Finite Element Modelling Simulated Meniscus Translocation and Deformation during Locomotion of the Equine Stifle
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Animal and Image Acquisition
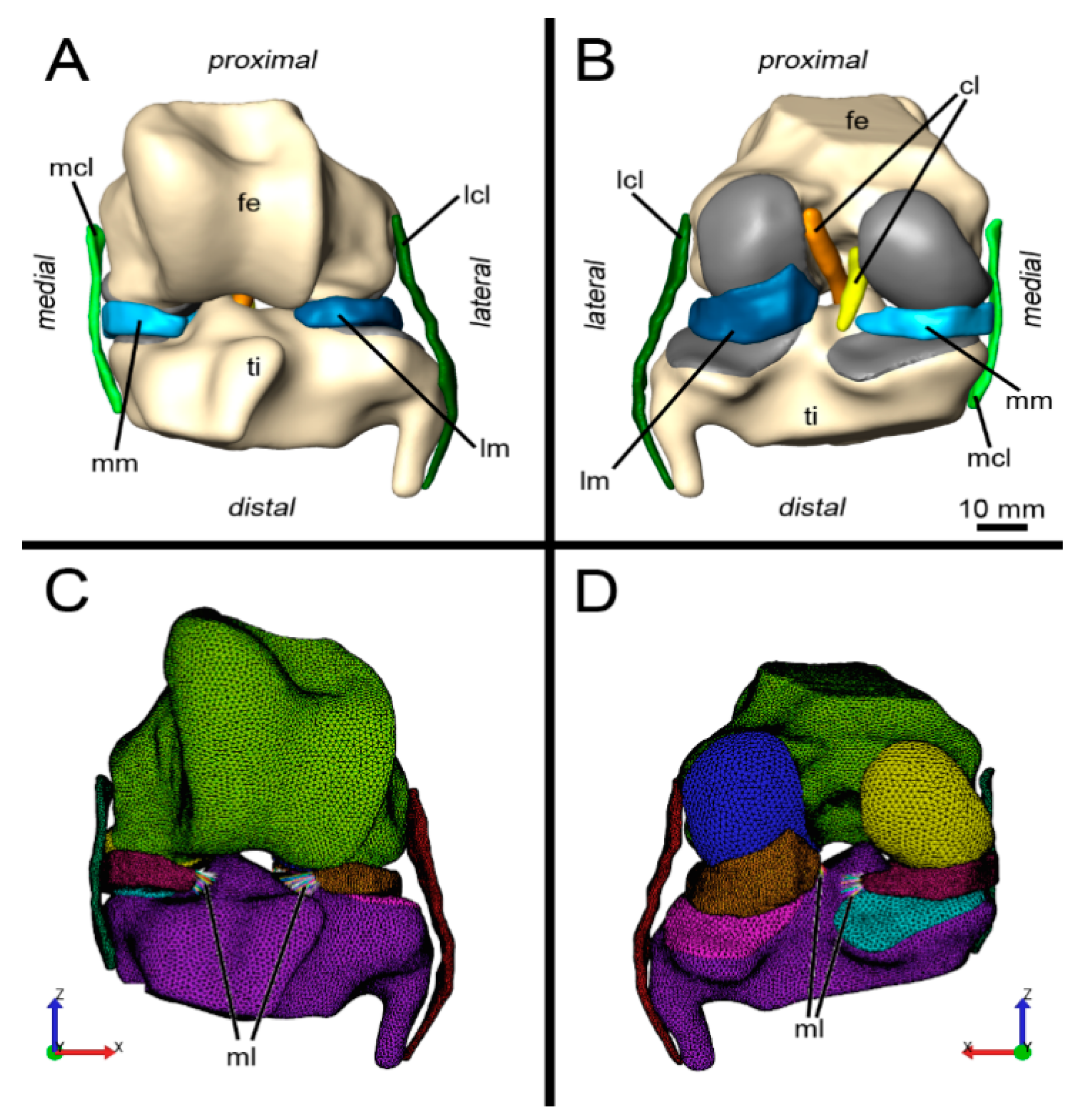
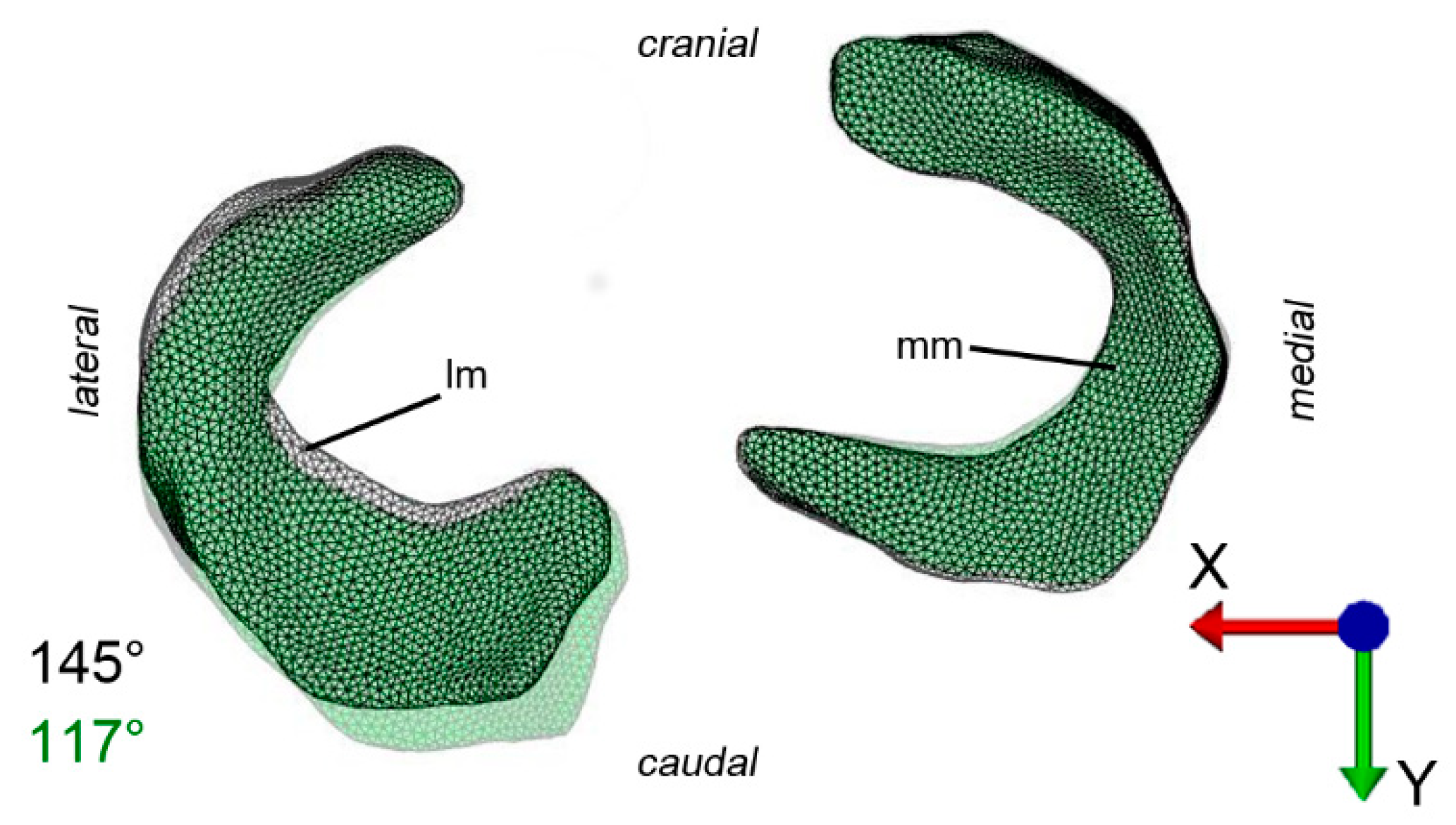
2.2. Image Processing, Image Segmentation and Generation of Polygon Surface Models
2.3. Computer Soft- and Hardware for Finite Element Analysis (FEA)
2.4. Finite Element Model
2.5. Boundary Conditions: Loads and Constraints
2.6. Simulation
3. Results
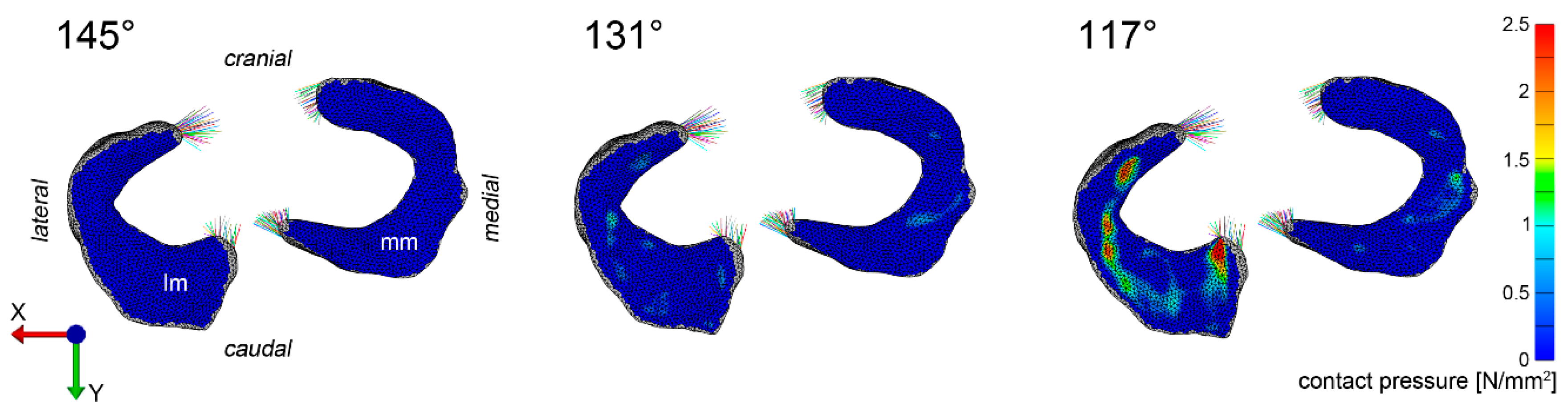
3.1. Pressure Distribution
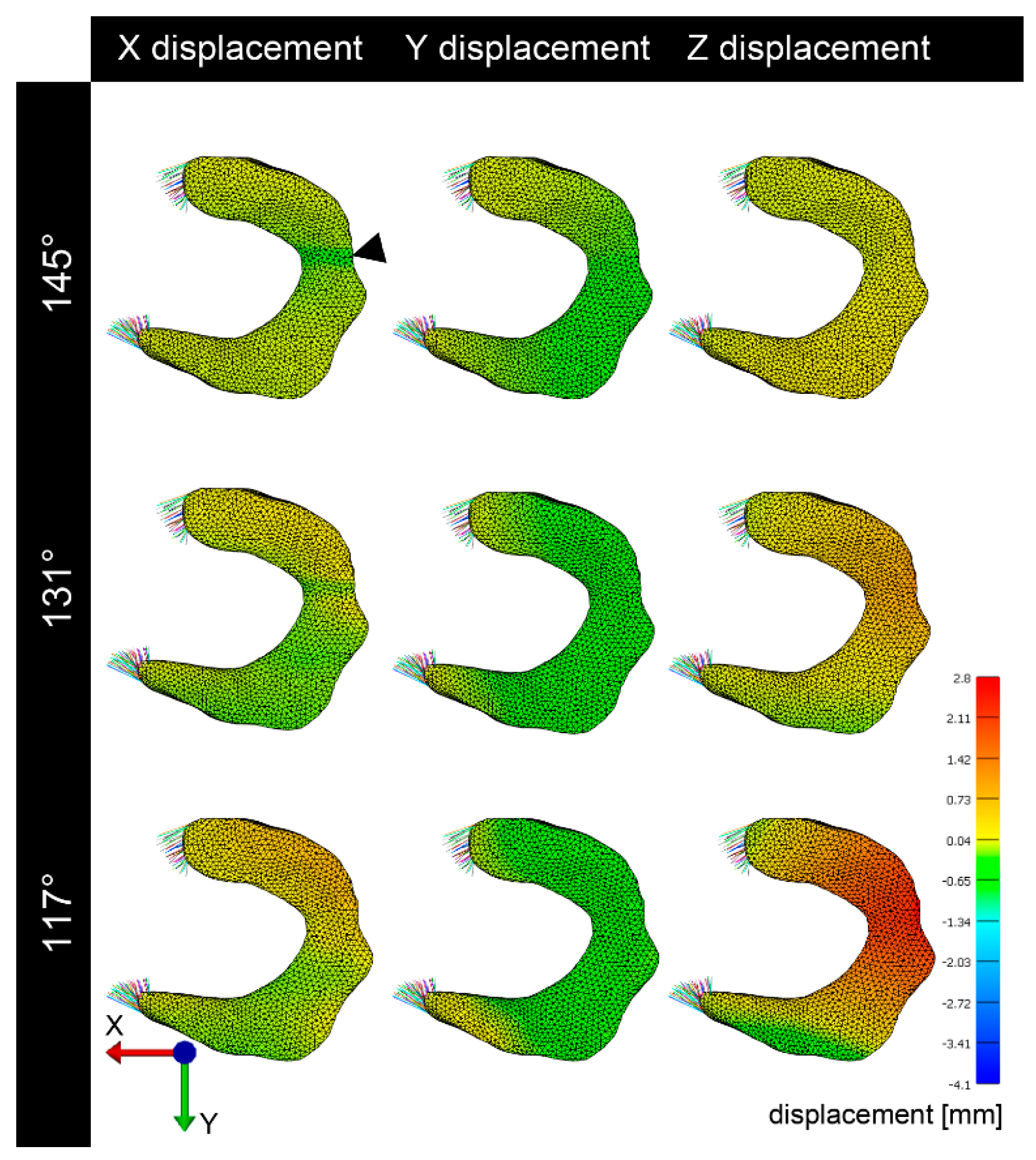
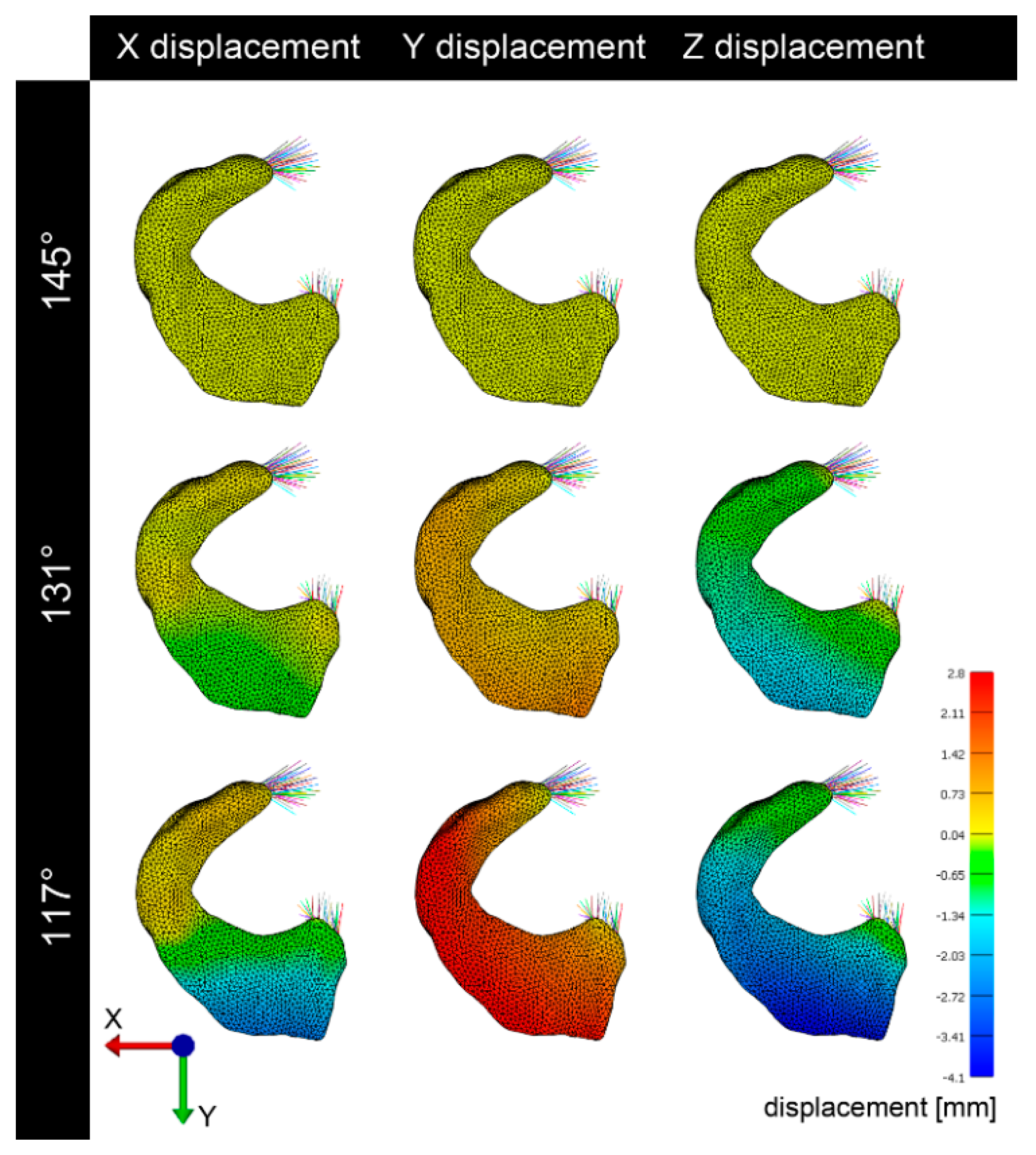
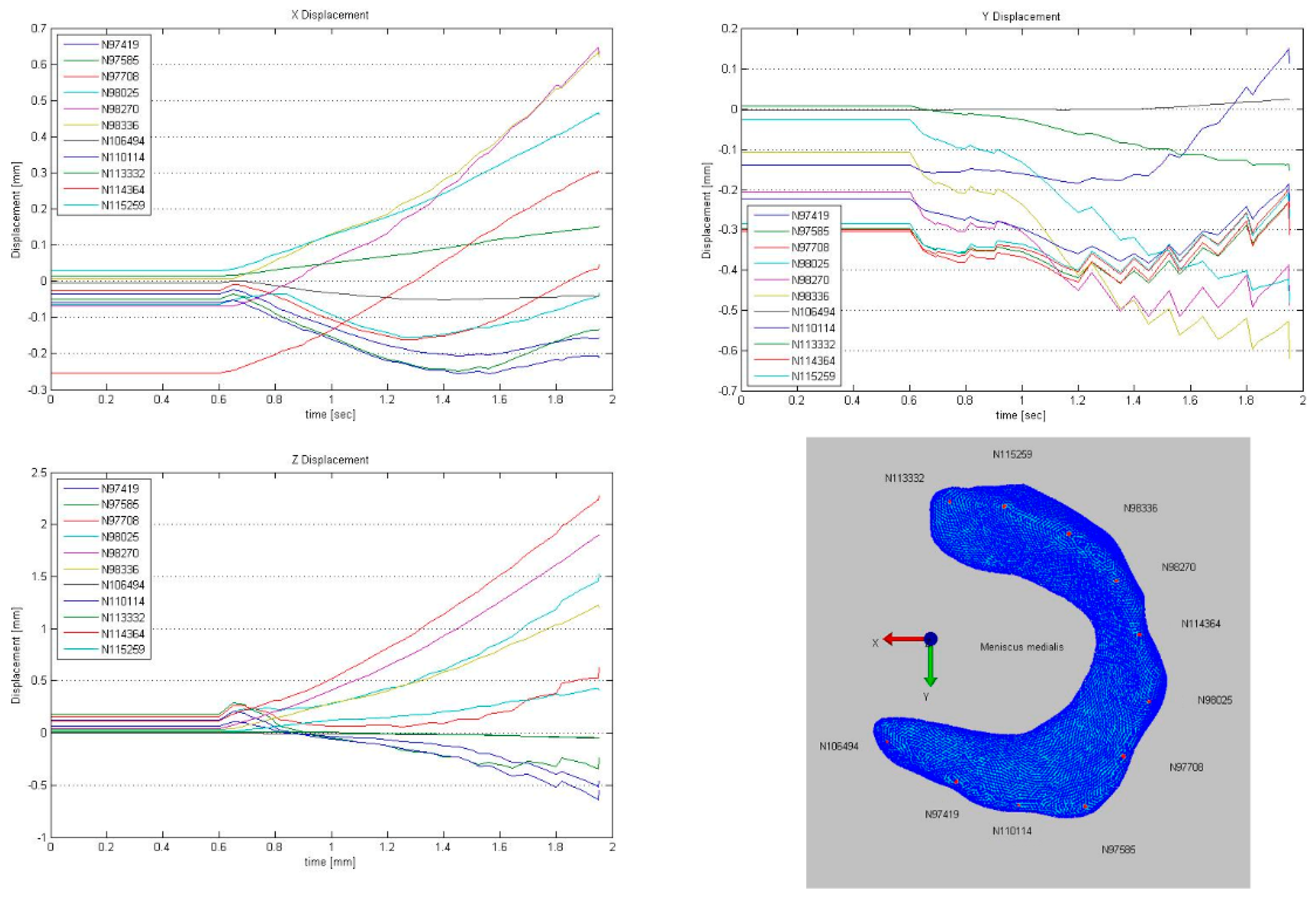
3.2. Deformation and Displacement
3.3. Visualization of Pressure Distribution and Deformation/Translocation of the Menisci
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Fowlie, J.G.; Arnoczky, S.P.; Stick, J.A.; Pease, A.P. Meniscal translocation and deformation throughout the range of motion of the equine stifle joint: An in vitro cadaveric study. Equine Vet. J. 2011, 43, 259–264. [Google Scholar] [CrossRef] [PubMed]
- Ellenberger, W.; Baum, H.; Zietzschmann, O. Handbuch der Vergleichenden Anatomie der Haustiere; Springer: Berlin, Germany, 1977. [Google Scholar]
- Fowlie, J.G. Stifle. In Equine Surgery, 4th ed.; Auer, Ed.; Elsevier: Amsterdam, The Netherlands, 2012; pp. 1419–1441. [Google Scholar]
- Neogi, T. The epidemiology and impact of pain in osteoarthritis. Osteoarthr. Cartil. 2013, 21, 1145–1153. [Google Scholar] [CrossRef] [PubMed]
- Makris, E.A.; Hadidi, P.; Athanasiou, K.A. The knee meniscus: Structure-function, pathophysiology, current repair techniques, and prospects for regeneration. Biomaterials 2011, 32, 7411–7431. [Google Scholar] [CrossRef] [PubMed]
