A Review of the Actions of Endogenous and Exogenous Vasoactive Substances during the Estrous Cycle and Pregnancy in Rats
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Endothelial Factors Involved in the Modulation of Vascular Tone
2.1.1. Angiotensin II (Ang-II)
2.1.2. Endothelin (ET)
2.1.3. Thromboxane A2 (TXA2)
2.1.4. Reactive Oxygen Species (ROS)
2.1.5. Nitric Oxide (NO)
2.1.6. Prostaglandins
2.1.7. Endothelium-dependent Hyperpolarizing Factor (EDHF)
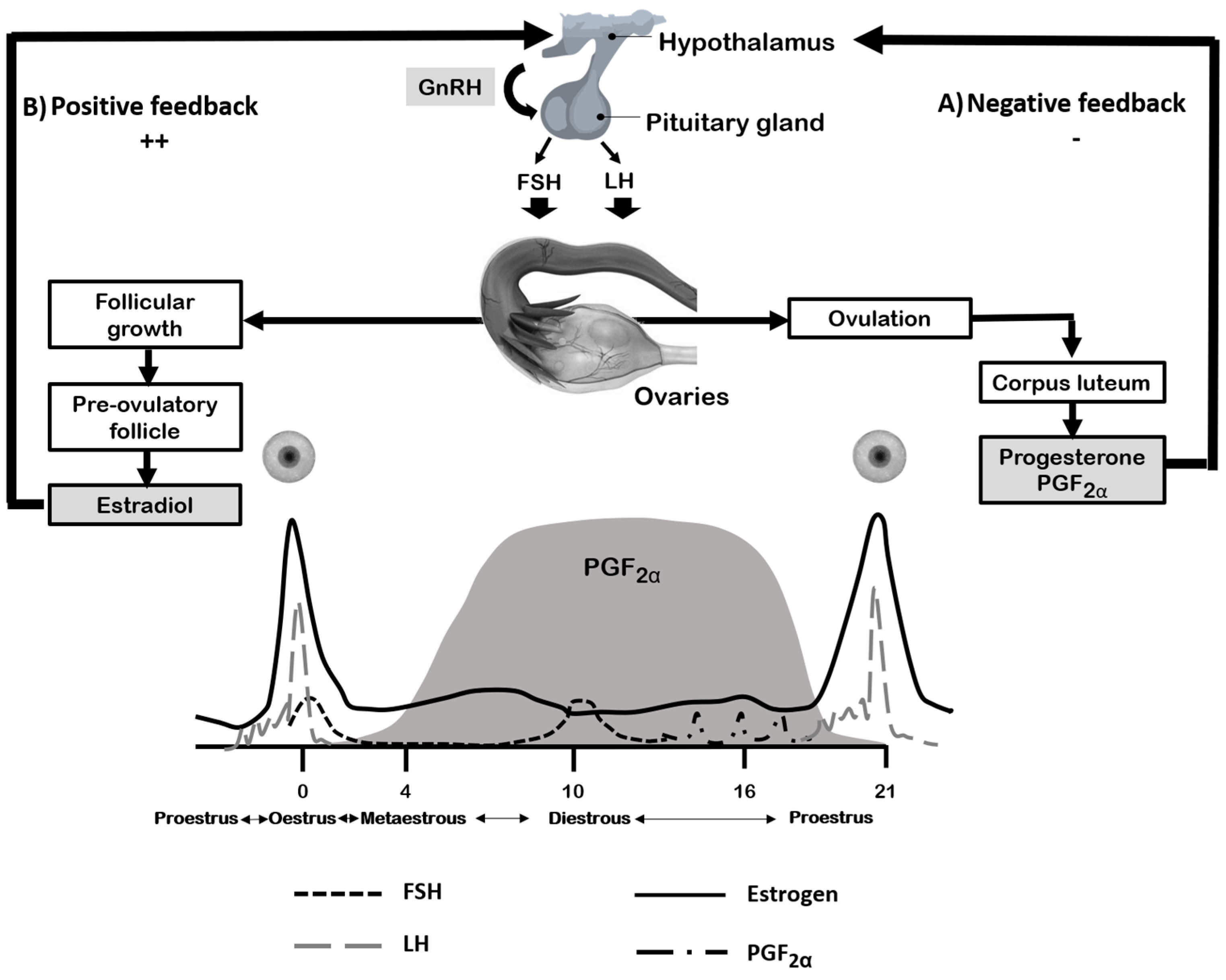
3. Estrous Cycle and Vascular Reactivity
4. Pregnancy and Vascular Reactivity
5. Actions of Exogenous Vasoactive Substances on The Endothelial Function during the Estrous Cycle and Pregnancy
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Aird, W.C. Phenotypic Heterogeneity of the Endothelium: I. Structure, Function and Mechanisms. Circ. Res. 2007, 100, 158–173. [Google Scholar] [CrossRef] [PubMed]
- Tran, Q.K.; Ohashi, K.; Watanabe, H. Calcium signaling in endothelial cells. Cardiovasc. Res. 2000, 48, 13–22. [Google Scholar] [CrossRef]
- Bonetti, P.O.; Lerman, L.O.; Lerman, A. Endothelial dysfunction. A marker of atherosclerotic risk. Art. Thromb. Vasc. Biol. 2003, 23, 168–175. [Google Scholar] [CrossRef]
- Vogel, R.A. Heads and hearts. The endotelial connection. Circulation 2003, 107, 2766–2768. [Google Scholar] [CrossRef]
- Furchgott, R.F.; Vanhoutte, P.M. Endothelium-derived relaxing and contracting factors. FASEB J. 1989, 3, 2007–2018. [Google Scholar] [CrossRef]
- Lüscher, T.F.; Vanhoutte, P.M. The Endothelium: Modulator of Cardiovascular Function, 1st ed.; CRC Press: Boca Ratón, FL, USA, 1990; pp. 1–23. ISBN 10084936468X. [Google Scholar]
- Lévesque, H.; Cailleux, N.; Courtois, H. Vasoactive peptides of endothelial origin. Therapeutic perspectives. Presse Med. 1994, 23, 1172–1177. [Google Scholar]
- Anderson, T.J. Assessment and treatment of endothelial dysfunction in humans. J. Am. Coll. Cardiol. 1999, 34, 631–638. [Google Scholar] [CrossRef]
- Behrendt, D.; Ganz, P. Endothelial function: From vascular biology to clinical applications. Am. J. Cardiol. 2002, 90, 40L–48L. [Google Scholar] [CrossRef]
- Celermajer, D.S. Endothelial dysfunction. Does it matter? Is it reversible? J. Am. Coll. Cardiol. 1997, 30, 325–333. [Google Scholar] [CrossRef]
- Vogel, R.A. Coronary risk factors, endothelial function, and atherosclerosis: A review. Clin. Cardiol. 1997, 20, 426–432. [Google Scholar] [CrossRef] [PubMed]
- Saadi, S.; Holzknecht, R.A.; Patte, C.P.; Stern, D.M.; Platt, J.L. Endothelial cell activation by pore-form structures. Pivotal role for interleukin-1 alpha. Circulation 2000, 101, 1867–1873. [Google Scholar] [CrossRef]
- Luther, T.; Mackman, N. Tissue factor in the heart. Multiple roles in hemostasis, thrombosis, and inflammation. Trends Cardiovasc. Med. 2001, 11, 307–312. [Google Scholar] [CrossRef]
- De la Serna, F. Insuficiencia Cardíaca Crónica, 3rd ed.; Editorial Federación Argentina de Cardiología: Buenos Aires, Argentina, 2010; pp. 114–171. [Google Scholar]
- Badimon, L.; Badimon, J.J.; Penny, W.; Webster, M.W.; Chesebro, J.H.; Fuster, V. Endothelium and atherosclerosis. J. Hypertens. 1992, 10, S43–S50. [Google Scholar]
- Badimon, L.; Martínez-González, J. Endotelio en la protección vascular: Nuevos conocimientos. Rev. Esp. Cardiol. 2002, 55, S17–S26. [Google Scholar]
- Badimon, L. Estatinas y función endotelial. Rev. Esp. Cardiol. 2003, 3, C25–C40. [Google Scholar]
- Teede, H.J. Sex hormones and the cardiovascular system: Effects on arterial function in women. Clin. Exp. Pharmacol. Physiol. 2007, 34, 672–676. [Google Scholar] [CrossRef]
- Villar, I.; Hobbs, A.J.; Ahluwalia, A. Sex differences in vascular function: Implication of endothelium-derived hyperpolarizing factor. J. Endocrinol. 2008, 197, 447–462. [Google Scholar] [CrossRef]
- Farhat, M.Y.; Lavigne, M.C.; Ramwell, P.W. The vascular protective effects of estrogen. FASEB J. 1996, 10, 615–624. [Google Scholar] [CrossRef]
- Joswig, M.; Hach-Wunderle, V.; Ziegler, R.; Nawroth, P.P. Postmenopausal hormone replacement therapy and the vascular wall: Mechanisms of 17 beta-estradiol’s effects on vascular biology. Exp. Clin. Endocrinol. Diabetes 1999, 107, 477–487. [Google Scholar] [CrossRef] [PubMed]
- Jones, R.D.; Hugh Jones, T.; Channer, K.S. The influence of testosterone upon vascular reactivity. Eur. J. Endocrinol. 2004, 151, 29–37. [Google Scholar] [CrossRef]
- Liu, P.Y.; Death, A.K.; Handelsman, D.J. Androgens and cardiovascular disease. Endocrinol. Rev. 2003, 24, 313–340. [Google Scholar] [CrossRef]
- Perusquia, M. Androgen-induced vasorelaxation: A potential vascular protective effect. Exp. Clin. Endocrinol. Diabetes 2003, 111, 55–59. [Google Scholar] [CrossRef]
- Littleton-Kearney, M.; Hurn, P.D. Testosterone as a modulator of vascular behavior. Biol. Res. Nurs. 2004, 5, 276–285. [Google Scholar] [CrossRef]
- Martorell, A. Influencia de las Hormonas Sexuales Endógenas en la Respuesta Vasodilatadora Inducida por Acetilcolina. Participación de Protanoides e Interacción con el Óxido Nítrico. Ph.D. Thesis, Universidad Autónoma de Madrid, Madrid, Spain, 2008. [Google Scholar]
- Netter, F.H. Sistema Reproductor, 2nd ed.; Editorial Salvat: Barcelona, Spain, 1990; pp. 85–105. ISBN 8445800477. [Google Scholar]
- Kalin, M.F.; Zumoff, B. Sex hormones and coronary disease: A review of the clinical studies. Steroids 1990, 55, 330–351. [Google Scholar] [CrossRef]
- Usandizaga, J.A.; de la Fuente, P. Tratado de Obstetricia y Ginecología, 1st ed.; Editorial McGraw-Hill Interamericana: Madrid, Spain, 1997; pp. 532–768. ISBN 8448605616. [Google Scholar]
- Vinet, R.; Knox, M.; Mascher, D.; Paredes-Carbajal, C.; Martinez, J.L. Isolated aorta model and its contribution to phytopharmacology. Bol. Latinoam. Caribe. Plant. Med. Aromat. 2012, 11, 35–45. [Google Scholar]
