Effect of Long-Term Contraception with Altrenogest in Dolphins (Tursiops truncatus)
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethical Statement
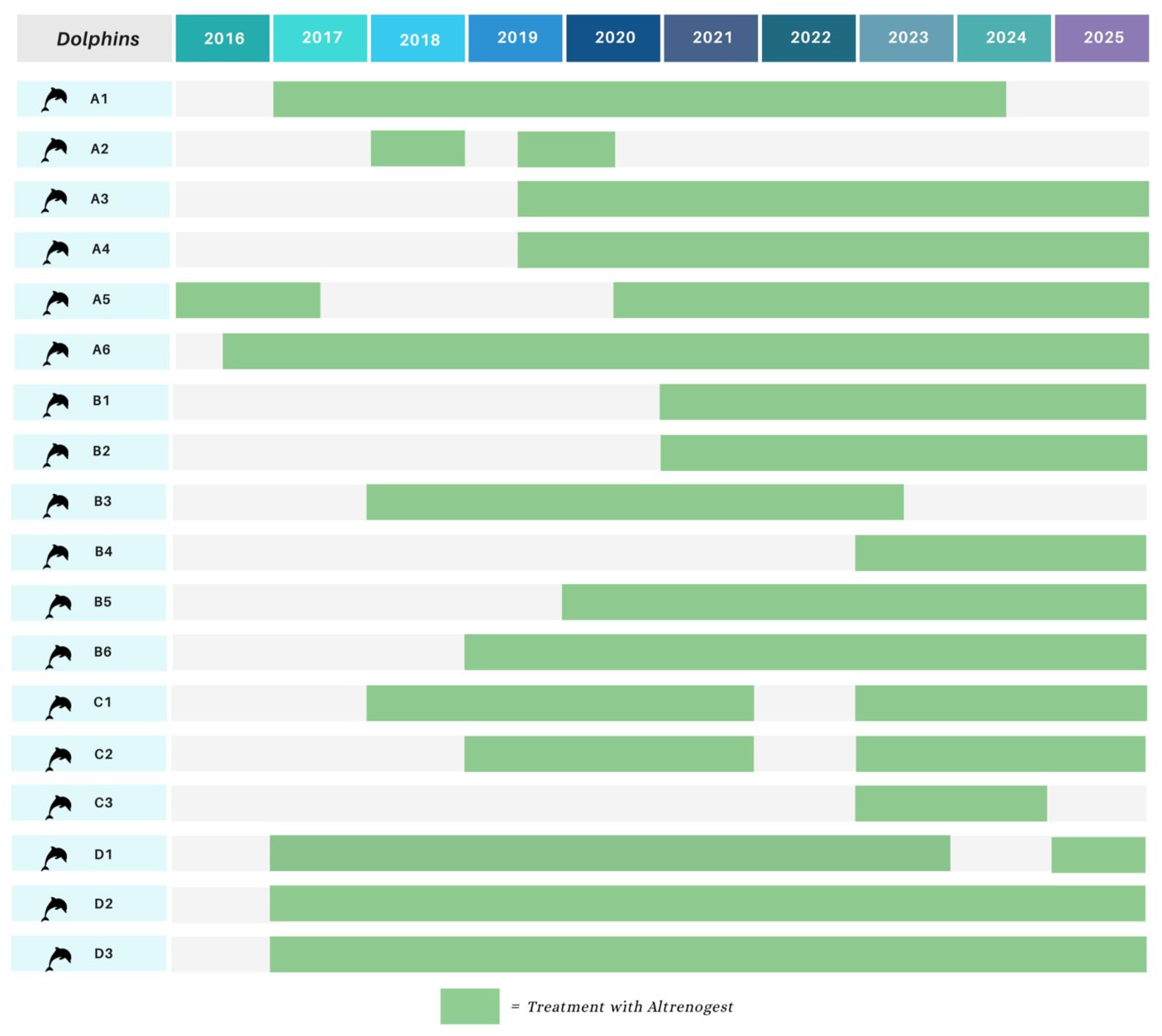
2.2. Animals and Experimental Design
- Serum progesterone and estradiol levels, obtained from routine hormone monitoring (at least twice per year).