- Englund, M. Osteoarthritis: Replacing the meniscus to prevent knee OA—Fact or fiction? Nat. Rev. Rheumatol. 2015, 11, 448–449. [Google Scholar] [CrossRef] [PubMed]
- Brzezinski, A.; Ghodbane, S.A.; Patel, J.M.; Perry, B.A.; Gatt, C.J.; Dunn, M.G. The Ovine Model for Meniscus Tissue Engineering: Considerations of Anatomy, Function, Implantation, and Evaluation. Tissue Eng. Part C Methods 2017, 23, 829–841. [Google Scholar] [CrossRef] [PubMed]
- Huiskes, R.; Chao, E.Y. A survey of finite element analysis in orthopedic biomechanics: The first decade. J. Biomech. 1983, 16, 385–409. [Google Scholar] [CrossRef]
- Prendergast, P.J. Finite element models in tissue mechanics and orthopaedic implant design. Clin. Biomech. 1997, 12, 343–366. [Google Scholar] [CrossRef]
- Snoeker, B.A.; Bakker, E.W.; Kegel, C.A.; Lucas, C. Risk factors for meniscal tears: A systematic review including meta-analysis. J. Orthop. Sports Phys. Ther. 2013, 43, 352–367. [Google Scholar] [CrossRef] [PubMed]
- Senter, C.; Hame, S.L. Biomechanical analysis of tibial torque and knee flexion angle: Implications for understanding knee injury. Sports Med. 2006, 36, 635–641. [Google Scholar] [CrossRef]
- Madry, H.; Ochi, M.; Cucchiarini, M.; Pape, D.; Seil, R. Large animal models in experimental knee sports surgery: Focus on clinical translation. J. Exp. Orthop. 2015, 2, 9. [Google Scholar] [CrossRef]
- Kremer, A.; Ribitsch, I.; Reboredo, J.; Durr, J.; Egerbacher, M.; Jenner, F.; Walles, H. 3D co-culture of meniscal cells and mesenchymal stem cells in collagen type I hydrogel on a small intestinal matrix—A pilot study towards equine meniscus tissue engineering. Tissue Eng. Part A 2017. [Google Scholar] [CrossRef] [PubMed]
