5.1. Behavioral Identification with Accelerometers
Information on how individual non-cage laying hens use space and the spatio-temporal variation in hens` space use is poorly understood. The dearth of information results from the methodological challenges inherent to describing individual animal behavior in group settings. By placing body-worn sensors on a representative proportion of the population and adopting analytical techniques used by other disciplines (e.g., Geographic Information Systems (GIS)), we may be able to better capture the responses of individuals housed in large groups. Modeling the spatial configuration of hen behaviors can provide insight into the general welfare of the individual. For example, feeding (consuming food from a feeder) and foraging (searching for and/or consuming food found in litter using the feet and beak) are both behaviors that hens are motivated to perform and are required for survival in natural settings [
32,
33]. Preening (
i.e., a maintenance and comfort behavior where feathers are cleaned with the beak) can be considered a comfort behavior and has been observed to be performed more often in the presence of familiar conspecifics [
34]. Additionally, based on previous studies, it is clear that hens make different choices with regard to where and when they choose to perform these welfare-relevant behaviors based upon their perception of the environment [
35,
36]. Technology that would allow identification of these behaviors in large groups (and their associated frequency) as well as influencing factors would allow for a paradigm shift in the way we evaluate animal welfare.
As one tool to identify behaviors remotely, accelerometers, or sensors that estimate acceleration along one or more axes, can be used to estimate velocity and displacement. Body-mounted accelerometers respond to acceleration resulting from movement of the individual as well as gravitational acceleration. When equipped appropriately, accelerometers have the potential to remotely detect performance of specific behaviors by individuals using movement data [
24]. Accelerometers have played an important role in detecting specific behaviors in animals including cattle [
37] and chickens [
38,
39] and in describing general activity levels in species such as dogs [
40], chickens [
41] and elephants [
42,
43].
The major challenge involved in using accelerometers to accurately detect the performance of specific behaviors in chickens is two-fold, both of which stem from the size of the subject. First, the size and weight of the sensor must not cause any significant change in the bird`s natural behavior, a topic that was discussed with greater detail in an earlier section (#4) of this manuscript. The limitation is not a problem in larger animals (e.g., cattle) and hence sensors equipped with better processing capabilities and larger batteries that increase the total size and weight can be used in these species without concern. Second, due to the relatively small size of hens and general movement patterns, the magnitude of acceleration produced by hens during movement is considerably smaller than that of larger animals and thus the state-space of accelerometer values obtained is relatively smaller. The brief and jittery movements produced by chickens result in a weak correlation between the accelerometer data and the activity of the individual and require their own unique protocols. To identify target sets of behavioral activities, specific machine learning mechanisms should be used on the features extracted from hens’ activity data.
Data from accelerometers have the following attributes: time, acceleration along the x-axis, acceleration along the y-axis, and possibly acceleration along the (third) z-axis though this review focuses on work conducted using a two-axis system. Two features, entropy and mean, can be extracted from each axis within a specified time window giving a total of four attributes for use in machine learning classification in a two-axis system. These two features are important for distinguishing different activities from one another by comparing their intensities and periodicities over time (
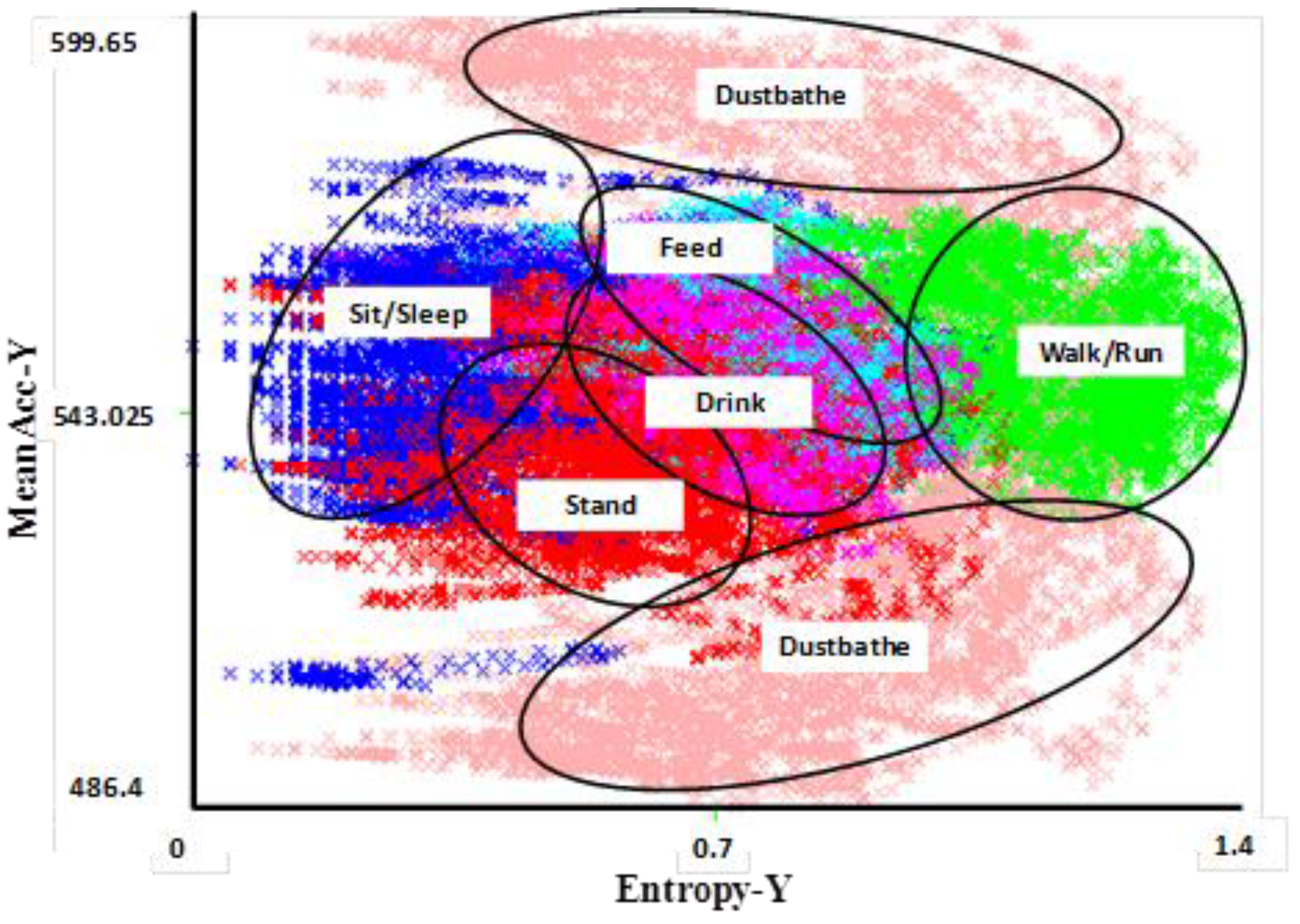
Figure 1). Generally, entropy represents the expected amount of information within a window and, the more intense the activity, the more entropy is recorded. The mean entropy is calculated as the average value within the time frame.
