Proteomic Profiles of the Longissimus Muscles of Entire Male and Castrated Pigs as Related to Meat Quality
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
3. Results
3.1. Carcass and Meat Physico-Chemical Traits
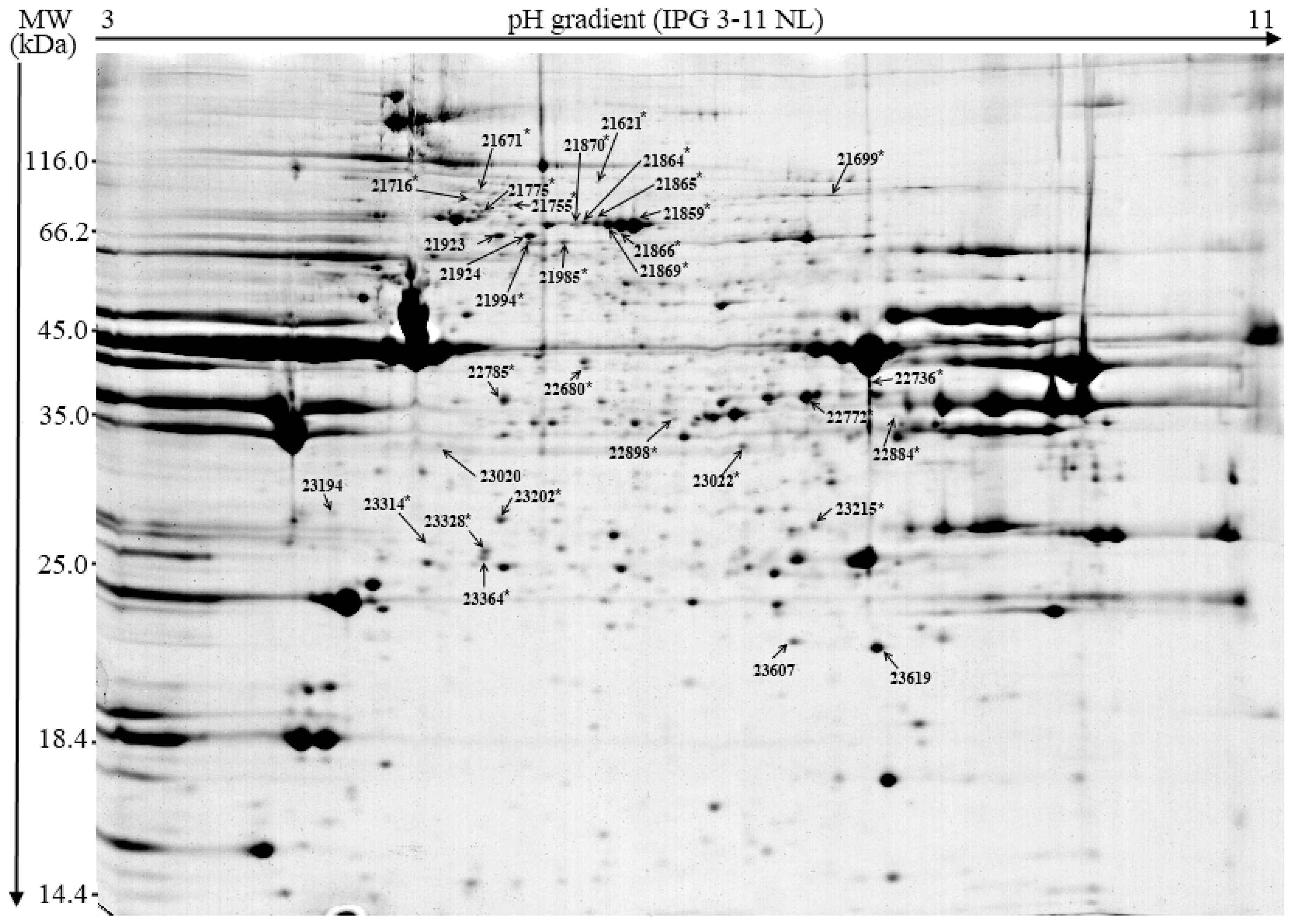
3.2. Proteomic Profile
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Babol, J.; Squires, E.J. Quality of meat from entire male pigs. Food Res. Int. 1995, 28, 201–212. [Google Scholar] [CrossRef]
- European Declaration on Alternatives to Surgical Castration of Pigs. Available online: http://ec.europa.eu/food/animals/welfare/practice/farm/pigs/castration_alternatives_en (accessed on 13 July 2017).
