Impact of Panax notoginseng Residue on Rumen Microbial Community, Blood Biochemical Parameters and Growth Performance in Cattle: A Preliminary Study on Its Potential as a Feed Resource
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Design
2.2. Rumen Microbial Sequencing Analysis
3. Results
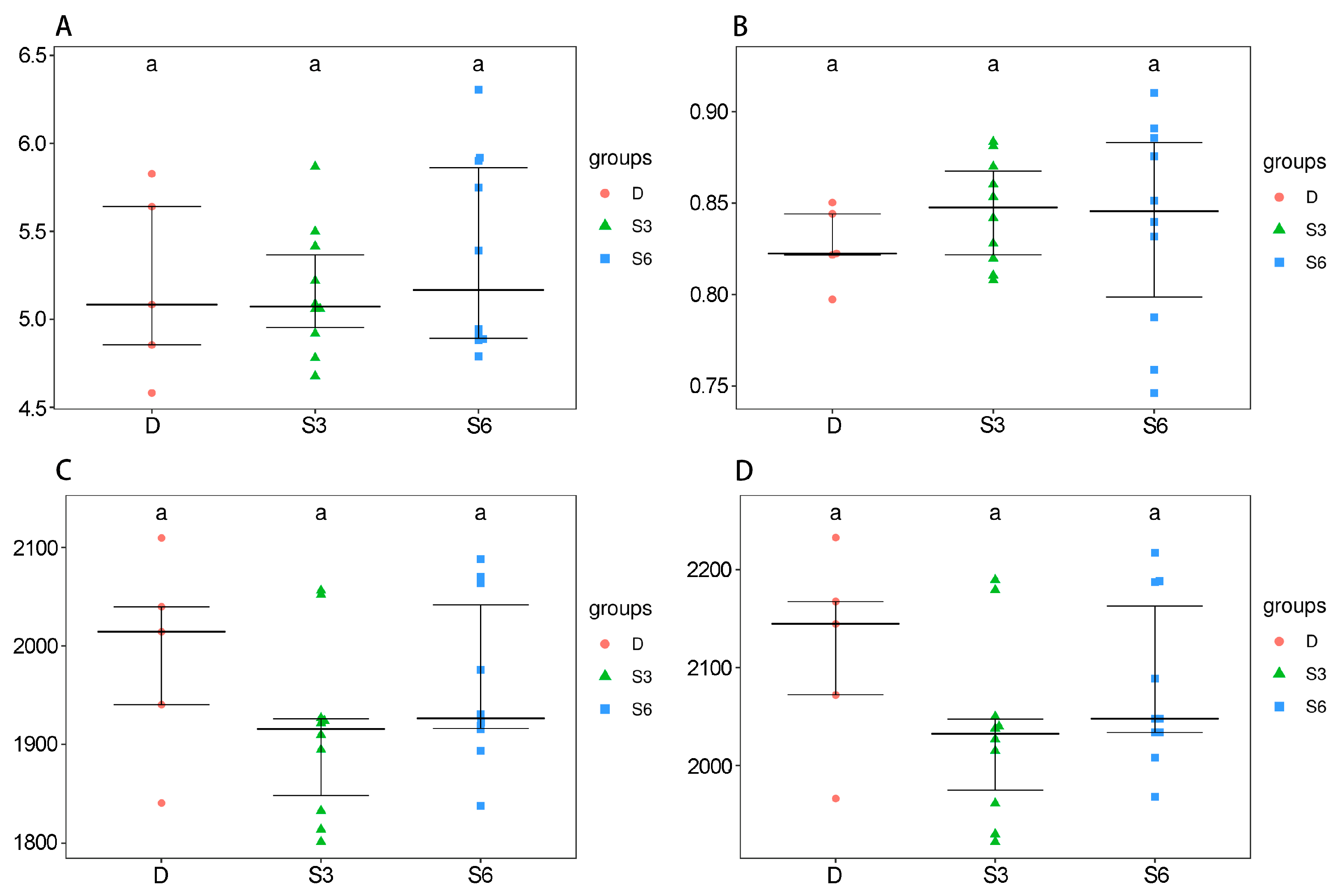
3.1. Effects of Panax notoginseng on Blood Biochemical Parameters and Body Weight of Wenshan Cattle
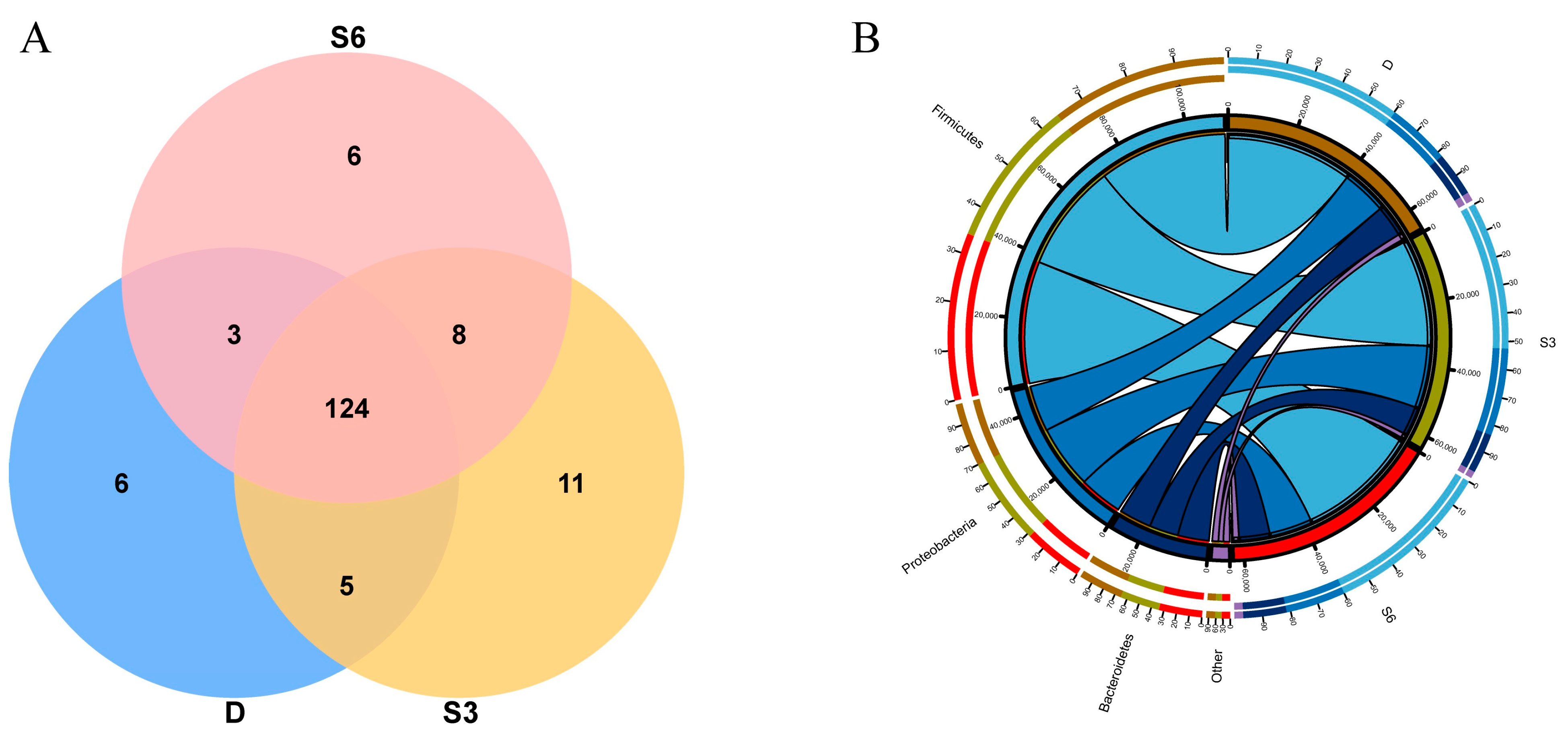
3.2. Rumen Microbial Sequencing Results
3.3. Alpha Diversity Analysis
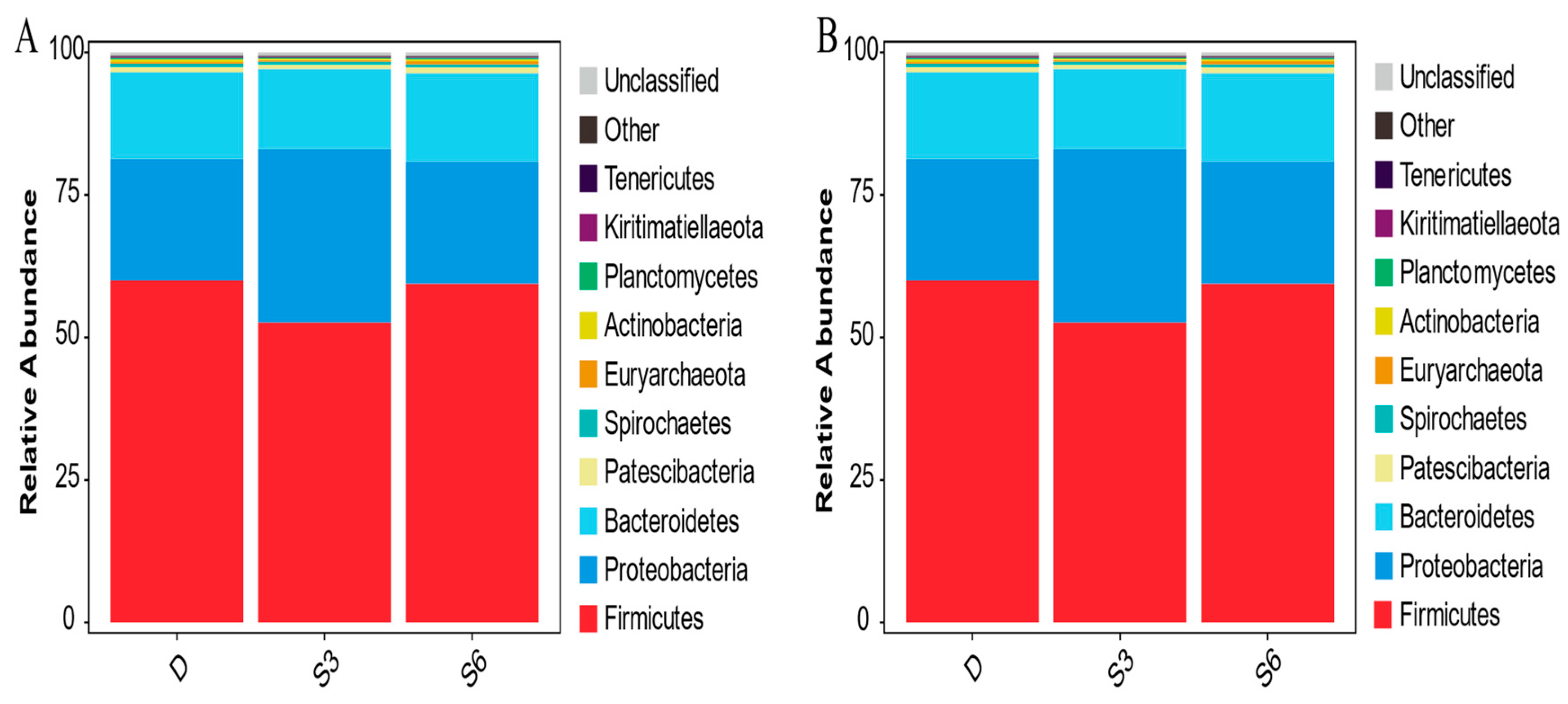
3.4. Analysis of Microbial Abundance and Composition in Rumen
3.5. Rumen Microbial Community Structure
3.6. Differential Microbiological Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhong, J.; Ni, K.; Yang, J.; Yu, Z.; Tao, Y. The present situation and prospect of the processing technology of forage grass in China. Chin. Sci. Bull. 2018, 63, 1677–1685. [Google Scholar] [CrossRef]
- Gerber, P.J.; Steinfeld, H. Worldwide growth of animal production and environmental consequences. Ecol. Future-Bulg. J. Ecol. Sci. 2008, 17–26. [Google Scholar]
- Warren, W.J. The Meat Question: Animals, Humans, and the Deep History of Food. Agric. Hist. 2021, 95, 380–382. [Google Scholar]
- Pinotti, L.; Manoni, M.; Fumagalli, F.; Rovere, N.; Luciano, A.; Ottoboni, M.; Ferrari, L.; Cheli, F.; Djuragic, O. Reduce, Reuse, Recycle for Food Waste: A Second Life for Fresh-Cut Leafy Salad Crops in Animal Diets. Animals 2020, 10, 1082. [Google Scholar] [CrossRef]
- An, X.; Zhang, S.; Li, T.; Chen, N.; Wang, X.; Zhang, B.; Ma, Y. Transcriptomics analysis reveals the effect of Broussonetia papyrifera L. fermented feed on meat quality traits in fattening lamb. PeerJ 2021, 9, e11295. [Google Scholar] [CrossRef]