- Fujime, M.; Tomimatsu, T.; Okaue, Y.; Koyama, S.; Kanagawa, T.; Taniguchi, T.; Kimura, T. Central aortic blood pressure and augmentation index during normal pregnancy. Hypertens. Res. 2012, 35, 633–638. [Google Scholar] [CrossRef]
- Khalil, A.; Jauniaux, E.; Cooper, D.; Harrington, K. Pulse wave analysis in normal pregnancy: A prospective longitudinal study. PLoS ONE 2009, 4, e6134. [Google Scholar] [CrossRef] [PubMed]
- Williams, B.; Mancia, G.; Spiering, W.; Rosei, E.A.; Azizi, M.; Burnier, M.; Clement, D.; Coca, A.; De Simone, G.; Dominiczak, A.; et al. Practice guidelines for the management of arterial hypertension of the European Society of Hypertension and the European Society of Cardiology. J. Hypertens. 2018, 36, 2284–2309. [Google Scholar] [CrossRef] [PubMed]
- Dostal, D.E.; Baker, K.M. The cardiac rennin-angiotensin system. Conceptual, or a regulator of cardiac function? Circ. Res. 1999, 85, 643–650. [Google Scholar] [CrossRef]
- Touyz, R.M.; Schiffrin, E.L. Signal transduction mechanisms mediating the physiological and pathophysiological actions of angiotensin II in vascular smooth muscle cells. Pharmacol. Rev. 2000, 52, 639–672. [Google Scholar]
- Dinh, D.T.; Fauman, A.G.; Johnston, C.I.; Fabiani, M.E. Angiotensin receptors: Distribution, signalling and function. Clin. Sci. 2001, 100, 482–492. [Google Scholar] [CrossRef]
- Heeneman, S.; Sluimer, I.J.; Daemen, M.J. Angiotensin converting enzyme and vascular remodeling. Circ. Res. 2007, 101, 441–454. [Google Scholar] [CrossRef]
- De Gasparo, M.; Whitebread, S.; Mele, M.; Motani, A.S.; Whitcombe, P.J.; Ramjoué, H.P.; Kamber, B. Biochemical characterization of two angiotensin II receptor subtypes in the rat. J. Cardiovasc. Pharmacol. 1990, 16, S31–S35. [Google Scholar] [CrossRef] [PubMed]
- Horiuchi, M.; Akishita, M.; Dzau, V.J. Recent progress in angiotensin II type 2 receptor research in the cardiovascular system. Hypertension 1999, 33, 613–621. [Google Scholar] [CrossRef] [PubMed]
- Escobar, E.; Rodríguez-Reyna, T.S.; Arrieta, O.; Sotelo, J. Angiotensin II, cell proliferation and angiogénesis regulator: Biologic and therapeutic implications in cancer. Curr. Vasc. Pharmacol. 2004, 2, 1–15. [Google Scholar] [CrossRef]
- Ferrario, C.M.; Brosnihan, K.B.; Diz, D.I.; Jaiswal, N.; Khosla, M.C.; Milsted, A.; Tallant, E.A. Angiotensin-(1-7): A new hormone of the angiotensin system. Hypertension 1991, 18, 126–133. [Google Scholar] [CrossRef]
- Laursen, J.B.; Rajagopalan, S.; Galis, Z.; Tarpey, M.; Freeman, B.A.; Harrison, D.G. Role of superoxide in angiotensin II iinduced but not catecholamine-induced hypertension. Circulation 1997, 95, 588–593. [Google Scholar] [CrossRef]
- Risler, N.; Miatello, R.; Montserrat, C.; Castro, C.; Bardi, V. Bases moleculares, genéticas y fisiopatológicas de la hipertensión arterial. Procardio 1998, 2, 19–63. [Google Scholar]
- Trindade, M.R.; Assuncao, H.C.R.; Torres, T.C.; Beertolina, J.S.; Fernandes, L. Venous endothelium reactivity to Angiotensine II: A study in primary endothelial cultures of rat vena cava and portal vein. Exp. Cell Res. 2018, 362, 188–194. [Google Scholar] [CrossRef]
- Inoue, A.; Yanagisawa, M.; Kimura, S.; Miyauchi, T.; Goto, K.; Masaki, T. The human endothelin family: Three structurally and pharmacologically distinct isopeptides predicted by three separate genes. Proc. Natl. Acad. Sci. USA 1989, 86, 2864–2867. [Google Scholar] [CrossRef] [PubMed]
- Davenport, A.P.; Hyndman, K.A.; Dhaun, N.; Southan, C.; Kohan, D.E.; Pollok, J.S.; Pollok, D.M.; Webb, D.J.; Maguire, J.J. Endothelin. Pharmacol. Rev. 2016, 68, 257–418. [Google Scholar] [CrossRef]
- Yanagisawa, M.; Masaki, T. Endothelin, a novel endothelium-derived peptide. Pharmacological activities, regulation and possible roles in cardiovascular control. Biochem. Pharmacol. 1989, 38, 1877–1883. [Google Scholar] [CrossRef] [PubMed]
- Leppaluoto, J.; Ruskoaho, H. Endothelin peptides: Biological activities, celular signaling and clinical significance. Ann. Med. 1992, 24, 153–161. [Google Scholar] [CrossRef]
- Haynes, W.G.; Ferro, C.E.; Webb, D.J. Physiologic role of endothelin in maintenance of vascular tone in humans. J. Cardiovasc. Pharmacol. 1995, 26, 183–185. [Google Scholar] [CrossRef]
- Balakrishnan, S.; Pandhi, P. Endothelins: A brief review. Ind. J. Pharmacol. 1997, 29, 281–288. [Google Scholar]
- Baltazares, M.; Rodríguez, H.; Ortega, J.; Sotres-Vega, A.; Baltazares, M.E. Sistema endotelina. Rev. Inst. Nac. Enf. Resp. Mex. 2005, 18, 308–320. [Google Scholar]
- Schorlemmer, A.; Matter, M.L.; Shohet, R.V. Cardioprotective signaling by endothelin. Trends Cardiovasc. Med. 2008, 18, 233–239. [Google Scholar] [CrossRef]
- Luscher, T.F.; Barton, M. Endothelins and endothelin receptor antagonists: Therapeutic considerations for a novel class of cardiovascular drugs. Circulation 2000, 102, 2434–2440. [Google Scholar] [CrossRef] [PubMed]
- Galie, N.; Manes, A.; Branzi, A. The endothelin systemnin pulmonary arterial hypertension. Cardiovasc. Res. 2004, 61, 227–237. [Google Scholar] [CrossRef]
- Balakrishnan, S.M.; Wang, H.D.; Gopalakrishnan, V.; Wilson, T.W.; McNeil, J.R. Effect of an endothelin antagonist on hemodynamic responses to angiotensin II. Hypertension 1996, 28, 806–809. [Google Scholar] [CrossRef]
- Savoia, C.; Schiffrin, E.L. Significance of recently identified peptides in hypertension: Endothelin, natriuretic peptides, adrenomedullin, leptin. Med. Clin. N. Am. 2004, 88, 39–62. [Google Scholar] [CrossRef]
- Malek, A.; Izumo, S. Physiological fluid shear stress causes down regulation of endothelin-1 mRNA in bovine aortic endothelium. Am. J. Physiol. 1992, 263, 389–396. [Google Scholar] [CrossRef] [PubMed]
- Gray, G.A.; Battistini, B.; Webb, D.J. Endothelins are potent vasoconstrictor, and much more besides. Trend Pharmacol. Sci. 2000, 21, 38–40. [Google Scholar] [CrossRef]