Banerjee
et al. [
38] sampled individual hen behavior using a body-mounted sensor at a rate of 10 Hz (
i.e., 100 ms sampling interval), and tested window sizes of 30 to 40 samples (
i.e., over 3 s and 4 s time periods, respectively, based on the sampling rate). A window was used for data analysis rather than raw accelerometer data, as windows can capture several cycles of the behavioral activity of interest within a single sample (e.g., multiple steps during walking or sequences of pecking during feeding or drinking). Overlapping windows can also improve detection accuracy. In their study, high intensity activities (e.g., walk/run) consistently reported higher entropy values than lower intensity activities (e.g., feed or drink). Similarly, there was a distinct difference in the entropy values between sitting/sleeping and feeding. The duration of individual data collection sessions varied from 30 s to 20 min depending upon the naturally occurring duration of the target activity. Data were collected until at least 15 min of data for each activity was obtained. For two behaviors that were difficult to induce in a timely manner and that occurred for very short durations, less data were obtained (dust bathing = data from 3 of 6 hens; drinking = 7 min total data). Data were labeled according to the activity being performed in a semiautomatic manner. In addition to data being collected by the sensor, a human observer recorded timing of the performance of specific activities. The acceleration data collected by the sensor between start and stop times noted by the observer for a certain activity were labeled accordingly. To avoid mislabeling of data due to human reaction time being slower than that of the sensor, data within 5 s of the start and end times of an activity were discarded. Labeled data from all hens were combined and 50% of the data was used to train the classifier. The remaining 50% of the data were used as a test data set.
Figure 1.
Visual clustering of activities in a 2D feature space for Y and X axis accelerometer data [
38]. The different behavioral activities appear as different distinct clusters, allowing them to accurately be distinguished from each other. The most difficult behaviors to distinguish are feeding and drinking, both of which involve a stationary body with pecking motion of the head (upwards in the case of drinking and downwards in the case of feeding). Data were collected from six sensor-wearing hens over several days to capture multiple performances of an activity by each hen. The clusters shown here correspond to classifications using the 50% test dataset.
Figure 1.
Visual clustering of activities in a 2D feature space for Y and X axis accelerometer data [
38]. The different behavioral activities appear as different distinct clusters, allowing them to accurately be distinguished from each other. The most difficult behaviors to distinguish are feeding and drinking, both of which involve a stationary body with pecking motion of the head (upwards in the case of drinking and downwards in the case of feeding). Data were collected from six sensor-wearing hens over several days to capture multiple performances of an activity by each hen. The clusters shown here correspond to classifications using the 50% test dataset.
In order to identify specific behaviors using entropy and acceleration, the accuracy of the software program used to classify the data as a particular behavior and the underlying criteria used to distinguish between behaviors of interest must be evaluated. In order to classify individual hen behavior, Banerjee
et al. [
38] evaluated the accuracy of multiple processing methodologies including: Decision Tree (J48), Neural Network, Radial Basis Function (RBF) Network and Naïve Bayes Tree. When behavioral classifications from these methodologies were compared with behavioral data obtained from watching video recordings of the sensor-wearing laying hens, the Neural Network approach using six hidden neurons provided the best results based on both windows of three and four seconds. This methodology was used for subsequent analyses of data generated by this sensor system.