- Ultrasound findings of the reproductive tract of the animals, concomitant with hormone monitoring.
- Any reproductive pathologies detected: Pyometra and follicular cyst. Pyometra has been defined as an infection of the uterus with echographically detectable fluid accumulation, thickening of the walls, and alterations in the blood inflammatory panel. Follicular cysts are defined as follicular structure ≥10 mm in diameter that persists for at least 10 days without evidence of ovulation [13].
- Behavioral observations made by the trainers.
- -
- Altrenogest: for conditioned dolphins.
- -
- Control: for unconditioned dolphins (considering a washout period of 60 days to encompass at least one complete estrous cycle and to allow the resolution of residual endocrine effects of the progestin on the hypothalamic–pituitary–gonadal axis).
2.3. Blood Sampling and Hormonal Analyses
2.4. Ultrasound
2.5. Behavior
2.6. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Robeck, T.R.; Steinman, K.J.; Gearhart, S.; Reidarson, T.R.; McBain, J.F.; Monfort, S.L. Reproductive Physiology and Development of Artificial Insemination Technology in Killer Whales (Orcinus orca). Biol. Reprod. 2004, 71, 650–660. [Google Scholar] [CrossRef] [PubMed]
- Steinman, K.J.; Montano, G.A.; Robeck, T.R. Characterization of Circulating Androgens, Cortisol and Estrogens During Normal, Abnormal and False Pregnancy in Bottlenose Dolphins (Tursiops truncatus) Under Managed Care. Front. Mar. Sci. 2021, 8, 737926. [Google Scholar] [CrossRef]
- Cornell, L.H.; Asper, E.D.; Antrim, J.E.; Searles, S.S.; Young, W.G.; Goff, T. Progress Report: Results of a Long-range Captive Breeding Program for the Bottlenose Dolphin, Tursiops truncatus and Tursiops truncatus Gilli. Zoo Biol. 1987, 6, 41–53. [Google Scholar] [CrossRef]
- Urian, K.W.; Duffield, D.A.; Read, A.J.; Wells, R.S.; Shell, E.D. Seasonality of Reproduction in Bottlenose Dolphins, Tursiops truncatus. J. Mammal. 1996, 77, 394–403. [Google Scholar] [CrossRef]
- Robeck, T.R.; Steinman, K.J.; Yoshioka, M.; Jensen, E.; O’Brien, J.K.; Katsumata, E.; Gili, C.; McBain, J.F.; Sweeney, J.; Monfort, S.L. Estrous Cycle Characterisation and Artificial Insemination Using Frozen-Thawed Spermatozoa in the Bottlenose Dolphin (Tursiops truncatus). Reproduction 2005, 129, 659–674. [Google Scholar] [CrossRef] [PubMed]
- Schroeder, J.P. Breeding Bottlenose Dolphins in Captivity. In The Bottlenose Dolphin; Elsevier: Amsterdam, The Netherlands, 1990; pp. 435–446. [Google Scholar]
- Atkinson, S. Reproductive Physiology of Dolphins. In The Physiology of Dolphins; Elsevier: Amsterdam, The Netherlands, 2024; pp. 227–242. [Google Scholar]