- De Roest, K.; Montanari, C.; Fowler, T.; Baltussen, W. Resource efficiency and economic implications of alternatives to surgical castration without anaesthesia. Animal 2009, 3, 1522–1531. [Google Scholar] [CrossRef] [PubMed]
- Von Borell, E.J.; Baumgartner, J.; Giershing, M.; Jäggin, N.; Prunier, A.; Tuyttens, F.A.M.; Edvards, S.A. Animal welfare implications of surgical castration and its alternatives in pigs. Animal 2009, 3, 1488–1496. [Google Scholar] [CrossRef] [PubMed]
- Lundström, K.; Matthews, K.R.; Haugen, J.-E. Pig meat quality from entire male pigs. Animal 2009, 3, 1497–1507. [Google Scholar] [CrossRef] [PubMed]
- Pauly, K.; Spring, P.; O’Doherty, J.V.; Ampuero Kragten, S.; Bee, G. Growth performance, carcass characteristics and meat quality of group-pened surgically castrated, immunocastrated (Improvac®) and entire male pigs and individually penned entire male pigs. Animal 2009, 3, 1057–1066. [Google Scholar] [CrossRef] [PubMed]
- Trefan, L.; Doeschl-Wilson, A.; Rooke, J.A.; Terulow, C.; Bünger, L. Meta-analysis of effects of gender in combination with carcass weight and breed on pork quality. J. Anim. Sci. 2013, 91, 1480–1492. [Google Scholar] [CrossRef] [PubMed]
- Batorek, N.; Škrlep, M.; Prunier, A.; Louveau, I.; Noblet, J.; Bonneau, M.; Čandek-Potokar, M. Effect of feed restriction on hormones, performance, carcass traits, and meat quality in immunocastrated pigs. J. Anim. Sci. 2012, 90, 4593–4603. [Google Scholar] [CrossRef] [PubMed]
- Škrlep, M.; Batorek, N.; Bonneau, M.; Prevolnik, M.; Kubale, V.; Čandek-Potokar, M. Effect of immunocastration in group-housed commercial fattening pigs on reproductive organs, malodorous compounds, carcass and meat quality. CZECH J. Anim Sci. 2012, 57, 290–299. [Google Scholar] [CrossRef]
- Aluwé, M.; Langendries, K.C.M.; Bekaert, K.M.; Tuyttens, F.A.M.; de Brabander, D.L.; de Smet, S.; Millet, S. Effect of surgical castration, immunocastration and chicory-diet on the meat quality and palatability of boars. Meat Sci. 2013, 94, 402–407. [Google Scholar] [CrossRef] [PubMed]
- Commission Decision of 18 February 2008 amending Decision 2005/879/EC authorising methods for grading pig carcases in Slovenia. OJEU 2008, L56/28, 28–30.
