Even Low Amounts of Amorphous Lignocellulose Affect Some Upper Gut Parameters, but They Do Not Modify Ileal Microbiota in Young Broiler Chickens
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Design and Dietary Treatments
2.2. Measurements and Samplings
2.3. DNA Extraction and Bacterial 16S rRNA Sequencing
2.4. Bioinformatics Analysis
2.5. Statistical Analysis
3. Results
3.1. Production Traits
3.2. Weight, Digesta Dry Matter, and Digesta Content of the Different Gut Segments
3.3. Digesta pH and Enzyme Activity of the Jejunal Content
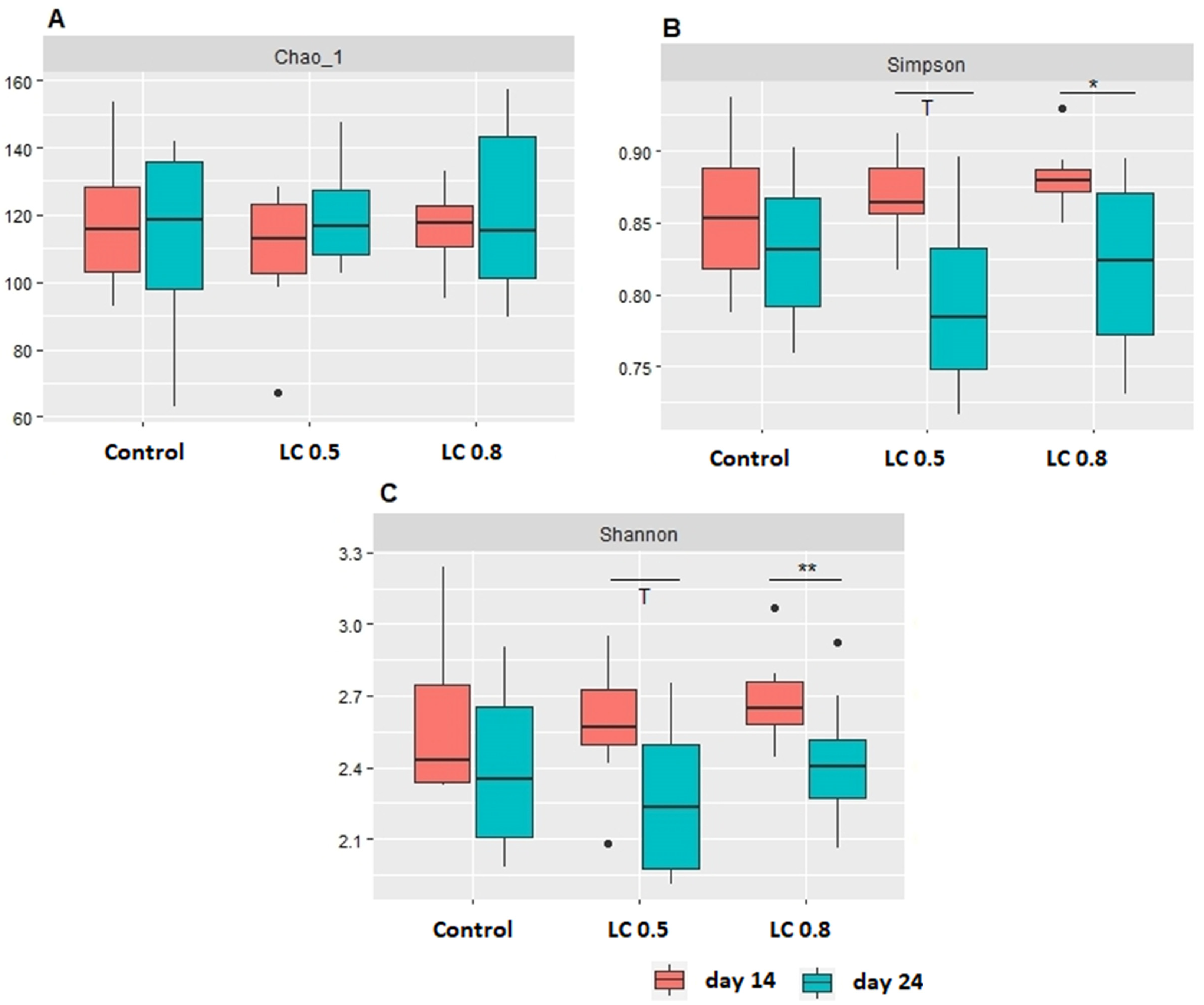
3.4. Microbial Diversity of Ileum
3.5. Ileal Microbial Abundances
4. Discussion
4.1. Production Traits
4.2. Gut Characteristics
4.3. Digesta pH and Enzyme Activity of the Jejunum
4.4. Microbial Diversity and Microbial Abundances of the Ileal Content
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mateos, G.G.; Jiménez-Moreno, E.; Serrano, M.P.; Lázaro, R.P. Poultry Response to High Levels of Dietary Fiber Sources Varying in Physical and Chemical Characteristics. J. Appl. Poult. Res. 2012, 21, 156–174. [Google Scholar] [CrossRef]
- Hetland, H.; Svihus, B.; Choct, M. Role of Insoluble Fiber on Gizzard Activity in Layers. J. Appl. Poult. Res. 2005, 14, 38–46. [Google Scholar] [CrossRef]
- Kheravii, S.K.; Morgan, N.K.; Swick, R.A.; Choct, M.; Wu, S.-B. Roles of Dietary Fibre and Ingredient Particle Size in Broiler Nutrition. Worlds Poult. Sci. J. 2018, 74, 301–316. [Google Scholar] [CrossRef]
- Jørgensen, H.; Zhao, X.-Q.; Eggum, B.O. The Influence of Dietary Fibre and Environmental Temoperature on the Development of the Gastrointestinal Tract, Digestibility, Degree of Fermentation in the Hind-Gut and Energy Metabolism in Pigs. Br. J. Nutr. 1996, 75, 365–378. [Google Scholar] [CrossRef] [PubMed]
- Choct, M.; Hughes, R.J.; Wang, J.; Bedford, M.R.; Morgan, A.J.; Annison, G. Increased Small Intestinal Fermentation Is Partly Responsible for the Anti-nutritive Activity of Non-starch Polysaccharides in Chickens. Br. Poult. Sci. 1996, 37, 609–621. [Google Scholar] [CrossRef] [PubMed]
- Raza, A.; Bashir, S.; Tabassum, R. An Update on Carbohydrases: Growth Performance and Intestinal Health of Poultry. Heliyon 2019, 5, e01437. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.K.; Bansal, S.; Mangal, M.; Dixit, A.K.; Gupta, R.K.; Mangal, A.K. Utilization of Food Processing By-Products as Dietary, Functional, and Novel Fiber: A Review. Crit. Rev. Food Sci. Nutr. 2016, 56, 1647–1661. [Google Scholar] [CrossRef]
- Macagnan, F.T.; da Silva, L.P.; Hecktheuer, L.H. Dietary Fibre: The Scientific Search for an Ideal Definition and Methodology of Analysis, and Its Physiological Importance as a Carrier of Bioactive Compounds. Food Res. Int. 2016, 85, 144–154. [Google Scholar] [CrossRef]
- Montagne, L.; Pluske, J.R.; Hampson, D.J. A Review of Interactions between Dietary Fibre and the Intestinal Mucosa, and Their Consequences on Digestive Health in Young Non-Ruminant Animals. Anim. Feed Sci. Technol. 2003, 108, 95–117. [Google Scholar] [CrossRef]