A layer-classification approach can also utilize a hierarchical approach to classify sensor output collected from hens across multiple days. Banerjee
et al. [
38] used a 2-layer approach where the top-layer (Layer 1) classification separated behaviors based on whether they were static, dynamic, or indicative of resource use (
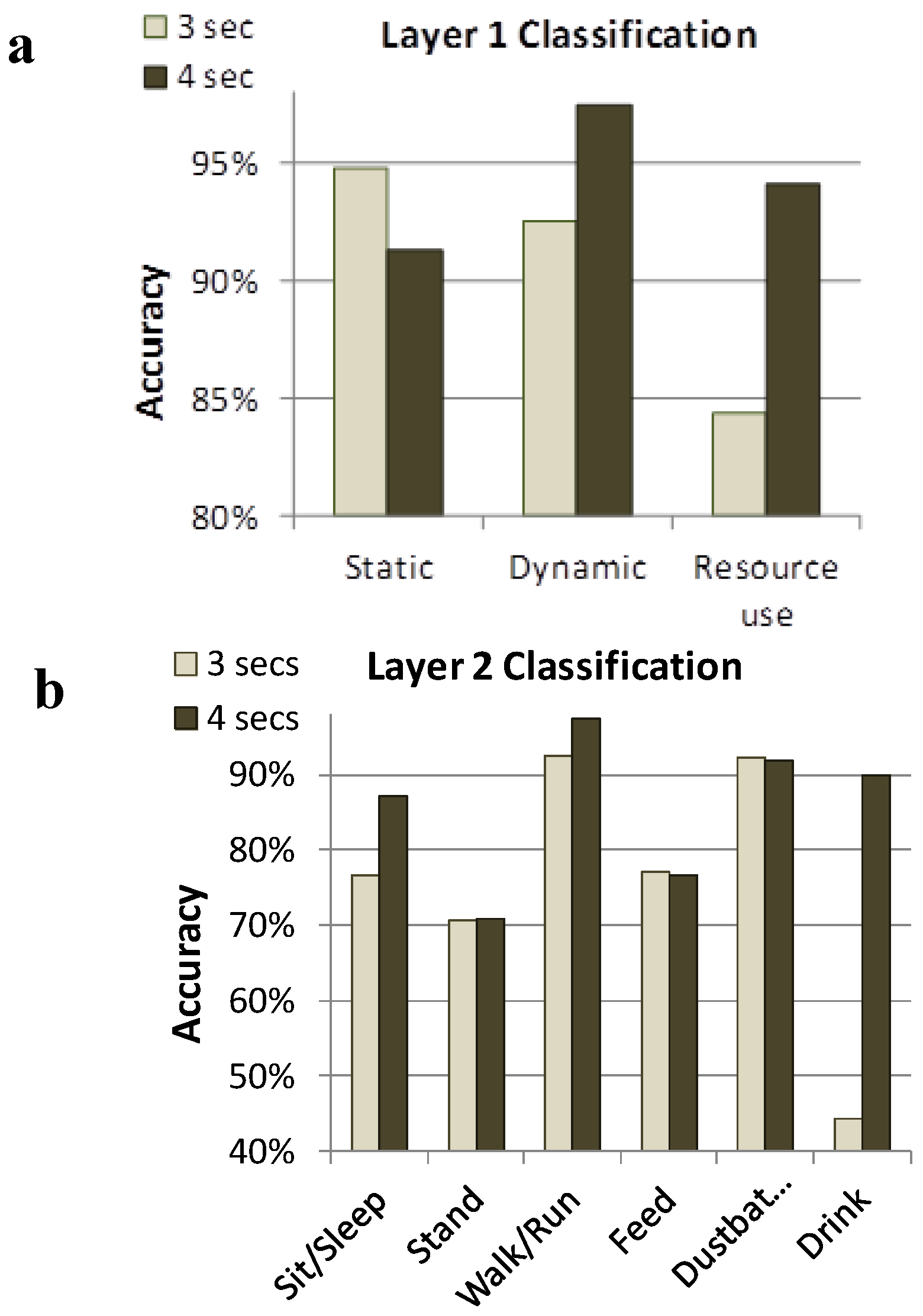
Figure 2). Within the three classifications used for Layer 1, a second layer (Layer 2) allowed discrimination of output into six separate activities: sit/sleep, stand, walk/run, feed, drink and dust bathe. In moving to Layer 2 classification, most of the misclassifications occurred within the individual classes identified in Layer 1, not between separate classes. For example, the majority of errors in detecting standing were caused by falsely classifying the behavior as sit, another behavior in the static class. Similarly, the majority of misclassifications of drinking data were as feed, another behavior in the resource use class. Using a tri-axis accelerometer to capture the z-axis would likely remedy some of these problems.
Figure 2.
Layer 1 (
a) and Layer 2 (
b) classification systems to remotely identify performance of behaviors within 3 and 4 seconds windows [
38]. Layer-1 accuracy was higher than Layer-2 accuracy, and in most cases, 4 s windows were more accurate than 3 s windows. Static behaviors (sit/sleep and stand) could accurately be distinguished from dynamic behaviors (walk and dust bathe) and both of these categories could be distinguished from resource use behaviors (feed and drink).
Figure 2.
Layer 1 (
a) and Layer 2 (
b) classification systems to remotely identify performance of behaviors within 3 and 4 seconds windows [
38]. Layer-1 accuracy was higher than Layer-2 accuracy, and in most cases, 4 s windows were more accurate than 3 s windows. Static behaviors (sit/sleep and stand) could accurately be distinguished from dynamic behaviors (walk and dust bathe) and both of these categories could be distinguished from resource use behaviors (feed and drink).
When combining the window size and classification criteria, Banerjee
et al. [
38] achieved a Layer 2 overall accuracy of 78.46% and 82.61% using the Neural Network in terms of correctly classifying the behaviors for the 3 s and 4 s windows, respectively. Therefore, accuracy increased with window size, however there is a cost that must be considered against the potential benefit. For example, if the sample window is too large, behaviors characterized by shorter durations may not be captured in isolation but the window will contain acceleration data related to fragments of preceding or following behaviors. Alternatively, if the window is too short, the sensor system may not generate enough information for accurate classification of the behavior by the software. Therefore, windows of varying length need to be tested when determining a sensor output algorithm that is appropriate for the target behavioral activities in question. In addition, there is some degree of variability in how individual hens perform even very fundamental motor patterns such as pecking and there is potential to misclassify behaviors with similar activity signatures (
Figure 2). Therefore, accuracy of behavioral detection by such a sensor may require further training of the system using more data from each individual.