- Christensen, L.B. Drip loss sampling in porcine m. longissimus dorsi. Meat Sci. 2003, 63, 469–477. [Google Scholar] [CrossRef]
- Traore, S.; Aubry, L.; Gatellier, P.; Przybylski, W.; Jaworska, D.; Kajak-Siemaszko, K.; Santé-Lhoutellier, V. Higher drip loss is associated with protein oxidation. Meat Sci. 2012, 90, 917–924. [Google Scholar] [CrossRef] [PubMed]
- Trout, G.R. A rapid method for measuring pigment concentration in porcine and other low pigmented muscles. In Proceedings of the 37th International Congress of Meat Science and Technology, Kulmbach, Germany, 1–6 September 1991; pp. 1198–1201. [Google Scholar]
- ISO 3496. Meat and Meat Products—Determination of Hydroxyproline Content; International Organization for Standardization: Genève, Switzerland, 1994. [Google Scholar]
- Škrlep, M.; Čandek-Potokar, M.; Mandelc, S.; Javornik, B.; Gou, P.; Chambon, C.; Santé-Lhoutellier, V. Proteomic profile of dry-cured ham relative to PRKAG3 or CAST genotype, level of salt and pastiness. Meat Sci. 2011, 88, 657–667. [Google Scholar]
- Dunshea, F.R.; Colantoni, C.; Howard, K.; McCauley, I.; Jackson, P.; Long, K.A.; Lopaticki, S.; Nugent, E.A.; Simons, J.A.; Walker, J.; et al. Vaccination of boars with GnRH vaccine (Improvac) eliminates boar taint and increases growth performance. J. Anim. Sci. 2001, 79, 2524–2535. [Google Scholar] [CrossRef] [PubMed]
- Pauly, K.; Luginbühl, W.; Ampuero, S.; Bee, G. Expected effects on carcass and pork quality when surgical castration is omitted. Meat Sci. 2012, 92, 858–862. [Google Scholar] [CrossRef] [PubMed]
- Gispert, M.; Oliver, M.A.; Velarde, A.; Suarez, P.; Perez, J.; Font i Furnols, M. Carcass and meat quality characteristics of immunocastrated male, surgically castrated male, entire male and female pigs. Meat Sci. 2010, 85, 664–670. [Google Scholar] [CrossRef] [PubMed]
- Seideman, S.C. Methods of expressing collagen characteristics and their relationship to meat tenderness and muscle fiber types. J. Food Sci. 1986, 51, 273–276. [Google Scholar] [CrossRef]
- Dikeman, M.E.; Reddy, G.B.; Arthaud, V.H.; Thuma, H.J.; Koch, R.M.; Mandigo, R.W.; Axe, J.B. Longissimus muscle quality, palatability and connective tissue histological characteristics of bulls and steers fed different energy levels and slaughtered at four ages. J. Anim. Sci. 1986, 63, 92–101. [Google Scholar] [CrossRef] [PubMed]
- Gerrard, D.E.; Jones, S.J.; Aberle, D.E.; Lemenager, R.P.; Dikeman, M.A.; Judge, M.D. Collagen stability, testosterone secretion and meat tenderness in growing bulls and steers. J. Anim. Sci. 1987, 65, 1236–1242. [Google Scholar] [CrossRef] [PubMed]
- Nold, R.A.; Romans, J.R.; Costello, W.J.; Libal, G.W. Characterization of muscles from boars, barrows and gilts slaughtered at 100 and 110 kilograms: Differences in fat moisture, colour, water-holding capacity and collagen. J. Anim. Sci. 1999, 77, 1746–1754. [Google Scholar] [CrossRef] [PubMed]
- Petersen, J.S.; Berge, P.; Henckel, P.; Soerensen, M.T. Collagen characteristics and meat texture of pigs exposed to different levels of physical activity. J. Muscle Foods 1997, 8, 47–61. [Google Scholar] [CrossRef]
- Vold, E.; Moen, R.A. A note on the effect of castration upon the development of the skin in the pig. Anim. Sci. 1972, 14, 253–254. [Google Scholar] [CrossRef]
