Triclosan Caused Oocyte Meiotic Arrest by Modulating Oxidative Stress, Organelle Dysfunctions, Autophagy, and Apoptosis in Pigs
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Culture of the Porcine Oocytes
2.3. Assessment of Maturation in Oocytes
2.4. Assessment of Mitochondrial and ER Distribution in Oocytes
2.5. Measurement of ROS, GSH, and Calcium Levels in Oocytes
2.6. Determination of ATP Levels
2.7. Mitochondrial Membrane Potential (ΔΨm) Assessment
2.8. Assessment of Early Apoptosis in Oocytes
2.9. Western Blotting
2.10. Statistical Analysis
3. Results
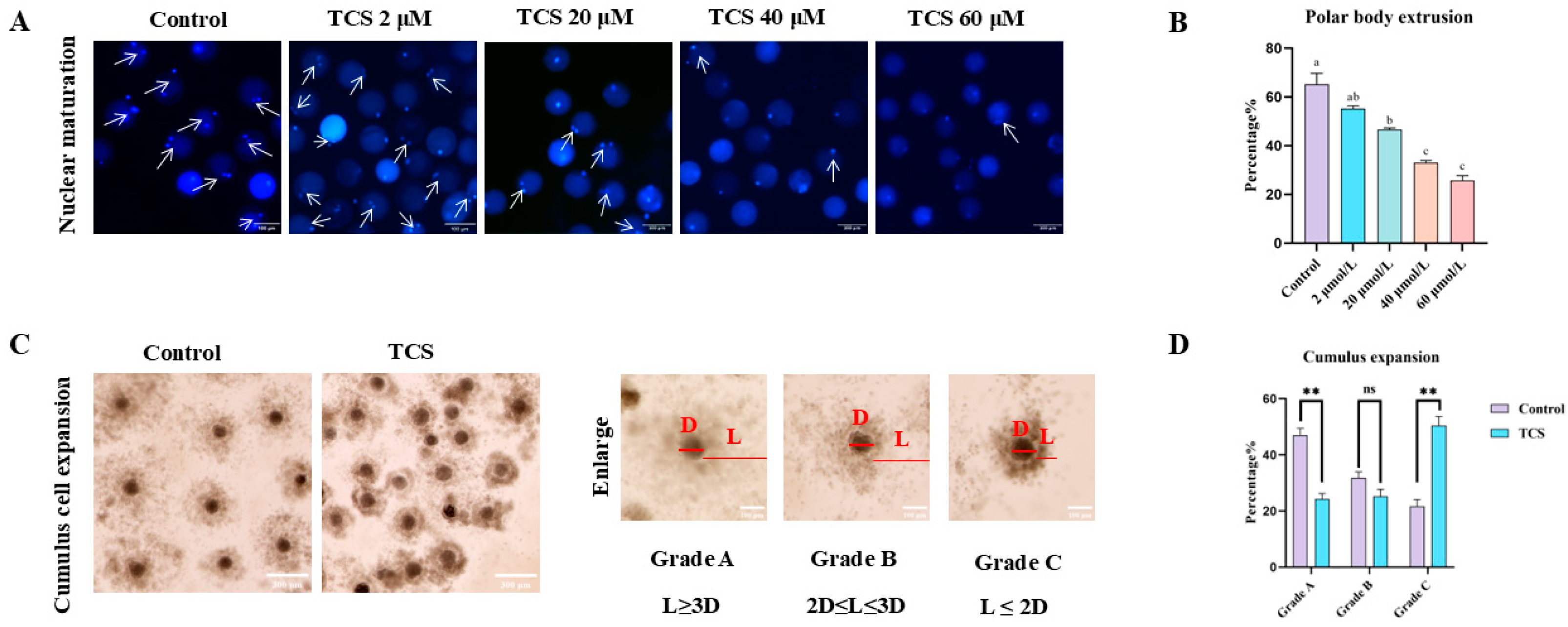
3.1. TCS Impedes Nuclear Maturation in Oocytes
3.2. TCS Exposure Reduced the Antioxidant Capacity in Oocytes
3.3. TCS Exposure Impairs Mitochondria Function in Oocytes
3.4. TCS Exposure Affects the Endoplasmic Reticulum Function of Porcine Oocytes
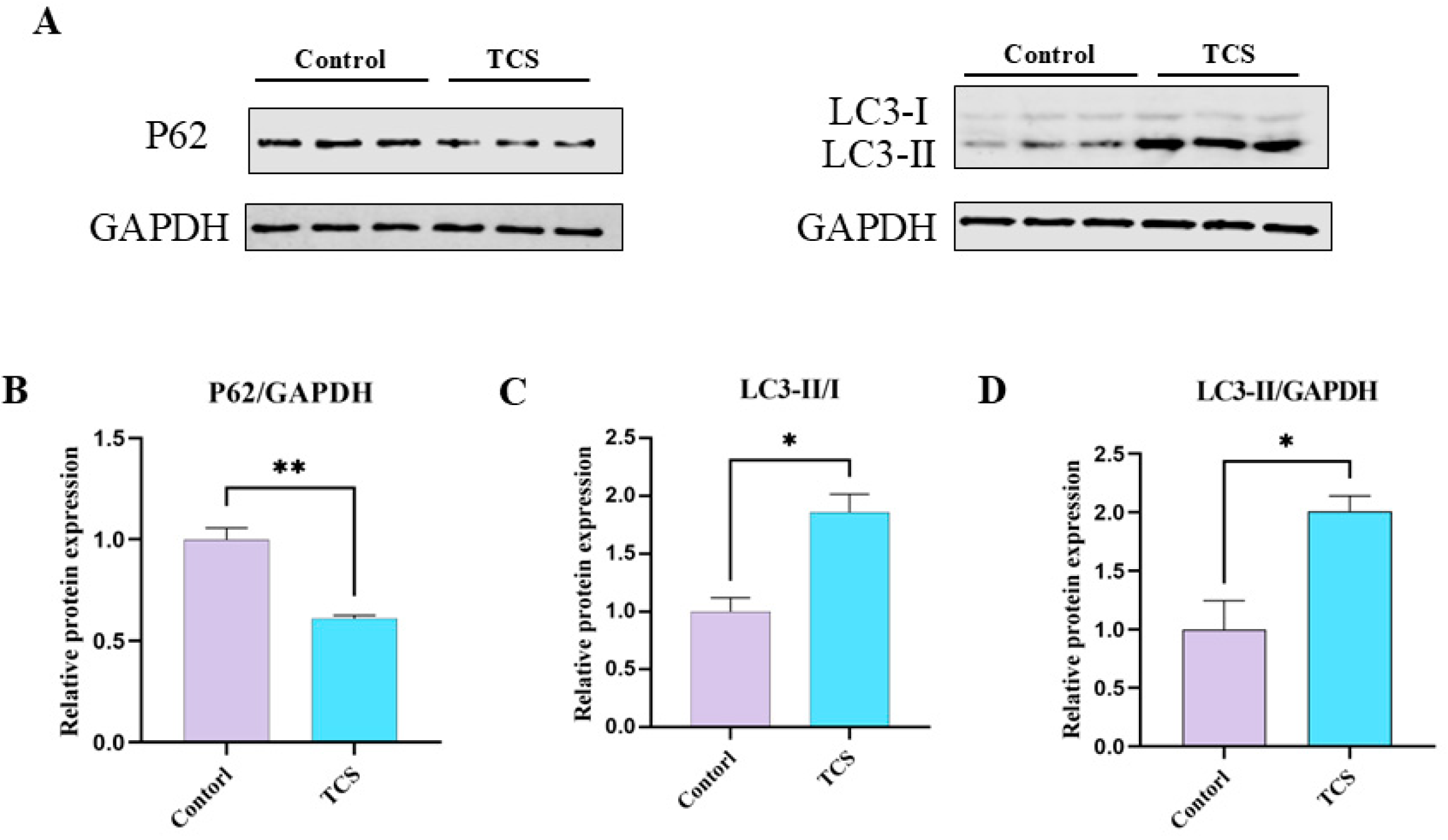
3.5. TCS Exposure Induces Autophagic Functions in Porcine Oocytes
3.6. TCS Promotes Early Apoptosis in Porcine Oocytes
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Motlík, J.; Fulka, J. Factors affecting meiotic competence in pig oocytes. Theriogenology 1986, 25, 87–96. [Google Scholar] [CrossRef]
- Moor, R.M.; Dai, Y.; Lee, C.; Fulka, J., Jr. Oocyte maturation and embryonic failure. Hum. Reprod. Update 1998, 4, 223–236. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.M.; Wang, N.; Hao, H.S.; Li, C.Y.; Zhao, Y.H.; Yan, C.L.; Wang, H.Y.; Du, W.H.; Wang, D.; Liu, Y.; et al. Melatonin improves the fertilization capacity and developmental ability of bovine oocytes by regulating cytoplasmic maturation events. J. Pineal Res. 2018, 64, e12445. [Google Scholar] [CrossRef]
- Seidler, E.A.; Moley, K.H. Metabolic determinants of mitochondrial function in oocytes. Semin. Reprod. Med. 2015, 33, 396–400. [Google Scholar] [CrossRef]
- Xing, C.H.; Chen, S.; Wang, Y.; Pan, Z.N.; Zou, Y.J.; Sun, S.C.; Ren, Z.L.; Zhang, Y. Glyphosate exposure deteriorates oocyte meiotic maturation via induction of organelle dysfunctions in pigs. J. Anim. Sci. Biotechnol. 2022, 13, 80–93. [Google Scholar] [CrossRef] [PubMed]
