Drinking Warm Water Improves Growth Performance and Optimizes the Gut Microbiota in Early Postweaning Rabbits during Winter
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethics Statement
2.2. Animal Management and Sample Collection
2.3. Intestinal Histological Analysis
2.4. SCFAs and NH3-N Determination
2.5. Extraction of RNA and Real-Time Quantitative PCR Analysis (qPCR)
2.6. Cecal Contents DNA Extraction and Sequencing
2.7. Bioinformatics Analysis
2.8. Statistical Analysis
3. Results
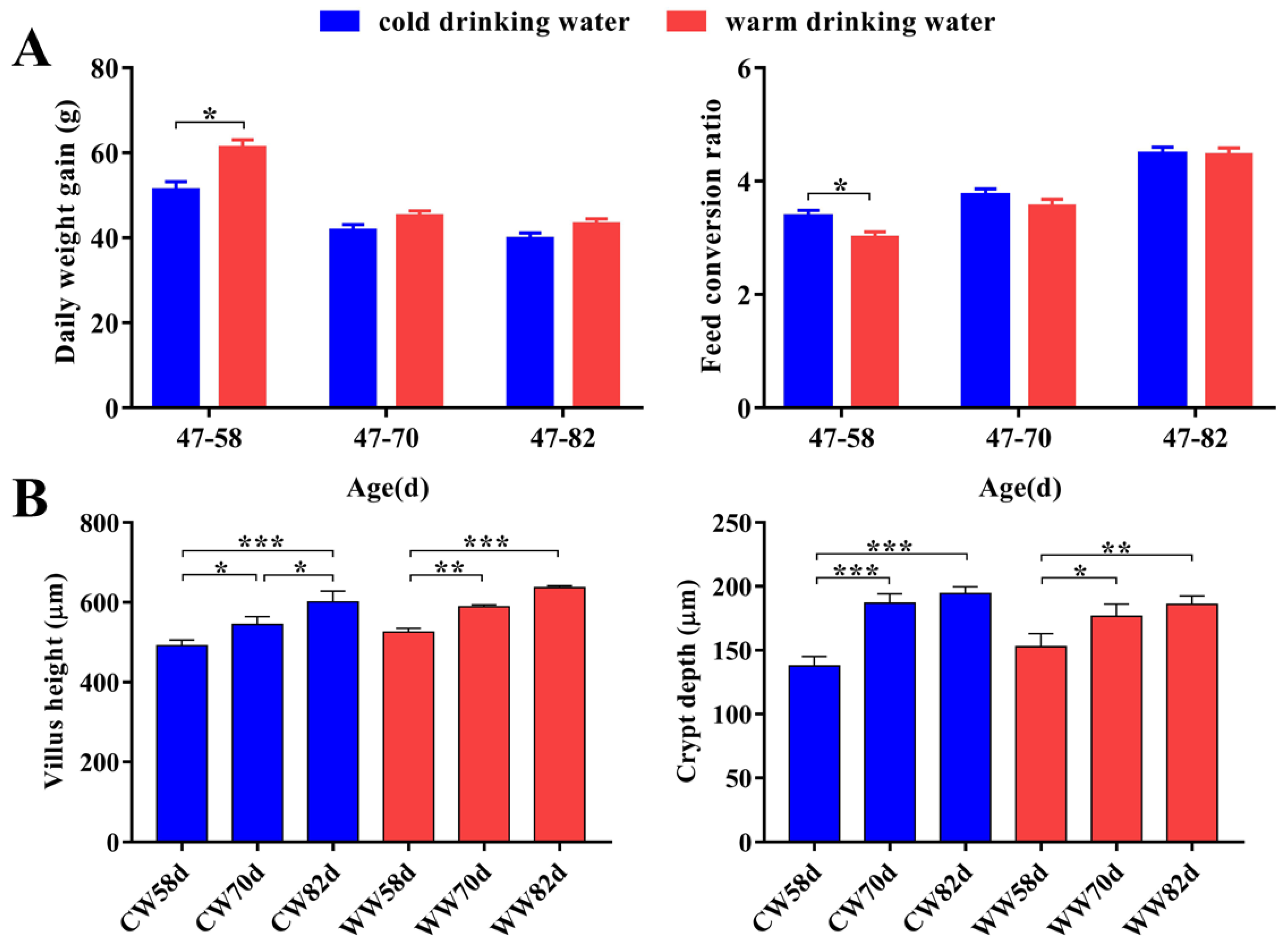
3.1. Growth Performance and Gut Morphology Changes within Warm- and Cold-Water Groups
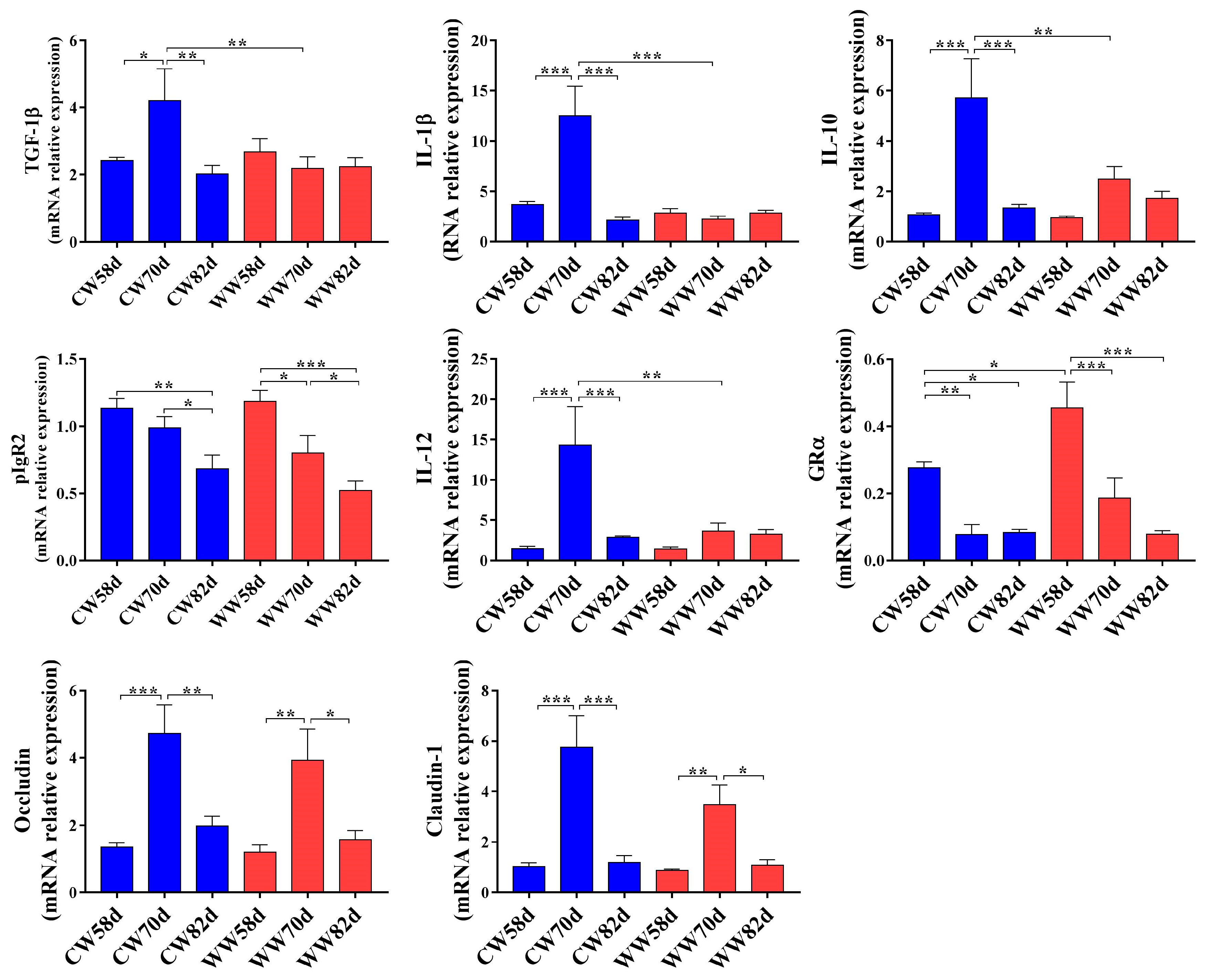
3.2. Expression of Intestinal Immunity and Tight Junction-Related Genes in Warm- and Cold-Water Groups
3.3. Fermentation Changes within Warm- and Cold-Water Groups
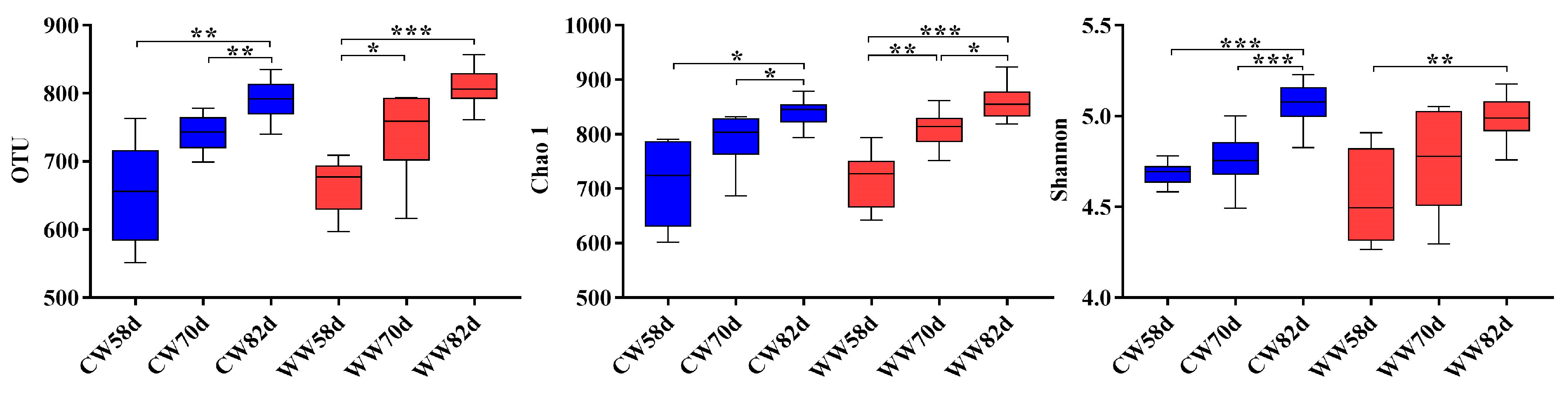
3.4. Microbial Diversity Changes within Warm- and Cold-Water Groups
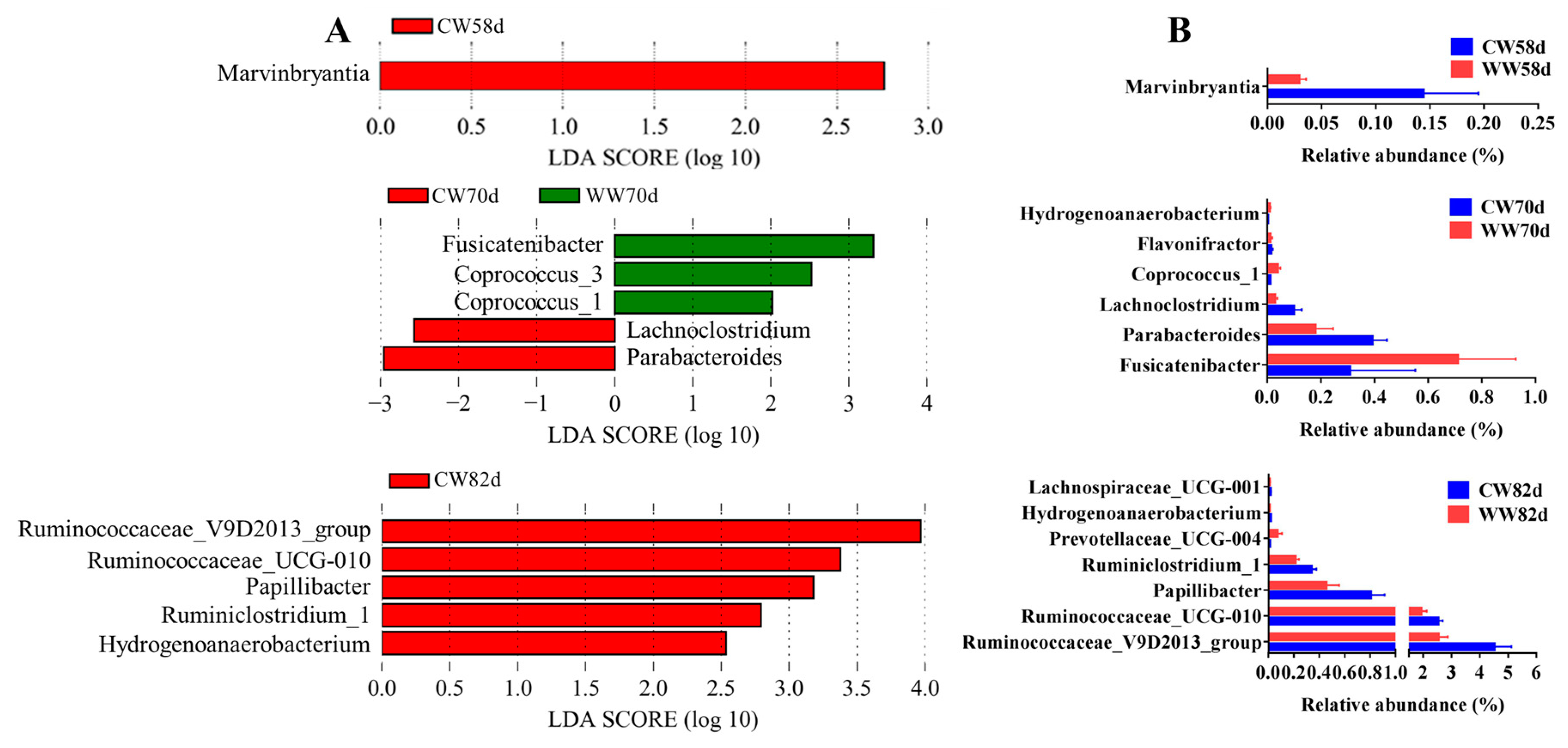
3.5. Microbial Community Composition Changes within Warm- and Cold-Water Groups
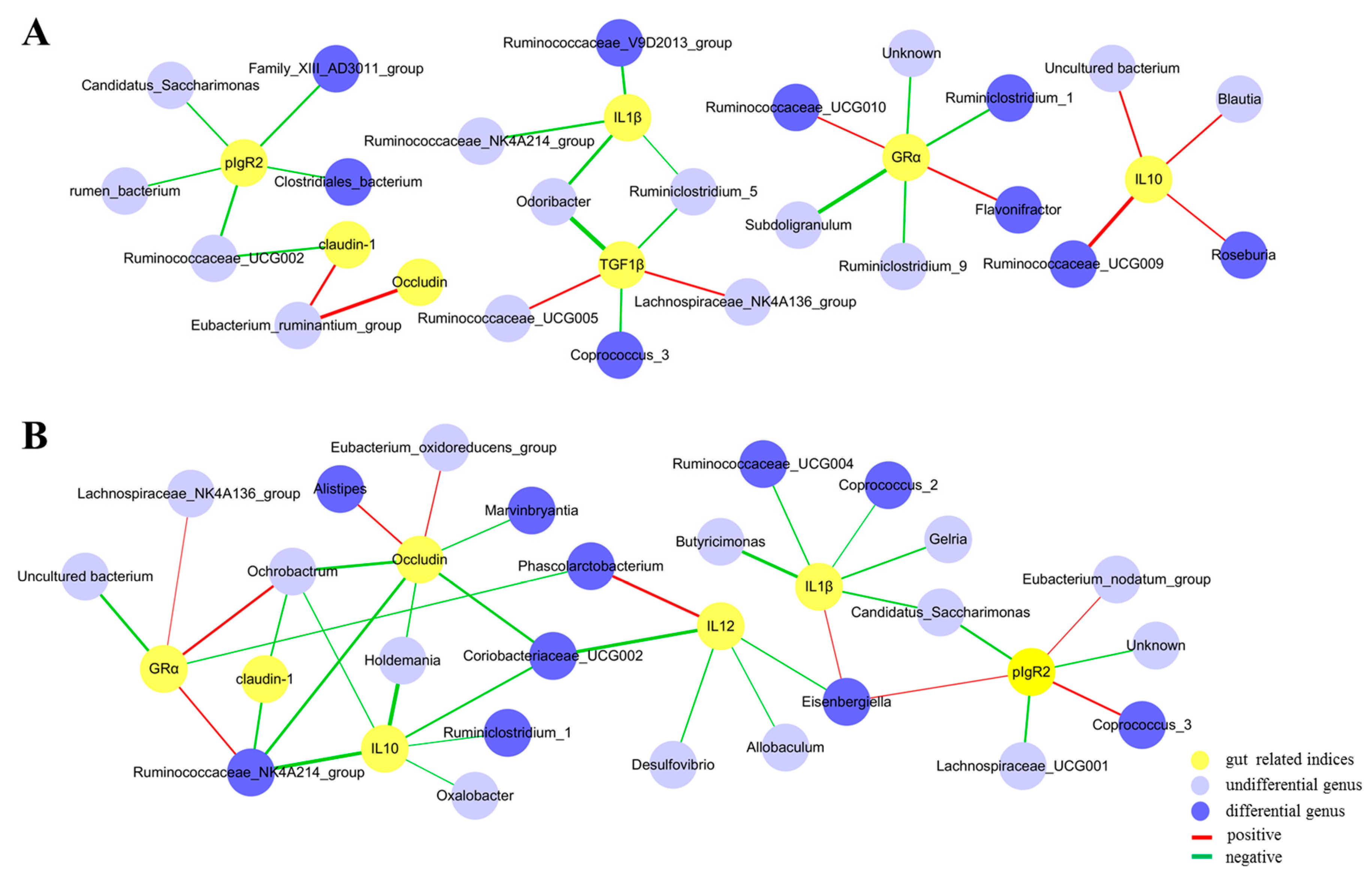
3.6. Correlations between the Gut Microbiota and Immunity-Related Genes
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ridaura, V.K.; Faith, J.J.; Rey, F.E.; Cheng, J.; Duncan, A.E.; Kau, A.L.; Griffin, N.W.; Lombard, V.; Henrissat, B.; Bain, J.R.; et al. Gut microbiota from twins discordant for obesity modulate metabolism in mice. Science 2013, 341, 1241214. [Google Scholar] [CrossRef] [PubMed]
- Frank, D.N.; St Amand, A.L.; Feldman, R.A.; Boedeker, E.C.; Harpaz, N.; Pace, N.R. Molecular-phylogenetic characterization of microbial community imbalances in human inflammatory bowel diseases. Proc. Natl. Acad. Sci. USA 2007, 104, 13780–13785. [Google Scholar] [CrossRef] [PubMed]
- Chevalier, C.; Stojanovic, O.; Colin, D.J.; Suarez-Zamorano, N.; Tarallo, V.; Veyrat-Durebex, C.; Rigo, D.; Fabbiano, S.; Stevanovic, A.; Hagemann, S.; et al. Gut Microbiota Orchestrates Energy Homeostasis during Cold. Cell 2015, 163, 1360–1374. [Google Scholar] [CrossRef] [PubMed]
- Worthmann, A.; John, C.; Ruhlemann, M.C.; Baguhl, M.; Heinsen, F.A.; Schaltenberg, N.; Heine, M.; Schlein, C.; Evangelakos, I.; Mineo, C.; et al. Cold-induced conversion of cholesterol to bile acids in mice shapes the gut microbiome and promotes adaptive thermogenesis. Nat. Med. 2017, 23, 839–849. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Huang, C.; Turner, L.R.; Su, H.; Qiao, Z.; Tong, S. Is diurnal temperature range a risk factor for childhood diarrhea? PLoS ONE 2013, 8, e64713. [Google Scholar] [CrossRef]