- Wood, J.D.; Enser, M.; Whittington, F.M.; Moncrieff, C.B.; Kempster, A.J. Backfat composition in pigs: Differences between fat thickness groups and sexes. Livestock Prod. Sci. 1989, 22, 351–362. [Google Scholar] [CrossRef]
- Claus, R.; Weiler, U.; Herzog, A. Physiological aspects of androstenone and skatole formation in the boar: A review with experimental data. Meat Sci. 1994, 38, 289–305. [Google Scholar] [CrossRef]
- Gandemer, G. Lipids and meat quality: Lipolysis, oxidation, maillard reaction and flavour. Sci. Aliments 1999, 19, 439–458. [Google Scholar]
- Estévez, M. Protein carbonyls in meat systems: A review. Meat Sci. 2011, 89, 259–279. [Google Scholar] [CrossRef] [PubMed]
- Mancini, R.A.; Hunt, M.C. Current research in meat color. Meat Sci. 2005, 71, 100–121. [Google Scholar] [CrossRef] [PubMed]
- Chelh, I.; Gatellier, P.; Santé-Lhoutellier, V. Characterization of fluorescent Schiff bases formed during oxidation of pig myofibrils. Meat Sci. 2007, 76, 210–215. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Carpena, J.G.; Morcuende, D.; Estévez, M. Avocado by-products as inhibitors of color deterioration and lipid and protein oxidation in raw porcine patties subjected to chilled storage. Meat Sci. 2011, 89, 166–173. [Google Scholar] [CrossRef] [PubMed]
- Škrlep, M.; Šegula, B.; Prevolnik, M.; Kirbiš, A.; Fazarinc, G.; Čandek-Potokar, M. Effect of immunocastration (Improvac®) in fattening pigs II: Carcass traits and meat quality. Slov. Vet. Res. 2010, 47, 65–72. [Google Scholar]
- Miyahara, M.; Matsuda, S.; Komaki, H.; Sakari, H.; Tsukise, A. Effects of sexual distinction on growth rate and meat production in three-way cross pigs. Jpn. J. Swine Sci. 2004, 41, 228–236. [Google Scholar] [CrossRef]
- Huff-Lonergan, E.; Mitsuhashi, T.; Beekman, D.D.; Parrish, F.C.; Olson, G.; Robson, R.M. Proteolysis of specific muscle structural proteins by μ-calpain at low pH and temperature is similar to degradation in postmortem bovine muscle. J. Anim. Sci. 1996, 74, 993–1008. [Google Scholar] [CrossRef] [PubMed]
- Lametsch, R.; Roepstorff, P.; Bendixen, E. Identification of protein degradation during post-mortem storage of pig meat. J. Agric. Food Chem. 2002, 50, 5508–5521. [Google Scholar] [CrossRef] [PubMed]
- Lametsch, R.; Karlsson, A.; Rosenvold, K.; Andersen, H.J.; Roepstorff, P.; Bendixen, E. Postmortem proteome changes of porcine muscle related to tenderness. J. Agric. Food Chem. 2003, 51, 6992–6997. [Google Scholar] [CrossRef] [PubMed]
- Morzel, M.; Chambon, C.; Hamelin, M.; Sante-Lhoutellier, V.; Sayd, T.; Monin, G. Proteome changes during pork meat ageing following use of two different pre-slaughter handling procedures. Meat Sci. 2004, 67, 689–696. [Google Scholar] [CrossRef] [PubMed]
- Hwang, I.H.; Park, B.Y.; Kim, J.H.; Cho, S.H.; Lee, J.M. Assessment of postmortem proteolysis by gel-based proteome analysis and its relationship to meat quality traits in pig longissimus. Meat Sci. 2005, 69, 79–91. [Google Scholar] [CrossRef] [PubMed]
- Stoeva, S.; Byrne, C.E.; Mullen, A.M.; Troy, D.J.; Voelter, W. Isolation and identification of proteolytic fragments from TCA soluble extracts of bovine M. longissimus dorsi. Food Chem. 2000, 69, 365–370. [Google Scholar] [CrossRef]
