Effects of Maternal Care During Rearing in White Leghorn and Brown Nick Layer Hens on Cognition, Sociality and Fear
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Housing
2.3. Testing
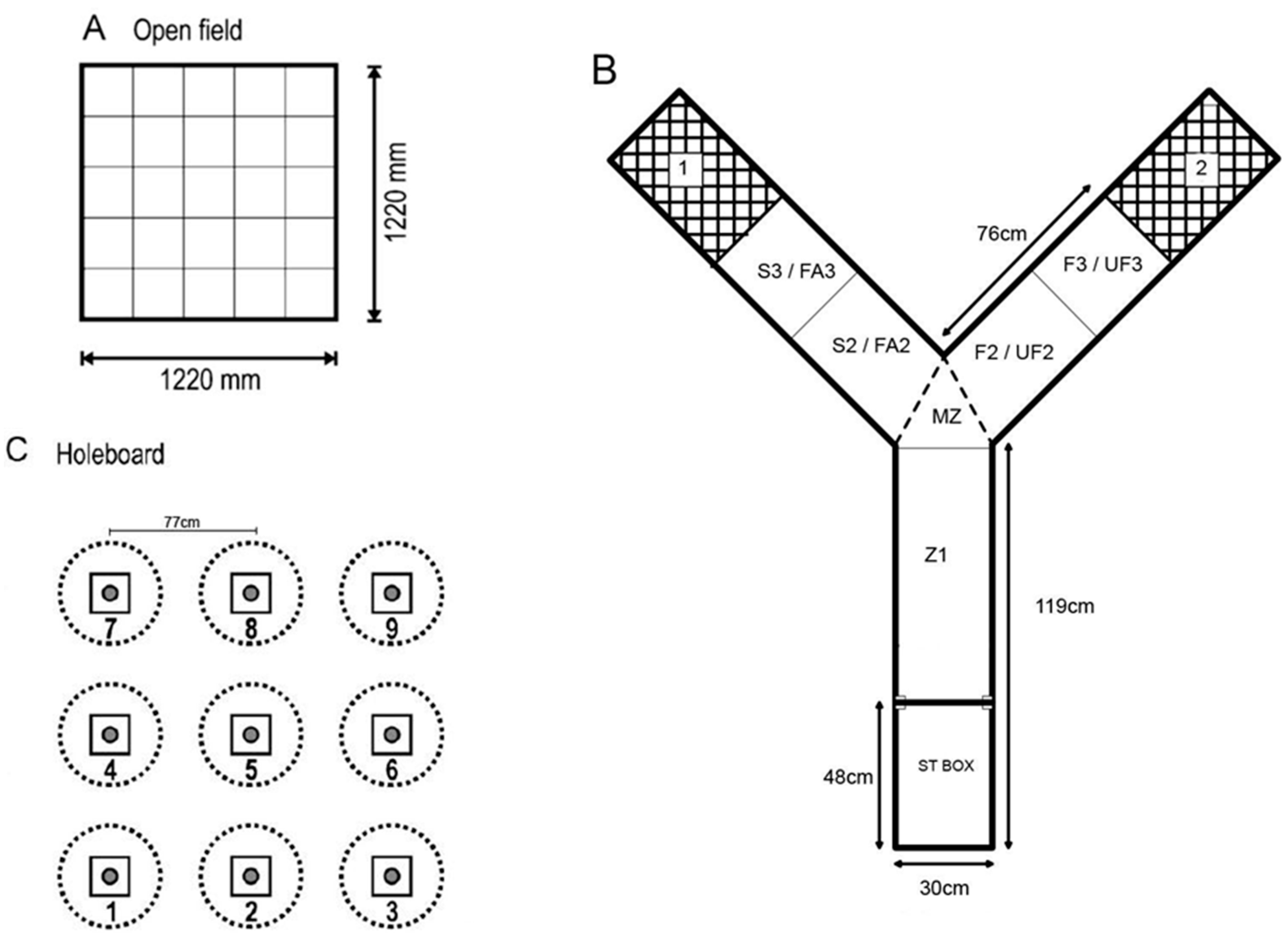
2.3.1. Open Field Test
2.3.2. Voluntary Human Approach Test
2.3.3. Social Versus Foraging Y-Maze
2.3.4. Social Recognition Y-Maze
2.3.5. Holeboard
2.3.6. Statistical Analysis
3. Results
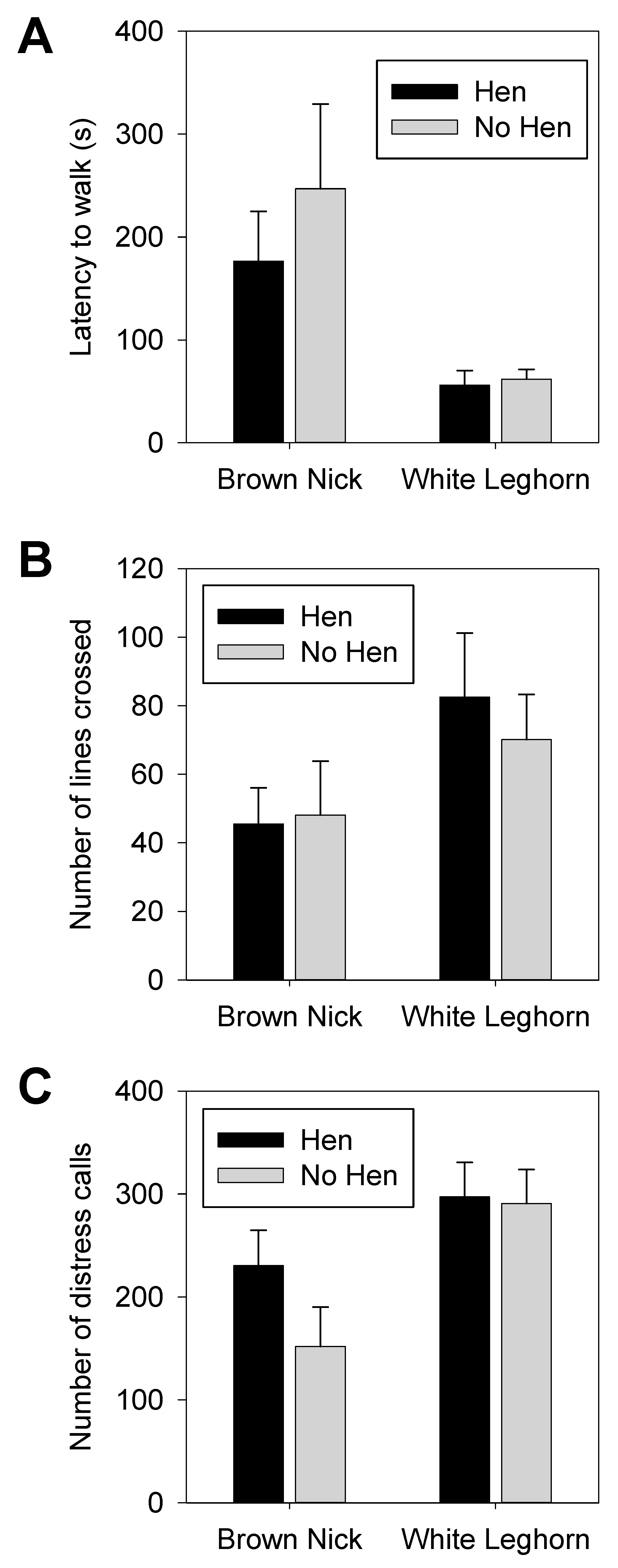
3.1. Open Field
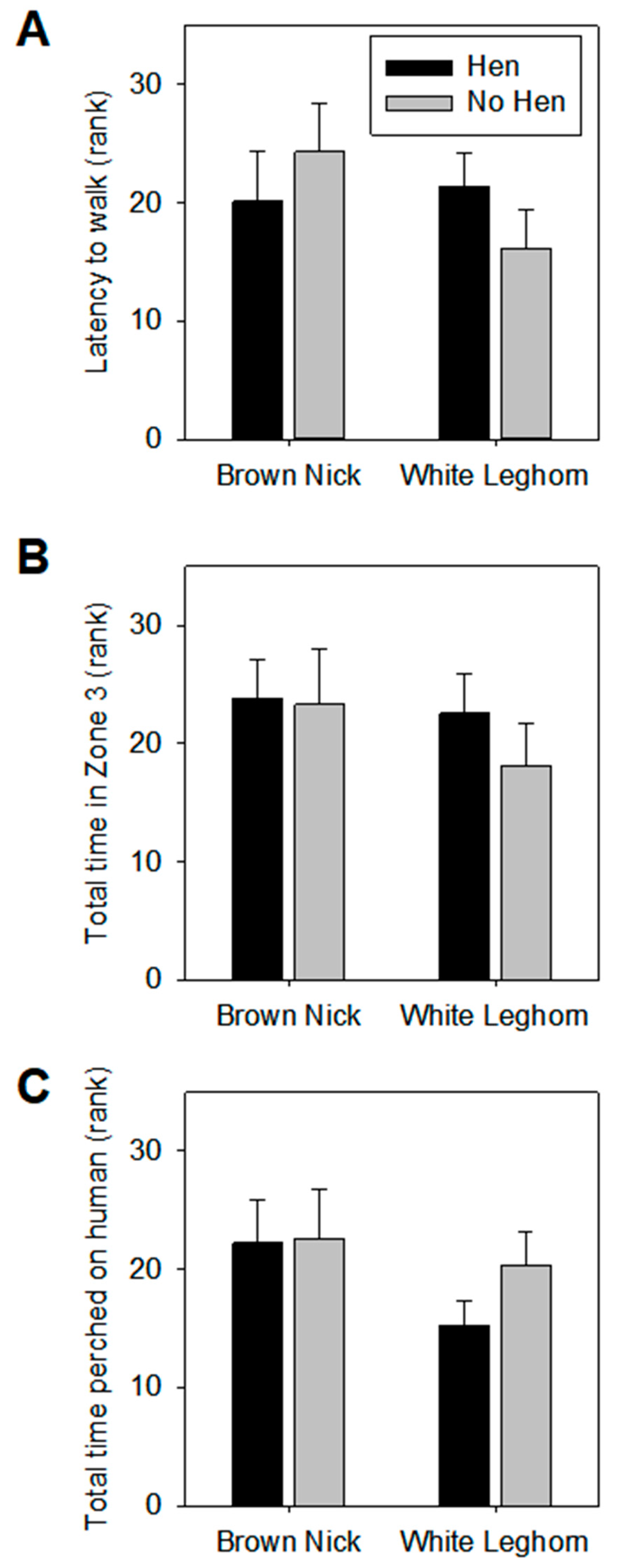
3.2. Voluntary Human Approach Test
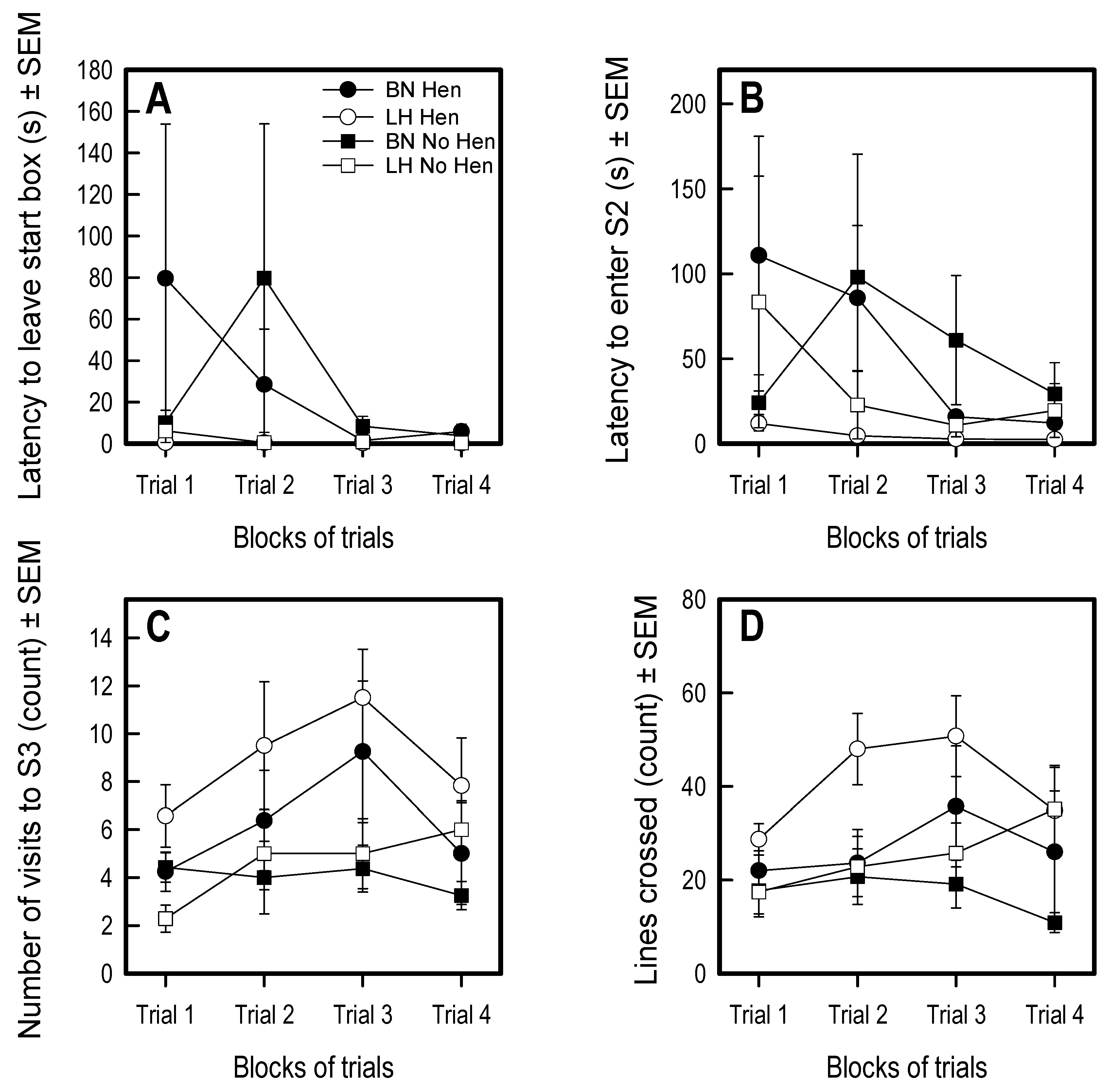
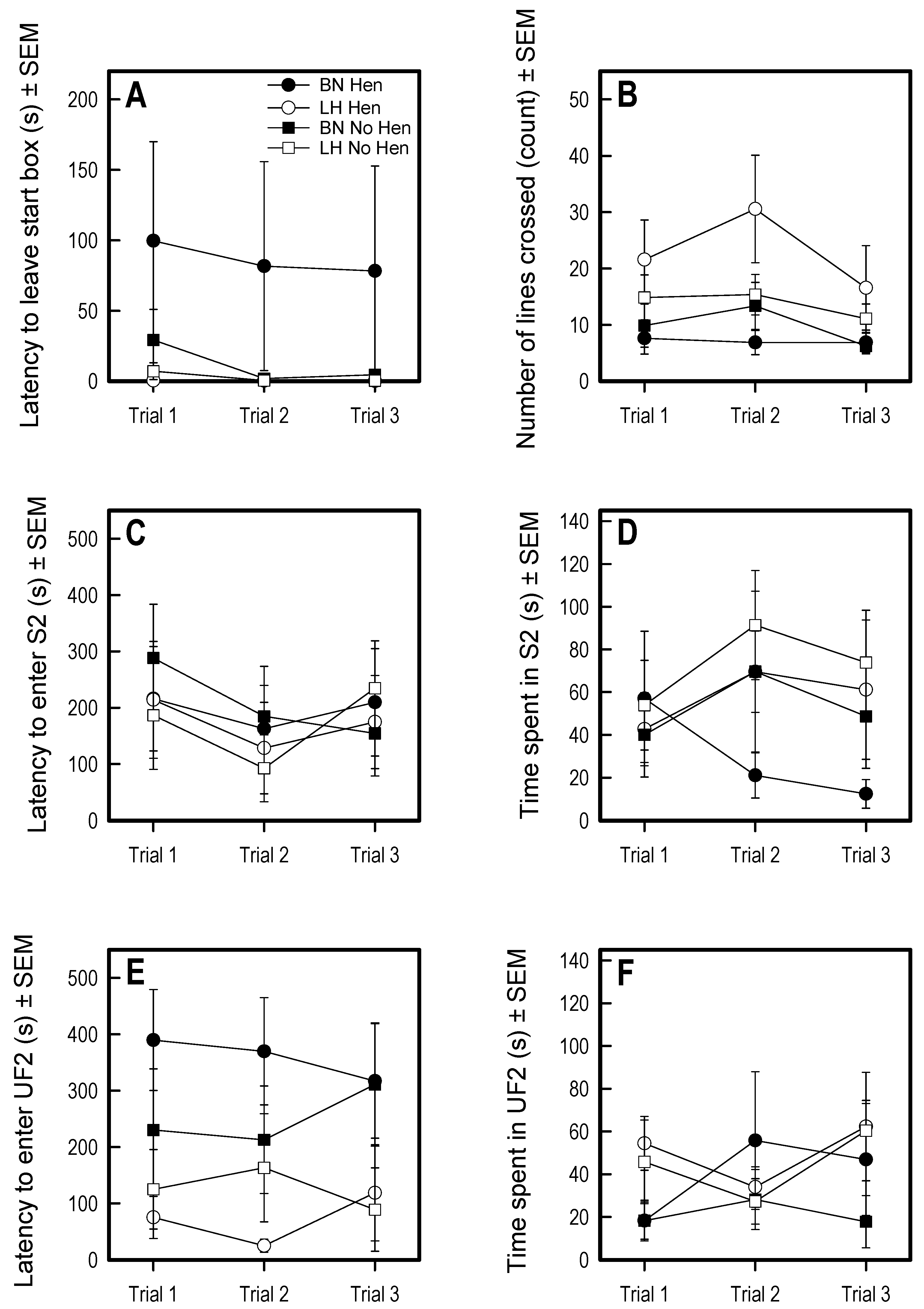
3.3. Social Versus Foraging Y-Maze
3.4. Social Recognition Y-Maze
3.5. Holeboard Spatial Working Memory Task
4. Discussion
4.1. Genetic Effects on Behavioral Reactions to Novel Situations
4.2. Genetic Effects on Exploratory and Social Behavior
4.3. Effects of Rearing on Sociality and Lack of Other Effects
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Nordquist, R.E.; van der Staay, F.J.; van Eerdenburg, F.J.C.M.; Velkers, F.C.; Fijn, L.; Arndt, S.S. Mutilating Procedures, Management Practices, and Housing Conditions That May Affect the Welfare of Farm Animals: Implications for Welfare Research. Animals 2017, 7, 12. [Google Scholar] [CrossRef] [PubMed]
- Pittet, F.; Coignard, M.; Houdelier, C.; Richard-Yris, M.-A.; Lumineau, S. Effects of maternal experience on fearfulness and maternal behaviour in a precocial bird. Anim. Behav. 2013, 85, 797–805. [Google Scholar] [CrossRef]
- Pittet, F.; Houdelier, C.; de Margerie, E.; Le Bot, O.; Richard-Yris, M.-A.; Lumineau, S. Maternal styles in a precocial bird. Anim. Behav. 2014, 87, 31–37. [Google Scholar] [CrossRef] [Green Version]
- Vallortigara, G.; Andrew, R.J.; Sertori, L.; Regolin, L. Sharply Timed Behavioral Changes During the First 5 Weeks of Life in the Domestic Chick (Gallus gallus). Bird Behav. 1997, 12, 29–40. [Google Scholar] [CrossRef]