- Thiam, S.; Diene, A.N.; Sy, I.; Winkler, M.S.; Schindler, C.; Ndione, J.A.; Faye, O.; Vounatsou, P.; Utzinger, J.; Cisse, G. Association between Childhood Diarrhoeal Incidence and Climatic Factors in Urban and Rural Settings in the Health District of Mbour, Senegal. Int. J. Environ. Res. Public Health 2017, 14, 1049. [Google Scholar] [CrossRef] [PubMed]
- Kelley, K.W. Stress and immune function: A bibliographic review. Ann. Rech. Vet. 1980, 11, 445–478. [Google Scholar] [CrossRef]
- Laine, T.M.; Lyytikainen, T.; Yliaho, M.; Anttila, M. Risk factors for post-weaning diarrhoea on piglet producing farms in Finland. Acta Vet. Scand. 2008, 50, 21. [Google Scholar] [CrossRef]
- LaVoy, E.C.; McFarlin, B.K.; Simpson, R.J. Immune responses to exercising in a cold environment. Wilderness Environ. Med. 2011, 22, 343–351. [Google Scholar] [CrossRef]
- Yang, X.J.; Li, W.L.; Feng, Y.; Yao, J.H. Effects of immune stress on growth performance, immunity, and cecal microflora in chickens. Poult. Sci. 2011, 90, 2740–2746. [Google Scholar] [CrossRef]
- Kaushik, S.; Kaur, J. Effect of chronic cold stress on intestinal epithelial cell proliferation and inflammation in rats. Stress 2005, 8, 191–197. [Google Scholar] [CrossRef] [PubMed]
- Rogier, E.W.; Frantz, A.L.; Bruno, M.E.; Wedlund, L.; Cohen, D.A.; Stromberg, A.J.; Kaetzel, C.S. Lessons from mother: Long-term impact of antibodies in breast milk on the gut microbiota and intestinal immune system of breastfed offspring. Gut Microbes 2014, 5, 663–668. [Google Scholar] [CrossRef] [PubMed]
- Bauerl, C.; Collado, M.C.; Zuniga, M.; Blas, E.; Perez Martinez, G. Changes in cecal microbiota and mucosal gene expression revealed new aspects of epizootic rabbit enteropathy. PLoS ONE 2014, 9, e105707. [Google Scholar] [CrossRef] [PubMed]
- Kogut, M.H.; Arsenault, R.J. Editorial: Gut health: The new paradigm in food animal production. Front. Vet. Sci. 2016, 3, 71. [Google Scholar] [CrossRef] [PubMed]
- Heaney, D.P.; Jnb, S. Effects of warm versus cold milk replacers and of free-choice hay postweaning on performance of artificially reared lambs. Can. J. Anim. Sci 1986, 65, 871–878. [Google Scholar] [CrossRef]
- Osborne, V.R.; Hacker, R.R.; Mcbride, B.W. Effects of heated drinking water on the production responses of lactating Holstein and Jersey cows. Can. J. Anim. Sci. 2002, 82, 267–273. [Google Scholar] [CrossRef]