- Di Luca, A.; Mullen, A.M.; Eila, G.; Davey, G.; Hamill, R.M. Centrifugal drip is an accessible source for protein indicators of pork ageing and water-holding capacity. Meat Sci. 2011, 88, 261–270. [Google Scholar] [CrossRef] [PubMed]
- Purintrapiban, J.; Wang, M.; Forsberg, N.E. Identification of glycogen phosphorilase and creatin kinase as calpain substrates in skeletal muscle. Int. J. Biochem. Cell Biol. 2001, 33, 531–540. [Google Scholar] [CrossRef]
- Lametsch, R.; Roepstoff, P.; Møller, H.S.; Bendixen, E. Identification of myofibrillar substrates for μ-calpain. Meat Sci. 2004, 68, 515–521. [Google Scholar] [CrossRef] [PubMed]
- Houbak, M.B.; Ertbjerg, P.; Therkildsen, M. In vitro study to evaluate the degradation of bovine muscle proteins post-mortem by proteasome and μ-calpain. Meat Sci. 2008, 79, 77–85. [Google Scholar] [CrossRef] [PubMed]
- Koohmaraie, M. Biochemical factors regulating the toughening and tenderization process of meat. Meat Sci. 1996, 43, S193–S201. [Google Scholar] [CrossRef]
- Baracos, V.E. Whole animal and tissue proteolysis in growing animals. In Biology of Metabolism in Growing Animals; Burrin, D., Mersmann, H.J., Eds.; Saunders Ltd.: Philadelphia, PA, USA, 2005; Volume 3, pp. 69–82. [Google Scholar]
- Kaltnekar, T.; Škrlep, M.; Batorek Lukač, N.; Tomažin, U.; Prevolnik Povše, M.; Labussière, E.; Demšar, L.; Čandek-Potokar, M. Effects of salting duration and boar taint level on quality of dry-cured hams. Acta Agric. Slov. 2016, 5, 132–137. [Google Scholar]
- Laville, E.; Sayd, T.; Terulow, C.; Chambon, C.; Damon, M.; Larzul, C.; Leroy, P.; Glénisson, J.; Chérel, P. Comparison of sarcoplasmic proteomes between two groups of pig muscles selected for shear force of cooked meat. J. Agric. Food Chem. 2007, 55, 5834–5841. [Google Scholar] [CrossRef] [PubMed]
- Kim, N.K.; Cho, S.; Lee, S.H.; Park, H.R.; Lee, C.S.; Cho, Y.M.; Choy, Y.H.; Yoon, D.; Im, S.K.; Park, E.W. Proteins in longissimus muscle of Korean native cattle and their relationship to meat quality. Meat Sci. 2008, 80, 1068–1073. [Google Scholar] [CrossRef] [PubMed]
- Laville, E.; Sayd, T.; Morzel, M.; Blinet, S.; Chambon, C.; Lepetit, J.; Renand, G.; Hocquette, J.F. Proteome changes during meat ageing in tough and tender beef suggest the importance of apoptosis and protein solubility for beef aging and tenderization. J. Agric. Food Chem. 2009, 57, 10755–10764. [Google Scholar] [CrossRef] [PubMed]
- Bjarnadottir, S.G.; Hollung, K.; Høy, M.; Bendixen, E.; Codrea, M.C.; Veiseth-Kent, E. Changes in protein abundance between tender and tough meat from bovine Longissimus thoracis muscle assessed by isobaric Tag for Relative and Absolute Quantitation (iTRAQ) and 2-dimmensional gel electrophoresis analysis. J. Anim. Sci. 2012, 90, 2035–2043. [Google Scholar] [CrossRef] [PubMed]
- Huff Lonergan, E.; Zhang, W.; Lonergan, S.M. Biochemistry of postmortem muscle—Lessons on mechanisms of meat tenderization. Meat Sci. 2010, 86, 184–195. [Google Scholar] [CrossRef] [PubMed]
- Shi, X.; Li, C.; Cao, C.; Xu, X.; Zhou, G.; Xiong, Y. Comparative proteomic analysis of longisimus dorsi muscle in immune- and surgically castrated male pigs. Food Chem. 2016, 199, 885–892. [Google Scholar] [CrossRef] [PubMed]
- Sayd, T.; Morzel, M.; Chambon, C.; Franck, M.; Figwer, P.; Larzul, C.; Le Roy, P.; Monin, G.; Cherel, P.; Laville, E. Proteome analysis of sarcoplastic fraction of pig semimembranosus muscle: Implications on meat colour development. J. Agric. Food Chem. 2006, 54, 2732–2734. [Google Scholar] [CrossRef] [PubMed]