- Liu, C.; Wang, H.; Karim, A.M.; Sun, J.; Wang, Y. Catalytic Fast Pyrolysis of Lignocellulosic Biomass. Chem. Soc. Rev. 2014, 43, 7594–7623. [Google Scholar] [CrossRef]
- Zhou, C.-H.; Xia, X.; Lin, C.-X.; Tong, D.-S.; Beltramini, J. Catalytic Conversion of Lignocellulosic Biomass to Fine Chemicals and Fuels. Chem. Soc. Rev. 2011, 40, 5588. [Google Scholar] [CrossRef] [PubMed]
- Waite, D.W.; Taylor, M.W. Characterizing the Avian Gut Microbiota: Membership, Driving Influences, and Potential Function. Front. Microbiol. 2014, 5, 223. [Google Scholar] [CrossRef] [PubMed]
- de Vries, S.; Pustjens, A.M.; Schols, H.A.; Hendriks, W.H.; Gerrits, W.J.J. Improving Digestive Utilization of Fiber-Rich Feedstuffs in Pigs and Poultry by Processing and Enzyme Technologies: A Review. Anim. Feed Sci. Technol. 2012, 178, 123–138. [Google Scholar] [CrossRef]
- de Vries, S. Dietary Fiber in Poultry Nutrition: Dispensable Diluent or Pivotal for Digesta Transit Regulation? In Proceedings of the 17th BOKU-Symposium Animal Nutrition, Vienna, Austria, 12 April 2018; Universität für Bodenkultur Wien: Vienna, Austria, 2018; pp. 18–24. [Google Scholar]
- Röhe, I.; Zentek, J. Lignocellulose as an Insoluble Fiber Source in Poultry Nutrition: A Review. J. Anim. Sci. Biotechnol. 2021, 12, 82. [Google Scholar] [CrossRef]
- Yokhana, J.S.; Parkinson, G.; Frankel, T.L. Effect of Insoluble Fiber Supplementation Applied at Different Ages on Digestive Organ Weight and Digestive Enzymes of Layer-Strain Poultry. Poult. Sci. 2016, 95, 550–559. [Google Scholar] [CrossRef]
- Sarikhan, M.; Shahryar, H.A.; Gholizadeh, B.; Hosseinzadeh, M.H.; Beheshti, B.; Mahmoodnejad, A. Effects of Insoluble Fiber on Growth Performance, Carcass Traits and Ileum Morphological Parameters on Broiler Chick Males. Int. J. Agric. Biol. 2010, 12, 531–536. [Google Scholar]
- Makivic, L.; Glisic, M.; Boskovic, M.; Djordjevic, J.; Markovic, R.; Baltic, M.; Sefer, D. Performances, Ileal and Cecal Microbial Populations and Histological Characteristics in Broilers Fed Diets Supplemented with Lignocellulose. Kafkas Univ. Vet. Fak. Derg. 2018, 25, 83–91. [Google Scholar] [CrossRef]
- Kheravii, S.K.; Swick, R.A.; Choct, M.; Wu, S.-B. Coarse Particle Inclusion and Lignocellulose-Rich Fiber Addition in Feed Benefit Performance and Health of Broiler Chickens. Poult. Sci. 2017, 96, 3272–3281. [Google Scholar] [CrossRef]
- Farran, M.T.; Pietsch, M.; Chabrillat, T. Effect of Lignocellulose on the Litter Quality and the Ready to Cook Carcass Yield of Male Broilers. In Proceedings of the Actes des 10èmes Journées de la Recherche Avicole et Palmipèdes à Foie Gras, France, Paris, 26–28 March 2013; Institut Technique de l’Aviculture: France, Paris, 2013; pp. 917–921. [Google Scholar]
- Bogusławska-Tryk, M.; Szymeczko, R.; Piotrowska, A.; Burlikowska, K.; Śliżewska, K. Ileal and Cecal Microbial Population and Short-Chain Fatty Acid Profile in Broiler Chickens Fed Diets Supplemented with Lignocellulose. Pak. Vet. J. 2015, 35, 212–216. [Google Scholar]
