Food Deprivation, Body Weight Loss and Anxiety-Related Behavior in Rats
Abstract
:Simple Summary
Abstract
1. Introduction
2. Experimental Section
2.1. Animals
2.2. Experimental Design
2.2.1. Food Deprivation and Fixed-Time Feeding Schedule
2.2.2. Body Weight Measures
2.2.3. Behavioral Experiments
2.2.4. Modified Open Field (mOF) Test
2.2.5. Elevated Plus Maze (EPM) Test
2.2.6. Data Presentation and Analysis
3. Results
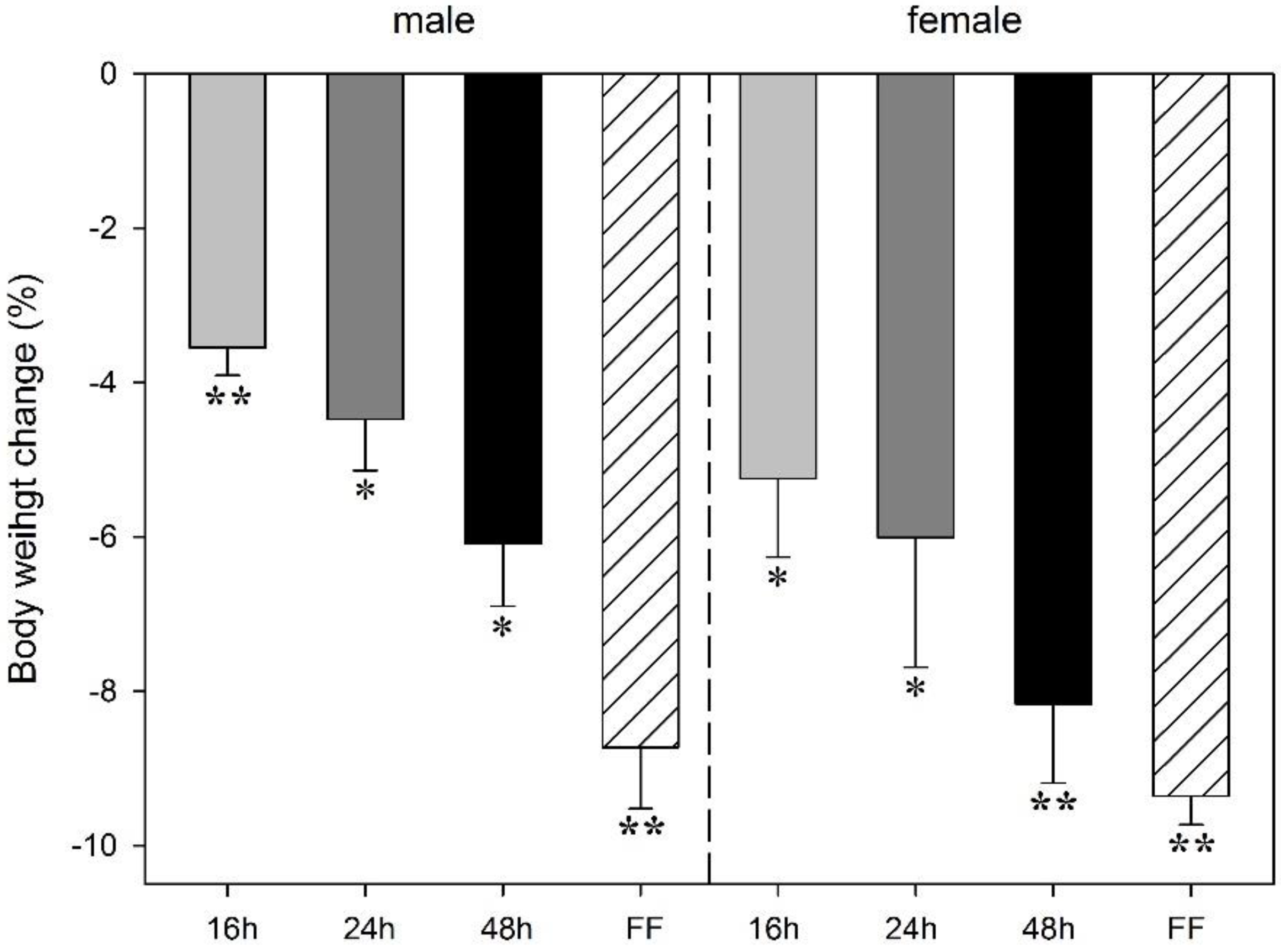
3.1. Effect of Food Deprivation and Fixed-Time Feeding Schedule on Body Weight Change
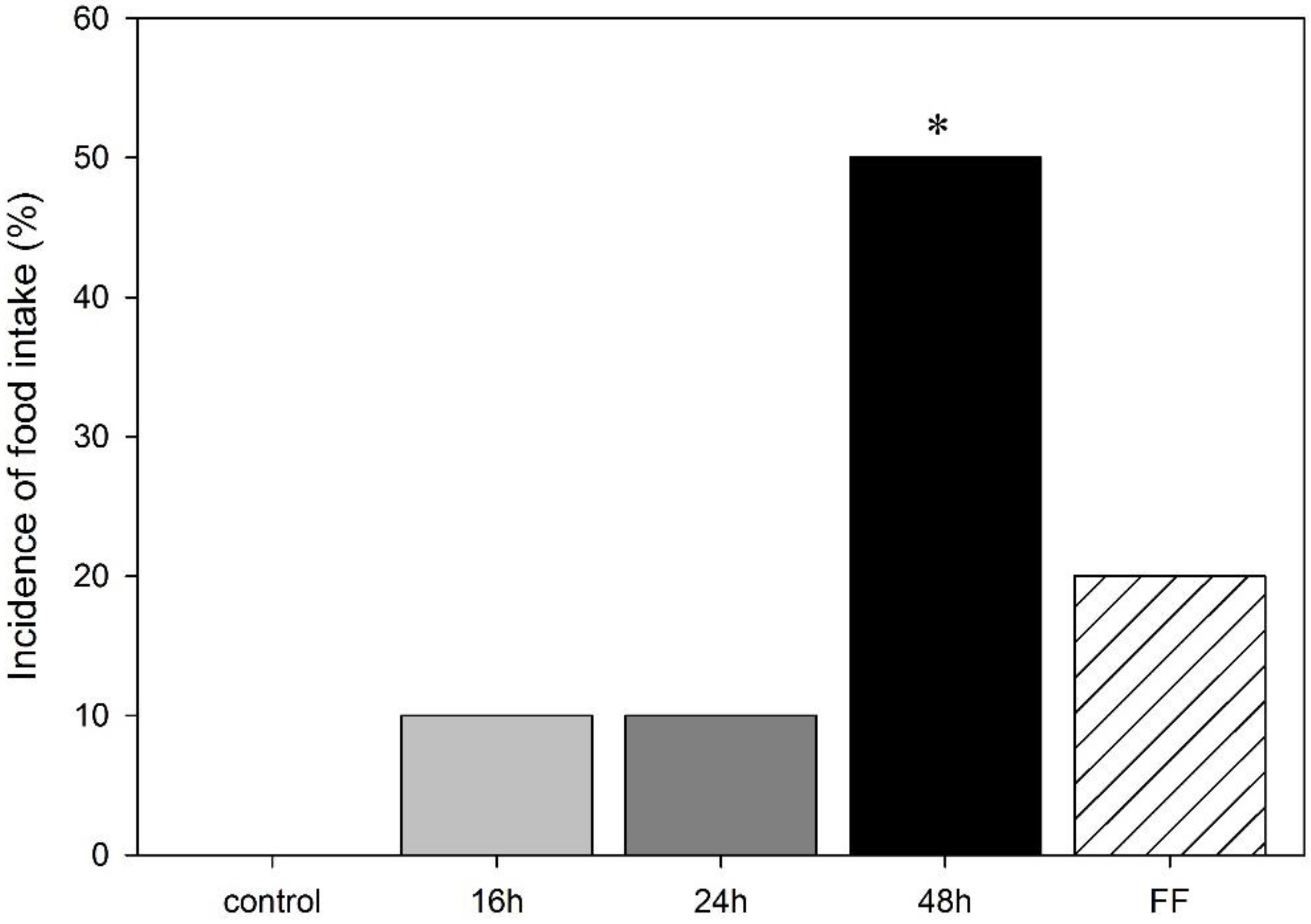
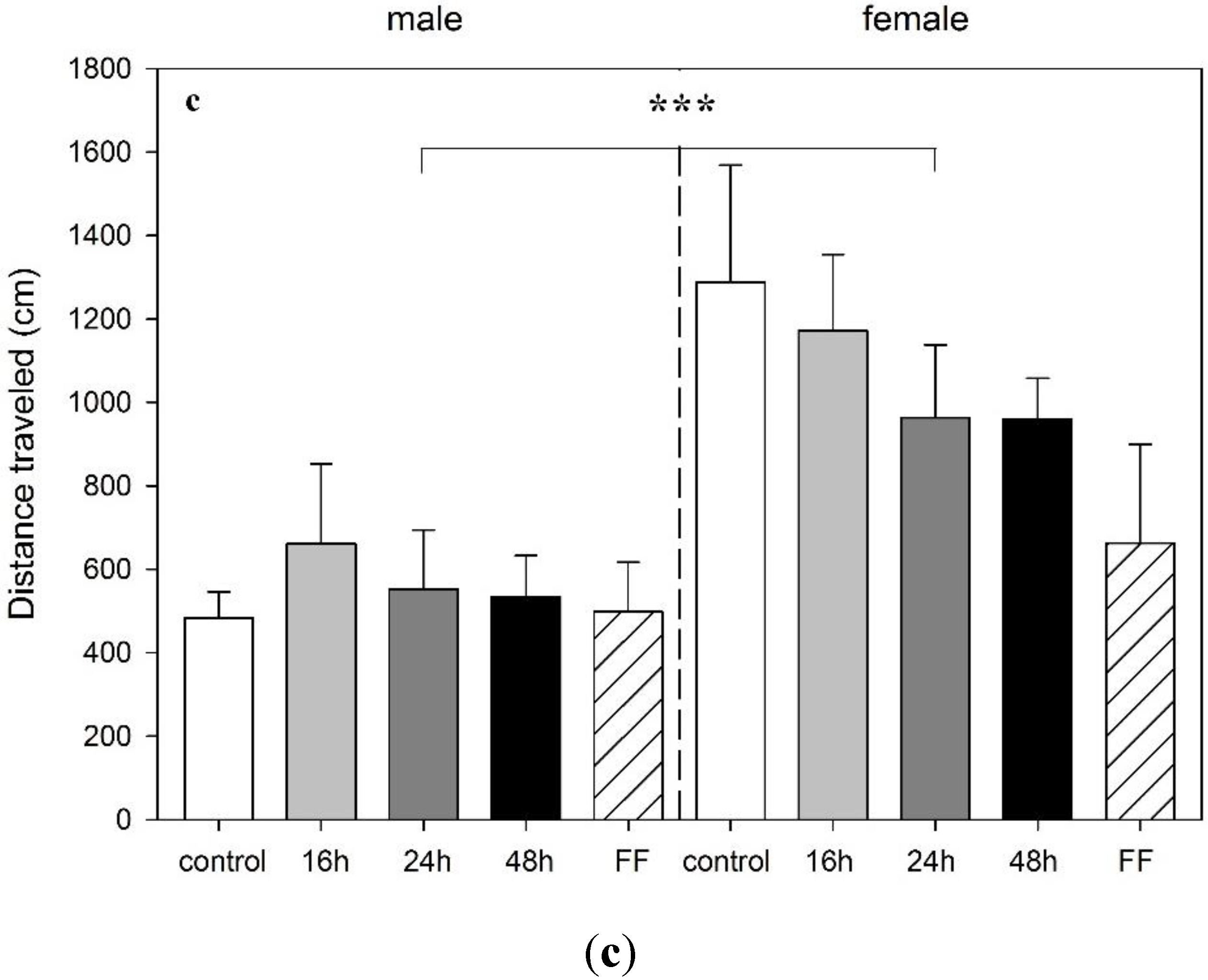
3.2. Effect of Food Deprivation and Fixed-Time Feeding Schedule on the Performance in the mOF Test
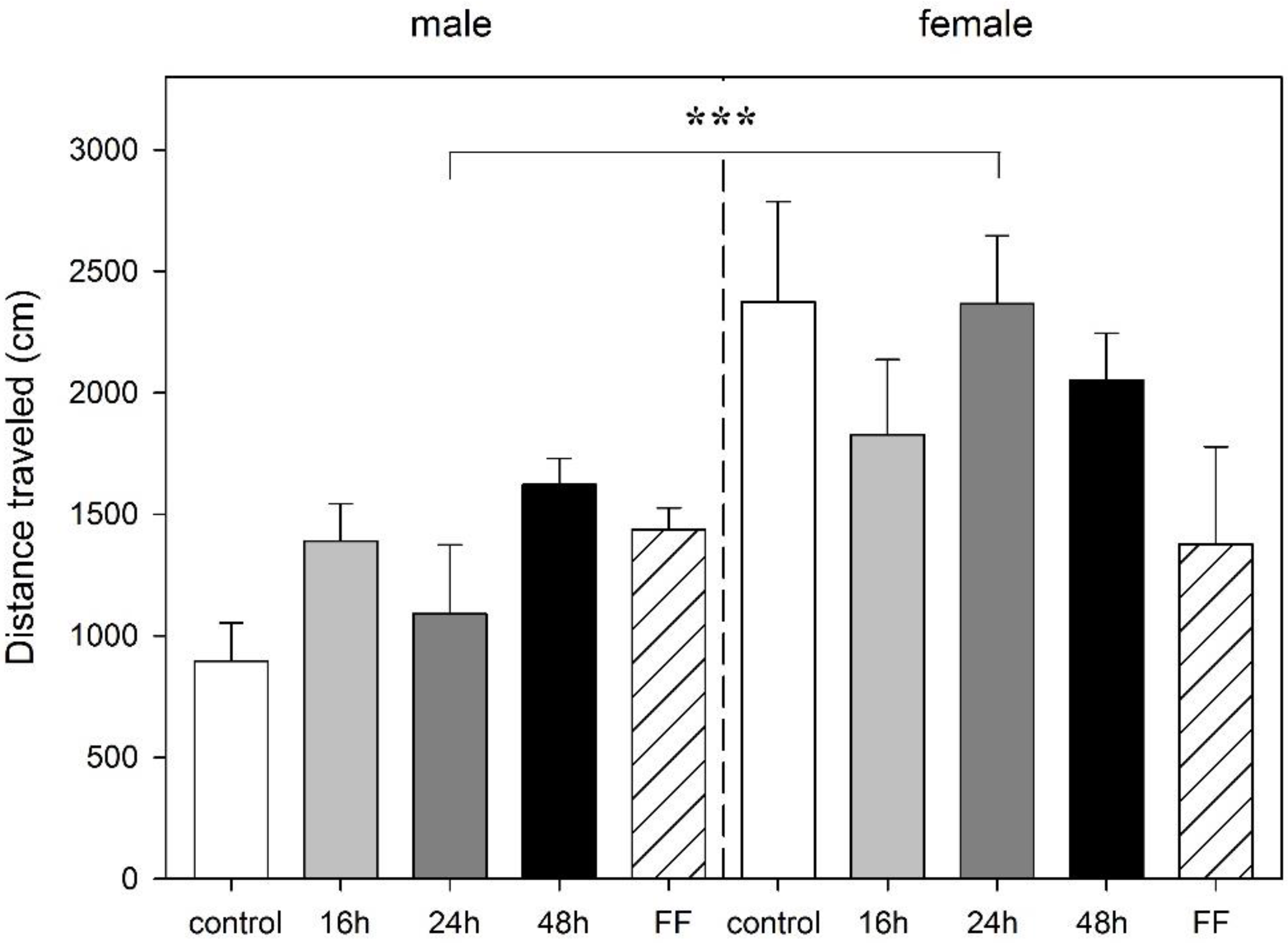
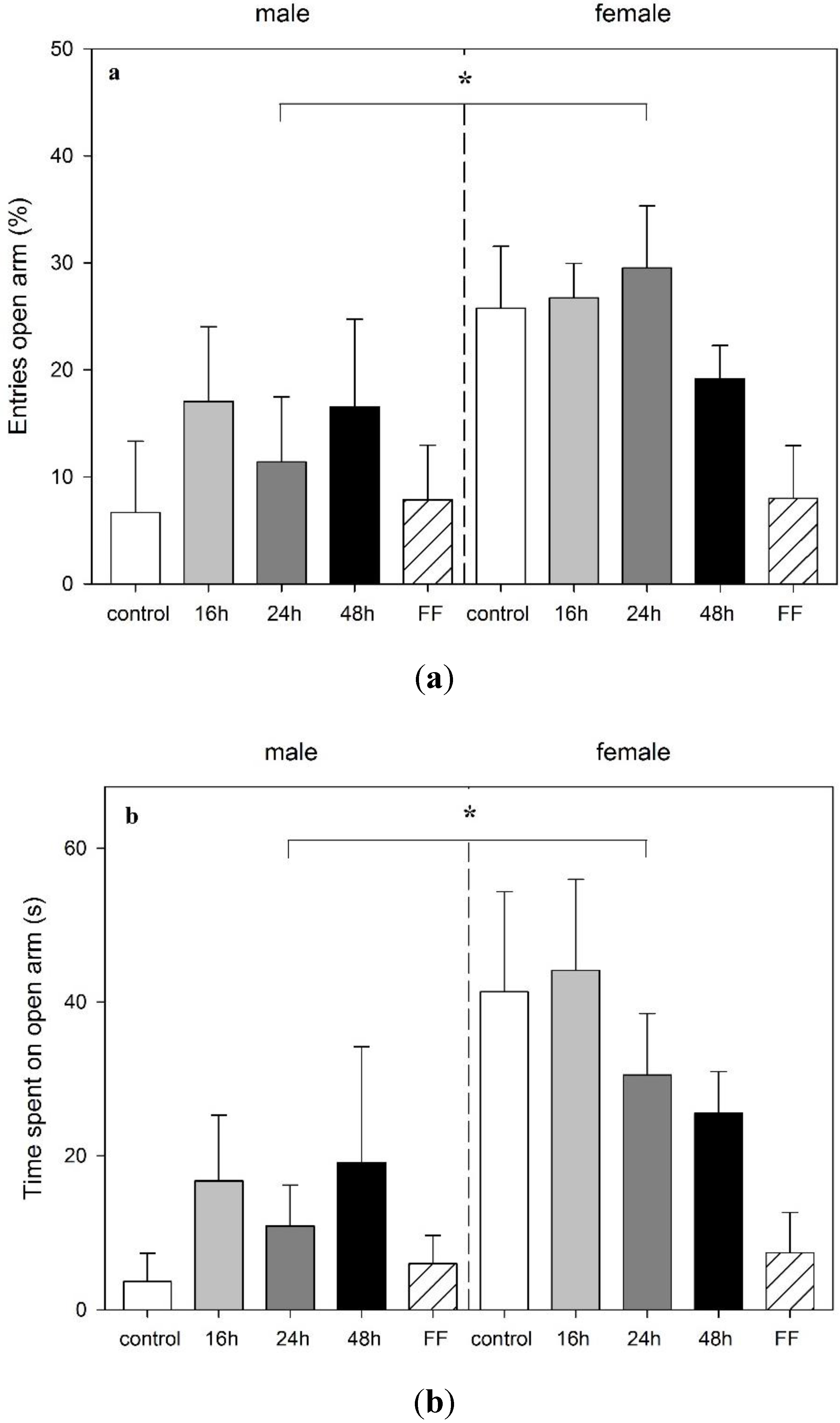
3.3. Effect of Food Deprivation and Fixed-Time Feeding Schedule on the Performance in the EPM Test
