Post-Fishing Ban Period: The Fish Diversity and Community Structure in the Poyang Lake Basin, Jiangxi Province, China
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
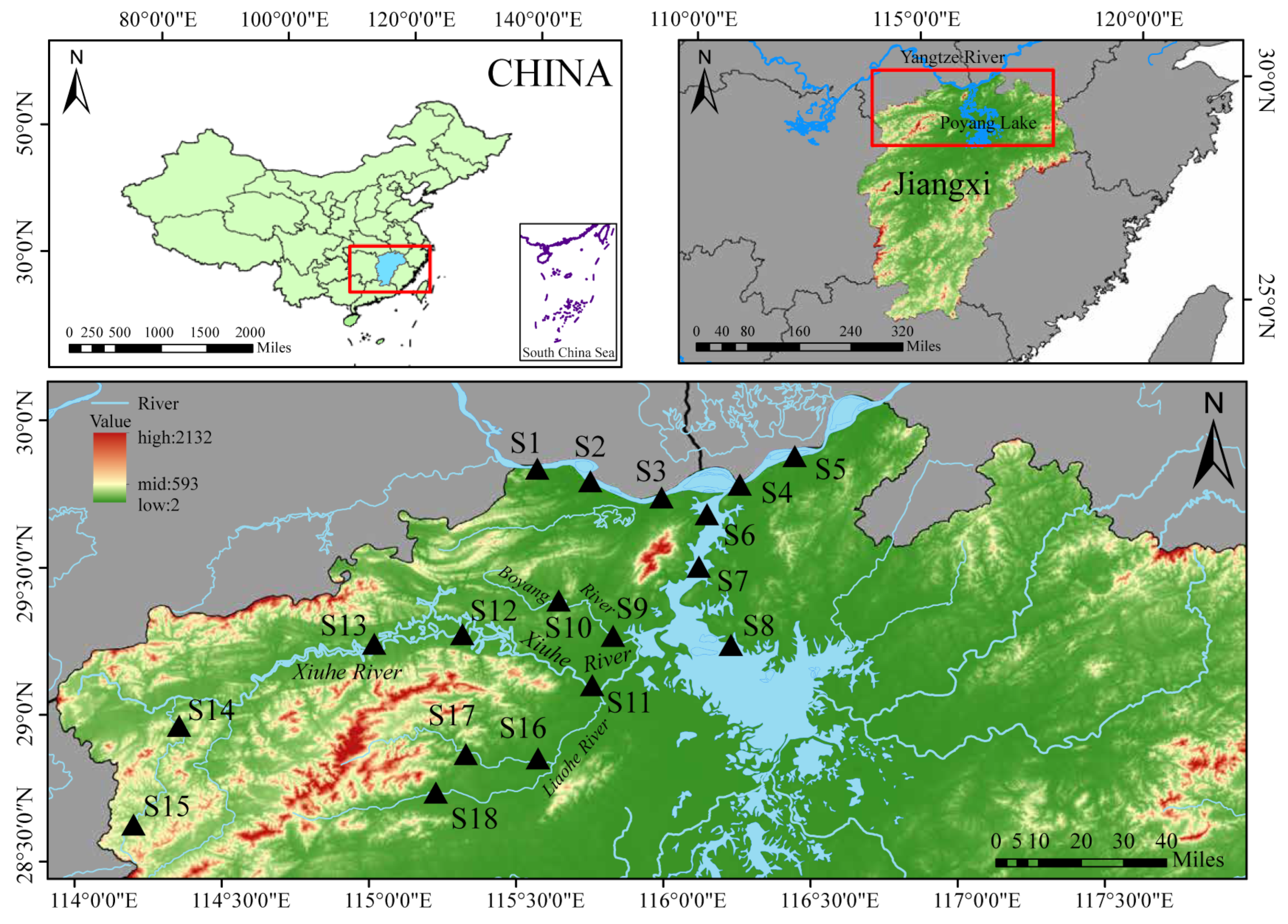
2.1. Study Area
2.2. Material Sources
2.3. Data Analysis
2.3.1. The Index of Relative Importance (IRI)
2.3.2. Similarity Analysis of Community Structure
2.3.3. ANOSIM and SIMPER Analysis
2.3.4. Calculation of the Diversity Index
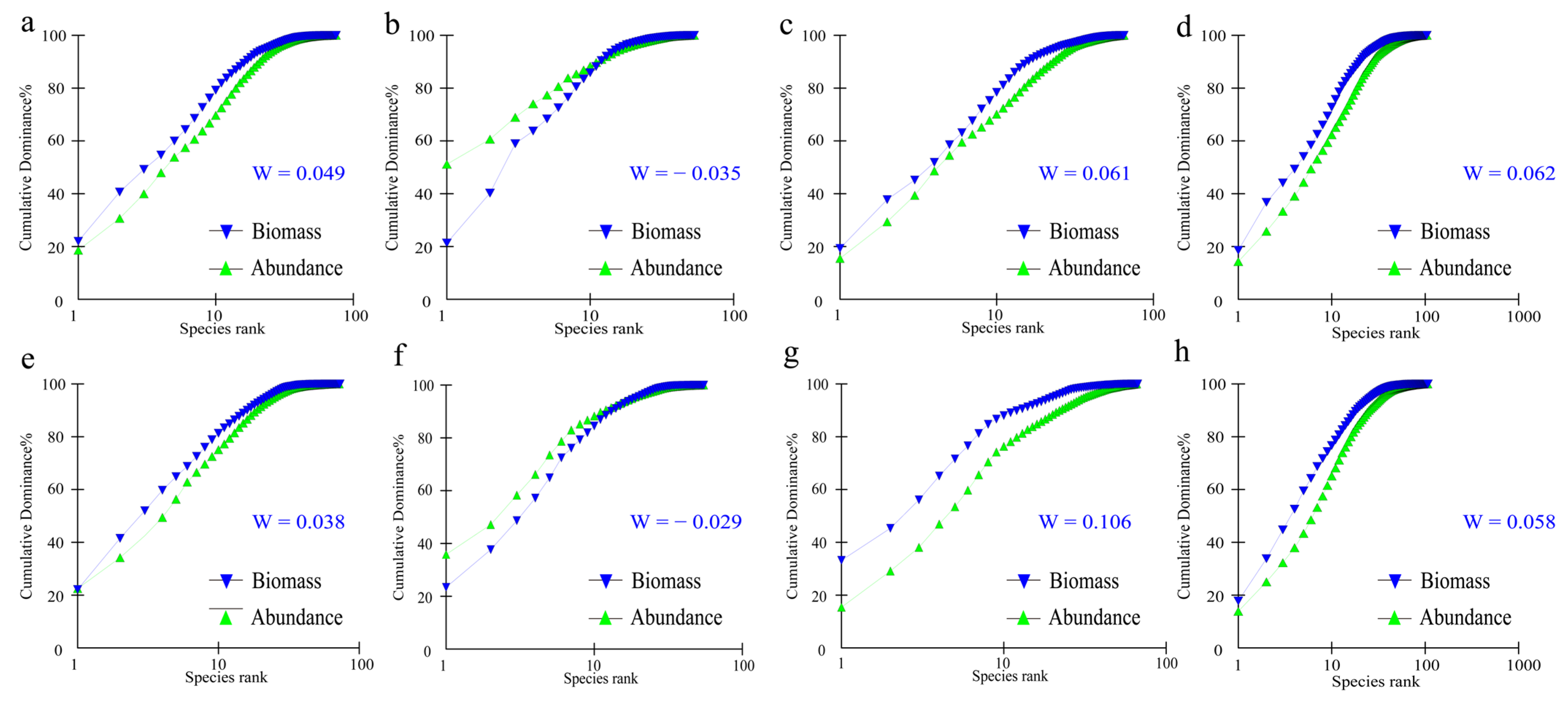
2.3.5. ABC Curve Analysis
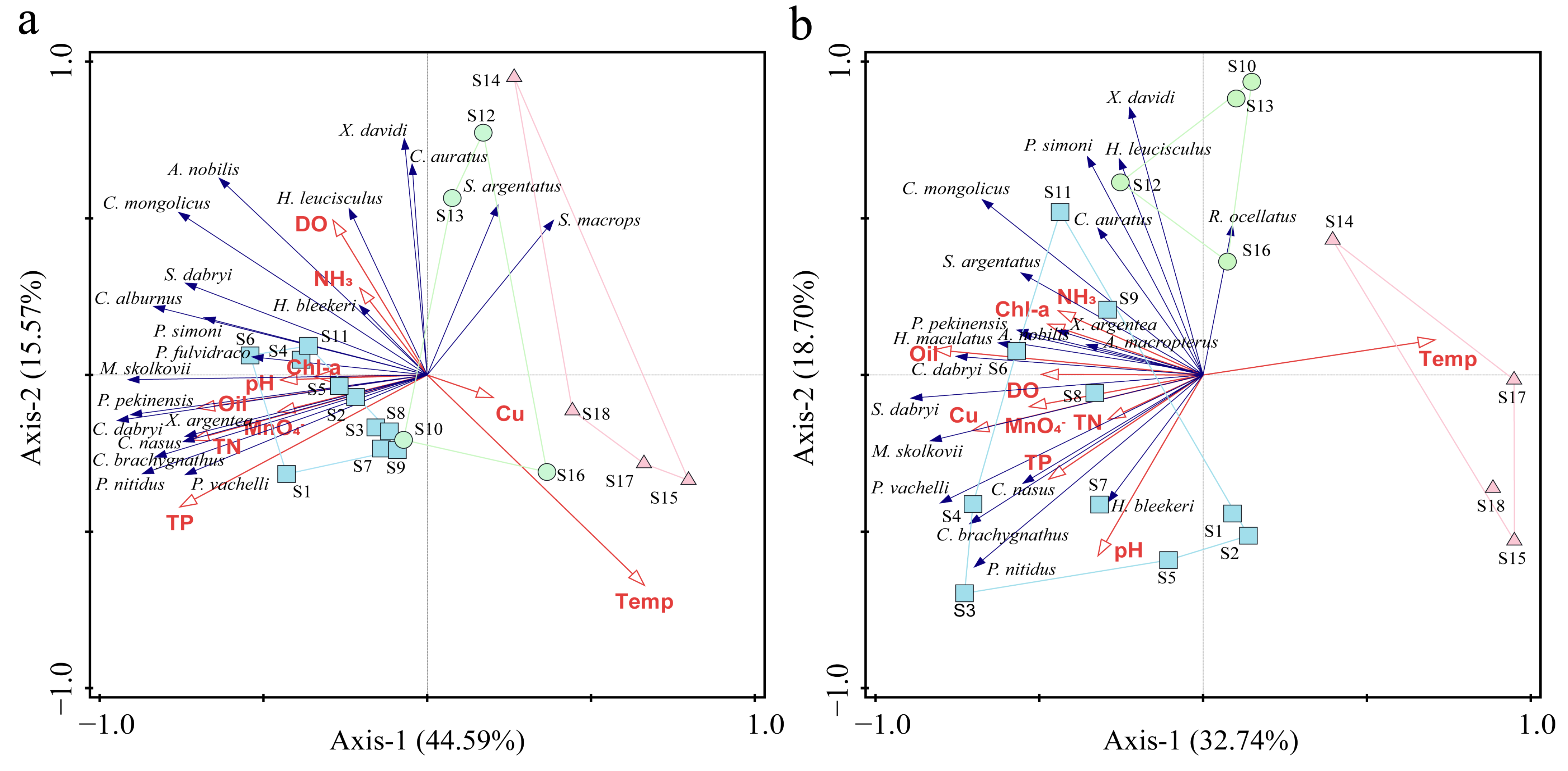
2.3.6. Redundancy Analysis, RDA
3. Results
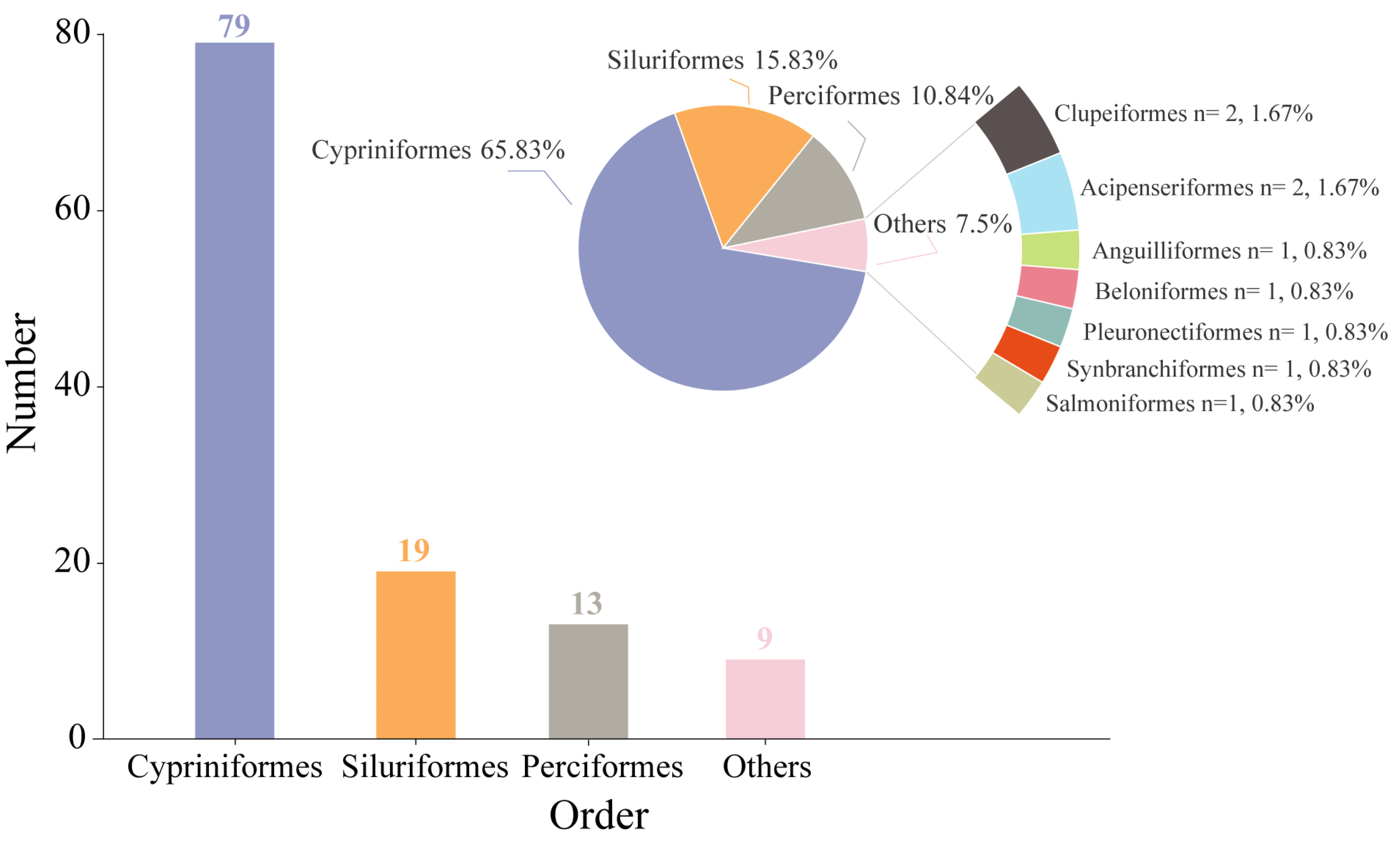
3.1. Species Composition and Dominant Species
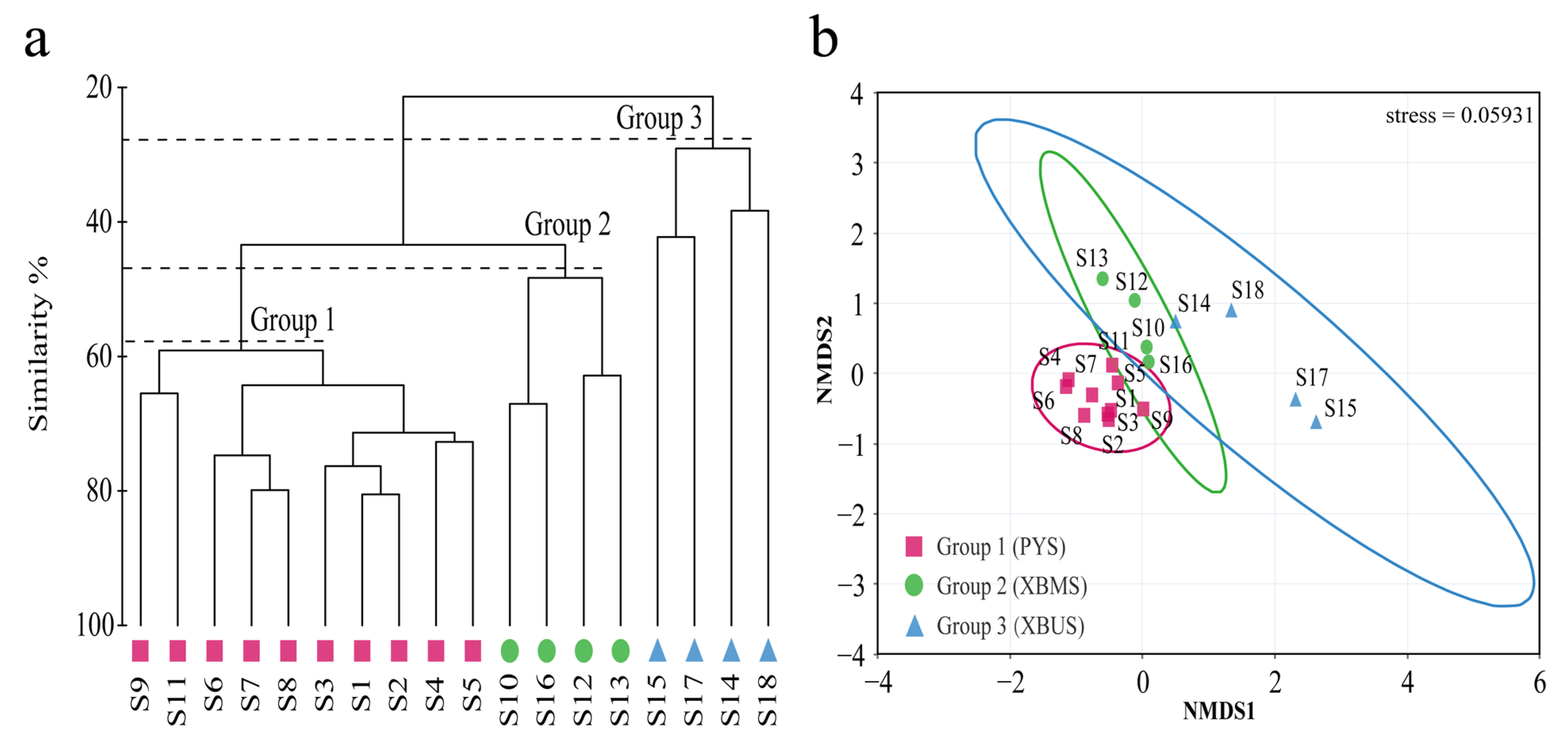
3.2. Community Similarity
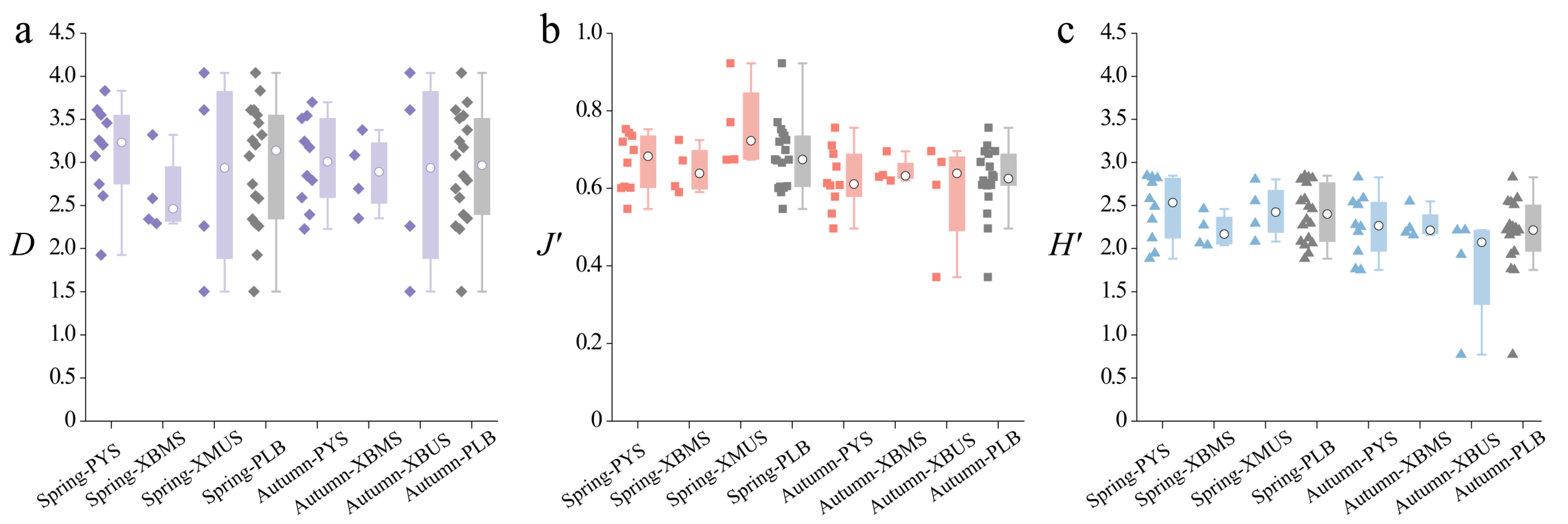
3.3. Diversity Indices
3.4. Community Stability
3.5. Correlation of Communities with Environmental Factors
4. Discussion
4.1. Characteristics of Fish Community Structure at the Early Stage of the Fishing Ban
4.2. Composition of Dominant Species and Regional Variations
4.3. Ecological Adaptation of Fish Communities
4.4. The Relationship Between Fish Communities and Environmental Factors
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Joint Investigation Group on Fishery Resources in Yangtze River. Fishery Resources in the Yangtze River; Ocean Press: Beijing, China, 1990; pp. 50–52. ISBN 7-5027-0985-1. [Google Scholar]
- Yang, H.L.; Shen, L.; He, Y.F.; Tian, H.W.; Gao, L.; Wu, J.M.; Mei, Z.G.; Wei, N.; Wang, L.; Zhu, T.B.; et al. Status of aquatic organisms resources and their environments in Yangtze river system (2017–2021). Aquacult. Fish. 2024, 9, 833–850. [Google Scholar] [CrossRef]
- Das, M.K.; Sharma, A.P.; Vass, K.K.; Tyagi, R.K.; Suresh, V.R.; Naskar, M.; Akolkar, A.B. Fish diversity, community structure and ecological integrity of the tropical River Ganges, India. Aquat. Ecosyst. Health Manag. 2013, 16, 395–407. [Google Scholar] [CrossRef]
- Lennox, R.J.; Crook, D.A.; Moyle, P.B.; Struthers, D.P.; Cooke, S.J. Toward a better understanding of freshwater fish responses to an increasingly drought-stricken world. Rev. Fish Biol. Fish. 2019, 29, 71–92. [Google Scholar] [CrossRef]
- Chen, X.J.; Wang, M.R.; Kroeze, C.; Chen, X.; Ma, L.; Chen, X.P.; Shi, X.J.; Strokal, M. Nitrogen in the Yangtze River Basin: Pollution reduction through coupling crop and livestock production. Environ. Sci. Technol. 2022, 56, 17591–17603. [Google Scholar] [CrossRef]
- Sun, R.; Yao, P.P.; Wang, W.; Yue, B.; Liu, G. Assessment of wetland ecosystem health in the Yangtze and Amazon River Basins. ISPRS Int. J. Geoinf. 2017, 6, 81. [Google Scholar] [CrossRef]
- Zahoor, I.; Mushtaq, A. Water pollution from agricultural activities: A critical global review. Int. J. Chem. Biochem. Sci. 2023, 23, 164–176. [Google Scholar]
- Onukogu, O.A.; Onyebuchi, C.N.; Scott, T.O.; Babawarun, T.; Neye-Akogo, C.; Olagunju, O.A.; Uzougbo, C.G. Impacts of industrial wastewater effluent on Ekerekana Creek and policy recommendations for mitigation. J. Eng. Exact Sci. 2023, 9, 15890-01e. [Google Scholar] [CrossRef]