- Kim, G.-D.; Jeong, J.-Y.; Hur, S.-J.; Yang, H.-S.; Jeon, J.-T.; Joo, S.-T. The relationship between meat colour (CIE L* and a*), myoglobin content, and their influence on muscle fiber characteristics and pork quality. Korean J. Food Sci. Anim. Resour. 2010, 30, 626–633. [Google Scholar] [CrossRef]
- Jia, X.H.; Veiseth-Kent, E.; Grove, H.; Kuziora, P.; Aass, L.; Hildrum, K.I.; Hollung, K. Peroxiredoxin-6—A potential protein marker for meat tenderness in bovine longissimus thoracis muscle. J. Anim. Sci. 2009, 87, 2391–2399. [Google Scholar] [CrossRef] [PubMed]
- Herrera-Mendez, C.H.; Becila, S.; Boudjellal, A.; Ouali, A. Meat ageing: Reconsideration of the current concept. Trends Food Sci. Technol. 2006, 17, 394–405. [Google Scholar] [CrossRef]
- Becila, S.C.; Herrera-Mendez, H.; Coulis, G.; Labas, R.; Astruc, T.; Picard, B.; Boudjellal, A.; Pelissier, P.; Bremaud, L.; Ouali, A. Postmortem muscle cells die through apoptosis. Eur. Food Res. Technol. 2010, 231, 458–493. [Google Scholar] [CrossRef]
- Lomiwes, D.; Farouk, M.M.; Wiklund, E.; Young, O.A. Small heat shock proteins and their role in meat tenderness: A review. Meat Sci. 2014, 96, 26–40. [Google Scholar] [CrossRef] [PubMed]
- Kwasiborski, A.; Sayd, T.; Chambon, C.; Santé-Lhoutellier, V.; Rocha, D.; Terulow, C. Pig longissimus lumborum proteome: Part II: Relationship between protein content and meat quality. Meat Sci. 2008, 80, 892–996. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Tang, S.; Bao, E.; Zhang, M.; Hao, Q.; Zue, Z. The effect of transportation on the expression of heat shock proteins and meat quality of M. longissimus dorsi in pigs. Meat Sci. 2009, 83, 474–478. [Google Scholar] [CrossRef] [PubMed]
- Lonergan, S.M.; Huff-Lonergan, E.; Rowe, L.J.; Kuhlers, D.L.; Jungst, S.B. Selection for lean growth efficiency in Duroc pigs influences pork quality. J. Anim. Sci. 2001, 79, 2075–2085. [Google Scholar] [CrossRef] [PubMed]
- Promeyrat, A.; Sayd, T.; Laville, E.; Chambon, C.; Lebret, B.; Gatellier, P. Early post-mortem sarcoplasmic proteome of porcine muscle related to protein oxidation. Food Chem. 2011, 127, 1097–1104. [Google Scholar] [CrossRef] [PubMed]
- Lefaucheur, L. A second look into fibre typing—Relation to meat quality. Meat Sci. 2010, 84, 257–270. [Google Scholar] [CrossRef] [PubMed]
- Musrati, R.A.; Kollárová, M.; Mernik, N.; Mikoulášová, D. Malate dehydrogenase: Distribution, function and properties. Gen. Physiol. Biophys. 1998, 17, 193–210. [Google Scholar] [PubMed]
- Thompson, C.B.; McDonough, A.A. Skeletal muscle Na, K-ATPase alpha and beta subunit protein levels respond to hypokalemic challenge with isoform and muscle type specificity. J. Biol. Chem. 1996, 271, 32653–32658. [Google Scholar] [CrossRef] [PubMed]
- Fowles, J.R.; Green, H.J.; Ouyang, J. Na+-K+-ATPase in rat skeletal muscle: Content, isoform, and activity characteristics. J. Appl. Physiol. 2004, 96, 306–326. [Google Scholar] [CrossRef] [PubMed]
- Dreyer, G.H.; Nande, R.T.; Henning, J.W.N.; Rossouw, E. The influence of breed, castration and age on muscle fibre type and diameter in Friesland and Afrikaner cattle. S. Afr. J. Anim. Sci. 1977, 7, 171–180. [Google Scholar]
