Keel Fracture Causes Stress and Inflammatory Responses and Inhibits the Expression of the Orexin System in Laying Hens
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Management
2.2. Assessment of Keel Fracture
2.3. Sample Collection
2.4. Determination of Serum Corticosterone Content
2.5. Total RNA Extraction, Reverse Transcription, and Quantitative Real-Time PCR (qRT-PCR) Analysis
2.6. Western Blotting
2.7. Statistical Analysis
3. Results
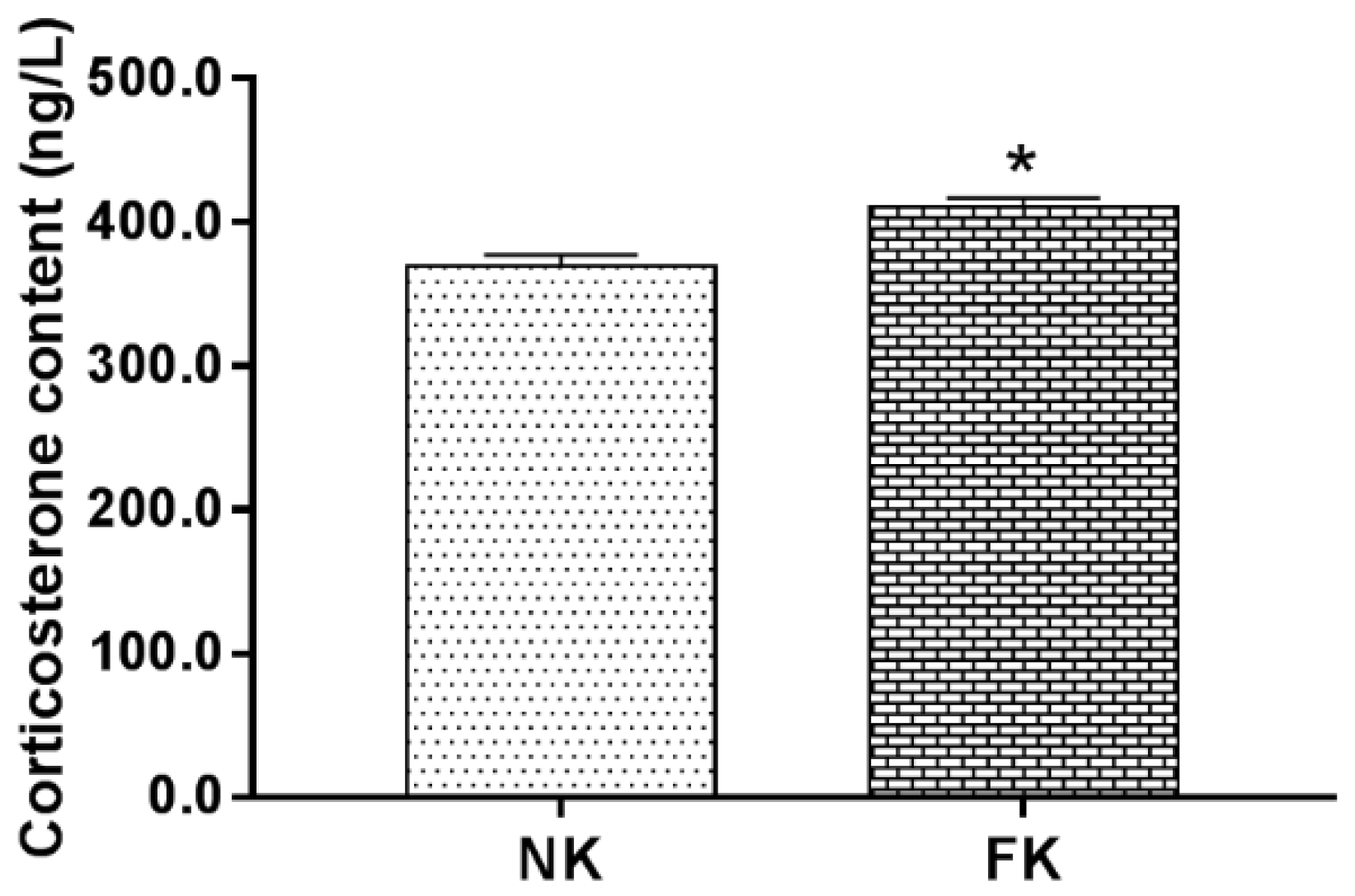
3.1. Determination of Serum Corticosterone Concentration
3.2. Expression Levels of Hsps in Hen Brain, Liver, and Muscle Tissues
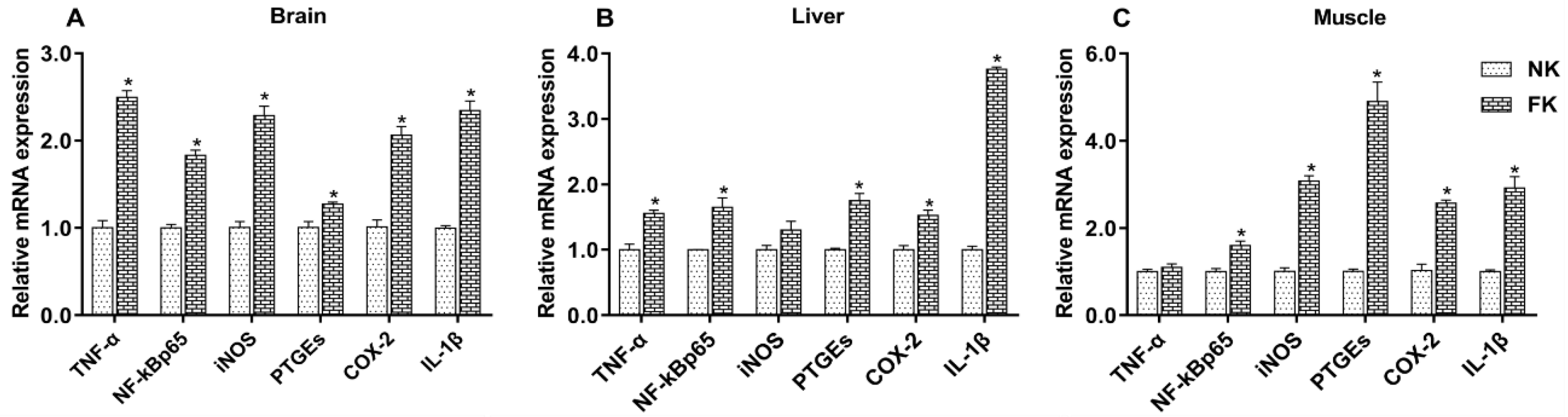
3.3. Expression Levels of Inflammatory Factors in Hen Brains, Livers, and Muscles
3.4. Expression Levels of Orexin and Orexin Receptors in Hen Brains, Livers, and Muscles
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Wilkins, L.J.; Brown, S.N.; Zimmerman, P.H.; Leeb, C.; Nicol, C.J. Investigation of palpation as a method for determining the prevalence of keel and furculum damage in laying hens. Vet. Rec. 2004, 155, 547–549. [Google Scholar] [CrossRef] [PubMed]
- Sherwin, C.M.; Richards, G.J.; Nicol, C.J. Comparison of the welfare of layer hens in 4 housing systems in the UK. Br. Poult. Sci. 2010, 51, 488–499. [Google Scholar] [CrossRef] [PubMed]
- Wilkins, L.J.; McKinstry, J.L.; Avery, N.C.; Knowles, T.G.; Brown, S.N.; Tarlton, J.; Nicol, C.J. Influence of housing system and design on bone strength and keel bone fractures in laying hens. Vet. Rec. 2011, 169, 414. [Google Scholar] [CrossRef] [PubMed]
- Petrik, M.T.; Guerin, M.T.; Widowski, T.M. On-farm comparison of keel fracture prevalence and other welfare indicators in conventional cage and floor-housed laying hens in Ontario, Canada. Poult. Sci. 2015, 94, 579–585. [Google Scholar] [CrossRef]
- Heerkens, J.L.T.; Delezie, E.; Rodenburg, T.B.; Kempen, I.; Zoons, J.; Ampe, B.; Tuyttens, F.A.M. Risk factors associated with keel bone and foot pad disorders in laying hens housed in aviary systems. Poult. Sci. 2016, 95, 482–488. [Google Scholar] [CrossRef]
- Nasr, M.A.F.; Murrell, J.; Wilkins, L.J.; Nicol, C.J. The effect of keel fractures on egg-production parameters, mobility and behaviour in individual laying hens. Anim. Welf. 2012, 21, 127–135. [Google Scholar] [CrossRef]
- Nasr, M.A.F.; Murrell, J.; Nicol, C.J. The effect of keel fractures on egg production, feed and water consumption in individual laying hens. Br. Poult. Sci. 2013, 54, 165–170. [Google Scholar] [CrossRef]