- Quehenberger, P.; Exner, M.; Sunder-Plassmann, R.; Ruzicka, K.; Bieglmayer, C.; Endler, G.; Muellner, C.; Speiser, W.; Wagner, O. Leptin induces endothelin-1 in endothelial cells in vitro. Circ. Res. 2002, 90, 711–718. [Google Scholar] [CrossRef] [PubMed]
- Clozel, M.; Flores, S. Endothelin receptor as drug targets in chronic cardiovascular disease: The rationale for dual antagonism. Drug Develop. Res. 2006, 67, 825–834. [Google Scholar] [CrossRef]
- Barton, M.; Yanagisawa, M. Endothelin: 20 years from discovery to therapy. Can. J. Physiol. Pharmacol. 2008, 86, 485–498. [Google Scholar] [CrossRef]
- Buzzard, C.J.; Pfister, S.L.; Campbell, W.B. Endothelium-dependent contractions in rabbit pulmonary artery are mediated by thromboxane A2. Circ. Res. 1993, 72, 1023–1034. [Google Scholar] [CrossRef]
- Nie, D.; Lamberti, M.; Zacharek, A.; Li, L.; Szekeres, K.; Tang, K.; Chen, Y.; Honn, K.V. Thromboxane A(2) regulation of endothelial cell migration, angiogenesis, and tumor metastasis. Biochem. Biophys. Res. Commun. 2000, 267, 245–251. [Google Scholar] [CrossRef]
- Mayeux, P.R.; Mais, D.E.; Carr, C.; Halushka, P.V. Human erythroleukemia cells express functional thromboxane A2/prostaglandin H2 receptors. J. Pharmacol. Exp. Ther. 1989, 250, 923–927. [Google Scholar]
- Hagemann, C.; Rapp, U.R. Isotype-specific functions of raf kinases. Exp. Cell Res. 1999, 253, 34–46. [Google Scholar] [CrossRef]
- Narumiya, S.; Sugimoto, Y.; Ushikubi, F. Prostanoid receptors: Structures, properties, and functions. Physiol. Rev. 1999, 79, 1193–1226. [Google Scholar] [CrossRef] [PubMed]
- Miggin, S.M.; Kinsella, B.T. Regulation of extracellular signal-regulated kinase cascades by alpha- and beta-isoforms of the human thromboxane A2 receptor. Mol. Pharmacol. 2002, 61, 817–831. [Google Scholar] [CrossRef]
- Manabe, K.; Shirahase, H.; Usui, H.; Kurahashi, K.; Fujiwara, M. Endothelium-dependent contractions induced by angiotensin I and angiotensin II in canine cerebral artery. J. Pharmacol. Exp. Ther. 1989, 251, 317–320. [Google Scholar] [PubMed]
- Taddei, S.; Vanhoutte, P.M. Endothelium-dependent contractions to endothelin in the rat aorta are mediated by thromboxane A2. J. Cardiovasc. Pharmacol. 1993, 22, S328–S331. [Google Scholar] [CrossRef]
- Srisawat, S.; Phivthong-Ngam, L.; Unchern, S.; Chantharaksri, U.; Govitrapong, P.; Sanvarinda, Y. Improvement of vascular function by chronic administration of a cyclo-oxygenase inhibitor in cholesterol-fed rabbits. Clin. Exp. Pharmacol. Physiol. 2003, 30, 405–412. [Google Scholar] [CrossRef]
- Gonzales, R.J.; Ghaffari, A.A.; Duckles, S.P.; Krause, D.N. Testosterone treatment increases thromboxane function in rat cerebral arteries. Am. J. Physiol. Heart Circ. Physiol. 2005, 289, 578–585. [Google Scholar] [CrossRef]
- Blanco-Rivero, J.; Balfagón, G.; Ferrer, M. Orchidectomy modulates alpha2-adrenoceptor reactivity in rat mesenteric artery through increased thromboxane A2 formation. J. Vasc. Res. 2006, 43, 101–108. [Google Scholar] [CrossRef]
- Fitzgerald, G.A. Mechanisms of platelet activation: Thromboxane A2 as an amplifying signal for other agonists. Am. J. Cardiol. 1991, 68, 11B–15B. [Google Scholar] [CrossRef]
- Shimokawa, H. Endothelial dysfunction in hypertension. J. Atheroscler. Thromb. 1998, 4, 118–127. [Google Scholar] [CrossRef] [PubMed]
- Matz, R.L.; de Sotomayor, M.A.; Schott, C.; Stoclet, J.C.; Andriantsitohaina, R. Vascular bed heterogeneity in age-related endothelial dysfunction with respect to NO and eicosanoids. Br. J. Pharmacol. 2000, 131, 303–311. [Google Scholar] [CrossRef] [PubMed]
- Dancs, P.T.; Ruisanchez, E.; Balogh, A.; Panta, C.R.; Miklos, Z.; Nüsing, R.M.; Aoki, J.; Chun, J.; Offermanns, S.; Tigyi, G.; et al. LPA1 receptor-mediated Thromboxane A2 release is responsible for lysophosphatidic acid-induced vascular smooth muscle contraction. FASEB J. 2017, 31, 1547–1555. [Google Scholar] [CrossRef] [PubMed]
- Cheeseman, K.H.; Slater, T.F. An introduction of free radical radical biochemistry. Br. Med. Bull. 1993, 49, 481–493. [Google Scholar] [CrossRef] [PubMed]
- Thomas, C. Oxygen Radicals and Disease Process, 1st ed.; Harwood Academic: Amsterdam, The Netherland, 1997; pp. 327–377. [Google Scholar]
- Tejero, J.; Shiva, S.; Gladwin, M. Sources of vascular nitric oxide and reactive oxygen species and their regulation. Physiol. Rev. 2019, 99, 311–379. [Google Scholar] [CrossRef]
- Pagano, P.J.; Ito, Y.; Tornheim, K.; Gallop, P.M.; Tauber, A.I.; Cohen, R.A. An NADPH oxidase superoxide generating system in the rabbit aorta. Am. J. Physiol. 1995, 268, H2274–H2280. [Google Scholar] [CrossRef]
- Halliwell, B. Mechanisms involved in the generation of free radicals. Pathol. Biol. 1996, 44, 6–13. [Google Scholar]
- Poli, G.; Leonarduzzi, G.; Biasi, F.; Chiarpotto, E. Oxidative stress and cell signalling. Curr. Med. Chem. 2004, 11, 1163–1182. [Google Scholar] [CrossRef]
- Zalba, G.; Beaumont, F.J.; San José, G.; Fortuño, A.; Fortuño, M.A.; Díez, J. Vascular oxidant stress: Molecular mechanisms and pathophysiological implications. J. Physiol. Biochem. 2000, 56, 57–64. [Google Scholar] [CrossRef]
- DeKeulenaer, G.W.; Alexander, R.W.; Ushio-Fukai, M.; Ishizaka, N.; Griendling, K.K. Tumor necrosis factor-α activates a p22phox-based NADH oxidase in vascular smooth muscle cells. Biochem. J. 1998, 329, 653–657. [Google Scholar] [CrossRef]
- Griendling, K.K.; Minieri, C.A.; Ollerenshaw, J.D.; Alexander, R.W. Angiotensin II stimulates NADH and NADPH oxidase activity in cultured vascular smooth muscle cells. Circ. Res. 1994, 74, 1141–1148. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.D.; Pagano, P.J.; Du, Y.; Cayatte, A.J.; Quinn, M.T.; Brecher, P.; Cohen, R.A. Superoxide anion from the adventitia of the rat thoracic aorta inactivates nitric oxide. Circ. Res. 1998, 82, 810–818. [Google Scholar] [CrossRef] [PubMed]
- Finkel, T. Oxygen radicals and signaling. Curr. Opin. Cell Biol. 1998, 10, 248–253. [Google Scholar] [CrossRef]