- Wu, H.; Chen, J.; Xu, J.; Zeng, G.M.; Sang, L.H.; Liu, Q.; Yin, Z.J.; Dai, J.; Yin, D.C.; Liang, J.; et al. Effects of dam construction on biodiversity: A review. J. Clean. Prod. 2019, 221, 480–489. [Google Scholar] [CrossRef]
- Li, B.; Yang, G.; Wan, R.; Lai, X.J.; Wagner, P.D. Impacts of hydrological alteration on ecosystem services changes of a large river-connected lake (Poyang Lake), China. J. Environ. Manag. 2022, 310, 114750. [Google Scholar] [CrossRef]
- Jackson, J.B.C.; Kirby, M.X.; Berger, W.H.; Bjorndal, K.A.; Botsford, L.W.; Bourque, B.J.; Bradbury, R.H.; Cooke, R.; Erlandson, J.; Estes, J.A.; et al. Historical overfishing and the recent collapse of coastal ecosystems. Science 2001, 293, 629–638. [Google Scholar] [CrossRef]
- Pinnegar, J.K.; Engelhard, G.H. The ‘shifting baseline’ phenomenon: A global perspective. Rev. Fish Biol. Fish. 2007, 18, 1–16. [Google Scholar] [CrossRef]
- Castello, L.; Arantes, C.C.; McGrath, D.G.; Stewart, D.J.; Sousa, F.S. Understanding fishing-induced extinctions in the Amazon. Aquat. Conserv. 2015, 25, 447–458. [Google Scholar] [CrossRef]
- Ma, J.; Li, B.; Zhao, J.; Wang, X.F.; Hodgdon, C.T.; Tian, S.Q. Environmental influences on the spatial—Temporal distribution of Coilia nasus in the Yangtze River Estuary. J. Appl. Ichthyol. 2020, 36, 315–325. [Google Scholar] [CrossRef]
- Xuan, Z.Y.; Jiang, T.; Liu, H.B.; Chen, X.B.; Yang, J. Mitochondrial DNA and microsatellite analyses reveal strong genetic differentiation between two types of estuarine tapertail anchovies (Coilia) in Yangtze River Basin, China. Hydrobiologia 2021, 848, 1409–1431. [Google Scholar] [CrossRef]
- Xi, J.P. Speech at the Symposium on Promoting the Development of the Yangtze River Economic Belt. Soc. Forum. 2019, 34, 5–9. [Google Scholar]
- Sun, C.; König, H.J.; Uthes, S.; Cheng, C.; Peng, L.; Karoline, H. Protection effect of overwintering water bird habitat and defining the conservation priority area in Poyang Lake wetland, China. Environ. Res. Lett. 2020, 15, 125013. [Google Scholar] [CrossRef]
- Yuan, L.; Wang, L.; Li, Z.H.; Zhang, M.Q.; Shao, W.; Sheng, G.P. Antibiotic resistance and microbiota in the gut of Chinese four major freshwater carp from retail markets. Environ. Pollut. 2019, 255, 113327. [Google Scholar] [CrossRef]
- Qian, X.E.; Huang, C.G.; Wang, Y.M.; Xiong, F. The status quo of fishery resources of the Poyang Lake and its environmental monitoring. Acta Hydrobiol. Sin. 2002, 26, 612–617. (In Chinese) [Google Scholar] [CrossRef]
- Dai, N.H.; Yao, Z.; You, X.; Huang, C.G.; Chen, F. Study on the utilization and protection of fishery resources in the Poyang Lake. In Proceedings of the Healthy Lakes and Beautiful China—The 3rd China Lakes Forum and the 7th Hubei Science and Technology Forum, Wuhan, China, 24–25 October 2013; pp. 961–969. [Google Scholar]
- Harrison, I.; Abell, R.; Darwall, W.; Thieme, M.L.; Tickner, D.; Timboe, I. The freshwater biodiversity crisis. Science 2018, 362, 1369. [Google Scholar] [CrossRef]
- Castello, L.; McGrath, D.G.; Hess, L.L.; Coe, M.T.; Lefebvre, P.A.; Petry, P.; Macedo, M.N.; Reno, V.F.; Arantes, C.C. The vulnerability of Amazon freshwater ecosystems. Conserv. Lett. 2013, 6, 217–229. [Google Scholar] [CrossRef]
