1. Introduction
In aquaponic systems, plants primarily obtain nutrients from fish waste [
1]. Aquaculture effluents are rich in nitrogen and phosphorus, derived from fish gill excretion, feces, and leftover feed [
2,
3]. However, despite the presence of these nutrients, certain mineral elements, particularly iron (Fe), are deficient in aquaponic systems, leading to plant growth deficiencies [
4,
5].
Iron deficiency in fish slows their growth and increases their susceptibility to anemia. Previous studies have demonstrated the critical role of iron in fish health. For instance, iron deficiency affects the lipid metabolism in yellow catfish
(Pelteobagrus fulvidraco) liver and reduces the activity of various antioxidant enzymes in the liver [
6]. Iron is also vital for immune activity in fish. Iron deficiency can impair phagocyte function; insufficient dietary iron increases the susceptibility of channel catfish(
Ictalurus punctatus) to bacterial infections [
7], whereas iron-supplemented diets significantly enhance macrophage migration in this species [
8]. Serum superoxide dismutase (SOD) activity correlates with serum iron levels [
9], and lysozyme activity serves as a reference for fish immunity [
10]. Iron-enriched feeds have been commercially adopted, with studies indicating that 580–1200 mg Fe/kg feed optimally promotes growth and improves hematological parameters in Nile tilapia (
Oreochromis niloticus) [
11]. However, exceeding 1500 mg/kg iron in feed negatively affects the growth and immunity of channel catfish (
Ictalurus punctatus) [
12].
The bioavailability of iron depends on its chemical form, such as Fe
3+ and Fe
2+, which can be from inorganic or organic complexes [
13]. Hence, the form of iron in feed profoundly affects its bioavailability. While fish can absorb iron through their gills, most of the iron absorption occurs in the gut [
13]. Excess iron can be transferred via transferrin to the gills and subsequently released into the water, where it becomes available for plant use in aquaponic systems [
14]. In bony fish, the gut serves as the primary absorption site for Fe, zinc, and copper, and intestinal regulation maintains the homeostasis of these metals [
15]. Craig et al. also highlighted the competitive interactions between iron and copper absorption in zebrafish (
Danio rerio) [
16].
In fish, serum transferrin serves as the primary transporter of iron, moving iron ions from storage sites to erythrocytes for hemoglobin synthesis or other iron-requiring tissues [
17]. The body lacks an active iron excretion mechanism and maintains the iron balance by regulating intestinal absorption, recycling iron from senescent erythrocytes, and releasing iron from mononuclear macrophages [
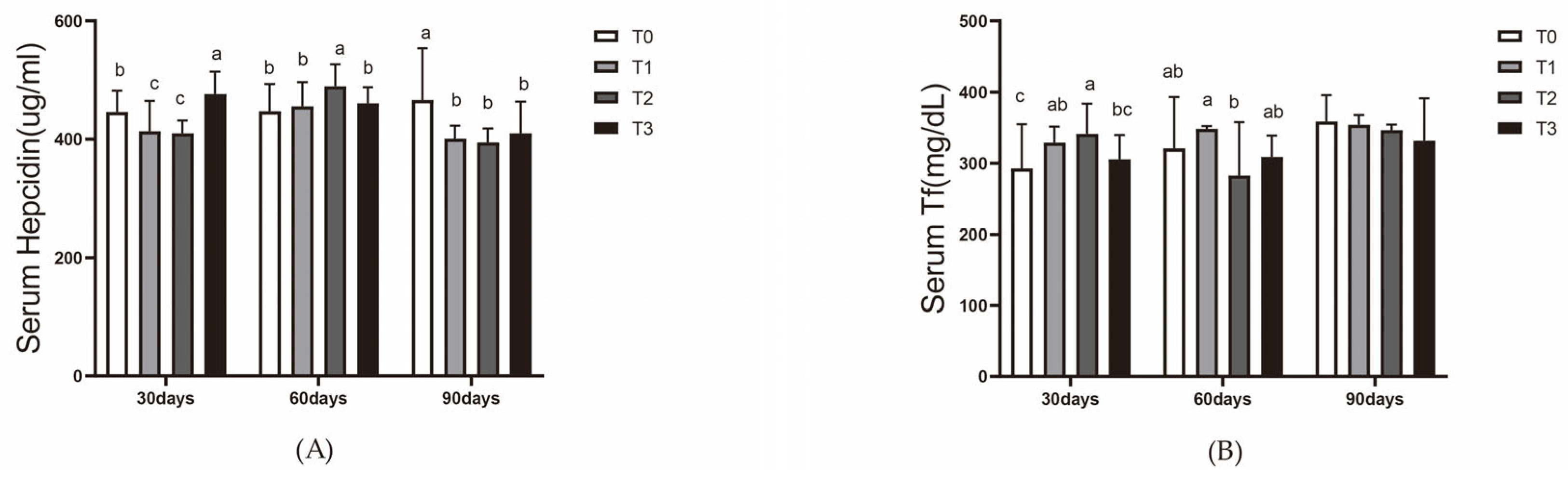
18]. Hepcidin, a hormone regulating iron homeostasis, senses and responds to changes in blood iron levels. Transferrin and hepcidin also play roles in fish immunity; for example, hepcidin has been identified as an antimicrobial peptide [
