Potential Impact of Construction Noise on Selected Zoo Animals
Simple Summary
Abstract
1. Introduction
- To what extent does exposure to construction noise affect activity budgets of focal zoo species?
- Is behavior affected differently by continuous or intermittent noise?
- Is exposure to construction noise associated with a significant increase in glucocorticoid concentration?
- Do focal animals move away from the noise source during exposure?
- Does noise exposure affect social spacing between individuals?
2. Materials and Methods
2.1. Experimental Approach
2.2. Animals and Facility
2.3. Experimental Protocol
Construction Noise Measurement and Exposure
2.4. Enclosure Noise Contour Mapping
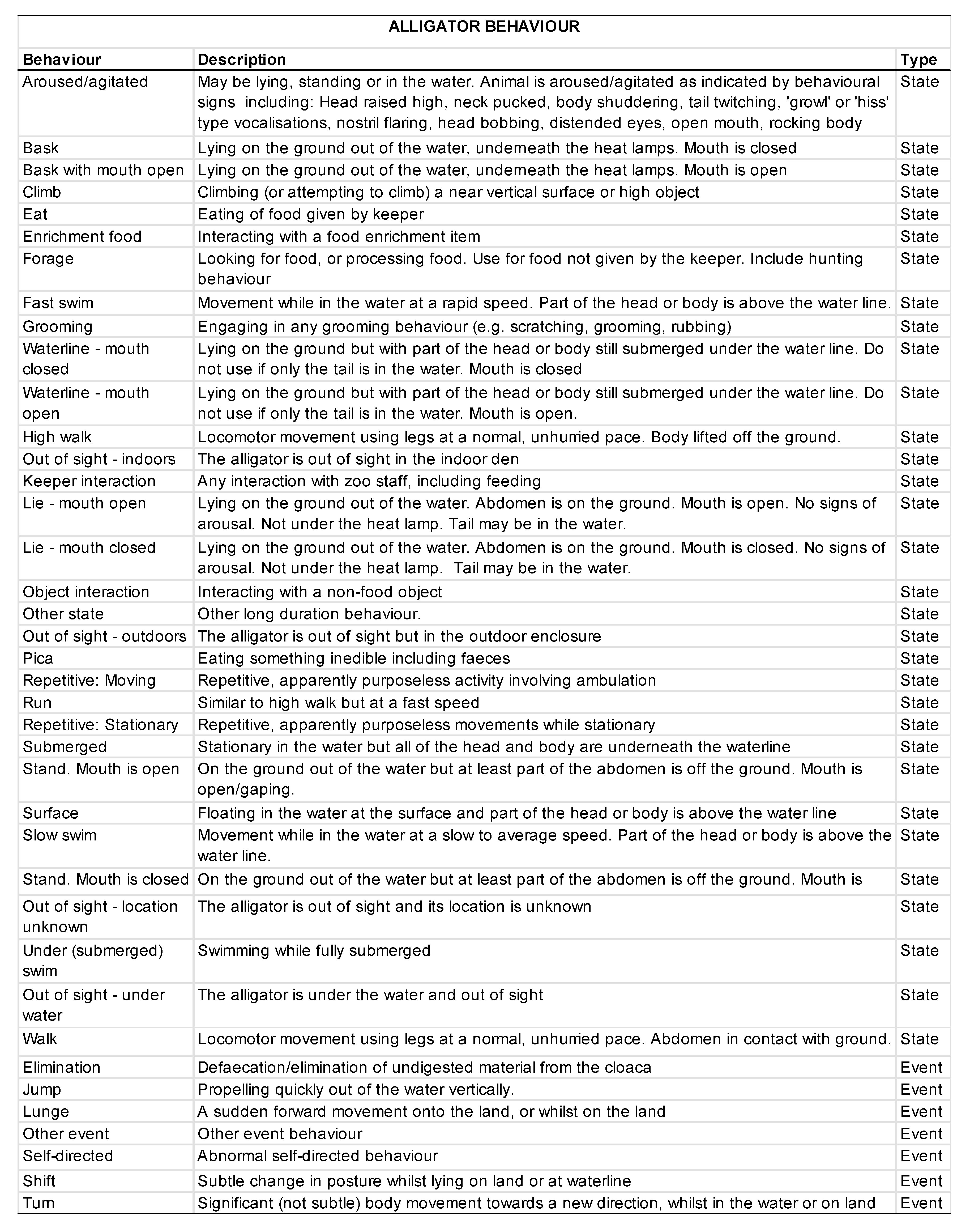
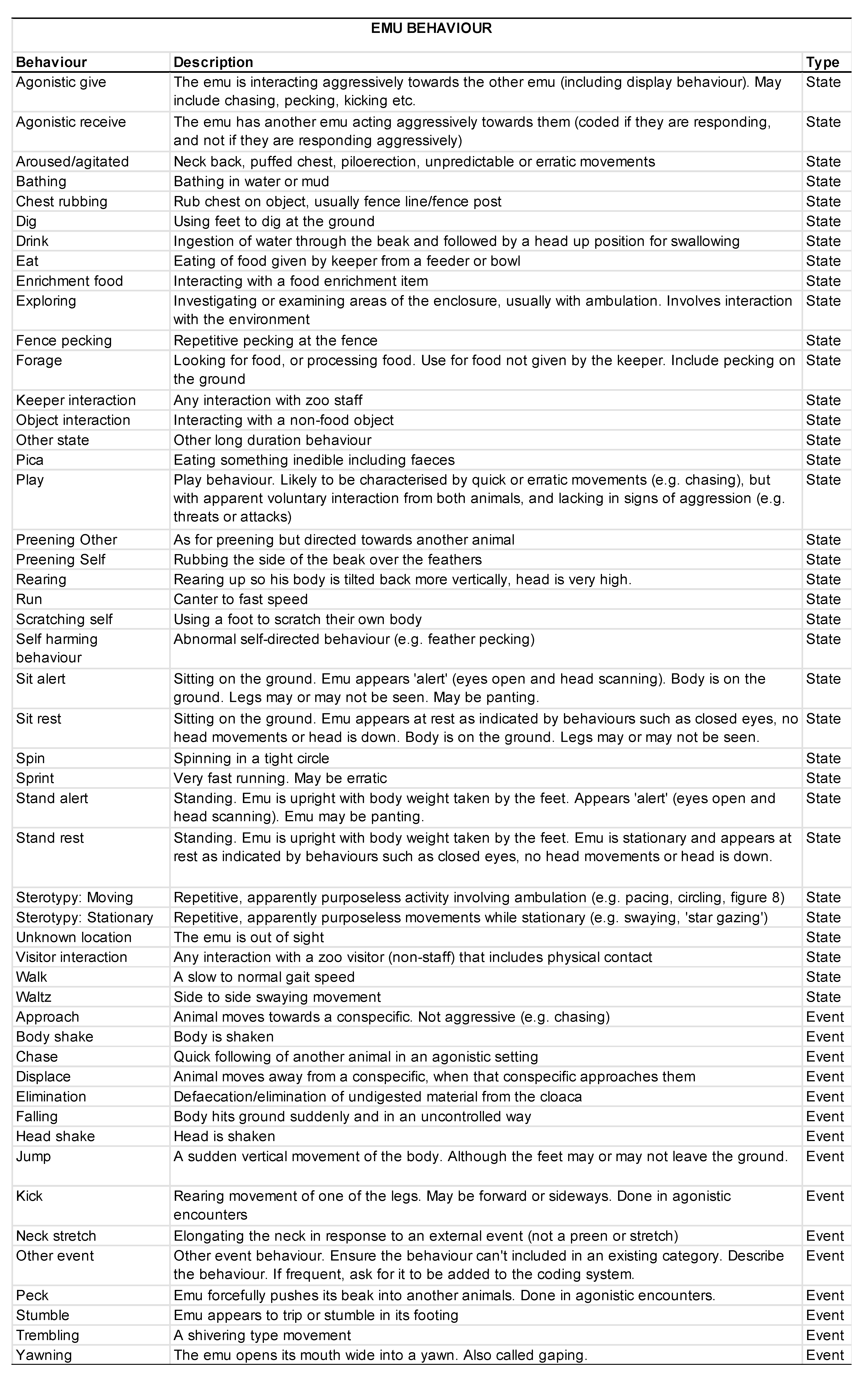
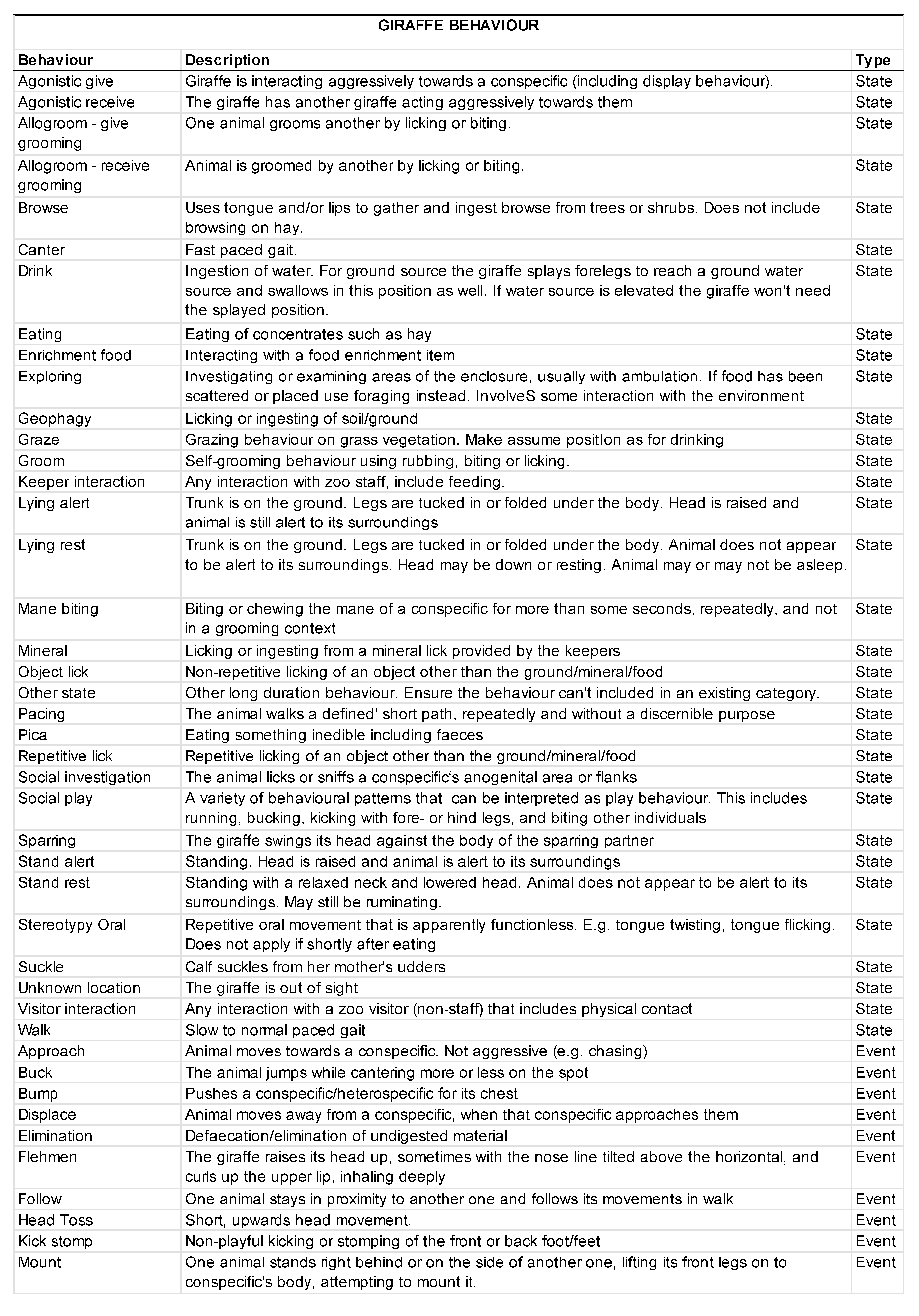
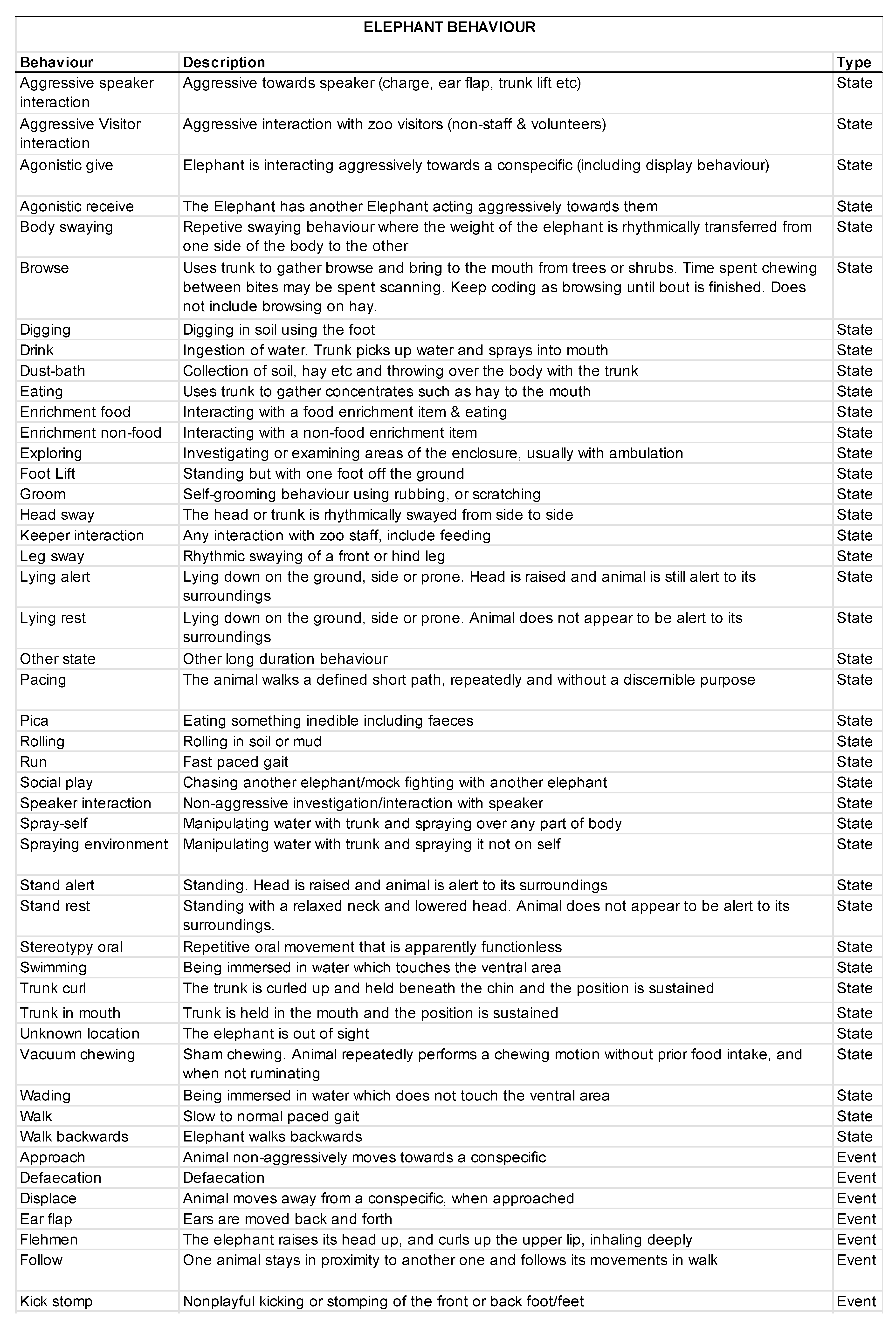
2.5. Behavioral Data Collection
2.6. Fecal Collection from Emus
2.7. Fecal Corticosterone Metabolite Measurement in Emus
2.8. Ethics
2.9. Data Treatment and Statistical Analysis
3. Results
3.1. Acoustic Measurements and Analysis
3.2. Animal Behavior
3.2.1. Activity Budgets
3.2.2. Combined Behavioral Categories
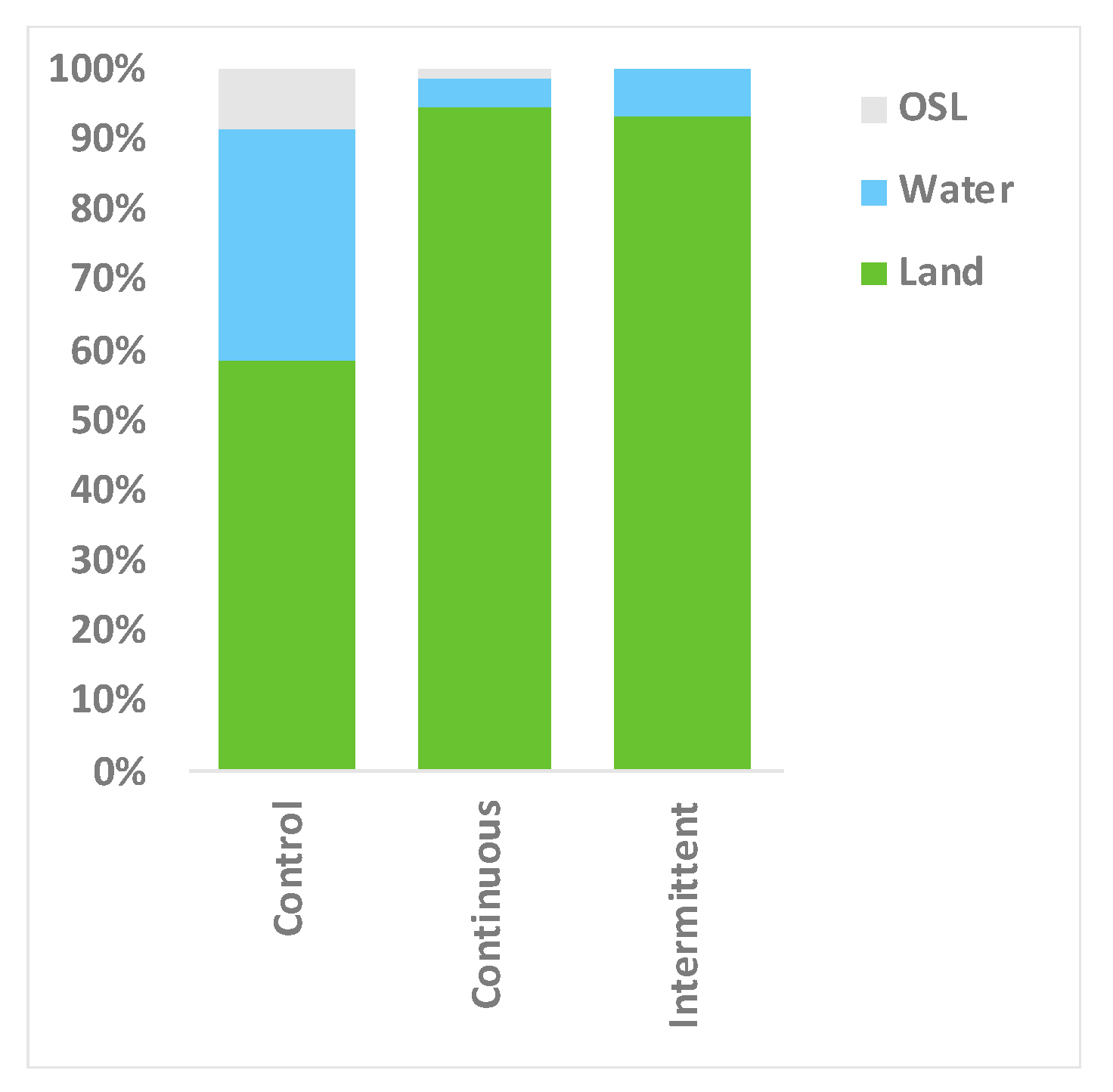
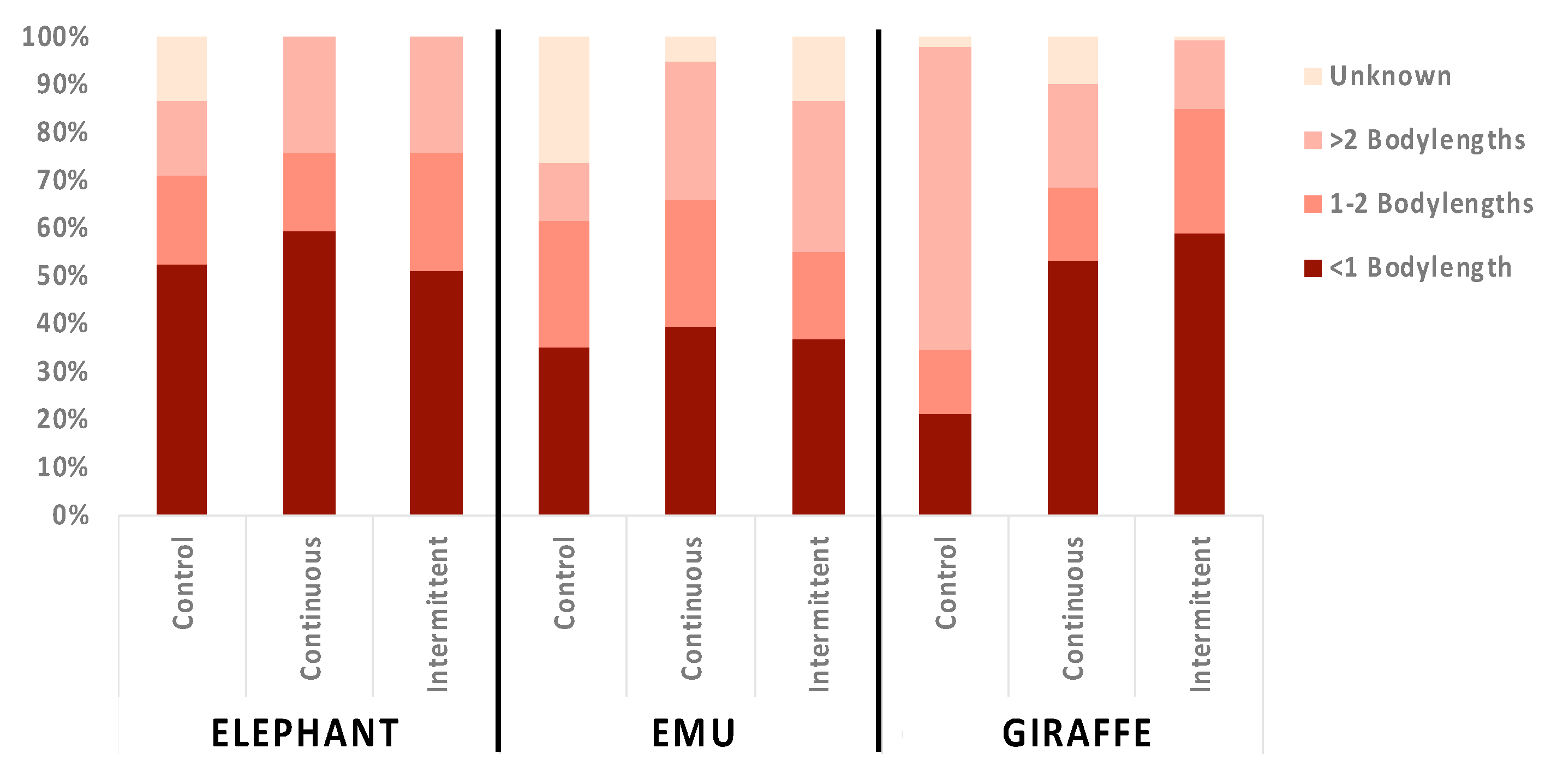
3.3. Location Changes and Social Proximity in Response to Sound
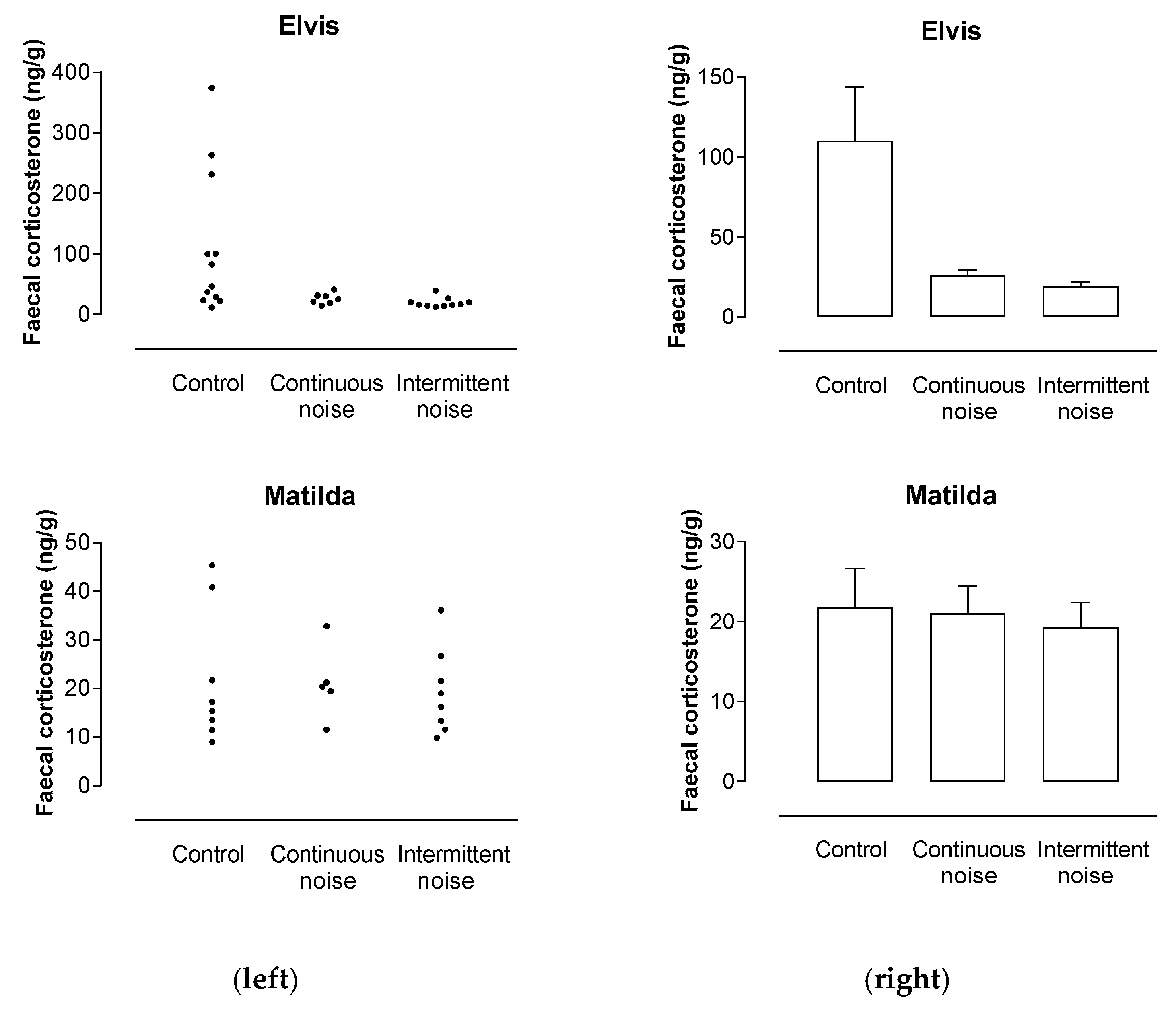
3.4. Emu Fecal Corticosterone
4. Discussion
5. Conclusions
- Scheduling the work to occur while animals were closely monitored by keepers.
- Keeper review of closed-circuit overnight video recordings to monitor elephant behaviour including sleep patterns.
- Timing specific construction activities to fit in with animal activity periods or behavioural cycles.
- Providing distraction for the animals via, for example, training activities (elephants) or provision of enrichment opportunities.
- Modifying routine husbandry procedures to allow individuals access to additonal or alternative parts of their enclosures.
- The application of a commercial sound-absorbant material (Hushtec® Acoustic Exterior Curtain, Duraflex Distribution) to construction site fencing adjacent to the emu enclosure.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A


References