- Thompson, W.O.; Thaete, F.L.; Fu, F.H.; Dye, S.F. Tibial meniscal dynamics using three-dimensional reconstruction of magnetic resonance images. Am. J. Sports Med. 1991, 19, 210–215. [Google Scholar] [CrossRef] [PubMed]
- Walmsley, J.P. Diagnosis and treatment of ligamentous and meniscal injuries in the equine stifle. Vet. Clin. N. Am. Equine Pract. 2005, 21, 651–672. [Google Scholar] [CrossRef] [PubMed]
- Buma, P.; Ramrattan, N.N.; Van Tienen, T.G.; Veth, R.P. Tissue engineering of the meniscus. Biomaterials 2004, 25, 1523–1532. [Google Scholar] [CrossRef]
- De Busscher, V.; Verwilghen, D.; Bolen, G.; Serteyn, D.; Busoni, V. Meniscal damage diagnosed by ultrasonography in horses: A retrospective study of 74 femorotibial joint ultrasonographic examinations (2000–2005). J. Equine Vet. Sci. 2006, 26, 453–461. [Google Scholar] [CrossRef]
- Tucker, B.; Khan, W.; Al-Rashid, M.; Al-Khateeb, H. Tissue engineering for the meniscus: A review of the literature. Open Orthop. J. 2012, 6, 348–351. [Google Scholar] [CrossRef] [PubMed]
- Cohen, J.M.; Richardson, D.W.; McKnight, A.L.; Ross, M.W.; Boston, R.C. Long-term outcome in 44 horses with stifle lameness after arthroscopic exploration and debridement. Vet. Surg. 2009, 38, 543–551. [Google Scholar] [CrossRef]
- Fowlie, J.G.; Arnoczky, S.P.; Lavagnino, M.; Stick, J.A. Stifle extension results in differential tensile forces developing between abaxial and axial components of the cranial meniscotibial ligament of the equine medial meniscus: A mechanistic explanation for meniscal tear patterns. Equine Vet. J. 2012, 44, 554–558. [Google Scholar] [CrossRef]