- Finkel, T. Signal transduction by mitochondrial oxidants. J. Biol. Chem. 2012, 287, 4434–4440. [Google Scholar] [CrossRef]
- Karbach, S.; Wenzel, P.; Waisman, A.; Munzel, T.; Daiber, A. eNOS uncoupling in cardiovascular diseases–the role of oxidative stress and inflammation. Curr. Pharm. Des. 2014, 20, 3579–3594. [Google Scholar] [CrossRef]
- Li, H.; Förstermann, U. Uncoupling of endothelial NO synthase in atherosclerosis and vascular disease. Curr. Opin. Pharmacol. 2013, 13, 161–167. [Google Scholar] [CrossRef]
- Siragusa, M.; Fleming, I. The eNOS signalosome and its link to endothelial dysfunction. Pflugers Arch. 2016, 468, 1125–1137. [Google Scholar] [CrossRef] [PubMed]
- Squadrito, G.L.; Pryor, W.A. The formation of peroxynitrite in vivo from nitric oxide and superoxide. Chem. Biol. Interact. 1995, 96, 203–206. [Google Scholar] [CrossRef]
- Munzel, T.; Heitzer, T.; Harrison, D.G. The physiology and pathophysiology of the nitric oxide/superoxide system. Herz 1997, 22, 158–172. [Google Scholar] [CrossRef]
- Halliwell, B.; Zhao, K.; Whiteman, M. Nitric oxide and peroxynitrite. The ugly, the uglier and the not so good: A personal view of recent controversies. Free Radic. Res. 1999, 31, 651–669. [Google Scholar] [CrossRef] [PubMed]
- Muller, G.; Morawietz, H. NAD(P)H oxidase and endothelial dysfunction. Horm. Metab. Res. 2008, 41, 152–158. [Google Scholar] [CrossRef] [PubMed]
- Ohara, Y.; Peterson, T.E.; Harrison, D.G. Hypercholesterolemia increases endothelial superoxide anion production. J. Clin. Investig. 1993, 91, 2546–2551. [Google Scholar] [CrossRef]
- Cai, H.; Harrison, D.G. Endothelial dysfunction in cardiovascular diseases: The role of oxidant stress. Circ. Res. 2000, 87, 840–844. [Google Scholar] [CrossRef]
- Hogg, N. Free radicals in disease. Semin. Reprod Endocrinol. 1998, 16, 241–248. [Google Scholar] [CrossRef] [PubMed]
- Wolin, M.S. Interaction of oxidants with vascular signaling system. Arterioscler. Thromb. Vasc. Biol. 2000, 20, 1430–1442. [Google Scholar] [CrossRef]
- Moncada, S.; Palmer, R.M.J.; Higgs, E.A. Nitric oxide: Physiology, pathophysiology, and pharmacology. Pharmacol. Rev. 1991, 43, 109–142. [Google Scholar]
- Kojda, G.; Harrison, D. Interactions between NO and reactive oxygen species: Pathophysiological importance in atherosclerosis, hypertension, diabetes and heart failure. Cardiovasc. Res. 1999, 43, 562–571. [Google Scholar] [CrossRef]
- Palmer, R.M.J.; Ferrige, A.G.; Moncada, S. Nitric oxide reléase accounts for the biological activity of endothelium-derived relaxing factor. Nature 1987, 327, 524–526. [Google Scholar] [CrossRef] [PubMed]
- Birks, E.J.; Yacoub, M.H. The role of nitric oxide and cytokines in heart failure. Coron. Artery Dis. 1997, 8, 389–402. [Google Scholar] [CrossRef] [PubMed]
- Marletta, M.A. Nitric oxide synthase: Aspects concerning structure and catalysis. Cell 1994, 78, 927–930. [Google Scholar] [CrossRef]
- Stuehr, D.J. Structure-function aspects in the nitric oxide synthases. Ann. Rev. Pharmacol. Toxicol. 1997, 37, 339–359. [Google Scholar] [CrossRef]
- Govers, R.; Rabelink, T.J. Cellular regulation of endothelial nitric oxide synthase. Am. J. Physiol. Renal. Physiol. 2001, 280, 193–206. [Google Scholar] [CrossRef] [PubMed]
- Pollock, J.S.; Klinghofer, V.; Forstermann, U.; Murad, F. Endothelial nitric oxide synthase is myristylated. FEBS Lett. 1993, 309, 402–404. [Google Scholar] [CrossRef]
- Bredt, D.S.; Hwang, P.M.; Snyder, S.H. Localization of nitric oxide synthase indicating a neural role for nitric oxide. Nature 1990, 347, 768–770. [Google Scholar] [CrossRef] [PubMed]
- Ferreiro, C.R.; Chagas, A.C.; Carvalho, M.H.; Dantas, A.P.; Scavone, C.; Souza, L.C.; Buffolo, E.; da Luz, P.L. Expression of inducible nitric oxide synthase is increased in patients with heart failure due to ischemic disease. Braz. J. Med. Biol. Res. 2004, 37, 1313–1320. [Google Scholar] [CrossRef] [PubMed]
- Pollock, J.S.; Forstermann, U.; Tracey, W.R.; Nakane, M. Nitric oxide synthase isozymes antibodies. Histochem. J. 1995, 27, 738–744. [Google Scholar] [CrossRef] [PubMed]
- Andrew, P.J.; Mayer, B. Enzymatic function of nitric oxide synthases. Cardiovasc. Res. 1999, 43, 521–531. [Google Scholar] [CrossRef]
- Alderton, W.K.; Cooper, C.E.; Knowles, R.G. Nitric oxide synthases: Structure, function and inhibition. Biochem. J. 2001, 357, 593–615. [Google Scholar] [CrossRef]
- Furchgott, R.F.; Zawadski, J.V. The obligatory role of endothelial cells in the relaxation of smooth muscle by acethilcholine. Nature 1980, 288, 373–376. [Google Scholar] [CrossRef]
- Rees, D.D.; Palmer, R.M.; Moncada, S. Role of endothelium-derived nitric oxide in the regulation of bllod pressure. Proc. Natl. Acad. Sci. USA 1989, 86, 3375–3378. [Google Scholar] [CrossRef]
- Raffetto, J.D.; Yu, P.; Reslan, O.M.; Xia, Y.; Khalil, R.A. Endothelium-dependent nitric oxide and hyperpolarization-mediated venous relaxation pathways in rat inferior vena cava. J. Vasc. Surg. 2012, 55, 1716–1725. [Google Scholar] [CrossRef]
- Vane, J.R.; Bakhle, Y.S.; Botting, R.M. Cyclooxygenases 1 and 2. Ann. Rev. Pharmacol. Toxicol. 1998, 38, 97–120. [Google Scholar] [CrossRef] [PubMed]
- Meirer, K.; Steinhilber, D.; Proschak, E. Inhibitors of the arachidonic acid cascade: Interfering with multiple pathways. Basic Clin. Pharmacol. Toxicol. 2014, 114, 83–91. [Google Scholar] [CrossRef] [PubMed]
- Davidge, S.T. Prostaglandin H synthase and vascular function. Circ Res. 2001, 89, 650–660. [Google Scholar] [CrossRef] [PubMed]
- Tanabe, T.; Tohnai, N. Cyclooxygenase isozymes and their gene structures and expression. Prostaglandins Other Lipid Mediat. 2002, 68, 95–114. [Google Scholar] [CrossRef]
- Ramsay, R.G.; Ciznadija, D.; Vanevski, M.; Mantamadiotis, T. Transcriptional regulation of cyclo-oxygenase expression: Three pillars of control. Int. J. Immunopathol. Pharmacol. 2003, 16, 59–67. [Google Scholar]