- Maynard, C.L.; Elson, C.O.; Hatton, R.D.; Weaver, C.T. Reciprocal interactions of the intestinal microbiota and immune system. Nature 2012, 489, 231–241. [Google Scholar] [CrossRef]
- Young, V.B. The intestinal microbiota in health and disease. Curr. Opin. Gastroenterol. 2012, 28, 63–69. [Google Scholar] [CrossRef]
- Combes, S.; Michelland, R.J.; Monteils, V.; Cauquil, L.; Soulie, V.; Tran, N.U.; Gidenne, T.; Fortun-Lamothe, L. Postnatal development of the rabbit caecal microbiota composition and activity. FEMS Microbiol. Ecol. 2011, 77, 680–689. [Google Scholar] [CrossRef]
- Kundu, P.; Blacher, E.; Elinav, E.; Pettersson, S. Our gut microbiome: The evolving inner self. Cell 2017, 171, 1481–1493. [Google Scholar] [CrossRef]
- Tremaroli, V.; Backhed, F. Functional interactions between the gut microbiota and host metabolism. Nature 2012, 489, 242–249. [Google Scholar] [CrossRef] [PubMed]
- Jobin, C. GPR109a: The missing link between microbiome and good health? Immunity 2014, 40, 8–10. [Google Scholar] [CrossRef] [PubMed]
- Singh, N.; Gurav, A.; Sivaprakasam, S.; Brady, E.; Padia, R.; Shi, H.; Thangaraju, M.; Prasad, P.D.; Manicassamy, S.; Munn, D.H.; et al. Activation of Gpr109a, receptor for niacin and the commensal metabolite butyrate, suppresses colonic inflammation and carcinogenesis. Immunity 2014, 40, 128–139. [Google Scholar] [CrossRef] [PubMed]
- Hamer, H.M.; Jonkers, D.; Venema, K.; Vanhoutvin, S.; Troost, F.J.; Brummer, R.J. Review article: The role of butyrate on colonic function. Aliment. Pharmacol. Ther. 2008, 27, 104–119. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Song, P.; Fan, P.; Hou, C.; Thacker, P.; Ma, X. Dietary sodium butyrate decreases postweaning diarrhea by modulating intestinal permeability and changing the bacterial communities in weaned piglets. J. Nutr. 2015, 145, 2774–2780. [Google Scholar] [CrossRef]
- Ma, X.; Fan, P.X.; Li, L.S.; Qiao, S.Y.; Zhang, G.L.; Li, D.F. Butyrate promotes the recovering of intestinal wound healing through its positive effect on the tight junctions. J. Anim. Sci. 2012, 90, 266–268. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.Y.; Sukhchuluun, G.; Bo, T.B.; Chi, Q.S.; Yang, J.J.; Chen, B.; Zhang, L.; Wang, D.H. Huddling remodels gut microbiota to reduce energy requirements in a small mammal species during cold exposure. Microbiome 2018, 6, 103. [Google Scholar] [CrossRef]