- Drosos, G.I.; Pozo, J.L. The causes and mechanisms of meniscal injuries in the sporting and non-sporting environment in an unselected population. Knee 2004, 11, 143–149. [Google Scholar] [CrossRef]
- Guess, T.M.; Razu, S. Loading of the medial meniscus in the ACL deficient knee: A multibody computational study. Med Eng. Phys. 2017, 41, 26–34. [Google Scholar] [CrossRef]
- Bendjaballah, M.Z.; Shirazi-Adl, A.; Zukor, D.J. Finite element analysis of human knee joint in varus-valgus. Clin. Biomech. 1997, 12, 139–148. [Google Scholar] [CrossRef]
- Donahue, T.L.; Hull, M.L.; Rashid, M.M.; Jacobs, C.R. A finite element model of the human knee joint for the study of tibio-femoral contact. J. Biomech. Eng. 2002, 124, 273–280. [Google Scholar]
- Li, G.; Gil, J.; Kanamori, A.; Woo, S.L. A validated three-dimensional computational model of a human knee joint. J. Biomech. Eng. 1999, 121, 657–662. [Google Scholar] [CrossRef]
- Li, G.; Lopez, O.; Rubash, H. Variability of a three-dimensional finite element model constructed using magnetic resonance images of a knee for joint contact stress analysis. J. Biomech. Eng. 2001, 123, 341–346. [Google Scholar] [CrossRef]
- Pena, E.; Calvo, B.; Martinez, M.A.; Doblare, M. A three-dimensional finite element analysis of the combined behavior of ligaments and menisci in the healthy human knee joint. J. Biomech. 2006, 39, 1686–1701. [Google Scholar] [CrossRef]
- Claeson, A.A.; Barocas, V.H. Computer simulation of lumbar flexion shows shear of the facet capsular ligament. Spine, J. 2017, 17, 109–119. [Google Scholar] [CrossRef]
- Fan, L.; Pei, S.; Lu, X.L.; Wang, L. A multiscale 3D finite element analysis of fluid/solute transport in mechanically loaded bone. Bone Res. 2016, 4, 16032. [Google Scholar] [CrossRef]