- Rilling, J.K.; Young, L.J. The biology of mammalian parenting and its effect on offspring social development. Science 2014, 345, 771–776. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maccari, S.; Krugers, H.J.; Morley-Fletcher, S.; Szyf, M.; Brunton, P.J. The Consequences of Early-Life Adversity: Neurobiological, Behavioural and Epigenetic Adaptations. J. Neuroendocrinol. 2014, 26, 707–723. [Google Scholar] [CrossRef]
- Curley, J.P.; Champagne, F.A. Influence of maternal care on the developing brain: Mechanisms, temporal dynamics and sensitive periods. Front. Neuroendocrinol. 2016, 40, 52–66. [Google Scholar] [CrossRef]
- Bradshaw, R.H. Conspecific discrimination and social preference in the laying hen. Appl. Anim. Behav. Sci. 1992, 33, 69–75. [Google Scholar] [CrossRef]
- Perré, Y.; Wauters, A.-M.; Richard-Yris, M.-A. Influence of mothering on emotional and social reactivity of domestic pullets. Appl. Anim. Behav. Sci. 2002, 75, 133–146. [Google Scholar] [CrossRef]
- Shimmura, T.; Kamimura, E.; Azuma, T.; Kansaku, N.; Uetake, K.; Tanaka, T. Effect of broody hens on behaviour of chicks. Appl. Anim. Behav. Sci. 2010, 126, 125–133. [Google Scholar] [CrossRef]
- Abe, H.; Nagao, K.; Nakamura, A.; Inoue-Murayama, M. Differences in responses to repeated fear-relevant stimuli between Nagoya and White Leghorn chicks. Behav. Process. 2013, 99, 95–99. [Google Scholar] [CrossRef] [PubMed]
- Dudde, A.; Krause, E.T.; Matthews, L.R.; Schrader, L. More Than Eggs—Relationship Between Productivity and Learning in Laying Hens. Front. Psychol. 2018, 9. [Google Scholar] [CrossRef] [PubMed]
- Dudde, A.; Schrader, L.; Weigend, S.; Matthews, L.R.; Krause, E.T. More eggs but less social and more fearful? Differences in behavioral traits in relation to the phylogenetic background and productivity level in laying hens. Appl. Anim. Behav. Sci. 2018, 209, 65–70. [Google Scholar] [CrossRef]