- Mellor, D.J. Operational details of the five domains model and its key applications to the assessment and management of animal welfare. Animals 2017, 7, 60. [Google Scholar] [CrossRef] [PubMed]
- Sherwen, S.L.; Hemsworth, L.M.; Beausoleil, N.J.; Embury, A.; Mellor, D.J. An Animal Welfare Risk Assessment Process for Zoos. Animals 2018, 8, 130. [Google Scholar] [CrossRef] [PubMed]
- Mellor, D.J.; Hunt, S.; Gusset, M. (Eds.) Caring for Wildlife: The World Zoo and Aquarium Animal Welfare Strategy; WAZA Executive Office: Gland, Switzerland, 2015; p. 87. [Google Scholar]
- Morgan, K.N.; Tromborg, C.T. Sources of stress in captivity. Appl. Anim. Behav. Sci. 2007, 102, 262–302. [Google Scholar] [CrossRef]
- Clubb, R.; Mason, G.J. Natural behavioural biology as a risk factor in carnivore welfare: How analysing species differences could help zoos improve enclosures. Appl. Anim. Behav. Sci. 2007, 102, 303–328. [Google Scholar] [CrossRef]
- Dielenberg, R.A.; Carrive, P.; McGregor, I.S. The cardiovascular and behavioral response to cat odor in rats: Unconditioned and conditioned effects. Brain Res. 2001, 897, 228–237. [Google Scholar] [CrossRef]
- Hillmann, E.; Mayer, C.; Schrader, L. Lying behaviour and adrenocortical response as indicators of the thermal tolerance of pigs of different weights. Anim. Welf. 2004, 13, 329–335. [Google Scholar]
- Maddocks, S.A.; Goldsmith, A.R.; Cuthill, I.C. Behavioural and physiological effects of absence of ultraviolet wavelengths on European starlings Sturnus vulgaris. J. Avian Biol. 2002, 33, 103–106. [Google Scholar] [CrossRef]
- Reinhardt, V.; Reinhardt, A. The Lower Row Monkey Cage: An Overlooked Variable in Biomedical Research. J. Appl. Anim. Welf. Sci. 2000, 3, 141–149. [Google Scholar] [CrossRef]
- Owen, M.A.; Hall, S.; Bryant, L.; Swaisgood, R.R. The Influence of Ambient Noise on Maternal Behavior in a Bornean Sun Bear (Helarctos malayanus euryspilus). Zoo Biol. 2014, 33, 49–53. [Google Scholar] [CrossRef]
- Owen, M.A.; Swaisgood, R.R.; Czekala, N.M.; Steinman, K.; Lindburg, D.G. Monitoring stress in captive giant pandas (Ailuropoda melanoleuca): Behavioral and hormonal responses to ambient noise. Zoo Biol. 2004, 23, 147–164. [Google Scholar] [CrossRef]