- Agazzi, A. The beneficial role of probiotics in monogastric animal nutrition and health. J. Dairy Vet. Anim. Res. 2015, 2. [Google Scholar] [CrossRef]
- Huff, G.R.; Huff, W.E.; Rath, N.C.; El-Gohary, F.A.; Zhou, Z.Y.; Shini, S. Efficacy of a novel prebiotic and a commercial probiotic in reducing mortality and production losses due to cold stress and Escherichia coli challenge of broiler chicks 1. Poult. Sci. 2015, 94, 918–926. [Google Scholar] [CrossRef]
- Schnupf, P.; Sansonetti, P.J. Quantitative RT-PCR profiling of the rabbit immune response: Assessment of acute Shigella flexneri infection. PLoS ONE 2012, 7, e36446. [Google Scholar] [CrossRef]
- Whiting, R.C.; Jenkins, R.K. Comparison of rabbit, beef, and chicken meats for functional-properties and frankfurter processing. J. Sci. Food Agric. 1981, 46, 1693–1696. [Google Scholar] [CrossRef]
- Zhu, Y.; Wang, C.; Li, F. Impact of dietary fiber/starch ratio in shaping caecal microbiota in rabbits. Can. J. Microbiol. 2015, 61, 771–784. [Google Scholar] [CrossRef] [PubMed]
- Larzul, C.; de Rochambeau, H. Selection for residual feed consumption in the rabbit. Livest. Prod. Sci. 2005, 95, 67–72. [Google Scholar] [CrossRef]
- Zanuzzi, C.N.; Fontana, P.A.; Barbeito, C.G.; Portiansky, E.L.; Gimeno, E.J. Paneth cells: Histochemical and morphometric study in control and Solanum glaucophyllum intoxicated rabbits. Eur. J. Histochem. 2008, 52, 93–100. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Yang, Q.; Sun, Z.; Chen, X.; Yang, C.; Ma, X. Editorial: Advance of interactions between exogenous natural bioactive peptides and intestinal barrier and immune responses. Curr. Protein Pept. Sci. 2015, 16, 574–575. [Google Scholar] [CrossRef]
- Broderick, G.A.; Kang, J.H. Automated simultaneous determination of ammonia and total amino acids in ruminal fluid and in vitro media. J. Dairy Sci. 1980, 63, 64–75. [Google Scholar] [CrossRef]
- Edgar, R.C.; Haas, B.J.; Clemente, J.C.; Quince, C.; Knight, R. UCHIME improves sensitivity and speed of chimera detection. Bioinformatics 2011, 27, 2194. [Google Scholar] [CrossRef]
- Edgar, R.C. Search and clustering orders of magnitude faster than BLAST. Bioinformatics 2010, 26, 2460. [Google Scholar] [CrossRef]
- Bokulich, N.A.; Subramanian, S.; Faith, J.J.; Gevers, D.; Gordon, J.I.; Knight, R.; Mills, D.A.; Caporaso, J.G. Quality-filtering vastly improves diversity estimates from Illumina amplicon sequencing. Nat. Methods 2013, 10, 57–59. [Google Scholar] [CrossRef]