- Desta, T.T. Phenotypic characteristic of junglefowl and chicken. World’s Poult. Sci. J. 2019, 75, 69–82. [Google Scholar] [CrossRef]
- Versace, E.; Fracasso, I.; Baldan, G.; Dalle Zotte, A.; Vallortigara, G. Newborn chicks show inherited variability in early social predispositions for hen-like stimuli. Sci. Rep. 2017, 7, 40296. [Google Scholar] [CrossRef] [PubMed]
- Madec, I.; Gabarrou, J.-F.; Pageat, P. Influence of a maternal odorant on copying strategies in chicks facing isolation and novelty during a standardized test. Neuroendocrinol. Lett. 2008, 29, 507–511. [Google Scholar] [PubMed]
- Riber, A.B.; Guzman, D.A. Effects of dark brooders on behavior and fearfulness in layers. Animals 2016, 6, 3. [Google Scholar] [CrossRef]
- Uitdehaag, K.A.; Rodenburg, T.B.; van Hierden, Y.M.; Bolhuis, J.E.; Toscano, M.J.; Nicol, C.J.; Komen, J. Effects of mixed housing of birds from two genetic lines of laying hens on open field and manual restraint responses. Behav. Process. 2008, 79, 13–18. [Google Scholar] [CrossRef]
- Fraisse, F.; Cockrem, J.F. Corticosterone and fear behaviour in white and brown caged laying hens. Br. Poult. Sci. 2006, 47, 110–119. [Google Scholar] [CrossRef]
- Schutz, K.E.; Kerje, S.; Jacobsson, L.; Forkman, B.; Carlborg, O.; Andersson, L.; Jensen, P. Major growth QTLs in fowl are related to fearful behavior: Possible genetic links between fear responses and production traits in a red junglefowl x white leghorn intercross. Behav. Genet. 2004, 34, 121–130. [Google Scholar] [CrossRef]
- Campler, M.; Jöngren, M.; Jensen, P. Fearfulness in red junglefowl and domesticated White Leghorn chickens. Behav. Process. 2009, 81, 39–43. [Google Scholar] [CrossRef] [PubMed]
- Rodenburg, T.B.; Bolhuis, J.E.; Koopmanschap, R.E.; Ellen, E.D.; Decuypere, E. Maternal care and selection for low mortality affect post-stress corticosterone and peripheral serotonin in laying hens. Physiol. Behav. 2009, 98, 519–523. [Google Scholar] [CrossRef] [PubMed]