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Russell, W.M.S.; Burch, L.R. The Principles of Humane Experimental Technique. In The Principles of Humane Experimental Technique; Methuen & Co.: London, UK, 1959. [Google Scholar]
- Burden, N.; Chapman, K.; Sewell, F.; Robinson, V. Pioneering better science through the 3Rs: An introduction to the national centre for the replacement, refinement, and reduction of animals in research (NC3Rs). J. Am. Assoc. Lab. Anim. Sci. 2015, 54, 198–208. [Google Scholar] [PubMed]
- Baumans, V. Use of animals in experimental research: An ethical dilemma? Gene Ther. 2004, 11, 64–66. [Google Scholar] [CrossRef] [PubMed]
- Bert, B.; Harms, S.; Langen, B.; Fink, H. Clomipramine and selegiline: Do they influence impulse control? J. Vet. Pharmacol. Ther. 2006, 29, 41–47. [Google Scholar] [CrossRef] [PubMed]
- Carlini, V.P.; Martini, A.C.; Schioth, H.B.; Ruiz, R.D.; Fiol de Cuneo, M.; de Barioglio, S.R. Decreased memory for novel object recognition in chronically food-restricted mice is reversed by acute ghrelin administration. Neuroscience 2008, 153, 929–934. [Google Scholar] [CrossRef] [PubMed]