- Röhe, I.; Urban, J.; Dijkslag, A.; te Paske, J.; Zentek, J. Impact of an Energy- and Nutrient-Reduced Diet Containing 10% Lignocellulose on Animal Performance, Body Composition and Egg Quality of Dual Purpose Laying Hens. Arch. Anim. Nutr. 2019, 73, 1–17. [Google Scholar] [CrossRef]
- Röhe, I.; Vahjen, W.; Metzger, F.; Zentek, J. Effect of a “Diluted” Diet Containing 10% Lignocellulose on the Gastrointestinal Tract, Intestinal Microbiota, and Excreta Characteristics of Dual Purpose Laying Hens. Poult. Sci. 2020, 99, 310–319. [Google Scholar] [CrossRef] [PubMed]
- Aviagen. Roos Broiler Management Handbook. 2018. Available online: www.aviagen.com (accessed on 3 February 2025).
- Bernfeld, P. Amylases, α and β. In Methods in Enzymology; Academic Press: Cambridge, MA, USA, 1955; pp. 149–158. [Google Scholar]
- Beisson, F.; Tiss, A.; Riviere, C.; Verger, R. Methods for Lipase Detection and Assay: Acritical Review. Eur. J. Lipid Sci. Technol. 2000, 102, 133–153. [Google Scholar] [CrossRef]
- Bergmeyer, H.U.; Gawehn, K.; Grassl, M. Enzymatic Assay of Trypsin. In Methods of Enzymatic Analysis; Bergmeyer, H.U., Ed.; Academic Press, Inc.: New York, NY, USA, 1974; Volume 1, pp. 515–516. [Google Scholar]
- Ohnishi, S.T.; Barr, J.K. A Simplified Method of Quantitating Protein Using the Biuret and Phenol Reagents. Anal. Biochem. 1978, 86, 193–200. [Google Scholar] [CrossRef] [PubMed]
- Klindworth, A.; Pruesse, E.; Schweer, T.; Peplies, J.; Quast, C.; Horn, M.; Glöckner, F.O. Evaluation of General 16S Ribosomal RNA Gene PCR Primers for Classical and Next-Generation Sequencing-Based Diversity Studies. Nucleic Acids Res 2013, 41, e1. [Google Scholar] [CrossRef]
- Estaki, M.; Jiang, L.; Bokulich, N.A.; McDonald, D.; González, A.; Kosciolek, T.; Martino, C.; Zhu, Q.; Birmingham, A.; Vázquez-Baeza, Y.; et al. QIIME 2 Enables Comprehensive End-to-End Analysis of Diverse Microbiome Data and Comparative Studies with Publicly Available Data. Curr. Protoc. Bioinform. 2020, 70, e100. [Google Scholar] [CrossRef] [PubMed]
- Bolyen, E.; Rideout, J.R.; Dillon, M.R.; Bokulich, N.A.; Abnet, C.C.; Al-Ghalith, G.A.; Alexander, H.; Alm, E.J.; Arumugam, M.; Asnicar, F.; et al. Reproducible, Interactive, Scalable and Extensible Microbiome Data Science Using QIIME 2. Nat. Biotechnol. 2019, 37, 852–857. [Google Scholar] [CrossRef] [PubMed]
- Amir, A.; McDonald, D.; Navas-Molina, J.A.; Kopylova, E.; Morton, J.T.; Zech Xu, Z.; Kightley, E.P.; Thompson, L.R.; Hyde, E.R.; Gonzalez, A.; et al. Deblur Rapidly Resolves Single-Nucleotide Community Sequence Patterns. mSystems 2017, 2, 10-1128. [Google Scholar] [CrossRef]
- Quast, C.; Pruesse, E.; Yilmaz, P.; Gerken, J.; Schweer, T.; Yarza, P.; Peplies, J.; Glöckner, F.O. The SILVA Ribosomal RNA Gene Database Project: Improved Data Processing and Web-Based Tools. Nucleic Acids Res. 2013, 41, D590–D596. [Google Scholar] [CrossRef]
