Storage Conditions of Sperm Samples and Gametic Characterization by Sperm Head Morphometry in Drones (Apis mellifera)
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Design
2.2. Semen Collection and Sperm Variables Evaluation
2.3. Supplementation with Catalase of the Drone Extender
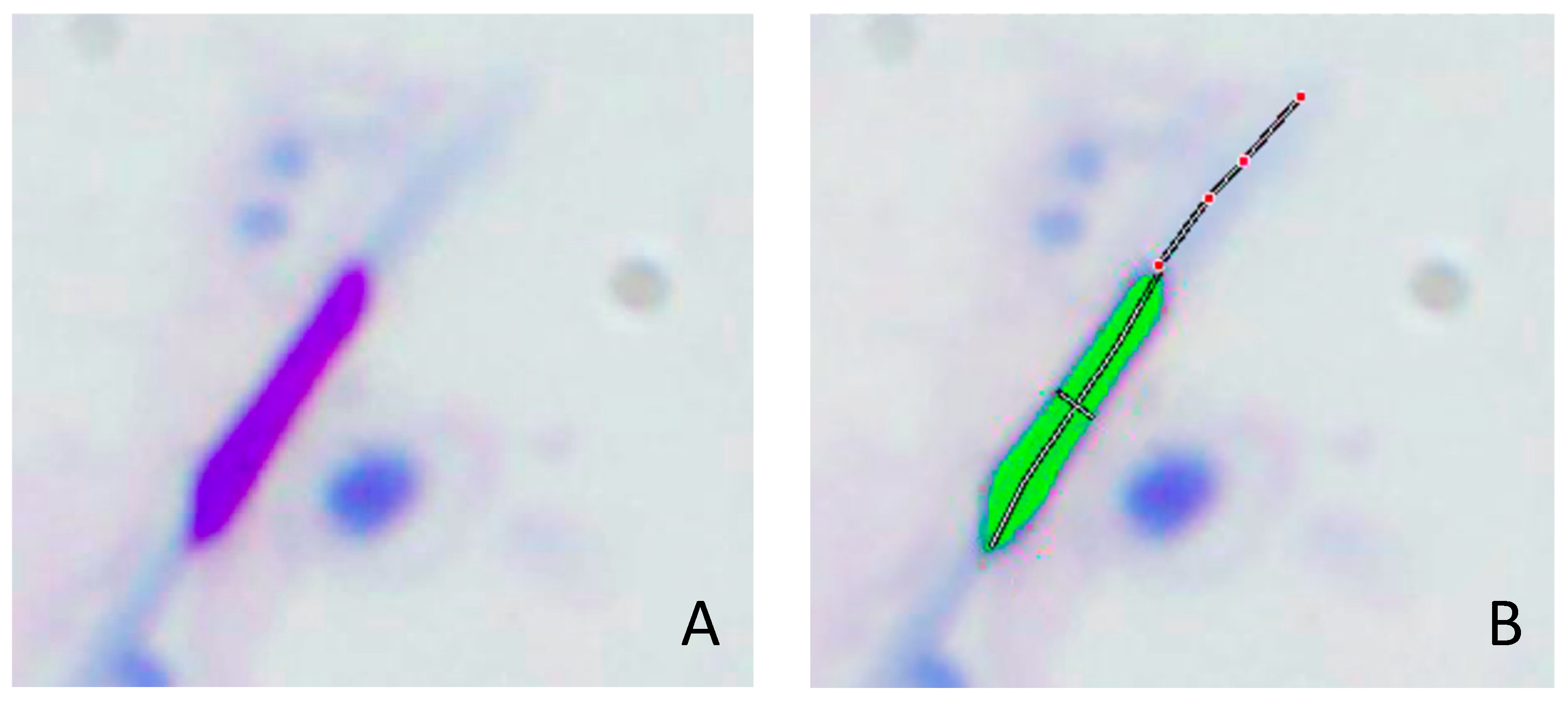
2.4. Morphometric Analysis of the Sperm Head
2.5. Statistical Analysis
3. Results
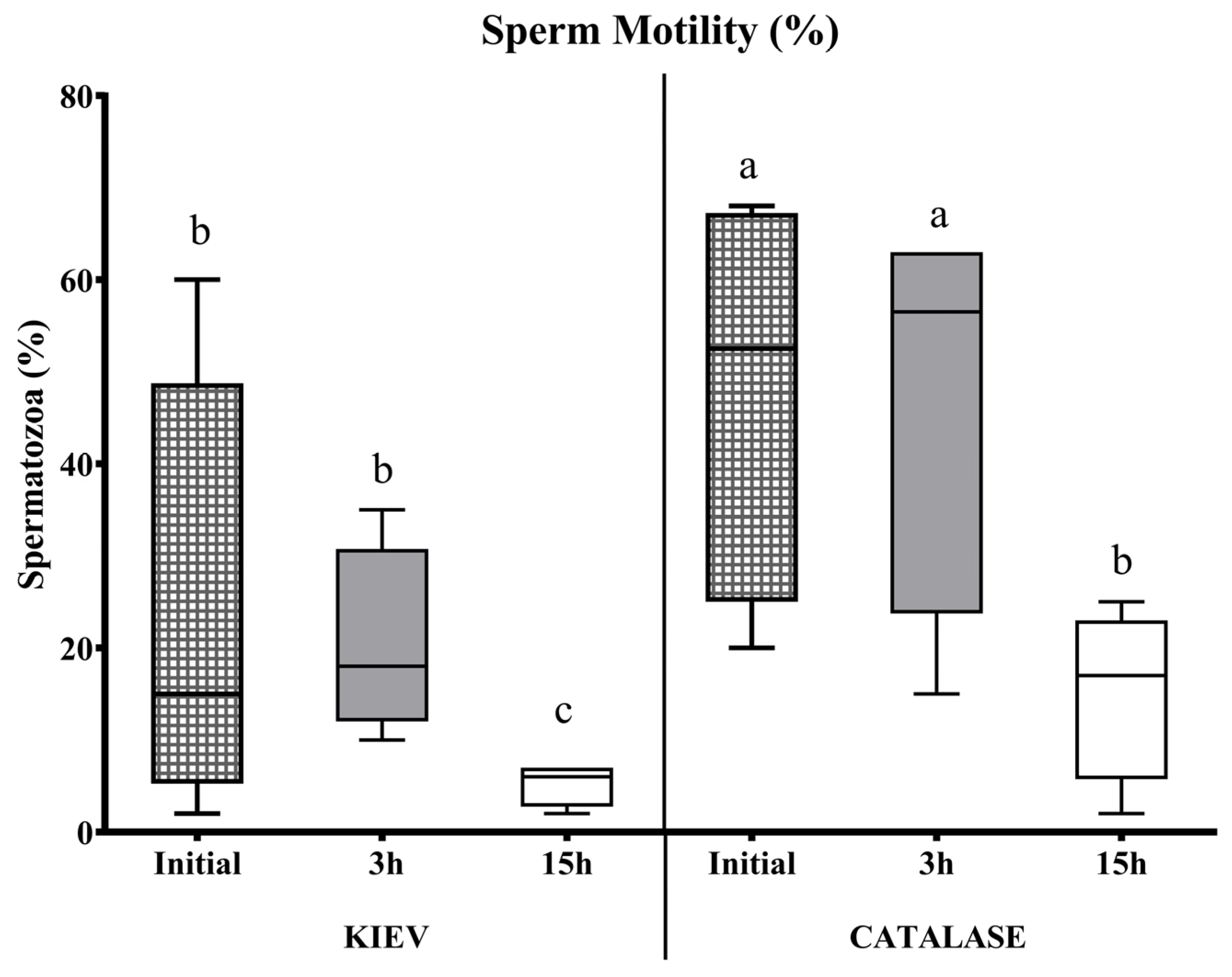
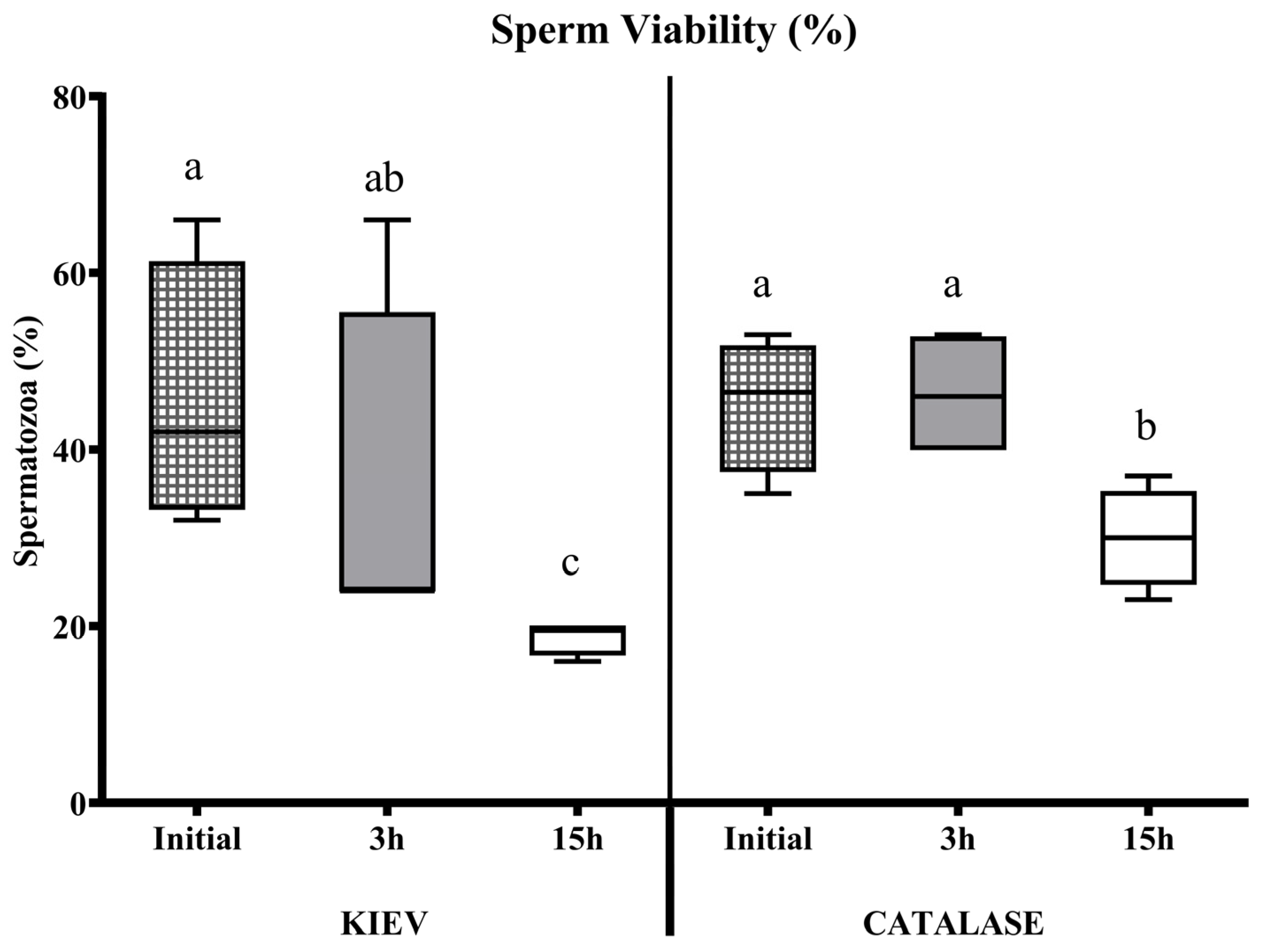
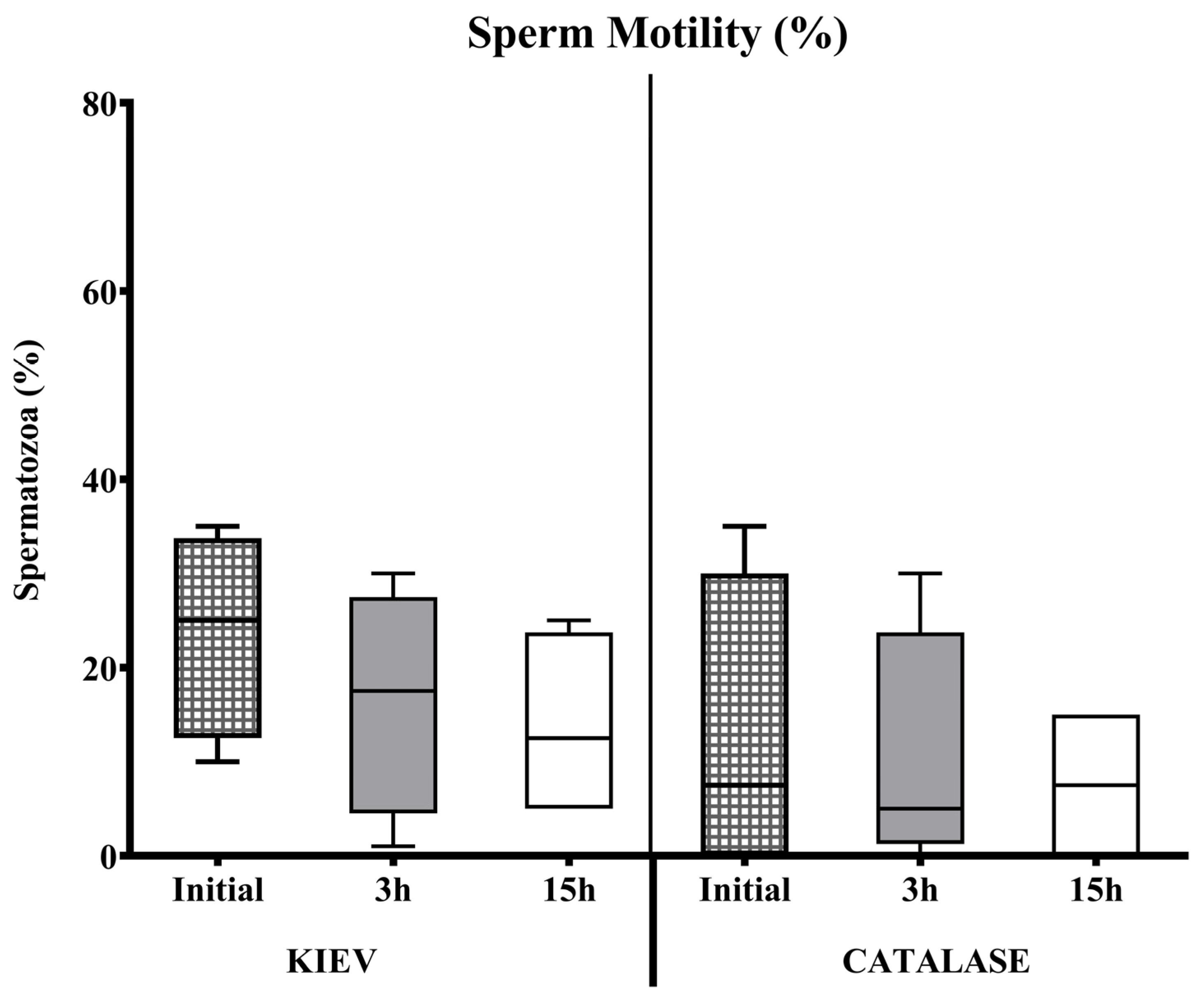
3.1. Experiment 1
3.2. Experiment 2
3.3. Experiment 3
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Grassl, J.; Holt, S.; Cremen, N.; Peso, M.; Hahne, D.; Baer, B. Synergistic effects of pathogen and pesticide exposure on honey bee (Apis mellifera) survival and immunity. J. Invertebr. Pathol. 2018, 159, 78–86. [Google Scholar] [CrossRef] [PubMed]
- Hristov, P.; Shumkova, R.; Palova, N.; Neov, B. Factors Associated with Honey Bee Colony Losses: A Mini-Review. Vet. Sci. 2020, 7, 166. [Google Scholar] [CrossRef] [PubMed]
- Brutscher, L.M.; Baer, B.; Niño, E.L. Putative Drone Copulation Factors Regulating Honey Bee (Apis mellifera) Queen Reproduction and Health: A Review. Insects 2019, 10, 8. [Google Scholar] [CrossRef]
- Murcia-Morales, M.; Heinzen, H.; Parrilla-Vázquez, P.; Gómez-Ramos, M.; Fernández-Alba, A.R. Presence and distribution of pesticides in apicultural products: A critical appraisal. TrAC Trends Anal. Chem. 2022, 146, 116506. [Google Scholar] [CrossRef]
- Benito-Murcia, M.; Botías, C.; Martín-Hernández, R.; Higes, M.; Soler, F.; Perez-Lopez, M.; Míguez-Santiyán, M.P.; Martinez-Morcillo, S. Biomarker responses and lethal dietary doses of tau-fluvalinate and coumaphos in honey bees: Implications for chronic acaricide toxicity. Environ. Toxicol. Pharmacol. 2024, 105, 104330. [Google Scholar] [CrossRef] [PubMed]
- Tavera-Mendoza, L.; Ruby, S.; Brousseau, P.; Fournier, M.; Cyr, D.; Marcogliese, D. Response of the amphibian tadpole (Xenopus laevis) to atrazine during sexual differentiation of the testis. Environ. Toxicol. Chem. 2002, 21, 527–531. [Google Scholar] [CrossRef]
