Effects of Follicular Fluid and Serum Supplementation on Cumulus Cell Expansion and Nuclear Progression of Guinea Pig Oocytes, Using a Baseline Medium Established with Bovine Oocytes
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Media
2.2. Experiment 1: Effects of Three In Vitro Maturation Media on Cumulus Cell Expansion and IVM Rate of Bovine Oocytes
2.2.1. Oocyte Collection and Classification
2.2.2. In Vitro Maturation (IVM)
2.2.3. Cumulus Oocyte Complex Expansion Evaluation
2.2.4. Fixation and Cell Staining
2.3. Experiment 2: Effects of Estrous Guinea Pig Serum and Estrous Guinea Pig Follicular Fluid on the IVM Rate
2.3.1. Estrous Guinea Pig Serum and Follicular Fluid
2.3.2. Guinea Pig Oocyte Collection and Classification
2.3.3. In Vitro Maturation
2.4. Statistical Analysis
3. Results
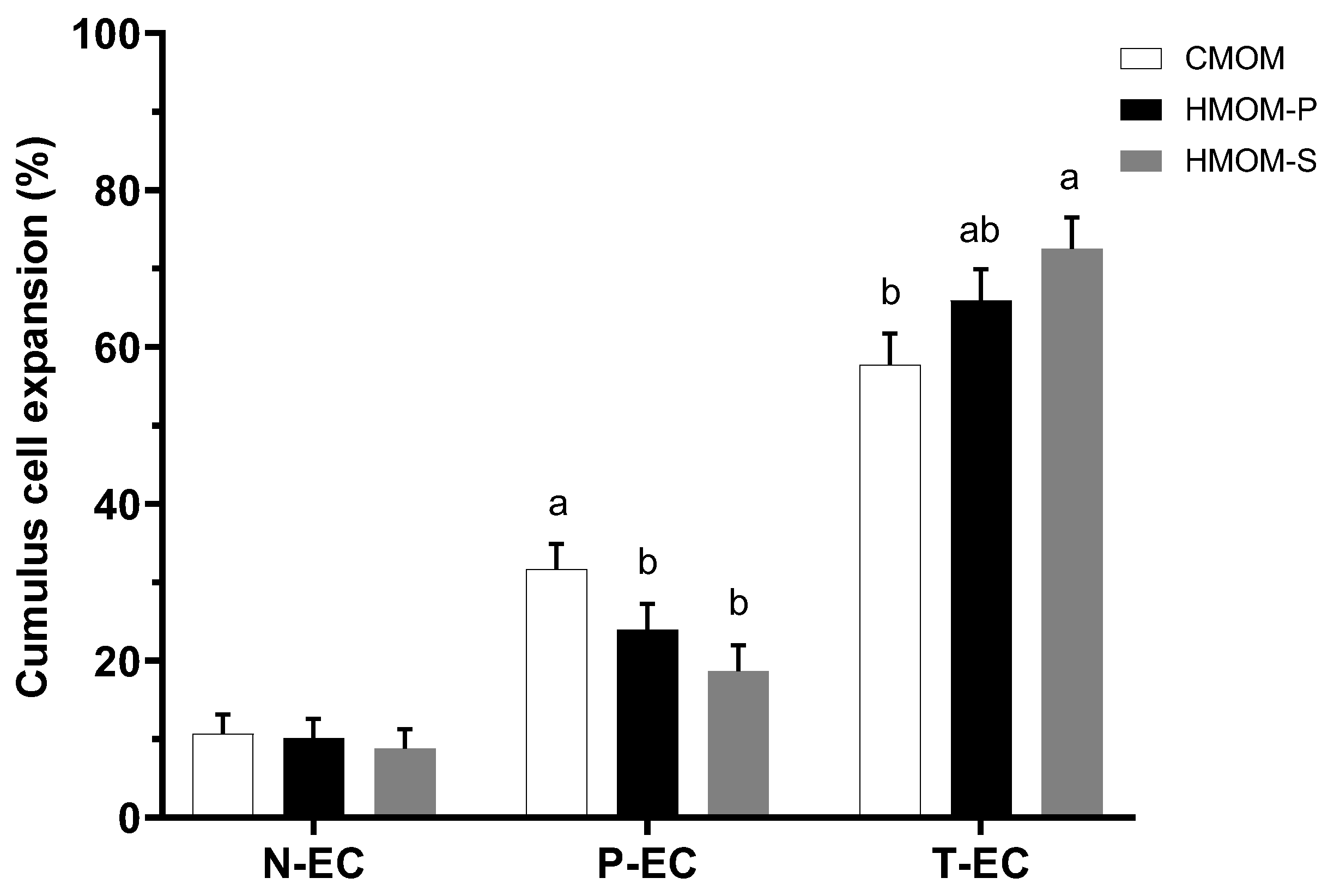
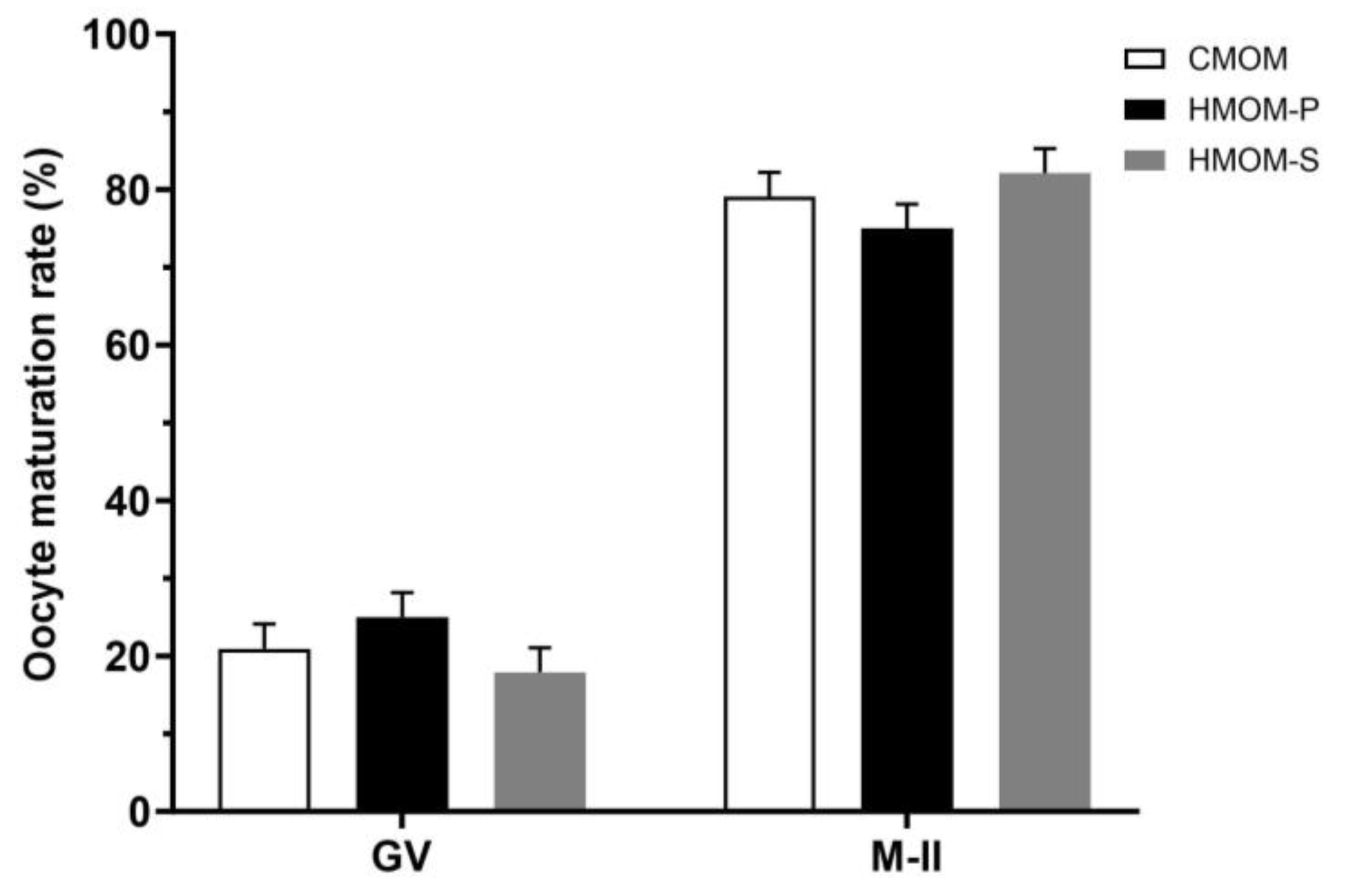
3.1. Effects of Three In Vitro Maturation Media on Cumulus Cell Expansion and IVM Rate of Bovine Oocytes
3.2. Experiment 2: Effects of Serum and Follicular Fluid from Estrous Guinea Pigs on Cumulus Cell Expansion and IVM Rate of Guinea Pig Oocytes
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sandweiss, D.; Wing, E. Ritual Rodents: The Guinea Pigs of Chincha, Peru. J. Field Archaeol. 1997, 24, 47–58. [Google Scholar] [CrossRef]
- Aphrodita, A.; Sentono, D.N.; Fitria, L. Analysis of Carcass Weight and Proximate Composition as Guinea Pig [Cavia Porcellus (Linnaeus, 1758)] Meat Quality Indicator. BIO Web Conf. 2024, 94, 06004. [Google Scholar] [CrossRef]
- Pinchao-Pinchao, Y.; Serna-Cock, L.; Osorio-Mora, O.; Tirado, D.F. Guinea Pig Breeding and Its Relation to Sustainable Food Security and Sovereignty in South America: Nutrition, Health, and Production Challenges. CyTA J. Food 2024, 22, 2392886. [Google Scholar] [CrossRef]