- Chong, J.; Liu, P.; Zhou, G.; Xia, J. Using MicrobiomeAnalyst for Comprehensive Statistical, Functional, and Meta-Analysis of Microbiome Data. Nat. Protoc. 2020, 15, 799–821. [Google Scholar] [CrossRef]
- Célino, A.; Fréour, S.; Jacquemin, F.; Casari, P. The Hygroscopic Behavior of Plant Fibers: A Review. Front. Chem. 2014, 1, 43. [Google Scholar] [CrossRef]
- Hetland, H.; Svihus, B. Effect of Oat Hulls on Performance, Gut Capacity and Feed Passage Time in Broiler Chickens. Br. Poult. Sci. 2001, 42, 354–361. [Google Scholar] [CrossRef]
- Carre, B.; Derouet, L.; Leclercq, B. The Digestibility of Cell-Wall Polysaccharides from Wheat (Bran or Whole Grain), Soybean Meal, and White Lupin Meal in Cockerels, Muscovy Ducks, and Rats. Poult. Sci. 1990, 69, 623–633. [Google Scholar] [CrossRef] [PubMed]
- Hetland, H.; Svihus, B.; Krogdahl, Å. Effects of Oat Hulls and Wood Shavings on Digestion in Broilers and Layers Fed Diets Based on Whole or Ground Wheat. Br. Poult. Sci. 2003, 44, 275–282. [Google Scholar] [CrossRef]
- Soltan, M.A.; Shewita, R.S.; Matroud, O.A.; Alkeridis, L.A.; Sayed, S.; Shukry, M.; El-Shobokshy, S.A. Lignocellulose and Probiotic Supplementation in Broiler Chicken Diet: Effect on Growth Performance, Digestive Health, Litter Quality, and Genes Expression. Poult. Sci. 2024, 103, 103735. [Google Scholar] [CrossRef] [PubMed]
- Rezaei, M.; Karimi Torshizi, M.A.; Wall, H.; Ivarsson, E. Body Growth, Intestinal Morphology and Microflora of Quail on Diets Supplemented with Micronised Wheat Fibre. Br. Poult. Sci. 2018, 59, 422–429. [Google Scholar] [CrossRef] [PubMed]
- Jha, R.; Mishra, P. Dietary Fiber in Poultry Nutrition and Their Effects on Nutrient Utilization, Performance, Gut Health, and on the Environment: A Review. J. Anim. Sci. Biotechnol. 2021, 12, 51. [Google Scholar] [CrossRef]
- González-Alvarado, J.M.; Jiménez-Moreno, E.; Lázaro, R.; Mateos, G.G. Effect of Type of Cereal, Heat Processing of the Cereal, and Inclusion of Fiber in the Diet on Productive Performance and Digestive Traits of Broilers. Poult. Sci. 2007, 86, 1705–1715. [Google Scholar] [CrossRef]
- Svihus, B. The Gizzard: Function, Influence of Diet Structure and Effects on Nutrient Availability. Worlds Poult. Sci. J. 2011, 67, 207–224. [Google Scholar] [CrossRef]
- Sacranie, A.; Svihus, B.; Denstadli, V.; Moen, B.; Iji, P.A.; Choct, M. The Effect of Insoluble Fiber and Intermittent Feeding on Gizzard Development, Gut Motility, and Performance of Broiler Chickens. Poult. Sci. 2012, 91, 693–700. [Google Scholar] [CrossRef]
- Jiménez-Moreno, E.; González-Alvarado, J.M.; de Coca-Sinova, A.; Lázaro, R.; Mateos, G.G. Effects of Source of Fibre on the Development and PH of the Gastrointestinal Tract of Broilers. Anim. Feed Sci. Technol. 2009, 154, 93–101. [Google Scholar] [CrossRef]
- Shang, Q.; Wu, D.; Liu, H.; Mahfuz, S.; Piao, X. The Impact of Wheat Bran on the Morphology and Physiology of the Gastrointestinal Tract in Broiler Chickens. Animals 2020, 10, 1831. [Google Scholar] [CrossRef] [PubMed]