- Rufener, C.; Baur, S.; Stratmann, A.; Toscano, M.J. Keel bone fractures affect egg laying performance but not egg quality in laying hens housed in a commercial aviary system. Poult. Sci. 2018, 9, 1589–1600. [Google Scholar] [CrossRef]
- Rørvang, M.V.; Hinrichsen, L.K.; Riber, A.B. Welfare of layers housed in small furnished cages on Danish commercial farms: The condition of keel bone, feet, plumage and skin. Br. Poult. Sci. 2019, 60, 1–7. [Google Scholar] [CrossRef]
- Wang, L.; Guo, T.Z.; Wei, T.; Li, W.W.; Shi, X.; Clark, J.D.; Kingery, W.S. Bisphosphonates inhibit pain, bone loss, and inflammation in a rat tibia fracture model of complex regional pain syndrome. Anesth. Analg. 2016, 123, 1033–1045. [Google Scholar] [CrossRef]
- Pesic, G.; Jeremic, J.; Nikolic, T.; Zivkovic, V.; Srejovic, I.; Vranic, A.; Bradic, J.; Ristic, B.; Matic, A.; Prodanovic, N.; et al. Interleukin-6 as possible early marker of stress response after femoral fracture. Mol. Cell. Biochem. 2017, 430, 191–199. [Google Scholar] [CrossRef] [PubMed]
- Rani, A.; Kumar, A.; Lal, A.; Pant, M. Cellular mechanisms of cadmium-induced toxicity: A review. Int. J. Environ. Health Res. 2014, 24, 378–399. [Google Scholar] [CrossRef] [PubMed]
- Flees, J.; Rajaei-Sharifabadi, H.; Greene, E.; Beer, L.; Hargis, B.M.; Ellestad, L.; Porter, T.; Donoghue, A.; Bottje, W.G.; Dridi, S. Effect of Morinda citrifolia (noni)-enriched diet on hepatic heat shock protein and lipid metabolism-related genes in heat stressed broiler chickens. Front. Physiol. 2017, 8, 919. [Google Scholar] [CrossRef] [PubMed]
- Wei, H.; Zhang, R.; Su, Y.; Bi, Y.; Li, X.; Zhang, X.; Li, J.; Bao, J. Effects of acute cold stress after long-term cold stimulation on antioxidant status, heat shock proteins, inflammation and immune cytokines in broiler heart. Front. Physiol. 2018, 9, 1589. [Google Scholar] [CrossRef]
- Nguyen, P.H.; Greene, E.; Kong, B.W.; Bottje, W.; Anthony, N.; Dridi, S. Acute heat stress alters the expression of orexin system in quail muscle. Front. Physiol. 2017, 8, 1079. [Google Scholar] [CrossRef] [PubMed]
- Su, Y.; Wei, H.; Bi, Y.; Wang, Y.; Zhao, P.; Zhang, R.; Li, X.; Li, J.; Bao, J. Pre-cold acclimation improves the immune function of trachea and resistance to cold stress in broilers. J. Cell. Physiol. 2019, 234, 7198–7212. [Google Scholar] [CrossRef]
- Marsell, R.; Einhorn, T.A. The biology of fracture healing. Injury 2011, 42, 551–555. [Google Scholar] [CrossRef]