- Caporaso, J.G.; Kuczynski, J.; Stombaugh, J.; Bittinger, K.; Bushman, F.D.; Costello, E.K.; Fierer, N.; Pena, A.G.; Goodrich, J.K.; Gordon, J.I.; et al. QIIME allows analysis of high-throughput community sequencing data. Nat. Methods 2010, 7, 335–336. [Google Scholar] [CrossRef]
- Arumugam, M.; Raes, J.; Pelletier, E.; Le Paslier, D.; Yamada, T.; Mende, D.R.; Fernandes, G.R.; Tap, J.; Bruls, T.; Batto, J.M.; et al. Enterotypes of the human gut microbiome. Nature 2011, 473, 174–180. [Google Scholar] [CrossRef] [PubMed]
- Schloss, P.D.; Westcott, S.L.; Ryabin, T.; Hall, J.R.; Hartmann, M.; Hollister, E.B.; Lesniewski, R.A.; Oakley, B.B.; Parks, D.H.; Robinson, C.J.; et al. Introducing mothur: Open-source, platform-independent, community-supported software for describing and comparing microbial communities. Appl. Environ. Microbiol. 2009, 75, 7537–7541. [Google Scholar] [CrossRef] [PubMed]
- Shannon, P.; Markiel, A.; Ozier, O.; Baliga, N.S.; Wang, J.T.; Ramage, D.; Amin, N.; Schwikowski, B.; Ideker, T. Cytoscape: A software environment for integrated models of biomolecular interaction networks. Genome Res. 2003, 13, 2498–2504. [Google Scholar] [CrossRef] [PubMed]
- Campbell, J.M.; Crenshaw, J.D.; Polo, J. The biological stress of early weaned piglets. J. Anim. Sci. Biotechnol. 2013, 4, 19. [Google Scholar] [CrossRef] [PubMed]
- Francino, M.P. Early development of the gut microbiota and immune health. Pathogens 2014, 3, 769–790. [Google Scholar] [CrossRef]
- Shen, X.; Miao, J.; Wan, Q.; Wang, S.; Li, M.; Pu, F.; Wang, G.; Qian, W.; Yu, Q.; Marotta, F.; et al. Possible correlation between gut microbiota and immunity among healthy middle-aged and elderly people in southwest China. Gut Pathog. 2018, 10, 4. [Google Scholar] [CrossRef]
- Shaw, K.A.; Bertha, M.; Hofmekler, T.; Chopra, P.; Vatanen, T.; Srivatsa, A.; Prince, J.; Kumar, A.; Sauer, C.; Zwick, M.E.; et al. Dysbiosis, inflammation, and response to treatment: A longitudinal study of pediatric subjects with newly diagnosed inflammatory bowel disease. Genome Med. 2016, 8, 75. [Google Scholar] [CrossRef]
- Ling, Z.; Liu, F.; Shao, L.; Cheng, Y.; Li, L. Dysbiosis of the Urinary Microbiota Associated With Urine Levels of Proinflammatory Chemokine Interleukin-8 in Female Type 2 Diabetic Patients. Front. Immunol. 2017, 8, 1032. [Google Scholar] [CrossRef]
- Qiu, Z.; Yang, H.; Rong, L.; Ding, W.; Chen, J.; Zhong, L. Targeted Metagenome Based Analyses Show Gut Microbial Diversity of Inflammatory Bowel Disease patients. Indian J. Microbiol. 2017, 57, 307–315. [Google Scholar] [CrossRef]