- Tatone, C.; Carbone, M.C.; Gallo, R.; Delle Monache, S.; Di Cola, M.; Alesse, E.; Amicarelli, F. Age-associated changes in mouse oocytes during postovulatory in vitro culture: Possible role for meiotic kinases and survival factor BCL2. Biol Reprod. 2006, 74, 395–402. [Google Scholar] [CrossRef]
- Jiang, Y.H.; Liu, L.Y.; Jin, B.; Liu, Y.; Liang, X.L. Critical review on the environmental behaviors and toxicity of triclosan and its removal technologies. Sci. Total Environ. 2024, 932, 173013. [Google Scholar] [CrossRef]
- Hipwell, A.E.; Kahn, L.G.; Factor-Litvak, P.; Porucznik, C.A.; Siegel, E.L.; Fichorova, R.N.; Hamman, R.F.; Klein-Fedyshin, M.; Harley, K.G. Exposure to non-persistent chemicals in consumer products and fecundability: A systematic review. Hum. Reprod. Update 2019, 25, 51–71. [Google Scholar] [CrossRef]
- Delbes, G.; Blázquez, M.; Fernandino, J.I.; Grigorova, P.; Hales, B.F.; Metcalfe, C.; Navarro-Martín, L.; Parent, L.; Robaire, B.; Rwigemera, A.; et al. Effects of endocrine disrupting chemicals on gonad development: Mechanistic insights from fish and mammals. Environ. Res. 2022, 204, 112040. [Google Scholar] [CrossRef]
- Yuan, G.X.; Ma, Y.; Zeng, Y.X.; Pan, H.B.; Liu, P.Y.; Liu, Y.; Liu, G.H.; Cheng, J.Q.; Guo, Y.S. Associations between low-dose triclosan exposure and semen quality in a Chinese population. Environ. Pollut. 2022, 299, 118926. [Google Scholar] [CrossRef]
- Raj, S.; Singh, S.S.; Singh, S.P.; Singh, P. Evaluation of triclosan-induced reproductive impairments in the accessory reproductive organs and sperm indices in the mice. Acta Histochem. 2021, 123, 151744–151752. [Google Scholar] [CrossRef] [PubMed]
- Zhu, W.T.; Xie, C.; Zhao, S.S.; Zhang, D.; Zhang, H. Environmental exposure to triclosan and male fecundity: A prospective study in China. Front. Public Health 2022, 10, 814927. [Google Scholar] [CrossRef]
- Qiao, Y.J.; He, J.Y.; Han, P.; Qu, J.B.; Wang, X.B.; Wang, J. Long-term exposure to environmental relevant triclosan induces reproductive toxicity on adult zebrafish and its potential mechanism. Sci. Total Environ. 2022, 826, 154026. [Google Scholar] [CrossRef]