- Powell, D.M.; Carlstead, K.; Tarou, L.R.; Brown, J.L.; Monfort, S.L. Effects of construction noise on behavior and cortisol levels in a pair of captive giant pandas (Ailuropoda melanoleuca). Zoo Biol. 2006, 25, 391–408. [Google Scholar] [CrossRef]
- Sulser, C.E.; Steck, B.L.; Baur, B. Effects of construction noise on behaviour of and exhibit use by Snow leopards Uncia uncia at Basel zoo. Int. Zoo Yearb. 2008, 42, 199–205. [Google Scholar] [CrossRef]
- Shepherdson, D.J.; Carlstead, K.C.; Wielebnowski, N. Cross-institutional assessment of stress responses in zoo animals using longitudinal monitoring of faecal corticoids and behaviour. Anim. Welf. 2004, 13, S105–S113. [Google Scholar]
- Segerstrom, S.C.; Miller, G.E. Psychological stress and the human immune system: A meta-analytic study of 30 years of inquiry. Psychol. Bull. 2004, 130, 601–630. [Google Scholar] [CrossRef] [PubMed]
- Dickens, M.J.; Bentley, G.E. Stress, captivity, and reproduction in a wild bird species. Horm. Behav. 2014, 66, 685–693. [Google Scholar] [CrossRef]
- Selye, H. 40 years of stress research—Prinicpal remaining problems and misconceptions. Can. Med. Assoc. J. 1976, 115, 53–56. [Google Scholar] [PubMed]
- Michelson, D.; Licinio, J.; Gold, P. Mediation of the Stress Response by the Hypothalamic-Pituitary-Adrenal Axis, in Neurobiological and Clinical Consequences of Stress: From Normal Adaptation to Post-Traumatic Stress Disorder; Friedman, M.J., Charney, D.S., Deutch, A.Y., Eds.; Lippincott-Raven: Philadelphia, PA, USA, 1995; pp. 225–239. [Google Scholar]
- Tromborg, C.T.; Coss, R.C. Denizens, Decibels, and Dens. In Annual Proceedings of the American Association of Zoos and Aquariums; American Association of Zoos and Aquariums: Seattle, WA, USA, 1995; pp. 521–528. [Google Scholar]
- Quadros, S.; Goulart, V.D.; Passos, L.; Vecci, M.A.; Young, R.J. Zoo visitor effect on mammal behaviour: Does noise matter? Appl. Anim. Behav. Sci. 2014, 156, 78–84. [Google Scholar] [CrossRef]
- Waser, P.M.; Brown, C.H. Habitat acoustics and primate communication. Am. J. Primatol. 1986, 10, 135–154. [Google Scholar] [CrossRef]
- Zannin, P.H.T.; Diniz, F.B.; Barbosa, W.A. Environmental noise pollution in the city of Curitiba, Brazil. Appl. Acoust. 2002, 63, 351–358. [Google Scholar] [CrossRef]
