The Social Rank of Zoo-Housed Japanese Macaques is a Predictor of Visitor-Directed Aggression
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
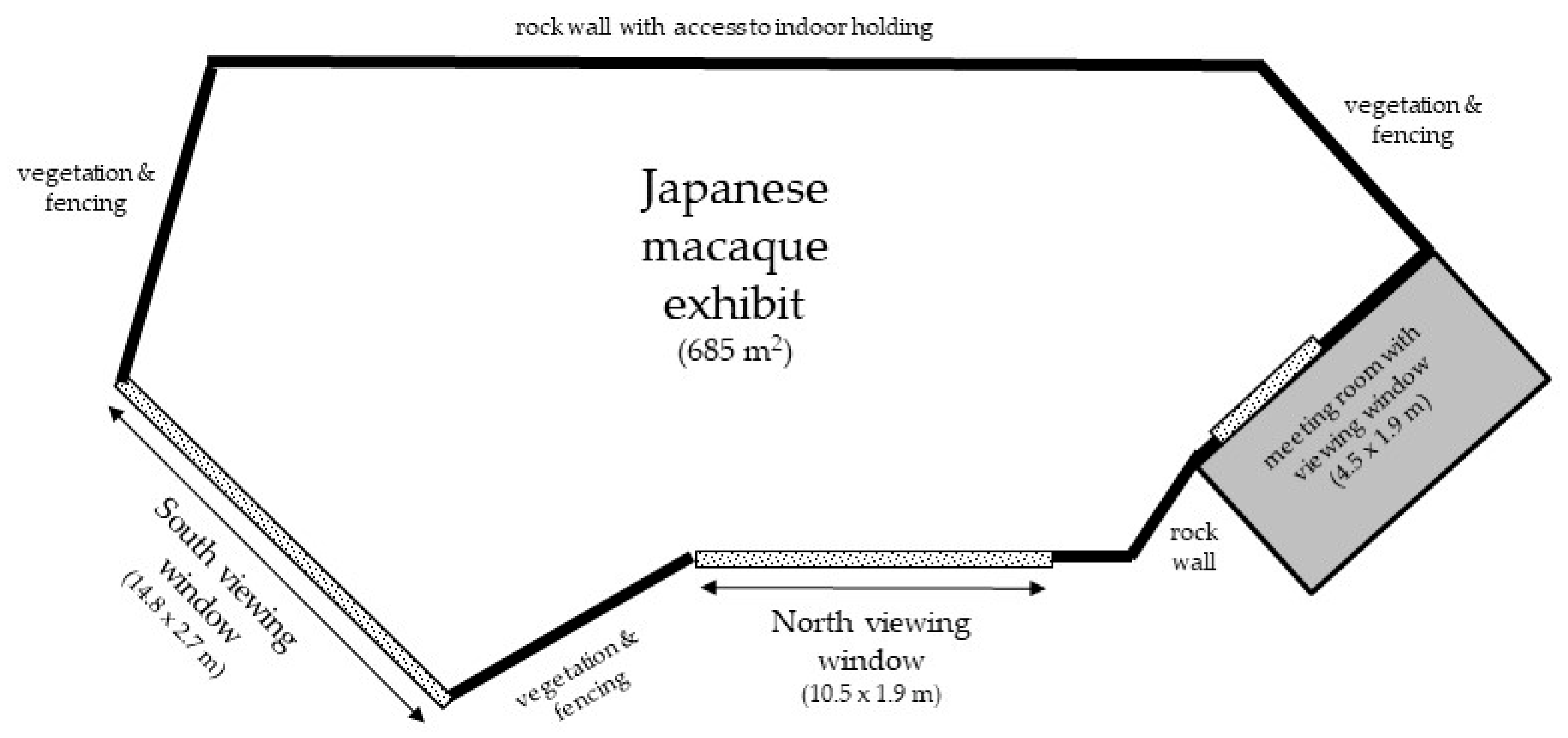
2.1. Subjects and Housing
2.2. Behavioral Data Collection
2.3. Calculation of Rank via Elo-Rating Scores
2.4. Statistical Analysis
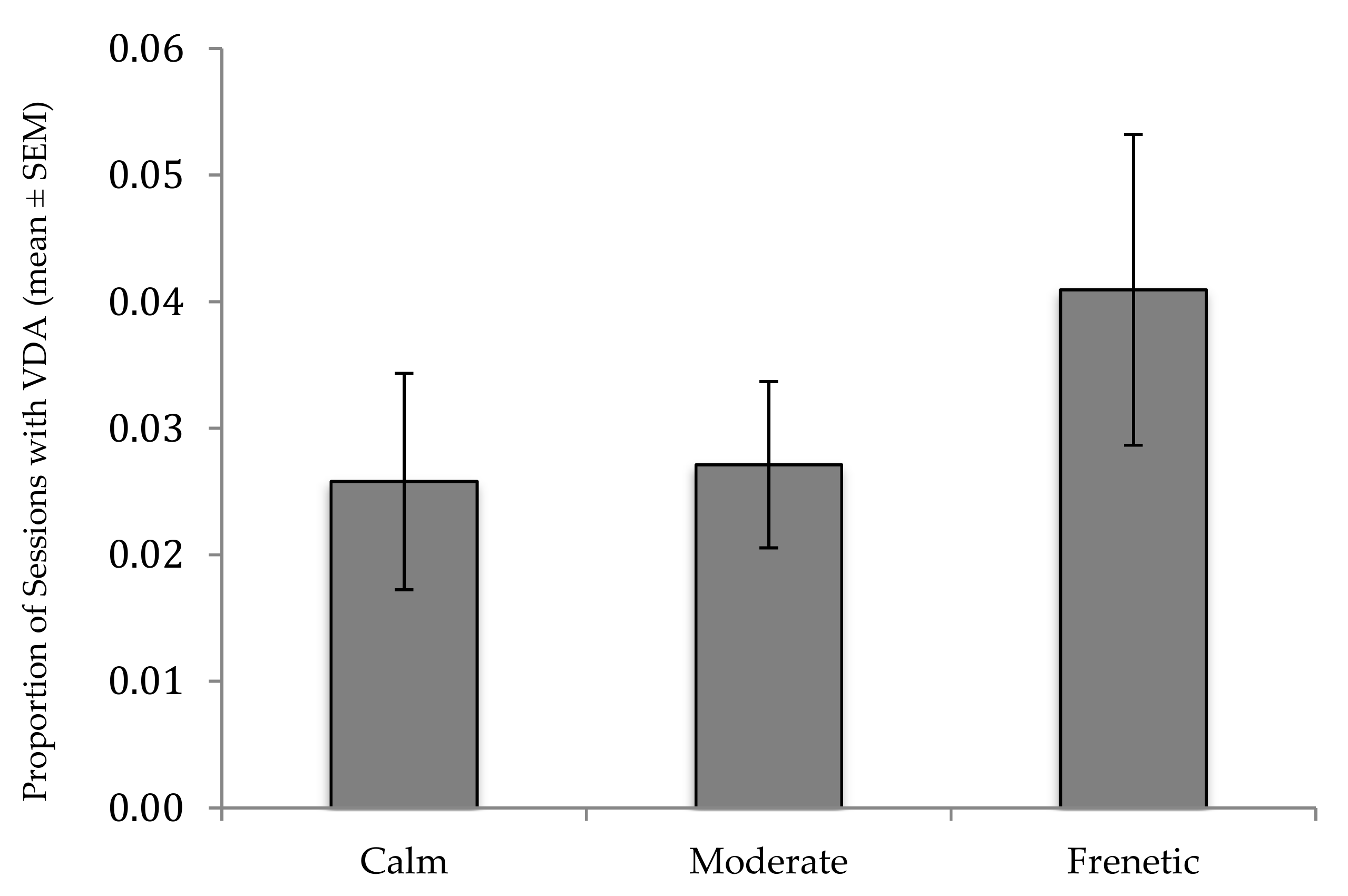
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Fernandez, E.J.; Tamborski, M.A.; Pickens, S.R.; Timberlake, W. Animal–visitor interactions in the modern zoo: Conflicts and interventions. Appl. Anim. Behav. Sci. 2009, 120, 1–8. [Google Scholar] [CrossRef]
- Hosey, G.R. Zoo animals and their human audiences: What is the visitor effect? Anim. Welf. 2000, 9, 343–357. [Google Scholar]
- Ross, S.R.; Lonsdorf, E.V.; Stoinski, T. Assessing the welfare implications of visitors in a zoo setting: A comment on wells (2005). Appl. Anim. Behav. Sci. 2007, 102, 130–133. [Google Scholar] [CrossRef]
- Fanson, K.; Wielebnowski, N. Effect of housing and husbandry practices on adrenocortical activity in captive Canada lynx (Lynx canadensis). Anim. Welf. 2013, 22, 159–165. [Google Scholar] [CrossRef]
- Bloomfield, R.C.; Gillespie, G.R.; Kerswell, K.J.; Butler, K.L.; Hemsworth, P.H. Effect of partial covering of the visitor viewing area window on positioning and orientation of zoo orangutans: A preference test: Orangutans position with regard to visitors. Zoo Biol. 2015, 34, 223–229. [Google Scholar] [CrossRef] [PubMed]
- Sherwen, S.L.; Harvey, T.J.; Magrath, M.J.L.; Butler, K.L.; Fanson, K.V.; Hemsworth, P.H. Effects of visual contact with zoo visitors on black-capped capuchin welfare. Appl. Anim. Behav. Sci. 2015, 167, 65–73. [Google Scholar] [CrossRef]
- Hosey, G.R.; Druck, P.L. The influence of zoo visitors on the behaviour of captive primates. Appl. Anim. Behav. Sci. 1987, 18, 19–29. [Google Scholar] [CrossRef]