- Castellanos, P.; Maroto-Morales, A.; García-Álvarez, O.; Garde, J.J.; Mateo, R. Identification of optimal concentrations and incubation times for the study of in vitro effects of Pb in ram spermatozoa. Bull. Environ. Contam. Toxicol. 2013, 91, 197–201. [Google Scholar] [CrossRef]
- Vallverdú-Coll, N.; Mougeot, F.; Ortiz-Santaliestra, M.E.; Castaño, C.; Santiago-Moreno, J.; Mateo, R. Effects of Lead Exposure on Sperm Quality and Reproductive Success in an Avian Model. Environ. Sci. Technol. 2016, 50, 12484–12492. [Google Scholar] [CrossRef]
- Schlüns, H.; Koeniger, G.; Koeniger, N.; Moritz, R.F.A. Sperm utilization pattern in the honey bee (Apis mellifera). Behav. Ecol. Sociobiol. 2004, 56, 458–463. [Google Scholar] [CrossRef]
- Collins, A.M.; Donoghue, A.M. Viability assessment of honey bee, Apis mellifera, sperm using dual fluorescent staining. Theriogenology 1999, 51, 1513–1523. [Google Scholar] [CrossRef]
- Gontarz, A.; Banaszewska, D.; Gryzinska, M.; Andraszek, K. Differences in drone sperm morphometry and activity at the beginning and end of the season. Turk. J. Vet. Anim. Sci. 2016, 40, 598–602. [Google Scholar] [CrossRef]
- Taber, S.; Blum, M.S. Preservation of Honey Bee Semen. Science 1960, 131, 1734–1735. [Google Scholar] [CrossRef]
- Collins, A.M. Survival of honey bee (Hymenoptera: Apidae) spermatozoa stored at above-freezing temperatures. J. Econ. Entomol. 2000, 93, 568–571. [Google Scholar] [CrossRef]
- Taylor, M.A.; Guzmán-Novoa, E.; Morfin, N.; Buhr, M.M. Improving viability of cryopreserved honey bee (Apis mellifera L.) Sperm with selected diluents, cryoprotectants, and semen dilution ratios. Theriogeniology 2009, 72, 149–159. [Google Scholar] [CrossRef]
- Aitken, R.J.; Gibb, Z.; Baker, M.A.; Drevet, J.; Gharagozloo, P. Causes and consequences of oxidative stress in spermatozoa. Reprod. Fertil. Dev. 2016, 28, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Blesbois, E.; Grasseau, I.; Blum, J.C. Effects of vitamin E on fowl semen storage at 4 °C. Theriogenology 1993, 39, 771–779. [Google Scholar] [CrossRef]