- Jones, B.; Hung, C.T.; Ateshian, G. Biphasic Analysis of Cartilage Stresses in the Patellofemoral Joint. J. Knee Surg. 2016, 29, 92–98. [Google Scholar] [CrossRef]
- MacLeod, A.R.; Rose, H.; Gill, H.S. A Validated Open-Source Multisolver Fourth-Generation Composite Femur Model. J. Biomech. Eng. 2016, 138, 124501. [Google Scholar] [CrossRef]
- Meng, Q.; An, S.; Damion, R.A.; Jin, Z.; Wilcox, R.; Fisher, J.; Jones, A. The effect of collagen fibril orientation on the biphasic mechanics of articular cartilage. J. Mech. Behav. Biomed. Mater. 2017, 65, 439–453. [Google Scholar] [CrossRef]
- Tadepalli, S.C.; Erdemir, A.; Cavanagh, P.R. Comparison of hexahedral and tetrahedral elements in finite element analysis of the foot and footwear. J. Biomech. 2011, 44, 2337–2343. [Google Scholar] [CrossRef]
- Maas, S.A.; Ellis, B.J.; Ateshian, G.A.; Weiss, J.A. FEBio: Finite elements for biomechanics. J. Biomech. Eng. 2012, 134, 011005. [Google Scholar] [CrossRef]
- Erdemir, A. Open Knee: A Pathway to Community Driven Modeling and Simulation in Joint Biomechanics. J. Med Devices 2013, 7, 0409101. [Google Scholar] [CrossRef]
- Erdemir, A.; Sibole, S. Open Knee: A Three-Dimensional Finite Element Representation of the Knee Joint; Version 1.0.0.; Open Knee User’s & Developer’s Guide: Cleaveland, OH, USA, 2010. [Google Scholar]
- Maas, S.A.; Ellis, B.J.; Rawlins, D.S.; Weiss, J.A. Finite element simulatio n of articular contact mechanics with quadratic tetrahedral elements. J. Biomech. 2016, 49, 659–667. [Google Scholar] [CrossRef]
- Villegas, D.F.; Maes, J.A.; Magee, S.D.; Donahue, T.L. Failure properties and strain distribution analysis of meniscal attachments. J. Biomech. 2007, 40, 2655–2662. [Google Scholar] [CrossRef]