- Mitchell, G.; Tromborg, C.T.; Kaufman, J.; Bargabus, S.; Simoni, R.; Geissler, V. More on the ‘influence’ of zoo visitors on the behaviour of captive primates. Appl. Anim. Behav. Sci. 1992, 35, 189–198. [Google Scholar] [CrossRef]
- Nimon, A.J.; Dalziel, F.R. Cross-species Interaction and communication: A study method applied to captive siamang (Hylobates syndactylus) and long-billed corella (Cacatua tenuirostris) contacts with humans. Appl. Anim. Behav. Sci. 1992, 33, 261–272. [Google Scholar] [CrossRef]
- Wood, W. Interactions among environmental enrichment, viewing crowds, and zoo chimpanzees (Pantroglodytes). Zoo Biol. 1998, 17, 211–230. [Google Scholar] [CrossRef]
- Stoinski, T.S.; Jaicks, H.F.; Drayton, L.A. Visitor effects on the behavior of captive western lowland gorillas: The importance of individual differences in examining welfare: Visitor effects on gorilla behaviour. Zoo Biol. 2012, 31, 586–599. [Google Scholar] [CrossRef] [PubMed]
- Fa, J.E. Visitor-directed aggression among the Gibraltar macaques. Zoo Biol. 1992, 11, 43–52. [Google Scholar] [CrossRef]
- Mitchell, G.; Obradovich, S.D.; Herring, F.H.; Dowd, B.; Tromborg, C. Threats to observers, keepers, visitors, and others by zoo mangabeys (Cercocebus galeritus chrysogaster). Primates 1991, 32, 515–522. [Google Scholar] [CrossRef]
- Clark, F.E.; Fitzpatrick, M.; Hartley, A.; King, A.J.; Lee, T.; Routh, A.; Walker, S.L.; George, K. Relationship between behavior, adrenal activity, and environment in zoo-housed western lowland gorillas (Gorilla gorilla gorilla). Zoo Biol. 2012, 31, 306–321. [Google Scholar] [CrossRef] [PubMed]
- Zumpe, D.; Michael, R.P. Redirected Aggression and Gonadal Hormones in Captive Rhesus Monkeys (Macaca mulatta). Anim. Behav. 1970, 18, 11–19. [Google Scholar] [CrossRef]
- Judge, P. Redirection of aggression based on kinship in a captive group of pigtail macaques. Int. J. Primatol. 1982, 3, 225–237. [Google Scholar]
- Smuts, B.B. Sex and Friendship in Baboons; Aldine: Hawthorne, NY, USA, 1985. [Google Scholar]