- Bilodeau, J.F.; Chatterjee, S.; Sirard, M.A.; Gagnon, C. Levels of antioxidant defenses are decreased in bovine spermatozoa after a cycle of freezing and thawing. Mol. Rep. Dev. 2000, 55, 282–288. [Google Scholar] [CrossRef]
- Mata-Campuzano, M.; Álvarez-Rodríguez, M.; Tamayo-Canul, J.; López-Urueña, E.; de Paz, P.; Anel, L.; Martínez-Pastor, F.; Álvarez, M. Refrigerated storage of ram sperm in presence of Trolox and GSH antioxidants: Effect of temperature, extender and storage time. Anim. Reprod. Sci. 2014, 151, 137–147. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Fan, X.; Zeng, Y.; Wang, L.; Zhu, Z.; Li, R.; Tian, X.; Wang, Y.; Lin, Y.; Wu, D.; et al. Resveratrol protects boar sperm in vitro via its antioxidant capacity. Zygote 2020, 28, 417–424. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Santos, M.R.; Domínguez-Rebolledo, A.E.; Esteso, M.C.; Garde, J.J.; Martínez-Pastor, F. Refrigerated storage of red deer epididymal spermatozoa in the epididymis, diluted and with vitamin C supplementation. Reprod. Domest. Anim. 2009, 44, 212–220. [Google Scholar] [CrossRef]
- Kowalczyk, A.M.; Klećkowska-Nawrot, J.; Łukaszewicz, E.T. Effect of selenium and vitamin E addition to the extender on liquid stored capercaillie (Tetrao urogallus) semen quality. Reprod. Domest. Anim. 2017, 52, 603–609. [Google Scholar] [CrossRef] [PubMed]
- Goodarzi, P.; Akhlaghi, A.; Zamiri, M.J.; Shirazi, M.R.J.; Akhlaghi, A.A.; Habibi, M.; Daryabari, H.; Saemi, F.; Peebles, E.D. Sperm characteristics of Chukar partridge (Alectoris chukar) breeders as affected by the addition of calcitriol to the semen extender. Poult. Sci. 2019, 98, 3292–3297. [Google Scholar] [CrossRef]
- Papas, A.M. Diet and antioxidant status. Food Chem. Toxicol. 1999, 37, 999–1007. [Google Scholar] [CrossRef] [PubMed]
- Garrido, N.; Meseguer, M.; Simon, C.; Pellicer, A.; Remohi, J. Pro-oxidative and anti-oxidative imbalance in human semen and its relation with male fertility. Asian J. Androl. 2004, 6, 59–65. [Google Scholar] [PubMed]