- Lanz, B.; Dietz, S.; Swanson, T. The expansion of modern agriculture and global biodiversity decline: An integrated assessment. Ecol. Econ. 2018, 144, 260–277. [Google Scholar] [CrossRef]
- Cai, Y.J.; Lu, Y.J.; Wu, Z.S.; Chen, Y.W.; Zhang, L.; Lu, Y. Community structure and decadal changes in macrozoobenthic assemblages in Lake Poyang, the largest freshwater lake in China. Knowl. Manag. Aquat. Ecosyst. 2014, 9, 2–21. [Google Scholar] [CrossRef]
- Sun, C.; Zhen, L.; Miah, M.G. Comparison of the ecosystem services provided by China’s Poyang Lake wetland and Bangladesh’s Tanguar Haor wetland. Ecosyst. Serv. 2017, 26, 411–421. [Google Scholar] [CrossRef]
- Schlosser, I.J. Fish community structure and function along two habitat gradients in a headwater stream. Ecol. Monogr. 1982, 52, 395–414. [Google Scholar] [CrossRef]
- Committee for the Protection of Urban Construction and Badlands of the Jiujiang People’s Congress. Record of the Xiuhe River; Jiangxi People’s Publishing House: Nanchang, China, 2011; pp. 2–86. ISBN 9787210047575. [Google Scholar]
- Wang, Y.K.; Xia, Z.Q.; Wang, D. Characterization of hydraulic suitability of Chinese sturgeon (Acipenser sinensis) spawning habitat in the Yangtze River. Hydrol. Process. 2012, 26, 3489–3498. [Google Scholar] [CrossRef]
- Yi, Y.J.; Yang, Z.F.; Zhang, S.H. Ecological influence of dam construction and river-lake connectivity on migration fish habitat in the Yangtze River basin, China. Procedia Environ. Sci. 2010, 2, 1942–1954. [Google Scholar] [CrossRef]
- Pinkas, L.M.; Oliphant, S.; Iverson, L.K. Food Habits of Albacore, Bluefin Tuna and Bonito in Californian Waters. Fish. Bull. 1971, 152, 1–105. [Google Scholar]
- Hart, R.K.; Calver, M.C.; Dickman, C.R. The Index of Relative Importance: An Alternative Approach to Reducing Bias in Descriptive Studies of Animal Diets. Wildl. Res. 2022, 29, 415–421. [Google Scholar] [CrossRef]
- Clarke, K.R. Non-parametric multivariate analyses of changes in community structure. Aust. J. Ecol. 1993, 18, 117–143. [Google Scholar] [CrossRef]
- Clarke, K.R.; Ainsworth, M. A method of linking multivariate community structure to environmental variables. Mar. Ecol. Prog. Ser. 1993, 92, 205–219. [Google Scholar] [CrossRef]
- Warwick, R.M.; Clarke, K.R.; Somerfield, P. K-Dominance Curve; Elsevier: Amsterdam, The Netherlands, 2008; pp. 2055–5057. [Google Scholar]
- Li, Y.Q.; Chen, G.B. Research status of marine fish communities in Chinese sea area. Mod. Fish. Inf. 2008, 23, 16–21. (In Chinese) [Google Scholar]
- Wilhm, J.L. Use of biomass units in Shannon’s formula. Ecology 1968, 49, 153–156. [Google Scholar] [CrossRef]
- Clarke, K.R.; Warwick, R.M. Change in Marine Communities: An Approach to Statistical Analysis and Interpretation, 2nd ed.; Plymouth Marine Laboratory: Devon, UK, 2001; Volume 1, pp. 121–134. ISBN 1855311402-9781855311404. [Google Scholar]
- Clarke, K.R. Comparisons of Dominance Curves. J. Exp. Mar. Biol. Ecol. 1990, 138, 143–157. [Google Scholar] [CrossRef]
- Lepš, J.; Šmilauer, P. Multivariate Analysis of Ecological Data Using CANOCO; Cambridge University Press: Cambridge, UK, 2003; pp. 1–253. ISBN 0-521-81409-X. [Google Scholar]
- Vannote, R.L.; Minshall, G.W.; Cummins, K.W.; Sedell, J.R.; Cushing, C.E. The river continuum concept. Can. J. Fish. Aquat. Sci. 1980, 37, 130–137. [Google Scholar] [CrossRef]