- Conrad, C.D. A critical review of chronic stress effects on spatial learning and memory. Prog. Neuropsychopharmacol. Biol. Psychiatry 2010, 34, 742–755. [Google Scholar] [CrossRef] [PubMed]
- Kant, G.J.; Yen, M.H.; D’Angelo, P.C.; Brown, A.J.; Eggleston, T. Maze performance: A direct comparison of food vs. water mazes. Pharmacol. Biochem. Behav. 1988, 31, 487–491. [Google Scholar] [CrossRef]
- Voigt, J.P.; Huston, J.P.; Voits, M.; Fink, H. Effects of cholecystokinin octapeptide (CCK-8) on food intake in adult and aged rats under different feeding conditions. Peptides 1996, 17, 1313–1315. [Google Scholar] [CrossRef]
- Buresova, O.; Bures, J. Learning and memory. In Techniques and Basic Experiments for the Study of Brain and Behavior; Elsevier: Amsterdam, The Netherlands, 1983; pp. 135–216. [Google Scholar]
- Moriyama, T.; Miyazawa, H.; Tomohiro, M.; Fujikake, N.; Samura, K.; Nishikibe, M. Beneficial effect of moderate food restriction in toxicity studies in rats. J. Toxicol. Sci. 2006, 31, 197–206. [Google Scholar] [CrossRef] [PubMed]