- Angevaare, M.J.; Prins, S.; van der Staay, F.J.; Nordquist, R.E. The effect of maternal care and infrared beak trimming on development, performance and behavior of Silver Nick hens. Appl. Anim. Behav. Sci. 2012, 140, 70–84. [Google Scholar] [CrossRef]
- Houdelier, C.; Lumineau, S.; Bertin, A.; Guibert, F.; De Margerie, E.; Augery, M.; Richard-Yris, M.-A. Development of fearfulness in birds: Genetic factors modulate non-genetic maternal influences. PLoS ONE 2011, 6, e14604. [Google Scholar] [CrossRef] [PubMed]
- Francis, D.D.; Meaney, M.J. Maternal care and the development of stress responses. Curr. Opin. Neurobiol. 1999, 9, 128–134. [Google Scholar] [CrossRef]
- Lévy, F.; Keller, M.; Poindron, P. Olfactory regulation of maternal behavior in mammals. Horm. Behav. 2004, 46, 284–302. [Google Scholar] [CrossRef] [PubMed]
- Menard, J.L.; Champagne, D.L.; Meaney, M.J.P. Variations of maternal care differentially influence ‘fear’ reactivity and regional patterns of cFos immunoreactivity in response to the shock-probe burying test. Neuroscience 2004, 129, 297–308. [Google Scholar] [CrossRef]
- Frankola, K.A.; Flora, A.L.; Torres, A.K.; Grissom, E.M.; Overstreet, S.; Dohanich, G.P. Effects of early rearing conditions on cognitive performance in prepubescent male and female rats. Neurobiol. Learn. Mem. 2010, 94, 91–99. [Google Scholar] [CrossRef]
- D’Amato, F.R.; Zanettini, C.; Sgobio, C.; Sarli, C.; Carone, V.; Moles, A.; Ammassari-Teule, M. Intensification of maternal care by double-mothering boosts cognitive function and hippocampal morphology in the adult offspring. Hippocampus 2011, 21, 298–308. [Google Scholar] [CrossRef]
- Lindeyer, C.M.; Meaney, M.J.; Reader, S.M. Early maternal care predicts reliance on social learning about food in adult rats. Dev. Psychobiol. 2013, 55, 168–175. [Google Scholar] [CrossRef]