- Luna, R.A.; Oezguen, N.; Balderas, M.; Venkatachalam, A.; Runge, J.K.; Versalovic, J.; Veenstra-VanderWeele, J.; Anderson, G.M.; Savidge, T.; Williams, K.C. Distinct microbiome-neuroimmune signatures correlate with functional abdominal pain in children with autism spectrum disorder. Cell. Mol. Gastroenterol. Hepatol. 2017, 3, 218–230. [Google Scholar] [CrossRef]
- Wu, A.; Duan, J.; Liu, S.; Meng, X.; Zhou, P.; Dou, Q.; Li, C. Gut microbiota composition related with clostridium difficile-positive diarrhea and C. difficile type (A+B+, A-B+, And A-B-) in ICU hospitalized patients. BioRxiv 2017. [Google Scholar] [CrossRef]
- De Cruz, P.; Kang, S.; Wagner, J.; Buckley, M.; Sim, W.H.; Prideaux, L.; Lockett, T.; McSweeney, C.; Morrison, M.; Kirkwood, C.D.; et al. Association between specific mucosa-associated microbiota in Crohn’s disease at the time of resection and subsequent disease recurrence: A pilot study. J. Gastroenterol. Hepatol. 2015, 30, 268–278. [Google Scholar] [CrossRef] [PubMed]
- Chang, L.; Neu, J. Early factors leading to later obesity: Interactions of the microbiome, epigenome, and nutrition. Curr. Probl. Pediatr. Adolesc. Health Care 2015, 45, 134–142. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Ames, N.P.; Tun, H.M.; Tosh, S.M.; Jones, P.J.; Khafipour, E. High molecular weight barley beta-glucan alters gut microbiota toward reduced cardiovascular disease risk. Front. Microbiol. 2016, 7, 129. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Xu, Y.; Chen, X.; Fang, C.; Zhao, L.; Chen, F. The Maturing development of gut microbiota in commercial piglets during the weaning transition. Front. Microbiol. 2017, 8, 1688. [Google Scholar] [CrossRef]
- Le Chatelier, E.; Nielsen, T.; Qin, J.; Prifti, E.; Hildebrand, F.; Falony, G.; Almeida, M.; Arumugam, M.; Batto, J.M.; Kennedy, S.; et al. Richness of human gut microbiome correlates with metabolic markers. Nature 2013, 500, 541–546. [Google Scholar] [CrossRef]
- Konopka, A. What is microbial community ecology? ISME J. 2009, 3, 1223–1230. [Google Scholar] [CrossRef]
- Reichardt, N.; Duncan, S.H.; Young, P.; Belenguer, A.; McWilliam Leitch, C.; Scott, K.P.; Flint, H.J.; Louis, P. Phylogenetic distribution of three pathways for propionate production within the human gut microbiota. ISME J. 2014, 8, 1323–1335. [Google Scholar] [CrossRef]