- Birke, L. Effects of browse, human visitors and noise on the behaviour of captive orang utans. Anim. Welf. 2002, 11, 189–202. [Google Scholar]
- Carder, G.; Semple, S. Visitor effects on anxiety in two captive groups of western lowland gorillas. Appl. Anim. Behav. Sci. 2008, 115, 211–220. [Google Scholar] [CrossRef]
- Maia, C.M.; Volpato, G.L.; Santos, E.F. A Case Study: The Effect of Visitors on Two Captive Pumas With Respect to the Time of the Day. J. Appl. Anim. Welf. Sci. 2012, 15, 222–235. [Google Scholar] [CrossRef] [PubMed]
- West, C.D. The relationship of the spiral turns of the cochlea and the length of the basilar membrane to the range of audible frequencies in ground dwelling mammals. J. Acoust. Soc. Am. 1985, 77, 1091–1101. [Google Scholar] [CrossRef] [PubMed]
- Wever, E.G. Hearing in the Crocodilia. Proc. Natl. Acad. Sci. USA 1971, 68, 1498–1500. [Google Scholar] [CrossRef] [PubMed]
- Manley, G.A.; Köppl, C.; Yates, G.K. Activity of primary auditory neurons in the cochlear ganglion of the emu Dromaius novaehollandiae: Spontaneous discharge, frequency tuning, and phase locking. J. Acoust. Soc. Am. 1997, 101, 1560–1573. [Google Scholar] [CrossRef] [PubMed]
- Corfield, J.R.; Kubke, M.F.; Köppl, C. Emu and kiwi: The ear and hearing in Paleognathous birds. In Insights from Comparative Hearing Research; Springer: New York, NY, USA, 2013; pp. 263–287. [Google Scholar]
- Baotic, A.; Sicks, F.; Stoeger, A.S. Nocturnal “humming” vocalizations: Adding a piece to the puzzle of giraffe vocal communication. BMC Res. Notes 2015, 8, 425. [Google Scholar] [CrossRef] [PubMed]
- Geisler, C.D. From Sound to Synapse: Physiology of the Mammalian Ear; Oxford University Press: New York, NY, USA, 1998. [Google Scholar]
- Griffin, D.R.; Webster, F.A.; Michael, C.R. The echolocation of flying insects by bats. Anim. Behav. 1960, 8, 141–154. [Google Scholar] [CrossRef]
- Pytte, C.L.; Ficken, M.S.; Moiseff, A. Ultrasonic singing by the blue-throated hummingbird: A comparison between production and perception. J. Comp. Physiol. A Neuroethol. Sens. Neural Behav. Physiol. 2004, 190, 665–673. [Google Scholar] [CrossRef]
- Sanchez, C. Stress-induced vocalisation in adult animals. A valid model of anxiety? Eur. J. Pharmacol. 2003, 463, 133–143. [Google Scholar] [CrossRef]