- Du, Z.; Yang, F.; Fang, J.; Yamasaki, S.; Oya, T.; Nguluve, D.; Kumagai, H.; Cai, Y. Silage preparation and sustainable livestock production of natural woody plant. Front. Plant Sci. 2023, 14, 1253178. [Google Scholar] [CrossRef]
- Cassir, N.; Benamar, S.; La Scola, B. Clostridium butyricum: From beneficial to a new emerging pathogen. Clin. Microbiol. Infect. 2016, 22, 37–45. [Google Scholar] [CrossRef]
- Goenaga, I.; Garcia-Rodriguez, A.; Goiri, I.; Leon-Ecay, S.; De Las Heras, J.; Aldai, N.; Insausti, K. Vegetable By-Products as Alternative and Sustainable Raw Materials for Ruminant Feeding: Nutritive Evaluation and Their Inclusion in a Novel Ration for Calf Fattening. Animals 2023, 13, 1391. [Google Scholar] [CrossRef]
- Şen Mutlu, M.I.; Yildirim, A. Effect of dietary supplementation of Panax ginseng leaf extract on production performance and egg quality of hens at the beginning of their laying period. Large Anim. Rev. 2020, 26, 341–348. [Google Scholar]
- Yldrm, A. Changes in quality characteristics during storage time of eggs from layer hens fed diet supplemented with Panax ginseng Meyer leaf extract. Prog. Nutr. 2017, 19, 197–204. [Google Scholar]
- Wang, T.; Guo, R.; Zhou, G.; Zhou, X.; Kou, Z.; Sui, F.; Li, C.; Tang, L.; Wang, Z. Traditional uses, botany, phytochemistry, pharmacology and toxicology of Panax notoginseng (Burk.) F.H. Chen: A review. J. Ethnopharmacol. 2016, 188, 234–258. [Google Scholar] [CrossRef] [PubMed]
- Liying, Z. Feed Analysis and Feed Quality Detection Technology; China Agricultural University Press: Beijing, China, 2016. [Google Scholar]