- Keenan, K.P.; Laroque, P.; Dixit, R. Need for dietary control by caloric restriction in rodent toxicology and carcinogenicity studies. J. Toxicol. Environ. Health B Crit. Rev. 1998, 1, 135–148. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Hryhorczuk, C.; Fulton, S. Progressive-ratio responding for palatable high-fat and high-sugar food in mice. J. Vis. Exp. 2012. [Google Scholar] [CrossRef] [PubMed]
- Claassen, V. Neglected Factors in Pharmacology and Neuroscience Research: Biopharmaceutics, Animal Characteristics, Maintenance, Testing Conditions; Elsevier: Amsterdam, The Netherlands, 1994. [Google Scholar]
- Garlick, P.J.; Millward, D.J.; James, W.P. The diurnal response of muscle and liver protein synthesis in vivo in meal-fed rats. Biochem. J. 1973, 136, 935–945. [Google Scholar] [CrossRef] [PubMed]
- CCAC. Guidelines on: Choosing an Appropriate Endpoint in Experiments Using Animals for Research, Teaching and Testing. 1998. Available online: http://www.ccac.ca/Documents/Standards/Guidelines/Appropriate_endpoint.pdf (accessed on 24 November 2015).
- OECD. Guidance Document on the Recognition, Assessment, and Use of Clinical Signs as Humane Endpoints for Experimental Animals Used in Safety Evaluation, 2000. Available online: http://www.oecd.org/officialdocuments/publicdisplaydocumentpdf/?cote=env/jm/mono(2000)7&doclanguage=en (accessed on 24 November 2015).
- IACUC. Human Intervention and Endpoints for Laboratory Animal Species, 2011. Available online: http://www.upenn.edu/regulatoryaffairs/Documents/iacuc/guidelines/iacucguideline-humaneendpoints-8%2023%2011.pdf (accessed on 24 November 2015).
- European Union. Directive 2010/63/EU of the European Parliament and of the Council of 22 September 2010 on the Protection of Animals Used for Scientific Purposes, 2010. Available online: http://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=OJ:L:2010:276:0033:0079:en:PDF (accessed on 24 November 2015).