- Cheney, D.L.; Seyfarth, R.M. Aggression and reconciliation among vervet monkeys, Cercopithecus aethiops. Behaviour 1989, 110, 258–275. [Google Scholar]
- Aureli, F.; Veenema, H.C.; van Panthaleon van Eck, C.J.; van Hooff, J.A.R.A.M. Reconciliation, consolation, and redirection in Japanese macaques (Macaca fuscata). Behaviour 1993, 124, 1–21. [Google Scholar]
- Butovskaya, M.L.; Meishvili, N.V.; Chalyan, V.G. Redirection of aggression and consolation in hamadryas baboons. Neurosci. Behav. Physiol. 2005, 45, 417–422. [Google Scholar] [CrossRef]
- Chamove, A.S.; Hosey, G.R.; Schaetzel, P. Visitors excite primates in zoos. Zoo Biol. 1988, 7, 359–369. [Google Scholar] [CrossRef]
- Kuhar, C.W. Group differences in captive gorillas’ reaction to large crowds. Appl. Anim. Behav. Sci. 2008, 110, 377–385. [Google Scholar] [CrossRef]
- Collins, C.K.; Marples, N.M. The Effects of zoo visitors on a group of Western lowland gorillas (Gorilla gorilla gorilla) before and after the birth of an infant at Dublin zoo. Int. Zoo Yearb. 2016, 50, 183–192. [Google Scholar] [CrossRef]
- Smith, J.J. Primates and people in the zoo: Implications of human–animal interactions and relationships. In Ethnoprimatology; Waller, M.T., Ed.; Springer International Publishing: Cham, Switzerland, 2016; pp. 371–398. [Google Scholar] [CrossRef]
- Sterck, E.H.M.; Watts, D.P.; van Schaik, C.P. The evolution of female social relationships in nonhuman primates. Behav. Ecol. Sociobiol. 1997, 41, 291–309. [Google Scholar] [CrossRef]
- Wrangham, R.W. An ecological model of female-bonded primate groups. Behaviour 1980, 75, 262–300. [Google Scholar] [CrossRef]
- Cowlishaw, G.; Dunbar, R.I. Dominance rank and mating success in male primates. Anim. Behav. 1991, 41, 1045–1056. [Google Scholar] [CrossRef]
- Thierry, B. Unity in diversity: Lessons from Macaque Societies. Evolut. Anthropol. Issues News Rev. 2007, 16, 224–238. [Google Scholar] [CrossRef]