- McCleary, B.V.; Monaghan, D.A. Measurement of resistant starch. J. AOAC Int. 1994, 77, 1062–1069. [Google Scholar] [CrossRef]
- Guo, M.; Wu, F.; Hao, G.; Qi, Q.; Li, R.; Li, N.; Wei, L.; Chai, T. Bacillus subtilis Improves Immunity and Disease Resistance in Rabbits. Front. Immunol. 2017, 8, 354. [Google Scholar] [CrossRef]
- Wellmann, K.B.; Kim, J.; Urso, P.M.; Smith, Z.K.; Johnson, B.J. Evaluation of the dietary vitamin A requirement of finishing steers via systematic depletion and repletion, and its effects on performance and carcass characteristics. J. Anim. Sci. 2020, 98, skaa266. [Google Scholar] [CrossRef]
- Nkrumah, J.D.; Crews, D.H., Jr.; Basarab, J.A.; Price, M.A.; Okine, E.K.; Wang, Z.; Li, C.; Moore, S.S. Genetic and phenotypic relationships of feeding behavior and temperament with performance, feed efficiency, ultrasound, and carcass merit of beef cattle. J. Anim. Sci. 2007, 85, 2382–2390. [Google Scholar] [CrossRef]
- Jiang, X.L.; Ma, G.F.; Zhao, B.B.; Meng, Y.; Chen, L.L. Structural characterization and immunomodulatory activity of a novel polysaccharide from Panax notoginseng. Front. Pharmacol. 2023, 14, 1190233. [Google Scholar] [CrossRef]
- Nkrumah, J.D.; Sherman, E.L.; Li, C.; Marques, E.; Crews, D.H., Jr.; Bartusiak, R.; Murdoch, B.; Wang, Z.; Basarab, J.A.; Moore, S.S. Primary genome scan to identify putative quantitative trait loci for feedlot growth rate, feed intake, and feed efficiency of beef cattle. J. Anim. Sci. 2007, 85, 3170–3181. [Google Scholar] [CrossRef][Green Version]
- Walsh, R.B.; Walton, J.S.; Kelton, D.F.; LeBlanc, S.J.; Leslie, K.E.; Duffield, T.F. The effect of subclinical ketosis in early lactation on reproductive performance of postpartum dairy cows. J. Dairy Sci. 2007, 90, 2788–2796. [Google Scholar] [CrossRef]