5.2. Background, Radio Frequency Identification
Potential applications for RFID systems are numerous and can be used in a variety of contexts of which two are examined in this review: registration of passage through a pop hole and egg-laying in a nest box. In brief, RFID systems use a uniquely coded identifying unit, typically referred to as a transponder, which when in proximity to a powered antenna, is registered by a reading unit and the transponder identity sent to a central computer. For the purpose of this review, we have focused on applications where the location of a hen is registered within a defined area (i.e., nest box) or by linking multiple antennae to record movement from one defined area to another (i.e., veranda/free-range). Specific RFID systems will have varying capacities, including the proximity with which the transponder can be identified by the antennae and whether multiple transponders can be identified simultaneously. In terms of simultaneous registration, a high frequency (like 13.56 MHz versus the low frequency 134.2 kHz) can detect several transponders simultaneously on the same antenna with the help of an additional protocol, commonly referred to as an anti-collision protocol (e.g., International Organization for Standardization (ISO) #15693). In brief, several variations exist at present. The first operates where the transponder responds in a slightly changed frequency and the reader goes through each frequency to identify them. A second variation allows the transponders to answer with a specific time lap during which the reader tries to read them one after the other. A last variation allows the reader to tell those transponders that have been read to shut down for the ongoing reading in order to read all remaining transponders.
The RFID systems function where the transponder with its antenna is energized by the electro-magnetic field produced by the reader antennae, which then sends a uniquely coded signal to the receiving unit that decodes the transponder number alongside the time and date. In this way, the RFID systems have a major advantage over other systems in that the transponders (located on the bird) do not require an external power source and can therefore be minimized in size and weight. Assuming that the antennae are provided with uninterrupted electrical power, the system can continue collecting data for extended periods of time, e.g., the approximately 50 weeks of a laying cycle [
30,
44]. The RFID systems can generally be used as a mobile system, allowing for relatively easy installation at varied locations for a period of time, after which they can be disassembled, cleaned, and moved to another location. The system’s mobility is reduced in locations where the presence of water or metal can dampen the signal requiring additional setup time to ensure the transponder is read with the required accuracy. The system’s ability to transmit through these mediums, and thus the ease in setup, will vary with the transmitting frequency used. Lastly, the speed of the animal being tracked (or the attached transponder) is also a major consideration in deciding what type of RFID system should be used and their arrangement. Speed is particularly relevant for tracking passage in/out of areas (such as the pop hole) and is more thoroughly discussed below.
5.3. Range Use with RFID
Multiple groups have independently used RFID technology in order to record ranging behavior and the associated occurrence of hens passing through a pop hole within a commercial setting with similar but variable approaches [
30,
44,
45,
46,
47,
48,
49,
50,
51]. The effort by Gebhard-Henrich
et al. [
45] is described here in the greatest detail as the accuracy of the system was validated in an accompanying paper [
22], the system registered actual passage of hens through the pop hole
vs. presence in the pop hole alone (as with [
30]), and the setup minimized changes to the pop hole itself (as with [
46,
47,
48,
49]). A second validated system, also described in detail, that did not require adjustments to the pop hole dimensions is “
the wide electronic pop hole” [
50,
51]. Relative benefits and drawbacks of each system are discussed below.
Specifically for the effort by Gebhardt-Henrich [
45], a low frequency RFID system (Gantner pigeon system, Benzing, Schruns, Austria) (125 kHz) was used to study ranging behavior in 12 flocks of laying hens where the aim was to monitor individual bird usage of outdoor areas (verandas and free range areas) without altering pop hole number, dimensions, or management. Across the various flocks, between 5 and 10% of the birds were tracked. Antennae were placed on either side of each pop hole linking the house/veranda and veranda/free-range. The width of the pop holes ranged from 1.2 to 4.6 m and, depending on the size of the pop hole, up to 12 antennae were put side-by-side to cover the entire width of both sides of the pop hole. The inclusion of antennae on either side of the pop hole was necessary to determine the direction of a hen`s transition between two areas and therefore required registration of two events—both entrance into the pop hole in one area (e.g., inside the house) followed by exit from the pop hole into a second area (e.g., to the veranda).
An alternative effort by Thurner
et al. [
50] which registered pop hole usage of all birds in the flock with a system termed the “wide electronic pop hole” used a high frequency (13.54 MHz) RFID technology setup. The system was required to be inserted into the pop hole and tuned when positioned (tuning board ISC.MAT-A, Feig Electronics, Weilburg, Germany). Compared to the system used by Gebhardt-Henrich
et al. [
45], “
the wide electronic pop hole” uses two antennae to determine the hens` direction and is designed as a tunnel with a length of 1.0 m, a cross section of 0.35 m by 0.70 m, and a width that can be changed according to the pop hole size. The identification reliability was evaluated using video recordings over several days from the whole flock and resulted in an identification reliability of 97.6% for a width of 70 cm (n = 3,113 passages), 99.3% for a width of 55 cm (n = 606 passages) and 99.8% for a width of 40 cm (n = 582 passages) [
50]. In this study, hens that had lost the transponder or had a defective transponder were included in the reliability evaluation.
While an arrangement with RFID antennae on either side of the pop holes provides a high degree of coverage to ensure accurate registration of hens, it requires a relatively high number of antennae and associated hardware that unfortunately increases the purchase costs. For instance, a typical barn with 25,000 hens may have 40 pop holes and if each of those pop holes requires 12 antennae, the cost quickly becomes prohibitive. As an alternative that allows the relative benefits of RFIDs with reduced costs, researchers have employed a methodology using antennae that were placed in the pop hole itself. Using this configuration, Richards
et al. [
27] assessed half of a single flock (approximately 6000 birds in the surveyed group) while Thurner
et al. [
50] assessed all bird in small groups between 300 and 400 hens per group. While this configuration reduces the number antennae needed, the major drawback is that the system is unable to identify the hen’s direction of motion when leaving the pop hole and thus allows only recording of the hen’s presence in the pop hole. In other words, when a hen was registered by the antennae, the authors had no way of identifying whether it originated from inside or outside the house or where it returned to that originating location. Despite this drawback, the effort by Richards
et al. [
30] and Thurner [
50] had results which seemed to parallel that of Gebhardt-Henrich
et al. [
45] (e.g., all found a bimodal distribution in range use), thus the arrangement is an attractive option if needed.
