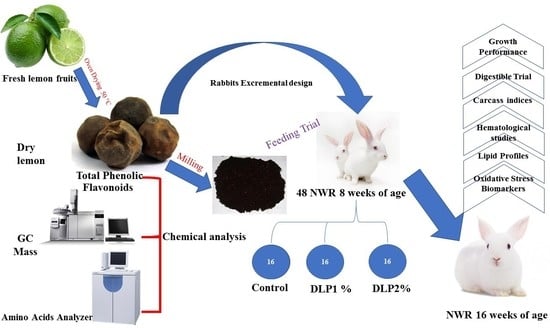
1. Introduction
Nutritional factors play an important role in the protection against the deleterious consequences of free radicals. Natural antioxidants-rich diets can significantly affect the enhancement of reactive antioxidants and reduce the risk of some diseases induced by free radicals generation. A high enough level of antioxidants in a diet is considered to encourage immunological processes and correctly elevate the defensive abilities of the cell [
1].
Over the years, plants have served as precious sources of bioactive compounds for humans and animals, thereby possessing great potential for use in the discovery of new drugs [
2].
Citrus fruit has the highest antioxidant activity [
3] as it contains many flavonoids, vitamin C, and carotenoids [
4]. There are numerous bioflavonoids in
Citrus limon such as hesperidin, rutin, and naringin, which exhibit many pharmacological actions in the body [
5]. Citrus fruits, especially
C. limon, have been scientifically proven to display many health benefits as well as limiting the risk of diseases due to their richness in vitamins and minerals and other active constituents [
6].
Numerous studies have proposed that
C. limon may display antifungal [
7] and anti-cancer [
8] activities. Also, some studies have suggested that its flavonoids possess numerous biological functions, including anti-inflammatory, antioxidant, antiviral, antiallergic, antiproliferative, antimutagenic, and antitumor activities [
9,
10,
11,
12]. Essential oils of
C. limon has also been found to prevent damage to the brain, lung, and intestine induced by aspirin toxicity [
13].
Several progenies of farm rabbits were known to produce a higher amount of meat at a rate greater than 10–15 times of their weight [
14]. Rabbit meat is preferred for its nutritional value compared with other meat products because of its low calorie content and high digestion [
15]. Proper care to maintain the physiological status of rabbits is an essential factor in the production of high-quality products that meat consumers expect. Nobakht [
16] demonstrated that different concentrations of dried lemon pulp in diets had marked influence on broilers performance. Azra et al. [
17] found that consuming lime peel or fresh lime juice induced the highest changes in serum antioxidant capacity. Flavonoids in
Citrus was found to protect against hyperglycemia via increasing liver glycolysis and lowering liver gluconeogenesis [
18]. Hesperidin and naringin, both
C. limon active compounds, were also found to significantly lowered the blood glucose level [
19]. Moreover, dietary dried citrus pulp supplementation may improve laying hens performance by their positive effects on lipid profile and blood glucose levels [
20].
Previous studies demonstrated the use of citrus pulp, juice, and peel meal as an animal feed additive without any accompanying side effects [
21]. In addition, dietary fresh or dry fruit of
C. limon was demonstrated to contribute to the maintenance of optimum health due to its health-promoting phytochemicals. To date, however, impactful investigations on dried
C. limon have not been performed to establish its beneficial effect and possible antioxidant activity with a rabbit model. Hence, this experiment was employed to explore the hemato-biochemical profiles, thyroid activity, and antioxidant responses of dried