- Vimalraj, S. Alkaline phosphatase: Structure, expression and its function in bone mineralization. Gene 2020, 754, 144855. [Google Scholar] [CrossRef]
- Mancuso, C. Panax notoginseng: Pharmacological Aspects and Toxicological Issues. Nutrients 2024, 16, 2120. [Google Scholar] [CrossRef]
- Yu, Q.; Zeng, K.W.; Ma, X.L.; Jiang, Y.; Tu, P.F.; Wang, X.M. Ginsenoside Rk1 suppresses pro-inflammatory responses in lipopolysaccharide-stimulated RAW264.7 cells by inhibiting the Jak2/Stat3 pathway. Chin. J. Nat. Med. 2017, 15, 751–757. [Google Scholar] [CrossRef] [PubMed]
- Tang, F.; Lu, M.; Yu, L.; Wang, Q.; Mei, M.; Xu, C.; Han, R.; Hu, J.; Wang, H.; Zhang, Y. Inhibition of TNF-alpha-mediated NF-kappaB Activation by Ginsenoside Rg1 Contributes the Attenuation of Cardiac Hypertrophy Induced by Abdominal Aorta Coarctation. J. Cardiovasc. Pharmacol. 2016, 68, 257–264. [Google Scholar] [CrossRef] [PubMed]
- Lan, R.X.; Park, J.W.; Lee, D.W.; Kim, I.H. Effects of Astragalus membranaceus, Codonopsis pilosula and allicin mixture on growth performance, nutrient digestibility, faecal microbial shedding, immune response and meat quality in finishing pigs. J. Anim. Physiol. Anim. Nutr. 2017, 101, 1122–1129. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Li, Z.; Liu, S.; Tang, T.; Chen, Q.; Yan, Z.; Peng, J.; Yang, Z.; Zhang, G.; Liu, Y.; et al. Fermented Chinese Herbal Medicine Promoted Growth Performance, Intestinal Health, and Regulated Bacterial Microbiota of Weaned Piglets. Animals 2023, 13, 476. [Google Scholar] [CrossRef]