- Such, N.; Farkas, V.; Csitári, G.; Pál, L.; Márton, A.; Menyhárt, L.; Dublecz, K. Relative Effects of Dietary Administration of a Competitive Exclusion Culture and a Synbiotic Product, Age and Sampling Site on Intestinal Microbiota Maturation in Broiler Chickens. Vet. Sci. 2021, 8, 187. [Google Scholar] [CrossRef] [PubMed]
- De Maesschalck, C.; Eeckhaut, V.; Maertens, L.; De Lange, L.; Marchal, L.; Daube, G.; Dewulf, J.; Haesebrouck, F.; Ducatelle, R.; Taminau, B.; et al. Amorphous Cellulose Feed Supplement Alters the Broiler Caecal Microbiome. Poult. Sci. 2019, 98, 3811–3817. [Google Scholar] [CrossRef] [PubMed]

| Starter Diets | Grower Diets | Finisher Diets | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Ingredient | C | LC 0.5 | LC 0.8 | C | LC 0.5 | LC 0.8 | C | LC 0.5 | LC 0.8 |
| Corn | 391.2 | 386.2 | 383.2 | 437.2 | 432.2 | 429.2 | 493.3 | 488.3 | 485.3 |
| Wheat | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 |
| Extracted soybean meal | 400 | 400 | 400 | 354 | 354 | 354 | 302 | 302 | 302 |
| Sunflower oil | 55 | 55 | 55 | 65 | 65 | 65 | 63 | 63 | 63 |
| Limestone | 18 | 18 | 18 | 15 | 15 | 15 | 15 | 15 | 15 |
| MCP | 16 | 16 | 16 | 14 | 14 | 14 | 13 | 13 | 13 |
| L-lysine | 4 | 4 | 4 | 2 | 2 | 2 | 2 | 2 | 2 |
| DL-methionine | 4 | 4 | 4 | 3 | 3 | 3 | 3 | 3 | 3 |
| L-threonine | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| L-valine | 1 | 1 | 1 | ||||||
| Premix 1 | 5 | 5 | 5 | 4 | 4 | 4 | 3.5 | 3.5 | 3.5 |
| Salt | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 |
| Sodium bicarbonate | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| Lignocellulose 2 | 5 | 8 | 5 | 8 | 5 | 8 | |||
| Coccidiostat (Maxiban) | 0.63 | 0.63 | 0.63 | ||||||
| Coccidiostat (Elancoban) | 0.55 | 0.55 | 0.55 | ||||||
| Phytase 3 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 |
| NSP degrading enzyme 4 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 |
| Measured nutrient content | |||||||||
| AMEn (MJ/kg) 5 | 12.74 | 12.61 | 12.59 | 12.81 | 12.60 | 12.63 | 12.87 | 12.8 | 12.97 |
| Crude protein | 221 | 225 | 219 | 210.9 | 207.9 | 210.6 | 184.7 | 186.6 | 184.8 |
| Crude fiber | 37.9 | 41.9 | 42.8 | 40.8 | 42.8 | 44.2 | 43.8 | 50.8 | 51.3 |
| Crude fat | 76.2 | 78.9 | 79.9 | 78.6 | 77.6 | 75.5 | 81.6 | 84.0 | 86.3 |
| Starch | 362.8 | 346.0 | 348.7 | 367.9 | 365.8 | 368.2 | 405.2 | 389.7 | 397.8 |
| Calcium | 10.4 | 11.0 | 11.1 | 9.8 | 9.7 | 10.0 | 8.8 | 9.1 | 9.2 |
| Total phosphorus | 7.2 | 7.5 | 7.5 | 7.1 | 7.2 | 7.1 | 6.1 | 6.1 | 6.2 |
| Lysine | 15.1 | 15.4 | 15.1 | 12.8 | 12.7 | 13.0 | 11.1 | 11.4 | 11.3 |
| Methionine | 6.5 | 6.9 | 6.7 | 6.0 | 5.7 | 5.8 | 5.5 | 5.7 | 5.4 |
| Cystine | 3.4 | 3.4 | 3.3 | 3.2 | 3.3 | 3.3 | 2.9 | 3.1 | 2.8 |
| Methionine + cystine | 9.9 | 10.3 | 10.0 | 9.2 | 9.0 | 9.1 | 8.4 | 8.7 | 8.2 |
| Threonine | 8.8 | 9.2 | 9.0 | 8.9 | 8.5 | 8.8 | 7.8 | 7.5 | 7.5 |
| Valine | 10.9 | 11.1 | 10.9 | 9.5 | 9.5 | 9.5 | 8.3 | 8.2 | 8.3 |
| Isoleucine | 9.3 | 9.4 | 9.4 | 8.7 | 7.6 | 8.9 | 7.5 | 7.8 | 7.5 |
| Arginine | 14.5 | 14.6 | 14.5 | 13.9 | 14.1 | 13.9 | 12.6 | 12.2 | 12.2 |