- M, S.A.; Nair, S.M.; Sudheesan, D.; Samanta, S.; Paul, S.K.; Bhowmick, S.; Kumar, V.; Das, B.K. Reconnoitre on ichthyofauna of Mahanadi River of India: Shifting diversity down the river continuum and linking ecological traits with patterns in biodiversity. Environ. Sci. Pollut. Res. 2024, 31, 5684–5698. [Google Scholar]
- Loreau, M. Linking biodiversity and ecosystems: Towards a unifying ecological theory. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2010, 365, 49–60. [Google Scholar] [CrossRef]
- Huang, L.L.; Wu, Z.Q.; Li, J.H. Fish fauna, biogeography and conservation of freshwater fish in Poyang Lake Basin, China. Environ. Biol. Fish. 2013, 96, 1229–1243. [Google Scholar] [CrossRef]
- Zhao, S.; Fang, J.; Peng, C.; Tang, Z.Y.; Piao, S.L. Patterns of fish species richness in China’s lakes. Glob. Ecol. Biogeogr. 2006, 15, 386–394. [Google Scholar] [CrossRef]
- Zhang, T.L.; Li, Z.J. Fish resources and fishery utilization of Lake Poyang. J. Lake Sci. 2007, 19, 434–444. [Google Scholar]
- Yang, J.; Yan, D.; Yang, Q.; Gong, S.H.; Shi, Z.M.; Qiu, Q.J.; Huang, S.L.; Zhou, S.F.; Hu, M.L. Fish species composition, distribution and community structure in the Fuhe River Basin, Jiangxi Province, China. Glob. Ecol. Conserv. 2021, 27, e01559. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, H.; Wu, Z.; Zhao, M.G.; Feng, G.P. Community Structure Characteristics and Changes in Fish Species at Poyang Lake after the Yangtze River Fishing Ban. Fishes 2024, 9, 281. [Google Scholar] [CrossRef]
- Chao, A. Nonparametric estimation of the number of classes in a population. Scand. J. Statist. 1984, 11, 265–270. [Google Scholar]
- Blanchard, F.; LeLoc’h, F.; Hily, C.; Boucher, J. Fishing effects on diversity, size and community structure of the benthic invertebrate and fish megafauna on the Bay of Biscay coast of France. Mar. Ecol. Prog. Ser. 2004, 280, 249–260. [Google Scholar] [CrossRef]
- Yin, S.L.; Yi, Y.J.; Liu, Q.; Luo, Q.Y.; Chen, K.B. A review on effects of human activities on aquatic organisms in the Yangtze River Basin since the 1950s. River 2022, 1, 104–119. [Google Scholar] [CrossRef]
- Chen, T.G.; Wang, Y.; Gardner, C.; Wu, F. Threats and protection policies of the aquatic biodiversity in the Yangtze River. J. Nat. Conserv. 2020, 58, 125931. [Google Scholar] [CrossRef]
- Guo, H.; Hu, Q.; Jiang, T. Annual and seasonal streamflow responses to climate and land-cover changes in the Poyang Lake basin, China. J. Hydrol. 2008, 355, 106–122. [Google Scholar] [CrossRef]
- Ficke, A.D.; Myrick, C.A.; Hansen, L.J. Potential impacts of global climate change on freshwater fisheries. Rev. Fish Biol. Fish. 2007, 17, 581–613. [Google Scholar] [CrossRef]
- Dermond, P.; Melián, C.J.; Brodersen, J. Size-dependent tradeoffs in seasonal freshwater environments facilitate differential salmonid migration. Mov. Ecol. 2019, 7, 40. [Google Scholar] [CrossRef]
- Ordeix, M.; Casals, F. Why and when do freshwater fish migrate? Observations of migration patterns of the native fishes from the Iberian Peninsula (SW Europe). Limnetica 2024, 43, 9–28. [Google Scholar] [CrossRef]