- Briones, A.M.; Salaices, M.; Vila, E. Ageing alters the production of nitric oxide and prostanoids after IL-1β exposure in mesenteric resistance arteries. Mech. Ageing Dev. 2005, 126, 710–721. [Google Scholar] [CrossRef] [PubMed]
- Ricciotti, E.; FitzGerald, G.A. Prostaglandins and Inflammation. Arterioscler. Thromb. Vasc. Biol. 2011, 31, 986–1000. [Google Scholar] [CrossRef] [PubMed]
- Dorris, S.L.; Peebles, R.S. PGI2 as a regulator of inflammatory diseases. Med. Inflamm. 2012, 2012, 926968–926969. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.S. Prostacyclin: A major prostaglandin in the regulation of adipose tissue development. J. Cell Physiol. 2019, 234, 3254–3262. [Google Scholar] [CrossRef] [PubMed]
- Bergstrom, S.; Danielsson, H.; Samuelsson, B. The enzymatic formation of prostaglandin E2 from arachidonic acid. Prostaglandins and related factors. Biochim. Biophys. Acta 1974, 90, 207–210. [Google Scholar] [CrossRef]
- Yamamoto, S.; Ogino, N.; Ohki, S.; Yoshimoto, T.; Bhat, S.G.; Oka, J.; Hayaishi, O. Enzymological studies on prostaglandin biosynthesis. In Biochemical Aspects of Prostaglandins and Thromboxanes, 1st ed.; Kharasch, N., Freid, J., Eds.; Academic: New York, NY, USA, 1977; p. 278. ISBN 9780323158510. [Google Scholar]
- Schulman, E.S.; Newball, H.H.; Demers, L.M.; Fitzpatrick, F.A.; Adkinson, N.F. Anaphylactic release of Thromboxane A2, Prostaglandin D2 and Prostacyclin from human lung parenchyma. Am. Rev. Respir. Dis. 1981, 124, 402–406. [Google Scholar] [CrossRef] [PubMed]
- Higgs, E.A.; Moncada, S. Prostacyclin-physiology and clinical uses. Gen. Pharmacol. 1983, 14, 7–11. [Google Scholar] [CrossRef]
- Lüsher, T.F.; Oemar, B.S.; Boulanger, C.M.; Hahn, A.W.A. Molecular and cellular biology of endothelin and its receptor. Part I. Hypertens. 1993, 11, 121–126. [Google Scholar]
- Parsaee, H.; Mcewan, J.R.; Joseph, S.; MacDermot, J. Differential sensitivities of the prostacyclin and nitric oxide biosynthetic pathways to cytosolic calcium in bovine aortic endothelial cells. Br. J. Pharmacol. 1992, 107, 1013–1019. [Google Scholar] [CrossRef]
- Williams, S.P.; Dorn, G.W.; Rapoport, R.M. Prostaglandin I2 mediates contraction and relaxation of vascular smooth muscle. Am. J. Physiol. 1994, 267, H796–H803. [Google Scholar] [CrossRef] [PubMed]
- Blanco-Rivero, J.; Cachofeiro, V.; Lahera, V.; Aras-Lopez, R.; Márquez-Rodas, I.; Salaices, M.; Xavier, F.E.; Ferrer, M.; Balfagón, G. Participation of prostacyclin in endothelial dysfunction induced by aldosterone in normotensive and hypertensive rats. Hipertension 2005, 46, 107–112. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, J.A.; De Nucci, G.; Warner, T.D.; Vane, J.R. Different patterns of release of endothelium-derived relaxing factor and prostacyclin. Br. J. Pharmacol. 1992, 105, 485–489. [Google Scholar] [CrossRef] [PubMed]
- Mollace, V.; Muscoli, C.; Masini, E.; Cuzzocrea, S.; Salvemini, D. Modulation of prostaglandin biosynthesis by nitric oxide and nitric oxide donors. Pharmacol. Rev. 2005, 57, 217–252. [Google Scholar] [CrossRef]
- Bachschmid, M.; Schildknecht, S.; Ullrich, V. Redox regulation of vascular prostanoid synthesis by the nitric oxide-superoxide system. Biochem. Biophys. Res. Commun. 2005, 338, 536–542. [Google Scholar] [CrossRef]
- Catella-Lawson, F.; Crofford, L.J. Cyclooxygenase inhibition and thrombogenicity. Am. J. Med. 2001, 110, 28S–32S. [Google Scholar] [CrossRef]
- Mukherjee, D.; Nissen, S.E.; Topol, E.J. Risk of cardiovascular events associated with selective COX-2 inhibitors. JAMA 2001, 286, 954–959. [Google Scholar] [CrossRef]
- Busse, R.; Förstermann, U.; Matsuda, H.; Pohl, U. The role of prostaglandins in the endothelium-mediated vasodilatory response to hypoxia. Pflügers Arch. 1984, 401, 77–83. [Google Scholar] [CrossRef]
- Krone, W.; Klass, A.; Nagele, H.; Behnke, B.; Greten, H. Effects of prostaglandins on LDL receptor activity and cholesterol synthesis in freshly isolated human mononuclear leukocytes. J. Lipid Res. 1988, 29, 1663–1669. [Google Scholar]
- Braun, M.; Hohlfeld, T.; Kienbaum, P.; Weber, A.A.; Sarbia, M.; Schror, K. Antiatherosclerotic effects of oral cicaprost in experimental hypercholesterolemia in rabbits. Atherosclerosis 1993, 103, 93–105. [Google Scholar] [CrossRef]
- Kowala, M.C.; Mazzucco, C.E.; Hartl, K.S.; Seiler, S.M.; Warr, G.A.; Abid, S.; Grove, R.I. Prostacyclin agonists reduce early atherosclerosis in hyperlipidemic hamsters. Octimibate and BMY 42393 suppress monocyte chemotaxis, macrophage cholesteryl ester accumulation, scavenger receptor activity, and tumor necrosis factor production. Arterioscler. Thromb. 1993, 13, 435–444. [Google Scholar] [CrossRef]
- Stables, M.J.; Gilroy, D.W. Old and new generation lipid mediators in acute inflammation and resolution. Progress Lipid Res. 2011, 50, 35–51. [Google Scholar] [CrossRef] [PubMed]
- Hwang, S.H.; Wecksler, A.T.; Wagner, K.; Hammock, B.D. Rationally designed multitarget. agents against inflammation and pain. Curr. Med. Chem. 2013, 20, 1783–1799. [Google Scholar] [CrossRef]
- Vanhoutte, P.M. Other endothelium-derived vasoactive factors. Circulation 1993, 87, V9–V17. [Google Scholar]
- Vanhoutte, P.M.; Boulanger, C.M.; Mombouli, J.V. Endothelium-derived relaxing factors and converting enzyme inhibition. Am. J. Cardiol. 1995, 76, 3E–12E. [Google Scholar] [CrossRef]
- Campbell, W.B.; Gebremedhin, D.; Pratt, P.F.; Harder, D.R. Identification of epoxyeicosatrienoic acids as endothelium-derived hyperpolarizing factors. Circ. Res. 1996, 78, 415–423. [Google Scholar] [CrossRef]
- Komori, K.; Suzuki, H. Heterogenous distribution of muscarinic receptors in the rabbit saphenous artery. Br. J. Pharmacol. 1987, 92, 657–664. [Google Scholar] [CrossRef]