19], and transferrin expression increases during parasitic infection in carp [
20]. Both serve as valuable indicators for evaluating dietary iron supplementation in this study.
Tomatoes are highly adaptable to environmental changes, with a root system that is well suited for hydroponic systems, allowing for efficient nutrient absorption from the water. In hydroponic systems, tomatoes can be cultivated vertically or in compact spaces, thereby significantly enhancing yield per unit area [
21]. Furthermore, under aquaponic conditions, tomatoes exhibit improved disease resistance, even in the absence of pesticides [
22]. Pak choi, with its short growth cycle and strong adaptability, thrives in a variety of hydroponic systems. It has relatively low demands for temperature and water quality, making it particularly well suited for hydroponic systems [
23]. Based on these characteristics, tomatoes and pak choi were chosen as the experimental vegetables for this study.
Ferric EDTA, or ferric sodium ethylenediaminetetraacetate, is a chelated form of iron with the chemical formula C
10H
12FeN
2NaO
8. Commonly found as a yellowish-brown powder, it is used as a foliar spray in agriculture or as a dietary supplement. Without proper chelating agents, ferric ions in food precipitate at pH levels above 3.5, limiting absorption. Studies confirm that ferric EDTA facilitates iron absorption by physiological pathways, as iron dissociates from the chelate before absorption. Compared to inorganic iron solutions, ferric EDTA exhibits superior performance as a food additive [
24,
25].
In summary, fish primarily acquire iron through dietary intake, with transferrin transporting and storing iron in the liver. Hepcidin regulates iron uptake, which directly affects growth, hematopoiesis, and immunity. However, the optimal amount of iron released into the system by fish in aquaponic systems has not yet been determined. This study aims to determine the optimal level of iron supplementation in feed by evaluating plant growth parameters, the growth of mirror carp (Cyprinus carpio var. specularis), hematological and immune parameters, as well as hepatopancreatic condition to simultaneously meet the growth requirements of mirror carp and ensure that the iron released into the system through carp metabolism is sufficient for plant growth. Ferric EDTA is used as the iron source in this study to address the iron requirements in aquaponic systems.
2. Materials and Methods
2.1. Experimental Design
The 90-day experiment was conducted in a plastic greenhouse at the Taigu campus of Shanxi Agricultural University, covered with a 50% light-transmitting shade net. Twelve wooden fish tanks (90 cm length × 60 cm width × 70 cm height) were used, with tanks 1–6 arranged in a row on the eastern side and tanks 7–12 on the western side of the greenhouse. To balance light exposure, tanks 1, 6, and 7 were set as the control group (T0); tanks 2, 3, and 12 formed experimental group T1; tanks 4, 8, and 9 formed experimental group T2; and tanks 5, 10, and 11 formed experimental group T3.