- Avilés, D.F.; Martínez, A.M.; Landi, V.; Delgado, J.V. El Cuy (Cavia porcellus): Un Recurso Andino de Interés Agroalimentario The Guinea Pig (Cavia Porcellus): An Andean Resource of Interest as an Agricultural Food Source. In Animal Genetic Resources; Cambridge University Press: Cambridge, UK, 2014; Volume 55, pp. 87–91. [Google Scholar] [CrossRef]
- Grégoire, A.; Allard, A.; Huamán, E.; León, S.; Silva, R.M.; Buff, S.; Berard, M.; Joly, T. Control of the Estrous Cycle in Guinea-Pig (Cavia porcellus). Theriogenology 2012, 78, 842–847. [Google Scholar] [CrossRef]
- Suzuki, O.; Koura, M.; Noguchi, Y.; Takano, K.; Yamamoto, Y.; Matsuda, J. Optimization of Superovulation Induction by Human Menopausal Gonadotropin in Guinea Pigs Based on Follicular Waves and FSH-Receptor Homologies. Mol. Reprod. Dev. 2003, 64, 219–225. [Google Scholar] [CrossRef]
- Wang, J.; Liu, Z.; Sun, Q.; Xia, S.; Cui, J.; Yang, L.; An, L.; Zhang, J.; Su, L.; Su, Y.; et al. Combined Treatment with Cysteamine and Leukemia Inhibitory Factor Promotes Guinea Pig Oocyte Meiosis in Vitro. Am. J. Transl. Res. 2019, 11, 7479–7491. [Google Scholar]
- Shi, F.; Mochida, K.; Suzuki, O.; Matsuda, J.; Ogura, A.; Tsonis, C.G.; Watanabe, G.; Suzuki, A.K.; Taya, K. Development of Embryos in Superovulated Guinea Pigs Following Active Immunization against the Inhibin A-Subunit. Endocr. J. 2000, 47, 451–459. [Google Scholar] [CrossRef][Green Version]
- Shi, F.; Watanabe, G.; Trewin, A.L.; Hutz, R.J.; Taya, K. Localization of Ovarian Inhibin/Activin Subunits in Follicular Dominance during the Estrous Cycle of Guinea Pigs. Zool. Sci. 2000, 17, 1311–1320. [Google Scholar] [CrossRef]
- Cañón-Beltrán, K.; García-García, R.M.; Cajas, Y.N.; Fierro, N.; Lorenzo, P.L.; Arias-Álvarez, M. Improvement of Oocyte Competence and in Vitro Oocyte Maturation with EGF and IGF-I in Guinea Pig Model. Theriogenology 2024, 214, 206–214. [Google Scholar] [CrossRef]
- Demetrio, D.; Benedetti, E.; Demetrio, C.G.B.; Fonseca, J.; Oliveira, M.; Magalhaes, A.; dos Santos, R.M. How Can We Improve Embryo Production and Pregnancy Outcomes of Holstein Embryos Produced in Vitro? (12 Years of Practical Results at a California Dairy Farm). Anim. Reprod. 2020, 17, e20200053. [Google Scholar] [CrossRef]