- Gluais, P.; Edwards, G.; Weston, A.H.; Falck, J.R.; Vanhoutte, P.M.; Félétou, M. Role of SK (Ca) and IK (Ca) in endothelium-dependent hyperpolarization of the guinea-pig isolated carotid artery. Br. J. Pharmacol. 2005, 144, 477–485. [Google Scholar] [CrossRef]
- Vázquez-Pérez, S.; Navarro-Cid, J.; De Las Heras, N.; Cediel, E.; Sanz-Rosa, D.; Ruilope, L.M.; Cachofeiro, V.; Lahera, V. Relevance of endothelium-derived hyperpolarizing factor in the effects of hypertension on rat coronary relaxations. J. Hypertens. 2001, 19, 539–545. [Google Scholar] [CrossRef]
- Luksha, L.; Agewall, S.; Kublickiene, K. Endothelium-derived hyperpolarizing factor in vascular physiology and cardiovascular disease. Atherosclerosis 2009, 202, 330–344. [Google Scholar] [CrossRef] [PubMed]
- Ellinsworth, D.F.C.; Sandow, S.L.; Shukla, A.; Liu, Y.; Jeremy, J.Y.; Gutterman, D.D. Endothelium-derived hyperpolarization and coronary vasodilation: Diverse and integrated roles of epoxyeicosatrienoic acids, hydrogen peroxide, and gap junctions. Microcirculation 2016, 23, 15–32. [Google Scholar] [CrossRef] [PubMed]
- Shimokawa, H.; Morikawa, K. Hydrogen peroxide is an endothelium-derived hyperpolarizing factor in animals and humans. J. Mol. Cell. Cardiol. 2005, 39, 725–732. [Google Scholar] [CrossRef] [PubMed]
- Sandow, S.L.; Tare, M. C-type natriuretic peptide: A new endothelium-derived hyperpolarizing factor? Trends Pharmacol. Sci. 2007, 28, 61–67. [Google Scholar] [CrossRef]
- Majed, B.H.; Khalil, R.A. Molecular mechanisms regulating the vascular prostacyclin pathways and their adaptation during pregnancy and in the newborn. Pharmacol. Rev. 2012, 64, 540–582. [Google Scholar] [CrossRef] [PubMed]
- Köhler, R.; Hoyer, J. The endothelium-derived hyperpolarizing factor: Insights from genetic animal models. Kidney Int. 2007, 72, 145–150. [Google Scholar] [CrossRef]
- Levine, J.E. Neuroendocrine control of the ovarian cycle of the rat. In Knobil and Neill’s Physiology of Reproduction, 4th ed.; Plant, T.M., Zeleznik, A.J., Eds.; Academic Press: Oxford, UK, 2015; Chapter 26; pp. 1199–1257. ISBN 978-0-12-397175-3. [Google Scholar]
- Leavitt, W.W.; Toft, D.O.; Strott, C.A.; O’Malley, B.W. A specific progesterone receptor in the Hamster uterus: Physiologic properties and regulation during the estrous cycle. Endocrinology 1974, 94, 1041–1053. [Google Scholar] [CrossRef]
- Goldman, J.M.; Murr, A.S.; Coper, R.L. The rodent estrous cycle: Characterization of vaginal cytology and its utility in toxicological studies. Birth Defects Res. 2007, 80, 84–97. [Google Scholar] [CrossRef]
- Rothchild, I. The regulation of the mammalian corpus luteum. Recent Prog. Horm. Res. 1981, 37, 183–298. [Google Scholar]
- Linares, N.; Millán, Y.; Garrido-Gracia, J.C.; Aguilar, R.; Gordon, A.; Sánchez-Criado, J.E.; Martín de las Mulas, J. Utilidad de los agonistas, moduladores selectivos y antagonistas puros del receptor de estrógenos en estudios morfofuncionales del útero de la rata. Anal. Real Acad. Cienc. Vet. Andalucía Orient. 2010, 23, 97–109. [Google Scholar]
- Belozertseva, I.V.; Merkulovs, D.D.; Vilitis, O.J.; Skryabin, B.V. Instrumental methods for determining the estrous cycle stages in small laboratory rodents. Lab. Anim. Sci. 2018, 4, 125–135. [Google Scholar] [CrossRef]
- Marcondes, F.K.; Miguel, K.; Melo, L.L.; Spadari-Bratfisch, R.C. Estrous cycle influences the response of female rats in the elevated plus-maze. Physiol. Behav. 2001, 74, 435–440. [Google Scholar] [CrossRef]
- Tanriverdi, F.; Silveira, L.; MacColl, G.; Bouloux, P.M. The hypothalamic-pituitary-gonadal axis: Immune function and autoimmunity. J. Endocrinol. 2003, 176, 293–304. [Google Scholar] [CrossRef]
- Lamb, G.C.; Smith, M.F.; Perry, G.A.; Atkins, J.A.; Risley, M.E.; Busch, D.C.; Patterson, D.J. Reproductive endocrinology and hormonal control of the estrous cycle. Bovine Practitioner 2010, 44, 18–26. [Google Scholar]
- Freeman, M.E. The ovarian cycle of the rat. In Knobil and Neill’s Physiology of Reproduction, 4th ed.; Plant, T.M., Zeleznik, A.J., Eds.; Academic Press: Oxford, UK, 2015; ISBN 978-0-12-397175-3. [Google Scholar]
- Herbison, A.E. Noradrenergic regulation of cyclic GnRH secretion. Rev. Reprod. 1997, 2, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Turkstra, J.; Meloen, R. Active immunization against gonadotropin-releasing hormone, an active tool to block the fertility axis in mammals. Vet. Sci. Tomorrow 2006. [Google Scholar]
- Fink, G. Gonadotropin secretion and its control. In Knobil and Neill’s Physiology of Reproduction, 4th ed.; Plant, T.M., Zeleznik, A.J., Eds.; Academic Press: Oxford, UK, 2015; ISBN 978-0-12-397175-3. [Google Scholar]
- Alonso, R.; Marín, F.; González, M.; Guelmes, P.; Bellido, C.; Hernández, G.; Marín, R.; Díaz, M.; Sánchez-Criado, J. The hypothalamus-pituitary-ovarian axis as a model system for the study of SERM effects: An overview of experimental and clinical studies. In Selective Estrogen Receptor Modulators. A New Brand of Multitarget Drugs, 1st ed.; Cano, A., Calaf i Alsina, J., Dueñas-Diez, J.L., Eds.; Springer: Berlin, Germany, 2006; Chapter 5; pp. 103–139. ISBN 9783540347422. [Google Scholar]
- Berne, R.M.; Levy, M.N. Physiology, 7th ed.; Elsevier Science: St. Louis, MO, USA, 1998; pp. 1–880. [Google Scholar]
- Simpson, E.R.; Clyne, C.; Rubin, G.; Boon, W.C.; Robertson, K.; Britt, K.; Speed, C.; Jones, M. Aromatase—A brief overview. Ann. Rev. Physiol. 2002, 64, 93–127. [Google Scholar] [CrossRef] [PubMed]
- Harada, N.; Sasano, H.; Murakami, H.; Ohkuma, T.; Nagura, H.; Takagi, Y. Localized expression of aromatase in human vascular tissues. Circ. Res. 1999, 84, 1285–1291. [Google Scholar] [CrossRef]
- Miller, V.M.; Duckles, S.P. Vascular actions of estrogens: Functional implications. Pharmacol Rev. 2008, 60, 210–241. [Google Scholar] [CrossRef]
- Miller, V.M.; Mulvagh, S.L. Sex steroids and endothelial function: Translating basic science to clinical practice. Trends Pharmacol. Sci. 2007, 28, 263–270. [Google Scholar] [CrossRef]
