D.; Robker, R.L.; Russell, D.L.; Gilchrist, R.B.; Thompson, J.G. Hemoglobin: A gas transport molecule that is hormonally regulated in the ovarian follicle in mice and humans. Biol. Reprod. 2015, 92, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Watson, C.S.; Jeng, Y.J.; Guptarak, J. Endocrine disruption via estrogen receptors that participate in nongenomic signaling pathways. J. Steroid Biochem. Mol. Biol. 2011, 127, 44–50. [Google Scholar] [CrossRef] [PubMed]
- Boonyaprakob, U.; Gadsby, J.E.; Hedgpeth, V.; Routh, P.A.; Almond, G.W. Expression and localization of hypoxia inducible factor-1alpha mRNA in the porcine ovary. Can. J. Vet. Res. 2005, 69, 215–222. [Google Scholar]
- West-Farrell, E.R.; Xu, M.; Gomberg, M.A.; Chow, Y.H.; Woodruff, T.K.; Shea, L.D. The mouse follicle microenvironment regulates antrum formation and steroid production: Alterations in gene expression profiles. Biol. Reprod. 2009, 80, 432–439. [Google Scholar] [CrossRef][Green Version]
- Xu, J.; Lawson, M.S.; Yeoman, R.R.; Pau, K.Y.; Barrett, S.L.; Zelinski, M.B.; Stouffer, R.L. Secondary follicle growth and oocyte maturation during encapsulated three-dimensional culture in rhesus monkeys: Effects of gonadotrophins, oxygen and fetuin. Reproduction 2011, 26, 1061–1072. [Google Scholar] [CrossRef]
- Richards, J.S.; Hernandez-Gonzalez, I.; Gonzalez-Robayna, I.; Teuling, E.; Lo, Y.; Boerboom, D.; Falender, A.E.; Doyle, K.H.; LeBaron, R.G.; Thompson, V. Regulated expression of ADAMTS family members in follicles and cumulus oocyte complexes: Evidence for specific and redundant patterns during ovulation. Biol. Reprod. 2005, 72, 1241–1255. [Google Scholar] [CrossRef]
- Hatipoglu, O.F.; Hirohata, S.; Cilek, M.Z.; Ogawa, H.; Miyoshi, T.; Obika, M.; Demircan, K.; Shinohata, R.; Kusachi, S.; Ninomiya, Y. ADAMTS1 is a unique hypoxic early response gene expressed by endothelial cells. J. Biol. Chem. 2009, 284, 16325–16333. [Google Scholar] [CrossRef] [PubMed]
- Rico, C.; Dodelet Devillers, A.; Paquet, M.; Tsoi, M.; Lapointe, E.; Carmeliet, P.; Boerboom, D. HIF1 Activity in Granulosa Cells Is Required for FSH-Regulated VEGFA Expression and Follicle Survival in Mice1. Biol. Reprod. 2014, 90, 135. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wang, S.F.; Zheng, J.D.; Zhao, C.B.; Zhang, Y.N.; Liu, L.L.; Huang, J.H. Effects of testosterone on the expression levels of AMH, VEGF and HIF-1α in mouse granulosa cells. Exp. Ther. Med. 2016, 12, 883–888. [Google Scholar] [CrossRef] [PubMed]
- De Francesco, E.M.; Lappano, R.; Santolla, M.F.; Marsico, S.; Caruso, A.; Maggiolini, M. HIF-1α/GPER signaling mediates the expression of VEGF induced by hypoxia in breast cancer associated fibroblasts (CAFs). Breast Cancer Res. 2013, 15, 64. [Google Scholar] [CrossRef] [PubMed]
- Klipper, E.; Levit, A.; Mastich, Y.; Berisha, B.; Schams, D.; Meidan, R. Induction of endothelin-2 expression by luteinizing hormone and hypoxia: Possible role in bovine corpus luteum formation. Endocrinology 2010, 151, 1914–1922. [Google Scholar] [CrossRef] [PubMed]