- Thierry, B.; Iwaniuk, A.N.; Pellis, S.M. The influence of phylogeny on the social behaviour of macaques (Primates: Cercopithecidae, genus Macaca). Ethology 2000, 106, 713–728. [Google Scholar] [CrossRef]
- Berman, C.M. Primate Social Relationships: An Integrated Approach; Blackwell: Boston, MA, USA, 1983. [Google Scholar]
- Davis, N.; Schaffner, C.M.; Smith, T.E. Evidence that zoo visitors influence HPA activity in spider monkeys (Ateles geoffroyii rufiventris). Appl. Anim. Behav. Sci. 2005, 90, 131–141. [Google Scholar] [CrossRef]
- Cronin, K.A.; Bethell, E.J.; Jacobson, S.L.; Egelkamp, C.; Hopper, L.M. Evaluating mood changes in response to anthropogenic noise with a response-slowing task in three species of zoo-housed primates. Anim. Behav. Cognit. 2018, 5, 209–221. [Google Scholar] [CrossRef]
- ZooMonitor, version 1; Mobile Application Software; Lincoln Park Zoo: Chicago, IL, USA, 2016. Available online: https://zoomonitor.org (accessed on 4 April 2019).
- Wark, J.D.; Cronin, K.A.; Niemann, T.; Shender, M.A.; Horrigan, A.; Kao, A.; Ross, M.R. Monitoring the behavior and habitat use of animals to enhance welfare using the ZooMoniotor app. Anim. Behav. Cognit. 2019, in press. [Google Scholar]
- Jacobson, S.L.; Kwiatt, A.C.; Ross, S.R.; Cronin, K.A. The Effects of cognitive testing on the welfare of zoo-housed Japanese macaques (Macaca fuscata). Appl. Anim. Behav. Sci. 2019, 212, 90–97. [Google Scholar] [CrossRef]
- Neumann, C.; Duboscq, J.; Dubuc, C.; Ginting, A.; Irwan, A.M.; Agil, M.; Widdig, A.; Engelhardt, A. Assessing dominance hierarchies: Validation and advantages of progressive evaluation with Elo-rating. Anim. Behav. 2011, 82, 911–921. [Google Scholar] [CrossRef]
- Newton-Fisher, N.E. Modeling Social Dominance: Elo-ratings, prior history, and the intensity of aggression. Int. J. Primatol. 2017, 38, 427–447. [Google Scholar] [CrossRef] [PubMed]
- R Core Team. R: A Language and Environment for Statistical Computing. 2017. Available online: https://www.R-project.org/ (accessed on 4 April 2019).