- Toth, L.A.; Gardiner, T.W. Food and water restriction protocols: Physiological and behavioral considerations. Contemp. Top. Lab. Anim. Sci. 2000, 39, 9–17. [Google Scholar] [PubMed]
- Roe, F.J. Historical histopathological control data for laboratory rodents: Valuable treasure or worthless trash? Lab. Anim. 1994, 28, 148–154. [Google Scholar] [CrossRef] [PubMed]
- Malatova, Z.; Ahlers, I. Diurnal rhythm corticosterone in fasted rats. Endocrinol. Exp. 1977, 11, 241–247. [Google Scholar] [PubMed]
- Heiderstadt, K.M.; McLaughlin, R.M.; Wright, D.C.; Walker, S.E.; Gomez-Sanchez, C.E. The effect of chronic food and water restriction on open-field behaviour and serum corticosterone levels in rats. Lab. Anim. 2000, 34, 20–28. [Google Scholar] [CrossRef] [PubMed]
- Moscarello, J.M.; Ben-Shahar, O.; Ettenberg, A. Effects of food deprivation on goal-directed behavior, spontaneous locomotion, and c-Fos immunoreactivity in the amygdala. Behav. Brain Res. 2009, 197, 9–15. [Google Scholar] [CrossRef] [PubMed]
- Iio, W.; Tokutake, Y.; Koike, H.; Matsukawa, N.; Tsukahara, T.; Chohnan, S.; Toyoda, A. Effects of chronic mild food restriction on behavior and the hypothalamic malonyl-CoA signaling pathway. Anim. Sci. J. 2015, 86, 181–188. [Google Scholar] [CrossRef] [PubMed]
- Moran, G. Severe food deprivation: Some thoughts regarding its exclusive use. Psychol. Bull. 1975, 82, 543–557. [Google Scholar] [CrossRef] [PubMed]
- Kalueff, A.V.; Zimbardo, P.G. Behavioral neuroscience, exploration, and K.C. Montgomery’s legacy. Brain Res. Rev. 2007, 53, 328–331. [Google Scholar] [CrossRef] [PubMed]
- Rex, A.; Voigt, J.P.; Voits, M.; Fink, H. Pharmacological evaluation of a modified open-field test sensitive to anxiolytic drugs. Pharmacol. Biochem. Behav. 1998, 59, 677–683. [Google Scholar] [CrossRef]
- Bodnoff, S.R.; Suranyi-Cadotte, B.; Aitken, D.H.; Quirion, R.; Meaney, M.J. The effects of chronic antidepressant treatment in an animal model of anxiety. Psychopharmacology 1988, 95, 298–302. [Google Scholar] [CrossRef]
- Britton, D.R.; Britton, K.T. A sensitive open field measure of anxiolytic drug activity. Pharmacol. Biochem. Behav. 1981, 15, 577–582. [Google Scholar] [CrossRef]
- Rodgers, R.J.; Cao, B.J.; Dalvi, A.; Holmes, A. Animal models of anxiety: An ethological perspective. Braz. J. Med. Biol. Res. 1997, 30, 289–304. [Google Scholar] [CrossRef] [PubMed]
- Pellow, S.; File, S.E. Anxiolytic and anxiogenic drug effects on exploratory activity in an elevated plus-maze: A novel test of anxiety in the rat. Pharmacol. Biochem. Behav. 1986, 24, 525–529. [Google Scholar] [CrossRef]
- Cruz, A.P.; Frei, F.; Graeff, F.G. Ethopharmacological analysis of rat behavior on the elevated plus-maze. Pharmacol. Biochem. Behav. 1994, 49, 171–176. [Google Scholar] [CrossRef]
- Carobrez, A.P.; Bertoglio, L.J. Ethological and temporal analyses of anxiety-like behavior: The elevated plus-maze model 20 years on. Neurosci. Biobehav. Rev. 2005, 29, 1193–1205. [Google Scholar] [CrossRef] [PubMed]
- Rodgers, R.J.; Dalvi, A. Anxiety, defence and the elevated plus-maze. Neurosci. Biobehav. Rev. 1997, 21, 801–810. [Google Scholar] [CrossRef]
- Schmitt, U.; Hiemke, C. Strain differences in open-field and elevated plus-maze behavior of rats without and with pretest handling. Pharmacol. Biochem. Behav. 1998, 59, 807–811. [Google Scholar] [CrossRef]
- Hogg, S. A review of the validity and variability of the elevated plus-maze as an animal model of anxiety. Pharmacol. Biochem. Behav. 1996, 54, 21–30. [Google Scholar] [CrossRef]
- Padovan, C.M.; Guimaraes, F.S. Restraint-induced hypoactivity in an elevated plus-maze. Braz. J. Med. Biol. Res. 2000, 33, 79–83. [Google Scholar] [CrossRef] [PubMed]
- Korte, S.M.; de Boer, S.F. A robust animal model of state anxiety: Fear-potentiated behaviour in the elevated plus-maze. Eur. J. Pharmacol. 2003, 463, 163–175. [Google Scholar] [CrossRef]
- Faul, F.; Erdfelder, E.; Lang, A.G.; Buchner, A. G* power 3: A flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav. Res. Methods 2007, 39, 175–191. [Google Scholar] [CrossRef] [PubMed]