- Wilson, D.R.; Hare, J.F. Ground squirrel uses ultrasonic alarms. Nature 2004, 430, 523. [Google Scholar] [CrossRef]
- Lammers, M.O.; Au, W.W.; Herzing, D.L. The broadband social acoustic signaling behavior of spinner and spotted dolphins. J. Acoust. Soc. Am. 2003, 114, 1629–1639. [Google Scholar] [CrossRef] [PubMed]
- Barklow, W.E. Some underwater sounds of the hippopotamus (Hippopotamus amphibius). Mar. Freshw. Behav. Physiol. 1997, 29, 237–249. [Google Scholar] [CrossRef]
- Mack, A.L.; Jones, J. Low-frequency vocalizations by cassowaries (Casuarius spp.). Auk 2003, 120, 1062–1068. [Google Scholar] [CrossRef]
- Vliet, K.A. Social displays of the American alligator (Alligator mississippiensis). Am. Zool. 1989, 29, 1019–1031. [Google Scholar] [CrossRef]
- Von Muggenthaler, E. Giraffe Helmholtz resonance. Proc. Meet. Acoust. Acoust. Soc. Am. 2013, 19, 010012. [Google Scholar]
- World Health Organization. ISO 9613-2: Acoustics-Attenuation of Sound Propagation Outdoors, Part 2: General Method of Calculation; International Organization for Standardization: Geneva, Switzerland, 1996. [Google Scholar]
- Bies, D.; Hansen, C. Engineering Noise Control: Theory and Practice, (Paperback); Taylor and Francis: New York, NY, USA, 2009. [Google Scholar]
- Keetman, L.; Schapira, C. The Welfare of Ostriches, Emus, Rheas and Marabou Storks in Mixed-Species Enclosures in Zoos in the Netherlands; Whitley Wildlife Conservation Trust: Leeuwarden, The Netherlands, 2014; p. 26. [Google Scholar]
- Blache, D.; Martin, G.B. Day length affects feeding behaviour and food intake in adult male (Dromaius novaehollandiae). Br. Poult. Sci. 1999, 40, 573–578. [Google Scholar] [CrossRef]
- Glatz, P.; Lunam, C.; Malecki, I. (Eds.) The Welfare of Farmed Ratites; Springer: Berlin/Heidelberg, Germany, 2011; pp. 111–130. [Google Scholar]
- Seeber, P.A.; Ciofolo, I.; Ganswindt, A. Behavioural inventory of the giraffe (Giraffa camelopardalis). BMC Res. Notes 2012, 5, 650. [Google Scholar] [CrossRef]
- Posta, B.; Huber, R.; Moore III, D. The effects of housing on zoo elephant behavior: A quantitative case study of diurnal and seasonal variation. Int. J. Comp. Psychol. 2013, 26, 37–52. [Google Scholar]