| Dietary Treatments | Feed Intake (g/Bird) | Weight Gain (g/Bird) | ||||||
|---|---|---|---|---|---|---|---|---|
| Starter | Grower | Finisher | Total | Starter | Grower | Finisher | Total | |
| C | 268 | 1277 A | 2650 | 4194 | 230 | 930 | 1728 | 2887 |
| LC 0.5 | 268 | 1239 B | 2573 | 4081 | 235 | 942 | 1696 | 2873 |
| LC 0.8 | 264 | 1237 B | 2681 | 4182 | 227 | 909 | 1733 | 2869 |
| Pooled SEM | 2.37 | 13.39 | 55.74 | 64.60 | 3.48 | 11.73 | 32.15 | 39.85 |
| p-Value | 0.464 | 0.087 | 0.391 | 0.408 | 0.299 | 0.152 | 0.686 | 0.942 |
| Feed-Conversion Ratio (g/g) | Mortality (Nb) | |||||||
| Starter | Grower | Finisher | Total | Starter | Grower | Finisher | Total | |
| C | 1.17 | 1.37 A | 1.53 | 1.45 | 2 | 3 | 0 | 5 (2.5%) |
| LC 0.5 | 1.14 | 1.32 B | 1.52 | 1.42 | 1 | 1 | 0 | 2 (1.0%) |
| LC 0.8 | 1.16 | 1.36 A | 1.55 | 1.46 | 1 | 0 | 3 | 4 (2.1%) |
| Pooled SEM | 0.02 | 0.02 | 0.02 | 0.01 | ||||
| p-Value | 0.551 | 0.068 | 0.523 | 0.146 | ||||
| Gut Segments | Dietary Treatments | Relative Weight of Digestive-Tract Parts (%) | DM Content of Digesta (%) | Digesta Contents of Digestive-Tract Parts (g DM) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Mean | Pooled SEM | p-Value | Mean | Pooled SEM | p-Value | Mean | Pooled SEM | p-Value | ||
| Crop | C | 0.39 | 0.056 | 0.226 | 42.6 a | 0.84 | 0.002 | 3.0 A | 0.39 | 0.083 |
| LC 0.5 | 0.49 | 39.7 ab | 3.2 A | |||||||
| LC 0.8 | 0.36 | 38.0 b | 2.0 B | |||||||
| Gizzard | C | 4.11 | 0.180 | 0.656 | 33.8 A | 0.59 | 0.090 | 4.0 | 0.17 | 0.491 |
| LC 0.5 | 4.28 | 32.1 B | 3.8 | |||||||
| LC 0.8 | 4.36 | 32.2 B | 3.7 | |||||||
| Duodenum | C | 1.22 B | 0.056 | 0.085 | 21.0 | 0.83 | 0.927 | 0.4 | 0.05 | 0.160 |
| LC 0.5 | 1.39 A | 21.5 | 0.5 | |||||||
| LC 0.8 | 1.36 A | 21.3 | 0.5 | |||||||
| Jejunum | C | 2.90 | 0.083 | 0.397 | 17.8 b | 0.24 | 0.000 | 1.8 | 0.07 | 0.120 |
| LC 0.5 | 3.07 | 19.4 a | 2.0 | |||||||
| LC 0.8 | 2.94 | 18.2 b | 1.9 | |||||||
| Ileum | C | 1.73 | 0.051 | 0.973 | 19.4 B | 0.20 | 0.073 | 2.1 | 0.07 | 0.174 |
| LC 0.5 | 1.70 | 19.8 AB | 2.2 | |||||||
| LC 0.8 | 1.71 | 20.1 A | 2.3 | |||||||
| Gut Segments | Dietary Treatment | Weight of Digestive-Tract Parts (g) | DM Content of the Gut Contents (%) | Digesta Contents of Digestive-Tract Parts (g DM) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Mean | Pooled SEM | p-Value | Mean | Pooled SEM | p-Value | Mean | Pooled SEM | p-Value | ||
| Crop | C | 0.54 | 0.066 | 0.454 | 37.9 | 0.85 | 0.196 | 2.1 | 0.57 | 0.580 |
| LC 0.5 | 0.64 | 38.0 | 1.3 | |||||||
| LC 0.8 | 0.57 | 35.7 | 1.4 | |||||||
| Gizzard | C | 2.22 | 0.118 | 0.201 | 33.5 a | 0.32 | 0.047 | 4.5 | 0.43 | 0.367 |
| LC 0.5 | 2.21 | 33.3 ab | 5.3 | |||||||
| LC 0.8 | 2.54 | 32.4 b | 5.1 | |||||||
| Duodenum | C | 0.99 | 0.058 | 0.612 | 17.4 | 0.80 | 0.877 | 0.5 | 0.04 | 0.393 |
| LC 0.5 | 0.91 | 16.9 | 0.5 | |||||||
| LC 0.8 | 0.91 | 17.0 | 0.4 | |||||||
| Jejunum | C | 1.71 | 0.053 | 0.237 | 20.2 a | 0.44 | 0.002 | 3.0 | 0.15 | 0.430 |