- Abraham, A.C.; Moyer, J.T.; Villegas, D.F.; Odegard, G.M.; Haut Donahue, T.L. Hyperelastic properties of human meniscal attachments. J. Biomech. 2011, 44, 413–418. [Google Scholar] [CrossRef]
- Liebich, H.G.; König, H.E. Veterinary Anatomy of Domestic Mammals, 6th ed.; Liebich, K.A., Ed.; Schattauer: Stuttgart, Germany, 2014; pp. 223–258. [Google Scholar]
- Dar, F.H.; Meakin, J.R.; Aspden, R.M. Statistical methods in finite element analysis. J. Biomech. 2002, 35, 1155–1161. [Google Scholar] [CrossRef]
- Walmsley, C.W.; McCurry, M.R.; Clausen, P.D.; McHenry, C.R. Beware the black box: Investigating the sensitivity of FEA simulations to modelling factors in comparative biomechanics. PeerJ 2013, 1, e204. [Google Scholar] [CrossRef]
- Mononen, M.E.; Jurvelin, J.S.; Korhonen, R.K. Implementation of a gait cycle loading into healthy and meniscectomised knee joint models with fibril-reinforced articular cartilage. Comput. Methods Biomech. Biomed. Eng. 2015, 18, 141–152. [Google Scholar] [CrossRef]
- Petersen, W.; Tillmann, B. Collagenous fibril texture of the human knee joint menisci. Anat. Embryol. 1998, 197, 317–324. [Google Scholar] [CrossRef]
- Danso, E.K.; Makela, J.T.; Tanska, P.; Mononen, M.E.; Honkanen, J.T.; Jurvelin, J.S.; Toyras, J.; Julkunen, P.; Korhonen, R.K. Characterization of site-specific biomechanical properties of human meniscus-Importance of collagen and fluid on mechanical nonlinearities. J. Biomech. 2015, 48, 1499–1507. [Google Scholar] [CrossRef]
- McNulty, A.L.; Guilak, F. Mechanobiology of the meniscus. J. Biomech. 2015, 48, 1469–1478. [Google Scholar] [CrossRef]