Each tank was equipped with a water pump connected to a mat of vine cotton placed over a filter cloth, both housed in a plastic basket fixed above the tank. Filtered water flowed back into the tanks. An air pump with multi-porous aeration tubes supplied oxygen to all tanks. The water level was marked with a marker for monitoring, and water was added daily to maintain consistent tank volumes. Filters and vine cotton were not cleaned during the experiment to ensure a “no water change” and “no fertilizer” aquaponics concept. Each tank was fitted with a foam board (60 cm × 45 cm), perforated with six large holes and six small holes for planting. Tomato seedlings, sourced from the Taigu Juxin Agricultural Base, were 20.43 ± 1.25 cm in height, with a stem diameter of 3.24 ± 0.21 mm and 28.33 ± 3.27 leaves. Each large hole housed one tomato (
Solanum lycopersicum) seedling secured in planting cotton. Pak choi (
Brassica rapa subsp.
chinensis) seeds were pre-germinated for seven days in damp planting cotton, then placed in the smaller holes. Daily foliar spraying prevented pest damage. A loose plastic mesh was fixed above each tank to prevent fish from damaging roots or jumping out. Foam boards floated on the water, ensuring all planting cotton was in contact with the water (
Figure 1). The experiment used 240 uniformly sized mirror carp (
Cyprinus carpio var. specularis) (average length: 9.98 ± 0.47 cm; average weight: 19.66 ± 2.37 g), with 20 fish per tank. Temperature, pH, and dissolved oxygen (DO) levels were measured every 15 days using a thermometer, pH meter, and DO meter.
2.2. Feeding Method and Feed Preparation
Commercial 2 mm extruded feed was used. Ferric EDTA of analytical grade was dissolved in 100 mL distilled water, then evenly sprayed onto the feed and air-dried at room temperature for 12 h. Sodium alginate (≥99% purity) was dissolved in distilled water at a content of 2% (
w/
v) and sprayed onto the feed to form a protective coating, preventing the ferric EDTA from dissolving in the water. Only sodium alginate was applied for the control group, T0. Feed nutritional content was analyzed, and feed for each group was weighed before feeding. Fish were fed twice daily, with a feed amount equivalent to 1% of the total body weight in the tank per feeding. A fixed sound signal was used during feeding to condition the fish. Observations ensured that all feed was consumed within 20 min, leaving no residuals in the tank. The nutritional composition of the commercial feed was as follows: crude protein content 31.76 ± 9.41%, crude fat content 6.67 ± 0.55%, moisture content 15.24 ± 0.96%, and ash content 10.31 ± 0.21%. Regarding trace elements, the iron content was 337.35 ± 9.41 mg/kg, and the copper content was 56.8 ± 1.61 mg/kg. Additionally, the iron (Fe) content in the experimental feed groups was as follows: T0 group, 337.35 ± 9.41 mg/kg; T1 group, 514.22 ± 19.74 mg/kg; T2 group, 752.80 ± 31.82 mg/kg; and T3 group, 1178.81 ± 39.98 mg/kg (
Table 1).
2.3. Sampling and Measurement of Vegetables and Fish
Tomatoes: Plant height (cm): measured from the root collar to the growth tip using a measuring tape. Stem diameter (mm): measured 2 cm above the root collar using calipers. Leaf count: total number of fully expanded leaves manually counted. On Days 60 and 90, the number of flowers and fruits were recorded: Flower count: number of fully open flowers per plant. Fruit count: number of formed fruits per plant. Pak Choi: harvested on Day 60, fresh weight (g) was measured using an electronic balance.