- Kim, J.Y.; Yi, B.R.; Go, R.E.; Hwang, K.A.; Nam, K.H.; Choi, K.C. Methoxychlor and triclosan stimulates ovarian cancer growth by regulating cell cycle- and apoptosis-related genes via an estrogen receptor-dependent pathway. Environ. Toxicol. Pharmacol. 2014, 37, 1264–1274. [Google Scholar] [CrossRef]
- Jurewicz, J.; Wielgomas, B.; Radwan, M.; Karwacka, A.; Klimowska, A.; Dziewirska, E.; Korczak, K.; Zajdel, R.; Radwan, P.; Hanke, W. Triclosan exposure and ovarian reserve. Reprod. Toxicol. 2019, 89, 168–172. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.H.; Huang, W.H.; Lin, J.B.; Fang, F.; Wang, X.D.; Wang, H.L. Triclosan-induced liver and brain injury in zebrafish (Danio rerio) via abnormal expression of miR-125 regulated by PKCα/Nrf2/p53 signaling pathways. Chemosphere 2020, 241, 125086. [Google Scholar] [CrossRef]
- Du, Y.T.; Wang, B.; Cai, Z.Z.; Zhang, H.H.; Wang, B.; Liang, W.; Zhou, G.D.; Ouyang, F.X.; Wang, W.Y. The triclosan-induced shift from aerobic to anaerobic metabolism link to increased steroidogenesis in human ovarian granulosa cells. Ecotoxicol. Environ. Saf. 2021, 220, 112389. [Google Scholar] [CrossRef] [PubMed]
- Stoker, T.E.; Gibson, E.K.; Zorrilla, L.M. Triclosan exposure modulates estrogen-dependent responses in the female wistar rat. Toxicol. Sci. 2010, 117, 45–53. [Google Scholar] [CrossRef]
- Duan, J.X.; Chen, H.L.; Xu, D.J.; Li, Y.; Li, X.Y.; Cheng, J.Y.; Hua, R.M.; Zhang, Z.L.; Yang, L.; Li, Q.W. 17β-estradiol improves the developmental ability, inhibits reactive oxygen species levels and apoptosis of porcine oocytes by regulating autophagy events. J. Steroid Biochem. Mol. Biol. 2021, 209, 105826. [Google Scholar] [CrossRef]
- Xiang, D.C.; Jia, B.Y.; Fu, X.W.; Guo, J.X.; Hong, Q.H.; Quan, G.B.; Wu, G.Q. Role of astaxanthin as an efficient antioxidant on the in vitro maturation and vitrification of porcine oocytes. Theriogenology 2021, 167, 13–23. [Google Scholar] [CrossRef]
- Duan, J.X.; Chen, H.L.; Li, Y.; Xu, D.J.; Li, X.Y.; Zhang, Z.L.; Cheng, J.Y.; Yang, L.; Li, Q.W. 17β-Estradiol Enhances Porcine Meiosis Resumption from Autophagy-Induced Gap Junction Intercellular Communications and Connexin 43 Phosphorylation via the MEK/ERK Signaling Pathway. J. Agric. Food Chem. 2021, 69, 11847–11855. [Google Scholar] [CrossRef] [PubMed]