- Melo, A.I.; Hernandez-Curiel, M.; Hoffman, K.L. Maternal and peer contact during the postnatal period participate in the normal development of maternal aggression, maternal behavior, and the behavioral response to novelty. Behav. Brain Res. 2009, 201, 14–21. [Google Scholar] [CrossRef] [PubMed]
- Menard, J.L.; Hakvoort, R.M. Variations of maternal care alter offspring levels of behavioural defensiveness in adulthood: Evidence for a threshold model. Behav. Brain Res. 2007, 176, 302–313. [Google Scholar] [CrossRef] [PubMed]
- Riber, A.B.; Guzman, D.A. Effects of different types of dark brooders on injurious pecking damage and production-related traits at rear and lay in layers. Poult. Sci. 2017, 96, 3529–3538. [Google Scholar] [CrossRef] [PubMed]
- Formanek, L.; Houdelier, C.; Lumineau, S.; Bertin, A.; Richard-Yris, M.-A. Maternal Epigenetic Transmission of Social Motivation in Birds. Ethology 2008, 114, 817–826. [Google Scholar] [CrossRef]
- Chokchaloemwong, D.; Prakobsaeng, N.; Sartsoongnoen, N.; Kosonsiriluk, S.; El Halawani, M.; Chaiseha, Y. Mesotocin and maternal care of chicks in native Thai hens (Gallus domesticus). Horm. Behav. 2013, 64, 53–69. [Google Scholar] [CrossRef]
- Hewlett, S.E.; Zeinstra, E.C.; van Eerdenburg, F.J.C.M.; Rodenburg, T.B.; van Kooten, P.J.S.; van der Staay, F.J.; Nordquist, R.E. Hypothalamic vasotocin and tyrosine hydroxylase levels following maternal care and selection for low mortality in laying hens. BMC Vet. Res. 2014, 10, 167. [Google Scholar] [CrossRef] [PubMed]
- Schütz, K.E.; Jensen, P. Effects of Resource Allocation on Behavioural Strategies: A Comparison of Red Junglefowl (Gallus gallus) and Two Domesticated Breeds of Poultry. Ethology 2001, 107, 753–765. [Google Scholar] [CrossRef]
- Hulshof, H.J.; Novati, A.; Sgoifo, A.; Luiten, P.G.M.; den Boer, J.A.; Meerlo, P. Maternal separation decreases adult hippocampal cell proliferation and impairs cognitive performance but has little effect on stress sensitivity and anxiety in adult Wistar rats. Behav. Brain Res. 2011, 216, 552–560. [Google Scholar] [CrossRef]