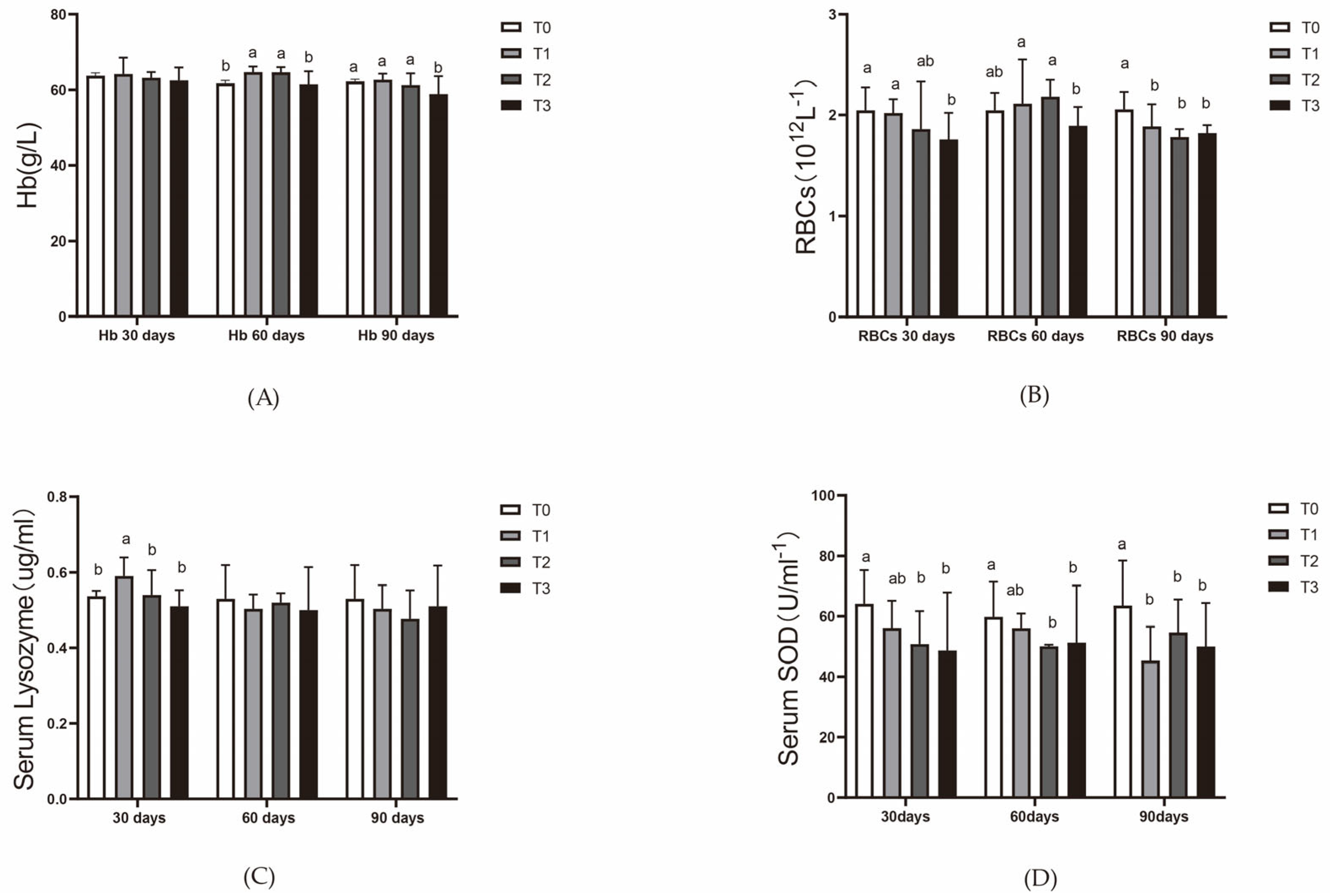
Every 30 days, four fish per tank were randomly sampled for length and weight measurements. The fish were anesthetized by placing them in an MS222 solution (25 g/m3, and measurements were taken once the fish no longer responded to stimuli. After measurements, the fish were immediately dissected. Blood was collected from the tail vein using a 1 mL syringe pre-washed with 2.7% EDTA and transferred to anticoagulant tubes. Blood was gently mixed and split into two parts: one part for red blood cell counts and hemoglobin content measurement, stored at 4 °C and analyzed on the same day, the other for serum separation via centrifugation (3500 rpm, 15 min, 4 °C). Serum was stored in sterile cryovials at −80 °C for subsequent analysis.