- Li, L.; Wang, H.; Zhang, N.; Zhang, T.; Ma, Y. Effects of alpha-glycerol monolaurate on intestinal morphology, nutrient digestibility, serum profiles, and gut microbiota in weaned piglets. J. Anim. Sci. 2022, 100, skac046. [Google Scholar] [CrossRef]
- Evans, N.J.; Brown, J.M.; Murray, R.D.; Getty, B.; Birtles, R.J.; Hart, C.A.; Carter, S.D. Characterization of novel bovine gastrointestinal tract Treponema isolates and comparison with bovine digital dermatitis treponemes. Appl. Environ. Microbiol. 2011, 77, 138–147. [Google Scholar] [CrossRef]
- Sharma, V.; Vashishtha, A.; Jos, A.L.M.; Khosla, A.; Basu, N.; Yadav, R.; Bhatt, A.; Gulani, A.; Singh, P.; Lakhera, S.; et al. Phylogenomics of the Phylum Proteobacteria: Resolving the Complex Relationships. Curr. Microbiol. 2022, 79, 224. [Google Scholar] [CrossRef]
- Couch, C.E.; Stagaman, K.; Spaan, R.S.; Combrink, H.J.; Sharpton, T.J.; Beechler, B.R.; Jolles, A.E. Diet and gut microbiome enterotype are associated at the population level in African buffalo. Nat. Commun. 2021, 12, 2267. [Google Scholar] [CrossRef]
- Lau, S.K.P.; Teng, J.L.L.; Chiu, T.H.; Chan, E.; Tsang, A.K.L.; Panagiotou, G.; Zhai, S.L.; Woo, P.C.Y. Differential Microbial Communities of Omnivorous and Herbivorous Cattle in Southern China. Comput. Struct. Biotechnol. J. 2018, 16, 54–60. [Google Scholar] [CrossRef]
- Zou, C.; Gu, Q.; Zhou, X.; Xia, Z.; Muhammad, W.I.; Tang, Q.; Liang, M.; Lin, B.; Qin, G. Ruminal microbiota composition associated with ruminal fermentation parameters and milk yield in lactating buffalo in Guangxi, China-A preliminary study. J. Anim. Physiol. Anim. Nutr. 2019, 103, 1374–1379. [Google Scholar] [CrossRef]