- Rowland, N.E. Food or fluid restriction in common laboratory animals: Balancing welfare considerations with scientific inquiry. Comp. Med. 2007, 57, 149–160. [Google Scholar] [PubMed]
- Armstrong, S.; Coleman, G.; Singer, G. Food and water deprivation: Changes in rat feeding, drinking, activity and body weight. Neurosci. Biobehav. Rev. 1980, 4, 377–402. [Google Scholar] [CrossRef]
- Palou, M.; Sanchez, J.; Rodriguez, A.M.; Priego, T.; Pico, C.; Palou, A. Induction of NPY/AgRP orexigenic peptide expression in rat hypothalamus is an early event in fasting: Relationship with circulating leptin, insulin and glucose. Cell Physiol. Biochem. 2009, 23, 115–124. [Google Scholar] [CrossRef] [PubMed]
- Gianotti, M.; Clapes, J.; Llado, I.; Palou, A. Effect of 12, 24 and 72 h fasting in thermogenic parameters of rat brown adipose tissue mitochondrial subpopulations. Life Sci. 1998, 62, 1889–1899. [Google Scholar] [CrossRef]
- Dohm, G.L.; Tapscott, E.B.; Barakat, H.A.; Kasperek, G.J. Influence of fasting on glycogen depletion in rats during exercise. J. Appl. Physiol. Respir. Environ. Exerc. Physiol. 1983, 55, 830–833. [Google Scholar] [PubMed]
- Bi, S.; Robinson, B.M.; Moran, T.H. Acute food deprivation and chronic food restriction differentially affect hypothalamic NPY mRNA expression. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2003, 285, 1030–1036. [Google Scholar] [CrossRef] [PubMed]
- Hurwitz, H.M.; Davis, H. Depriving rats of food: A reappraisal of two techniques. J. Exp. Anal. Behav. 1983, 40, 211–213. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Hadjimarkou, M.M.; Singh, A.; Kandov, Y.; Israel, Y.; Pan, Y.X.; Rossi, G.C.; Pasternak, G.W.; Bodnar, R.J. Opioid receptor involvement in food deprivation-induced feeding: Evaluation of selective antagonist and antisense oligodeoxynucleotide probe effects in mice and rats. J. Pharmacol. Exp. Ther. 2004, 311, 1188–1202. [Google Scholar] [CrossRef] [PubMed]
- El Fazaa, S.; Somody, L.; Gharbi, N.; Kamoun, A.; Gharib, C.; Gauquelin-Koch, G. Effects of acute and chronic starvation on central and peripheral noradrenaline turnover, blood pressure and heart rate in the rat. Exp. Physiol. 1999, 84, 357–368. [Google Scholar] [CrossRef] [PubMed]
- Li, J.B.; Wassner, S.J. Effects of food deprivation and refeeding on total protein and actomyosin degradation. Am. J. Physiol. 1984, 246, 32–37. [Google Scholar]
- Deacon, R.M.; Rawlins, J.N. Hippocampal lesions, species-typical behaviours and anxiety in mice. Behav. Brain Res. 2005, 156, 241–249. [Google Scholar] [CrossRef] [PubMed]
- Olszewski, P.K.; Ulrich, C.; Ling, N.; Allen, K.; Levine, A.S. A non-peptide oxytocin receptor agonist, WAY-267,464, alleviates novelty-induced hypophagia in mice: Insights into changes in c-Fos immunoreactivity. Pharmacol. Biochem. Behav. 2014, 124, 367–372. [Google Scholar] [CrossRef] [PubMed]
- Rex, A.; Sondern, U.; Voigt, J.P.; Franck, S.; Fink, H. Strain differences in fear-motivated behavior of rats. Pharmacol. Biochem. Behav. 1996, 54, 107–111. [Google Scholar] [CrossRef]
- Bert, B.; Fink, H.; Sohr, R.; Rex, A. Different effects of diazepam in Fischer rats and two stocks of Wistar rats in tests of anxiety. Pharmacol. Biochem. Behav. 2001, 70, 411–420. [Google Scholar] [CrossRef]
- Inoue, K.; Zorrilla, E.P.; Tabarin, A.; Valdez, G.R.; Iwasaki, S.; Kiriike, N.; Koob, G.F. Reduction of anxiety after restricted feeding in the rat: Implication for eating disorders. Biol. Psychiatry 2004, 55, 1075–1081. [Google Scholar] [CrossRef] [PubMed]
- Genn, R.F.; Tucci, S.A.; Thomas, A.; Edwards, J.E.; File, S.E. Age-associated sex differences in response to food deprivation in two animal tests of anxiety. Neurosci. Biobehav. Rev. 2003, 27, 155–161. [Google Scholar] [CrossRef]
- Pego, J.M.; Sousa, J.C.; Almeida, O.F.; Sousa, N. Stress and the neuroendocrinology of anxiety disorders. Curr. Top. Behav. Neurosci. 2010, 2, 97–117. [Google Scholar] [PubMed]
- Hatori, M.; Vollmers, C.; Zarrinpar, A.; DiTacchio, L.; Bushong, E.A.; Gill, S.; Leblanc, M.; Chaix, A.; Joens, M.; Fitzpatrick, J.A.; et al. Time-restricted feeding without reducing caloric intake prevents metabolic diseases in mice fed a high-fat diet. Cell Metab. 2012, 15, 848–860. [Google Scholar] [CrossRef] [PubMed]