Fish were fasted for 24 h prior to health parameter analysis. The visceral mass was extracted, and the liver was divided into two parts: one for enzyme activity measurement at −80 °C and the other for histological sectioning.
Growth Performance Metrics:
The growth parameters of mirror carp were calculated based on sampling data from days 0–30, 31–60, and 61–90. Specifically, the initial weight on day 60 was the final weight recorded on day 30, and the initial weight on day 90 was the final weight recorded on day 60.
2.4. Determination of Fish Nutritional Composition
The contents of moisture, crude protein, crude fat, and ash in fish samples were analyzed according to standard laboratory protocols (AOAC). Crude protein was measured using the Kjeldahl nitrogen determination method [
26].Crude fat was determined by the Soxhlet extraction method [
27]. Moisture content and ash content were evaluated following standard procedures [
28,
29].
2.5. Determination of Elemental Content in Water and Mirror Carp (Cyprinus carpio var. specularis) Liver
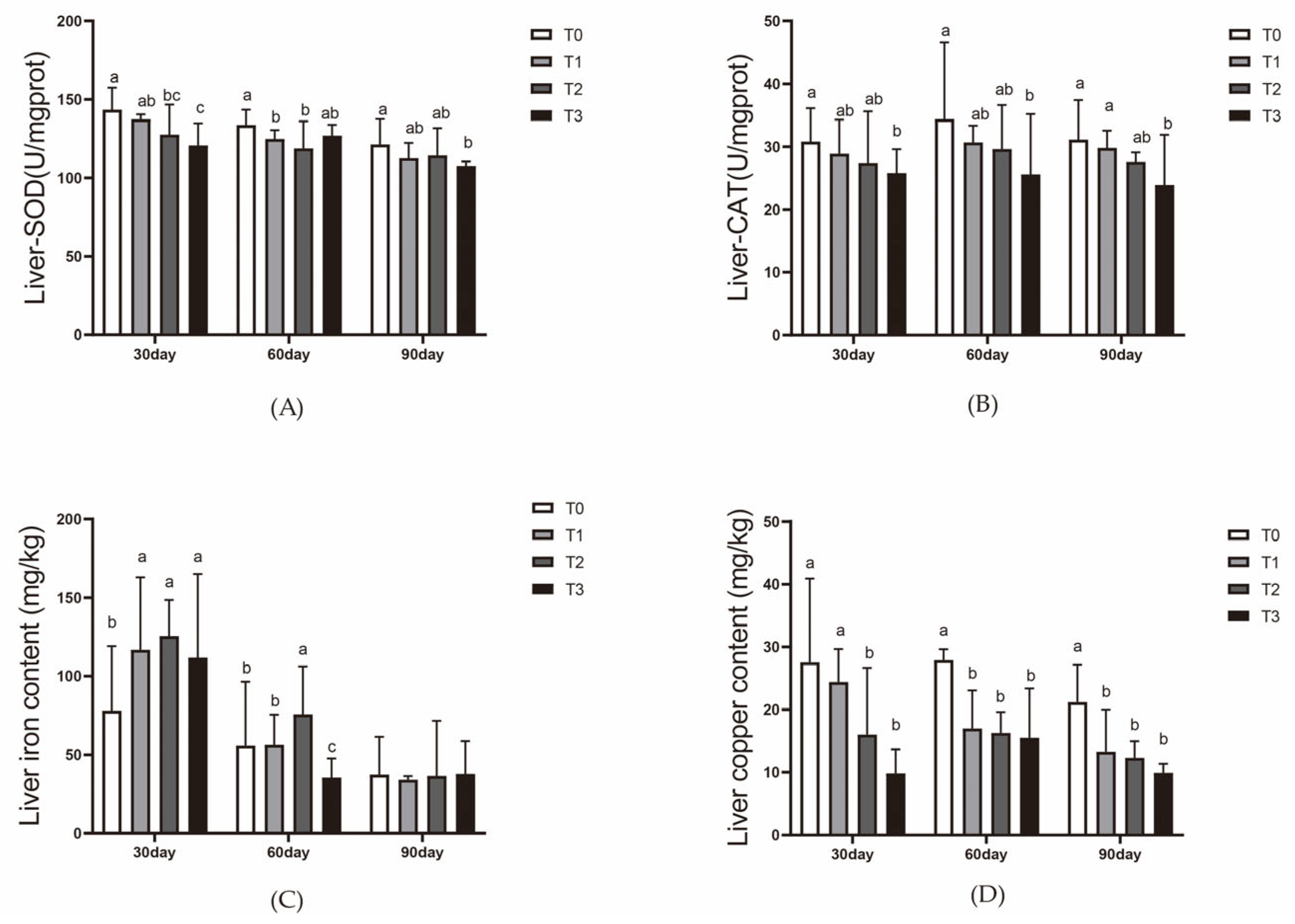
Elemental content in liver and water samples was quantified using an inductively coupled plasma optical emission spectrometer (ICP-OES) manufactured by Shimadzu, Kyoto, Japan. Samples were prepared using microwave digestion [