- Jin, L.; Huang, Y.; Yang, S.; Wu, D.; Li, C.; Deng, W.; Zhao, K.; He, Y.; Li, B.; Zhang, G.; et al. Diet, habitat environment and lifestyle conversion affect the gut microbiomes of giant pandas. Sci. Total Environ. 2021, 770, 145316. [Google Scholar] [CrossRef]

| Items | D | S3 | S6 |
|---|---|---|---|
| Corn | 15.60 | 15.60 | 15.60 |
| Soybean meal | 5.00 | 5.00 | 5.00 |
| Barley | 3.30 | 3.30 | 3.30 |
| Wheat bran | 2.10 | 2.10 | 2.10 |
| Premix (1) | 3.00 | 3.00 | 3.00 |
| Molasses | 3.00 | 3.00 | 3.00 |
| NaCl | 1.00 | 1.00 | 1.00 |
| NaHCO3 | 1.00 | 1.00 | 1.00 |
| Corn silage | 50.00 | 48.00 | 46.00 |
| Dry rice straw | 16.00 | 15.00 | 14.00 |
| PNR | 0.00 | 3.00 | 6.00 |
| Total | 100.00 | 100.00 | 100.00 |
| Nutrient levels (2) | |||
| NEmf/(MJ/kg) | 10.92 | 10.88 | 10.85 |
| CP | 13.41 | 13.30 | 13.26 |
| EE | 4.31 | 4.28 | 4.25 |
| Starch | 26.19 | 25.67 | 25.17 |
| NDF | 34.26 | 33.30 | 34.52 |
| ADF | 17.51 | 17.53 | 17.60 |
| Ca | 0.92 | 0.87 | 0.90 |
| P | 0.56 | 0.54 | 0.50 |
| Items | Content (%) |
|---|---|
| Ash | 8.35 |
| CP | 8.75 |
| EE | 0.53 |
| Starch | 16.00 |
| CF | 15.3 |
| ADF | 25.3 |
| NDF | 45.1 |
| Crude polysaccharide | 1.20 |
| Total flavones | <0.01 |
| Total saponins | 0.01 |
| Index | D | S3 | S6 | p-Value |
|---|---|---|---|---|
| CHOL (mmol/L) | 2.79 ± 0.89 ab | 3.20 ± 0.39 a | 2.59 ± 0.32 b | 0.047 |
| LDL-CH (mmol/L) | 0.80 ± 0.35 ab | 1.08 ± 0.12 a | 0.61 ± 0.25 b | 0.043 |
| TG (mmol/L) | 0.24 ± 0.10 a | 0.12 ± 0.04 b | 0.21 ± 0.10 a | 0.041 |
| HDL-CH (mmol/L) | 1.67 ± 0.37 | 1.76 ± 0.24 | 1.73 ± 0.24 | 0.807 |
| GGT (U/L) | 16.64 ± 6.86 a | 16.61 ± 6.36 a | 21.52 ± 2.83 b | 0.037 |
| ALB (g/L) | 38.91 ± 3.55 | 38.90 ± 1.60 | 39.13 ± 1.81 | 0.987 |
| ALT (U/L) | 27.82 ± 2.81 b | 31.16 ± 4.59 a | 24.92 ± 3.06 c | 0.050 |