- Thompson, D.L.; Godke, R.A.; Squires, E.L. Testosterone Effects on Mares during Synchronization with Altrenogest: FSH, LH, Estrous Duration and Pregnancy Rate. J. Anim. Sci. 1983, 56, 678–686. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.; Xu, X.; Song, Y.; Xiao, L.; Wen, J.; Ding, H.; Zhao, S.; Qiao, D.; Zhang, B.; Niu, A.; et al. Effect of Altrenogest Treatment before Weaning on Reproductive Performance and Production Efficiency in Primiparous and Multiparous Sows. Porc. Health Manag. 2024, 10, 25. [Google Scholar] [CrossRef] [PubMed]
- Robeck, T.R.; Montano, G.A.; Steinman, K.J.; Smolensky, P.; Sweeney, J.; Osborn, S.; O’Brien, J.K. Development and Evaluation of Deep Intra-Uterine Artificial Insemination Using Cryopreserved Sexed Spermatozoa in Bottlenose Dolphins (Tursiops truncatus). Anim. Reprod. Sci. 2013, 139, 168–181. [Google Scholar] [CrossRef] [PubMed]
- Robeck, T.R.; Steinman, K.J.; Greenwell, M.; Ramirez, K.; Van Bonn, W.; Yoshioka, M.; Katsumata, E.; Dalton, L.; Osborn, S.; O’Brien, J.K. Seasonality, Estrous Cycle Characterization, Estrus Synchronization, Semen Cryopreservation, and Artificial Insemination in the Pacific White-Sided Dolphin (Lagenorhynchus obliquidens). Reproduction 2009, 138, 391–405. [Google Scholar] [CrossRef] [PubMed]
- Saviano, P.; Fiorucci, L.; Grande, F.; Macrelli, R.; Troisi, A.; Polisca, A.; Orlandi, R. Pregnancy and Fetal Development: Cephalic Presentation and Other Descriptive Ultrasonographic Findings from Clinically Healthy Bottlenose Dolphins (Tursiops truncatus) under Human Care. Animals 2020, 10, 908. [Google Scholar] [CrossRef] [PubMed]
- Dierauf, L.; Gulland, F.M.D. (Eds.) CRC Handbook of Marine Mammal Medicine, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2001; ISBN 9780429123887. [Google Scholar]
- Brook, F.M. Ultrasonographic Imaging of the Reproductive Organs of the Female Bottlenose Dolphin, Tursiops truncatus Aduncas. Reproduction 2001, 121, 419–428. [Google Scholar] [CrossRef] [PubMed]
- Kraeling, R.R.; Dziuk, P.J.; Pursel, V.G.; Rampacek, G.B.; Webel, S.K. Synchronization of Estrus in Swine with Allyl Trenbolone (RU-2267). J. Anim. Sci. 1981, 52, 831–835. [Google Scholar] [CrossRef] [PubMed]
- Fedorka, C.E.; Ball, B.A.; Walker, O.F.; Conley, A.J.; Corbin, C.J.; Lu, K.G.; Hanneman, J.M.; Troedsson, M.H.T.; Adams, A.A. Alteration of the Mare’s Immune System by the Synthetic Progestin, Altrenogest. Am. J. Reprod. Immunol. 2019, 82, e13145. [Google Scholar] [CrossRef] [PubMed]
- Baird, D.T.; Smith, K.B. Inhibin and Related Peptides in the Regulation of Reproduction. Oxf. Rev. Reprod. Biol. 1993, 15, 191–232. [Google Scholar] [PubMed]
- Machnik, M.; Hegger, I.; Kietzmann, M.; Thevis, M.; Guddat, S.; Schänzer, W. Pharmacokinetics of Altrenogest in Horses. J. Vet. Pharmacol. Ther. 2007, 30, 86–90. [Google Scholar] [CrossRef] [PubMed]
- Xavier, R.G.C.; Santana, C.H.; de Castro, Y.G.; de Souza, T.G.V.; do Amarante, V.S.; Santos, R.L.; Silva, R.O.S. Canine Pyometra: A Short Review of Current Advances. Animals 2023, 13, 3310. [Google Scholar] [CrossRef] [PubMed]