30].
2.6. Processing of Blood and Tissue Samples
Red blood cell count was performed using a hemocytometer. Hemoglobin content, serum SOD activity, serum lysozyme activity, serum transferrin levels, and serum hepcidin levels were determined using commercial kits provided by Nanjing Jiancheng Bioengineering Institute, Nanjing, China. Hepatic SOD activity and CAT activity were also assessed using these kits.
2.7. Tissue Sectioning
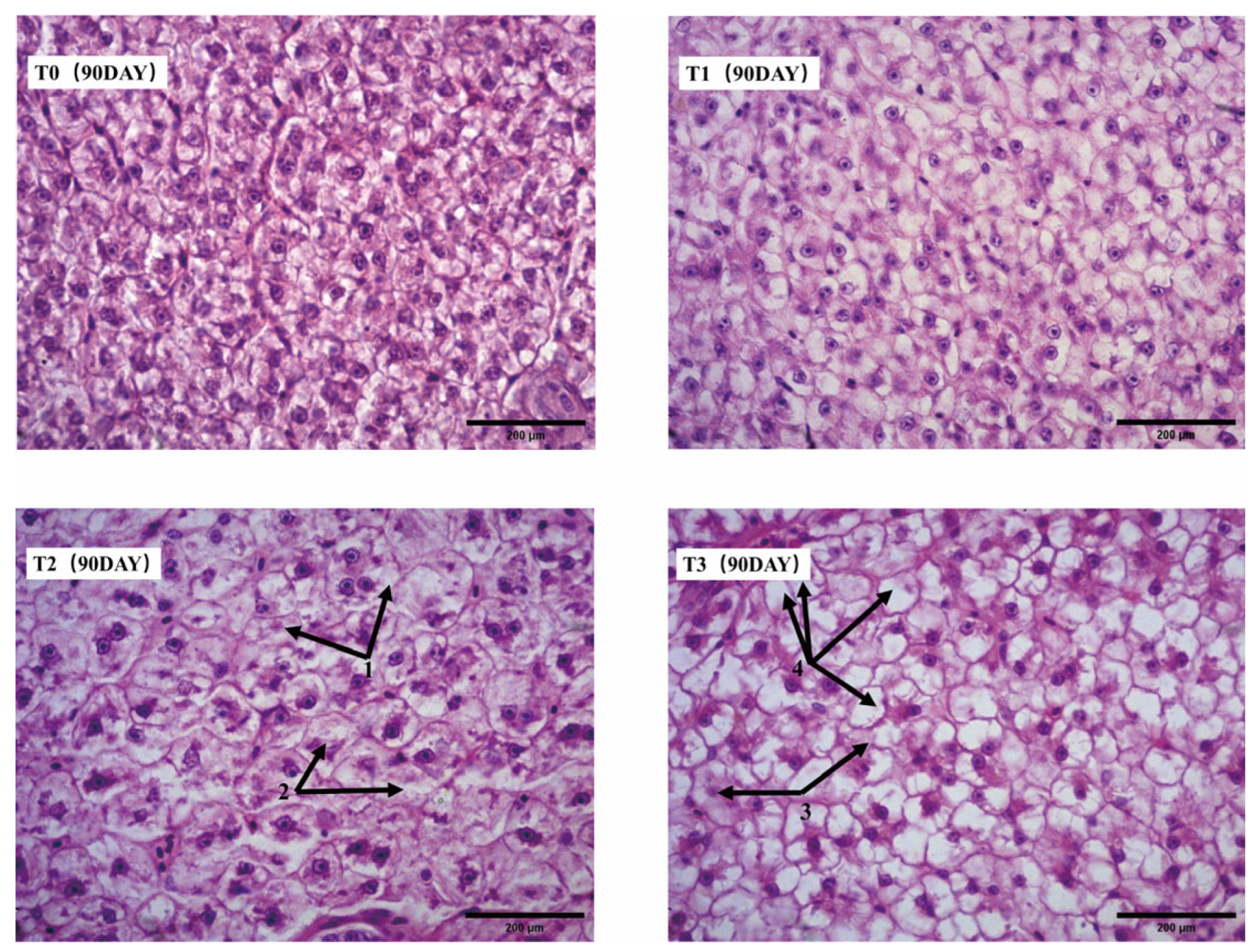
Liver tissue fragments were fixed in 4% paraformaldehyde solution, embedded in paraffin, sectioned into 5 μm slices, and mounted on glass slides. After dehydration, the sections were stained with hematoxylin and eosin (H&E) to observe histological changes. Image analysis and measurement were performed using IMAGE J 1.5.3 software.
2.8. Data Analysis Methods
All data are expressed as mean ± S.E.M. One-way analysis of variance (ANOVA) was performed using SPSS 26.0 (IBM) for Windows to determine significance levels. Duncan’s multiple range test was employed for multiple comparisons. Statistical significance was set at p < 0.05. Graphs were created using GraphPad Prism 6.0 (GraphPad Software, San Diego, CA, USA).
5. Conclusions
In this 90-day experiment, the addition of ferric EDTA to the feed significantly promoted the growth of vegetables (tomatoes and pak choi) in the aquaponic system. Adequate iron supplementation also mitigated the effects of root acidification caused by iron deficiency, which reduces the impact of lowered pH on fish. On day 30, the iron content in the liver of the mirror carp (Cyprinus carpio var. specularis) in the T1 (200 mg/kg) and T2 (400 mg/kg) groups reached its peak, while liver copper levels were consistently lower in the experimental groups than in the control group throughout the 90-day period. After 60 days of feeding, the T1 and T2 groups showed enhanced hematopoiesis and improved immunity in the mirror carp. However, by day 90, slight degeneration of hepatopancreatic cells was observed in the T2 group, while the T3 group, fed with 800 mg/kg ferric EDTA, exhibited growth suppression and liver and pancreas tissue damage.
Overall, these results suggest that the T2 group—which received 200 mg/kg of EDTA iron in the feed, with a total iron content of 514.22 mg/kg in the feed over 60 days in a non-water-changing aquaponic system—produced optimal fish and vegetable yield. These findings provide valuable insights for optimizing ferric EDTA supplementation in floating valve-based aquaponic systems.