| IBIL (umol/L) | 1.93 ± 0.68 a | 2.32 ± 0.76 b | 2.42 ± 0.60 b | 0.033 |
| ALP (U/L) | 2.60 ± 1.86 a | 1.34 ± 0.93 c | 1.90 ± 1.20 b | 0.028 |
| GLOB (g/L) | 41.03 ± 3.01 | 37.55 ± 4.86 | 39.41 ± 2.74 | 0.354 |
| AST (U/L) | 104.52 ± 13.88 a | 103.84 ± 27.90 a | 90.80 ± 16.87 b | 0.049 |
| DBIL (umol/L) | 1.90 ± 0.73 | 1.78 ± 0.57 | 2.19 ± 0.85 | 0.671 |
| TBIL (umol/L) | 3.83 ± 0.98 | 4.10 ± 1.31 | 4.60 ± 1.38 | 0.613 |
| TP (g/L) | 79.94 ± 3.21 | 76.45 ± 5.51 | 78.53 ± 1.86 | 0.381 |
| UREA (mmol/L) | 4.56 ± 0.80 | 4.36 ± 0.58 | 4.49 ± 0.46 | 0.897 |
| GLU (mmol/L) | 8.06 ± 1.74 a | 6.17 ± 1.31 b | 6.53 ± 2.09 b | 0.031 |
| Items | D | S3 | S6 | p-Value |
|---|---|---|---|---|
| Pretrial weight (kg) | 368.64 ± 6.25 | 370.85 ± 7.68 | 369.54 ± 5.76 | 0.832 |
| Post-test weight (kg) | 438.84 ± 7.61 b | 448.25 ± 5.38 a | 443.34 ± 4.56 ab | 0.026 |
| Average daily feed intake (kg/d) | 9.10 ± 0.62 | 9.00 ± 0.79 | 9.08 ± 0.46 | 0.675 |
| ADG (kg/d) | 0.78 ± 0.07 b | 0.86 ± 0.05 a | 0.82 ± 0.08 ab | 0.031 |
| Item | D | S3 | S6 | p-Value |
|---|---|---|---|---|
| Solibacillus | 36.60 ± 5.73 a | 25.39 ± 14.98 b | 38.13 ± 7.79 a | 0.049 |
| Acinetobacter | 20.69 ± 14.86 b | 33.68 ± 11.43 a | 15.97 ± 4.88 c | 0.026 |
| Rikenellaceae_RC9_gut_group | 5.63 ± 1.36 | 5.31 ± 1.30 | 6.32 ± 0.92 | 0.656 |
| Lysinibacillus | 5.17 ± 2.50 | 7.22 ± 6.24 | 5.10 ± 2.17 | 0.081 |
| Christensenellaceae_R-7_group | 4.35 ± 0.81 | 3.82 ± 0.40 | 4.94 ± 0.83 | 0.161 |
| Prevotella_1 | 2.91 ± 1.19 | 1.92 ± 0.52 | 2.43 ± 0.23 | 0.199 |
| Saccharofermentans | 1.59 ± 0.46 | 1.47 ± 0.30 | 1.89 ± 0.28 | 0.268 |
| Succiniclasticum | 1.56 ± 0.84 | 1.03 ± 0.23 | 1.54 ± 0.39 | 0.228 |