- Nathan, L.; Chaudhuri, G. Estrogens and atherosclerosis. Ann. Rev. Pharmacol. Toxicol. 1997, 37, 477–515. [Google Scholar] [CrossRef]
- Herrington, D.M.; Reboussin, D.M.; Brosnihan, K.B.; Sharp, P.C.; Shumaker, S.A.; Snyder, T.E.; Furberg, C.D.; Kowalchuk, G.J.; Stuckey, T.D.; Rogers, W.J.; et al. Effects of estrogen replacement on the progression of coronary-artery atherosclerosis. N. Engl. J. Med. 2000, 343, 522–529. [Google Scholar] [CrossRef] [PubMed]
- Mendelsohn, M.E.; Karas, R.H. The protective effects of estrogen on the cardiovascular system. N. Engl. J. Med. 1999, 340, 1801–1811. [Google Scholar] [CrossRef]
- Nasr, A.; Breckwoldt, M. Estrogen replacement therapy and cardiovascular protection: Lipid mechanisms are the tip of an iceburg. Gynecol. Endocrinol. 1998, 12, 43–59. [Google Scholar] [CrossRef]
- Raddino, R.; Pela, G.; Uberti, D.; Portera, C.; Ferrari, R.; Scarabelli, T.M.; Cooper, T.J.; Manca, C. Estrogen derivative relaxes rabbit aorta via the endotelial receptor system. Ital. Heart J. 2001, 2, 49–54. [Google Scholar] [PubMed]
- Wild, R.A.; Reis, S.E. Estrogens, progestins, selective estrogen receptor modulators, and the arterial tree. Am. J. Obstet. Gynecol. 2001, 184, 1031–1039. [Google Scholar] [CrossRef]
- Mendelsohn, M.E. Mechanisms of estrogen action in the cardiovascular system. J. Steroid Biochem. Mol. Biol. 2000, 74, 337–343. [Google Scholar] [CrossRef]
- Hayashi, T.; Yamada, K.; Esaki, T.; Kuzuya, M.; Satake, S.; Ishikawa, T.; Hidaka, H.; Iguchi, A. Estrogen increases endothelial nitric oxide by a receptor-mediated system. Biochem. Biophys. Res. Commun. 1995, 214, 847–855. [Google Scholar] [CrossRef]
- Virdis, A.; Ghiadoni, L.; Pinto, S.; Lombardo, M.; Petraglia, F.; Gennazzani, A.; Buralli, S.; Taddei, S.; Salvetti, A. Mechanisms responsible for endothelial dysfunction associated with acute estrogen deprivation in normotensive women. Circulation 2000, 101, 2258–2263. [Google Scholar] [CrossRef] [PubMed]
- Zamorano, B.; Bruzzone, M.E.; Martínez, J.L. Influence of estrous cycle on norepinephrine-induced contraction in rat aorta: Relationship to vascular prostanoids biosynthesis. Biol. Res. 1994, 27, 209–215. [Google Scholar] [PubMed]
- Martinez, J.L.; Bruzzone, M.E.; Morales, B.; Palacios, J.; Knox, M.; Vinet, R.; Laurido, C. Effects of indomethacin on the norepinephrine-induced contraction in vascular smooth muscle in different stages of the estrous cycle. Pak. Vet. J. 2019, in press. [Google Scholar]
- Conrad, K.P.; Colpoys, M.C. Evidence against the hypothesis that prostaglandins are the vasodepressor agents of pregnancy. Serial studies in chronically instrumented, conscious rats. J. Clin. Investig. 1986, 77, 236–245. [Google Scholar] [CrossRef] [PubMed]
- Debrah, D.O.; Novak, J.; Matthews, J.E.; Ramirez, R.J.; Shroff, S.G.; Conrad, K.P. Relaxin is essential for systemic vasodilation and increased global arterial compliance during early pregnancy in conscious rats. Endocrinology 2006, 147, 5126–5131. [Google Scholar] [CrossRef]
- Gilson, G.J.; Mosher, M.D.; Conrad, K.P. Systemic hemodynamics and oxygen transport during pregnancy in chronically instrumented, conscious rats. Am. J. Physiol. Heart Circ. Physiol. 1992, 263, H1911–H1918. [Google Scholar] [CrossRef] [PubMed]
- Slangen, B.F.; Out, I.C.; Verkeste, C.M.; Peeters, L.L. Hemodynamic changes in early pregnancy in chronically instrumented, conscious rats. Am. J. Physiol. Heart Circ. Physiol. 1996, 270, H1779–H1784. [Google Scholar] [CrossRef]
- Iacobaeus, C.; Andolf, E.; Thorsell, M.; Bremme, K.; Jörneskog, G.; Östlund, E.; Kahan, T. Longitudinal study of vascular structure and function during normal pregnancy. Ultrasound Obstet. Gynecol. 2017, 49, 46–53. [Google Scholar] [CrossRef]
- Clapp, J.F.; Capeless, E. Cardiovascular function before, during, and after the first and subsequent pregnancies. Am. J. Cardiol. 1997, 80, 1469–1473. [Google Scholar] [CrossRef]
- Sladek, S.M.; Magness, R.R.; Conrad, K.P. Nitric oxide and pregnancy. Am. J. Physiol. Regul. Integr. Comp. Physiol. 1997, 272, R441–R463. [Google Scholar] [CrossRef]
- Lenton, E.A.; Neal, L.M.; Sulaiman, R. Plasma concentrations of human chorionic gonadotropin from the time of implantation until the second week of pregnancy. Fertil Steril. 1982, 37, 773–778. [Google Scholar] [CrossRef]
- Mahendru, A.A.; Everett, T.R.; Wilkinson, I.B.; Lees, C.C.; McEniery, C.M. Maternal cardiovascular changes from pre-pregnancy to very early pregnancy. J. Hypertens. 2012, 30, 2168–2172. [Google Scholar] [CrossRef] [PubMed]
- Weiner, C.P.; Thompson, L.P. Nitric oxide and pregnancy. Semin. Perinatol. 1997, 21, 367–380. [Google Scholar] [CrossRef]
- Osol, G.; Cipolla, M. Pregnancy-induced changes in the three-dimensional mechanical properties of pressurized rat uteroplacental (radial) arteries. Am. J. Obstet. Gynecol. 1993, 168, 268–274. [Google Scholar] [CrossRef]
- Goulopoulou, S. Maternal vascular physiology in preeclampsia. Hypertension 2017, 70, 1076–1083. [Google Scholar] [CrossRef]
- Conrad, K.P. Maternal vasodilation in pregnancy: The emerging role of relaxin. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2011, 301, R267–R275. [Google Scholar] [CrossRef]
- Zhang, Y.; Stewart, K.; Davidge, S. Endogenous estrogen mediates vascular reactivity and distensibility in pregnant rat mesenteric arteries. Am. J. Physiol. Heart Circ. Physiol. 2001, 280, H956–H961. [Google Scholar] [CrossRef]
- Geary, G.G.; Krause, D.N.; Duckles, S.P. Estrogen reduces myogenic tone through a nitric oxide-dependent mechanism in rat cerebral arteries. Am. J. Physiol. Heart Circ. Physiol. 1998, 275, H292–H300. [Google Scholar] [CrossRef]
- Magness, R.R.; Parker, C.R.; Rosenfeld, C.R. Systemic and uterine responses to chronic infusion of estradiol-17beta. Am. J. Physiol. Endocrinol. Metab. 1993, 265, E690–E698. [Google Scholar] [CrossRef]