- Yang, G.; Zhang, Q.; Wan, R.; Lai, X.J.; Jiang, X.; Li, L.; Dai, H.C.; Lei, G.C.; Chen, J.C.; Lu, Y.J. Lake hydrology, water quality and ecology impacts of altered river–lake interactions: Advances in research on the middle Yangtze river. Hydrol. Res. 2016, 47, 1–7. [Google Scholar] [CrossRef]
- Mondal, R.; Bhat, A. Temporal and environmental drivers of fish-community structure in tropical streams from two contrasting regions in India. PLoS ONE 2020, 15, e0227354. [Google Scholar] [CrossRef] [PubMed]
- Smith, M.D.; Knapp, A.K. Dominant species maintain ecosystem function with non-random species loss. Ecol. Lett. 2003, 6, 509–517. [Google Scholar] [CrossRef]
- Vilizzi, L.; Tarkan, A.S. Experimental evidence for the effects of common carp (Cyprinus carpio L., 1758) on freshwater ecosystems: A narrative review with management directions for Turkish inland waters. J. Limnol. Freshw. Fish. Res. 2015, 1, 123–149. [Google Scholar]
- Park, J.Y.; Kim, C.H. Habitats and air uptake based on analysis of skin structure of two Korean bullheads, Pseudobagrus brevicorpus and P. koreanus (Pisces; Bagridae). Integr. Biosci. 2007, 11, 155–160. [Google Scholar] [CrossRef]
- Jeppesen, E.; Meerhoff, M.; Holmgren, K.; González-Bergonzoni, I.; Teixeira-de Mello, F.; Declerck, S.A.J.; Meester, L.D.; Søndergaard, M.; Lauridsen, T.L.; Bjerring, R.; et al. Impacts of climate warming on lake fish community structure and potential effects on ecosystem function. Hydrobiologia 2010, 646, 73–90. [Google Scholar] [CrossRef]
- Yan, T.M.; He, J.Y.; Yang, D.Y.; Ma, Z.J.; Chen, H.J.; Zhang, Q.; Deng, F.Q.; Ye, L.Y.; Pu, Y.; Zhang, M.W.; et al. Fish community structure and biomass particle-size spectrum in the upper reaches of the Jinsha River (China). Animals 2022, 12, 3412. [Google Scholar] [CrossRef]
- Li, R.H.; Liu, S.M.; Li, Y.W.; Zhang, G.L.; Ren, J.L.; Zhang, J. Nutrient dynamics in tropical rivers, lagoons, and coastal ecosystems of eastern Hainan Island, South China Sea. Biogeosciences 2014, 11, 481–506. [Google Scholar] [CrossRef]
- Elosegi, A.; Díez, J.; Mutz, M. Effects of hydromorphological integrity on biodiversity and functioning of river ecosystems. Hydrobiologia 2010, 657, 199–215. [Google Scholar] [CrossRef]
- Pretty, J.L.; Harrison, S.S.C.; Shepherd, D.J.; Smith, C.; Hildrew, A.G.; Hey, R.D. River rehabilitation and fish populations: Assessing the benefit of instream structures. J. Appl. Ecol. 2003, 40, 251–265. [Google Scholar] [CrossRef]
- Badyaev, A.V.; Foresman, K.R. Extreme environmental change and evolution: Stress-induced morphological variation is strongly concordant with patterns of evolutionary divergence in shrew mandibles. Proc. R. Soc. Lond. Ser. B Biol. Sci. 2000, 267, 371–377. [Google Scholar] [CrossRef]
- Chen, J.; Chen, J. Hydrological Characteristics of the Yangtze River. In Evolution and Water Resources Utilization of the Yangtze River; Springer: Singapore, 2019; pp. 123–162. ISBN 978-981-13-7872-0. [Google Scholar]
- Webb, P.W. Control of posture, depth, and swimming trajectories of fishes. Integr. Comp. Biol. 2002, 42, 94–101. [Google Scholar] [CrossRef] [PubMed]
- Bracciali, C.; Guzzo, G.; Giacoma, C.; Dean, J.M.; Sarà, G. Fish functional traits are affected by hydrodynamics at small spatial scale. Mar. Environ. Res. 2016, 113, 116–123. [Google Scholar] [CrossRef] [PubMed]