- Rees, P.A. Activity Budgets and the Relationship Between Feeding and Stereotypic Behaviors in Asian Elephants (Elephas maximus) in a Zoo. Zoo Biol. 2009, 28, 79–97. [Google Scholar] [CrossRef]
- Fraisse, F.; Cockrem, J.F. Corticosterone and fear behaviour in white and brown caged laying hens. Br. Poult. Sci. 2006, 47, 110–119. [Google Scholar] [CrossRef]
- Cockrem, J.F.; Satterlee, D.G.; Candy, E.J.; Castille, S.A. Fecal corticosterone metabolites and plasma corticosterone in Japanese quail selected for low or high plasma corticosterone responses to brief restraint. Domest. Anim. Endocrinol. 2012, 42, 249–255. [Google Scholar] [CrossRef] [PubMed]
- Cockrem, J.F.; Barrett, D.P.; Candy, E.J.; Potter, M.A. Corticosterone responses in birds: Individual variation and repeatability in Adelie penguins (Pygoscelis adeliae) and other species, and the use of power analysis to determine sample sizes. Gen. Comp. Endocrinol. 2009, 163, 158–168. [Google Scholar] [CrossRef] [PubMed]
- Government, N.Z. Animal Welfare Act; New Zealand Government: Wellington, New Zealand, 1999. [Google Scholar]
- Government, N.Z. Animal Welfare (Zoos) Code of Welfare 2005; New Zealand Government: Wellington, New Zealand, 2005. [Google Scholar]
- Kilkenny, C.; Browne, W.J.; Cuthill, I.C.; Emerson, M.; Altman, D.G. Improving bioscience research reporting: The ARRIVE guidelines for reporting animal research. Osteoarthr. Cartil. 2012, 20, 256–260. [Google Scholar] [CrossRef] [PubMed]
- Periquet, S.; Valeix, M.; Loveridge, A.J.; Madzikanda, H.; Macdonald, D.W.; Fritz, H. Individual vigilance of African herbivores while drinking: The role of immediate predation risk and context. Anim. Behav. 2010, 79, 665–671. [Google Scholar] [CrossRef]
- Larsen, M.J.; Sherwen, S.L.; Rault, J.L. Number of nearby visitors and noise level affect vigilance in captive koalas. Appl. Anim. Behav. Sci. 2014, 154, 76–82. [Google Scholar] [CrossRef]
- Cameron, E.Z.; du Toit, J.T. Social influences on vigilance behaviour in giraffes, Giraffa camelopardalis. Anim. Behav. 2005, 69, 1337–1344. [Google Scholar] [CrossRef]
- Muller, Z.; Cantor, M.; Cuthill, I.C.; Harris, S. Giraffe social preferences are context dependent). Anim. Behav. 2018, 146, 37–49. [Google Scholar] [CrossRef]