| Ruminococcaceae_NK4A214_group | 1.12 ± 0.24 | 0.94 ± 0.09 | 1.17 ± 0.27 | 0.298 |
| Comamonas | 0.21 ± 0.31 c | 1.54 ± 2.08 a | 0.53 ± 0.97 b | 0.045 |
| Other | 10.57 ± 2.31 | 9.30 ± 2.14 | 11.88 ± 1.56 | 0.360 |
| Unclassified | 9.58 ± 2.76 | 8.38 ± 0.71 | 10.11 ± 1.56 | 0.069 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, D.; Wang, K.; Lu, Y.; Gao, Z.; Chong, Y.; Hong, J.; Wu, J.; Deng, W.; He, X.; Xi, D. Impact of Panax notoginseng Residue on Rumen Microbial Community, Blood Biochemical Parameters and Growth Performance in Cattle: A Preliminary Study on Its Potential as a Feed Resource. Animals 2025, 15, 788. https://doi.org/10.3390/ani15060788
Wu D, Wang K, Lu Y, Gao Z, Chong Y, Hong J, Wu J, Deng W, He X, Xi D. Impact of Panax notoginseng Residue on Rumen Microbial Community, Blood Biochemical Parameters and Growth Performance in Cattle: A Preliminary Study on Its Potential as a Feed Resource. Animals. 2025; 15(6):788. https://doi.org/10.3390/ani15060788
Chicago/Turabian StyleWu, Dongwang, Kai Wang, Ying Lu, Zhendong Gao, Yuqing Chong, Jieyun Hong, Jiao Wu, Weidong Deng, Xiaoming He, and Dongmei Xi. 2025. "Impact of Panax notoginseng Residue on Rumen Microbial Community, Blood Biochemical Parameters and Growth Performance in Cattle: A Preliminary Study on Its Potential as a Feed Resource" Animals 15, no. 6: 788. https://doi.org/10.3390/ani15060788
APA StyleWu, D., Wang, K., Lu, Y., Gao, Z., Chong, Y., Hong, J., Wu, J., Deng, W., He, X., & Xi, D. (2025). Impact of Panax notoginseng Residue on Rumen Microbial Community, Blood Biochemical Parameters and Growth Performance in Cattle: A Preliminary Study on Its Potential as a Feed Resource. Animals, 15(6), 788. https://doi.org/10.3390/ani15060788