- Falchi, L.; Ledda, S.; Zedda, M.T. Embryo Biotechnologies in Sheep: Achievements and New Improvements. Reprod. Domest. Anim. 2022, 57, 22–33. [Google Scholar] [CrossRef]
- Garcia-Canovas, M.; Parrilla, I.; Cuello, C.; Gil, M.A.; Martinez, E.A. Swine in Vitro Embryo Production: Potential, Challenges, and Advances. Anim. Reprod. Sci. 2024, 270, 107600. [Google Scholar] [CrossRef]
- Hamze, J.G.; Peris-Frau, P.; Galiano-Cogolludo, B.; Tomás-Almenar, C.; Santiago-Moreno, J.; Bermejo-Álvarez, P. Efficient and Repeatable in Vitro Fertilization in Rabbits. Theriogenology 2024, 217, 64–71. [Google Scholar] [CrossRef]
- Lee, S.H.; Liu, X.; Jimenez-Morales, D.; Rinaudo, P.F. Murine Blastocysts Generated by in Vitro Fertilization Show Increased Warburg Metabolism and Altered Lactate Production. Elife 2022, 11, e79153. [Google Scholar] [CrossRef]
- Yao, M.; Cheng, W.; Liu, L.; Zheng, H.; Gu, W.; Miao, F.; Zhang, J.; Wang, L.; Su, Y.; Liu, Y.; et al. Relationship between Chromatin Configuration and in Vitro Maturation Ability in Guinea Pig Oocytes. Vet. Med. Sci. 2021, 7, 2410–2417. [Google Scholar] [CrossRef]
- Yao, M.; Gong, Z.; Xu, W.; Shi, X.; Liu, X.; Tang, Y.; Xuan, S.; Su, Y.; Xu, X.; Luo, M.; et al. Establishment and Optimization of an in Vitro Guinea Pig Oocyte Maturation System. PLoS ONE 2023, 18, e0285016. [Google Scholar] [CrossRef]
- Krisher, R.L. In Vivo and In Vitro Environmental Effects on Mammalian Oocyte Quality. Annu. Rev. Anim. Biosci. 2013, 1, 393–417. [Google Scholar] [CrossRef]
- Rizos, D.; Ward, F.; Duffy, P.; Boland, M.P.; Lonergan, P. Consequences of Bovine Oocyte Maturation, Fertilization or Early Embryo Development in Vitro versus in Vivo: Implications for Blastocyst Yield and Blastocyst Quality. Mol. Reprod. Dev. 2002, 61, 234–248. [Google Scholar] [CrossRef]