- Pratt, D.M.; Anderson, V.H. Giraffe cow-calf relationships and social development of the calf in the Serengeti. Z. Tierpsychol. 1979, 51, 233–251. [Google Scholar] [CrossRef]
- Bercovitch, F.B.; Bashaw, M.J.; Penny, C.G.; Rieches, R.G. Maternal Investment in Captive Giraffes. J. Mammal. 2004, 85, 428–431. [Google Scholar] [CrossRef]
- Dawkins, M.S. Using behaviour to assess animal welfare. Anim. Welf. 2004, 13, S3–S7. [Google Scholar]
- Buchanan-Smith, H.M.; Badihi, I. The psychology of control: Effects of control over supplementary light on welfare of marmosets. Appl. Anim. Behav. Sci. 2012, 137, 166–174. [Google Scholar] [CrossRef]
- Castles, D.L.; Whiten, A.; Aureli, F. Social anxiety, relationships and self-directed behaviour among wild female olive baboons. Anim. Behav. 1999, 58, 1207–1215. [Google Scholar] [CrossRef] [PubMed]
- Mohiyeddini, C.; Bauer, S.; Semple, S. Neuroticism and stress: The role of displacement behavior. Anxiety Stress Coping 2015, 28, 391–407. [Google Scholar] [CrossRef] [PubMed]
- De Marco, A.; Cozzolino, R.; Dessì-Fulgheri, F.; Thierry, B. Conflicts induce affiliative interactions among bystanders in a tolerant species of macaque (Macaca tonkeana). Anim. Behav. 2010, 80, 197–203. [Google Scholar] [CrossRef]
- Mohiyeddini, C.; Semple, S. Displacement behaviour regulates the experience of stress in men. Stress 2013, 16, 163–171. [Google Scholar] [CrossRef] [PubMed]
- Mason, G.J.; Veasey, J.S. How should the psychological well-being of zoo elephants be objectively investigated? Zoo Biol. 2010, 29, 237–255. [Google Scholar] [CrossRef] [PubMed]
- O’Connell-Rodwell, C.E. Keeping an “ear” to the ground: Seismic communication in elephants. Physiology 2007, 22, 287–294. [Google Scholar] [CrossRef] [PubMed]
- Poole, J.; Granli, P. Mind and Movement: Meeting the Interests of Elephants; Forthman, D.L., Kane, F.L., Hancocks, D., Waldau, P.F., Eds.; Center for Animals and Public Policy, Cummings School of Veterinary Medicine, Tufts University: Medford, MA, USA, 2009; pp. 2–27. [Google Scholar]
- Hall, C.; Goodwin, D.; Heleski, C.; Randle, H.; Waran, N. Is there evidence of learned helplessness in horses? J. Appl. Anim. Welf. Sci. 2008, 11, 249–266. [Google Scholar] [CrossRef] [PubMed]
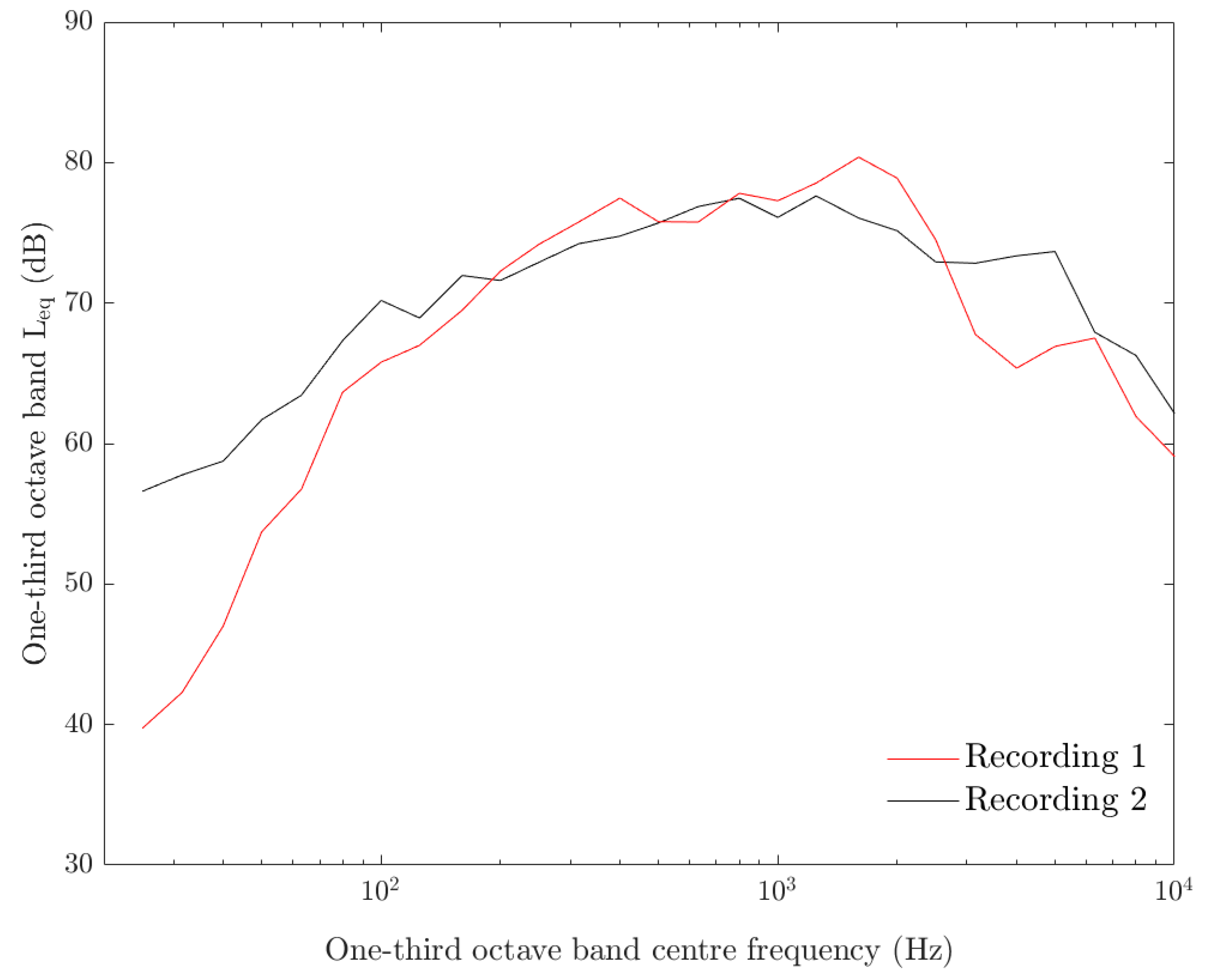

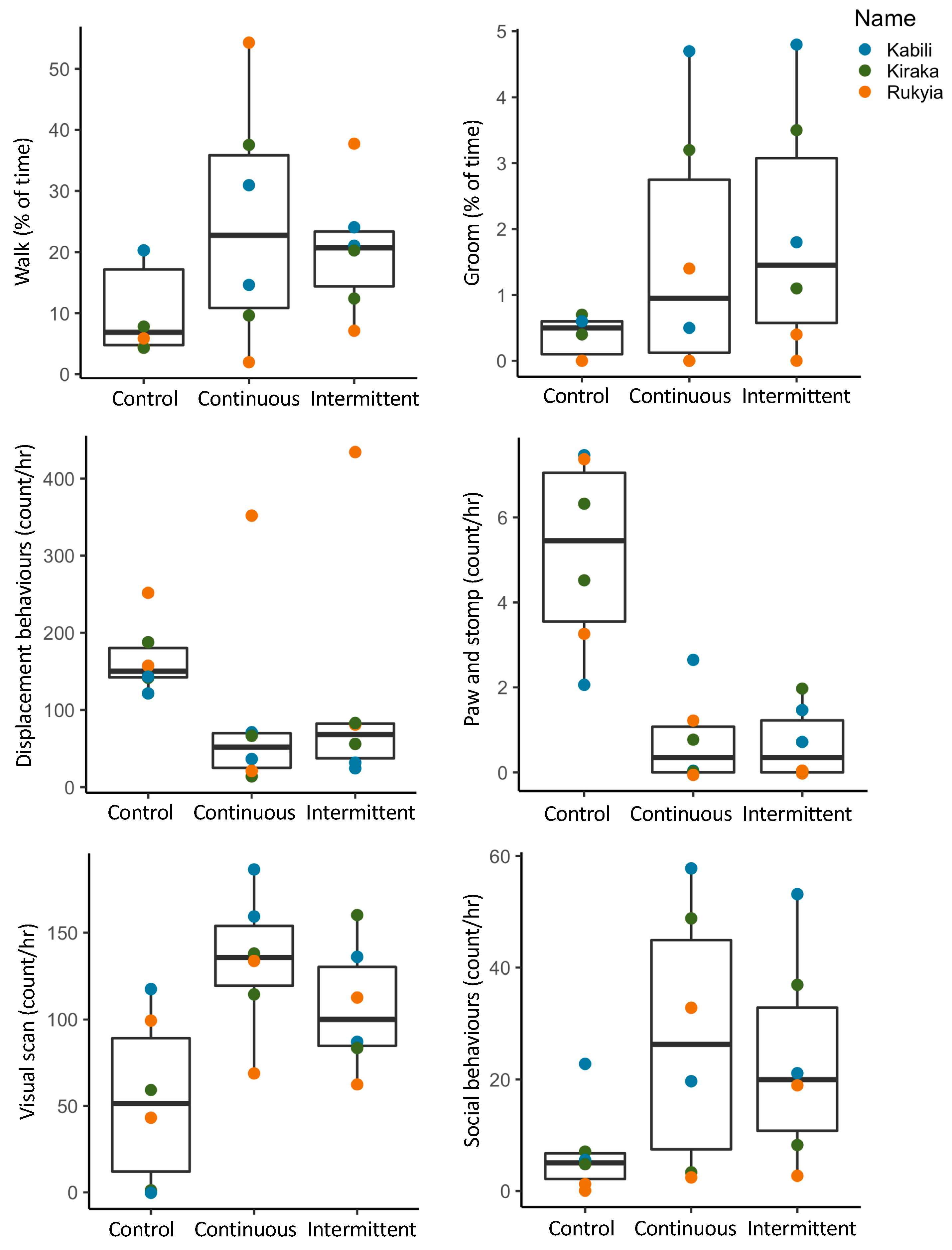