- Barros, R.G.; Lima, P.F.; Soares, A.C.S.; Sanches, L.; Price, C.A.; Buratini, J. Fibroblast growth factor 2 regulates cumulus differentiation under the control of the oocyte. J. Assist. Reprod. Genet. 2019, 36, 905–913. [Google Scholar] [CrossRef]
- Xu, Y.; Sun, M.H.; Li, X.H.; Ju, J.Q.; Chen, L.Y.; Sun, Y.R.; Sun, S.C. Modified hydrated sodium calcium aluminosilicate-supplemented diet protects porcine oocyte quality from zearalenone toxicity. Environ. Mol. Mutagen. 2021, 62, 124–132. [Google Scholar] [CrossRef] [PubMed]
- Joan, K.L.; Angelica Van, G.; Kristen, W.; Taylor, H.; Jasmine, F.; Chaohui, D. Importance of the pig as a human biomedical model. Sci. Transl. Med. 2021, 31, eabd5758. [Google Scholar] [CrossRef]
- Mesbah, F.; Kafi, M.; Nili, H. Cumulus cell expansion and first polar body extrusion during in vitro oocyte maturation in relation to morphological and morphometric characteristics of the dromedary camel ovary. Reprod. Domest. Anim. 2016, 51, 916–923. [Google Scholar] [CrossRef]
- Park, H.J.; Song, B.S.; Kim, J.W.; Yang, S.G.; Kim, S.U.; Koo, D.B. Exposure of triclosan in porcine oocyte leads to superoxide production and mitochondrial-mediated apoptosis during in vitro maturation. Int. J. Mol. Sci. 2020, 21, 3050. [Google Scholar] [CrossRef]
- Ufer, C.; Wang, C.C. The Roles of Glutathione Peroxidases during Embryo Development. Front. Mol. Neurosci. 2011, 4, 12. [Google Scholar] [CrossRef]
- Masgras, I.; Carrera, S.; de Verdier, P.J.; Brennan, P.; Majid, A.; Makhtar, W.; Tulchinsky, E.; Jones, G.D.D.; Roninson, I.B.; Macip, S. Reactive oxygen species and mitochondrial sensitivity to oxidative stress determine induction of cancer cell death by p21. J. Biol. Chem. 2012, 287, 9845–9854. [Google Scholar] [CrossRef]
- Szychowski, K.A.; Wnuk, A.; Rzemieniec, J.; Kajta, M.; Leszczyńska, T.; Wójtowicz, A.K. Triclosan-evoked neurotoxicity involves nmdar subunits with the specific role of glun2a in caspase-3-dependent apoptosis. Mol. Neurobiol. 2019, 56, 1–12. [Google Scholar] [CrossRef]
- Basini, G.; Grasselli, F.; Quintavalla, F.; Bussolati, S.; Andreoli, V.; Carrillo Heredero, A.M.; Bertini, S. Redox status, estrogen and progesterone production by swine granulosa cells are impaired by triclosan. Animals 2022, 12, 3559. [Google Scholar] [CrossRef]