- Glass, G.E.; Chan, J.K.; Freidin, A.; Feldmann, M.; Horwood, N.J.; Nanchahal, J. TNF-α promotes fracture repair by augmenting the recruitment and differentiation of muscle-derived stromal cells. Proc. Natl. Acad. Sci. USA 2011, 108, 1585–1590. [Google Scholar] [CrossRef]
- Lange, J.; Sapozhnikova, A.; Lu, C.; Hu, D.; Li, X.; Miclau, T., III; Marcucio, R.S. Action of IL-1β during fracture healing. J. Orthop. Res. 2010, 28, 778–784. [Google Scholar]
- Simon, A.M.; Manigrasso, M.B.; O’Connor, J.P. Cyclo-oxygenase 2 function is essential for bone fracture healing. J. Bone Miner. Res. 2002, 17, 963–976. [Google Scholar] [CrossRef]
- De Lecea, L.; Kilduff, T.S.; Peyron, C.; Gao, X.B.; Foye, P.E.; Danielson, P.E.; Fukuhara, C.; Battenberg, E.L.F.; Gautvik, V.T.; Bartlett, F.S., II; et al. The hypocretins: Hypothalamus-specific peptides with neuroexcitatory activity. Proc. Natl. Acad. Sci. USA 1998, 95, 322–327. [Google Scholar] [CrossRef] [PubMed]
- Kukkonen, J.P.; Leonard, C.S. Orexin/hypocretin receptor signalling cascades. Br. J. Pharmacol. 2014, 171, 314–331. [Google Scholar] [CrossRef] [PubMed]
- Carter, M.E.; Adamantidis, A.; Ohtsu, H.; Deisseroth, K.; de Lecea, L. Sleep homeostasis modulates hypocretin-mediated sleep-to-wake transitions. J. Neurosci. 2009, 29, 10939–10949. [Google Scholar] [CrossRef] [PubMed]
- Boutrel, B.; Cannella, N.; de Lecea, L. The role of hypocretin in driving arousal and goal-oriented behaviors. Brain Res. 2010, 1314, 103–111. [Google Scholar] [CrossRef] [PubMed]
- Lassiter, K.; Greene, E.; Piekarski, A.; Faulkner, O.B.; Hargis, B.M.; Bottje, W.; Dridi, S. Orexin system is expressed in avian muscle cells and regulates mitochondrial dynamics. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2015, 308, 173–187. [Google Scholar] [CrossRef]
- Blankenship, K.; Gilley, A.; Piekarski, A.; Orlowski, S.; Greene, E.; Bottje, W.; Anthony, N.; Dridi, S. Differential expression of feeding-related hypothalamic neuropeptides in the first generation of quails divergently selected for low or high feed efficiency. Neuropeptides 2016, 58, 31–40. [Google Scholar] [CrossRef]
- Greene, E.; Khaldi, S.; Ishola, P.; Bottje, W.; Ohkubo, T.; Anthony, N.; Dridi, S. Heat and oxidative stress alter the expression of orexin and its related receptors in avian liver cells. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2016, 191, 18–24. [Google Scholar] [CrossRef]
- Scholz, B.; Rönchen, S.; Hamann, H.; Hewicker-Trautwein, M.; Distl, O. Keel bone condition in laying hens: A histological evaluation of macroscopically assessed keel bones. Berl. Munch. Tierarztl. Wochenschr. 2008, 121, 89–94. [Google Scholar]