- Yalu, R.; Oyesiji, A.E.; Eisenberg, I.; Imbar, T.; Meidan, R. HIF1A-dependent increase in endothelin 2 levels in granulosa cells: Role of hypoxia, LH/cAMP, and reactive oxygen species. Reproduction 2015, 149, 11–20. [Google Scholar] [CrossRef]
- Na, G.; Bridges, P.J.; Koo, Y.; Ko, C. Role of hypoxia in the regulation of periovulatory EDN2 expression in the mouse. Can. J. Physiol. Pharm. 2008, 86, 310. [Google Scholar] [CrossRef]
- Li, Y.; Liu, Y.; Lu, Y.; Zhao, B. Inhibitory effects of 17β-estradiol or a resveratrol dimer on hypoxia-inducible factor-1α in genioglossus myoblasts: Involvement of ERα and its downstream p38 MAPK pathways. Int. J. Mol. Med. 2017, 40, 1347–1356. [Google Scholar] [CrossRef]
| Gene | Forward Primers (5′–3′) | Reverse Primers (5′–3′) |
|---|---|---|
| β-ACTIN | ATATCGCTGCGCTGGTCGTC | GCTGCCTCAACACCTCAACCC |
| ERα | AATTCTGACAATCGACGCCAG | GTGCTTCAACATTCTCCCTCCTC |
| FSHR | GCTCCCAATGCAGACCTCTT | CCTCCAGTTTGCAAAGGCAC |
| LHCGR | TGCCTTTGACAACCTCCTCA | AGCATCTGGTTCAGGAGCAC |
| IGF1 | CTTGAAGCAGGTGAAGATGCC | AGAGCATCCACCAACTCAGC |
| HIF1A | AGATCTCGGCGAAGCAAAGAGT | CGGCATCCAGAAGTTTTCTCACAC |
| ADAMTS1 | TGCTCCAAGACATGCGGCTCAG | TGGTACTGGCTGGCTTCACTTCC |
| VEGFA | CTGTGCAGGCTGCTGTAACG | GTTCCCGAAACCCTGAGGAG |
| EDN2 | CTGGCCTGAAGCTGTGGT | ATAGGGGCCAGCCATGAT |
| Items | Day 1 | Day 2 | Day 3 | Day 4 | Day 5 | Day 6 |
|---|---|---|---|---|---|---|
| Control (μm) | 145.33 ± 6.53 | 175.45 ± 7.84 A | 238.36 ± 10.67 Ba | 281.70 ± 15.55 Ca | 316.67 ± 8.59 Da | 342.26 ± 11.86 Ea |
| Hypoxia (μm) | 146.26 ± 8.31 | 173.63 ± 5.48 A | 205.48 ± 9.22 Bb | 235.87 ± 12.30 Cb | 258.16 ± 8.65 Db | 276.37 ± 7.52 Ec |
| Hypoxia + E2 (μm) | 146.33 ± 4.21 | 172.34 ± 4.78 A | 230.25 ± 7.61 Ba | 275.07 ± 10.24 Ca | 307.61 ± 9.85 Da | 321.66 ± 8.11 Eb |
| Hypoxia + E2 + ICI (μm) | 146.26 ± 8.31 | 174.36 ± 8.45 A | 211.36 ± 2.92 Bb | 240.78 ± 3.12 Cb | 261.65 ± 6.81 Db | 281.67 ± 5.27 Ec |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, L.; Wang, L.; Gao, H.; Liu, N.; Zheng, Y.; Gao, Y.; Liu, S.; Jiang, Z. Hypoxia Limits the Growth of Bovine Follicles in Vitro by Inhibiting Estrogen Receptor α. Animals 2019, 9, 551. https://doi.org/10.3390/ani9080551
Ma L, Wang L, Gao H, Liu N, Zheng Y, Gao Y, Liu S, Jiang Z. Hypoxia Limits the Growth of Bovine Follicles in Vitro by Inhibiting Estrogen Receptor α. Animals. 2019; 9(8):551. https://doi.org/10.3390/ani9080551
Chicago/Turabian StyleMa, Lizhu, Liqiang Wang, Huimin Gao, Ning Liu, Yuxin Zheng, Yan Gao, Shujie Liu, and Zhongliang Jiang. 2019. "Hypoxia Limits the Growth of Bovine Follicles in Vitro by Inhibiting Estrogen Receptor α" Animals 9, no. 8: 551. https://doi.org/10.3390/ani9080551
APA StyleMa, L., Wang, L., Gao, H., Liu, N., Zheng, Y., Gao, Y., Liu, S., & Jiang, Z. (2019). Hypoxia Limits the Growth of Bovine Follicles in Vitro by Inhibiting Estrogen Receptor α. Animals, 9(8), 551. https://doi.org/10.3390/ani9080551