C. limon as a powder in growing rabbits.
4. Discussion
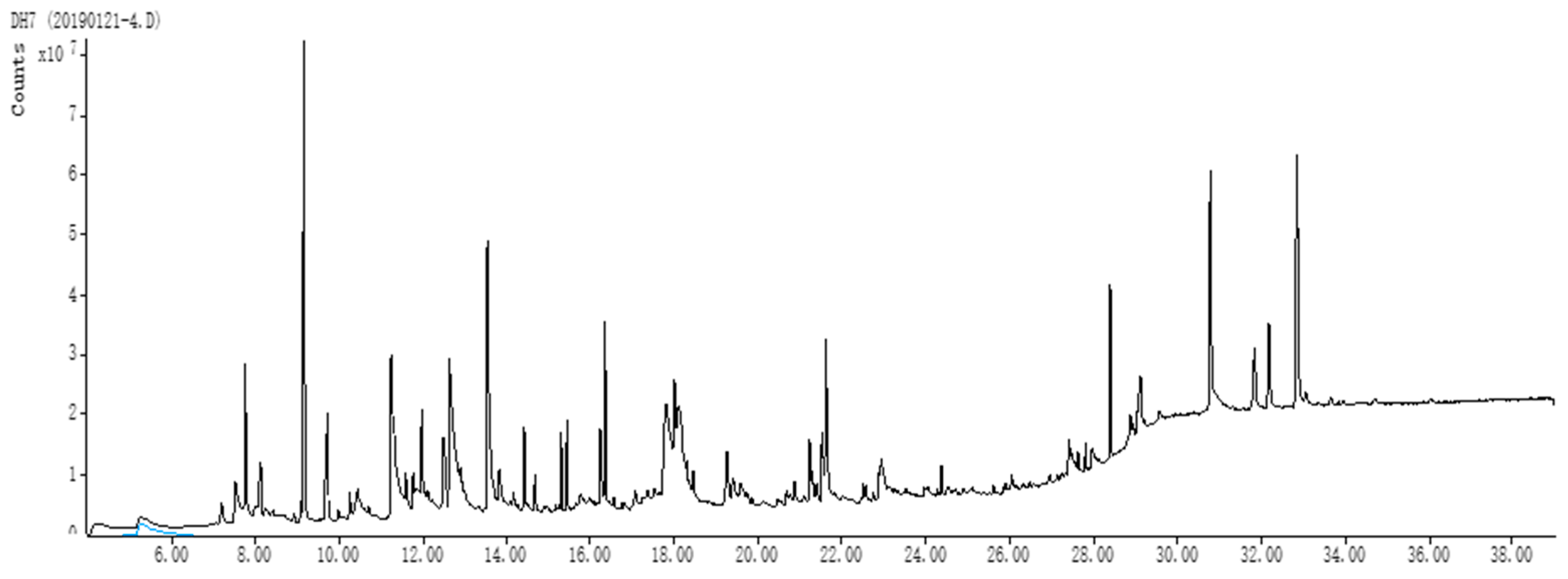
Dietary antioxidant supplements can enhance the physiological and productive statuses of animals by modifying metabolic processes. The current study aimed to investigate the influences of dry lemon on the hemato-biochemical alterations, and antioxidant indices of growing rabbits. Firstly, we sought to investigate in vitro phytochemical compounds of dry lemon (total phenolics, total flavonoids content) as well as total antioxidant capacity. The presence of phenolic compounds and flavonoids in the diet play an important role in cancer chemoprevention. Additionally, the health benefits of phenolic compounds depend on their consumption and bioavailability [
34]. Phenolic compounds have a wide range of biological and chemical activities, including radical scavenging properties. Total phenolic content and total flavonoids content were 120.57 mg/g and 31.28 mg/g, respectively. In contrast, Sultana et al. [
35] reported respective values of 158.79 mg/g and 29.33 mg/g. The differences between these findings and our data may be attributed to several factors including the regions and seasons of the sample collection, sections (peels, fruits), used for analysis, and the extraction procedure.
Secondly, the DPPH method was employed to assess the antioxidant activity of the dried lemon methanolic extract.
Through phytochemical analysis, the percentage of DPPH radicals inhibition of
C. limon extract was found to be 49.12%. A similar, antioxidant activity (43.60%) of lemon peel was reported by Reyhan et al. [
36] and may have been due to its high phenolic content. Our results also demonstrated that
C. limon extracts possess a high concentration of total polyphenols (120.57 mg/g) and flavonoids (31.28 mg/g).
The antioxidant activity of lemon fruits might be due to the occurrence of ascorbic acid content and biologically active secondary metabolites, which have been known to display significant antioxidant activity [
37]. Data of the present study showed that dry lemon methanolic extract contains marked content of total phenolics, flavonoids, and antioxidant activity. Several phytochemical molecules were identified by dry lemon extract GC analysis.
Generally, terpenoids, glycosides, and coumarins give the dry lemon extract their antioxidant and antimicrobial properties [
38]. The significance and activities of these molecules attribute to their lipophilic character of hydrocarbon skeleton and functional groups [
39]. GC analysis revealed that dry lemon methanolic extract had a relatively high content of 5-(Hydroxymethyl)-2-(dimethoxymethyl) furan, imidazole, and limonene, which are known to possess crucial antioxidant activity. Interactions among the components of the dry lemon methanolic extract could also affect their activity because of the synergistic effect of their main constituents [
40]. Increasing terpenes levels makes essential oils more effective [
41]. A substantial number of studies reported that phenolic compounds not only cause the antioxidant activity, but that all the phytochemical molecules are responsible for this activity [
42].