- Casey-Trott, T.M.; Heerkens, J.L.T.; Petrik, M.; Regmi, P.; Schrader, L.; Toscano, M.J.; Widowski, T.M. Methods for assessment of keel bone damage in poultry. Poult. Sci. 2015, 94, 2339–2350. [Google Scholar] [CrossRef]
- Schmittgen, T.D.; Livak, K.J. Analyzing real-time PCR data by the comparative CT method. Nat. Protoc. 2008, 3, 1101–1108. [Google Scholar] [CrossRef]
- Casey-Trott, T.M.; Widowski, T.M. Behavioral differences of laying hens with fractured keel bones within furnished cages. Front. Vet. Sci. 2016, 3, 42. [Google Scholar] [CrossRef] [PubMed]
- Odihambo Mumma, J.; Thaxton, J.P.; Vizzier-Thaxton, Y.; Dodson, W.L. Physiological stress in laying hens. Poult. Sci. 2006, 85, 761–769. [Google Scholar] [CrossRef] [PubMed]
- Backus, B.L.; McGlone, J.J.; Guay, K. Animal Welfare: Stress, Global Issues, and Perspectives. In Encyclopedia of Agriculture and Food Systems; Alfen, N.K.V., Ed.; Elsevier: San Diego, CA, USA, 2014; Volume 3, pp. 387–402. [Google Scholar]
- Moberg, G.P. Biological response to stress: Implications for animal welfare. In The Biology of Animal Stress: Basic Principles and Implications for Animal Welfare; Moberg, G.P., Mench, J.A., Eds.; CABI Publishing: Oxon, UK, 2000; pp. 1–12. [Google Scholar]
- DeRoos, R. The corticoids of the avian adrenal gland. Gen. Comp. Endocrinol. 1961, 1, 494–512. [Google Scholar] [CrossRef]
- Pitk, M.; Tilgar, V.; Kilgas, P.; Mänd, R. Acute stress affects the corticosterone level in bird eggs: A case study with great tits (Parus major). Horm. Behav. 2012, 62, 475–479. [Google Scholar] [CrossRef] [PubMed]
- Houshmand, M.; Azhar, K.; Zulkifli, I.; Bejo, M.H.; Kamyab, A. Effects of prebiotic, protein level, and stocking density on performance, immunity, and stress indicators of broilers. Poult. Sci. 2012, 91, 393–401. [Google Scholar] [CrossRef] [PubMed]
- Najafi, P.; Zulkifli, I.; Jajuli, N.A.; Farjam, A.S.; Ramiah, S.K.; Amir, A.A.; O’Reily, E.; Eckersall, D. Environmental temperature and stocking density effects on acute phase proteins, heat shock protein 70, circulating corticosterone and performance in broiler chickens. Int. J. Biometeorol. 2015, 59, 1577–1583. [Google Scholar] [CrossRef] [PubMed]
- Bernabò, P.; Rebecchi, L.; Jousson, O.; Martínez-Guitarte, J.L.; Lencioni, V. Thermotolerance and hsp70 heat shock response in the cold-stenothermal chironomid Pseudodiamesa branickii (NE Italy). Cell Stress Chaperones 2011, 16, 403–410. [Google Scholar] [CrossRef]