- Pakkasmaa, S.; Piironen, J. Water velocity shapes juvenile salmonids. Evol. Ecol. 2000, 14, 721–730. [Google Scholar] [CrossRef]
- Deng, J.; Zhang, X.C.; Yao, X.Y.; Rao, J.H.; Dai, F.; Wang, H.; Wang, Y.C.; Jiang, W. eDNA metabarcoding reveals differences in fish diversity and community structure in Danjiang River. Sci. Rep. 2024, 14, 29460. [Google Scholar] [CrossRef]
- Sun, L.; Liao, L.; Chen, M.; Li, J.; An, R. Relation between fish morphological differentiation and pressure drag difference. Ecol. Indic. 2023, 156, 111071. [Google Scholar] [CrossRef]
- Reznick, D.; Butler-IV, M.J.; Rodd, H. Life-history evolution in guppies. VII. The comparative ecology of high-and low-predation environments. Am. Nat. 2001, 157, 126–140. [Google Scholar] [CrossRef]
- Yin, H.; Wang, S.; Yang, J.; Chen, S.W.; Hua, Q.; Wang, L.Y.; Yang, Q.J.; Hu, M.L. Fish community and its relationships with environmental variables in the channel connecting Poyang Lake and the Yangtze River. Aquat. Sci. 2024, 86, 59. [Google Scholar] [CrossRef]
- Plagányi, É. Climate change impacts on fisheries. Science 2019, 363, 930–931. [Google Scholar] [CrossRef]
- Zhang, C.; Zhang, Y.Z.; García-Girón, J.; Tan, K.; Wang, L.; Ge, Y.H.; Yan, Y.Z. Signals of potential species associations offer clues about community organisation of stream fish across seasons. Animals 2022, 12, 1721. [Google Scholar] [CrossRef]
- Keppeler, F.W.; Souza, A.C.; Hallwass, G.; Begossi, A.; Almeida, M.C.; Isaac, V.J.; Silvano, R.A.M. Ecological influences of human population size and distance to urban centres on fish communities in tropical lakes. Aquat. Conserv. 2018, 28, 1030–1043. [Google Scholar] [CrossRef]
- Matono, P.; Batista, T.; Sampaio, E.; llhéu, M. Effects of agricultural land use on the ecohydrology of small-medium Mediterranean river basins: Insights from a case study in the south of Portugal. In Land Use—Assessing the Past, Envisioning the Future; IntechOpen: London, UK, 2019; pp. 30–51. ISBN 978-1-83962-073-7. [Google Scholar]
- Pei, Z.; Kang, Y.; Long, F. Research and Development of Inland Green and Smart Ship Technologies in China. Appl. Sci. 2024, 14, 2316. [Google Scholar] [CrossRef]
- Vilizzi, L.; Tarkan, A.S.; Copp, G.H. Experimental evidence from causal criteria analysis for the effects of common carp Cyprinus carpio on freshwater ecosystems: A global perspective. Rev. Fish. Sci. Aquac. 2015, 23, 253–290. [Google Scholar] [CrossRef]
- Helminen, H.; Karjalainen, J.S.; Kurkilahti, M.; Rask, M.; Sarvala, J. Eutrophication and fish biodiversity in Finnish lakes. Verh. Int. Ver. Theor. Angew. Limnol. 2000, 27, 194–199. [Google Scholar] [CrossRef]
- Pulliam, H.R. Sources, sinks, and population regulation. Am. Nat. 1988, 132, 652–661. [Google Scholar] [CrossRef]
- Öckinger, E.; Schweiger, O.; Crist, T.O.; Debinski, D.M.; Krauss, J.; Kuussaari, M.; Petersen, J.D.; Pöyry, J.; Settele, J.; Summerville, K.S.; et al. Life-history traits predict species responses to habitat area and isolation: A cross-continental synthesis. Ecol. Lett. 2010, 13, 969–979. [Google Scholar] [CrossRef]