- Field, S.E.; Rickard, N.S.; Toukhsati, S.R.; Gibbs, M.E. Maternal hen calls modulate memory formation in the day-old chick: The role of noradretialine. Neurobiol. Learn. Mem. 2007, 88, 321–330. [Google Scholar] [CrossRef]
- Nordquist, R.E.; Zeinstra, E.C.; Rodenburg, T.B.; van der Staay, F.J. Effects of maternal care and selection for low mortality on tyrosine hydroxylase concentrations and cell soma size in hippocampus and nidopallium caudolaterale in adult laying hen. J. Anim. Sci. 2013, 91, 137–146. [Google Scholar] [CrossRef]
- Tommasi, L.; Vallortigara, G. Searching for the center: Spatial cognition in the domestic chick (Gallus gallus). J. Exp. Psychol. Anim. Behav. Process. 2000, 26, 477–486. [Google Scholar] [CrossRef] [PubMed]
- Freire, R.; Munro, U.; Rogers, L.J.; Sagasser, S.; Wiltschko, R.; Wiltschko, W. Different responses in two strains of chickens (Gallus gallus) in a magnetic orientation test. Anim. Cogn. 2008, 11, 547–552. [Google Scholar] [CrossRef] [PubMed]
- Denzau, S.; Nießner, C.; Wiltschko, R.; Wiltschko, W. Different responses of two strains of chickens to different training procedures for magnetic directions. Anim. Cogn. 2013, 16, 395–403. [Google Scholar] [CrossRef] [PubMed]
- Richard-Yris, M.A.; Garnier, D.H.; Leboucher, G. Induction of maternal behavior and some hormonal and physiological correlates in the domestic hen. Horm. Behav. 1983, 17, 345–355. [Google Scholar] [CrossRef]
- Richard-Yris, M.-A.; Leboucher, G.; Chadwick, A.; Garnier, D.H. Induction of maternal behavior in incubating and non-incubating hens: Influence of hormones. Physiol. Behav. 1987, 40, 193–199. [Google Scholar] [CrossRef]
- Brown Nick Brown Egg Layers Management Guide. 2019; Available online: http://www.feedonline.ir/aa18.pdf (accessed on 5 July 2019).