- Ferreira, E.M.; Vireque, A.A.; Adona, P.R.; Meirelles, F.V.; Ferriani, R.A.; Navarro, P.A.A.S. Cytoplasmic Maturation of Bovine Oocytes: Structural and Biochemical Modifications and Acquisition of Developmental Competence. Theriogenology 2009, 71, 836–848. [Google Scholar] [CrossRef]
- Lonergan, P.; Fair, T. Maturation of Oocytes in Vitro. Annu. Rev. Anim. Biosci. 2016, 4, 255–268. [Google Scholar] [CrossRef]
- Ali, A.; Benkhalifa, M.; Miron, P. In-Vitro Maturation of Oocytes: Biological Aspects. Reprod. Biomed. Online 2006, 13, 437–446. [Google Scholar] [CrossRef] [PubMed]
- Chronopoulou, E.; Harper, J.C. IVF Culture Media: Past, Present and Future. Hum. Reprod. Update 2015, 21, 39–55. [Google Scholar] [CrossRef] [PubMed]
- Somfai, T.; Inaba, Y.; Watanabe, S.; Geshi, M.; Nagai, T. Follicular Fluid Supplementation during in Vitro Maturation Promotes Sperm Penetration in Bovine Oocytes by Enhancing Cumulus Expansion and Increasing Mitochondrial Activity in Oocytes. Reprod. Fertil. Dev. 2012, 24, 743. [Google Scholar] [CrossRef] [PubMed]
- Leivas, F.G.; Brum, D.S.; Fialho, S.S.; Saliba, W.P.; Alvim, M.T.T.; Bernardi, M.L.; Rubin, M.I.B.; Silva, C.A.M. Fetal Calf Serum Enhances in Vitro Production of Bos Taurus Indicus Embryos. Theriogenology 2011, 75, 429–433. [Google Scholar] [CrossRef]
- Richter, K.S. The Importance of Growth Factors for Preimplantation Embryo Development and In-Vitro Culture. Curr. Opin. Obstet. Gynecol. 2008, 20, 292–304. [Google Scholar] [CrossRef]
- Ali, A.; Sirard, M.-A. The Effects of 17β-Estradiol and Protein Supplement on the Response to Purified and Recombinant Follicle Stimulating Hormone in Bovine Oocytes. Zygote 2002, 10, 65–71. [Google Scholar] [CrossRef]
- Jagiello, G.M. Some Cytologic Aspects of Meiosis in Female Guinea Pig. Chromosoma 1969, 27, 95–101. [Google Scholar] [CrossRef]
- Yanagimachi, R. Maturation and Fertilization in Vitro of Guinea-Pig Ovarian Oocytes. Reproduction 1974, 38, 485–488. [Google Scholar] [CrossRef][Green Version]