- Mehmood, M.A.; Qadri, H.; Bhat, R.A.; Rashid, A.; Ganie, S.A.; Dar, G.H.; Rehman, S.U. Heavy metal contamination in two commercial fish species of a trans-Himalayan freshwater ecosystem. Environ. Assess. 2019, 191, 104. [Google Scholar] [CrossRef]
- Guiherme, G.S.; Eric, C.; Torsten, W.; Rüdiger, R.; Ann, A.; Martin, P. Plath Water pollution affects fish community structure and alters evolutionary trajectories of invasive guppies (Poecilia reticulata). Sci. Total Environ. 2020, 730, 138912. [Google Scholar]
| Year | Survey Seasons | Number Stations | Multi-Mesh Gillnets | Tandem Ground Cage | Downtime | ||||
|---|---|---|---|---|---|---|---|---|---|
| Net Length (m) | Net Height (m) | Mesh Size (cm) | Net Length (m) | Net Width\Height (m) | Mesh Size (cm) | ||||
| 2022, 2023 | Spring, Autumn | 18 | 50 | 2 | 2.0 | 18 | 0.45\0.33 | 0.8 | 12 h |
| 50 | 2 | 6.0 | 12 h | ||||||
| 50 | 2 | 10.0 | 12 h | ||||||
| 50 | 2 | 14.0 | 12 h | ||||||
| Species | Spring | Autumn | Total | ||||||
|---|---|---|---|---|---|---|---|---|---|
| N | W | IRI | N | W | IRI | N | W | IRI | |
| Megalobrama skolkovii | 1355 | 738.58 | 1985.67 | 1266 | 540.07 | 1930.99 | 2621 | 1278.66 | 1962.48 |
| Aristichthys nobilis | 366 | 723.07 | 1415.20 | 224 | 331.24 | 856.33 | 590 | 1054.31 | 1171.73 |
| Hemiculter leucisculus | 3680 | 112.62 | 1237.21 | 2615 | 63.51 | 661.89 | 6295 | 176.13 | 1275.56 |
| Hypophthalmichthys molitrix | 245 | 292.93 | 692.86 | 233 | 487.32 | 1324.73 | 478 | 780.26 | 1007.57 |
| Coilia brachygnathus | 2947 | 86.10 | 756.42 | 3255 | 81.41 | 1011.70 | 6202 | 167.50 | 916.35 |
| Xenocypris davidi | 762 | 162.41 | 508.65 | 1213 | 210.23 | 873.17 | 1975 | 372.64 | 828.91 |
| Cyprinus carpio | 208 | 207.85 | 536.19 | 204 | 238.67 | 727.06 | 412 | 446.52 | 640.06 |
| Pelteobagrus nitidus | 1945 | 33.42 | 653.17 | 1700 | 23.91 | 535.59 | 3645 | 57.32 | 639.85 |
| Culter mongolicus | 850 | 133.87 | 555.63 | 711 | 143.66 | 646.60 | 1561 | 277.53 | 633.63 |
| Parabramis pekinensis | 560 | 190.43 | 580.17 | 496 | 136.26 | 513.26 | 1056 | 326.69 | 566.85 |
| Culter dabryi | 953 | 136.20 | 554.28 | 438 | 68.87 | 298.57 | 1391 | 205.08 | 447.24 |
| Squalidus argentatus | 1300 | 17.86 | 519.49 | 695 | 10.62 | 276.03 | 1995 | 28.48 | 421.35 |
| Xenocypris argentea | 542 | 47.14 | 237.68 | 1333 | 57.82 | 589.98 | 1875 | 104.96 | 412.79 |
| Coilia nasus | 1478 | 116.73 | 433.86 | 941 | 91.59 | 351.29 | 2419 | 208.32 | 394.31 |
| Carassius auratus | 455 | 64.34 | 338.52 | 475 | 42.71 | 343.05 | 930 | 107.05 | 341.61 |
| Ctenopharyngodon idellus | 248 | 171.94 | 381.70 | 62 | 43.44 | 94.10 | 310 | 215.38 | 308.29 |
| Saurogobio dabryi | 487 | 10.75 | 180.23 | 1104 | 18.89 | 444.01 | 1591 | 29.65 | 304.73 |
| Pseudobrama simoni | 708 | 19.26 | 269.61 | 658 | 15.52 | 239.46 | 1366 | 34.77 | 272.70 |
| Siniperca chuatsi | 124 | 140.30 | 267.20 | 82 | 61.18 | 157.55 | 206 | 201.49 | 255.89 |
| Culter alburnus | 436 | 71.87 | 311.15 | 122 | 37.83 | 127.48 | 558 | 109.70 | 239.78 |
| Pelteobagrus vachelli | 477 | 47.90 | 271.78 | 322 | 26.99 | 175.86 | 799 | 74.89 | 239.25 |
| Pelteobagrus fulvidraco | 569 | 39.46 | 320.29 | 223 | 13.68 | 85.60 | 792 | 53.14 | 236.73 |
| Acheilognathus macropterus | 243 | 2.36 | 78.05 | 841 | 6.36 | 295.13 | 1084 | 8.72 | 206.92 |