- Roca, J.; Rodríguez, M.J.; Gil, M.A.; Carvajal, G.; García, E.M.; Cuello, C.; Vázquez, J.M.; Martínez, E.A. Survival and in vitro fertility of boar spermatozoa frozen in the presence of superoxide dismutase and/or catalase. J. Androl. 2005, 26, 15–24. [Google Scholar] [CrossRef]
- Michael, A.; Alexopoulos, C.; Pontiki, E.; Hadjipavlou-Litina, D.; Saratsis, P.; Boscos, C. Effect of antioxidant supplementation on semen quality and reactive oxygen species of frozen-thawed canine spermatozoa. Theriogenology 2007, 68, 204–212. [Google Scholar] [CrossRef] [PubMed]
- Silvestre, M.A.; Yániz, J.L.; Peña, F.J.; Santolaria, P.; Castelló-Ruiz, M. Role of Antioxidants in Cooled Liquid Storage of Mammal Spermatozoa. Antioxidants 2021, 10, 1096. [Google Scholar] [CrossRef] [PubMed]
- García-Cuenca Ariati, L.; Pérez Fuentes, J.; Cuéllar Cariñanos, C.; Fontanillas Pérez, J.C. Características espermáticas del zángano (Apis mellifera L.). Mundo Ganad. 1991, 3, 49–53. [Google Scholar]
- Chalah, T.; Brillard, J.P. Comparison of assessment of fowl sperm viability by eosin-nigrosin and dual fluorescence (SYBR-14/PI). Theriogenology 1998, 50, 487–493. [Google Scholar] [CrossRef]
- Villaverde-Morcillo, S.; Esteso, M.C.; Castaño, C.; Toledano Díaz, A.; López- Sebastián, A.; Campo, J.L.; Santiago-Moreno, J. Influence of staining method on the values of avian sperm head morfometry variables. Reprod. Domest. Anim. 2015, 50, 750–755. [Google Scholar] [CrossRef]
- Collins, A.M.; Williams, V.; Evans, J.D. Sperm storage and antioxidative enzyme expression in the honey bee, Apis Mellifera. Insect Mol. Biol. 2004, 13, 141–146. [Google Scholar] [CrossRef] [PubMed]
- Tarliyah, L.; Boediono, A.; Walujo, D. Motility of honey bee Apis mellifera L. (Hymenoptera: Apidae) Spermatozoa in various storage temperature in dilution media containing different glucose levels. Media Vet. 1999, 6, 15–20. [Google Scholar]
- Harbo, J.R.; Williams, J.L. Effect of above-freezing temperatures on temporary storage of honeybee spermatozoa. J. Apic Res. 1987, 26, 53–55. [Google Scholar] [CrossRef]