- Management Guide: Alternative Systems. 2018; Available online: https://www.hyline.com/userdocs/pages/B_ALT_COM_ENG.pdf (accessed on 5 July 2019).
- Väisänen, J.; Jensen, P. Responses of Young Red Jungle Fowl (Gallus gallus) and White Leghorn Layers to Familiar and Unfamiliar Social Stimuli. Poult. Sci. 2004, 83, 335–343. [Google Scholar] [CrossRef] [PubMed]
- van der Staay, F.J.; Gieling, E.T.; Pinzon, N.E.; Nordquist, R.E.; Ohl, F. The appetitively motivated “cognitive” holeboard: A family of complex spatial discrimination tasks for assessing learning and memory. Neurosci. Biobehav. Rev. 2011. [Google Scholar] [CrossRef]
- Vallortigara, G.; Cailotto, M.; Zanforlin, M. Sex differences in social reinstatement motivation of the domestic chick (Gallus gallus) revealed by runway tests with social and nonsocial reinforcement. J. Comp. Psychol. 1990, 104, 361–367. [Google Scholar] [CrossRef]
- Vallortigara, G. Affiliation and aggression as related to gender in domestic chicks (Gallus gallus). J. Comp. Psychol. 1992, 106, 53–57. [Google Scholar] [CrossRef]
- Jones, R.B. Fear and adaptability in poultry: Insights, implications and imperatives. Worlds Poult. Sci. J. 1996, 52, 131–174. [Google Scholar] [CrossRef]
- Forkman, B.; Boissy, A.; Meunier-Salauen, M.C.; Canali, E.; Jones, R.B. A critical review of fear tests used on cattle, pigs, sheep, poultry and horses. Physiol. Behav. 2007, 92, 340–374. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koolhaas, J.M.; Bartolomucci, A.; Buwalda, B.; de Boer, S.F.; Flugge, G.; Korte, S.M.; Meerlo, P.; Murison, R.; Olivier, B.; Palanza, P.; et al. Stress revisited: A critical evaluation of the stress concept. Neurosci. Biobehav. Rev. 2011, 35, 1291–1301. [Google Scholar] [CrossRef] [PubMed]
- Koolhaas, J.M.; DeBoer, S.F.; DeRuiter, A.J.H.; Meerlo, P.; Sgoifo, A. Social stress in rats and mice. Acta Physiol. Scand. 1997, 161, 69–72. [Google Scholar]
- Suarez, S.D.; Gallup, G.G. Social reinstatement and open-field testing in chickens. Anim. Learn. Behav. 1983, 11, 119–126. [Google Scholar] [CrossRef] [Green Version]
- Koolhaas, J.M.; Korte, S.M.; De Boer, S.F.; Van Der Vegt, B.J.; Van Reenen, C.G.; Hopster, H.; De Jong, I.C.; Ruis, M.A.W.; Blokhuis, H.J. Coping styles in animals: Current status in behavior and stress-physiology. Neurosci. Biobehav. Rev. 1999, 23, 925–935. [Google Scholar] [CrossRef]
- Römkens, D.M.J.M. Assessment of Emotional Reactivity, Learning and Memory—Open Field & Hole-Board. Available online: http://igitur-archive.library.uu.nl/student-theses/2009-1125-200120/UUindex.html (accessed on May 30 2019).