| LC 0.5 | 1.60 | 18.1 b | 2.7 | |||||||
| LC 0.8 | 1.78 | 18.3 b | 2.8 | |||||||
| Ileum | C | 1.29 | 0.052 | 0.832 | 20.0 A | 0.28 | 0.069 | 3.0 | 0.20 | 0.739 |
| LC 0.5 | 1.26 | 19.1 B | 2.8 | |||||||
| LC 0.8 | 1.27 | 19.7 AB | 3.0 | |||||||
| Dietary Treatments | DM Content of Excreta (%) | |||||
|---|---|---|---|---|---|---|
| Day 14 | Day 24 | |||||
| Mean | Pooled Sem | p-Value | Mean | Pooled SEM | p-Value | |
| C | 18.8 | 0.228 | 0.279 | 16.7 | 0.347 | 0.931 |
| LC 0.5 | 17.8 | 16.9 | ||||
| LC 0.8 | 18.2 | 16.6 | ||||
| Gut Segments | Dietary Treatment | Digesta pH on Day 14 | Digesta pH on Day 24 | ||||
|---|---|---|---|---|---|---|---|
| Mean | Pooled SEM | p-Value | Mean | Pooled SEM | p-Value | ||
| Crop | C | 5.97 | 0.08 | 0.237 | 5.36 | 0.06 | 0.617 |
| LC 0.5 | 5.83 | 5.43 | |||||
| LC 0.8 | 5.77 | 5.38 | |||||
| Gizzard | C | 3.69 a | 0.09 | 0.011 | 3.75 | 0.10 | 0.524 |
| LC 0.5 | 3.35 b | 3.79 | |||||
| LC 0.8 | 3.70 a | 3.90 | |||||
| Duodenum | C | 6.37 b | 0.02 | 0.045 | 6.31 a | 0.02 | 0.011 |
| LC 0.5 | 6.44 ab | 6.24 b | |||||
| LC 0.8 | 6.45 a | 6.27 b | |||||
| Jejunum | C | 6.30 | 0.03 | 0.249 | 6.25 b | 0.03 | 0.010 |
| LC 0.5 | 6.23 | 6.32 ab | |||||
| LC 0.8 | 6.30 | 6.38 a | |||||
| Ileum | C | 7.17 a | 0.07 | 0.027 | 7.11 | 0.11 | 0.936 |
| LC 0.5 | 6.90 b | 7.16 | |||||
| LC 0.8 | 7.02 a | 7.13 | |||||
| Dietary Treatments | Day 14 | Day 24 | ||||
|---|---|---|---|---|---|---|
| Trypsin Units/mg Protein | Lipase Units/mg Protein | α-Amylase Units/mg Protein | Trypsin Units/mg Protein | Lipase Units/mg Protein | α-Amylase Units/mg Protein | |
| C | 89.50 | 0.20 | 8.18 | 81.52 | 0.17 | 5.81 ab |
| LC 0.5 | 82.87 | 0.21 | 9.07 | 76.74 | 0.19 | 7.90 a |
| LC 0.8 | 89.51 | 0.20 | 8.65 | 79.51 | 0.17 | 5.14 b |
| Pooled SEM | 5.760 | 0.018 | 0.681 | 7.447 | 0.014 | 0.716 |
| p-Value | 0.649 | 0.784 | 0.659 | 0.902 | 0.411 | 0.033 |
| Treatments | Age | FDR p-Values | |||||
|---|---|---|---|---|---|---|---|
| D14 | D24 | Mean (Dietary Treatment) | Mean (Dietary Treatment) | Mean (Age) | Interaction | ||
| Actinobacteria | C | 3.60 | 4.83 | 4.22 | 0.977 | 1.210 | |
| LC 0.5 | 4.29 | 4.40 | 4.34 | ||||
| LC 0.8 | 5.30 | 5.45 | 5.38 | ||||
| Mean (age) | 4.40 | 4.90 | 0.705 | ||||
| Bacteroidetes | C | 0.00 | 0.00 | 0.00 | 0.788 | 1.122 | |
| LC 0.5 | 0.00 | 0.00 | 0.00 | ||||
| LC 0.8 | 0.01 | 0.01 | 0.01 | ||||
| Mean (age) | 0.01 | 0.01 | 0.808 | ||||
| Cyanobacteria | C | 0.11 | 0.08 | 0.10 | 0.708 | 0.840 | |
| LC 0.5 | 0.09 | 0.16 | 0.12 | ||||
| LC 0.8 | 0.08 | 0.16 | 0.12 | ||||
| Mean (age) | 0.09 | 0.13 | 0.243 | ||||
| Firmicutes | C | 95.31 | 94.78 | 95.04 | 0.836 | 0.967 | |
| LC 0.5 | 95.38 | 95.08 | 95.23 | ||||
| LC 0.8 | 94.00 | 94.10 | 94.05 | ||||
| Mean (age) | 94.90 | 94.65 | 0.812 | ||||
| Patescibacteria | C | 0.10 | 0.13 | 0.11 | 0.914 | 0.660 | |
| LC 0.5 | 0.07 | 0.17 | 0.12 | ||||
| LC 0.8 | 0.10 | 0.11 | 0.10 | ||||
| Mean (age) | 0.09 | 0.14 | 0.182 | ||||
| Proteobacteria | C | 0.88 | 0.17 | 0.00 | 0.686 | 0.740 | |