Data revealed that adding both levels of DLP (1% or 2%) to the diet of growing rabbit diets significantly increased their body weight, weight gain, and carcass weight. The improvement of rabbit performance by adding DLP to their diets may be due to the use of lemon as whole fruit, which led to an increase in the appetite and a higher consumption of feed. Also, lemon fruits were enriched with the most essential amino acids, potent antioxidant activity, total phenolic and total flavonoids compounds including anthocyanins, coumarins, flavonoids, flavones, isoflavones, catechins, isocatechins, and lignans, which might be represent the optimum antioxidant activity of dry lemons. A similar study conducted by Chaudry et al. [
43] revealed that adding 5% of citrus pulp into broiler diets did not have any significant effects on feed consumption. This has been confirmed in the present study. Furthermore, our results concerning feed conversion differed from those of Mourao et al. [
44] who found that boilers fed 5% or 10% citrus pulp had significantly higher FCR than the control birds. However, Wang et al. [
21] added 0%, 4%, 8%, 12%, or 16% DCP to a geese diet until 70 d of age, and they found that geese that were fed on diets containing 4% DCP resulted in higher average daily gain than other groups. Additionally, diets containing 16% DCP led to an elevation in the average daily feed intake and a higher FCR compared to control and 12%, respectively. Diet of geese supplement with DCP showed no effects on carcass traits across the treatment groups [
45]. However, rabbits fed the control diet had the highest values for the absolute and proportions of the liver, kidney, and heart compared to treated groups. The highest values of carcass and dressing percentages were observed when rabbits were fed the higher amount of DLP (2%) than the lower amount (1% DLP). No effect was detected in the absolute and proportion of the kidney based on addition amount. The previous results disagree with the study conducted by Petracci et al. [
15], which showed that gizzard weight and abdominal fat percentages were significantly affected by the addition of DLP in broiler diets. However, numerically, DLP had beneficial effects on some carcass traits.
Following DLP supplementation, a significant improvement in all hematological parameters was found. Moreover, 2% DLP led to the highest values for RBCs, PCV, Hb, MCV, and MCH (5.96%, 8.57%, 8.56%, 2.46% and 2.41%, respectively) compared to the control group. The enhancement in most hematological parameters might be due to the presence of many active compounds such as vitamin C, flavonoids, iron, and pyridoxine. Azra et al. [
17] reported similar results, as they found that different
C. limon doses (0.2, 0.4, and 0.6 mL/kg) could significantly affect different hematological parameters in healthy rabbits.
The results of the current study showed that
Citrus limon significantly increased the thyroid hormone levels (T3, T4, and T3/T4 ratio) compared to those found for the control group. This finding is supported by Miler et al. [
45], who found that
Citrus flavanones increased serum TSH without changing T4 levels. Moreover, by supplementing rabbit diets with 1% or 2% DLP showed a significant reduction in serum triglycerides, cholesterol, LDL-c, and VLDL-c were significantly reduced, while the level of HDL-c level statistically increased compared to the level in control animals. The hypolipidemic effect of
C. limon may be due to its flavonoids content, which can reduce blood cholesterol, triglycerides, and LDL-c levels based on its antioxidant effect [
46]. Complete methoxyylation of ring A with Citrus flavonoids seems to be an optimal structural change that exhibits substantial effects on the modulation of hepatic lipid metabolism via the suppressed secretion of lipoproteins with apoB in HepG2 cells [
47]. Other optimal molecular structures, such as tangeretin and nobiletin, exhibited a lower effect on blood cholesterol levels and triglyceride concentration. Also,
Citrus flavonoids, such as hesperidin and naringin content lacking a fully methoxylated ring, can virtually exhibit little or no reduction in the effects of lipids in humans [
48].
In the current study, a significant elevation in the levels of serum HDL was detected [
49], suggesting that
C. limon may serve as a valuable supplement in rabbit diet. Other compounds, such as pectin, a component of the plant cell wall, was reported to play a role in reducing cholesterol. This is due to the structure of flavonoids containing numerous OH groups, which can supply H atoms to quench free radicals, ultimately contributing to its potent antioxidant property [
50].
Three major levels of defense mechanisms were found in the antioxidant system of living cells. The activities of SOD, GSH-PX, and CAT is the first, which act to prevent free radical formation [
51]. The second level act by chain-breaking antioxidants (vitamin E, C, carotenoids) that disrupt chain multiplication and formation. The third level restores or eliminates damaged molecules from the cell by the help of specific enzymes [
52].
More importantly, this study showed that the activities of antioxidant enzymes such as GST CAT, SOD, and GSH were increased, while that of MDA was markedly decreased in the rabbits of the DLP treatment group compared to those in the control group. The increase in activities of antioxidant enzymes may be related to active DLP constituents such as ascorbic acid, flavonoids, and phenolic compounds and agree with many previous studies [
53,
54,
55], where citrus extracts, such as citrus peel,
C. karna, and
C. limetta were demonstrated to potentially have antioxidant bioactivity. The most important defense mechanism of dry lemon phenolic compounds is based on the absorption and neutralization of free radicals [
56]. However, previous reports have placed more emphasis on the antioxidant capacity of
C. limon [
4,
57,
58].
C. limon also contains several constituents such as 3-carene, imidazole, D-limonene, geraniol, and squalene as well as other compounds reported as potent antioxidants that can prevent microsomal lipid peroxide and protein degradation of ROS [
59,
60,
61,
62,
63], These data are in accordance with our phytochemical study results which highlighted
C. limon as a powerful scavenging agent.