- Kim, K.S.; Mitsumizo, N.; Fujita, K.; Utsumi, K. The Effects of Follicular Fluid on in Vitro Maturation, Oocyte Fertilization and the Development of Bovine Embryos. Theriogenology 1996, 45, 787–799. [Google Scholar] [CrossRef]
- Dell’Aquila, M.E.; Cho, Y.S.; Minoia, P.; Traina, V.; Lacalandra, G.M.; Maritato, F. Effects of Follicular Fluid Supplementation of In-Vitro Maturation Medium on the Fertilization and Development of Equine Oocytes after in- Vitro Fertilization or Intracytoplasmic Sperm Injection. Hum. Reprod. 1997, 12, 2766–2772. [Google Scholar] [CrossRef]
- Park, J.-E.; Lee, S.-H.; Hwangbo, Y.; Park, C.-K. Porcine Follicular Fluid Derived from > 8 Mm Sized Follicles Improves Oocyte Maturation and Embryo Development during in Vitro Maturation of Pigs. Zygote 2021, 29, 27–32. [Google Scholar] [CrossRef] [PubMed]
- Hawk, H.W.; Wall, R.J. Improved Yields of Bovine Blastocysts from in Vitro-Produced Oocytes. I. Selection of Oocytes and Zygotes. Theriogenology 1994, 41, 1571–1583. [Google Scholar] [CrossRef]
- Ayala Guanga, L.; Samaniego Campoverde, J.; Argudo Garzón, D.; Perea Brugal, M.; Perea Ganchou, F.; Rodas Carpio, E.; Nieto Escandón, P. El Intervalo de Tiempo Entre La Estimulación Ovárica Con FSH/LH y La Colecta Afecta La Cantidad, Calidad y Capacidad de Desarrollo de Los Ovocitos Recuperados de Novillas Criollas Ecuatorianas. Rev. De Investig. Vet. Del Perú 2020, 31, e17571. [Google Scholar] [CrossRef]
- Lorenzo, P.L.; Illera, M.J.; Illera, J.C.; Illera, M. Enhancement of Cumulus Expansion and Nuclear Maturation during Bovine Oocyte Maturation in Vitro by the Addition of Epidermal Growth Factor and Insulin-like Growth Factor I. Reproduction 1994, 101, 697–701. [Google Scholar] [CrossRef]
- Di Rienzo, J.A.; Casanoves, F.; Balzarini, M.G.; González, L.; Tablada, M.; Robledo, C.W. InfoStat, Versión 2008, Primera Edición, Manual del Usuario; Editorial Brujas, Grupo InfoSat, Facultad de Ciencias Agropecuarias, Universidad Nacional de Córdoba: Córdoba, Argentina, 2008.