- Numan, M.; Woodside, B. Maternity: Neural Mechanisms, Motivational Processes, and Physiological Adaptations. Behav. Neurosci. 2010, 124, 715–741. [Google Scholar] [CrossRef]
- Nordquist, R.E.; Heerkens, J.L.T.; Rodenburg, T.B.; Boks, S.; Ellen, E.D.; van der Staay, F.J. Laying hens selected for low mortality: Behaviour in tests of fearfulness, anxiety and cognition. Appl. Anim. Behav. Sci. 2011, 131, 110–122. [Google Scholar] [CrossRef]
- Aigueperse, N.; Pittet, F.; de Margerie, E.; Nicolle, C.; Houdelier, C.; Lumineau, S. Brood size can influence maternal behaviour and chick’s development in precocial birds. Behav. Process. 2017, 138, 96–104. [Google Scholar] [CrossRef]
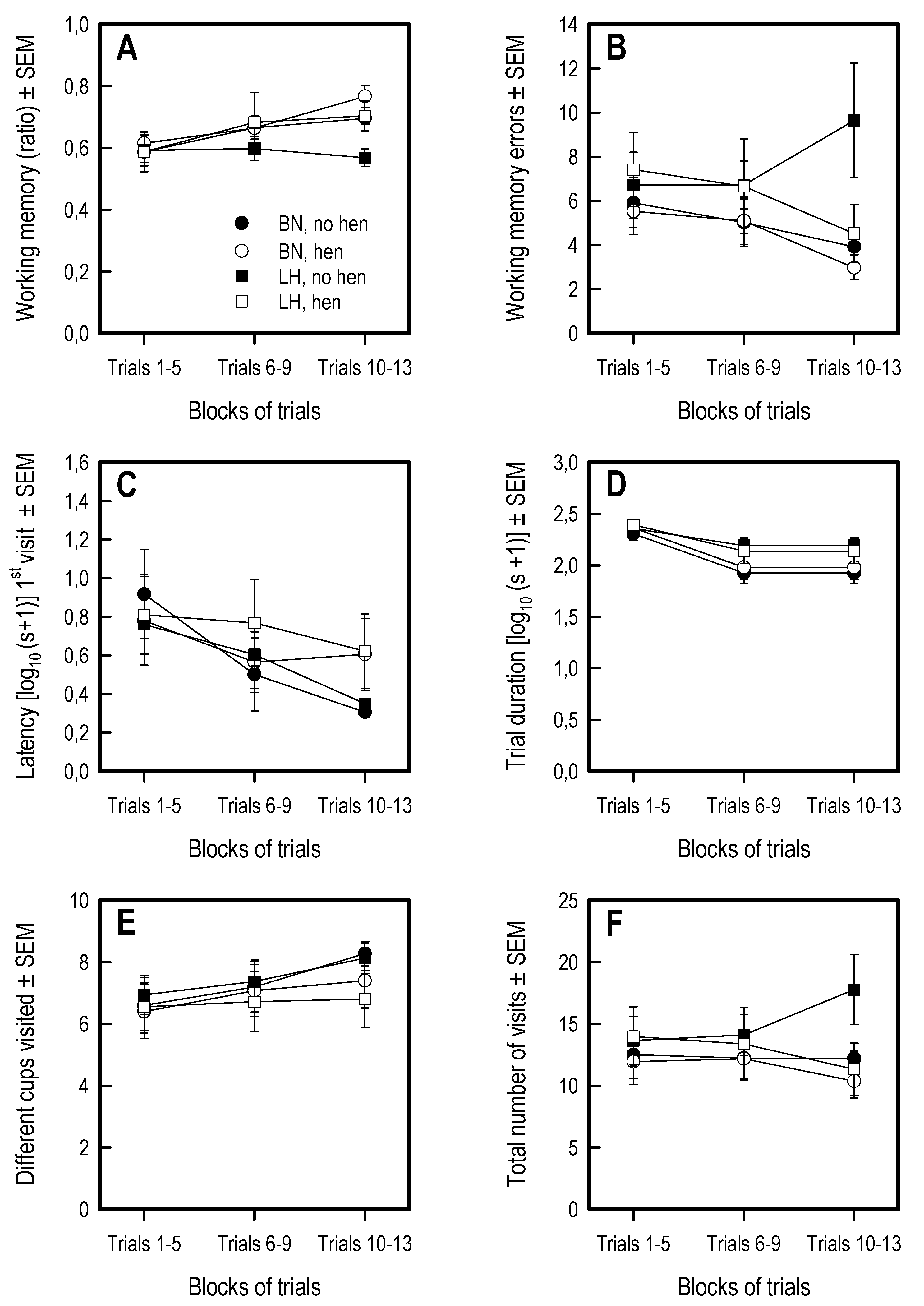
| Measure | Description |
|---|---|
| Number of different holes visited | Reflects exploratory motivation (and efficacy of exploring) |
| Number of revisits | Working memory errors |
| Spatial working memory | Number of rewarded visits/Total number of visits within a trial |
| Latency to first cup | Time into the circle of the first cup visited after release into the arena |
| Reflects anxiety and foraging motivation | |
| Trial duration | Time elapsed to find all baits or maximum trial duration (300 s) |
| Total visits | Reflect both exploratory motivation and poor memory |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hewlett, S.E.; Nordquist, R.E. Effects of Maternal Care During Rearing in White Leghorn and Brown Nick Layer Hens on Cognition, Sociality and Fear. Animals 2019, 9, 454. https://doi.org/10.3390/ani9070454
Hewlett SE, Nordquist RE. Effects of Maternal Care During Rearing in White Leghorn and Brown Nick Layer Hens on Cognition, Sociality and Fear. Animals. 2019; 9(7):454. https://doi.org/10.3390/ani9070454
Chicago/Turabian StyleHewlett, Susie E., and Rebecca E. Nordquist. 2019. "Effects of Maternal Care During Rearing in White Leghorn and Brown Nick Layer Hens on Cognition, Sociality and Fear" Animals 9, no. 7: 454. https://doi.org/10.3390/ani9070454
APA StyleHewlett, S. E., & Nordquist, R. E. (2019). Effects of Maternal Care During Rearing in White Leghorn and Brown Nick Layer Hens on Cognition, Sociality and Fear. Animals, 9(7), 454. https://doi.org/10.3390/ani9070454