- Ockerman, H.W.; Jaworek, D.; Van Stavern, B.; Parrett, N.; Pierson, C.J. Castration and sire effects on carcass traits, meat palatability and muscle fiber characteristics in angus cattle. J. Anim. Sci. 1984, 59, 981–990. [Google Scholar] [CrossRef]
- Brandstetter, A.M.; Picard, B.; Geay, I. Muscle fibre characteristics in four muscles of growing male cattle II. Effect of castration and feeding level. Livestock Prod. Sci. 1998, 53, 25–36. [Google Scholar] [CrossRef]
- Brandstetter, A.M.; Sauerwein, H.; Veerkamp, J.H.; Geay, Y.; Hocquette, J.F. Effects of muscle type, castration, age and growth rate on H-FABP expression in bovine skeletal muscle. Livestock Prod. Sci. 2002, 75, 199–208. [Google Scholar] [CrossRef]
- Guillemin, N.; Jurie, C.; Cassar-Malek, I.; Hocquette, J.-F.; Renand, G.; Piccard, B. Variations in the abundance of 24 protein biomarkers of beef tenderness according to muscle and animal type. Animal 2011, 5, 885–894. [Google Scholar] [CrossRef] [PubMed]
- Cai, Z.; Zhang, L.; Jiang, X.; Sheng, Y.; Xu, N. Differential miRNA expression profiles in the longissimus dorsi muscle between intact and castrated male pigs. Res. Vet. Sci. 2015, 99, 99–104. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Gariépy, C.; Jin, Y.; Font i Furnols, M.; Fortin, J.; Rocha, L.M.; Faucitano, L. Effects of ractopamine administration and castration method on muscle fiber characteristics and sensory quality of the longissimus muscle in two Piétrain pig genotypes. Meat Sci. 2015, 102, 27–34. [Google Scholar] [CrossRef] [PubMed]

| Live Weight and Carcass Traits | EMs | SCs | RMSE | p-Value |
|---|---|---|---|---|
| Live weight, kg | 132.8 | 132.3 | 7.9 | 0.818 |
| Carcass weight, kg | 100.8 | 105.9 | 5.8 | 0.043 |
| Back fat, mm | 9.8 | 13.9 | 3.8 | 0.016 |
| Muscle thickness, mm | 71.2 | 81.1 | 6.9 | 0.002 |
| Lean meat % | 62.4 | 60.5 | 3.1 | 0.157 |
| Chemical Traits | EMs | SCs | RMSE | p-Value |
|---|---|---|---|---|
| IMF, % | 1.8 | 3.1 | 1.4 | 0.033 |
| Proteins, % | 24.1 | 23.6 | 0.6 | 0.118 |
| Moisture, % | 74.0 | 73.2 | 0.6 | 0.008 |
| Collagen, mg/g | 8.09 | 3.63 | 2.59 | <0.001 |
| Collagen solubility, % | 22.8 | 11.5 | 8.8 | 0.005 |
| Myoglobin, mg/g | 1.26 | 1.45 | 0.28 | 0.118 |
| Carbonyl, nmol/g protein | 1.67 | 0.82 | 0.39 | <0.001 |
| Meat Quality Traits | EMs | SCs | RMSE | p-Value |
|---|---|---|---|---|
| L* | 55.4 | 52.7 | 2.4 | 0.011 |
| a* | 7.3 | 8.3 | 1.2 | 0.065 |
| b* | 3.4 | 2.1 | 1.0 | 0.006 |
| Ultimate pH | 5.35 | 5.37 | 0.08 | 0.417 |
| Drip loss after 24 h, % | 7.1 | 3.9 | 1.9 | <0.001 |
| Thawing loss, % | 13.8 | 11.7 | 3.6 | 0.176 |
| Cooking loss, % | 34.1 | 28.8 | 3.7 | 0.002 |
| Shear force, N | 160.6 | 123.0 | 32.2 | 0.009 |
| ID | Consensus Protein Identity | UniProt ID a | Mascot Score | % SC/MP b | Theoretical Mr/Pi c | Protein Integrity d |
|---|---|---|---|---|---|---|
| Enzymes | ||||||
| 21621 | Na/K-transporting ATPase subunit alpha-1 | P05024 | 23 | 1/1 | 113920/5.36 | Entire |
| 21755 | Na/K-transporting ATPase subunit alpha-1 | P05024 | 18 | 1/1 | 113920/5.36 | Fragment |
| 22736 | Beta enolase | Q1KYT0 | 152 | 8/3 | 47443/8.05 | Fragment |
| 22884 | Creatine kinase M-type | Q5XLD3 | 151 | 8/3 | 43260/6.61 | Fragment |
| 23215 | Creatine kinase M-type | Q5XLD3 | 177 | 8/3 | 43260/6.61 | Fragment |