- Verma, L.R. An ionic basis for a possible mechanism of sperm survival in the spermatheca of the queen honey bee (Apis mellifera L.). Comp. Biochem. Physiol. Part A Physiol. 1973, 44, 1325–1331. [Google Scholar] [CrossRef]
- Falchi, L.; Galleri, G.; Zedda, M.T.; Pau, S.; Bogliolo, L.; Ariu, F.; Ledda, S. Liquid storage of ram semen for 96 h: Effects on kinematic parameters, membranes and DNA integrity, and ROS production. Livest. Sci. 2018, 207, 1–6. [Google Scholar] [CrossRef]
- Sadeghi, S.; Del Gallego, R.; García-Colomer, B.; Gómez, E.A.; Yániz, J.L.; Gosálvez, J.; López-Fernández, C.; Silvestre, M.A. Effect of Sperm Concentration and Storage Temperature on Goat Spermatozoa during Liquid Storage. Biology 2020, 9, 300. [Google Scholar] [CrossRef]
- Miyake, M.; Ito, Y.; Suzuki, H.; Tomizawa, M.; Sato, H.; Liu, M.; Okamura, A.; Nakajima, T.; Ohtani, K.; Takino, H.; et al. Epididymal phospholipidosis is a possible mechanism for spermatotoxicity induced by the organophosphorus insecticide fenitrothion in rats. Toxicol. Lett. 2018, 285, 27–33. [Google Scholar] [CrossRef] [PubMed]
- Verma, L.R. Biology of honeybee (Apis mellifera L.) spermatozoa. Effect of different diluents on motility and survival. Apidologie 1978, 9, 167–174. [Google Scholar] [CrossRef]
- Moritz, R.F. The effect of diluents on the insemination success in the honey bee using mixed semen. J. Apic. Res. 1984, 23, 164–167. [Google Scholar] [CrossRef]
- Aitken, R.J.; Bennetts, L. Reactive Oxygen Species: Friend or Foe. In The Sperm Cell; De Jonge, C., Barratt, C., Eds.; University of Cambridge Press: Cambridge, UK, 2006; pp. 170–193. [Google Scholar] [CrossRef]
- Gonzalez, A.N.; Ing, N.; Rangel, J. Upregulation of antioxidant genes in the spermathecae of honey bee (Apis mellifera) queens after mating. Apidologie 2018, 49, 224–234. [Google Scholar] [CrossRef]
- Baer, B.; Heazlewood, J.L.; Taylor, N.L.; Eubel, H.; Millar, A.H. The seminal fluid proteome of the honeybee Apis mellifera. Proteomics 2009, 9, 2085–2097. [Google Scholar] [CrossRef] [PubMed]
- Weirich, G.; Collins, A.; Williams, V. Antioxidant enzymes in the honey bee, Apis mellifera. Apidologie 2002, 33, 3–14. [Google Scholar] [CrossRef]
