4.1. Minerals Balance
Based on the values obtained in this study, dietary treatments had significant effects on Cu, Se, Zn, and Fe balance among treatment groups. The feeding of 5% corn along with 70.3% PKC or 10% corn with 65.3% PKC resulted in higher Cu intake, apparent absorption, retention, apparent mineral digestibility, and mineral balance compared to no corn diets (T1). The increased consumption of the diets is due to the rise in Cu intake and this finding is in line with Oladokun et al. [
11]. The increase in dietary Cu caused the groups difference to diminish over time, indicating that any group distinction in the metabolic process is probably saturated with high Cu intakes [
12]. With regards to the Cu balance, the amount of its excretion in fecal matter was greater than in urine due to the primary route of trace mineral excretion being through the feces [
13]. Generally, the quantity of Cu excreted in the fecal matter of lambs fed on T2 and T3 could transform Cu into a complex compound of protozoa, resulting in it being excreted less by lambs than lambs not fed the corn diet. Ivan et al. [
14] clarified that the increase in Cu bioavailability due to the elimination of ciliate protozoa from the rumen may amount to between 15% and 50%. However, Suttle [
15] reported that sulfur could be converted to sulfide by ruminal bacteria and could have a similar adverse effects on the bioavailability of Cu.
In our experiment, the intake, absorption, retention, apparent mineral digestibility, and mineral balance of Se was high in T3, which is a marked diet effect, since the high content of PKC improves the availability of Se [
16,
17]. However, the low absorption of Se in lambs is the result of a reduction of dietary Se to insoluble forms such as elemental Se or hydrogen selenide in the rumen environment [
17]. The differences in the absorption among the groups depends on the chemical form of Se and the composition of the diet [
16]. The amount of Se did not cause toxicity problems, and the maximum tolerable amount is 5 mg Se/kg DM [
18]. Most of the inorganic Se is not used immediately in the liver for selenoprotein synthesis and is quickly excreted via the urine [
17]. This explains the increase in the level of Se secretion in the urine of lambs in T1.
In the present experiment, Zn consumption, feces Zn excretion, apparent absorption, and retention in lambs fed with the corn supplemented diet were lower than that of the control group, but the apparent mineral digestibility of Zn (58.31%) and Zn balance (60.69%) was higher due to the increase in fecal Zn excretion and the overall mean of Zn excretion. However, the high intake of divalent cations such as Cu, Fe, and Ca reduced Zn absorption [
18]. The amount of Zn excreted via urine excretion was similar between lambs fed with corn and those on control diets. The results also show that the urinary excretion of Zn by lambs were generally higher than 1 mg/d with no effect on secretion due to its concentration in these diets [
18]. It was previously reported that dietary Zn reduced the accumulation of hepatic Cu and promotes the formation of relatively non-toxic forms of Cu in the liver, such as metallothionein, which is involved in the storage and detoxification of Cu and other heavy metals [
19,
20]. In the present experiment, the supplemented corn diet resulted in relatively low dietary Zn concentrations. Additionally, it was much less than 1 g/kg DM, leading to the lower feed intake and growth of lambs [
21,
22].
The intake of Fe by lambs fed with the treated diet decreased dramatically. McDowell [
23] reported that the massive amount of Zn and Fe intake could affect Cu utilization. High dietary Zn and Fe can reduce Cu absorption in cattle and sheep [
24,
25]. The primary routes of Fe excretion are via feces and urine [
23]. In this study, corn supplementation had an effect on the dietary level of Fe, which altered its absorption amount, resulting in a decrease in its absorbable intake. In any event, the increase in apparent Fe uptake tends to be associated with high Fe retention. It is likely that there exists a mineral binding protein in the PKC diet treated with corn, which is responsible for the decreased absorption of Fe via the process of maintaining its ions in a soluble form during its transfer from the lumen of the intestine into the cell of the intestinal wall. The concentration of Fe in PKC is known to range between 800 and 6000 mg/kg DM and was 640–1800 mg/kg DM in this study. The study by Al-Kirshi et al. [
26] indicated the probability of using a Fe diet to reduce the bioavailability of dietary Cu because of the high PKC content.
4.2. Hematological and Biochemical Parameters
The average concentrations of hemogram and biochemical indicators in the blood of lambs remained normal at the beginning of the experiment, which is based on average values for RBC concentrations in sheep (8–13 (× 10
6) RBC/mL) [
27]. The MCV values of the treatments were within the normal range of 23–48 fl [
28]. The variations observed agree with Daramola et al. [
29] who reported that age was a practical factor that has a significant effect on the Hb and RBC of goats, suggesting that the oxygen-carrying capacity of the blood was high in adult goats.