- Magness, R.R.; Phernetton, T.M.; Zheng, J. Systemic and uterine blood flow distribution during prolonged infusion of 17beta estradiol. Am. J. Physiol. Heart Circ. Physiol. 1998, 275, H731–H743. [Google Scholar] [CrossRef]
- Zhang, Y.; Davidge, S.T. Effect of estrogen replacement on vasoconstrictor responses in rat mesenteric arteries. Hypertension 1999, 34, 1117–1122. [Google Scholar] [CrossRef] [PubMed]
- Conrad, K.P.; Mosher, M.D.; Brinck-Johnsen, T.; Colpoys, M.C. Effects of 17b-estradiol and progesterone on pressor responses in conscious ovariectomized rats. Am. J. Physiol. Regul. Integr. Comp. Physiol. 1994, 266, R1267–R1272. [Google Scholar] [CrossRef] [PubMed]
- Zamorano, B.; Bruzzone, M.E.; Martínez, J.L. Vascular smooth muscle reactivity to norepinephrine in ovariectomized rats: Relationship to vascular PGE2/PGF2α ratio. Gen. Pharmac. 1995, 26, 1613–1618. [Google Scholar] [CrossRef]
- Dalle Lucca, J.J.; Adeagbo, A.S.O.; Alsip, N.L. Influence of estrous cycle and pregnacy on the reactivity of yhe rat mesenteric vascular bed. Hum. Rep. 2000, 15, 961–968. [Google Scholar] [CrossRef]
- Cooke, C.L.M.; Davidge, S.T. Pregnancy-induced alterations of vascular function in mouse mesenteric and uterine arteries. Biol. Reprod. 2003, 68, 1072–1077. [Google Scholar] [CrossRef] [PubMed]
- Oluyemi, A.; Okwuonu, C.; Baxter, D.; Oyesola, T.O. Toxic effects of methanolic extract of Aspilia africana leaf on the estrous cycle and uterine tissues of Wistar rats. Int. J. Morphol. 2007, 25, 609–614. [Google Scholar] [CrossRef]
- Parada, M.; Valenzuela-Barra, G.; Delporte, C.; Lara, H. Estrous cycle disruptor effect of an ethanolic extract from Buddleja globosa leaves and its main component (verbascoside). Bol. Latinoam. Caribe Plant. Med. Aromat. 2014, 13, 189–197. [Google Scholar]

| Vasoactive Factors | |
| Vasodilators | -Nitric oxide (NO) |
| -Endothelium-derived hyperpolarizing factor (EDHF) | |
| -Prostacyclin (PGI2) | |
| Vasoconstrictors | -Endothelin 1 (ET-1) |
| -Angiotensin II (Ang II) | |
| -Thromboxane A2 (TXA2) | |
| Growth modulators | |
| Growth promoters | -Platelet-derived growth factor (PDGF) |
| -Basic fibroblast growth factor (bFGF) | |
| -Somatomedin-1 (IGF-1) | |
| -Endothelin 1 (ET-1) | |
| -Angiotensin II (Ang II) | |
| -Heparan sulfate (HPS) | |
| Growth inhibitors | -Transforming growth factor (TGF) |
| -Nitric oxide (NO) | |
| -Prostacyclin (PGI2) | |
| Modulators of inflammation | |
| Adhesion molecules | -Endothelial leukocyte adhesion molecule (ELAM) -Intercellular adhesion molecule (ICAM) -Vascular cell adhesion molecule (VCAM) |
| Hemostatic and thrombolytic factors | |
| -Tissue plasminogen activator (t-PA) -Plasminogen activator inhibitor 1 (PAI-1) -Thrombomodulin | |
| Vasoactive Substance | Model | Tissue | Actions in Endothelium | References |
|---|---|---|---|---|
| Norepinephrine (NE) | Control group: Cyclic rats Experimental group: Ovariectomized rats (OVX) | Aorta (vascular smooth muscle) Treated in isometric myography system | -The vascular synthesis of both PGE2 and PGF2a was significantly higher in the group of OVX rats compared to the proestrous. -The vascular response to NE was significantly higher in OVX rats, compared to normal cycling rats during proestrous. | [205] |
| Cirazoline (α1-Adrenergic agonist) | Pregnant, proestrous and diestrous rats | Mesenteric vascular bed Treated by perfusion | -The tone induced by cirazoline was lower in the proestrous and pregnant groups, but the increase in the tone of L-NA is higher in pregnant compared to proestrous and diestrous group. The participation of EDRF in this effect is suggested. | [206] |
| Methacholine (Muscarinic agonist, endothelium-dependent vasodilator) | Control group: Experimental group: Pregnant mice | Uterine and mesenteric arteries Treated in isometric myography system | -The relaxation induced by methacholine was higher in pregnant mice, in both uterine artery and mesenteric vessels, with a more pronounced effect on the uterine vasculature. -Modulation of relaxation is endothelium-dependent PGHS or NOS pathways is reinforced in the uterine arteries. | [207] |
| Aspilia africana (Ethanolic extract) | Cyclic rats | Uterine endothelium Treatment by using an oropharyngeal cannula and calibrated hypodermic syringe | -Dose-dependent decrease in duration of estrous cycle and histoarchitecture of the uterus. | [208] |
| Buddleja globosa (Ethanolic extract) | Cyclic rats Ovariectomized rats (OVX) | Uterine endothelium Treatment administered subcutaneously with hypodermic syringe | Anti-estrogenic effect of extract of Buddleja globosa at the highest dose evaluated. | [209] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jaimes, L.; Vinet, R.; Knox, M.; Morales, B.; Benites, J.; Laurido, C.; Martínez, J.L. A Review of the Actions of Endogenous and Exogenous Vasoactive Substances during the Estrous Cycle and Pregnancy in Rats. Animals 2019, 9, 288. https://doi.org/10.3390/ani9060288
Jaimes L, Vinet R, Knox M, Morales B, Benites J, Laurido C, Martínez JL. A Review of the Actions of Endogenous and Exogenous Vasoactive Substances during the Estrous Cycle and Pregnancy in Rats. Animals. 2019; 9(6):288. https://doi.org/10.3390/ani9060288
Chicago/Turabian StyleJaimes, Luisauris, Raúl Vinet, Marcela Knox, Bernardo Morales, Julio Benites, Claudio Laurido, and José L. Martínez. 2019. "A Review of the Actions of Endogenous and Exogenous Vasoactive Substances during the Estrous Cycle and Pregnancy in Rats" Animals 9, no. 6: 288. https://doi.org/10.3390/ani9060288
APA StyleJaimes, L., Vinet, R., Knox, M., Morales, B., Benites, J., Laurido, C., & Martínez, J. L. (2019). A Review of the Actions of Endogenous and Exogenous Vasoactive Substances during the Estrous Cycle and Pregnancy in Rats. Animals, 9(6), 288. https://doi.org/10.3390/ani9060288