- Shaliutina-Kolešová, A.; Gazo, I.; Cosson, J.; Linhart, O. Protection of common carp (Cyprinus carpio L.) spermatozoa motility under oxidative stress by antioxidants and seminal plasma. Fish. Phys. Biochem. 2014, 40, 1771–1781. [Google Scholar] [CrossRef] [PubMed]
- Ali, L.; Hassan Andrabi, S.M.; Ahmed, H.; Hussain Shah, A.A. Freezability of water buffalo spermatozoa is improved with the addition of catalase in cryodiluent. Cryo Lett. 2017, 38, 145–154. [Google Scholar] [PubMed]
- Del Prete, C.; Stout, T.; Montagnaro, S.; Pagnini, U.; Uccello, M.; Florio, P.; Ciani, F.; Tafuri, S.; Palumbo, V.; Pasolini, M.P.; et al. Combined addition of superoxide dismutase, catalase and glutathione peroxidase improves quality of cooled stored stallion semen. Anim. Reprod. Sci. 2019, 210, 106195. [Google Scholar] [CrossRef] [PubMed]
- Sekoni, V.O.; Gustafsson, B.K. Seasonal variations in the incidence of sperm morphological abnormalities in dairy bulls regularly used for artificial insemination. Br. Vet. J. 1987, 143, 312–317. [Google Scholar] [CrossRef] [PubMed]
- Gravance, C.; Lewis, K.M.; Casey, P.J. Computer automated sperm head morphometry analysis (ASMA) of goat spermatozoa. Theriogenology 1995, 44, 989–1002. [Google Scholar] [CrossRef]
- Ball, B.A.; Mohammed, H.O. Morphometry of stallion spermatozoa by computer-assisted image analysis. Theriogenology 1995, 44, 367–377. [Google Scholar] [CrossRef]
- Brito, L.F.C. Evaluation of Stallion Sperm Morphology. Clin. Tech. Equine Pract. 2007, 6, 249–264. [Google Scholar] [CrossRef]
- Esteso, M.C.; Rodríguez, E.; Toledano-Díaz, A.; Castaño, C.; Pradiee, J.; López-Sebastián, A.; Santiago-Moreno, J. Descriptive analysis of sperm head morphometry in Iberian ibex (Capra pyrenaica): Optimum sampling procedure and staining methods using Sperm-Class Analyzer®. Anim. Reprod. Sci. 2015, 155, 42–49. [Google Scholar] [CrossRef] [PubMed]