Another variation that substituted placement of antennae on either side of the pop hole was employed by Hernandez
et al. [
46] who used light beam sensors in combination with an RFID configuration to register passage. The inclusion of the light beam sensors (PD70CNT12, Carlo Gavazzi Automation S.p.A, Italy) installed inside and outside of the pop hole passageways allowed for recording direction of hen movement (
i.e., in or out of the barn by registering a blockage of the beam) with a single antenna in groups of 200 [
46,
49] and 600 (unpublished data) laying hens. During the initial setup, it became apparent that the high motivation to gain access to the range area caused multiple problems. A large number of hens rushed to go through the pop holes at the same time creating a bottleneck, resulting in several missed RFID readings that were identified by parallel video observations. Comparing video recordings and RFID output found that, when birds got stuck in front of the pop holes, some birds at the back jumped over those stuck at the front resulting in a distance beyond the antennae`s reading range. Furthermore, when birds ran (rather than walked) through the pop holes, some failed to step on the antenna with the leg containing the transponder and were not registered. These problems were addressed in the final set-up by altering increasing the height and reducing the width of the pop holes. By setting the pop hole height at 30 cm above the ground, “bird jumping” was limited and encouraged birds to hop into the pop hole and land with both feet directly on the antenna ensuring an RFID reading. In addition, reducing the width of the pop hole limited access so that only one bird at a time could pass through the pop hole. Increasing the height and reducing the width of the pop holes increased the bottleneck effect, but since bird passage through the pop holes was better controlled, the data more accurately reflected bird movement. Besides affecting registration of hens, pop hole sizes may also affect the behavior of the hens. Thurner [
48] found with a system termed the “
narrow electronic pop hole”, which allowed only passage of a single bird at a time, that hens did not use these narrow pop holes to the same extent as relatively wider pop holes.
Other problems encountered with using RFID systems are birds that stay within reading distance of the antennas for extended periods of time, which can result in several hundred readings from the same bird during a single visit to the pop hole. The problem can be addressed with software modifications by reading RFID tags only once while a hen remains within the reading range [
46] or by restricting data to one reading every minute for each bird [
30]. The protocol can also be modified so that the collected data is filtered at a stage subsequent to actual transponder registration (
i.e., all registrations are recorded) allowing collected data to be continually re-assessed for the most appropriate filtration. A final problem is that a bird may pass through the pop hole but return (without leaving the reading range of the antenna), an event that would appear as the bird going outside several times but not coming back in, which relates to the pop hole usage problem described earlier. However, in this scenario, the use of light sensors can allow the operator to exclude consecutive OUT readings that do not have matched IN readings (or
vice versa). Another way to solve this problem is to have two RFID antennae with high reading rates and an algorithm that can identify when the bird is entering and leaving the pop hole from the same side [
48].
More generally, a major limitation of RFID systems used for research purposes such as these (with rapid movement of hens) is the velocity of the moving transponders over the antennae. In validation testing, accuracy of registration began to fall sharply for tags moving faster than 1.5 m/s, though adapting to a 32-bit system could increase the maximum speed to 3.2 m/s [
22]. In order to put this speed into context for likely applications, the median calculated speed for tagged brown hens on one particular farm when passing the pop holes was 1.5 m/s, but some hens reached more than 4.5 m/s [
22]. Thus, with a 32-bit system, most laying hen movements would be expected to register. The greater speed of hens could also decrease the likelihood of registration where wider gaits of fast-moving-hens might have moved the transponder outside the recording range of the 300 mm wide antenna, a problem mentioned earlier [
46]. In the work of Gebhardt-Henrich
et al. [
22], the probability of registration was higher when hens were leaving the house or the veranda than when they returned (leaving the house: 94.3%, returning: 83.5%, overall in terms of days where outdoor access was possible; Wilcoxon test
p < 0.0005, N = 10; from the veranda to the pasture: 94.8%, back to the veranda: 83%, Wilcoxon test
p < 0.0005, N = 10; [
22]). The speed of the hens passing the pop holes was probably higher when they returned to the veranda or the house because of frightening events or because the farmer chased them inside at the end of the day.
Probability of registration was also influenced by other attributes of the pop holes. For instance, hens were more likely registered when pop holes were reached through ramps than when they were on the same level as the floor of the house. Possible solutions include laying two antennas side-by-side (creating, in principle, a larger antennae), fixing transponders on both legs to increase the probability of registration, or use of an RFID system with a higher frequency. Furthermore, larger transponders and antennae have larger reading ranges, and the time the transponder needs to be read will vary with the frequency and protocol. Therefore, a person should calculate the time the transponder is within the antenna field and whether this time is long enough to complete one reading cycle.