- Kosińska, K.; Szychowski, K.A. Current state of knowledge of triclosan (TCS)-dependent reactive oxygen species (ROS) production. Environ. Res. 2024, 250, 118532–118545. [Google Scholar] [CrossRef] [PubMed]
- Xu, D.J.; Wu, L.; Jiang, X.H.; Yang, L.; Cheng, J.Y.; Chen, H.L.; Hua, R.M.; Geng, G.X.; Yang, L.L.; Li, Q.W.; et al. SIRT2 inhibition results in meiotic arrest, mitochondrial dysfunction, and disturbance of redox homeostasis during bovine oocyte maturation. Int. J. Mol. Sci. 2019, 20, 1365. [Google Scholar] [CrossRef]
- Dumollard, R.; Duchen, M.; Carroll, J. The role of mitochondrial function in the oocyte and embryo. Curr. Top. Dev. Biol. 2007, 77, 21–49. [Google Scholar] [CrossRef]
- Weatherly, L.M.; Shane, H.L.; Friend, S.A.; Lukomska, E.; Baur, R.; Anderson, S.E. Topical application of the antimicrobial agent triclosan induces nlrp3 inflammasome activation and mitochondrial dysfunction. Toxicol. Sci. 2020, 176, 147–161. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.Y.; Feng, R.; Wang, D.; Huo, T.G.; Jiang, H. Triclosan-induced glycolysis drives inflammatory activation in microglia via the Akt/mTOR/HIF 1α signaling pathway. Ecotoxicol. Environ. Saf. 2021, 224, 112664. [Google Scholar] [CrossRef] [PubMed]
- Guzel, E.; Arlier, S.; Guzeloglu-Kayisli, O.; Tabak, M.S.; Ekiz, T.; Semerci, N.; Larsen, K.; Schatz, F.; Lockwood, C.J.; Kayisli, U.A. Endoplasmic reticulum stress and homeostasis in reproductive physiology and pathology. Int. J. Mol. Sci. 2017, 18, 792. [Google Scholar] [CrossRef]
- Deng, S.W.; Li, C.F.; Chen, J.Q.; Cui, Z.; Lei, T.; Yang, H.J.; Chen, P. Effects of triclosan exposure on stem cells from human exfoliated deciduous teeth (SHED) fate. Sci. Total Environ. 2023, 905, 167053. [Google Scholar] [CrossRef]
- Malhotra, J.D.; Kaufman, R.J. ER stress and its functional link to mitochondria: Role in cell survival and death. Cold Spring Harb. Perspect. Biol. 2011, 3, a004424. [Google Scholar] [CrossRef]
- Li, X.Y.; Duan, J.X.; Wang, S.Y.; Cheng, J.Y.; Chen, H.L.; Zhang, Z.L.; Yang, L.; Hua, R.M.; Li, Q.W. Isorhamnetin protects porcine oocytes from zearalenone-induced reproductive toxicity through the PI3K/Akt signaling pathway. J. Anim. Sci. Biotechnol. 2023, 14, 22–35. [Google Scholar] [CrossRef]