- Ziecik, A.J.; Drzewiecka, K.; Gromadzka-Hliwa, K.; Klos, J.; Witek, P.; Knapczyk-Stwora, K.; Gajewski, Z.; Kaczmarek, M.M. Altrenogest Affects the Development and Endocrine Milieu of Ovarian Follicles in Prepubertal and Mature Gilts. Biol. Reprod. 2020, 103, 1069–1084. [Google Scholar] [CrossRef] [PubMed]
- Hagman, R. Pyometra in Small Animals. Vet. Clin. North Am. Small Anim. Pract. 2018, 48, 639–661. [Google Scholar] [CrossRef] [PubMed]




| Parameter | Group | SEM | p-Value | |
|---|---|---|---|---|
| Control | Altrenogest | |||
| Estradiol | 9.03 A | 14.11 B | 0.63 | <0.0001 |
| Progesterone | 3.01 A | 0.31 B | 0.29 | <0.0001 |
| Group | Time | SEM | p-Value | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0 | 6m | 1y | 1y6m | 2y | 2y6m | 3y | 3y6m | 4y | 4y6m | 5y | G | T | G × T | ||
| Estradiol | |||||||||||||||
| Control | 9.86 X (n = 18) | 9.54 X | 9.38 X | 9.03 X | 8.61 X | 8.69 X | 9.57 X | 8.97 X | 8.74 X | 8.54 X | 8.46 X | 2.09 | <0.0001 | 0.9935 | 0.9993 |
| Altrenogest | 14.91 Y (n = 18) | 13.55 Y | 13.82 Y | 13.00 Y | 13.11 Y | 13.17 Y | 15.28 Y | 16.35 Y | 15.47 Y (n = 17) | 13.23 Y (n = 17) | 13.29 Y (n = 14) | ||||
| Progesterone | |||||||||||||||
| Control | 1.50 X (n = 18) | 3.57 X | 4.21 X | 3.79 X | 7.53 X | 3.05 X | 1.62 X | 1.18 X | 2.02 X | 3.01 X | 1.69 X | 0.97 | <0.0001 | 0.0782 | 0.0731 |
| Altrenogest | 0.29 Y (n = 18) | 0.31 Y | 0.34 Y | 0.29 Y | 0.29 Y | 0.24 Y | 0.26 Y | 0.29 Y | 0.33 Y (n = 17) | 0.34 Y (n = 17) | 0.42 Y (n = 14) | ||||
| ID_Dolphin | Age (Years) | Pathologies | Duration of Treatment with Altrenogest Prior to Diagnosis of Pathology (Years) |
|---|---|---|---|
| A1 | 42 | Pyometra | 7 |
| A2 | 57 | Follicular Cyst | 2 |
| B1 | 29 | Follicular Cyst | 5 |
| B3 | 52 | Pyometra | 5 |
| B6 | 17 | Follicular Cyst | 9 |
| C3 | 50 | Pyometra | 2 |
| D1 | 16 | Pyometra | 7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2026 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license.
Share and Cite
Cicirelli, V.; Carbonari, A.; Forte, L.; Carreca, R.; Canales, R.; Fernandes, T.; Fiorucci, L.; Rizzo, A. Effect of Long-Term Contraception with Altrenogest in Dolphins (Tursiops truncatus). Animals 2026, 16, 399. https://doi.org/10.3390/ani16030399
Cicirelli V, Carbonari A, Forte L, Carreca R, Canales R, Fernandes T, Fiorucci L, Rizzo A. Effect of Long-Term Contraception with Altrenogest in Dolphins (Tursiops truncatus). Animals. 2026; 16(3):399. https://doi.org/10.3390/ani16030399
Chicago/Turabian StyleCicirelli, Vincenzo, Alice Carbonari, Lucrezia Forte, Roberta Carreca, Rocio Canales, Teresa Fernandes, Letizia Fiorucci, and Annalisa Rizzo. 2026. "Effect of Long-Term Contraception with Altrenogest in Dolphins (Tursiops truncatus)" Animals 16, no. 3: 399. https://doi.org/10.3390/ani16030399
APA StyleCicirelli, V., Carbonari, A., Forte, L., Carreca, R., Canales, R., Fernandes, T., Fiorucci, L., & Rizzo, A. (2026). Effect of Long-Term Contraception with Altrenogest in Dolphins (Tursiops truncatus). Animals, 16(3), 399. https://doi.org/10.3390/ani16030399