- Richani, D.; Gilchrist, R.B. The Epidermal Growth Factor Network: Role in Oocyte Growth, Maturation and Developmental Competence. Hum. Reprod. Update 2018, 24, 1–14. [Google Scholar] [CrossRef]
- Bao, B.; Wang, J.; Li, Y.; Feng, F.; Ji, Z.; Luoreng, Z.; Wang, X. Molecular Regulation Mechanism of Oocyte Maturation in Beef Cattle. Biocell 2023, 47, 1509–1518. [Google Scholar] [CrossRef]
- Cañón-Beltrán, K.; Cajas, Y.; Garcia-Garcia, R.; Lorenzo, P.; Carrera, R.; Rebollar, P.; Arias-Álvarez, M. Effect of Epidermal Growth Factor on Nuclear and Cytoplasmic in Vitro Maturation of Guinea Pig Oocytes. In Proceedings of the 29th Annual Meeting of the Brazilian Embryo Technology Society (SBTE), Gramado, RS, Brazil, 20–23 August 2015; Volume 12, p. 793. [Google Scholar]
- Turathum, B.; Gao, E.M.; Chian, R.C. The Function of Cumulus Cells in Oocyte Growth and Maturation and in Subsequent Ovulation and Fertilization. Cells 2021, 10, 2292. [Google Scholar] [CrossRef]
- Lee, D.Y.; Lee, S.Y.; Yun, S.H.; Jeong, J.W.; Kim, J.H.; Kim, H.W.; Choi, J.S.; Kim, G.D.; Joo, S.T.; Choi, I.; et al. Review of the Current Research on Fetal Bovine Serum and the Development of Cultured Meat. Food Sci. Anim. Resour. 2022, 42, 775. [Google Scholar] [CrossRef]
- Liu, S.; Yang, W.; Li, Y.; Sun, C. Fetal Bovine Serum, an Important Factor Affecting the Reproducibility of Cell Experiments. Sci. Rep. 2023, 13, 1–8. [Google Scholar] [CrossRef]
- Puri, G.; Chaudhary, S.S.; Singh, V.K.; Sharma, A.K. Effects of Fetal Bovine Serum and Estrus Buffalo Serum on Maturation of Buffalo (Bubalus Bubalis) Oocytes in Vitro. Vet. World 2015, 8, 143. [Google Scholar] [CrossRef]
- Chen, L.; Mao, S.J.T.; Larsen, W.J. Identification of a Factor in Fetal Bovine Serum That Stabilizes the Cumulus Extracellular Matrix. A Role for a Member of the Inter-Alpha-Trypsin Inhibitor Family. J. Biol. Chem. 1992, 267, 12380–12386. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Mao, S.J.T.; McLean, L.R.; Powers, R.W.; Larsen, W.J. Proteins of the Inter-Alpha-Trypsin Inhibitor Family Stabilize the Cumulus Extracellular Matrix through Their Direct Binding with Hyaluronic Acid. J. Biol. Chem. 1994, 269, 28282–28287. [Google Scholar] [CrossRef] [PubMed]
- Lonergan, P.; Carolan, C.; Van Langendonckt, A.; Donnay, I.; Khatir, H.; Mermillod, P. Role of Epidermal Growth Factor in Bovine Oocyte Maturation and Preimplantation Embryo Development in Vitro. Biol. Reprod. 1996, 54, 1420–1429. [Google Scholar] [CrossRef] [PubMed]
- Del Collado, M.; Saraiva, N.Z.; Lopes, F.L.; Gaspar, R.C.; Padilha, L.C.; Costa, R.R.; Rossi, G.F.; Vantini, R.; Garcia, J.M. Influence of Bovine Serum Albumin and Fetal Bovine Serum Supplementation during in Vitro Maturation on Lipid and Mitochondrial Behaviour in Oocytes and Lipid Accumulation in Bovine Embryos. Reprod. Fertil. Dev. 2016, 28, 1721–1732. [Google Scholar] [CrossRef]