- Fung, K.Y.; Cosgrove, L.; Lockett, T.; Head, R.; Topping, D.L. A review of the potential mechanisms for the lowering of colorectal oncogenesis by butyrate. Br. J. Nutr. 2012, 108, 820–831. [Google Scholar] [CrossRef]
- Wilson, A.J.; Chueh, A.C.; Togel, L.; Corner, G.A.; Ahmed, N.; Goel, S.; Byun, D.S.; Nasser, S.; Houston, M.A.; Jhawer, M.; et al. Apoptotic sensitivity of colon cancer cells to histone deacetylase inhibitors is mediated by an Sp1/Sp3-activated transcriptional program involving immediate-early gene induction. Cancer Res. 2010, 70, 609–620. [Google Scholar] [CrossRef]
- Blas, E.; Gidenne, T. Digestion of starch and sugars. In The Nutrition of the Rabbit, 2nd ed.; De Blas, C., Wiseman, J., Eds.; CABI Publishing: Wallingford, UK, 2010; pp. 273–274. [Google Scholar]
- Degen, A.A.; Young, B.A. Effects of ingestion of warm, cold and frozen water on heat-balance in cattle. Can. J. Anim. Sci 1984, 64, 73–80. [Google Scholar] [CrossRef]
- Nicol, A.M.; Young, B.A. Short-term thermal and metabolic responses of sheep to ruminal cooling: Effects of level of cooling and physiological state. Can. J. Anim. Sci. 1990, 70, 833–843. [Google Scholar] [CrossRef]
- Louis, P.; Duncan, S.H.; McCrae, S.I.; Millar, J.; Jackson, M.S.; Flint, H.J. Restricted distribution of the butyrate kinase pathway among butyrate-producing bacteria from the human colon. J. Bacteriol. 2004, 186, 2099–2106. [Google Scholar] [CrossRef]
- Bedford, A.; Gong, J. Implications of butyrate and its derivatives for gut health and animal production. Anim. Nutr. 2018, 4, 151–159. [Google Scholar] [CrossRef]
- Sleeth, M.L.; Thompson, E.L.; Ford, H.E.; Zac-Varghese, S.E.; Frost, G. Free fatty acid receptor 2 and nutrient sensing: A proposed role for fibre, fermentable carbohydrates and short-chain fatty acids in appetite regulation. Nutr. Res. Rev. 2010, 23, 135–145. [Google Scholar] [CrossRef]
- Flynn, N.E.; Wu, G. Glucocorticoids play an important role in mediating the enhanced metabolism of arginine and glutamine in enterocytes of postweaning pigs. J. Nutr. 1997, 127, 732–737. [Google Scholar] [CrossRef]
- Silverman, M.N.; Sternberg, E.M. Glucocorticoid regulation of inflammation and its functional correlates: From HPA axis to glucocorticoid receptor dysfunction. Ann. N. Y. Acad. Sci. 2012, 1261, 55–63. [Google Scholar] [CrossRef]
- Lacey, J.M.; Wilmore, D.W. Is glutamine a conditionally essential amino acid? Nutr. Rev. 2010, 48, 297–309. [Google Scholar] [CrossRef]
- Zhang, X. Molecular sensors and modulators of thermoreception. Channels 2015, 9, 73–81. [Google Scholar] [CrossRef]