- Gravance, C.G.; Liu, I.K.; Davis, R.O.; Hughes, J.P.; Casey, P.J. Quantification of normal head morphometry of stallion spermatozoa. J. Reprod. Fertil. 1996, 108, 41–46. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Buendía, P.; Soler, C.; Paolicchi, F.; Gago, G.; Urquieta, B.; Pérez-Sánchez, F.; Bustos-Obregón, E. Morphometric characterization and classification of alpaca sperm heads using the sperm-class analyzer computer-assisted system. Theriogenology 2002, 57, 1207–1218. [Google Scholar] [CrossRef] [PubMed]
- Núñez-Martínez, I.; Moran, J.M.; Peña, F.J. Do computer-assisted, morphometric-derived sperm characteristics reflect DNA status in canine spermatozoa? Reprod. Domest. Anim. 2005, 40, 537–543. [Google Scholar] [CrossRef]
- Esteso, M.C.; Toledano-Díaz, A.; Castaño, C.; Pradiee, J.; López-Sebastián, A.; Santiago-Moreno, J. Effect of two cooling protocols on the post-thaw characteristics of Iberian ibex sperms. Cryobiology 2018, 80, 12–17. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Liu, Z.; Pan, Q.; Chen, X.; Wang, H.; Guo, H.; Liu, S.; Lu, H.; Tian, S.; Li, R.; et al. Genomic analyses reveal demographic history and temperate adaptation of the newly discovered honey bee subspecies Apis mellifera sinisxinyuan n. ssp. Mol. Biol. Evol. 2016, 33, 1337–1348. [Google Scholar] [CrossRef]
- De la Rúa, P.; Jaffé, R.; Dall’Olio, R.; Muñoz, I.; Serrano, J. Biodiversity, conservation and current threats to European honeybees. Apidologie 2009, 40, 263–284. [Google Scholar] [CrossRef]
- Bouga, M.; Alaux, C.; Bienkowska, M.; Büchler, R.; Carreck, N.L.; Cauia, E.; Chlebo, R.; Dahle, B.; Dall’Olio, R.; De la Rúa, P.; et al. A review of methods for discrimination of honey bee populations as applied to European beekeeping. J. Apic. Res. 2011, 50, 51–84. [Google Scholar] [CrossRef]
- Muñoz, I.; De la Rúa, P. Wide genetic diversity in Old World honey bees threaten by introgression. Apidologie 2021, 52, 200–217. [Google Scholar] [CrossRef]
| Sperm parameters after 3 h incubation | 5 °C | 15 °C | 37 °C + 5% CO2 |
| Motility (%) | 1.5 ± 0.5 ab | 10.0 ± 5.0 b | 0.0 ± 0.0 a |
| Score (0–5) | 0.5 ± 0.0 | 0.8 ± 0.3 | 0.0 ± 0.0 |
| Viability (%) | 10.0 ± 8.0 | 12.5 ± 2.5 | 0.5 ± 0.5 |
| Sperm parameters after 15 h incubation | 5 °C | 15 °C | 37 °C + 5% CO2 |