- Fernández-Montoro, A.; Angel-Velez, D.; Benedetti, C.; Azari-Dolatabad, N.; Pascottini, O.B.; Van Soom, A.; Pavani, K.C. Alternative Culture Systems for Bovine Oocyte In Vitro Maturation: Liquid Marbles and Differentially Shaped 96-Well Plates. Animals 2023, 13, 1635. [Google Scholar] [CrossRef] [PubMed]
- Sithole, S.M.; Mphaphathi, M.L.; Sebopela, M.D.; Nedambale, T.L. Comparison of in Vitro Maturation Media on Cattle Oocytes after in Vitro Embryo Production. Am. J. Anim. Vet. Sci. 2023, 18, 27–39. [Google Scholar] [CrossRef]
- Avery, B.; Strøbech, L.; Jacobsen, T.; Bøgh, I.B.; Greve, T. In Vitro Maturation of Bovine Cumulus-Oocyte Complexes in Undiluted Follicular Fluid: Effect on Nuclear Maturation, Pronucleus Formation and Embryo Development. Theriogenology 2003, 59, 987–999. [Google Scholar] [CrossRef]
- Andrade, G.M.; del Collado, M.; Meirelles, F.V.; da Silveira, J.C.; Perecin, F. Intrafollicular Barriers and Cellular Interactions during Ovarian Follicle Development. Anim. Reprod. 2019, 16, 485. [Google Scholar] [CrossRef]
- Azari-Dolatabad, N.; Raes, A.; Pavani, K.C.; Asaadi, A.; Angel-Velez, D.; Van Damme, P.; Leroy, J.L.M.R.; Van Soom, A.; Pascottini, O.B. Follicular Fluid during Individual Oocyte Maturation Enhances Cumulus Expansion and Improves Embryo Development and Quality in a Dose-Specific Manner. Theriogenology 2021, 166, 38–45. [Google Scholar] [CrossRef]
- Zhang, Y.; He, C.; He, Y.; Zhu, Z. Follicular Fluid Metabolomics: Tool for Predicting IVF Outcomes of Different Infertility Causes. Reprod. Sci. 2024, 31, 1–14. [Google Scholar] [CrossRef]
- Revelli, A.; Piane, L.D.; Casano, S.; Molinari, E.; Massobrio, M.; Rinaudo, P. Follicular Fluid Content and Oocyte Quality: From Single Biochemical Markers to Metabolomics. Reprod. Biol. Endocrinol. 2009, 7, 40. [Google Scholar] [CrossRef] [PubMed]
- Eppig, J.J. Coordination of Nuclear and Cytoplasmic Oocyte Maturation in Eutherian Mammals. Reprod. Fertil. Dev. 1996, 8, 485–489. [Google Scholar] [CrossRef] [PubMed]
- Dell’Aquila, M.E.; Caillaud, M.; Maritato, F.; Martoriati, A.; Gérard, N.; Aiudi, G.; Minoia, P.; Goudet, G. Cumulus Expansion, Nuclear Maturation and Connexin 43, Cyclooxygenase-2 and FSH Receptor MRNA Expression in Equine Cumulus-Oocyte Complexes Cultured in Vitro in the Presence of FSH and Precursors for Hyaluronic Acid Synthesis. Reprod. Biol. Endocrinol. 2004, 2, 44. [Google Scholar] [CrossRef] [PubMed][Green Version]
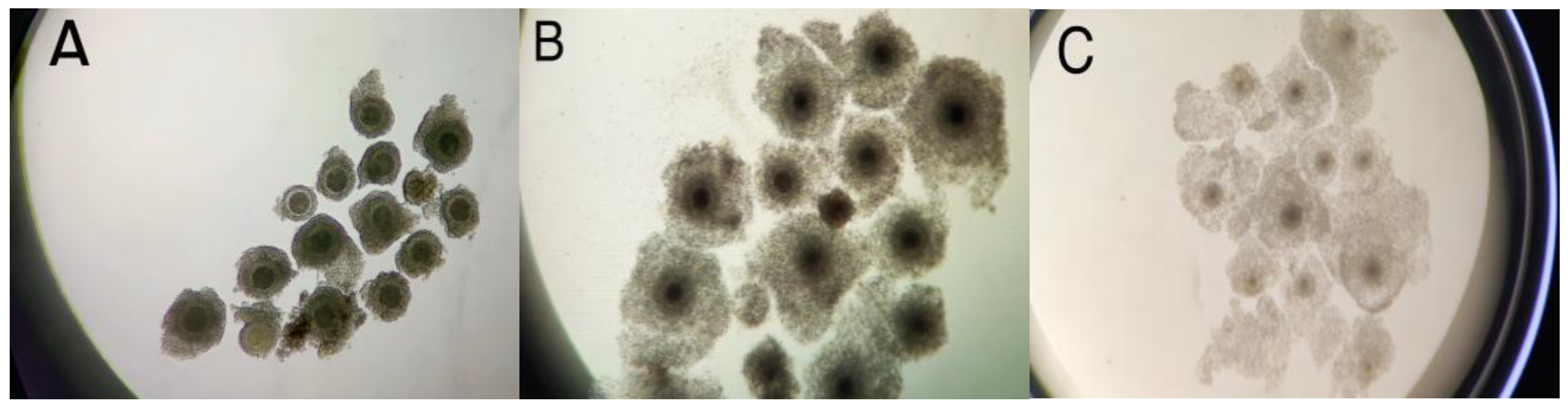
| Component | CMOM (Commercial) | HMOM-P | HMOM-S |
|---|---|---|---|
| Base medium | Not specified | TCM-199 with Earle’s salts | TCM-199 with Earle’s salts |
| FBS (10%) | Not specified | ✓ | ✓ |
| Sodium pyruvate (0.2 mM) | Not specified | ✓ | ✓ |
| FSH (25 μg/mL) | Not specified | ✓ | ✓ |
| LH (5 μg/mL) | Not specified | ✓ | ✓ |
| Estradiol-17β (5 μg/mL) | Not specified | ✓ | ✓ |