- Danso, E.K.; Oinas, J.M.; Saarakkala, S.; Mikkonen, S.; Toyras, J.; Korhonen, R.K. Structure-function relationships of human meniscus. J. Mech. Behav. Biomed. Mater. 2017, 67, 51–60. [Google Scholar] [CrossRef]
- Gray, H.A.; Guan, S.; Thomeer, L.T.; Schache, A.G.; de Steiger, R.; Pandy, M.G. Three-dimensional motion of the knee-joint complex during normal walking revealed by mobile biplane x-ray imaging. J. Orthop. Res. 2019. [Google Scholar] [CrossRef]
- Guess, T.M.; Razu, S.; Jahandar, H.; Stylianou, A. Predicted loading on the menisci during gait: The effect of horn laxity. J. Biomech. 2015, 48, 1490–1498. [Google Scholar] [CrossRef]
- De Filippo, M.; Bertellini, A.; Pogliacomi, F.; Sverzellati, N.; Corradi, D.; Garlaschi, G.; Zompatori, M. Multidetector computed tomography arthrography of the knee: Diagnostic accuracy and indications. Eur. J. Radiol. 2009, 70, 342–351. [Google Scholar] [CrossRef]
- Vekens, E.V.; Bergman, E.H.; Vanderperren, K.; Raes, E.V.; Puchalski, S.M.; Bree, H.J.; Saunders, J.H. Computed tomographic anatomy of the equine stifle joint. Am. J. Vet. Res. 2011, 72, 512–521. [Google Scholar] [CrossRef]
- Shirazi, R.; Shirazi-Adl, A.; Hurtig, M. Role of cartilage collagen fibrils networks in knee joint biomechanics under compression. J. Biomech. 2008, 41, 3340–3348. [Google Scholar] [CrossRef]
- Kazemi, M.; Li, L.P.; Buschmann, M.D.; Savard, P. Partial meniscectomy changes fluid pressurization in articular cartilage in human knees. J. Biomech. Eng. 2012, 134, 021001. [Google Scholar] [CrossRef]
- Gu, K.B.; Li, L.P. A human knee joint model considering fluid pressure and fiber orientation in cartilages and menisci. Med Eng. Phys. 2011, 33, 497–503. [Google Scholar] [CrossRef]