- Moberg, G.P.; Bellinger, L.L.; Mendel, V.E. Effect of meal feeding on daily rhythms of plasma corticosterone and growth hormone in the rat. Neuroendocrinology 1975, 19, 160–169. [Google Scholar] [CrossRef] [PubMed]
- Patton, D.F.; Parfyonov, M.; Gourmelen, S.; Opiol, H.; Pavlovski, I.; Marchant, E.G.; Challet, E.; Mistlberger, R.E. Photic and pineal modulation of food anticipatory circadian activity rhythms in rodents. PLoS ONE 2013. [Google Scholar] [CrossRef] [PubMed]
- Verwey, M.; Amir, S. Food-entrainable circadian oscillators in the brain. Eur. J. Neurosci. 2009, 30, 1650–1657. [Google Scholar] [CrossRef] [PubMed]
- Poulin, A.M.; Timofeeva, E. The dynamics of neuronal activation during food anticipation and feeding in the brain of food-entrained rats. Brain Res. 2008, 1227, 128–141. [Google Scholar] [CrossRef] [PubMed]
- Nowland, M.H.; Hugunin, K.M.; Rogers, K.L. Effects of short-term fasting in male Sprague-Dawley rats. Comp. Med. 2011, 61, 138–144. [Google Scholar] [PubMed]
- Johansson, A.; Fredriksson, R.; Winnergren, S.; Hulting, A.L.; Schioth, H.B.; Lindblom, J. The relative impact of chronic food restriction and acute food deprivation on plasma hormone levels and hypothalamic neuropeptide expression. Peptides 2008, 29, 1588–1595. [Google Scholar] [CrossRef] [PubMed]
- Pirke, K.M.; Spyra, B. Catecholamine turnover in the brain and the regulation of luteinizing hormone and corticosterone in starved male rats. Acta Endocrinol. 1982, 100, 168–176. [Google Scholar] [CrossRef] [PubMed]
- De Boer, S.F.; Koopmans, S.J.; Slangen, J.L.; van der Gugten, J. Effects of fasting on plasma catecholamine, corticosterone and glucose concentrations under basal and stress conditions in individual rats. Physiol. Behav. 1989, 45, 989–994. [Google Scholar] [CrossRef]
- Chandler-Laney, P.C.; Castaneda, E.; Pritchett, C.E.; Smith, M.L.; Giddings, M.; Artiga, A.I.; Boggiano, M.M. A history of caloric restriction induces neurochemical and behavioral changes in rats consistent with models of depression. Pharmacol. Biochem. Behav. 2007, 87, 104–114. [Google Scholar] [CrossRef] [PubMed]
- Johnston, A.L.; File, S.E. Sex differences in animal tests of anxiety. Physiol. Behav. 1991, 49, 245–250. [Google Scholar] [CrossRef]
- Nasello, A.G.; Machado, C.; Bastos, J.F.; Felicio, L.F. Sudden darkness induces a high activity-low anxiety state in male and female rats. Physiol. Behav. 1998, 63, 451–454. [Google Scholar] [CrossRef]
- Warneke, W.; Klaus, S.; Fink, H.; Langley-Evans, S.C.; Voigt, J.P. The impact of cafeteria diet feeding on physiology and anxiety-related behaviour in male and female Sprague-Dawley rats of different ages. Pharmacol. Biochem. Behav. 2014, 116, 45–54. [Google Scholar] [CrossRef] [PubMed]
- De Lorge, J.; Bolles, R.C. Effects of food deprivation on exploratory behavior in a novel situation. Psychol. Rep. 1961, 9, 599–606. [Google Scholar] [CrossRef]
- Pierre, P.J.; Skjoldager, P.; Bennett, A.J.; Renner, M.J. A behavioral characterization of the effects of food deprivation on food and nonfood object interaction: An investigation of the information-gathering functions of exploratory behavior. Physiol. Behav. 2001, 72, 189–197. [Google Scholar] [CrossRef]
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dietze, S.; Lees, K.R.; Fink, H.; Brosda, J.; Voigt, J.-P. Food Deprivation, Body Weight Loss and Anxiety-Related Behavior in Rats. Animals 2016, 6, 4. https://doi.org/10.3390/ani6010004
Dietze S, Lees KR, Fink H, Brosda J, Voigt J-P. Food Deprivation, Body Weight Loss and Anxiety-Related Behavior in Rats. Animals. 2016; 6(1):4. https://doi.org/10.3390/ani6010004
Chicago/Turabian StyleDietze, Silke, Katarina R. Lees, Heidrun Fink, Jan Brosda, and Jörg-Peter Voigt. 2016. "Food Deprivation, Body Weight Loss and Anxiety-Related Behavior in Rats" Animals 6, no. 1: 4. https://doi.org/10.3390/ani6010004
APA StyleDietze, S., Lees, K. R., Fink, H., Brosda, J., & Voigt, J.-P. (2016). Food Deprivation, Body Weight Loss and Anxiety-Related Behavior in Rats. Animals, 6(1), 4. https://doi.org/10.3390/ani6010004