- Rana, S.V.S. Endoplasmic reticulum stress induced by toxic elements-a review of recent developments. Biol. Trace Elem. Res. 2020, 196, 10–19. [Google Scholar] [CrossRef]
- Duerrschmidt, N.; Zabirnyk, O.; Nowicki, M.; Ricken, A.; Hmeidan, F.A.; Blumenauer, V.; Borlak, J.; Spanel-Borowski, K. Lectin-like oxidized low-density lipoprotein receptor-1-mediated autophagy in human granulosa cells as an alternative of programmed cell death. Endocrinology 2006, 147, 3851–3860. [Google Scholar] [CrossRef]
- Shen, X.H.; Jin, Y.X.; Liang, S.; Kwon, J.W.; Zhu, J.W.; Lei, L.; Kim, N.H. Autophagy is required for proper meiosis of porcine oocytes maturing in vitro. Sci. Rep. 2018, 8, 12581–12592. [Google Scholar] [CrossRef]
- Lee, S.; Hiradate, Y.; Hoshino, Y.; Tanemura, K.; Sato, E. Quantitative analysis in LC3-II protein in vitro maturation of porcine oocyte. Zygote 2014, 22, 404–410. [Google Scholar] [CrossRef] [PubMed]
- Jin, J.; Chen, N.W.; Pan, H.; Xie, W.H.; Xu, H.; Lei, S.Y.; Guo, Z.Q.; Ding, R.Y.; He, Y.; Gao, J.L. Triclosan induces ROS-dependent cell death and autophagy in A375 melanoma cells. Oncol. Lett. 2020, 20, 73–82. [Google Scholar] [CrossRef] [PubMed]
- Mariño, G.; Niso-Santano, M.; Baehrecke, E.H.; Kroemer, G. Self-consumption: The interplay of autophagy and apoptosis. Nat. Rev. Mol. Cell Biol. 2014, 15, 81–94. [Google Scholar] [CrossRef] [PubMed]
- Livesey, K.M.; Kang, R.; Vernon, P.; Buchser, W.; Loughran, P.; Watkins, S.C.; Zhang, L.; Manfredi, J.J.; Zeh, H.J., 3rd; Li, L.; et al. P53/HMGB1 complexes regulate autophagy and apoptosis. Cancer Res. 2012, 72, 1996–2005. [Google Scholar] [CrossRef]
- Szychowski, K.A.; Skóra, B.; Bar, M.; Piechowiak, T. Triclosan (TCS) affects the level of DNA methylation in the human oral squamous cell carcinoma (SCC-15) cell line in a nontoxic concentration. Biomed. Pharmacother. 2022, 149, 112815. [Google Scholar] [CrossRef]
- Ali, D.; Tripathi, A.; Al Ali, H.; Shahi, Y.; Mishra, K.K.; Alarifi, S.; Alkahtane, A.A.; Manohardas, S. ROS-dependent Bax/Bcl2 and caspase 3 pathway-mediated apoptosis induced by zineb in human keratinocyte cells. OncoTargets Ther. 2018, 11, 489–497. [Google Scholar] [CrossRef]