| 22898 | Malate dehydrogenase, cytoplasmic | P11708 | 93 | 6/2 | 36716/6.16 | Entire |
| Blood plasma | ||||||
| 21699 | Serotransferrin | P09571 | 122 | 6/3 | 78971/6.93 | Entire |
| 21859 | Serum albumin | P08835 | 613 | 14/8 | 71643/6.08 | Entire |
| 21864 | Serum albumin | P08835 | 226 | 6/3 | 71643/6.08 | Entire |
| 21865 | Serum albumin | P08835 | 561 | 17/9 | 71643/6.08 | Entire |
| 21866 | Serum albumin | P08835 | 605 | 14/8 | 71643/6.08 | Entire |
| 21869 | Serum albumin | P08835 | 599 | 14/8 | 71643/6.08 | Entire |
| 21924 | Serum albumin | P08835 | 61 | 3/2 | 71643/6.08 | Entire |
| 21923 | Coagulation factor VIII | P12263 | 26 | 0/1 | 240467 | Fragment |
| 21985 | Coagulation factor VIII | P12263 | 23 | 0/1 | 240467 | Fragment |
| 21994 | Coagulation factor VIII | P12263 | 25 | 0/1 | 240467 | Fragment |
| Chaperone | ||||||
| 21870 | Heat shock 70kDa protein 6 | Q04967 | 358 | 9/6 | 71522/5.77 | Entire |
| 23607 | Alpha crystallin B chain | Q7M2W6 | 479 | 41/6 | 20116/6.76 | Entire |
| 23619 | Alpha crystallin B chain | Q7M2W6 | 246 | 23/5 | 20116/6.76 | Entire |
| Myofibrillar | ||||||
| 22772 | Troponin T, fast skeletal muscle | Q75NG9 | 200 | 13/3 | 32157/6.05 | Entire |
| 23022 | Troponin T, slow skeletal muscle | Q75ZZ6 | 146 | 12/3 | 31224/5,92 | Entire |
| 21671 | Myosin heavy chain 2a | Q9TV63 | 104 | 1/2 | 223924/5.64 | Fragment |
| 21716 | Myosin heavy chain 2a | Q9TV63 | 41 | 0/1 | 223924/5.64 | Fragment |
| 21755 | Myosin heavy chain 2a | Q9TV63 | 114 | 1/2 | 223924/5.64 | Fragment |
| 22680 | Actin alpha, skeletal muscle | P68137 | 295 | 15/5 | 42366/5.23 | Fragment |
| 22785 | Actin alpha, skeletal muscle | P68137 | 378 | 15/4 | 42366/5.23 | Fragment |
| 23020 | Actin alpha, skeletal muscle | P68137 | 199 | 10/3 | 42366/5.23 | Fragment |
| 23194 | Actin alpha, skeletal muscle | P68137 | 169 | 8/3 | 42366/5.23 | Fragment |
| 23202 | Actin alpha, skeletal muscle | P68137 | 281 | 10/3 | 42366/5.23 | Fragment |
| 23314 | Actin alpha, skeletal muscle | P68137 | 160 | 10/3 | 42366/5.23 | Fragment |
| 23328 | Actin alpha, skeletal muscle | P68137 | 276 | 10/3 | 42366/5.23 | Fragment |
| 23364 | Actin alpha, skeletal muscle | P68137 | 279 | 10/3 | 42366/5.23 | Fragment |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Škrlep, M.; Tomažin, U.; Lukač, N.B.; Poklukar, K.; Čandek-Potokar, M. Proteomic Profiles of the Longissimus Muscles of Entire Male and Castrated Pigs as Related to Meat Quality. Animals 2019, 9, 74. https://doi.org/10.3390/ani9030074
Škrlep M, Tomažin U, Lukač NB, Poklukar K, Čandek-Potokar M. Proteomic Profiles of the Longissimus Muscles of Entire Male and Castrated Pigs as Related to Meat Quality. Animals. 2019; 9(3):74. https://doi.org/10.3390/ani9030074
Chicago/Turabian StyleŠkrlep, Martin, Urška Tomažin, Nina Batorek Lukač, Klavdija Poklukar, and Marjeta Čandek-Potokar. 2019. "Proteomic Profiles of the Longissimus Muscles of Entire Male and Castrated Pigs as Related to Meat Quality" Animals 9, no. 3: 74. https://doi.org/10.3390/ani9030074
APA StyleŠkrlep, M., Tomažin, U., Lukač, N. B., Poklukar, K., & Čandek-Potokar, M. (2019). Proteomic Profiles of the Longissimus Muscles of Entire Male and Castrated Pigs as Related to Meat Quality. Animals, 9(3), 74. https://doi.org/10.3390/ani9030074