5.4. Tracking Egg-Laying with RFID Systems
Although attempts have been made to develop nests for individual productivity recording of laying hens [
52,
53,
54], to the authors` knowledge, individual productivity within large layer flocks over extended periods has only been successfully tested with the use of trap nests or funnel nest boxes [
55,
56]. The funnel nest box is a single nest box that can be used in group housing systems for recording the individual laying behavior and performance as well as individual egg quality parameters since it allows an assignment of each egg to the hen (
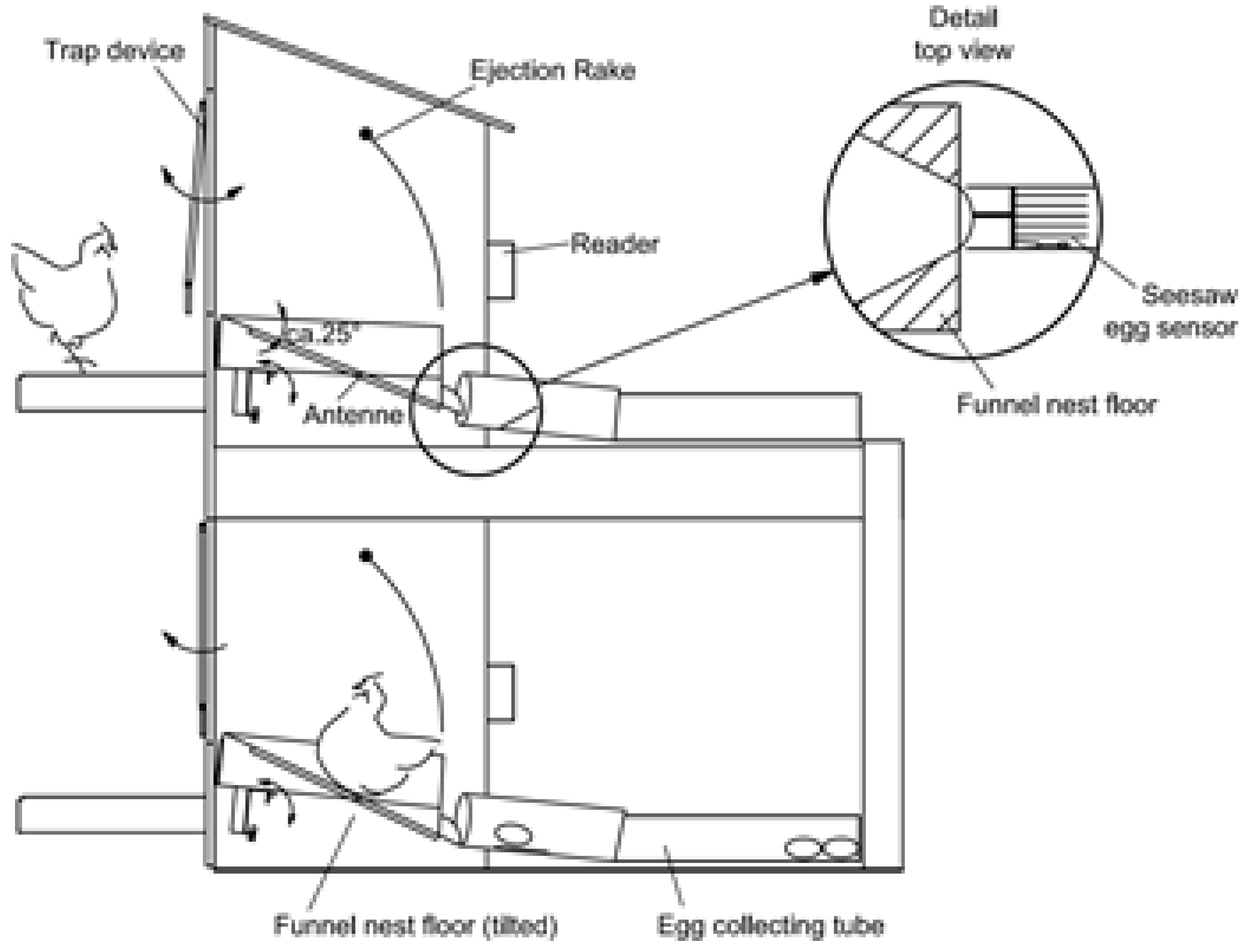
Figure 3). The funnel nest box is based on low frequency RFID technology (134.2 kHz) as described above (
Section 5.2). A trapezoid shaped antenna (length 240 mm, upper width 210 mm, lower width 100 mm) that reads the 23-mm glass transponder (Texas Instruments, HDX, ISO 11784/11785) on the hens’ leg is placed under the funnel floor. Each antenna is powered by a single, synchronized RFID-module, which initiates a reading at ten times per second. Four RFID-modules together with eight input channels for sensors (seesaw egg sensor and for double nest occupations, double tilt sensor) are combined to a fourfold reader unit [
57]. Up to 50 reader units can be connected via a RS485 bus-system to a central computer that sends registered data. The central computer controls the reader units, collects and analyses data with software packages specifically developed by researchers within the “Bayerische Landesanstalt für Landwirtschaft” in Germany. Though not available commercially, the software is being used for research purposes only.
Figure 3.
Schematic diagram of the funnel nest box [
55].
Figure 3.
Schematic diagram of the funnel nest box [
55].
When the system is operational, the hen enters the nest box via a trap device that is built with an entranceway composed of six aluminum rods which are suspended above the nest box in such a way that they can swing in and out of the nest box (
Figure 3). The trap device is responsible for separating the hens and for locking the nest box while occupied. After a hen has entered the nest box, the funnel floor tilts to the back, far enough to lock the trap device. Once closed, the trap can be opened (by the hen inside the nest box) by pushing it towards the outside of the nest box, a mechanism that also prevents hens from outside entering the locked nest box. A transponder, attached to the hen`s leg using a commercially available leg band (RoxanID, UK), is read by the antenna while the hen is in the nest box. The transponder signals are used to determine the number of nest box visits per day by each hen and the duration of each nest box visit. The system is capable of recognizing (by mass and sequence of transponder readings) when the nest is occupied by more than one hen. For this condition, the double tilting floor has to be adjusted to the lowest weight of a producing hen in the flock and will then register automatically if there are two or more hens in the nest box. The floor will tilt the first time when one hen is in the nest box locking the trap device. As soon as there is more than one hen in the nest box, the double tilting floor will tilt a second time (due to the greater weight) and press an attached spring sensor generating a signal that is recorded by the reader. Combining this signal and the sequence of transponder readings, up to 60% of the double nest occupations can be detected. From the undetected double nest occupations, 25% were critical, meaning that there was an egg laid. By choosing the rate of signals from the spring sensor or the minimum duration of the altering transponder readings, the researcher is able to make appropriate adjustments. The funnel-shaped nest floor is well tolerated by the hen and encourages her to assume a position with her head directed towards the nest exit causing the egg to roll from the nest immediately after laying (
Figure 4). The exit of the egg ensures that the egg of the current hen is not confused with that of the next hen.