- Ribitsch, I.; Peham, C.; Ade, N.; Durr, J.; Handschuh, S.; Schramel, J.P.; Vogl, C.; Walles, H.; Egerbacher, M.; Jenner, F. Structure-Function Relationships of Equine Menisci. PLoS ONE 2018, 13, e0194052. [Google Scholar] [CrossRef] [PubMed]

| Ligament | Density | C1 | C2 X | K | C3 | C4 | C5 | Λm |
|---|---|---|---|---|---|---|---|---|
| Lig. coll. Lat. | 1.5 × 10−9 | 1.44 | 0 | 397 | 0.57 | 48 | 467.1 | 1.063 |
| Lig. coll. Med. | 1.5 × 10−9 | 1.44 | 0 | 397 | 0.57 | 48 | 467.1 | 1.063 |
| Density | E1 | E2 | E3 | v12 | v23 | v31 | G12 | G23 | G31 | c | K |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1.5 × 10−9 | 125.0 | 27.5 | 27.5 | 0.1 | 0.33 | 0.1 | 2.0 | 12.5 | 2.5 | 1.0 | 10.0 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zellmann, P.; Ribitsch, I.; Handschuh, S.; Peham, C. Finite Element Modelling Simulated Meniscus Translocation and Deformation during Locomotion of the Equine Stifle. Animals 2019, 9, 502. https://doi.org/10.3390/ani9080502
Zellmann P, Ribitsch I, Handschuh S, Peham C. Finite Element Modelling Simulated Meniscus Translocation and Deformation during Locomotion of the Equine Stifle. Animals. 2019; 9(8):502. https://doi.org/10.3390/ani9080502
Chicago/Turabian StyleZellmann, Pasquale, Iris Ribitsch, Stephan Handschuh, and Christian Peham. 2019. "Finite Element Modelling Simulated Meniscus Translocation and Deformation during Locomotion of the Equine Stifle" Animals 9, no. 8: 502. https://doi.org/10.3390/ani9080502
APA StyleZellmann, P., Ribitsch, I., Handschuh, S., & Peham, C. (2019). Finite Element Modelling Simulated Meniscus Translocation and Deformation during Locomotion of the Equine Stifle. Animals, 9(8), 502. https://doi.org/10.3390/ani9080502