- Wang, D.; Liu, J.Y.; Jiang, H. Triclosan regulates the Nrf2/HO-1 pathway through the PI3K/Akt/JNK signaling cascade to induce oxidative damage in neurons. Environ. Toxicol. 2021, 36, 1953–1964. [Google Scholar] [CrossRef]
- Escobar Sánchez, M.L.; Echeverría Martínez, O.M.; Vázquez-Nin, G.H. Immunohistochemical and ultrastructural visualization of different routes of oocyte elimination in adult rats. Eur. J. Histochem. 2012, 56, e17. [Google Scholar] [CrossRef]
- Priyanka; Trivedi, A.; Maske, P.; Mote, C.; Dighe, V. Gestational and lactational exposure to triclosan causes impaired fertility of F1 male offspring and developmental defects in F2 generation. Environ. Pollut. 2020, 257, 113617. [Google Scholar] [CrossRef] [PubMed]
- Ning, T.; Peihong, F.; Li, C.; Xiaogang, Y.; Wenjuan, W.; Weiye, W.; Fengxiu, O. The Effect of Early Life Exposure to Triclosan on Thyroid Follicles and Hormone Levels in Zebrafish. Front. Endocrinol. 2022, 13, 850231. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, N.; Xu, A.; Yang, J.; Zhao, J.; Xie, J.; Li, B.; Duan, J.; Cao, G. Triclosan Caused Oocyte Meiotic Arrest by Modulating Oxidative Stress, Organelle Dysfunctions, Autophagy, and Apoptosis in Pigs. Animals 2025, 15, 802. https://doi.org/10.3390/ani15060802
Zhao N, Xu A, Yang J, Zhao J, Xie J, Li B, Duan J, Cao G. Triclosan Caused Oocyte Meiotic Arrest by Modulating Oxidative Stress, Organelle Dysfunctions, Autophagy, and Apoptosis in Pigs. Animals. 2025; 15(6):802. https://doi.org/10.3390/ani15060802
Chicago/Turabian StyleZhao, Ning, Anli Xu, Jingxian Yang, Jianan Zhao, Junhao Xie, Bugao Li, Jiaxin Duan, and Guoqing Cao. 2025. "Triclosan Caused Oocyte Meiotic Arrest by Modulating Oxidative Stress, Organelle Dysfunctions, Autophagy, and Apoptosis in Pigs" Animals 15, no. 6: 802. https://doi.org/10.3390/ani15060802
APA StyleZhao, N., Xu, A., Yang, J., Zhao, J., Xie, J., Li, B., Duan, J., & Cao, G. (2025). Triclosan Caused Oocyte Meiotic Arrest by Modulating Oxidative Stress, Organelle Dysfunctions, Autophagy, and Apoptosis in Pigs. Animals, 15(6), 802. https://doi.org/10.3390/ani15060802