Figure 4.
View of a hen entering the funnel nestbox (left) and positioning herself to lay an egg (right).
Figure 4.
View of a hen entering the funnel nestbox (left) and positioning herself to lay an egg (right).
After the egg exits the nest, it is registered by a seesaw egg sensor that is integrated into the egg collecting tube (100 mm diameter, closed pipe on either side of the seesaw egg sensor) where the eggs are stored in the order of lay for each nest box until the end of the daily laying time. The seesaw egg sensor also activates an attached spring sensor that is linked to a sensor input channel of the reader unit and thus registers the oviposition time for each egg. At the end of the daily laying time, the nest boxes are locked using a paddle inside the nest box. Using this system, each egg is assigned to the respective hen by combining the data from the transponder, seesaw egg sensor, and their relative order in the collecting tube.
To validate the system, video recordings were made of the hens while entering or leaving the funnel nest box and manually evaluated. Across the number of single nest boxes and size of the group housing section (4, 24 or 48 nest boxes per section), hen to nest box ratio (5.3 to 10.5 hens per nest box), and double occupation rate (0.0 to 10.5% of all recorded nesting events within a section), the reliability of correctly recording the hen’s nest entrance and exit varied between 76.1% and 97.8% (n = 2676 nest entrances and exits). The greatest reliability was associated with a hen to nest box ratio of less than seven and in flocks with less than a 5% double occupation rate. Adapting the system to allow identification of double nest occupations resulted in a correct assignment of the egg to the actual hen that exceeded 94% for all tested flocks which improved upon other efforts [
56].
It is possible that the system described [
55] may have influenced nesting behavior. For instance, laying hens using the funnel nest box appeared to stay longer in the nest box for each nest visit and show a lower number of nest box visits per day compared to a group nest box which is common in commercial housing systems (e.g., [
58]). Furthermore, the number of floor eggs can be on a higher but still acceptable level (
i.e., up to 5% but in extreme cases up to 10%) when using the funnel nest box compared to other designs [
56].
5.5. Radio Signal Strength (RSS)
Radio signal strength technology employs active transmission of radio frequency sensors equipped with radio signal strength indicators (RSSI) [
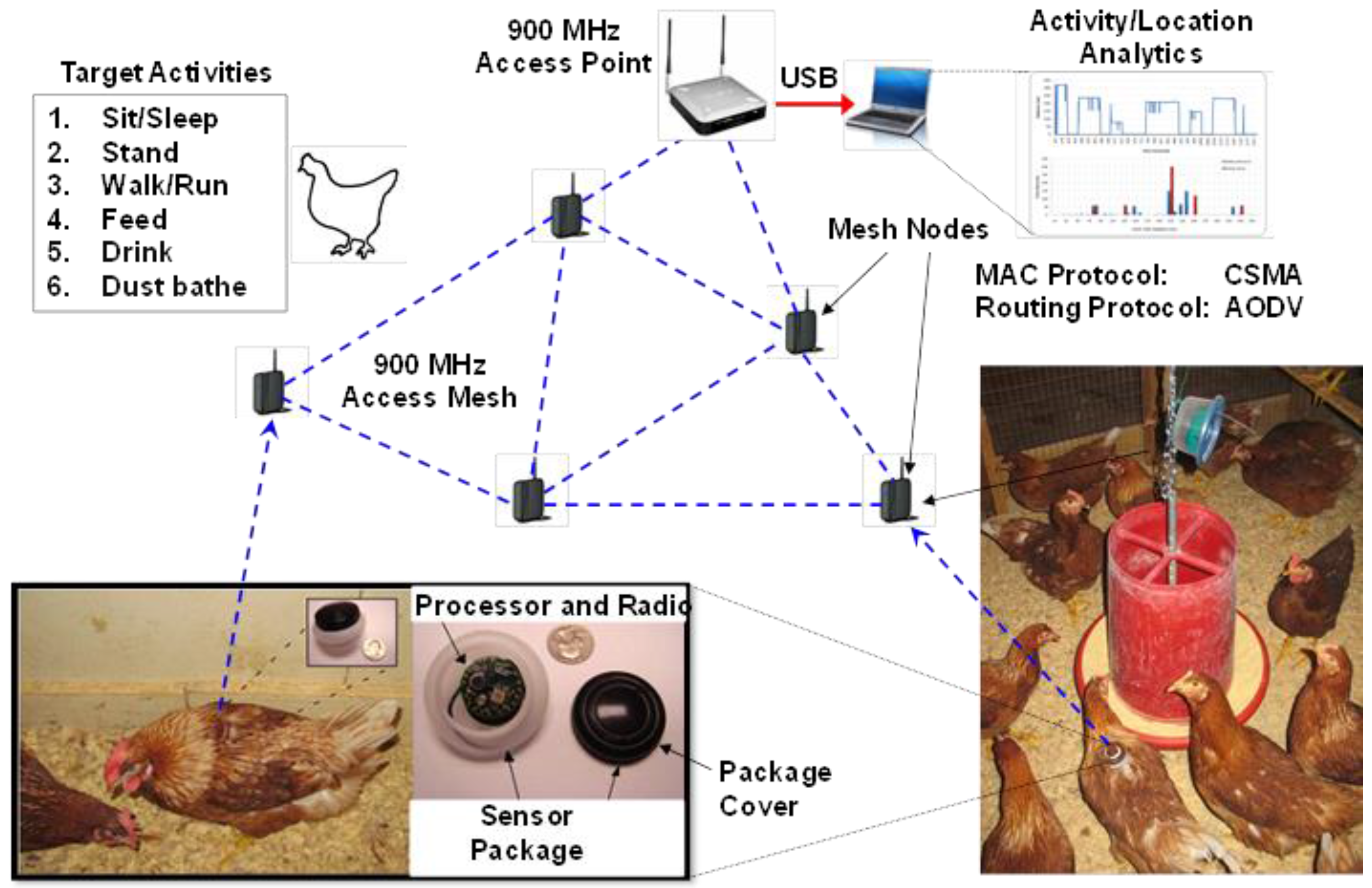
41]. In general, the system operates by devices (mounted on the backs of birds with a special harness) emitting a signal that is sensed by receiving units that register the signal strength which can then be used to estimate a hen`s relative location. The system discussed her used a 900 MHz radio frequency channel and a sensor card in a body-mountable package within a network arrangement (
Figure 5). The total weight of the sensor package was approximately 10 g and mounted on a hen’s back within a molded casing using a figure-eight nylon harness. The casing was colored to match hen feather color and painted with a unique number for easy visual identification. After experimenting with a number of other attachments, it was found that mounting the sensor on the back of the hen resulted in maximum sensor stability while maintaining sufficient 900 MHz radio signal quality and avoiding any tissue damage to the hen or changes in behavior [
24]. After attaching the sensor housing on the back, hens were allowed to acclimatize to wearing it for 48 h prior to beginning experiments. The sensor nodes ran on a 560 mAh button cell, which was able to support the system for over 50 hours on a fresh battery when used at an output radio transmission power of 5 dBm and sampling rate of 10 Hz (
i.e., a 100 ms sampling interval).
Figure 5.
Arrangement of the various components within the body-worn RSSI sensor system. Hens wear small (10 g) sensors on their backs attached via a figure eight harness. These sensors actively transmit information to stationary receivers, which communicate with a base station. Activity and location analyses are then performed using analytical software to classify accelerometer data into specific behavioral activities and locations relative to other hens and the stationary receivers.