| LC 0.5 | 0.17 | 0.16 | 0.00 | ||||
| LC 0.8 | 0.51 | 0.12 | 0.00 | ||||
| Mean (age) | 0.52 | 0.34 | 0.182 | ||||
| Verrucomicrobia | C | 0.00 | 0.01 | 0.00 | 0.917 | 0.917 | |
| LC 0.5 | 0.00 | 0.03 | 0.02 | ||||
| LC 0.8 | 0.00 | 0.04 | 0.02 | ||||
| Mean (age) | 0.00 b | 0.03 a | 0.000 | ||||
| Treatments | Age | FDR p-Values | |||||
|---|---|---|---|---|---|---|---|
| D14 | D24 | Mean (Dietary Treatment) | Mean (Dietary Treatment) | Mean (Age) | Interaction | ||
| Lactobacillus | C | 67.44 | 85.97 | 76.70 | 0.701 | 0.986 | |
| LC 0.5 | 75.07 | 85.62 | 80.34 | ||||
| LC 0.8 | 64.46 | 81.44 | 72.95 | ||||
| Mean (age) | 68.99 b | 84.34 a | 0.000 | ||||
| Staphylococcus | C | 9.65 | 1.08 | 5.36 | 0.950 | 0.987 | |
| LC 0.5 | 9.25 | 0.94 | 5.09 | ||||
| LC 0.8 | 10.98 | 1.57 | 6.28 | ||||
| Mean (age) | 9.96 a | 1.20 b | 0.000 | ||||
| Streptococcus | C | 5.68 | 3.54 | 4.61 | 0.701 | 0.986 | |
| LC 0.5 | 3.35 | 5.80 | 4.57 | ||||
| LC 0.8 | 6.03 | 7.62 | 6.83 | ||||
| Mean (age) | 5.02 | 5.65 | 0.770 | ||||
| Corynebacterium_1 | C | 2.56 | 3.57 | 3.07 | 0.886 | 0.986 | |
| LC 0.5 | 3.28 | 3.57 | 3.43 | ||||
| LC 0.8 | 3.86 | 3.73 | 3.80 | ||||
| Mean (age) | 3.24 | 3.63 | 0.710 | ||||
| Enterococcus | C | 5.91 | 0.46 | 3.19 | 0.701 | 0.986 | |
| LC 0.5 | 3.23 | 0.31 | 1.77 | ||||
| LC 0.8 | 6.17 | 0.38 | 3.27 | ||||
| Mean (age) | 5.10 a | 0.38 b | 0.000 | ||||
| Candidatus_ Arthromitus | C | 2.47 | 0.10 | 1.29 | 0.886 | 0.986 | |
| LC 0.5 | 1.59 | 0.25 | 0.92 | ||||
| LC 0.8 | 2.49 | 0.11 | 1.30 | ||||
| Mean (age) | 2.18 a | 0.15 b | 0.000 | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Farkas, V.; Mayer, A.; Poór, J.; Farkas, E.P.; Tewelde, K.G.; Kiss, B.; Such, N.; Pál, L.; Csitári, G.; Dublecz, K. Even Low Amounts of Amorphous Lignocellulose Affect Some Upper Gut Parameters, but They Do Not Modify Ileal Microbiota in Young Broiler Chickens. Animals 2025, 15, 851. https://doi.org/10.3390/ani15060851
Farkas V, Mayer A, Poór J, Farkas EP, Tewelde KG, Kiss B, Such N, Pál L, Csitári G, Dublecz K. Even Low Amounts of Amorphous Lignocellulose Affect Some Upper Gut Parameters, but They Do Not Modify Ileal Microbiota in Young Broiler Chickens. Animals. 2025; 15(6):851. https://doi.org/10.3390/ani15060851
Chicago/Turabian StyleFarkas, Valéria, András Mayer, Judit Poór, Eszter Péterné Farkas, Kesete Goitom Tewelde, Brigitta Kiss, Nikoletta Such, László Pál, Gábor Csitári, and Károly Dublecz. 2025. "Even Low Amounts of Amorphous Lignocellulose Affect Some Upper Gut Parameters, but They Do Not Modify Ileal Microbiota in Young Broiler Chickens" Animals 15, no. 6: 851. https://doi.org/10.3390/ani15060851
APA StyleFarkas, V., Mayer, A., Poór, J., Farkas, E. P., Tewelde, K. G., Kiss, B., Such, N., Pál, L., Csitári, G., & Dublecz, K. (2025). Even Low Amounts of Amorphous Lignocellulose Affect Some Upper Gut Parameters, but They Do Not Modify Ileal Microbiota in Young Broiler Chickens. Animals, 15(6), 851. https://doi.org/10.3390/ani15060851