- Itoh, H.; Komatsuda, A.; Ohtani, H.; Wakui, H.; Imai, H.; Sawada, K.I.; Otaka, M.; Ogura, M.; Suzuki, A.; Hamada, F. Mammalian HSP60 is quickly sorted into the mitochondria under conditions of dehydration. Eur. J. Biochem. 2002, 269, 5931–5938. [Google Scholar] [CrossRef]
- Gupta, S.C.; Sharma, A.; Mishra, M.; Mishra, R.K.; Chowdhuri, D.K. Heat shock proteins in toxicology: How close and how far? Life Sci. 2010, 86, 377–384. [Google Scholar] [CrossRef]
- Chen, J.X.; Meyrick, B. Hypoxia increases Hsp90 binding to eNOS via PI3K-Akt in porcine coronary artery endothelium. Lab. Invest. 2004, 84, 182. [Google Scholar] [CrossRef]
- Yang, Z.; Liu, C.; Zheng, W.; Teng, X.; Li, S. The functions of antioxidants and heat shock proteins are altered in the immune organs of selenium-deficient broiler chickens. Biol. Trace. Elem. Res. 2016, 169, 341–351. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.S.; Wu, R.W.; Ko, J.Y.; Tai, M.H.; Ke, H.C.; Yeh, D.W.; Wu, S.L.; Chen, M.W. Heat shock protein 60 protects skeletal tissue against glucocorticoid-induced bone mass loss by regulating osteoblast survival. Bone 2011, 49, 1080–1089. [Google Scholar] [CrossRef] [PubMed]
- Yao, Y.; Watson, A.D.; Ji, S.; Boström, K.I. Heat shock protein 70 enhances vascular bone morphogenetic protein-4 signaling by binding matrix Gla protein. Circ. Res. 2009, 105, 575–584. [Google Scholar] [CrossRef] [PubMed]
- Price, J.T.; Quinn, J.M.; Sims, N.A.; Vieusseux, J.; Waldeck, K.; Docherty, S.E.; Myers, D.; Nakamura, A.; Waltham, M.C.; Gillespie, M.T.; et al. The heat shock protein 90 inhibitor, 17-allylamino-17-demethoxygeldanamycin, enhances osteoclast formation and potentiates bone metastasis of a human breast cancer cell line. Cancer Res. 2005, 65, 4929–4938. [Google Scholar] [CrossRef]
- Einhorn, T.A. The science of fracture healing. J. Orthop. Trauma 2005, 19, 4–6. [Google Scholar] [CrossRef]
- Xie, C.; Ming, X.; Wang, Q.; Schwarz, E.M.; Guldberg, R.E.; O’Keefe, R.J.; Zhang, X. COX-2 from the injury milieu is critical for the initiation of periosteal progenitor cell mediated bone healing. Bone 2008, 43, 1075–1083. [Google Scholar] [CrossRef]
- Miranda, B.; Esposito, V.; de Girolamo, P.; Sharp, P.J.; Wilson, P.W.; Dunn, I.C. Orexin in the chicken hypothalamus: Immunocytochemical localisation and comparison of mRNA concentrations during the day and night, and after chronic food restriction. Brain Res. 2013, 1513, 34–40. [Google Scholar] [CrossRef]
- De Oliveira, C.V.; Ciriello, J. Cardiovascular responses to hypocretin-1 in nucleus ambiguus of the ovariectomized female rat. Brain Res. 2003, 986, 148–156. [Google Scholar] [CrossRef]