The unchanged PCV, MCV, and MCHC in lambs fed diets with or without corn is supported by the findings of Galıp [
30]. The variations in the blood profile occurred as time elapsed, which is due to changes in physiology resulting in the growth of ruminants [
29,
31] where it was found that the age of farm animals affects their hematological parameters.
The values of WBC, which can be used as an indicator of inflammation, were significantly different among groups. The mean concentration of WBC of the groups remained within the normal range (4000–12,000 × 10
9/L) [
27,
28]. The absence of any effect on B Neuts and S Neuts indicated that the source of protein has no effect on the blood profile. The results of this study showed that using PKC as a protein source had no adverse effects on the blood profile and this finding is supported by Nelson and Watkins [
32], who indicated that variations in protein sources might not influence the homeostatic mechanism. The lymphocytes, monocytes, and basophils were affected by the treatment, which increased in the group fed on T3. The reason for this is not clear and no corresponding information is available.
The serum ALT, ALP, and AST levels did not indicate significant differences between the treatment means. Total cholesterol levels were significantly different, and there was an increasing trend in T2 and T3, suggesting a dietary influence although the HDL and VLDL in the serum did not register any significant difference among the treatments, while LDL increased significantly in T3. This observation is explained by the fact that the cholesterol content in the serum has been used to assess the changes in lipid metabolism by feeding oil diets. The simultaneous increase in cholesterol and HDL levels in the serum of lambs with a corn-supplemented PKC diet in this study indicates that the inclusion was clearly reflected in the serum cholesterol level and that the increased cholesterol was mainly due to the increase in HDL level [
31,
33,
34]. The serum level of triglycerides and glucose in lambs fed with corn diets recorded non-significant variations among treatments. In addition, observed blood glucose concentrations were similar to those overseved by Turner et al. [
35], although variations in them could be affected by physiological status. In this study, the findings indicate that lambs fed on diets with corn in the PKC were in the normal energy status range. This could be a contributory factor for the lack of differences among treatments, and there were no negative effects on feed intake or the metabolism of the lambs. The serum metabolite values seen in this study were all within the normal range for sheep serum, as reported by Pampori [
36]. However, in spite of the higher figures recorded in this study, the corn-fed PKC diets did not have adverse effects on the health conditions of the lambs.
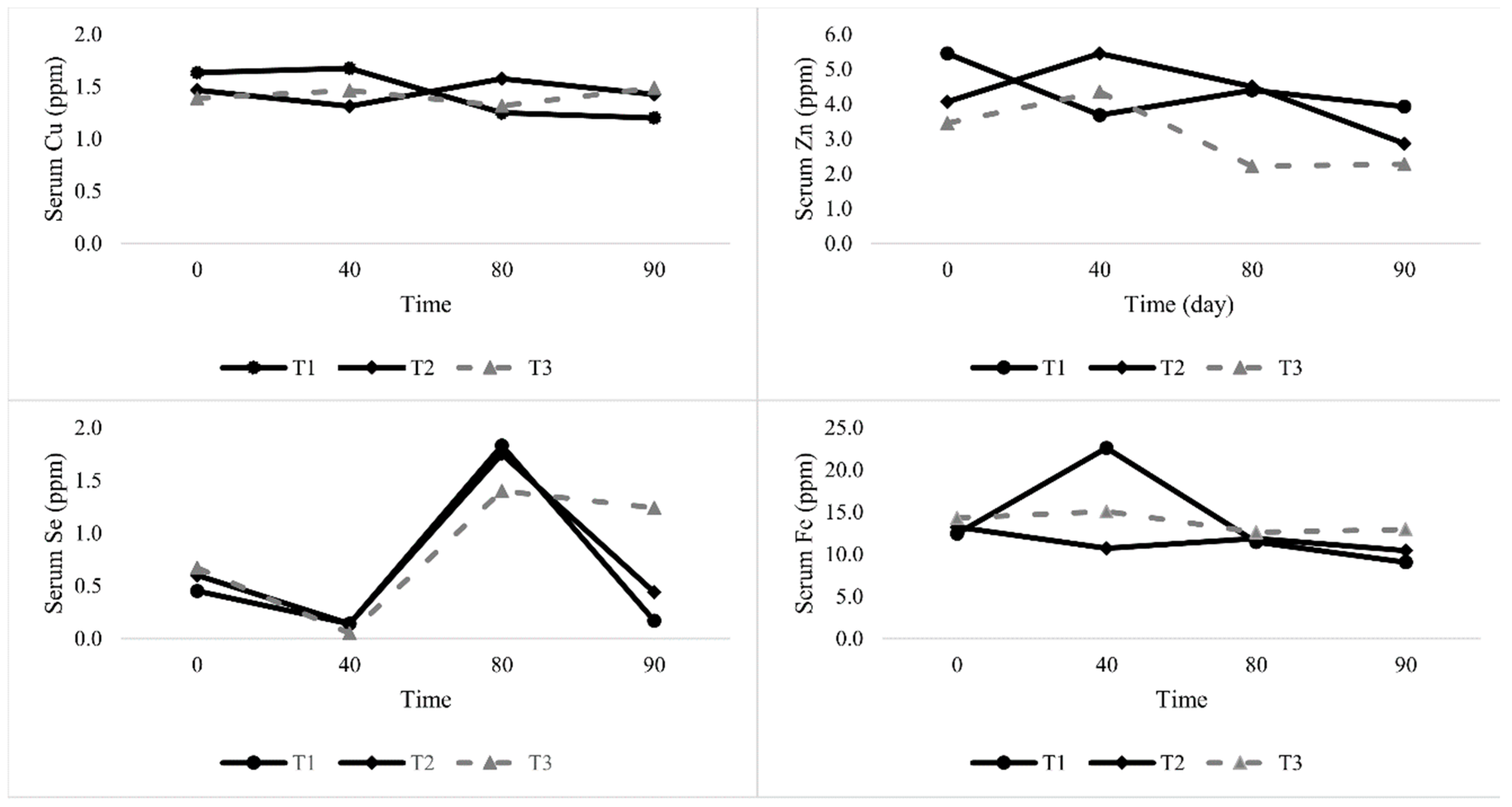
4.3. Mineral Serum