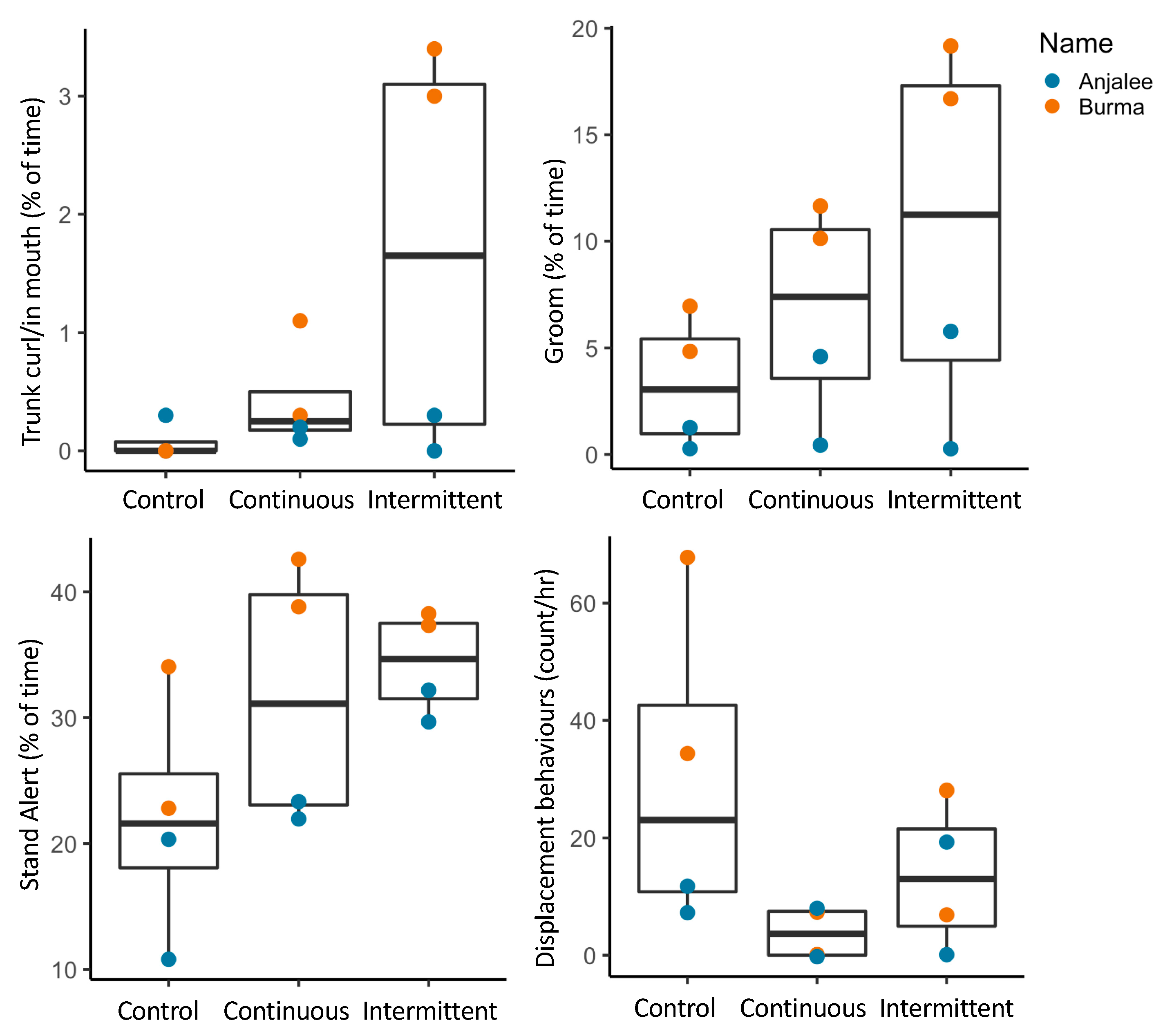
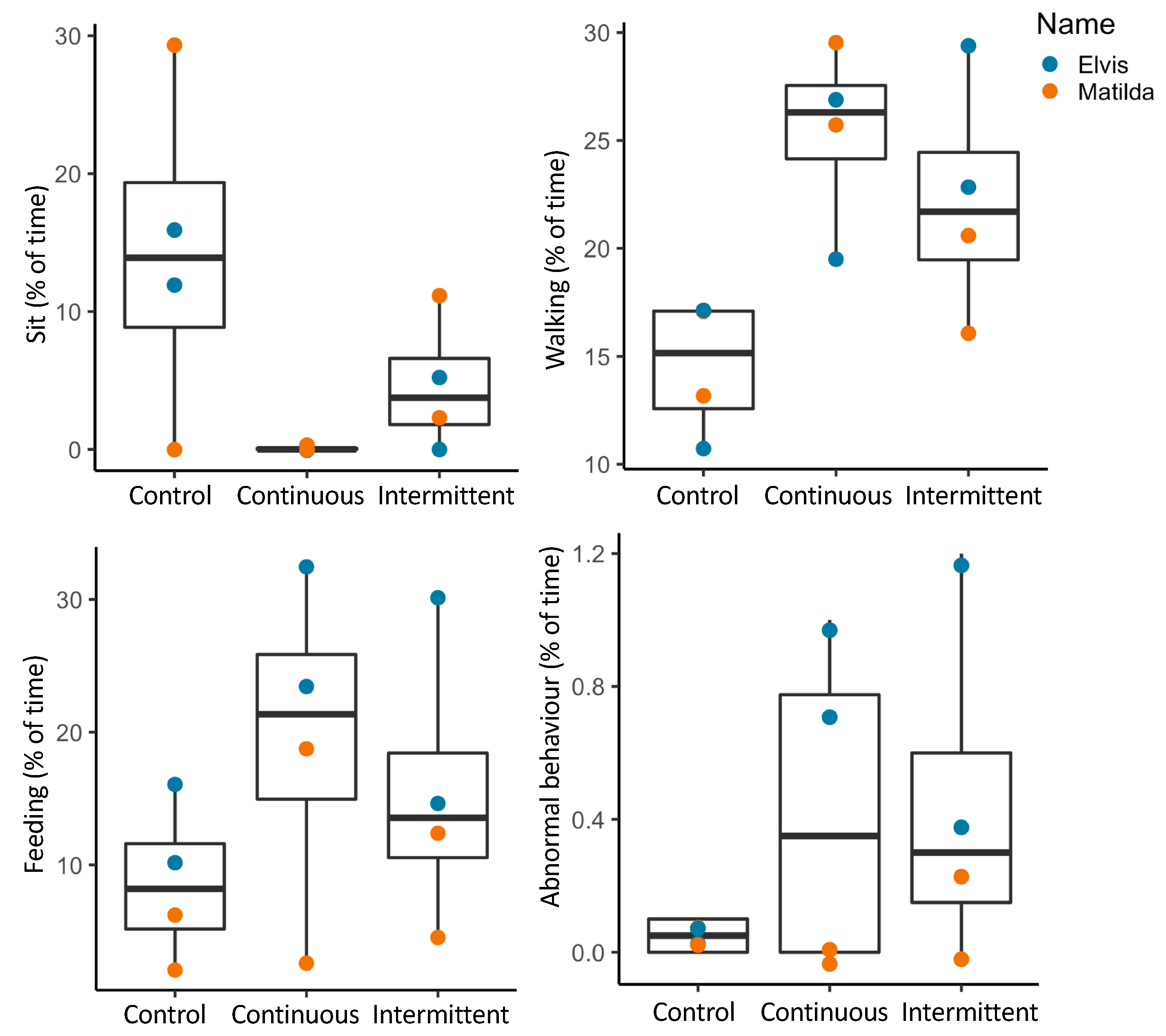


| Species (N) | Time | Control 1 | Control 2 | Contin 1 | Contin 2 | Inter 1 | Inter 2 |
|---|---|---|---|---|---|---|---|
| Giraffe (3) | 09:45–11:15 | 15-Feb | 22-Feb | 21-Aug | 23-Aug | 28-Aug | 7-Sep |
| Alligator (2) | 11:30–13:00 | 7-Feb, 14-Feb | 27-Feb | 20-Sep | 13-Sep | 5-Sep | 19-Sep |
| Emu (2) | 08:00–09:30 | 7-Feb, 21-Feb | 14-Feb | 15-Aug | 17-Aug | 22-Aug | 24-Aug |
| Elephant (2) | 09:30–11:00 | 25-Jul | 26-Jul | 16-Aug | 1-Sep | 25-Aug | 29-Aug |
| Behavior | Control | Continuous | Intermittent | Friedman’s Tests |
|---|---|---|---|---|
| Standing (%) | 42.6 ± 7.7 | 48.7 ± 5.8 | 36.8 ± 3.6 | χ2 = 2, df = 2, p = 0.37 |
| Feeding (%) | 25.9 ± 6.3 | 16.9 ± 3.8 | 28.3 ± 4.9 | χ2 = 0.9, df = 2, p = 0.65 |
| Grooming (%) | 3.3 ± 1.6 | 3.9 ± 0.9 | 4.6 ± 1.3 | χ2 = 3.7, df = 2, p = 0.16 |
| Locomotion (%) | 13.5 ± 2.2 a | 23.5 ± 1.4 b | 18.2 ± 2.3 a | χ2 = 8.9, df = 2, p = 0.01 |
| Abnormal (%) | 0.23 ± 0.21 | 0.5 ± 0.3 | 0.3 ± 0.1 | χ2 = 0.7, df = 2, p = 0.69 |
| Displacement (rate/hr) | 86.0 ± 30.3 | 44.15 ± 24.8 | 62.7 ± 33.3 | χ2 = 5.4, df = 2, p = 0.07 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jakob-Hoff, R.; Kingan, M.; Fenemore, C.; Schmid, G.; Cockrem, J.F.; Crackle, A.; Van Bemmel, E.; Connor, R.; Descovich, K. Potential Impact of Construction Noise on Selected Zoo Animals. Animals 2019, 9, 504. https://doi.org/10.3390/ani9080504
Jakob-Hoff R, Kingan M, Fenemore C, Schmid G, Cockrem JF, Crackle A, Van Bemmel E, Connor R, Descovich K. Potential Impact of Construction Noise on Selected Zoo Animals. Animals. 2019; 9(8):504. https://doi.org/10.3390/ani9080504
Chicago/Turabian StyleJakob-Hoff, Richard, Michael Kingan, Chiaki Fenemore, Gian Schmid, John F. Cockrem, Amanda Crackle, Emily Van Bemmel, Rebecca Connor, and Kris Descovich. 2019. "Potential Impact of Construction Noise on Selected Zoo Animals" Animals 9, no. 8: 504. https://doi.org/10.3390/ani9080504
APA StyleJakob-Hoff, R., Kingan, M., Fenemore, C., Schmid, G., Cockrem, J. F., Crackle, A., Van Bemmel, E., Connor, R., & Descovich, K. (2019). Potential Impact of Construction Noise on Selected Zoo Animals. Animals, 9(8), 504. https://doi.org/10.3390/ani9080504