- Galindo-Villegas, J.; Garcia-Moreno, D.; de Oliveira, S.; Meseguer, J.; Mulero, V. Regulation of immunity and disease resistance by commensal microbes and chromatin modifications during zebrafish development. Proc. Natl. Acad. Sci. USA 2012, 109, E2605–E2614. [Google Scholar] [CrossRef]

| Age (d) | Number of Rabbits with Diarrhea/Total Number of Rabbits | Odds Ratio | 95% CI 3 | p4 | |
|---|---|---|---|---|---|
| WW 1 | CW 2 | ||||
| Day 47 to 58 | 16/90 | 20/90 | 0.76 | 0.36–1.58 | 0.46 |
| Day 59 to 70 | 29/74 | 31/75 | 0.92 | 0.48–1.76 | 0.79 |
| Day 71 to 82 | 2/59 | 14/60 | 0.12 | 0.03–0.53 | <0.01 |
| Item | Main Effects Two-Way ANOVA | ||
|---|---|---|---|
| Age | Water Temperature | Interaction | |
| Acetic acid | *** | NS | NS |
| Propionic acid | *** | NS | NS |
| Butyric acid | *** | NS | * |
| Isobutyric acid | *** | ** | NS |
| Valeric acid | * | NS | NS |
| Isovaleric acid | * | NS | ** |
| NH3-N | ** | NS | NS |
| pH | NS | NS | NS |
| Alpha Diversity | Main Effects Two-Way ANOVA | ||
|---|---|---|---|
| Age | Water Temperature | Interaction | |
| OTUs | *** | NS | NS |
| ACE | *** | NS | NS |
| Chao | *** | NS | NS |
| Simpson | ** | NS | NS |
| Shannon | *** | NS | NS |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Q.; Fu, W.; Guo, Y.; Tang, Y.; Du, H.; Wang, M.; Liu, Z.; Li, Q.; An, L.; Tian, J.; et al. Drinking Warm Water Improves Growth Performance and Optimizes the Gut Microbiota in Early Postweaning Rabbits during Winter. Animals 2019, 9, 346. https://doi.org/10.3390/ani9060346
Wang Q, Fu W, Guo Y, Tang Y, Du H, Wang M, Liu Z, Li Q, An L, Tian J, et al. Drinking Warm Water Improves Growth Performance and Optimizes the Gut Microbiota in Early Postweaning Rabbits during Winter. Animals. 2019; 9(6):346. https://doi.org/10.3390/ani9060346
Chicago/Turabian StyleWang, Qiangjun, Wei Fu, Yao Guo, Yuhan Tang, Haoxuan Du, Meizhi Wang, Zhongying Liu, Qin Li, Lei An, Jianhui Tian, and et al. 2019. "Drinking Warm Water Improves Growth Performance and Optimizes the Gut Microbiota in Early Postweaning Rabbits during Winter" Animals 9, no. 6: 346. https://doi.org/10.3390/ani9060346
APA StyleWang, Q., Fu, W., Guo, Y., Tang, Y., Du, H., Wang, M., Liu, Z., Li, Q., An, L., Tian, J., Li, M., & Wu, Z. (2019). Drinking Warm Water Improves Growth Performance and Optimizes the Gut Microbiota in Early Postweaning Rabbits during Winter. Animals, 9(6), 346. https://doi.org/10.3390/ani9060346