This research shows that the dietary inclusion with different corn levels affected the Cu, Se, Zn, and Fe content in the blood serum of lambs. It shows that the Cu concentration in the lambs’ serum was below critical levels, indicative of a sufficient dietary treatment. Therefore, the micro minerals’ mean value of serum Cu is similar to the findings of Hidiroglou et al. [
37] and Karim and Verma [
38]. The average Cu in the serum from experimental animals was near the reference values. The previous study showed no significant effect of feeding TMR with or without PKC on the Cu concentration in the serum [
4].
However, wide variations have been observed in the Se concentration among lambs fed on T3 and this is generally considered an indicator of body health. This linear relationship between blood Se and Se intake suggests that the former may be an adequate marker for Se status over a range of intakes. Chalabis–Mazurek and Walkuska [
39] noticed that Se supplementation distinctly depressed the Cu and Fe content in the serum and liver in lambs. Increased feed intake in T3, leading to high concentrations of Se in lambs, seems to be beneficial with respect to its function as an oxidant contained in glutathione peroxidase, an activity that is conditioned by the presence of Se.
Feeding PKC significantly increased Zn and Fe concentrations in the serum and this finding is in line with Abdelrahman et al. [
4]. No difference was seen in lambs receiving the experimental diets (T2 and T3) at day 40 and 80, but for lambs that were fed on T1 for 120 days, serum Zn concentrations increased, while being depressed in the treatment diets. The decline in Zn levels in T2 and T3 could be due to improvements in the immune body of the lambs fed on T2 and T3. Kargin et al. [
40] noted that Zn is an essential element required for the immunity function to play a role in responding to diseases. Active, synergistic, or antagonistic interrelationships between Fe and other minerals such as Cu and Zn have been documented [
41,
42,
43,
44]. Throughout the experiment, the content of the serum Fe fluctuated, showing an increasing tendency after 10 weeks of the supplementation treatment. However, statistically significant changes were noted in the lamb group that was given 10% corn supplementation. Accordingly, high intakes of Fe by lambs fed PKC could affect their hormonal activities, and consequently, affect their metabolism and health [
4]. This finding could provide a good indicator of the body health of animals. It has been reported that a high level intake of Cu, Mg, and Zn contributed to reducing the absorption of Fe [
45]. Further, trace minerals like Fe and Mn have also been shown to be Se antagonists [
46].
The concentration of all trace minerals reported in this study falls within the normal range, except for Fe values, which were found to be above the normal range in lambs fed with PKC with or without corn. Variations in the mineral composition of PKC among different scientists might be due to the type of soil, water, sampling techniques, and assay procedures [
47].