| Gentamicin (50 μg/mL) | Not specified | ✓ | ✓ |
| L-glutamine (2 mM) | Not specified | ✓ | ✗ |
| Cysteamine (100 μM) | Not specified | ✓ | ✗ |
| EGF (30 ng/mL) | Not specified | ✗ | ✓ |
| Oocyte Type | Control (no egpFF) | egpFF 5% | egpFF 10% | egpFF 20% |
|---|---|---|---|---|
| A | 43.3 ± 4.82 b | 68.8 ± 6.22 A; a | 76.3 ± 5.39 A; a | 80.9 ± 6.22 A; a |
| B | 43.5 ± 4.82 b | 55.1 ± 6.22 A; b | 70.6 ± 5.39 A; a | 65.9 ± 6.22 A; a |
| C | 42.9 ± 4.82 | 46.7 ± 6.22 B | 44.1 ± 5.39 B | 53.1 ± 6.22 B |
| Oocyte Type | Control (no egpFF) | egpFF 5% | egpFF 10% | egpFF 20% |
|---|---|---|---|---|
| A | 23.8 ± 3.14 b | 41.8 ± 3.51 A; a | 51.4 ± 3.51 A; a | 52.2 ± 4.06 A; a |
| B | 14.1 ± 3.14 | 24.3 ± 4.06 B | 27.0 ± 3.51 B | 29.4 ± 4.06 B |
| C | 13.4 ± 3.14 | 12.1 ± 4.06 B | 17.3 ± 3.51 B | 18.7 ± 4.97 B |
| Oocyte Type | Control (no egpS) | egpS 5% | egpS 10% | egpS 20% |
|---|---|---|---|---|
| A | 69.7 ± 3.63 A | 84.7 ± 3.98 A | 84.5 ± 3.63 A | 87.4 ± 3.98 A |
| B | 65.9 ± 3.63 A | 85.8 ± 3.98 A | 81.9 ± 3.63 A | 86.4 ± 3.98 A |
| C | 40.1 ± 3.36 B | 38.9 ± 3.36 B | 39.8 ± 3.63 B | 34.0 ± 3.98 B |
| Oocyte Type | Control (no egpS) | egpS 5% | egpS 10% | egpS 20% |
|---|---|---|---|---|
| A | 8.7 ± 2.79 b | 25.9 ± 3.06 A; a | 26.9 ± 2.79 a | 32.5 ± 3.06 a |
| B | 10.0 ± 2.79 b | 25.7 ± 3.06 A; a | 25.7 ± 2.79 a | 29.1 ± 3.06 a |
| C | 6.5 ± 2.79 b | 10.2 ± 2.79 B; b | 18.0 ± 2.79 a | 21.5 ± 3.06 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Samaniego, J.X.; Pesantez, J.L.; Ayala, L.E.; Perea, F.P.; Galarza, D.A.; Dutan, J.B.; Ruiz, S. Effects of Follicular Fluid and Serum Supplementation on Cumulus Cell Expansion and Nuclear Progression of Guinea Pig Oocytes, Using a Baseline Medium Established with Bovine Oocytes. Animals 2025, 15, 666. https://doi.org/10.3390/ani15050666
Samaniego JX, Pesantez JL, Ayala LE, Perea FP, Galarza DA, Dutan JB, Ruiz S. Effects of Follicular Fluid and Serum Supplementation on Cumulus Cell Expansion and Nuclear Progression of Guinea Pig Oocytes, Using a Baseline Medium Established with Bovine Oocytes. Animals. 2025; 15(5):666. https://doi.org/10.3390/ani15050666
Chicago/Turabian StyleSamaniego, Jorge X., José L. Pesantez, Luis E. Ayala, Fernando P. Perea, Diego A. Galarza, Jorge B. Dutan, and Salvador Ruiz. 2025. "Effects of Follicular Fluid and Serum Supplementation on Cumulus Cell Expansion and Nuclear Progression of Guinea Pig Oocytes, Using a Baseline Medium Established with Bovine Oocytes" Animals 15, no. 5: 666. https://doi.org/10.3390/ani15050666
APA StyleSamaniego, J. X., Pesantez, J. L., Ayala, L. E., Perea, F. P., Galarza, D. A., Dutan, J. B., & Ruiz, S. (2025). Effects of Follicular Fluid and Serum Supplementation on Cumulus Cell Expansion and Nuclear Progression of Guinea Pig Oocytes, Using a Baseline Medium Established with Bovine Oocytes. Animals, 15(5), 666. https://doi.org/10.3390/ani15050666