Figure 5.
Arrangement of the various components within the body-worn RSSI sensor system. Hens wear small (10 g) sensors on their backs attached via a figure eight harness. These sensors actively transmit information to stationary receivers, which communicate with a base station. Activity and location analyses are then performed using analytical software to classify accelerometer data into specific behavioral activities and locations relative to other hens and the stationary receivers.
Using this technology, associated data analysis has been developed to track the location of non-cage laying hens [
38,
41]. This particular technology has also been used in conjunction with accelerometers to identify behaviors remotely, an application discussed in
Section 5.1. For the location detection as described by Quwaider
et al. [
41], the system was constantly transferring information to a base station and connected computer and thus no data was stored on the sensors worn by the hens. The constant stream of data was useful for providing instantaneous information about the hen’s location, but may not be practical in commercial implementation or where locations over large areas are monitored. Within this configuration, the area of detection for the bird-worn sensors with respect to stationary receivers was calibrated at one meter, though the stationary elements of the system are capable of a much larger range. The range was restricted to limit packet loss (
i.e., sets of data sent together from the mobile sensor to the base station) and missing data caused by interference of metal in the environment which radio signals are unable to penetrate. However, since detection fields radiate in a circular fashion from the center, stationary receivers sometimes had to be placed in such a way that areas of detection overlapped to avoid leaving areas where no detection was possible. This overlap created some confusion when analyzing locational output, as a hen could sometimes be detected in two locations simultaneously. In order to minimize this problem and ensure accurate signal transfer and detection, the antennae were suspended from the ceiling using PVC piping so they were one meter above the hens in a stable orientation with the antennae facing down towards the hens.
An additional problem with this body-mounted system was that the sensors worn by the birds were active transponders and therefore required a power source. Due to the small size of the hen, the sensor and power source must be small and lightweight, such as a watch battery, which provides limited usefulness. The longest data collection sessions were 52 hours, and, to achieve this duration, the sensor was programmed to collect data at a reduced frequency during the times when the hen was known to be less active (e.g., during the nighttime when the lights were off). In contrast, the transponders of the RFID system do not require a power source but provide a restrictive detection range that is limited to the near field of the antennae where the transponder is powered and read.
Several logistical challenges were also related to actually attaching the bird-worn sensor. The same hen would have to be identified and caught without a large flock disturbance each time the sensor system was deployed, and multiple sensor-wearing hens within a flock were needed to obtain a representative sample size. Placing the sensor on the hen’s head was an ideal location for technical success, however, behaviorally and practically, this was not feasible. The second location tested for sensor placement was around the wing, however, this orientation of the sensor caused the sensor to give a very strong signal when the hen was facing in one direction, and a weak signal when facing the opposite direction. In addition, the sensor would occasionally flip under the wing inhibiting the ability of the sensor to transmit information. Placing the sensor on the back of the hen was the most pragmatic solution, as this location provided maximum stability and signal quality without impacting the behavior or social interactions of the hen [
24]. However, care had to be taken that the sensor was oriented properly within the sensor casing to ensure good signal quality and accurate interpretation of the 3D space.