| Hemiculter bleekeri | 772 | 21.17 | 176.60 | 1011 | 3.38 | 147.36 | 1783 | 24.55 | 198.72 |
| Culter oxycephaloides | 85 | 10.76 | 26.70 | 160 | 39.49 | 121.10 | 245 | 50.24 | 74.19 |
| Hemibarbus maculatus | 43 | 7.13 | 17.32 | 309 | 13.75 | 127.83 | 352 | 20.89 | 73.18 |
| Species | Spring | Autumn | ||||
|---|---|---|---|---|---|---|
| PYS | XBMS | XBUS | PYS | XBMS | XBUS | |
| Coilia brachygnathus | 6.42 | 8.80 | ||||
| Pelteobagrus nitidus | 6.12 | 7.23 | ||||
| Megalobrama skolkovii | 6.10 | 4.77 | 6.83 | |||
| Culter mongolicus | 4.89 | 4.38 | 4.26 | 5.29 | ||
| Culter dabryi | 4.83 | |||||
| Coilia nasus | 4.58 | |||||
| Parabramis pekinensis | 4.42 | 4.23 | ||||
| Pelteobagrus vachelli | 4.10 | 4.68 | ||||
| Pseudobrama simoni | 10.02 | 7.38 | ||||
| Squalidus argentatus | 9.20 | 15.67 | 6.55 | |||
| Saurogobio dabryi | 7.51 | 7.42 | 5.90 | |||
| Acheilognathus macropterus | 6.10 | 6.77 | ||||
| Carassius auratus | 5.93 | 10.60 | 5.71 | 19.81 | ||
| Xenocypris davidi | 5.78 | 8.51 | ||||
| Hemiculter leucisculus | 5.72 | 4.14 | ||||
| Culter alburnus | 4.29 | |||||
| Cyprinus carpio | 4.01 | 4.19 | ||||
| Rhodeus ocellatus | 12.18 | |||||
| Opsariichthys bidens | 9.72 | 4.66 | ||||
| Pelteobagrus fulvidraco | 5.98 | 4.19 | ||||
| Sinibrama macrops | 5.30 | 4.10 | ||||
| Acrossocheilus parallens | 4.89 | 12.43 | ||||
| Zacco platypus | 4.16 | |||||
| Acrossocheilus kreyenbergii | 4.02 | 18.80 | ||||
| Siniperca chuatsi | 4.24 | |||||
| Xenocypris argentea | 4.13 | |||||
| Pseudorasbora parva | 21.83 | |||||
| Species | Spring | Autumn | ||||
|---|---|---|---|---|---|---|
| PYS and XBMS | PYS and XBUS | XBMS and XBUS | PYS and XBMS | PYS and XBUS | XBMS and XBUS | |
| Coilia brachygnathus | 5.77 | 4.05 | 5.74 | 4.81 | ||
| Coilia nasus | 4.72 | |||||
| Pseudobrama simoni | 4.15 | 4.53 | ||||
| Hemiculter leucisculus | 4.14 | 4.10 | 4.15 | |||
| Xenocypris davidi | 4.15 | 4.23 | ||||
| Pelteobagrus nitidus | 4.85 | |||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kong, C.; Luo, Y.; Xu, Q.; Zhang, B.; Gao, X.; Wang, X.; Luo, Z.; Luo, Z.; Li, L.; Gong, X. Post-Fishing Ban Period: The Fish Diversity and Community Structure in the Poyang Lake Basin, Jiangxi Province, China. Animals 2025, 15, 433. https://doi.org/10.3390/ani15030433
Kong C, Luo Y, Xu Q, Zhang B, Gao X, Wang X, Luo Z, Luo Z, Li L, Gong X. Post-Fishing Ban Period: The Fish Diversity and Community Structure in the Poyang Lake Basin, Jiangxi Province, China. Animals. 2025; 15(3):433. https://doi.org/10.3390/ani15030433
Chicago/Turabian StyleKong, Chiping, Yulan Luo, Qun Xu, Bao Zhang, Xiaoping Gao, Xianyong Wang, Zhen Luo, Zhengli Luo, Lekang Li, and Xiaoling Gong. 2025. "Post-Fishing Ban Period: The Fish Diversity and Community Structure in the Poyang Lake Basin, Jiangxi Province, China" Animals 15, no. 3: 433. https://doi.org/10.3390/ani15030433
APA StyleKong, C., Luo, Y., Xu, Q., Zhang, B., Gao, X., Wang, X., Luo, Z., Luo, Z., Li, L., & Gong, X. (2025). Post-Fishing Ban Period: The Fish Diversity and Community Structure in the Poyang Lake Basin, Jiangxi Province, China. Animals, 15(3), 433. https://doi.org/10.3390/ani15030433