| Motility (%) | 2.5 ± 2.5 | 6.5 ± 3.5 | 0.0 ± 0.0 |
| Score (0–5) | 0.25 ± 0.25 | 0.5 ± 0.0 | 0.0 ± 0.0 |
| Viability (%) | 5.0 ± 3.0 | 10.5 ± 4.5 | 4.5 ± 3.5 |
| CV (%) | ||||||
|---|---|---|---|---|---|---|
| Origin | L | W | A | P | LAcros | |
| UMU | Within-drone | 4.18 | 13.03 | 11.03 | 3.78 | 9.42 |
| Between-drones | 3.29 | 7.90 | 9.49 | 3.04 | 4.80 | |
| CIAPA-IRIAF | Within-drone | 4.98 | 12.88 | 9.27 | 4.14 | 10.84 |
| Between-drones | 2.04 | 5.82 | 6.79 | 1.96 | 4.43 | |
| FAUNIA | Within-drone | 4.96 | 16.71 | 18.35 | 5.21 | 12.04 |
| Between-drones | 3.79 | 13.95 | 11.72 | 4.52 | 5.39 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Esteso, M.C.; Toledano-Díaz, A.; Castaño, C.; Higes, M.; Martín-Hernández, R.; López-Goya, A.; De la Rúa, P.; Martínez-Madrid, B.; Santiago-Moreno, J. Storage Conditions of Sperm Samples and Gametic Characterization by Sperm Head Morphometry in Drones (Apis mellifera). Animals 2025, 15, 672. https://doi.org/10.3390/ani15050672
Esteso MC, Toledano-Díaz A, Castaño C, Higes M, Martín-Hernández R, López-Goya A, De la Rúa P, Martínez-Madrid B, Santiago-Moreno J. Storage Conditions of Sperm Samples and Gametic Characterization by Sperm Head Morphometry in Drones (Apis mellifera). Animals. 2025; 15(5):672. https://doi.org/10.3390/ani15050672
Chicago/Turabian StyleEsteso, Milagros Cristina, Adolfo Toledano-Díaz, Cristina Castaño, Mariano Higes, Raquel Martín-Hernández, Agustin López-Goya, Pilar De la Rúa, Belén Martínez-Madrid, and Julián Santiago-Moreno. 2025. "Storage Conditions of Sperm Samples and Gametic Characterization by Sperm Head Morphometry in Drones (Apis mellifera)" Animals 15, no. 5: 672. https://doi.org/10.3390/ani15050672
APA StyleEsteso, M. C., Toledano-Díaz, A., Castaño, C., Higes, M., Martín-Hernández, R., López-Goya, A., De la Rúa, P., Martínez-Madrid, B., & Santiago-Moreno, J. (2025). Storage Conditions of Sperm Samples and Gametic Characterization by Sperm Head Morphometry in Drones (Apis mellifera). Animals, 15(5), 672. https://doi.org/10.3390/ani15050672