- Neumann, C.; Kulik, L. EloRating: Animal dominance hierarchies by Elo rating. R Package Version 2014, 43, 1–26. [Google Scholar]
- Symonds, M.R.E.; Moussalli, A. A brief guide to model selection, multimodel inference and model averaging in behavioural ecology using Akaike’s information criterion. Behav. Ecol. Sociobiol. 2011, 65, 13–21. [Google Scholar] [CrossRef]
- Bates, D.; Maechler, M.; Bolker, B.; Walker, S. lme4: Linear mixed-effects models using Eigen and S4. R Package Version 2015, 1, 1–7. Available online: https://CRAN.R-project.org/package=lme4 (accessed on 4 April 2019).
- Mazerolle, M. AICcmodavg: Model selection and multimodel inference based on (Q)AIC(c). R Package Version 2013, 1, 35. [Google Scholar]
- Hothorn, T.; Bretz, F.; Westfall, P.; Heiberger, R.M.; Schuetzenmeister, A.; Scheibe, S.; Hothorn, M.T. Package ‘multcomp’. 2017. Available online: https://cran.r-project.org/web/packages/multcomp/index.html (accessed on 4 April 2019).
- Thierry, B. Covariation of conflict management patterns across macaque species. In Natural Conflict Resolution; Aureli, F., de Waal, F.B.M., Eds.; University of California Press: Oakland, CA, USA, 2000; pp. 106–128. ISBN 978-0-520-22346-2. [Google Scholar]
- Bygott, J.D. Agonistic Behaviour and Dominance in Wild Chimpanzees. Ph.D. Thesis, University of Cambridge, Cambridge, UK, June 1974. [Google Scholar]
- Sapolsky, R.M. Why Zebras Don’t Get Ulcers: An Updated Guide to Stress, Stress Related Diseases, and Coping, 2nd ed.; Holt Paperbacks: New York, NY, USA, 1998; p. 356. ISBN 978-0-7167-3210-5. [Google Scholar]
- Castles, D.L.; Whiten, A. Post-conflict behaviour of wild olive baboons. I. Reconciliation, redirection and consolation. Ethology 1998, 104, 126–147. [Google Scholar] [CrossRef]
- Birke, L. Effects of browse, human visitors and noise on the behaviour of captive orangutans. Anim. Welf. 2002, 11, 189–202. [Google Scholar]

| Factors Included in Model | AICc | Model Rank |
|---|---|---|
| Rank + Crowd Activity | 7072.6 | 1 (best) |
| Rank + Crowd Size + Crowd Activity | 7074.4 | 2 |
| Rank | 7075.0 | 3 |
| Rank + Crowd Size | 7075.9 | 4 |
| Crowd Activity | 7103.4 | 5 |
| Crowd Activity + Crowd Size | 7103.9 | 6 |
| Crowd Size | 7106.7 | 7 (worst) |
| Null Model | χ2 | p-Value |
|---|---|---|
| Model excluding RANK | 29.42 | <0.0001 |
| Model excluding CROWD SIZE | 0.18 | 0.6755 |
| Model excluding CROWD ACTIVITY | 5.43 | 0.0663 |
| Fixed Factors | Beta | Lower-95 | Upper-95 | Std. Error |
|---|---|---|---|---|
| Intercept | −4.313 | −4.495 | −4.130 | 0.093 |
| Crowd Activity (Frenetic) | 0.552 | 0.143 | 0.960 | 0.209 |
| Crowd Activity (Moderate) | 0.075 | −0.109 | 0.258 | 0.094 |
| Random Factor | SD | |||
| Rank | 1.272 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Woods, J.M.; Ross, S.R.; Cronin, K.A. The Social Rank of Zoo-Housed Japanese Macaques is a Predictor of Visitor-Directed Aggression. Animals 2019, 9, 316. https://doi.org/10.3390/ani9060316
Woods JM, Ross SR, Cronin KA. The Social Rank of Zoo-Housed Japanese Macaques is a Predictor of Visitor-Directed Aggression. Animals. 2019; 9(6):316. https://doi.org/10.3390/ani9060316
Chicago/Turabian StyleWoods, Jocelyn M., Stephen R. Ross, and Katherine A. Cronin. 2019. "The Social Rank of Zoo-Housed Japanese Macaques is a Predictor of Visitor-Directed Aggression" Animals 9, no. 6: 316. https://doi.org/10.3390/ani9060316
APA StyleWoods, J. M., Ross, S. R., & Cronin, K. A. (2019). The Social Rank of Zoo-Housed Japanese Macaques is a Predictor of Visitor-Directed Aggression. Animals, 9(6), 316. https://doi.org/10.3390/ani9060316