- Ciriello, J.; Li, Z.; de Oliveira, C.V. Cardioacceleratory responses to hypocretin-1 injections into rostral ventromedial medulla. Brain Res. 2003, 991, 84–95. [Google Scholar] [CrossRef]




| Gene | Reference Sequence | Primer Sequences (5′-3′) |
|---|---|---|
| Hsp60 | NM_001012916.1 | Forward: AGCCAAAGGGCAGAAATG Reverse: TACAGCAACAACCTGAAGACC |
| Hsp70 | NM_001006685.1 | Forward: CGGGCAAGTTTGACCTAA Reverse: TTGGCTCCCACCCTATCTCT |
| Hsp90 | NM_001109785.1 | Forward: TCCTGTCCTGGCTTTAGTTT Reverse: AGGTGGCATCTCCTCGGT |
| TNF-α | NM_204267 | Forward: GCCCTTCCTGTAACCAGATG Reverse: ACACGACAGCCAAGTCAACG |
| COX-2 | NM_001167718 | Forward: TGTCCTTTCACTGCTTTCCAT Reverse: TTCCATTGCTGTGTTTGAGGT |
| PTGEs | NM_001194983.1 | Forward: GTTCCTGTCATTCGCCTTCTAC Reverse: CGCATCCTCTGGGTTAGCA |
| NF-κBp65 | NM_205134 | Forward: TCAACGCAGGACCTAAAGACAT Reverse: GCAGATAGCCAAGTTCAGGATG |
| iNOS | NM_204961.1 | Forward: CCTGGAGGTCCTGGAAGAGT Reverse: CCTGGGTTTCAGAAGTGGC |
| IL-1β | NM_204524.1 | Forward: ACTGGGCATCAAGGGCTACA Reverse: GCTGTCCAGGCGGTAGAAGA |
| ORX | AB056748 | Forward: CCAGGAGCACGCTGAGAAG Reverse: CCCATCTCAGTAAAAGCTCTTTGC |
| ORXR1 | NM_205505 | Forward: TGCGCTACCTCTGGAAGGA Reverse: GCGATCAGCGCCCATTC |
| ORXR2 | AF408407 | Forward: AAGTGCTGAAGCAACCATTGC Reverse: AAGGCCACACTCTCCCTTCTG |
| GAPDH | NM_204305.1 | Forward: GCACGCCATCACTATCTTCC Reverse: CATCCACCGTCTTCTGTGTG |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wei, H.; Li, C.; Xin, H.; Li, S.; Bi, Y.; Li, X.; Li, J.; Zhang, R.; Bao, J. Keel Fracture Causes Stress and Inflammatory Responses and Inhibits the Expression of the Orexin System in Laying Hens. Animals 2019, 9, 804. https://doi.org/10.3390/ani9100804
Wei H, Li C, Xin H, Li S, Bi Y, Li X, Li J, Zhang R, Bao J. Keel Fracture Causes Stress and Inflammatory Responses and Inhibits the Expression of the Orexin System in Laying Hens. Animals. 2019; 9(10):804. https://doi.org/10.3390/ani9100804
Chicago/Turabian StyleWei, Haidong, Chun Li, Hongwei Xin, Shuang Li, Yanju Bi, Xiang Li, Jianhong Li, Runxiang Zhang, and Jun Bao. 2019. "Keel Fracture Causes Stress and Inflammatory Responses and Inhibits the Expression of the Orexin System in Laying Hens" Animals 9, no. 10: 804. https://doi.org/10.3390/ani9100804
APA StyleWei, H., Li, C., Xin, H., Li, S., Bi, Y., Li, X., Li, J., Zhang, R., & Bao, J. (2019). Keel Fracture Causes Stress and Inflammatory Responses and Inhibits the Expression of the Orexin System in Laying Hens. Animals, 9(10), 804. https://doi.org/10.3390/ani9100804






